Abstract
The National Institute of health and Care Excellence issued guidelines on familial breast cancer screening in 2004. Such guidelines should be uniformly implemented to ensure that members of the same family with the same level of risk, but living in different areas, have the same access to screening. We assessed uptake by creating a short, six question online survey designed to assess compliance in each regional area. We used this to conduct a survey of all 22 regional genetics services. There was a 100% response to the survey allowing a complete map to be created. The devolved nations had near complete compliance with the sole exception of SW Scotland, but in England the picture was fragmented with regions representing a combined population of 26.6 million (48%) not implementing the full NICE recommendations. Fifteen years after the publication of the original guidelines, major inequity in provision for screening still occurs and a postcode lottery exists for the management of women from families with a history of breast cancer. We estimate that up to 73 preventable breast cancer deaths occur each year due to the current inequity of access. It may be time to consider alternative funding and implementation models to ensure consistent access across the country.
Subject terms: Health policy, Health services
Background
Guidelines are an important mechanism to ensure equitable and evidence-based delivery of medical care. In the UK (England & Wales) the National Institute of health and Care Excellence (NICE) was created 20 years ago with a strapline of ‘Improving health and social care through evidence-based guidance’. When the term ‘offer’ is utilised this infers that health commissioners should implement the recommendation as it has usually passed the test of being cost effective. Although guidelines are just ‘guidance’, the purpose of NICE guidelines was to avoid postcode lotteries where an individual in one part of a country was able to access an aspect of healthcare, whereas in another part they would be refused access. While some have intimated that that individual guidance could be ignored if it was not in the patient’s interest to follow it,1 even if it was NICE guidance, this is not the same as health commissioners deciding whether or not to provide the financial requirements to deliver guidance for large groups of people.
Breast screening is an important element of what can be offered to women at increased risk of breast cancer by virtue of their family history. Targeting such screening at those at higher risk reduces the negative elements of screening such as concerns regarding what is often termed ‘overdiagnosis’. Women who have seen a mother or sister die from breast cancer before 50 years of age will be concerned about having to wait until age 50, the usual starting age for national programmes to undergo mammography screening.2 Many countries have produced guidelines including frequently updated guidance in the USA,3 Netherlands4 and France.5 The first NICE guideline group for familial breast cancer was constituted in 2002 and produced its first report in May 2004.6 This recommended that women at moderate (lifetime risk 17–29%; 10-year risk aged 40 of ≥3%) and high risk (lifetime risk ≥30%; 10-year risk aged 40 of ≥8%) should be ‘offered’ annual mammography screening aged 40–49 years of age. It was acknowledged that a national study (FH01) was in progress and would inform a future update.7,8 A short update in 2006,6 recommended MRI screening for women at very high risk including carriers of pathogenic variants in BRCA1, BRCA2 and TP53 aged 30–49 years based at least in part on the UK MARIBS study results.9 The 2013 update6 was able to include the results of the FH01 mammography screening trial in women aged 40–49 and strengthen the recommendation to offer annual mammography aged 40–49 for moderate-risk and 40–59 for high-risk women. The FH01 study8 showed a projected mortality advantage for annual mammography surveillance compared to age matched women with no screening based on tumour characteristics at diagnosis. Approximately 3% of women aged 40 years are at moderate or high-risk of breast cancer based on family history alone,9 and at least half of these come forward with concerns.10
Some women at moderate risk were still able to access mammographic screening aged 40–49 years at least through the FH01 trial8 that included 76 centres across the UK, but anecdotal evidence between 2006–2011 pointed to patchy implementation of MRI screening. As a result, the National Health Service Breast Screening Programme (NHSBSP) was asked to deliver the MRI aspects of NICE guidance. In January 2012 the Advisory Committee on Breast Cancer Screening agreed to implement this and there is now equitable provision of MRI screening (aged 30–49) and annual mammography screening from 40-69 years in BRCA1, BRCA2 and TP53 carriers (no mammography for TP53) in England.11 The protocol for MRI screening included those at ‘equivalent’ high risk to a BRCA1/BRCA2 mutation carrier. This was misinterpreted by a number of clinics to include all those at high-risk of breast cancer. In reality only about 8–10% of high-risk women qualify for the NHSBSP higher risk programme10,12 and the protocols have now been rewritten to clarify this. However, this uncertainty has led to many family history clinics (FHCs) not being commissioned to deliver the NICE defined high-risk annual screening to age 59 years.
These concerns over the reduction in availability of NICE recommended mammography screening led us to survey all UK genetics centres delivering cancer genetics services.
Methods
A short, six question online survey was designed (supplementary table) to assess compliance in each regional area. The invitation was sent out on 14 March 2019 to all cancer genetics leads from the Cancer Genetics Group (http://www.ukcgg.org/) covering 22 regional centres.
Results
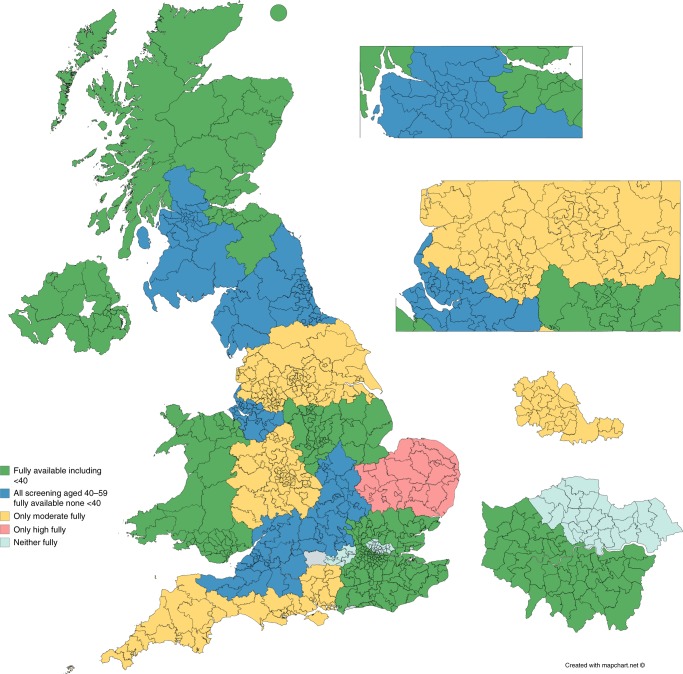
There was a 100% response to the survey allowing a complete map to be created. The devolved nations of Wales, Northern Ireland and Scotland had near complete compliance with the NICE recommended mammography screening ‘offer’ with the sole exception of parts of SW Scotland that only screened moderate-risk women 2-yearly (Fig. 1; Table 1). In England the picture was far patchier with regions representing a combined total population of 26.6 million (48% of the total population) not supplying all NICE recommended mammography screening. Regions covering 10.4 million people did not have coverage for Moderate-risk screening and in some areas moderate-risk screening was only available to those carrying pathogenic variants in “moderate risk” genes such as ATM or CHEK2, which represent only a tiny proportion of all those potentially eligible.
Fig. 1.
Map of mammographic screening provision by regional genetics centre. CCGs within each region are not shown, nor is the variation in provision by CCGs within a region. It is therefore possible that a CCG within a “non-compliant” region may be fully compliant and vice versa.
Table 1.
Provision of NICE approved mammography screening for familial breast cancer.
| Mammography screening provision | Fully available including <40 | All screening aged 40–59 fully available none <40 | Only moderate fully | Only high fully | neither fully | Total Population |
|---|---|---|---|---|---|---|
| South East Scotland | North West Thames | Peninsula | East Anglia | South West Thames | ||
| South Yorkshire, North Derbyshire, North Notts | Thames Valley | Wessex | London NE | |||
| South East London, Kent, East Sussex | Leicester, Leicestershire and Rutland | West Midlands and surrounding counties | ||||
| Northern Ireland | North East and North Cumbria | Yorkshire and Humber | ||||
| North of Scotland | Cheshire & Merseyside | Manchester/ North West England | ||||
| Tayside | Bristol and Somerset | |||||
| Wales | West of Scotland | |||||
| Population | 14,550,000 | 19,200,000 | 19,200,000 | 3,000,000 | 7,400,000 | 63,350,000 |
Discussion
NICE guidelines are considered as providing high-level evidence and are often utilised around the world.13 We have shown that 15 years after the original NICE guidance to offer annual mammography screening to women at moderate-risk aged 40–49 there is still patchy provision across England in particular. Based on the results we can attempt to estimate the possible impact of the observed discrepancies in screening. From Table 1, 10.4 million individuals (7.4 million + 3 million) live in areas not receiving the recommended surveillance for moderate risk. Thus, we estimate that up to 16.4% of eligible women (10.4/63.35 million) are not receiving the appropriate moderate-risk surveillance. Of the 4.3 million women aged 40–49 in the UK, it is estimated that 8% have ten-year risk in excess of 3% at age 40, that is 344,000 women.9 From the above, up to 16.4% of these are not receiving the recommended annual mammography surveillance, i.e. 56,416 women. In the FH01 study, it was estimated that 7.8 breast cancer deaths are prevented in this population per 10,000 surveillance episodes.14 Thus, up to 44 breast cancer deaths year would be prevented if the guidelines were fully adhered to.
The provision for high-risk screening in women aged 40–59 is even worse meaning that women at higher levels of breast cancer risk in their 40’s are unable to access screening. This is despite strengthening in the evidence base in the 2006 and 2013 updates to the familial breast cancer guidance.6 From Table 1, it is estimated that up to 42% of the population (26.6/63.35) live in areas not covered by the currently recommended high-risk surveillance. Of the 8.85 million women aged 40–59, it is estimated that 1%, 88,500, are eligible, and that up to 42% of these (37,167 women) are not receiving the appropriate surveillance. Although we do not have formal estimates of the absolute benefit of this surveillance, it is reasonable to assume that it is at least as effective as the moderate-risk surveillance. Thus, we estimate that up to 29 deaths per year would be prevented in the high-risk population if the guidelines for surveillance were followed.
With the recent publication of evidence that annual mammography screening in women aged 35–39 years at enhanced breast cancer risk appears equally effective at early detection12 compared to age 40–49,8 NICE is likely to evaluate this at the next update.6 However, one would have little confidence that any recommendation to offer screening aged 35–39 years would be fully implemented, despite the fact that women from regions covering a population of around 14.5 million appear to already get this (Table 1).
In the USA in 2007, the American Cancer Society (ACS) published recommendations for annual breast MRI along with annual mammography screening for women who have ≥20% lifetime risk for developing breast cancer incorporating both the majority of the UK moderate risk and all of the high-risk group.15 A survey in 2012 showed low access to MRI, although, understandably this was higher amongst those with health insurance.16 Overall there is relatively little published on adherence to breast screening guidelines internationally. That may be because it is difficult to audit or that it has not been explored. The Clinical Commissioning Groups (CGGs) look to save money and therefore are not willing to adopt all NICE guidelines. Not implementing this guidance would save on not having to provide funding for family history clinics for women to be assessed for BC risk. While CGGs appear to be able to ignore guidance, there are precedents to suggest that they could be successfully sued for not implementing NICE guidance without good reason (https://www.nice.org.uk/news/article/court-warns-ccg-over-disagreeing-with-nice-guidance). If CGGs were forced to adopt NICE guidelines this would reduce inequalities of access. Alternatively, all familial breast screening could be incorporated into the NHSBSP, which would also lead to more uniform access. Finally, it is important to educate and inform all women to have a breast cancer risk assessment by age 40 years, either through their GP or by developing tailored online tools to enable a self-assessment.
Conclusion
We have shown that there has been only partial compliance with NICE approved mammographic screening surveillance in women at familial risk with less than half of England is now fully compliant. This has created a postcode lottery where women in one part of the UK get access to surveillance that their sister at the same risk cannot. It is a concern that even in a fully funded public health system such as the UK that major inequity in provision occurs even with the highest levels of evidence provided by NICE guidelines.
Supplementary information
Acknowledgments
Cancer Genetics Group clinical leads
Julian Adlard, Munaza Ahmed, Julian Barwell, Angela Brady, Paul Brennan, Carole Brewer, Jacqueline Cook, Tabib Dabir, Rosemarie Davidson, Alan Donaldson, Angela George, David Goudie, Lynn Greenhalgh, Dorothy Halliday, Helen Hanson, Rachel Harrison, Anju Kulkarni, Fiona Lalloo, Kai ren Ong, Zosia Miedzybrodzka, Alex Murray, Mary Porteous, Lucy Side and Katie Snape
Author contributions
D.G.E. and S.W.D. devised the survey with the help of M.T. The survey was e-mailed to all cancer genetics clinical leads for each region through the UK Cancer Genetics Group which is currently chaired by M.T. M.E. collated the survey results. D.G.E. was chair of the 2004 and 2006 NICE guideline group (CG14, CG41) and clinical lead for the 2013 and 2017 updates.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This was not required.
Funding
D.G.E. is supported by the NIHR Manchester Biomedical Research centre (IS-BRC-1215-20007). M.T. is supported by the NIHR Cambridge Biomedical Research centre.
Data availability
All data are contained within the manuscript.
Footnotes
The members of the Cancer Genetics Group clicnical leads are listed above the Author contributions
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. Gareth Evans, Email: gareth.evans@mft.nhs.uk.
Marc Tischkowitz, Phone: +44(0)1223 216446, Email: mdt33@cam.ac.uk.
Cancer Genetics Group clinical leads:
Julian Adlard, Munaza Ahmed, Julian Barwell, Angela Brady, Paul Brennan, Carole Brewer, Jacqueline Cook, Tabib Dabir, Rosemarie Davidson, Alan Donaldson, Angela George, David Goudie, Lynn Greenhalgh, Dorothy Halliday, Helen Hanson, Rachel Harrison, Anju Kulkarni, Fiona Lalloo, Kai ren Ong, Zosia Miedzybrodzka, Alex Murray, Mary Porteous, Lucy Side, and Katie Snape
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-019-0631-2.
References
- 1.Colbrook, P. Can you ignore guidelines? BMJ330, s143 (2005).
- 2.Breast Screening Programme 2017–18. NHS Breast Screening Programme Report 2017–18. (2019). https://digitalnhsuk/data-and-information/publications/statistical/breast-screening-programme.
- 3.Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc Netw. 2018;16:1362–1389. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 4.NABON-CBO. Guideline for breast cancer. http://www.oncolinenl/mammacarcinoom (2012).
- 5.Eisinger, F., Alby, N., Bremond, A., Dauplat, J., Espie, M. & Janiaud, P. et al. INSERM-FNCLCC collective expertise. Recommendations for medical management of women with genetic risk of developing breast and/or ovarian cancer. Ann. Genet.42, 51–64 (1999). [PubMed]
- 6.McIntosh, A. S., C.; Evans, G. Clinical Guidelines and Evidence Review for The Classification and Care of Women at Risk of Familial Breast Cancer, London: National Collaborating Centre for Primary Care/University of Sheffield. NICE guideline CG14, CG41,CG164. updated 2006, 2013, 2017, www.nice.org.uk (2004).
- 7.Mackay J, Rogers C, Fielder H, Blamey R, Macmillan D, Boggis C, et al. Development of a protocol for evaluation of mammographic surveillance services in women under 50 with a family history of breast cancer. J. Epidemiol. Biostat. 2001;6:365–369. doi: 10.1080/135952201753337086. [DOI] [PubMed] [Google Scholar]
- 8.FH01-teams. Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol. 2010;11:1127–1134. doi: 10.1016/S1470-2045(10)70263-1. [DOI] [PubMed] [Google Scholar]
- 9.Evans DG, Brentnall AR, Harvie M, Dawe S, Sergeant JC, Stavrinos P, et al. Breast cancer risk in young women in the national breast screening programme: implications for applying NICE guidelines for additional screening and chemoprevention. Cancer Prev. Res (Philos.). 2014;7:993–1001. doi: 10.1158/1940-6207.CAPR-14-0037. [DOI] [PubMed] [Google Scholar]
- 10.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66646-9. [DOI] [PubMed] [Google Scholar]
- 11.NHSBSP74. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/766128/nhsbsp74.pdf. (2013).
- 12.Evans DG, Thomas S, Caunt J, Burch A, Brentnall AR, Roberts L, et al. Final results of the prospective FH02 mammographic surveillance study of women aged 35–39 at increased familial risk of breast cancer. EClinicalMedicine. 2019;7:39–46. doi: 10.1016/j.eclinm.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlton EW, Pickering JW, Greenslade J, Cullen L, Than M, Kendall J, et al. Assessment of the 2016 National Institute for Health and Care Excellence high-sensitivity troponin rule-out strategy. Heart. 2018;104:665–672. doi: 10.1136/heartjnl-2017-311983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy, S. W., Mackay, J., Thomas, S., Anderson, E., Chen, T. H., Ellis, I. et al. Evaluation of mammographic surveillance services in women aged 40-49 years with a moderate family history of breast cancer: a single-arm cohort study. Health Technol. Assess. 17, 1–95 (2013). [DOI] [PMC free article] [PubMed]
- 15.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 16.Miller JW, Sabatino SA, Thompson TD, Breen N, White MC, Ryerson AB, et al. Breast MRI use uncommon among U.S. women. Cancer Epidemiol. Biomark. Prev. 2013;22:159–166. doi: 10.1158/1055-9965.EPI-12-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.