Abstract
Classical signaling lymphocyte activating molecule (SLAM) family receptors are abundant within many types of immune cells, whereas the nonclassical SLAM family receptors SLAMF8 and SLAMF9, which uniquely lack cytoplasmic signaling motifs, are highly expressed by myeloid cells. Due to the potential redundancy, whether these two receptors regulate macrophage function remains largely unknown. Here, we show that SLAMF8 and SLAMF9 co-regulate macrophage-mediated liver inflammation. To overcome the redundancy, we generated mice that simultaneously lacked SLAMF8 and SLAMF9 using CRISPR-Cas9 technology. Although macrophage differentiation was not altered by the combined deficiency of SLAMF8 and SLAMF9, the loss of these two receptors significantly protected against lipopolysaccharide (LPS)-induced liver injury. SLAMF8 and SLAMF9 double-deficient mice had a prolonged survival rate and less infiltration of inflammatory cells. The depletion of macrophages using clodronate liposomes abolished the effects of SLAMF8 and SLAMF9 deficiencies on LPS-induced liver injury, which demonstrates that these receptors are required for macrophage activation following LPS challenge. Moreover, the deficiency of SLAMF8 and SLAMF9 suppressed the secretion of inflammatory cytokines by downregulating the expression of Toll-like receptor-4 (TLR4), a receptor that specifically binds LPS, which led to decreased mitogen-activated protein kinases (MAPK) signaling activation. Notably, combined injections of truncated extracellular SLAMF8 and SLAMF9 proteins significantly alleviated LPS-induced liver injury. Thus, our findings provide insights into the role of SLAMF8 and SLAMF9 in endotoxin-induced liver injury and suggest that SLAMF8 and SLAMF9 are potential therapeutic targets for acute hepatic injury.
Keywords: Nonclassical SLAM family receptors, Liver inflammation, Macrophage, TLR4, MAPK
Subject terms: Inflammation, Innate immunity
Introduction
Acute endotoxin-induced liver damage is characterized by severe hepatic dysfunction with high mortality, which leads to a clinical syndrome of coagulation disorder and subsequent multiple organ failure.1,2 The liver damage is a type of inflammatory injury manifested by hepatic infiltration of many inflammatory macrophages, T cells, and neutrophils.3 The precise mechanisms that underlie the immunopathology of endotoxin-induced liver injury are not fully understood, and effective therapeutic strategies with minimal side effects remain inadequate.
Lipopolysaccharide (LPS) has been identified as one of the major factors that induce acute hepatocyte damage through macrophage activation.4 The LPS/d-galactosamine (GalN) model is widely used as an experimental model for mimicking endotoxin-induced liver damage in humans.5,6 In this model, LPS specifically binds to Toll-like receptor-4 (TLR4) and promotes pro-inflammatory cytokine secretion by hepatic macrophages, including IL-1β, IL-6, and tumor necrosis factor (TNF)-α, which, in turn, stimulate intrahepatic reactive oxygen species (ROS) production.5,7 The increased ROS production induces the activation of mitogen-activated protein kinases (MAPKs).8 Persistent activation of MAPK family members, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, leads to the production of a large number of pro-inflammatory factors.9 In general, the expression level of TLR4 on macrophages largely determines the extent of LPS-induced liver injury; thus, TLR4-deficient mice are highly resistant to an LPS challenge.10 However, how the TLR4 expression is regulated remains unknown.
The signaling lymphocyte activating molecule (SLAM) family is a group of hematopoietic cell-specific receptors. This family comprises nine structurally related members. These receptors are homophilic receptors, and they function as self-ligands. Based on the cytoplasmic signaling motif, the SLAM family can be divided into two classes, classical and nonclassical.11 Classical SLAM family members, including SLAMF1–7, typically possess two to four immunoreceptor tyrosine switch motifs (ITSMs), which are able to recruit SH2 domain-containing molecules, such as SLAM-associated protein (SAP) and other phosphatases.12,13 These classical SLAM family receptors play critical roles in multiple immune events, such as NK cell activation, NK-T cell development and follicular T helper cell differentiation.14,15 Thus, mutations of the human SAP gene lead to X-linked lymphoproliferative (XLP), a syndrome with severe immunodeficiency.16,17
Nonclassical SLAM family members mainly refer to SLAMF8 and SLAMF9. In contrast to the classical SLAMF receptors, SLAMF8, and SLAMF9 have no signaling motifs in their short cytoplasmic tail.11,18 They are strictly expressed by various myeloid cells, such as neutrophils, macrophages, and dendritic cells.19 In contrast to other classical SLAM receptors, the roles of SLAMF8 and SLAMF9 are poorly understood. Limited studies have indicated that SLAMF8 can negatively regulate ROS production by macrophages.20 Both SLAMF8 and SLAMF9 are positioned at chromosome 1, very close to the locus of classical SLAM family members.12 We previously showed the redundancy of classical SLAM family receptors in the regulation of NK cell activity and NK-T cell development.14,15 Thus, we wondered whether there is redundancy in the nonclassical SLAMF8 and SLAMF9. In this study, we generated mice doubly deficient in SLAMF8 and SLAMF9. We showed that these nonclassical SLAM receptors play a critical role in macrophage activation by maintaining TLR4 expression following LPS exposure. Thus, mice that lack SLAMF8 and SLAMF9 are highly resistant to LPS-induced liver injury. Our data unveil a role of nonclassical SLAM family receptors and suggest that SLAMF8 and SLAMF9 are likely therapeutic targets for acute hepatic injury.
Materials and methods
Mice
Mice that simultaneously lacked SLAMF8 and SLAMF9 (SLAMF8/9−/−) were generated using CRISPR-Cas9-based genome editing as previously described.14 C57BL/6 (B6, H-2b) mice were obtained from Jackson Laboratory. SAP-deficient mice have been previously described.21 The triple knockout mice that lacked SLAMF8, SLAMF9 and SAP were obtained by crossing SLAMF8/9−/−mice with SAP-deficient mice. All mice used in our experiments were maintained under the C57BL/6 background and in the SPF facility of Tsinghua University. All procedures involving animals were approved by the Animal Ethics Committee of Tsinghua University.
Culture of Bone Marrow-Derived macrophages (BMDMs)
Bone marrow cells were differentiated in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 30% L929 supernatant that contained macrophage-stimulating factor (M-CSF) for 7 days. Cells were harvested with cold 0.2% EDTA and plated at a density of 4 × 105/mL in DMEM supplemented with 10% FBS for at least 12 h prior to stimulation.
Flow cytometry
Flow cytometry was performed on BD LSRII flow cytometers (BD Biosciences). Monoclonal antibodies against mouse CD3, B220, NK1.1, CD117, CD135, CD11b, F4/80, Ly6C, CD115, MHCII, CXCR5, GL7, IgD, PD1, TLR4 and streptavidin were purchased from eBioscience (San Diego, CA). Antibodies against CD138, CD11c and Fas were purchased from BD Biosciences. Antibodies against CD206 were purchased from BioLegend. CD1d-PBS157 tetramer was kindly provided by the NIH tetramer facility. For the surface marker analysis, cells were stained in PBS that contained 2% FBS with the indicated antibodies. For the intracellular staining assay, the cells were fixed and permeabilized using a Cytofix/Cytoperm Plus kit (BD Pharmingen) after the surface markers were labeled, and the intracellular molecules were subsequently stained.
Generation of SLAMF8-deficient or SLAMF9-deficient RAW264.7 variants using the CRISPR-Cas9 technique
Two single-guide RNAs (sgRNAs) targeting exon1 of SLAMF8 or SLAMF9 were designed by the CRISPR design website. The oligos were annealed and cloned into the lentiCRISPR v2 vector. The transfection mixture, including lentiCRISPR v2 vector inserted with sgRNA, packaging plasmid psPAX2, envelope plasmid VSV-G and lipofectamine 3000, was incubated at room temperature for 30 min and then carefully transferred into a cell culture dish with HEK 293T cells. The viruses were concentrated and then infected RAW264.7 cells supplemented with polybrene (Sigma-Aldrich, St. Louis, MO, USA). The infected cells were selected in media using puromycin (Sigma-Aldrich, St. Louis, MO, USA), and the resulting cells were examined by western blotting.
Endotoxin-induced liver injury
All experiments were performed with age-matched (6–8 week) and sex-matched mice. The LPS/GalN-induced mouse liver injury was created via an intraperitoneal injection of LPS (3 μg/kg body weight, Sigma-Aldrich, St. Louis, MO, USA) and GalN (300 mg/kg body weight, Sigma-Aldrich, St. Louis, MO, USA) dissolved in phosphate-buffered saline. To induce lethal liver inflammation, mice were intraperitoneally injected with LPS (10 μg/kg body weight) and GalN (500 mg/kg body weight).
Survival rate analysis
The mouse survival was monitored for 7 days after the LPS/GalN challenge. The number of dead mice was determined at intervals of 6 h, and the cumulative survival curves were created using the Kaplan–Meier method.
Histological analysis
The liver tissues were collected 6 h after LPS/GalN treatment. The tissue samples were subsequently fixed in 10% formalin and embedded in paraffin. The paraffin-embedded samples were sectioned (4 μm) and stained with hematoxylin and eosin for histopathological evaluation under a light microscope. Images were acquired on a Leica DM3000 microscope.
Detection of serum aminotransferase activity
Blood samples were collected 6 h after LPS/GalN injection. The liver injury in LPS/GalN-induced acute hepatitis was quantified by the measurement of the serum enzyme activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using a commercially available test kit (Rongsheng Biotech, Shanghai, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
The concentrations of TNF-α, IL-1β, and IL-6 in plasma samples or BMDM supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) using commercial kits from eBioscience (San Diego, CA) according to the manufacturer’s instructions.
RNA extraction and quantitative real-time PCR
Total RNA from liver samples, BMDMs or FACS-sorted immune cells was extracted using a Trizol Kit (Invitrogen) and reverse transcribed using a reverse transcription system (Promega, A3500) according to the manufacturer’s instructions. Quantitative PCR was performed with SYBR green PCR Master Mix. The expression level of the indicated genes was determined as their relative expression to GAPDH. The primer sequences are listed in Supplementary Table 1.
Macrophage depletion
Clodronate liposomes (200 μL/mouse, Formu Max Scientific, Sunnyvale, USA) were intravenously injected 48 h before LPS/GalN administration to deplete macrophages. The efficiency of depletion was confirmed by flow cytometric analysis of liver macrophages.
Immunofluorescence staining
BMDMs prestimulated with LPS (100 ng/mL) for 6 h were fixed/permeabilized and then incubated with a TLR4 antibody (Santa Cruz, CA, USA) overnight at 4 °C, followed by staining with Alexa 647-conjugated anti-rat IgG. The expression of TLR4 was visualized under a confocal microscope (Zeiss, Oberkochen, Germany).
Western blot
Total protein was extracted according to the method described in the protein extraction kit (P0013C, Beyotime, Shanghai). Protein extracts were separated by SDS-PAGE on 12% polyacrylamide gels and were subsequently electrically transferred to a PVDF membrane. After blocking, the membranes were incubated with the appropriate specific primary antibodies anti-p-p38, anti-p38, anti-p-ERK, anti-ERK, anti-p-JNK, anti-JNK, anti-p-p65, anti-p65, anti-p-IKBα, anti-IKBα, anti-TLR4, (Cell Signaling Technology, Beverly, MA), anti-SLAMF8 and anti-SLAMF9 (R&D, Minneapolis, USA) at 4 °C overnight, followed by incubation with HRP-conjugated secondary antibodies (Beyotime, Shanghai; or sheep #ab6747, Abcam, Cambridge, UK). Detection was performed using an enhanced chemiluminescence kit (Thermo Scientific, Hudson, NH, USA) according to the manufacturer’s instructions.
Co-Immunoprecipitation
BMDMs were collected and lysed with cell lysis buffer (P0013, Beyotime, Shanghai). To avoid nonspecific binding, the protein sample was mixed with species-specific IgG and protein A+G Agarose (P2019, Beyotime, Shanghai) at 4 °C for 2 h. The mix was centrifuged at 2500 rpm for 5 min, and the supernatant was collected. The indicated primary antibody was added to the supernatant and then slowly shook at 4 °C for 24 h. Then, 30 µL protein A+G agarose were added and shook at 4 °C for 3 h. The sample was centrifuged, and the supernatant was discarded. The sample was washed with PBS 5 times, followed by the addition of 30 µL SDS-PAGE loading buffer. The sample was then ready for further SDS-PAGE and western blot analyses.
Generation of mouse SLAMF8 and SLAMF9 extracellular domain proteins
Mouse cDNA that encoded the extracellular portion of SLAMF8 or SLAMF9 was cloned into a pET-32a vector. The pET-32a-SLAMF8 or SLAMF9 plasmid was transformed into competent BL21 E. coli. Protein expression was induced by the addition of isopropyl-β-d-thiogalactoside (IPTG). The recombinant proteins were purified with nickel ion affinity chromatography. Eluted proteins were collected and dialyzed using a 3000-unit dialysis bag following the removal of LPS using a ToxinEraserTM kit (Genscript Biotechnology, Nanjing, China).
Statistical analyses
A log-rank test was used to compare the Kaplan–Meier survival curves between the different groups of mice generated in GraphPad Prism 5. Unpaired Student’s t-tests (two-tailed) were performed using Prism software. A P-value < 0.05 was considered significant. *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
SLAMF8 and SLAMF9 are uniquely expressed on myeloid cells, particularly on macrophages
Nonclassical SLAMF8 and SLAMF9 are closely linked and positioned on chromosome 1, which suggests that both may have a similar function. To investigate their roles in immune regulation, we investigated their expression profiles on several types of hematopoietic cells. We first evaluated their transcripts by analyzing published RNA sequencing data from other groups.19,22 Compared with hematopoietic progenitors, including hematopoietic stem cells (HSCs), common lymphocyte progenitors (CLPs), and lymphocytes (T, B, and NK cells), SLAMF8 and SLAMF9 were highly expressed by macrophages, while SLAMF8, but not SLAMF9, was moderately distributed on monocytes. We subsequently performed real-time RT-PCR to verify these data. The results confirmed that SLAMF8 and SLAMF9 were largely expressed on macrophages, but not on lymphocytes (Fig. 1a). Moreover, we found that the expression of SLAMF8 and SLAMF9 transcripts was significantly upregulated after the LPS challenge (Fig. 1b). Thus, in contrast to the other SLAM family members, SLAMF8 and SLAMF9 are uniquely expressed on myeloid cells, particularly on macrophages, and LPS stimulation could significantly increase their expression levels.
Fig. 1.
SLAMF8 and SLAMF9 are highly expressed on macrophages. a Quantitative RT-PCR was used to analyze the expression levels of SLAMF8 and SLAMF9 in sorted T cells, B cells, and NK cells from the spleen, neutrophil, and macrophages from liver of WT mice. b RT-PCR was used to analyze the expression levels of SLAMF8 and SLAMF9 in BMDMs of WT mice at the indicated time after LPS stimulation. The data represent three independent experiments. Data are shown as the mean ± SEM
SLAMF8- and SLAMF9-deficiency blunt macrophage activation
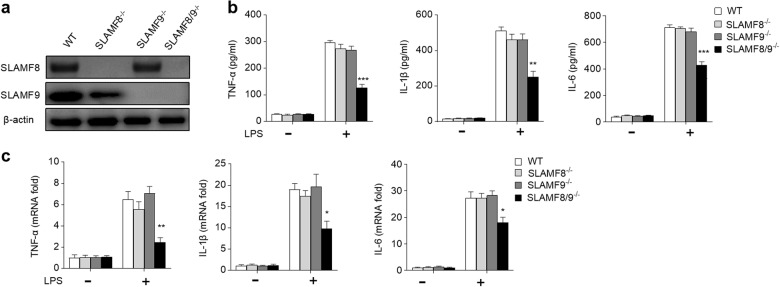
To examine whether SLAMF8 or SLAMF9 is required for macrophage activation, RAW264.7 (a mouse macrophage cell line) variants that lacked SLAM8 and/or SLAMF9 were generated using the CRISPR-Cas9 technique (Fig. 2a). We found that SLAMF8- and SLAMF9- deficient RAW264.7 cells secreted less pro-inflammatory cytokines following LPS stimulation, including TNF-α, IL-1β, and IL-6 (Fig. 2b). Their transcripts were also markedly reduced (Fig. 2c). However, these dramatic changes were not observed in the RAW264.7 cells that lacked SLAMF8 or SLAMF9. Given that SLAMF8 and SLAMF9 have a similar expression pattern and chromosome location, as well as a homotypic recognition way,11 these findings suggest that SLAMF8 and SLAMF9 play a redundant role in macrophage activation.
Fig. 2.
SLAMF8 and SLAMF9 deficiency blunt macrophage activation. a The expression levels of SLAMF8 and SLAMF9 in RAW264.7 cell line variants were detected using western blot analysis. b WT, SLAMF8-/-, SLAMF9-/- and SLAMF8/9−/− RAW264.7 cell lines were stimulated with (+) or without (−) LPS for 6 h. The cultured supernatants were harvested, and the concentrations of TNF-α, IL-1β, and IL-6 were measured using ELISA. c Quantitative RT-PCR was used to analyze the expression levels of TNF-α, IL-1β, and IL-6. All data represent at least three independent experiments. Data are shown as the mean ± SEM
Mice lacking SLAMF8 and SLAMF9 are generated using the CRISPR technique
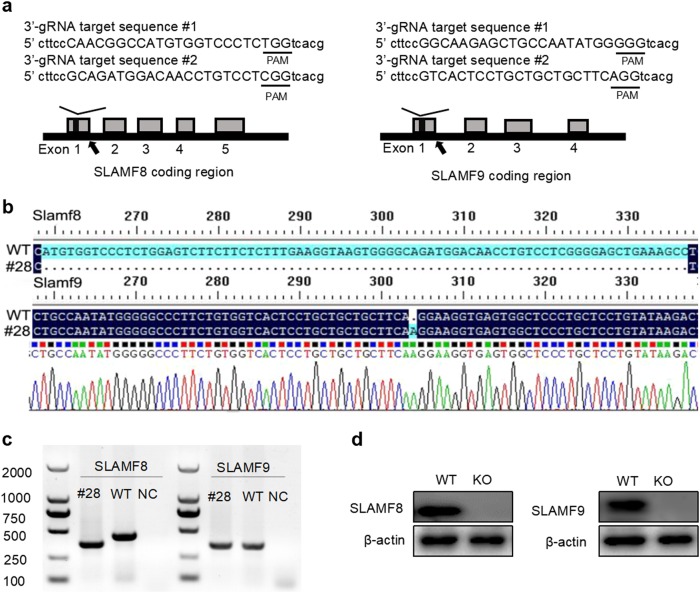
To explore whether SLAMF8 and SLAMF9 redundantly regulate the function of macrophages, we aimed to generate SLAMF8 and SLAMF9 double-deficient mice. However, because SLAMF8 and SLAMF9 are tightly linked on the same locus of chromosome 1, it was almost impossible to obtain the mice through conventional gene targeting. Fortunately, we successfully obtained mice that simultaneously lacked SLAMF8 and SLAMF9 using the CRISPR-based technique. In brief, a mixture of two pairs of synthetic guide RNAs targeting each exon1 of SLAMF8 and SLAMF9 together with the enzyme Cas9 were injected into blastocysts isolated from pure C57BL/6 mice (Fig. 3a). The injected embryos were transferred into recipient mice at the two-cell stage. Consequently, 29 founder pups were born. After genotyping and DNA sequencing, 20.6% (6/29) of these pups had double mutations at both loci of SLAMF8 and SLAMF9. Pup #28, which had a 79-base pair (bp) deletion at exon1 of SLAMF8 and a 1-bp insertion at exon1 of SLAMF9, was chosen for the following study (Fig. 3b). The mice bred normally and produced offspring according to the expected Mendelian ratio. We confirmed the loss of specific DNA fragments in SLAMF8, 9 doubly deficient mice (Fig. 3c). Their proteins were not detectable in the BMDMs from SLAMF8, 9 doubly deficient mice (Fig. 3d).
Fig. 3.
Mice lacking SLAM8 and 9 are generated using the CRISPR-Cas9 technique. a Schematic representation of the SLAMF8 and SLAMF9 loci; a portion of the genomic structure of the SLAMF8 and SLAMF9 loci is shown. The gray boxes represent exons of SLAMF8 and SLAMF9, and the sgRNA sequences targeting SLAMF8 and SLAMF9 are shown. b DNA sequencing data of mutations in mouse #28. c Genomic PCR was used to identify the mutations of SLAMF8 and SLAMF9 in mouse #28, NC: negative control. d The deficiency of SLAMF8 and SLAMF9 proteins in SLAMF8/9−/− mice was confirmed using western blot analysis
The homeostasis of lymphocyte and myeloid cells is not altered in SLAMF8- and SLAMF9-deficient mice
The combined deficiency of classical SLAM family members leads to abnormal lymphocyte development and differentiation.14,15 Thus, to exclude the possibility that SLAMF8 and SLAMF9 double deficiency can disturb the development or differentiation of immune cells, particularly macrophages, we performed extensive experiments to analyze the proportion and absolute numbers of lymphocytes and myeloid cells. Three types of lymphocytes from the mice that lacked SLAMF8 and SLAMF9, including T, B, and NK cells, as well as T cell subset NK-T cells, appeared normally in the SLAMF8-deficient and SLAMF9-deficient mice (Fig. S1a-c). Moreover, the percentage and absolute numbers of myeloid lineage cells, such as neutrophils, macrophages, and dendritic cells, in the spleen, bone marrow and liver from the SLAMF8 and SLAMF9 double knockout mice were nearly comparable with those from the wild-type (WT) mice (Fig. S2a-c). Finally, SLAMF8 and SLAMF9 deficiency did not affect the percentage and absolute number of monocytes, granulocyte-macrophage progenitors (GMPs), monocytes/macrophages or dendritic cell precursors (MDPs) and common monocyte precursors (MOPs) in the bone marrow (Fig. S2d, e). These results indicate that SLAMF8 and SLAMF9 are not essential for lymphocyte and myeloid cell homeostasis and development.
SLAMF8 and SLAMF9 are not required for humoral immunity
To determine whether deficiency of the two nonclassical SLAM family receptors compromises humoral immunity, SLAMF8-deficient and SLAMF9-deficient mice were immunized with sheep red blood cells (SRBCs), which are able to trigger T-dependent and T-independent germinal center (GC) reactions. As expected, the loss of SLAMF8 and SLAMF9 did not impair the percentage of Fas+ GL7+ GC B cells, plasma cells and follicular T helper (TFH) cells in the spleen and lymph nodes (Fig. S3a-c). This conclusion was further confirmed by immunization with OVA, which is a T-dependent antigen (Fig. S3a-c). These data suggest that the finding that humoral immunity was intact in mice that lacked all classical SLAM family receptors, which we recently reported, is not due to compensation by nonclassical SLAM family receptors.15 To further investigate whether SLAMF8 and SLAMF9 play an inhibitory role in SAP-deficient mice, SLAMF8-deficient and SLAMF9-deficient mice were intercrossed with SAP-knockout mice. Although SAP deficiency causes severe defects in humoral immunity, the additional removal of SLAMF8 and SLAMF9 from SAP-deficient mice failed to correct the defective generation of GC B cells, plasma cells and TFH cells after SRBC immunization (Fig. S3a-c). Similar results were obtained when OVA immunization was employed (Fig. S3a-c). These data indicate that nonclassical SLAMF8 and SLAMF9 are not only dispensable for SAP-dependent humoral immunity but they also have no inhibitory signaling even when SAP is missing.
Loss of SLAMF8 and SLAMF9 prevents endotoxin-induced liver damage in vivo
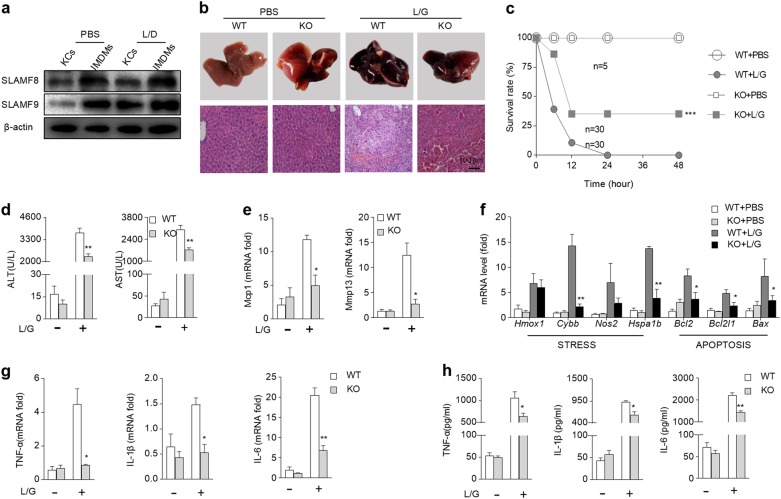
Hepatic macrophages play an important role in endotoxin-induced liver damage; thus, we further detected the expression of SLAMF8 and SLAMF9 on liver infiltrated monocyte-derived macrophages (IMDMs) and Kupffer cells (KCs). We found that the amount of SLAMF8 and SLAMF9 was substantially higher on IMDMs than that on KCs (Fig. 4a). To confirm the requirement of SLAMF8 and SLAMF9 for macrophage activation following LPS stimulation, mice were intraperitoneally injected with LPS in combination with GalN to mimic endotoxin-induced liver damage. In the WT mice administered LPS/GalN, massive hemorrhagic necrosis and hepatocyte apoptosis were observed, together with prominent vascular congestion and inflammatory cell infiltration. Notably, the loss of SLAMF8 and SLAMF9 remarkably improved the gross liver appearance, with an absence of the typical signs of hemorrhage. The histological results also showed markedly reduced hepatic necrosis compared with the WT mice (Fig. 4b). This protective effect of SLAMF8 and SLAMF9 deficiency on endotoxin-induced liver injury was further confirmed by analysis of the survival rate of mice challenged with lethal doses of LPS/GlaN. In the WT group, the mice began to die 6 h after LPS/GalN injection, and the mortality reached 89% at 12 h. However, the simultaneous loss of SLAMF8 and SLAMF9 dramatically enhanced the survival rates (Fig. 4c). We also detected the serum activities of alanine transaminase (ALT) and aspartate transaminase (AST) as indicators of hepatocyte necrosis. As expected, SLAMF8 and SLAMF9 deficiency downregulated the concentrations of serum ALT and AST (Fig. 4d). LPS/GalN injection also led to a remarkable elevation in the hepatic mRNA transcripts for Mcp1, Mmp13, and genes associated with cellular stress, including Heme oxygenase 1 (Hmox-1), cytochrome b-245 heavy chain (Cybb), nitric oxide synthase 2 (Nos2), heat-shock family member 70 (Hspa1b), apoptosis B-cell CLL Lymphoma 2 (Bcl-2), BCL2-like 1 (Bcl2l1), and BCL-2 associated-X protein (Bax), in the WT mice, whereas the loss of SLAMF8 and SLAMF9 tremendously prevented their transcriptions (Fig. 4e, f). Because pro-inflammatory cytokines, such as TNF-α and IL-1β, are critical factors for liver damage induced by LPS/GalN, we subsequently determined whether the loss of SLAMF8 and SLAMF9 diminished the production of these cytokines. LPS/GalN injection led to a remarkable elevation in the hepatic mRNA transcripts for TNF-α, IL-1β, and IL-6 in the WT mice (Fig. 4g), whereas in the SLAMF8,9-deficient mice, they were significantly inhibited. Similar results were also obtained at the protein level when measuring the serum TNF-α, IL-1β, and IL-6 (Fig. 4h). These results indicate that SLAMF8 and SLAMF9 deficiency protects the liver from injury, likely by suppressing the production of cytokines.
Fig. 4.
Loss of SLAMF8 and SLAMF9 prevents endotoxin-induced liver damage. a Western blot was used to detect the expression levels of SLAMF8 and SLAMF9 in kuppfer cells (KCs) and infiltrated monocyte-derived macrophages (IMDMs) in the liver from WT mice with or without LPS/GalN shock. b Macroscopic appearance of representative liver samples and HE staining of samples from WT and SLAMF8/9−/− (KO) mice 6 h after L/G challenge. c Survival rates were observed at various time points within 48 h after injection with a lethal dose of L/G. d The concentrations of ALT and AST in serum were measured 6 h after L/G challenge. e The relative hepatic mRNA levels of Mcp1 and Mmp13 were determined using qRT-PCR. f The relative hepatic mRNA levels of the indicated genes associated with stress and apoptosis were determined using qRT-PCR. g The relative hepatic mRNA levels of TNF-α, IL-1β, and IL-6 were determined using qRT-PCR. h The serum levels of TNF-α, IL-1β, and IL-6 were measured by ELISA. All data represent at least three independent experiments, and calculated data are shown as the means ± SEM
SLAMF8 and SLAMF9 are required for macrophage-mediated endotoxin-induced liver injury
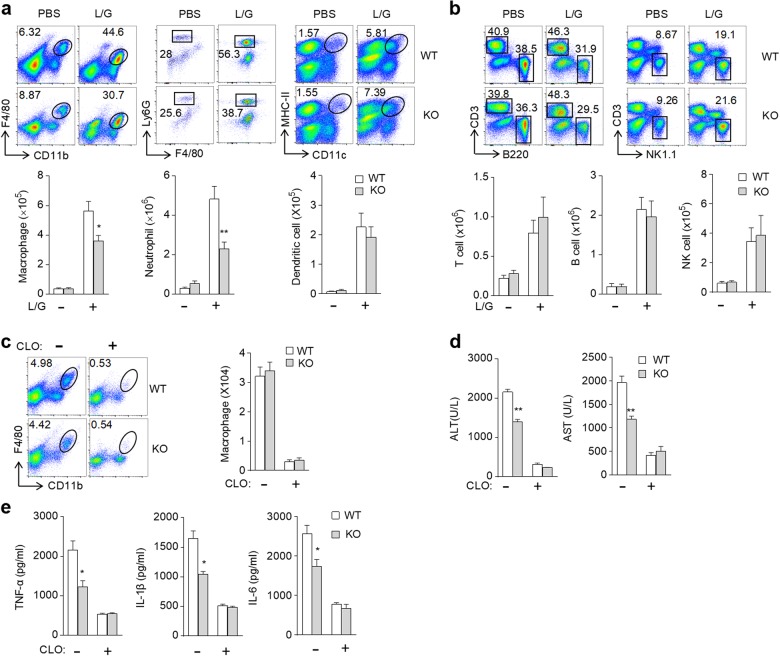
Because hepatic macrophages are the primary sources of these inflammatory cytokines when subjected to LPS challenge, we subsequently examined the accumulation of myeloid cells and lymphocytes in liver tissues after LPS/GalN treatment. The results showed that the percentage and absolute number of the detected myeloid cells (macrophages, neutrophils and dendritic cells) and lymphocytes (NK cells, T cells and B cells) in the liver were significantly increased after LPS/GalN challenge (Fig. 5a, b). The loss of SLAMF8 and SLAMF9 did not affect the infiltration of lymphocytes, whereas it inhibited the infiltration of macrophages and neutrophils in the liver after LPS/GalN administration (Fig. 5a, b). Therefore, the loss of SLAMF8 and SLAMF9 exerts a hepatoprotective effect in LPS/GalN-induced liver injury, partly due to the inhibition of myeloid cell accumulation. Because SLAMF8 and SLAMF9 were not restrictedly expressed on macrophages, we subsequently sought to determine whether the protective effect of SLAMF8 and SLAMF9 deficiency on LPS-induced liver injury was mostly attributed to macrophages. Mice were intravenously injected with a single dose of clodronate liposome to deplete macrophages 48 h prior to LPS/GalN administration. As expected, approximately 90% of mature macrophages in the liver were eliminated by clodronate liposome (Fig. 5c). After LPS/GalN administration, the levels of serum ALT, AST, and inflammatory cytokines in the macrophage-depleted WT mice were comparable with those in the SLAMF8- and SLAMF9-deficient mice that were depleted of macrophages (Fig. 5d, e). Therefore, the removal of macrophages abolished the protective role of SLAMF8 and SLAMF9 deficiency in LPS-induced liver injury. These results suggest that SLAMF8 and SLAMF9 promote endotoxin-induced liver injury by activating macrophages.
Fig. 5.
SLAMF8 and SLAMF9 promote LPS-induced liver injury via macrophages. Representative flow cytometry profiles and the absolute number of macrophages (CD45+Ly6G−F4/80+CD11b+), neutrophils (CD45+Ly6C+F4/80−Ly6G+CD11b+), DCs (CD45+MHCII+CD11c+) (a), T cells (CD45+CD3+B220−), B cells (CD45+CD3−B220+) and NK cells (CD45+CD3−NK1.1+) (b). c Representative flow cytometry profiles and the absolute number of macrophages in the liver of WT and SLAMF8/9-deficient mice injected with PBS or clodronate liposome prior to LPS/GalN injection. d The serum activities of ALT and AST were measured 6 h after LPS/GalN challenge in the control and macrophage-deletion mice. e The serum levels of TNF-α, IL-1β, and IL-6 were measured by ELISA 6 h after LPS/GalN challenge. All data represent at least three independent experiments, and calculated data are shown as the means ± SEM
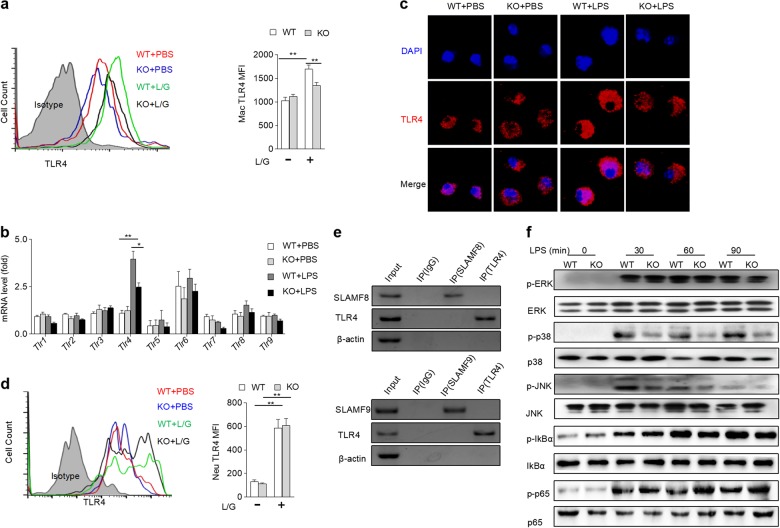
SLAMF8 and SLAMF9 maintain TLR4 expression on macrophages and promote LPS-induced MAPK activation
TLR4 is the main receptor for sensing LPS. Because both SLAMF8 and SLAMF9 have no signaling activity, we wondered whether the deficiency of SLAMF8 and SLAMF9 interferes with macrophage activation via TLR4. LPS increased the expression of TLR4 on hepatic macrophages; however, the effect was markedly attenuated by SLAMF8/9 deficiency (Fig. 6a). To confirm the intrinsic role of SLAMF8/9 in stabilizing TLR4 expression, BMDMs were stimulated by LPS in vitro. This treatment also substantially elevated TLR4, while the mRNA transcript levels of other TLR family members in BMDMs were intact. However, the loss of SLAMF8/9 inhibited the upregulated expression of TLR4 induced by LPS in BMDMs (Fig. 6b). To confirm this finding, the cellular distribution of the TLR4 expression was further detected with immunofluorescence. In a resting state, the amount of TLR4 on macrophages was comparable between the two genotypes. Nevertheless, LPS treatment increased the level of TLR4 on the macrophages from the WT mice but not on those from the SLAMF8/9-deficient mice (Fig. 6c). Although the loss of SLAMF8/9 decreased neutrophil accumulation in the liver after LPS/GalN shock, the expression of TLR4 on neutrophils was not affected (Fig. 6d). To investigate whether SLAMF8 and SLAMF9 stabilize TLR4 expression through direct binding, a coimmunoprecipitation assay was performed. The results showed that SLAMF8 or SLAMF9 did not directly interact with TLR4 (Fig. 6e). Thus, SLAM8 and SLAMF9 promote the activation of macrophages, likely through upregulating TLR4 expression.
Fig. 6.
SLAMF8 and SLAMF9 indirectly maintain TLR4 expression and regulate MAPK activation on macrophages. a The TLR4 expression level on liver macrophages from WT and KO mice was detected with flow cytometry after LPS/GalN (L/G) shock. b BMDMs from WT and KO mice were stimulated with 100 ng/mL LPS for 6 h, and TLR1-9 mRNA expression level was detected using qRT-PCR. c BMDMs from WT and SLAMF8,9-deficient mice were stimulated with LPS (100 ng/mL) for 6 h, and the expression level of TLR4 was detected using immunofluorescence. Blue staining indicates DAPI, and red staining indicates TLR4. d The TLR4 expression level on liver neutrophils from WT and KO mice was detected with flow cytometry. e The interaction of SLAMF8 or SLAMF9 with TLR4 was examined by coimmunoprecipitation. f BMDMs from WT and SLAMF8,9-deficient mice were stimulated with LPS (50 ng/mL) for the indicated time points, and the activities of p38, JNK, ERK, IkBα, and p65 were examined using western blot analysis. The total protein levels of p38, JNK, ERK, IkBα, and p65 were also measured. All data represent at least three independent experiments, and calculated data are shown as the means ± SEM
LPS binding to TLR4 can activate downstream signaling, such as MAPK and nuclear factor-κB signaling, which subsequently triggers the gene transcription of various inflammatory cytokines.23 To further understand whether the failure to upregulate TLR4 receptors caused by SLAMF8/9 deficiency can negatively affect TLR4 signaling transduction, the phosphorylation of major downstream molecules was detected in LPS-treated BMDMs. As expected, LPS stimulation increased the amount of phosphorylated p38 and JNK, two major MAPKs. However, the loss of SLAMF8/9 abolished this effect (Fig. 6e). These results indicate that SLAMF8 and SLAMF9 promote macrophage activation, likely via the stabilization of TLR4 expression and subsequent maintenance of MAPK activation.
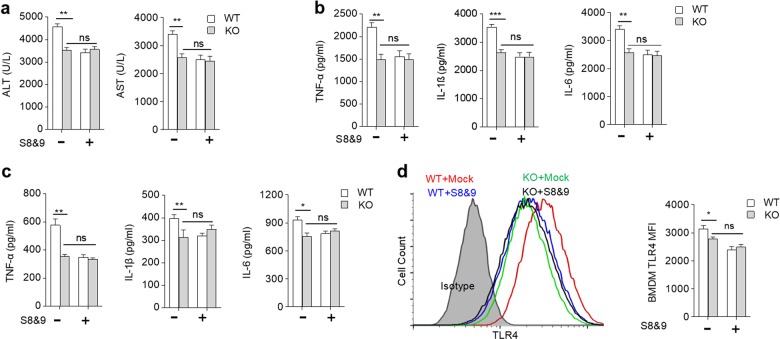
Blockage of SLAMF8 and SLAMF9 with their extracellular soluble protein can prevent LPS/GalN-induced liver injury
Previous studies confirmed that SLAMF8 and SLAMF9 are homophilic receptors.12,24 We thus attempted to explore whether blocking SLAMF8- and SLAMF9-mediated interactions with the soluble extracellular domain of SLAMF8 and SLAMF9 can prevent LPS-induced liver injury. The truncated SLAMF8 and SLAMF9 proteins were obtained using a prokaryotic expression system. We found that the WT mice treated with soluble SLAMF8 and 9 protein prior to LPS injection exhibited lower ALT and AST levels. This effect was not observed in the SLAMF8/9-deficient mice (Fig. 7a). The administration of soluble SLAMF8 and 9 protein significantly decreased the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in the WT mice, but not in the SLAMF8/9−/−mice (Fig. 7b). This treatment could also inhibit BMDM secretion of pro-inflammatory cytokines in vitro (Fig. 7c). Thus, these results suggest that truncated SLAMF8 and SLAMF9 protein can prevent LPS-induced liver injury through self-blocking. We also found that treatment with SLAMF8 and SLAMF9 proteins could minimize TLR4 expression on BMDMs (Fig. 7d). Taken together, the blockage of nonclassical SLAM family receptors attenuates LPS-induced inflammation by attenuating TLR4 expression and macrophage activation.
Fig. 7.
Blockage of SLAMF8 and SLAMF9 alleviates LPS/GalN-induced liver injury. a ALT and AST activities were measured 6 h after LPS/GalN challenge in mice treated with SLAMF8 plus SLAMF9 (S8&9) truncated protein. b The concentrations of TNF-α, IL-1β, and IL-6 in serum were measured using ELISA. c WT and SLAMF8,9-deficient BMDMs were treated with 20 µg/mL S8&9 for 16 h and then stimulated with LPS (100 ng/mL) for 6 h; the concentrations of TNF-α, IL-1β, and IL-6 were measured using ELISA. d WT and SLAMF8,9-deficient BMDMs were treated with 20 µg/mL S8&9 or mock protein for 16 h and then stimulated with LPS (100 ng/mL) for 6 h. The TLR4 expression level was detected using FCM. All data represent at least three independent experiments, and calculated data are shown as the means ± SEM
Discussion
In contrast to classical SLAMF receptors, SLAMF8 and SLAMF9 have no signaling motifs in their short cytoplasmic tail. They are strictly expressed by various myeloid cells, such as neutrophils, macrophages, and dendritic cells. To date, there are few reports regarding the characteristics of SLAMF8 and SLAMF9. We demonstrate that SLAMF8 and SLAMF9 co-regulate macrophage-mediated liver inflammation by maintaining TLR4 expression following LPS exposure, which suggests that SLAMF8 and SLAMF9 are potential therapeutic targets for acute hepatic injury.
Both SLAMF8 and SLAMF9 are positioned at chromosome 1, very close to the locus of classical SLAM family members. By analyzing the RNA-sequencing data reported by other research groups, combined with the results of our experiments,19,22 we determined that SLAMF8 and SLAMF9 are mainly expressed on myeloid cells, particularly on macrophages. Given that SLAMF8 and SLAMF9 have a similar expression pattern and chromosome location, as well as recognition site, these two receptors are probably functionally redundant. In this study, we generated SLAMF8 and SLAMF9 double-deficient mice. In a resting state, the loss of SLAMF8 and SLAMF9 did not impair immune cell homeostasis. There were normal numbers of lymphocytes and myeloid cells in the spleen and liver tissues. As expected, nonclassical SLAMF8 and SLAMF9 are not only dispensable for SAP-dependent humoral immunity but they also exhibit no inhibitory signaling even when SAP is missing. It remains to be determined whether these two receptors can transmit positive signaling mediated by EAT-2, a homolog of SAP, or inhibitory signaling by other SH2-domain containing phosphatases, such as SHP1 and SHP2.
Previous reports have demonstrated that macrophages play an important role in LPS/GalN-induced acute liver injury5, and our results confirmed that macrophage deletion inhibited LPS/GalN-induced acute hepatic failure. Given that SLAMF8 and SLAMF9 are mainly expressed on macrophages, we further investigated the role of SLAMF8 and SLAMF9 in macrophage-mediated LPS/GalN-induced fulminant hepatic injury. Although macrophage differentiation was not altered by the combined deficiency of SLAMF8 and SLAMF9, the loss of these two receptors significantly protected against lipopolysaccharide (LPS)-induced liver injury. SLAMF8 and 9 double-deficient mice had a prolonged survival rate and less infiltration of inflammatory cells in the liver. The depletion of macrophages using clodronate liposome abolished the effect of SLAMF8 and SLAMF9 deficiencies on endotoxin-induced liver injury, which demonstrates that these receptors are required for macrophage activation following LPS challenge.
TLR4 is the main receptor for sensing LPS. Because SLAMF8 or SLAMF9 do not have signaling activity, we investigated whether the deficiency affects TLR4 expression. Activated TLR4 stimulates MAPK and nuclear factor-kB signaling, thereby stimulating the release of inflammatory cytokines. MAPK signaling regulates the inflammatory response and is a target of anti-inflammatory drugs.25–27 As expected, the current data demonstrated that the loss of SLAMF8 and SLAMF9 prevented the elevated expression of TLR4 in liver macrophages and BMDMs after LPS treatment. The subsequent activation of MAPK signaling was also depressed in SLAMF8- and SLAMF9-deficient macrophages after stimulation by LPS. These data may explain why there were lower concentrations of plasma pro-inflammatory cytokines in SLAMF8- and SLAMF9-deficient mice than that in the WT control group.
Because SLAMF8 and SLAMF9 are homophilic receptors8,9, the soluble extracellular domain of SLAMF8 and SLAMF9 was used to block SLAMF8- and SLAMF9-mediated interactions. The results indicated that both SLAMF8 and SLAMF9 blockage protected against LPS/GalN-induced liver injury by inhibiting the expression of TLR4 and thus inhibiting the production of proinflammatory cytokines. Thus, the blockage of nonclassical SLAM family receptors likely represents a novel therapeutic approach for curing endotoxin-induced liver damage. In summary, our studies provide important insights into the regulation of innate immune responses by SLAMF8, 9 through the MAPK pathways. We have shown that SLAMF8, 9 positively regulate TLR4 expression, LPS shock and proinflammatory cytokine production in vivo and in vitro. We propose that SLAMF8, 9 may be potential therapeutic targets in LPS-induced sepsis. The results provide insights into the role of SLAMF8 and SLAMF9 in LPS/GalN-induced liver failure and suggest that SLAMF8 and SLAMF9 may be potential therapeutic targets for acute hepatic injury.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81725007, 81771666, and 81471523), Natural Science Foundation of Beijing Municipality (5172018) and 111 Project (B16201).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meixiang Yang, Phone: +86 13422034040, Email: yangmxqilu@163.com.
Zhongjun Dong, Phone: +86-10-62798536, Email: dongzj@biomed.tsinghua.edu.cn.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0191-z) contains supplementary material.
References
- 1.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, et al. Complement and the alternative pathway play an important role in LPS/D-GalN-induced fulminant hepatic failure. PLoS One. 2011;6:e26838. doi: 10.1371/journal.pone.0026838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmocker C, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45:864–869. doi: 10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 4.Ma K, Zhang Y, Zhu D, Lou Y. Protective effects of asiatic acid against d-galacto-samine/lipopolysaccharide-induced hepatotoxicity in hepatocytes and kupffer cells co-cultured system via redox-regulated leukotriene C4 synthase expression pathway. Eur. J. Pharmacol. 2009;603:98–107. doi: 10.1016/j.ejphar.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui H, Nishiguchi S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int. J. Mol. Sci. 2014;15:7711–7730. doi: 10.3390/ijms15057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl Acad. Sci. USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu FL, et al. Glycine attenuates endotoxin-induced liver injury by downregulating TLR4 signaling in Kupffer cells. Am. J. Surg. 2008;196:139–148. doi: 10.1016/j.amjsurg.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 9.Chi H, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl Acad. Sci. USA. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino K, et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 11.Calpe S, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 12.Van Driel BJ, Liao G, Engel P, Terhorst C. Responses to microbial challenges by SLAMF receptors. Front. Immunol. 2016;7:4. doi: 10.3389/fimmu.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Calisto J, et al. SAP-dependent and -independent regulation of innate T cell development involving SLAMF receptors. Front. Immunol. 2014;5:186. doi: 10.3389/fimmu.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, et al. The self-specific activation receptor SLAM family is critical for NK cell education. Immunity. 2016;45:292–304. doi: 10.1016/j.immuni.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, et al. Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J. Exp. Med. 2017;214:475–489. doi: 10.1084/jem.20161312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 17.Wu CB, et al. Genomic organization and characterization of mouse SAP, the gene that is altered in X-linked lymphoproliferative disease. Immunogenetics. 2000;51:805–815. doi: 10.1007/s002510000215. [DOI] [PubMed] [Google Scholar]
- 18.Fennelly JA, Tiwari B, Davis SJ, Evans EJ. CD2F-10: a new member of the CD2 subset of the immunoglobulin superfamily. Immunogenetics. 2001;53:599–602. doi: 10.1007/s002510100364. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Astiaso D, et al. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, et al. Cutting edge: Slamf8 is a negative regulator of Nox2 activity in macrophages. J. Immunol. 2012;188:5829–5832. doi: 10.4049/jimmunol.1102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Z, et al. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity. 2012;36:974–985. doi: 10.1016/j.immuni.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, et al. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappa B pathways. Sci. Rep. 2017;7:44252. doi: 10.1038/srep44252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang GX, et al. Migration of myeloid cells during inflammation is differentially regulated by the cell surface receptors Slamf1 and Slamf8. PLoS One. 2015;10:e0121968. doi: 10.1371/journal.pone.0121968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajaiah R, Perkins DJ, Ireland DD, Vogel SN. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc. Natl Acad. Sci. USA. 2015;112:8391–8396. doi: 10.1073/pnas.1424980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, et al. Differential role for p120-catenin in regulation of TLR4 signaling in macrophages. J. Immunol. 2014;193:1931–1941. doi: 10.4049/jimmunol.1302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh M, Subramani J, Rahman MM, Shapiro LH. CD13 restricts TLR4 endocytic signal transduction in inflammation. J. Immunol. 2015;194:4466–4476. doi: 10.4049/jimmunol.1403133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.