Abstract
Background
As genome-wide approaches prove difficult with genetically heterogeneous orphan diseases, we developed a new approach to identify candidate genes. We applied this to Emery-Dreifuss muscular dystrophy (EDMD), characterised by early onset contractures, slowly progressive muscular wasting, and life-threatening heart conduction disturbances with wide intra- and inter-familial clinical variability. Roughly half of EDMD patients are linked to six genes encoding nuclear envelope proteins, but the disease mechanism remains unclear because the affected proteins function in both cell mechanics and genome regulation.
Methods
A primer library was generated to test for mutations in 301 genes from four categories: (I) all known EDMD-linked genes; (II) genes mutated in related muscular dystrophies; (III) candidates generated by exome sequencing in five families; (IV) functional candidates — other muscle nuclear envelope proteins functioning in mechanical/genome processes affected in EDMD. This was used to sequence 56 unlinked patients with EDMD-like phenotype.
Findings
Twenty-one patients could be clearly assigned: 18 with mutations in genes of similar muscular dystrophies; 3 with previously missed mutations in EDMD-linked genes. The other categories yielded novel candidate genes, most encoding nuclear envelope proteins with functions in gene regulation.
Interpretation
Our multi-pronged approach identified new disease alleles and many new candidate EDMD genes. Their known functions strongly argue the EDMD pathomechanism is from altered gene regulation and mechanotransduction due to connectivity of candidates from the nuclear envelope to the plasma membrane. This approach highlights the value of testing for related diseases using primer libraries and may be applied for other genetically heterogeneous orphan diseases.
Funding
The Wellcome Trust, Muscular Dystrophy UK, Medical Research Council, European Community's Seventh Framework Programme “Integrated European –omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS)”.
Keywords: Emery-Dreifuss muscular dystrophy, Nuclear envelope, Nuclear envelope transmembrane protein, primer library, Orphan disease
Research in context.
Evidence before this study
Emery-Dreifuss muscular dystrophy (EDMD) is a genetically heterogeneous orphan disease with clinical variability presenting even in family members carrying the same mutation. The lack of large pedigrees in combination with its genetic heterogeneity (~50% of patients are solved with mutations in 6 genes), clinical variability, several known modifier genes, limited patient numbers, and an unsolved pathomechanism make the usage of genome-wide association sequencing studies ineffective. EDMD also presents a conundrum in that all previously known alleles are widely expressed nuclear envelope (NE) proteins, so how a muscle focused pathology is achieved remains obscure. Moreover the proteins involved have known functions in nuclear/cellular mechanical stability, genome regulation, and cell cycle regulation, so that the EDMD pathomechanism remains unclear.
Added value of this study
The new fully solved and candidate alleles encode proteins that form a physical connection from the plasma membrane to the nuclear envelope, further elaborating genome regulation and mechanosignal transduction as the pathomechanism and supporting the validity of new candidates. This connectivity together with that several of the new candidate alleles are muscle-specific resolves the conundrum of how a muscle-focused pathology could come from widely-expressed proteins. The range of proteins that can be disrupted through these connections moreover can help explain the clinical variability and also the overlaps to similar muscular dystrophies. Notably, the larger set of candidates from the exome sequencing step in our approach likely contains also modifying alleles that may be revealed with further use of the primer library.
Implications of all the available evidence
If this patient cohort is representative of the general patient population this approach likely solves >90% of the ~50% remaining genetically unsolved patients, and in doing so strongly implicates altered gene regulation and mechanotransduction as its pathomechanism. Furthermore, in addition to the potential application of this approach to other genetically heterogeneous orphan diseases, the primer library once generated provides a better and cheaper sequencing strategy for newly diagnosed patients compared to traditional Sanger sequencing of one linked gene at a time. The EDMD patient association has noted a problem with the time from clinical presentation to diagnosis that will be resolved with the wider application of this primer library and allow earlier interventions to improve disease management.
Alt-text: Unlabelled box
1. Introduction
Emery-Dreifuss muscular dystrophy (EDMD) is a rare neuromuscular disorder affecting ~0.3–0.4 in 100,000 people [1,2]. EDMD patients present typically in childhood with early contractures of elbows and Achilles’ tendons, progressive wasting of lower leg and upper arm muscles, and later development of cardiac conduction defects and, in a proportion of cases, dilated cardiomyopathy [3]. Features vary considerably in clinical presentation, leading to the usage 'Emery-Dreifuss-like syndromes' [4,5]: patients from the same pedigree can show remarkable phenotypic variation [6]. Consistent with this variation, EDMD is also genetically variable: ~half of Emery–Dreifuss-like syndrome cases are linked to mutations in genes encoding 6 nuclear envelope proteins (emerin, lamin A, nesprin 1, nesprin 2, SUN1 and FHL1 [7], [8], [9], [10], [11]). Variants in desmin and the nuclear envelope proteins SUN1 and SUN2 have been reported to modify the EDMD phenotype [10,12]. Roughly half of clinically diagnosed patients remain unlinked [13].
The strong nuclear envelope link raised the possibility that remaining unlinked patients might also have mutations in nuclear envelope proteins. The nuclear envelope is linked to >30 inherited diseases and syndromes [14], each with distinct tissue-specific pathologies: for example different lamin A mutations cause muscular dystrophies, neuropathy, lipodystrophy, and multisystemic disorders. How these widely expressed nuclear envelope proteins yield tissue-specific pathologies remains unresolved, but one hypothesis is that tissue-specific nuclear envelope partners mediate the tissue-specificity of effects [15].
We previously identified several muscle-specific nuclear envelope transmembrane proteins (NETs) [16]. Of the previously linked proteins emerin, nesprin 1, nesprin 2, SUN1, SUN2, and Tmem43 are all NETs, but these are widely expressed. Several of the muscle-specific NETs identified could contribute muscle specificity to either of the two principly hypothesized EDMD pathomechanisms: mechanical instability and disruption of gene expression. NETs Tmem214 and KLHL31 track with microtubules on the nuclear surface [16] while NET5/Samp1 contributes actin and centrosome interactions [17]. NETs Tmem38A, WFS1, NET39/PLPP7 and, again, Tmem214 and NET5/Samp1 all affect 3D gene positioning with corresponding effects on gene expression [18,19]. Many of the genes under muscle-specific NET regulation are recruited to the nuclear periphery to be more tightly shut down during myogenesis and encode proteins that are antagonistic to myogenesis or are from alternative differentiation pathways such as adipogenesis. Knockdown of the muscle-specific NETs results in these genes being de-repressed, suggesting a possible gene misregulation mechanism to disease pathology. The potential of gene mispositioning contributing to disease is further underscored by knockdown of Tmem38A, WFS1, and NET39/PLPP7 blocking myotube fusion [18]. Functional overlap of these muscle-specific NETs supports the possibility of their working in a common pathway towards EDMD pathophysiology, making them good candidates for mediators of EDMD muscle pathology at the same time as being novel candidates for causative EDMD alleles.
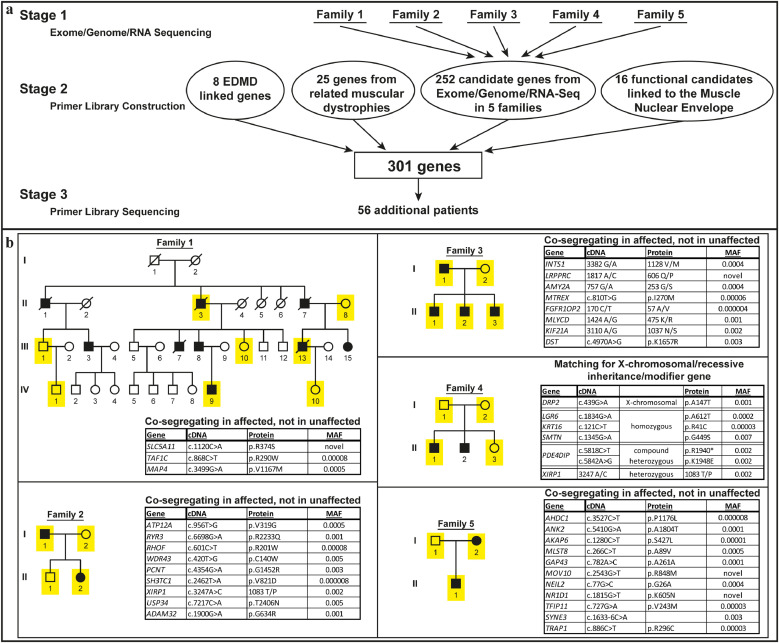
Therefore, we elected to sequence the genes encoding these muscle-specific NETs in unlinked EDMD patients using a primer library. However, for greater surety, we expanded this primer library to also re-check previously linked genes with complete gene sequencing for possible promoter mutations and to test for mutations in genes linked to related muscular dystrophies. Finally, to also search for candidate alleles in a completely unbiased manner, we performed exome sequencing in families for which material from enough members was available for linkage analysis and added these candidates also to the primer library (Fig. 1a).
Fig. 1.
Methodology and top candidate mutations from exome sequencing. (a) Flow chart describing the study methodology. (b) Pedigrees of the 5 families used for the initial exome sequencing with top candidate mutations listed in the adjacent boxes (MAF = minor allele frequency). The presented top candidate mutations were identified in all sequenced affected family members, but not in unaffected family members. Sequenced individuals: yellow; males: square; females: circles; patients: filled black. The presented top candidate mutations were identified in all sequenced affected family members, but not in unaffected family members.
2. Materials and methods
2.1. Patient materials
All patient DNA used for sequencing was obtained from blood samples. RNA was obtained from deltoid muscle from the family 1 index patient and matching control. The sources of patient samples were: the Muscle Tissue Culture Collection (MTCC) at the Friedrich-Baur-Institut (Department of Neurology, Ludwig-Maximilians-University, Munich, Germany); the Institute of Human Genetics, University of Newcastle upon Tyne, Newcastle upon Tyne, UK; the MRC Centre for Neuromuscular Disorders Biobank (CNDB) in London; the Department of pediatric Neurology, Developmental Neurology and Social Pediatrics at the University of Essen; the Rare Diseases biological samples biobank at the Dubowitz Neuromuscular Centre, Great Ormond Street Hospital for Children NHS Trust, London, UK.
2.2. Ethical approval and consent to participate
All materials were obtained with written informed consent of the donor at the CIND, the CNDB or the MTCC. Ethical approval for the Newcastle MRC Centre Biobank for Neuromuscular Diseases is covered by REC reference 08/H0906/28+5 and IRAS ID 118,436 and MTA CT-2166, that of the Rare Diseases biological samples biobank for research to facilitate pharmacological, gene and cell therapy trials in neuromuscular disorders is covered by REC reference 06/Q0406/33 with MTA reference CNMDBBL63 CT-2925/CT-1402, and for this particular study was obtained from the West of Scotland Research Ethics Service (WoSRES) with REC reference 15/WS/0069 and IRAS project ID 177,946.
2.3. Exome, RNA, and genome sequencing
Genome: 15X clean depth coverage using 90PE Illumina HiSeq2000 technology. RNA-Seq: total RNA from biopsy tissue with rRNA depletion and random-primed cDNA preparation and PE100 sequencing on a Hi-Seq2000 platform with 20 million reads minimum (Otogenetics Corporation, Norcoss, USA).
Exome: Sequencing was performed on the Illumina HiSeq and raw data processed with CASAVA 1.8.
2.4. Fluorescence in situ hybridisation
Mutations were generated by Agilent Site-Directed mutagenesis. Plasmids encoding tagged Tmem38a, PLPP7 and mutants were transfected using Lipofectamine 3000 (Invitrogen) into C2C12 cells (ATCC, VA, USA) cultured at 37 °C, 5% CO2 in DMEM containing 20% FBS, 50 U/mL penicillin and 10 mg/mL streptomycin. Fluorescent in situ hybridisation (FISH) experiments were performed as described in [20].
2.5. Primer library construction, processing and sequencing
A SureSelectXT Custom 1.638 Mbp target enrichment library (5190–4817) containing 25,036 oligonucleotide probes against H. sapiens hg19 GRCh37 sequence as of February 2009 was prepared by Agilent for use with Illumina multiplexed sequencing platforms. Patient genomic DNA was isolated from blood and prepared for sequencing using the SureSelectQXT Reagent Kit (G9681B) according to the manufacturer's instructions. Recommended minimum sequencing per sample was 327.793 Mbp and an average of 3427,092 was obtained with a range from 442,125 to 7066,507 using 125 base paired-end sequencing on a Hi-Seq2500.
2.6. Bioinformatics and analysis
Variant analysis was performed using the Genome Analysis toolkit [GATK] v2.7–2 [21] and picard tools v1.74 (http://broadinstitute.github.io/picard/) using GATK Best Practices recommendations [22,23] against human genome assembly hg19. The allele frequencies of variants were cross-referenced with gnomAD version 2.1 [24] using both the genome and exome datasets.
RNA-Seq: STAR v2.1.1 [25] was used to map reads to the hg19 reference genome, samtools v0.1.19 [26] was used for file conversion. Deeptools v1.5.1 [27] was used for downstream analysis.
3. Results
3.1. Sequencing EDMD families
Whole exome sequencing was performed in 12 EDMD patients and 12 unaffected individuals from 5 families with large enough pedigrees for linkage analysis (Fig. 1b), finding over 250,000 variants compared to the reference sequence. Variants were filtered using criteria: (a) phenotype co-segregation and modes of inheritance for each family; (b) selecting for SNP frequencies <1%, and filtering for <0.05%; (c) affecting coding sequence; (d) function/tissue-expression of the encoded protein e.g. >2-fold higher expression in muscle compared to other tissues. Filtering yielded 213 candidate genes for families 2–5 (Supplemental Table S1).
Family 1 yielded no convincing candidates. As this family had the largest pedigree, we postulated that an unaffected individual was a carrier who had not yet presented or had a distinct sporadic form of disease. Dropping younger individuals who may have not yet presented clinically failed to yield candidates; therefore, genome and transcriptome sequencing was performed on the index patient, resulting in 33 additional candidates (Supplemental Tables S2 and S3). The combined exome, genome and RNA sequencing yielded a total of 252 candidates from the five families.
3.2. Primer library sequencing
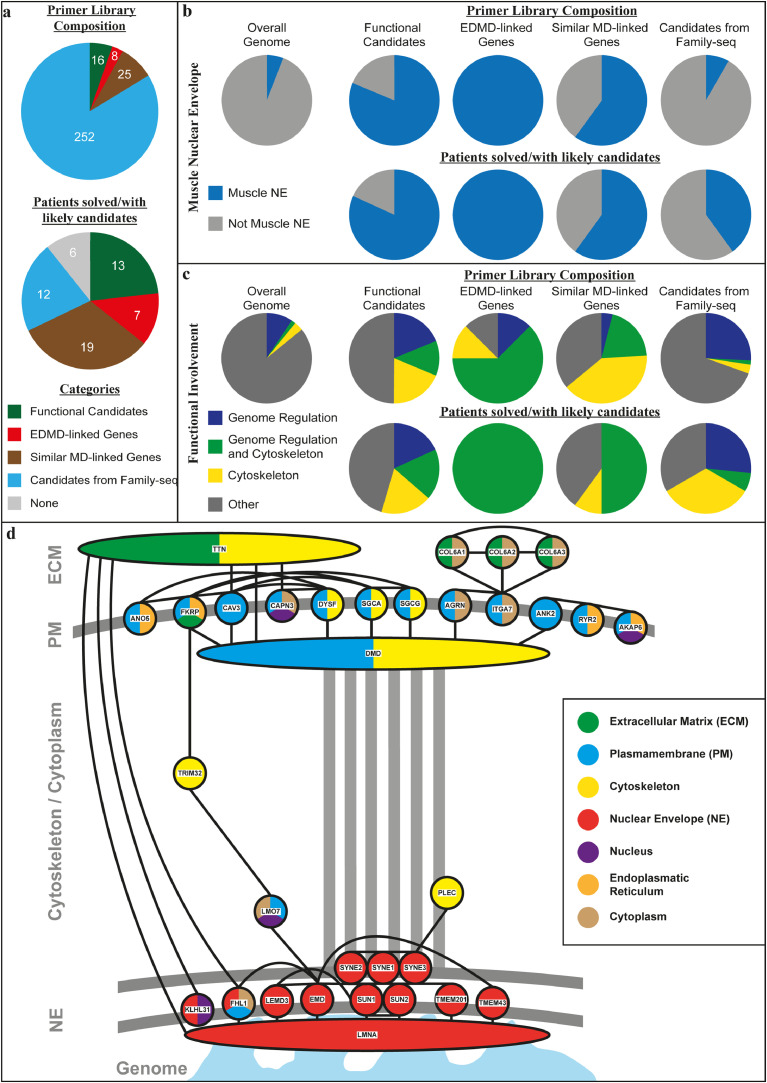
A primer library was generated containing (I) the 8 previously-linked EDMD gene ORFs plus the whole genes for LMNA and EMD (that together account for ~40% of linked alleles), (II) 25 genes from similar muscular dystrophies, (III) the 252 exome sequencing candidates, and (IV) 16 functional candidates, mostly muscle-specific nuclear envelope proteins (Fig. 2a; Supplemental Table S4). Sequencing was performed on 56 additional unlinked clinically diagnosed EDMD patients unrelated to each other, obtaining on average 3427,092 reads per patient. The data were analysed for genes carrying mutations that changed the coding sequence (nonsense, missense, splice sites) with expected altered protein function (e.g. non-conservative substitutions) and SNP frequencies <0.05% (Supplemental Table S5).
Fig. 2.
Primer library composition and gene ontology (GO) functions/localisations of all candidate genes from the four categories contributing to the primer library construction and for the top candidates identified after primer library sequencing. (a) Composition of the primer library with number of genes from each of the four categories used in its construction (upper panel) and number of patients solved/with likely candidates from the different categories after primer library sequencing (lower panel). (b) Presence in muscle nuclear envelopes for the starting library in comparison to the overall genome (upper panel) and of the remaining candidate genes after primer library sequencing (lower panel) in percent (based on GO-localisation terms and/or experimental evidence from appearance in nuclear envelope proteomics datasets20,28). (c) GO-terms for genome organisation, cytoskeleton, and genome organisation and cytoskeleton combined functions involvement for the starting library in comparison to the overall genome (upper panel) and of the remaining candidate genes after primer library sequencing (lower panel) in percent, showing an enrichment for the combined category in the top candidate alleles. (d) Interactive network of remaining candidate genes after library sequencing based on STRING (Search Tool for the Retrieval of Interacting Genes/Proteins, https://string-db.org/) interactions (high confidence) showing that most candidates are linked to other candidates and that these form connections from the nuclear envelope to the plasma membrane. These connections are consistent with possible mechanotransduction from the extracellular region to the nuclear envelope being the core disrupted function in EDMD. Different described localisations of proteins are displayed by colour-coding (based on GO-terms and/or experimental evidence through identification in muscle nuclear envelope proteomics datasets20).
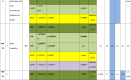
Candidate mutations were found in all four categories. Of category I previous EDMD-linked genes, LMNA had mutations in three patients that were missed in standard diagnostics (p.R41H, p.R249Q, p.G535fs*; Table 1). These mutations were determined as causative based on similarity to previously linked LMNA mutations. Previously EDMD-associated genes SYNE1, SYNE2, SUN1 and TMEM43 also had mutations; however, minor allele frequencies and their combination with other mutations made them unlikely as causative alleles excepting SYNE1. Modifying effects, nonetheless, cannot be excluded. No mutations were found in LMNA or EMD non-coding regions.
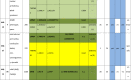
Table 1.
Solved Patients.
 |
 |
 |
 |
 |
 |
 |
 |
Dark green: known disease associated genes (EDMD or similar MDs) with likely disease causing mutation (category 2 and 3).
Light green: known disease associated genes (EDMD or similar MDs) with unlikely disease causing mutation or two genes of similar likelihood to be the causative disease allele (category 2 and 3).
Yellow: functional candidate gene mutations (category 1).
Purple: mutations in genes from the family sequencing (category 4).
Gene category II of related muscular dystrophies yielded 18 patients with mutations considered causative. Four of these patients had combinations of a missense and frameshift mutation in CAPN3 (Table 1). GBE1 mutations were found in four other patients: three missense and one splice-site. VCP and likely recessive TTN were mutated in two patients each; however, TTN mutation patients also carried SYNE1 mutations. Genes with one patient carrying likely disease-causing mutations were COL6A1, CAV3, DMD, ANO5, DYSF and POMT1. The DMD mutation created a stop codon at codon three, resulting in possible usage of an alternative start codon and a milder phenotype than Duchenne [28]. For ANO5, DYSF and POMT1 the respective patients had two mutations, consistent with the reported inheritance (autosomal recessive for MD-20/ANO5 and unknown for MD-21/DYSF and MD-23/POMT1; Table 1). However, lacking DNA from the parents we could not perform segregation studies.
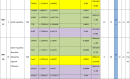
Category III genes from exome sequencing were elevated to "likely-causative" candidates if also mutated in multiple patients within the primer library cohort based on the assumption that causative genes will be independently mutated in multiple patients. The top candidates were INTS1, ANK2, XIRP1 and USP34. Heterozygous ANK2 mutations were identified in family 5 plus six cohort patients with no other obvious disease-causing mutations and so were most likely causative (Table 2). Causation is similarly likely for other genes; however, in some patients there were additional candidate alleles identified. Heterozygous INTS1 was mutated in four members of family 3 plus five cohort patients, four of whom had no mutation in already associated genes (Table 2). The last patient, MD-23, additionally carried two POMT1 mutations; however, it is unclear if the likely recessive POMT1 mutations affected one allele or both so causation remains undetermined. Other good category IV candidates were USP34 (heterozygous mutations in exome sequenced family 2) and XIRP1 (mutated in families 2 and 4), each with mutations in an additional five patients. Some patients had additional mutations in already associated genes, but if these other mutations were causative then modifying effects for the new candidates are still possible.
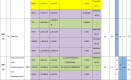
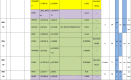
Table 2.
Patients with Candidate Genes.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
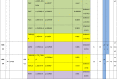 |
Light green: known disease associated genes (EDMD or similar MDs) with unlikely disease causing mutation or two genes of similar likelihood to be the causative disease allele (category 2 and 3).
Yellow: functional candidate gene mutations (category 1).
Purple: mutations in genes from the family sequencing (category 4).
Several category IV functional/tissue candidate genes were mutated in 16 of the 56 primer library cohort patients. These were WFS1 (4 patients), TMEM201 (3 patients), TMEM38A (3 patients), PLPP7 (2 patients), TMEM214 (2 patients), LPCAT3 (1 patient), KLHL31 (1 patient), and BVES (1 patient). Of these, three patients with TMEM38A mutations, two patients with TMEM214 mutations, one patient with an LPCAT3 mutation and one patient with a BVES mutation were clearly the top candidates with no other reasonable candidates identified and patient MD-32 carried mutations in both TMEM38A and PLPP7. Other mutations identified were in association with other possible candidates that included likely causative mutations in GBE1, COL6A1, LMNA and TTN (details in Table 1). The patient with the combined LMNA and TMEM201 mutations had a very early age of onset (1 year), suggesting that both mutations contribute to the more severe (congenital) phenotype as the LMNA mutation has not been associated with congenital muscular dystrophy.
All in all, sequencing the 56 additional patients with the primer library found mutations in only a subset of the 252 candidates from the exome sequencing and this subset is expected to be much higher confidence because causative genes are more likely to be also mutated in other EDMD patients. In contrast, mutations were found in 19 of 25 related muscular dystrophies and in 11 of 16 functional candidates; so a strong enrichment for these candidate pools was observed (Fig. 2a).
3.3. Nuclear envelope links
All previously linked EDMD genes encode nuclear envelope proteins. The functional candidates were also biased towards genes identified in the nuclear envelope by proteomics; however, there was no bias towards the nuclear envelope when selecting genes for the primer library from similar muscular dystrophies or from exome sequencing. Nonetheless, the majority of genes from similar muscular dystrophies encode proteins for which at least a subpopulation associates with the nuclear envelope (Fig. 2b). Interestingly, just considering the candidates from the exome sequencing in which mutations were also found in other patients from the primer library sequencing, the nuclear envelope portion increased from less than 10% to more than 40% - considerably more than the overall genome portion of 5.9% (Fig. 2b). Of note, the proteins encoded by genes linked to other muscular dystrophies such as COL6A1, CAV3, DYSF, DMD, TTN, and VCP and the strongest family sequencing candidates INTS1 and ANK2 were all found in nuclear envelope proteomics datasets [16,29]. While these could reflect either a separate pool in the nuclear envelope or connections that were maintained during nuclear envelope isolation, this suggests at least an indirect physical connection of these candidates to the nuclear envelope.
The two top argued mechanisms for how mutations in nuclear envelope proteins can cause pathology are mechanical instability and genome misregulation. Genes in different candidate categories contained Gene Ontology (GO)-terms for functions in gene regulation, cytoskeleton, and both together. Interestingly, the likely candidates from all categories were enriched for genes simultaneously linked to both gene regulation and cytoskeleton GO-terms compared to the overall genome (Fig. 2c). Such genes may be involved in mechanosignal transduction to the genome. Consistent with this idea, most of the proteins encoded by the final candidate genes interact with other candidates according to interactome studies and these interactions form a chain of connectivity between the nuclear envelope and the plasma membrane via cytoskeletal proteins that could support mechanotransduction to the genome (Fig. 2d).
3.4. Confirmation of novel EDMD alleles
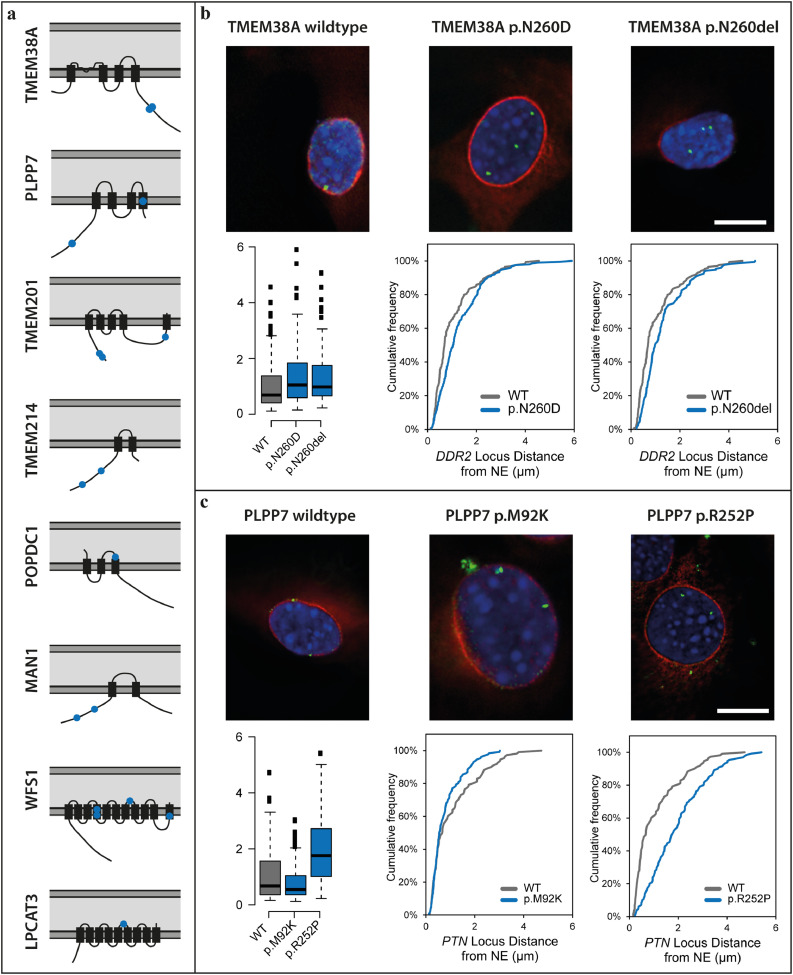
Thus far only the three LMNA mutations, the CAV3 and one of the CAPN3 (MD-43) mutations have been fully confirmed as insufficient numbers of family members have come to clinic for linkage analysis. Therefore, to test the likelihood that other mutations identified cause EDMD disease pathology, we tested two of the gene regulating NETs to determine if the mutations identified disrupt their normal functions in myogenic gene regulation. In keeping with this idea, for the 8 out of 16 functional NET candidates where mutations were found (6 of which have known gene regulation functions), nearly all mutations identified faced the nucleoplasm or were positioned where they could alter membrane topology (Fig. 3a). The two muscle-specific NETs we chose to test were PLPP7/NET39 and Tmem38A. Both recruit largely non-overlapping sets of genes to the nuclear periphery to enhance their repression and many of the genes targeted are antagonistic to myogenesis or from alternate (non-muscle) differentiation pathways [18]. Combined knockdown of PLPP7/NET39, TMEM38A and WFS1 blocked myogenesis, providing a logical route from their disruption to muscle disease pathology. Therefore, the PLPP7 and TMEM38A mutations were exogenously expressed in C2C12 myoblast cells to determine if they could perform the previously shown gene positioning function of the wild-type in recruiting specific gene targets to the nuclear periphery for enhanced repression. Tmem38A normally repositions the DDR2 gene locus to the nuclear envelope to repress it during myogenesis, but with mutations p.N260D and p.N260del it fails to do so (Fig. 3b). Similarly, PLPP7/NET39 normally recruits the PTN gene locus to the nuclear envelope to repress it during myogenesis, but with mutation p.R252P it could not. PLPP7/NET39 mutation p.M92K also affected the gene positioning, though apparently in the opposite direction which might also affect expression (Fig. 3c). Testing effects of both PLPP7 and TMEM38A mutations on nuclear morphology [30] did show that only TMEM38A p.N260D had a slight increase in numbers of abnormally shaped nuclei (Supplementary Fig. 1).
Fig. 3.
Mutations in muscle gene-repositioning NETs affect their ability to recruit genes to the nuclear envelope (NE). (a) Schematic presentation of the topology of further muscle NETs and their mutations identified by the primer library sequencing. The lipid bilayers of the nuclear envelope are shown in dark grey and the lumen of the nuclear envelope in light grey. Transmembrane segments are thicker black rectangles and point mutations identified are shown in blue. The mutations identified are all positioned in nucleoplasmic regions where they could either interact with the genome or at transmembrane spans where they could disrupt protein topology and hence also genome interactions. (b) FISH showing the localisation of the DDR2 gene (green) in C2C12 mouse myoblasts upon the expression of RFP-tagged wild type and mutant TMEM38A that can be seen in both cases to target to the nuclear envelope (red, upper panel). The cumulative frequency of the distance of the gene loci to the NE for each mutation compared to the wild type is shown under each image of the cells expressing the mutant NETs and a whisker plot summary for the distance to the NE of all mutations is given in the lower left corner. Both mutations block the ability of TMEM38A to reposition the DDR2 locus to the NE. (c) FISH showing the localisation of the PTN gene (green) in C2C12 mouse myoblasts upon the expression of GFP-tagged wild type and mutant PLPP7 (red, upper panel). Cumulative frequency plots of the distance of the gene loci to the NE for each mutation and the summary for the distance to the NE of all mutations are given as in B. The mutations also affect the gene repositioning function of PLPP7. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Failure of high throughput genomic approaches to identify new disease alleles can at least in some cases be overcome by our multistage approach. This approach pinpointed candidates in part based on the preferential tissue focus of pathology and in part on the subcellular localisation of known alleles. Similarly applying filters in focusing candidates for such a multipronged approach can be applied to other genetically heterogeneous diseases.
With nearly half of EDMD cases previously linked to genes encoding 6 nuclear envelope proteins it was clear that EDMD is a nuclear envelope disease. This is strengthened by enrichment for nuclear envelope proteins amongst our top new candidate alleles. COL6A1, CAV3, DYSF, DMD, TTN, and VCP gene products were found in muscle nuclear envelopes [16]. As most of these proteins have previously been associated with the cytoskeleton or plasma membrane, their association with the nuclear envelope may be indirect through lamin-cytoskeletal connections. However, this association could also be due to splice variants that target to the nuclear envelope or specific translocation to the nucleus under certain conditions as has been shown for CAV3 family member caveolin 2. In this case, a caveolin 2 subpopulation translocates to the nucleus and interacts with lamin A to regulate histone modifications and gene expression [31].
The gene positioning defects for TMEM38A and PLPP7 mutations not only further link the nuclear envelope to EDMD, but also strengthen the idea that misregulation of myogenic gene expression is the primary cause of EDMD pathology. In addition to Tmem38A and Plpp7, the muscle NETs Tmem214, WFS1, and NET5/Samp1 all have gene-repositioning functions that contribute to gene regulation and the NET MAN1 affects gene regulation through its interactions with Smads as well as binding several chromatin partners [32]. The involvement of these muscle gene repositioning NETs, not only as novel causative alleles but also in mediating EDMD pathology caused by mutations in widely expressed nuclear envelope proteins, is further supported by WFS1, Tmem214, Tmem38A, and NET5/Samp1 being mislocalised in isolated differentiating EDMD muscle cells or muscle biopsy sections [33].
Of the previously EDMD-linked nuclear envelope proteins, Lamin A has both cytoskeletal and genome regulation functions; so its mutation could support both mechanical instability and genome misregulation hypotheses for EDMD pathophysiology [34], [35], [36], [37]. Emerin interacts with actin supporting a cytoskeletal role, but it also has many reported contributions to genome regulation through its binding DNA condensing factors BAF and HDAC3, splicing factors, the transcription factor Lmo7, and the transcriptional repressor germ cell-less [38]. FLH1 is linked to signal transduction and splice variant FHL1B targets specifically to the nuclear envelope [39]. Moreover, FHL1 has been linked to other myopathies such as X-linked myopathy with postural muscle atrophy (XMPMA) [40] via its signal transduction function.
As signalling functions could affect both gene regulation and the cytoskeleton, these mechanisms toward pathology were considered equally likely; however, a gene mis-regulation mechanism is much more likely now with the new gene-repositioning candidate alleles identified. Though there are some other disparate functions reported for several of these NETs [16,41,42], WFS1, Tmem38A/TRIC-A, NET39/ PLPP7, Tmem214 and NET5/Samp1 are all at the nuclear envelope preferentially in muscle and all share a common function in directing gene-repositioning for regulation of gene expression during myogenesis [18]. That some of these muscle-specific NETs had overlap in their functions further supports the possibility of their working in a common pathway towards EDMD pathophysiology. Interestingly, Tmem214 regulated genes exhibited considerable overlap with WFS1, Tmem38A, and NET39 regulated genes, suggesting it functions in mechanosignal transduction, while the others each had principally unique gene targets. The links of candidate alleles to gene misregulation are the more compelling in this context because the different sets of genes regulated by WFS1, Tmem38A, and NET39 — all important in myogenesis — thus support the clinical variation observed in EDMD.
Our sequencing in patients diagnosed with an EDMD-like phenotype identified mutations in several genes linked to muscular dystrophies that share clinical features with EDMD. This might reflect incorrect diagnoses or their involvement in EDMD. The latter case seems likely, considering that COL6A1, CAV3, DYSF, DMD, TTN, and VCP gene products link to the nuclear envelope. Indeed, many of these gene products interact with one another in a way that could form a chain from the plasma membrane to the nuclear envelope (Fig. 2d). This also is compelling for the gene regulation pathomechanism as this chain could enable mechanosignal transduction to the nucleus.
Finally, as the families chosen for exome sequencing all had differences in presentation, there are likely additional mutations picked up in the primer library that may be disease modifiers and so might eventually be used as predictors of severity or other aspects of presentation such as cardiac involvement once further sequencing reveals better correlations. Thus it would be beneficial to continue using this primer library diagnostically both to find these correlations and because it is cheaper and faster than standard iterative Sanger sequencing for such a genetically variable disease to identify mutations in known linked genes. In general, this iterative multipronged approach, combining into a primer library a set of preliminary candidates from exome sequencing in which only sufficient pedigrees exist for partial linkage analysis together with candidates from related disorders and candidates specific to the tissue where pathology is manifested that are associated with linked organelles and functions, might be applied to a wider range of genetically heterogeneous orphan diseases where insufficient numbers of patients are available for standard genome and exome approaches to be effective. A further benefit of this primer library is the possibility to add additional genes at a later time point. This might become necessary when a better understanding of EDMD identifies more causative or modifying genes involved in the disease pathology.
Funding sources
Funding for this work was principally provided by Wellcome Trust Grants 095209, Muscular Dystrophy UK grant 18GRO-PG24-0248, and MRC grant MR/R018073/1 to ECS and 092076 for the Centre for Cell Biology. Funding was also provided by the European Community's Seventh Framework Programme (FP7/2007-2013) “Integrated European –omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS)” (grant agreement no. 2012-305121); the Muscular Dystrophy UK Grant on Gene Identification to FM, and the support of the MRC Neuromuscular Centre Biobank at UCL is also gratefully acknowledged. The funders played no role in study design, data collection, data analysis, interpretation, and writing of the report.
Declaration of Competing Interest
Dr. Meinke reports personal fees from Greenovation Biopharmaceuticals, outside the submitted work; Dr. Harris reports grants from Muscular Dystrophy-UK, during the conduct of the study; Dr. Koelbel reports personal fees from Santhera, personal fees from Sarepta, personal fees from Biogen, personal fees from Avexis, outside the submitted work; Dr Schara reports personal fees for consulting from Alexion, Santhera, PTC, Ipsen, Biogen, Sarepta, Dynacure, Novartis, Avexis and Sanofi; Dr. Muntoni reports personal fees from Pfizer, personal fees from Roche, personal fees from Biogen, personal fees from Avexis, personal fees from Sarepta, grants from Sarepta, outside the submitted work; Dr. Schoser reports Advisory boards: Amicus Therapeutics, Audentes Therapeutics, Nexien Biopharm, Lupin therapeutics, Sanofi Genzyme. Contracted research: Amicus therapeutics, Greenovation Biopharm, Sanofi Genzyme. Honoraria: Kedrion. Travel expenses: Sanofi Genzyme. Dr. Kerr, Dr. Czapiewski, Dr. de las Heras, Mr. Dixon, Dr. Straub, Dr. Wehnert and Dr. Schirmer have nothing to disclose.
Acknowledgements
We dedicate this study in honour of Alan E.H. Emery who not only described X-linked EDMD as a distinct disease in 1966 but also helped instigate the longest standing regular Laminopathy meeting in 2006. We further want to thank all patients and their families for their valuable contribution.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.048.
Appendix. Supplementary materials
References
- 1.Hughes M.I., Hicks E.M., Nevin N.C., Patterson V.H. The prevalence of inherited neuromuscular disease in Northern Ireland. Neuromuscul Disord. 1996;6(1):69–73. doi: 10.1016/0960-8966(94)00017-4. [DOI] [PubMed] [Google Scholar]
- 2.Norwood F.L., Harling C., Chinnery P.F., Eagle M., Bushby K., Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132(11):3175–3186. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery A.E., Dreifuss F.E. Unusual type of benign x-linked muscular dystrophy. J Neurol Neurosurg Psychiatry. 1966;29(4):338–342. doi: 10.1136/jnnp.29.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery A.E. Emery-Dreifuss syndrome. J Med Genet. 1989;26(10):637–641. doi: 10.1136/jmg.26.10.637. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoblauch H., Geier C., Adams S., Budde B., Rudolph A., Zacharias U. Contractures and hypertrophic cardiomyopathy in a novel FHL1 mutation. Ann Neurol. 2010;67(1):136–140. doi: 10.1002/ana.21839. [DOI] [PubMed] [Google Scholar]
- 6.Bonne G., Mercuri E., Muchir A., Urtizberea A., Becane H.M., Recan D. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000;48(2):170–180. [PubMed] [Google Scholar]
- 7.Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8(4):323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 8.Bonne G., Di Barletta M.R., Varnous S., Becane H.M., Hammouda E.H., Merlini L. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21(3):285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q., Bethmann C., Worth N.F., Davies J.D., Wasner C., Feuer A. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16(23):2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 10.Meinke P., Mattioli E., Haque F., Antoku S., Columbaro M., Straatman K.R. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014;10(9) doi: 10.1371/journal.pgen.1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gueneau L., Bertrand A.T., Jais J.P., Salih M.A., Stojkovic T., Wehnert M. Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2009;85(3):338–353. doi: 10.1016/j.ajhg.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntoni F., Bonne G., Goldfarb L.G., Mercuri E., Piercy R.J., Burke M. Disease severity in dominant Emery Dreifuss is increased by mutations in both emerin and desmin proteins. Brain. 2006;129(5):1260–1268. doi: 10.1093/brain/awl062. [DOI] [PubMed] [Google Scholar]
- 13.Meinke P. John Wiley & Sons, Ltd; Chichester: 2018. Molecular genetics of Emery–Dreifuss muscular dystrophy. [Google Scholar]
- 14.Meinke P., Schirmer E.C. The increasing relevance of nuclear envelope myopathies. Curr Opin Neurol. 2016;29(5):651–661. doi: 10.1097/WCO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 15.Wilkie G.S., Schirmer E.C. Guilt by association: the nuclear envelope proteome and disease. Mol Cell Proteomics. 2006;5(10):1865–1875. doi: 10.1074/mcp.R600003-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Wilkie G.S., Korfali N., Swanson S.K., Malik P., Srsen V., Batrakou D.G. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics. 2011;10(1) doi: 10.1074/mcp.M110.003129. M110 003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osorio D.S., Gomes E.R. Connecting the nucleus to the cytoskeleton for nuclear positioning and cell migration. Adv Exp Med Biol. 2014;773:505–520. doi: 10.1007/978-1-4899-8032-8_23. [DOI] [PubMed] [Google Scholar]
- 18.Robson M.I., de Las Heras J.I., Czapiewski R., Le Thanh P., Booth D.G., Kelly D.A. Tissue-Specific gene repositioning by muscle nuclear membrane proteins enhances repression of critical developmental genes during myogenesis. Mol Cell. 2016;62(6):834–847. doi: 10.1016/j.molcel.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuleger N., Boyle S., Kelly D.A., de las Heras J.I., Lazou V., Korfali N. Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol. 2013 15;14(2):R14. doi: 10.1186/gb-2013-14-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuleger N., Boyle S., Kelly D.A., de las Heras J.I., Lazou V., Korfali N. Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol. 2013;14(2):2013–2014. doi: 10.1186/gb-2013-14-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A. The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A. From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43 doi: 10.1002/0471250953.bi1110s43. 11 0 1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 2019 [Google Scholar]
- 25.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez F., Dundar F., Diehl S., Gruning B.A., Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanigan K.M., von Niederhausern A., Dunn D.M., Alder J., Mendell J.R., Weiss R.B. Rapid direct sequence analysis of the dystrophin gene. Am J Hum Genet. 2003;72(4):931–939. doi: 10.1086/374176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korfali N., Wilkie G.S., Swanson S.K., Srsen V., de Las Heras J., Batrakou D.G. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3(6):552–564. doi: 10.4161/nucl.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippi-Chiela E.C., Oliveira M.M., Jurkovski B., Callegari-Jacques S.M., da Silva V.D., Lenz G. Nuclear morphometric analysis (NMA): screening of senescence, apoptosis and nuclear irregularities. PLoS ONE. 2012;7(8):e42522. doi: 10.1371/journal.pone.0042522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong K., Kwon H., Lee J., Jang D., Pak Y. Insulin-response epigenetic activation of Egr-1 and JunB genes at the nuclear periphery by A-type lamin-associated pY19-Caveolin-2 in the inner nuclear membrane. Nucleic Acids Res. 2015;43(6):3114–3127. doi: 10.1093/nar/gkv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Lee K.K., Segura-Totten M., Neufeld E., Wilson K.L., Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(8):4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattioli E., Columbaro M., Jafferali M.H., Schena E., Hallberg E., Lattanzi G. Samp1 mislocalization in Emery-Dreifuss muscular dystrophy. Cells. 2018;7(10) doi: 10.3390/cells7100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broers J.L., Kuijpers H.J., Ostlund C., Worman H.J., Endert J., Ramaekers F.C. Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization. Exp Cell Res. 2005;304(2):582–592. doi: 10.1016/j.yexcr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Lammerding J., Hsiao J., Schulze P.C., Kozlov S., Stewart C.L., Lee R.T. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170(5):781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowat A.C., Lammerding J., Ipsen J.H. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006;91(12):4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakelien A.M., Delbarre E., Gaustad K.G., Buendia B., Collas P. Expression of the myodystrophic R453W mutation of lamin A in C2C12 myoblasts causes promoter-specific and global epigenetic defects. Exp Cell Res. 2008;314(8):1869–1880. doi: 10.1016/j.yexcr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Holaska J.M., Wilson K.L. An emerin "proteome": purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46(30):8897–8908. doi: 10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziat E., Mamchaoui K., Beuvin M., Nelson I., Azibani F., Spuler S. FHL1B interacts with lamin A/C and emerin at the nuclear lamina and is misregulated in Emery-Dreifuss muscular dystrophy. J Neuromuscul Dis. 2016;3(4):497–510. doi: 10.3233/JND-160169. [DOI] [PubMed] [Google Scholar]
- 40.Windpassinger C., Schoser B., Straub V., Hochmeister S., Noor A., Lohberger B. An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. Am J Hum Genet. 2008;82(1):88–99. doi: 10.1016/j.ajhg.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buch C., Lindberg R., Figueroa R., Gudise S., Onischenko E., Hallberg E. An integral protein of the inner nuclear membrane localizes to the mitotic spindle in mammalian cells. J Cell Sci. 2009;122(12):2100–2107. doi: 10.1242/jcs.047373. [DOI] [PubMed] [Google Scholar]
- 42.Tao S., Yamazaki D., Komazaki S., Zhao C., Iida T., Kakizawa S. Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing TRIC-A channels. J Biol Chem. 2013;288(22):15581–15589. doi: 10.1074/jbc.M112.435396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.