Abstract
Introduction
enchondromas rarely exceed 3–6 cm in long bones. Although the risk of developing secondary chondrosarcoma has been reported up to 4% in solitary lesions, it is not known if size represents a risk factor for transformation.
Objective
to describe three exceptional cases of enchondromas of the entire femur whereof one dedifferentiated in chondrosarcoma.
Results
two patients present stable disease at 5 and 6 years of follow-up; the third, already diagnosed with a dedifferentiated chondrosarcoma, died 14 months after the index surgery for systemic disease.
Conclusion
based on these observations, our hypothesis is that lesion size is an important risk factor for malignant transformation.
Keywords: Giant aggressive enchondroma, Atypic chondromatous tumor, Secondary chondrosarcoma
Abbreviations: CS, chondrosarcoma; EC, Enchondroma
1. Introduction
Enchondromas (ECs) are benign primary bone tumors composed of chondrocytes producing matrix of cartilage, usually asymptomatic and involving the medullary cavity of the bone. It is widely postulated that ECs arise from the displacement of embryonic rests of cartilage from the growth plate into the metaphysis. Involvement of multiple growth plates is thought to result in enchondromatosis as in Ollier disease and Maffucci syndrome.1
ECs usually occur in the 3rd to 4th decade with the small bones of the hands that represent the most common location, followed by the long bones with preference for the femur, the humerus and tibia. The reported incidence of ECs is about 5% of all tumors, however, since most of these lesions are asymptomatic and are often found incidentally, their real incidence remains debated.2 They usually occur as a solitary lesion and rarely exceed 3–6 cm in size in the long bones.3
The true rate of malignant transformation is not well known as most of ECs are asymptomatic, however the risk of developing secondary chondrosarcoma (CS) has been reported up to 4% in solitary lesions whereas in Ollier disease and Maffucci syndrome it rises up to 10–40%.4, 5, 6
Moreover, it is not known if the size of the lesion may represent a risk factor for malignant transformation.
The WHO classification7 identifies three grades of CS with an increased malignant metastatic activity. Grade1 (G1), also known as atypic chondromatous tumor, has a recurrence rate described in literature of about 9% and a metastasis rate of 1%; for Grade 2 (G2) CS the local recurrence and metastasis rate is about 19% and 15% respectively; Grade 3 (G3) CS has a local recurrence rate of about 26% and metastasis rate of 32% respectively.8
Beside those, there is also the dedifferentiated form which is often metastatic at diagnosis.
If distinguishing a G1 CS from a G2, and a G2 CS from a G3 is quite easy, the differential diagnosis between a low-grade chondrosarcoma (G1) and an EC can be very difficult and sometimes impossible.
We report three exceptional cases where ECs were extended to the entire femur whereof in one case a dedifferentiated CS arisen on it.
2. Methods
All cases with a histological diagnosis of benign chondroid lesion exceeding 15 cm, underwent observation at our Oncological Orthopaedics Department, from 2004 till 2016, were included in the study series.
Clinical characteristics, imaging and metabolic activity were analyzed to propose a possible approach for these exceptional cases. At the end of the study population analysis, three cases of giant aggressive enchondromas, all females patients with an age ranging between the fourth and sixth decade, were selected (See Table 1).
Table 1.
Demographic and clinical characteristics.
| Pt | Age | Symptoms | Diagnosis | Size | Scalloping/calcifications | Malignant Transformation | Follow-up |
|---|---|---|---|---|---|---|---|
| DB | 31 | Local pain | Aggressive EC | 23 cm | Y/Y | N | 36 months: occasional local pain |
| MS | 51 | Asymptomatic; discovered because of trauma | Aggressive EC | 31 cm | Y/Y | N | 29 months: asymptomatic |
| SD | 60 | Not-painful mass | Aggressive EC with dedifferentiated CS areas |
20 cm | Y/Y | Y | 29 months: Died at 19 months from the index surgery |
3. Results
Case 1
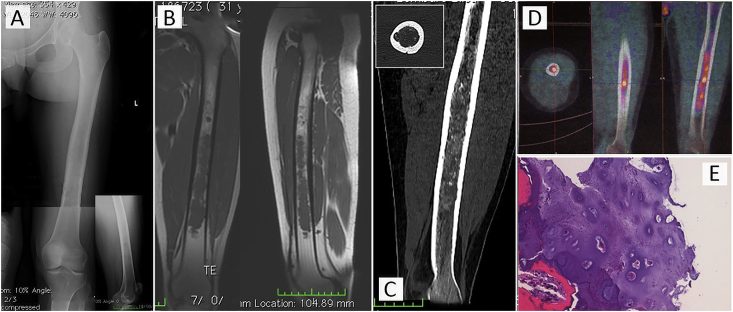
a 31-years-old female patient who presented at our observation for pain localized at the level of the left middle third of the thigh. X-rays showed osteolytic lesion extended to the whole diaphysis with cortical escalloping (Fig. 1A). The MRI evidenced a suspected cartilaginous lesion extended from the subtrochanteric area to just about the distal metaphysis of the femur for 23 cm (Fig. 1B). The following CT scan showed the cortical escalloping and calcifications in the context of the disease (Fig. 1C). Considering the high extension of the lesion a 18F-FDG-PET/CT scan was performed looking for areas with high metabolic activity (SUVmax = 3,2) (Fig. 1D) and biopsy was performed in three areas considered more representative. The relative histology evidenced the presence of an aggressive EC (Fig. 1E).
Considering the impact of a surgical treatment on the quality of life the patient was addressed to follow-up with gadolinium-contrasted MRI performed every 4 months for the first 2 years and every 6 months successively.
At six years from diagnosis the patient is in good health condition with a stable disease.
Case 2
a 51-years-old female presented to our observation because a huge lesion involving the diaphysis of the right femur (Fig. 2A) was revealed in an X-ray performed for trauma. Although she was asymptomatic, considering the unusual aspect and the extension of the lesion further exams were performed. The following MRI and CT scan evidenced a suspected chondroid lesion of 34 cm of length in the medulla of the right femur with cortical scalloping and calcifications in the matrix (Fig. 2B and C).
Due to the high suspicious of low-grade CS a 18F-FDG-PET/CT was performed (Fig. 2D) and a CT-guided biopsy (Fig. 2D upper side) revealed an EC (Fig. 2E).
By balancing the probable high risk of malignant progression based on the aggressive aspect, with the impact of surgery on the quality of life and its risks, the patient was addressed to intensive follow-up.
At five years from diagnosis, the lesion is stable and the patient asymptomatic.
Case 3
a 60-years-old female presented to our observation because of the presence of a not-painful mass in the postero-lateral aspect of the right thigh. The patient had performed an X-ray showing an osteolytic lesion with calcifications and cortical erosion (Fig. 3A). The following MRI and CT scan evidenced the presence of a chondroid lesion of about 20 cm of length with an extraosseous spread (Fig. 3B and C). Considering the dimension of the disease and the imaging high suggestive for malignancy a 18F-FDG PET/CT was performed to identify possible foci of high glucose metabolism in order to perform a CT-guide biopsy (Fig. 3D). The PET/CT showed an overall low-metabolic activity (SUVmax = 3,3) within the lesion except for an area of high glucose uptake (SUVmax = 8,8) in the extraosseous component. The relative histology showed a dedifferentiated CS.
The patient underwent neoadjuvant chemotherapy, resection of the middle and distal third of the right femur and reconstruction with megaprosthesis. The definitive histology evidenced a complete necrosis of the dedifferentiated areas but the presence of a vital enchondroma (Fig. 3E). The patient had brain metastases after six months from surgery occurred during adjuvant chemotherapy; brain lesions were surgically removed, but after further six months she developed liver metastases and she was addressed to palliative chemotherapy; she died 14 months after the index surgery.
Fig. 1.
Case 1; A) anteroposterior e lateral radiographs show an diaphyseal lesion of the left femur with mixed characteristics with prevalence of lytic component. B) Coronal and sagittal T1-weighted MR image reveal a hypointense lesion extending to the femoral diaphysis with cortical escalloping. C) Axial Ct scan and coronal CT scan reconstruction shows the endosteal escalloping with focal deep cortical penetration and allows a better detection and characterization of matrix mineralization. D) Nodule of enchondroma with uniform and small condrocytes encased by a rim of reactive bone. E)18F-FDG PET/TC scan: transaxial (left), sagittal (middle) and coronal (right) views. Moderately FDG uptake is detectable within the tumour with an area of relative higher uptake in the middle aspect of the lesion.
Fig. 2.
Case 2; A) anteroposterior X-ray demonstrates a diaphyseal lesion of the right femur with cortical escalloping and radiopaque flocculent pattern of calcifications of chondroid matrix; B) Coronal T2-weighted MR image reveals multilobulated hyperintense lesion extending along the entire femoral diaphysis. C) Coronal and sagittal CT scan reconstruction shows multiple areas of calcifications and allows better visualization of extensive longitudinal endosteal escalloping. D) inferior square: 18F-FDG PET/TC scan (sagittal view). Moderate and irregular FDG uptake can be observed within the tumour; the CT-guided biopsy performed where the histology should have been more representative; E) Nodule of mineralized hyaline cartilage in enchondroma framed by a rim of reactive bone.
Fig. 3.
Case 3; A) Anteroposterior e lateral X-rays show an extensive diaphyseal lesion of the right femur with areas of chondroid matrix mineralization, escalloping and cortical remodeling; B) Coronal T2-weighted fat saturation MR image and coronal gadolinium enhanced T1-weighted MR image with fat saturation reveals endosteal escalloping extending through the cortex with soft tissue extension; C) Coronal CT scan reconstruction shows endosteal escalloping and flocculent calcifications of chondroid matrix; D)18F-FDG PET/TC scan: sagittal (left) and coronal (right) views. This large tumor showed an inhomogeneous, faint FDG uptake except for an area of intense accumulation arising from subcortical side in the middle of the lesion which extends in the soft tissue of the posterior and lateral aspect of the thigh; E) Dedifferentiated chondrosarcoma show abrupt transition between the chondroid and the high-grade sarcoma component.
All the patients gave their consensus for publication of their cases.
4. Discussion
In this study, three cases of giant ECs with aggressive aspect involving the entire femur are reported. Usually in long bones this kind of lesions rarely exceeds 3–6 cm [1]. Bigger lesions are highly suspected for low-grade CS. Some radiological findings, such as the localized thickness of the cortex and the cortex scalloping, are possible signs of malignancy, nevertheless they can be present in ECs as well.9
Although the risk of malignant transformation is estimated around 4% of cases of solitary lesion, it is reasonable to sustain that it could be related to biological activity and size. Chondromas with aggressive radiological aspect, and giant lesions probably have a major risk of malignant progression than classic small enchondromas. No evidences are present in literature correlating size of the chondroma and malignant progression risk.
Moreover, histological evaluation represents a real challenge for the pathologist in the grading and in the distinction of benign or malignant lesions.10
In addition to this, different areas of the lesion can have different grades of malignancy and benign EC may coexist with highly malignant tumors.11
For these reasons, most of the authors sustain the uselessness of biopsy as they have low reliability in accurately predicting the specific grade in cartilaginous tumors.
In current literature, the reported correspondence between the grade obtained in biopsy and the resected specimen is 40–60%.12 The use of gadolinium contrast-enhanced MRI or 18F-FDG-PET/TC may represent a useful guide for the identification of the correct site of biopsy, revealing high-grade malignant areas.
Basing on this feature, diagnosis has to be based also on clinical symptoms and radiological aspect. Pain, interval enlargement, endosteal scalloping, disappearance or changes of pre-existing calcifications, cortical thinning, and pathological fractures may suggest malignancy.13,14 In this scenario, even if no consensus is present in literature about the cut-off value to distinguish between a EC and low grade chondrosarcoma, 18F-FDG PET/TC could be helpful since significant diffuse or focal increase of glucose uptake within the lesion could predict a sarcomatous transformation and to drive biopsy.15 Moreover, areas of very high FDG uptake as observed in Case 3 are usually related to tumor dedifferentiation thus helping for prognostication and to plan a possible neoadjuvant chemotherapy.
Although some authors suggest intralesional surgery for low-grade chondrosarcoma located in the limb,11,16,17 our group sustains wide surgery, particularly in big lesions where the risk of grade underestimatation by preoperative biopsy is consistent. Otherwise, an intralesional curettage performed without biopsy, as sustained by other authors, could be considered more dangerous because, there is the possibility to undertreat G2 and G3 CSs and overtreated benign ECs18; moreover, a recent review reported the absence of evidences in literature about the correct approach and in absence of those, the general rule to resect every bone malignancy could be considered.19
In the first two presenting cases, where a diagnosis of aggressive EC was done, considering the high risk of malignant progression, the approach was widely debated: a resection was immediately excluded considering the high impact on the quality of life and the associated risk; an aggressive curettage was valued nevertheless it presented the risk to undertreat a more aggressive chondrosarcoma which could have been present considering the size of the lesion; moreover the impact of the surgery should have been important.
Intense follow-up was then considered the best approach to detect a precocious malignant transformation and to proceed to a treatment just in that case. The patients were informed about risks and benefits and they agree with the proposal. Dedifferentiation and precocious metastases is the main risk as occurred in the last presented case.
Indeed, in Case 3, definitive histology demonstrated a giant EC with an area of low-grade chondrosarcoma and another of dedifferentiated chondrosarcoma that was suspected at PET/CT and confirmed at biopsy.
5. Conclusions
It is then possible to assume that size is an important risk factor for malignant transformation but considering the impact on the quality of life of a preventive surgery, an intensive follow-up could be considered reasonable. A gadolinium-contrasted MRI, performed every 4 months for the first 2 years and then every 6 months, should be considered a possible approach. In one of three cases a dedifferentiated CS arose on preexisting EC, thus undergoing resection of the entire femur. Our hypothesis is that the size of the lesion may represent a risk factor for malignant transformation.
Author contributions
C. Zoccali designed the study and wrote the paper;
J. Baldi contributed to write the paper and contributed to discussion;
V. Anelli performed the radiological studies and contributed to discussion;
A. Annovazzi performed and interpreted the PET scans and contributed to discussion;
A. Scotto di Uccio contribute to write the manuscript, performed the literature research and revised the English language because she is an English natural speaker;
A. Barile and F. Arrigoni performed and interpreted the radiological studies and contributed to discussion;
C. Masciocchi performed and interpreted the radiological studies, gave his supervision to the study.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
References
- 1.Kumar A., Jain V.K., Bharadwaj M., Arya R.K. Ollier disease: pathogenesis, diagnosis, and management. Orthopedics. 2015;38(6):e497–506. doi: 10.3928/01477447-20150603-58. [DOI] [PubMed] [Google Scholar]
- 2.Unni K.K., Inwards C.Y. Lippincott-Williams & Wilkins; 2009. Dahlin's Bone Tumors: General Aspects and Data on 10,165 Cases. [Google Scholar]
- 3.Douis H., Saifuddin A. The imaging of cartilaginous bone tumours. I. Benign lesions. Skelet Radiol. 2012;41(10):1195–1212. doi: 10.1007/s00256-012-1427-0. [DOI] [PubMed] [Google Scholar]
- 4.Altay M., Bayrakci K., Yildiz Y., Erekul S., Saglik Y. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007;12(5):415–423. doi: 10.1007/s00776-007-1152-z. [DOI] [PubMed] [Google Scholar]
- 5.Verdegaal S.H., Bovée J.V., Pansuriya T.C. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. The Oncologist. 2011;16(12):1771–1779. doi: 10.1634/theoncologist.2011-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brien E.W., Mirra J.M., Kerr R. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skelet Radiol. 1997;26(6):325–353. doi: 10.1007/s002560050246. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. fourth ed. IARC; Lyon: 2013. Dedifferentiated chondrosarcoma; p. 269. [Google Scholar]
- 8.Angelini A., Guerra G., Mavrogenis A.F., Pala E., Picci P., Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106(8):929–937. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 9.Choi B.B., Jee W.H., Sunwoo H.J. MR differentiation of low-grade chondrosarcoma from enchondroma. Clin Imaging. 2013;37(3):542–547. doi: 10.1016/j.clinimag.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Campanacci M. second ed. Springer; New York: 1999. Bone and Soft Tissue Tumors. [Google Scholar]
- 11.Gitelis S., Bertoni F., Picci P., Campanacci M. Chondrosarcoma of bone. The experience at the istituto ortopedico rizzoli. J Bone Joint Surg Am. 1981;63(8):1248–1257. [PubMed] [Google Scholar]
- 12.Etchebehere M., de Camargo O.P., Croci A.T., Oliveira C.R., Baptista A.M. Relationship between surgical procedure and outcome for patients with grade I chondrosarcomas. Clinics (Sao Paulo) 2005;60(2):121–126. doi: 10.1590/s1807-59322005000200007. [DOI] [PubMed] [Google Scholar]
- 13.Guide Line S.I.O.T. Study Group La diagnosi e il trattamento del condrosarcoma. GIOT. 2011;37:18–26. [Google Scholar]
- 14.Gelderblom H., Hogendoorn P.C., Dijkstra S.D. The clinical approach towards chondrosarcoma. The Oncologist. 2008;13(3):320–329. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 15.Purandare N.C., Rangarajan V., Agarwal M. Integrated PET/CT in evaluating sarcomatous transformation in osteochondromas. Clin Nucl Med. 2009;34(6):350–354. doi: 10.1097/RLU.0b013e3181a34525. [DOI] [PubMed] [Google Scholar]
- 16.Chow W.A. Update on chondrosarcomas. Curr Opin Oncol. 2007;19(4):371–376. doi: 10.1097/CCO.0b013e32812143d9. [DOI] [PubMed] [Google Scholar]
- 17.Campanacci D.A., Scoccianti G., Franchi A. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol. 2013;14(2):101–107. doi: 10.1007/s10195-013-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Camargo O.P., Baptista A.M., Atanásio M.J., Waisberg D.R. Chondrosarcoma of bone: lessons from 46 operated cases in a single institution. Clin Orthop Relat Res. 2010;468(11):2969–2975. doi: 10.1007/s11999-010-1368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoccali C., Baldi J., Attala D. Intralesional vs. extralesional procedures for low-grade central chondrosarcoma: a systematic review of the literature. Arch Orthop Trauma Surg. 2018;138(7):929–937. doi: 10.1007/s00402-018-2930-0. [DOI] [PubMed] [Google Scholar]