Abstract
The overall prognosis of patients with follicular lymphoma has substantially improved over the last decades with a 10-year overall survival of around 80% for the majority of patients. However, for most patients follicular lymphoma it is still a relapsing and remitting disease. Furthermore, certain subsets of patients still have much shorter survival. Currently, there is no established standard how to treat high-risk follicular lymphoma. With advances in the understanding of the biology and pathogenesis of B cell malignancies, a plethora of new compounds have been investigated in FL. These compounds have the potential to increase efficacy if added to current regimens or even replace them. The implementation of these compounds in treatment algorithms is another unsolved issue. This overview highlights major controversies in the treatment of follicular lymphoma and discusses the most recent and relevant clinical trials.
Introduction
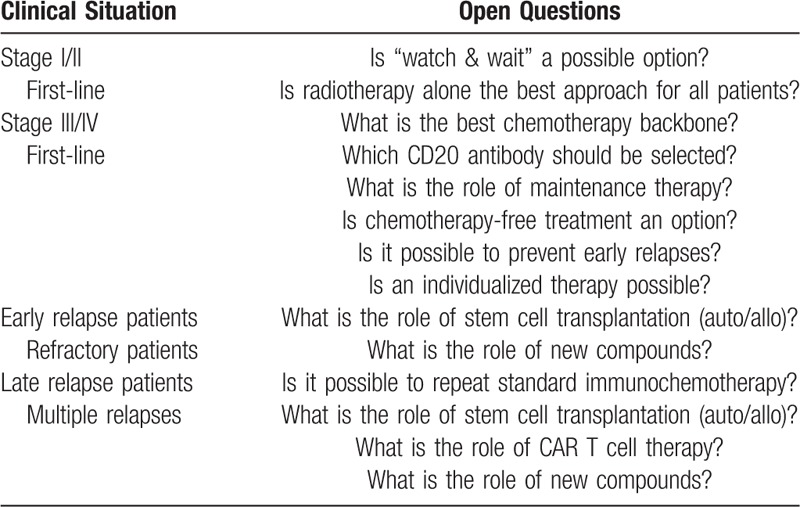
Follicular lymphoma (FL) is the second most common non-Hodgkin's lymphoma (NHL) in the Western world, representing 20% to 30% of all NHL cases and an annual incidence of 5/100,000.1,2 It is genetically characterized by an upregulation of B-cell lymphoma 2 (BCL2) of the originated B cell, which develops via the t(14;18) translocation to a proliferating clone.3 Patients usually presents with advanced and incurable disease; however, in the past 10 to 15 years, the introduction of new treatment approaches have remarkable improved the overall outcome of follicular lymphoma grade I-IIIa. Nowadays, there is a 5-year overall survival (OS) of more than 90% and a median OS approaching 20 years, making FL a chronic disease for the majority of patients.4,5 Despite these improvements, the major cause of death for patients with FL remains the lymphoma with a cumulative incidence of 10.3% at 10 years, followed by treatment-related mortality (3.0%).6 Certain subgroups still have a poor prognosis, and the optimal therapeutic approach in first line and relapsing disease is still a matter of debate. Major open questions in the treatment of FL are summarized in Table 1.
Table 1.
Major Questions for Clinical Trials in Follicular Lymphoma.
First-line treatment
Stage I/II limited disease
Less than 20% of patients with FL are diagnosed with stage I/II disease, and the vast majority of these patients are treated out of the setting of a clinical trial. The reason for this is not only the limited number of patients with localized FL but also that it is considered as curable and often treated with radiotherapy alone (usually as involved field radiation therapy (IFRT)), making it less incentive for research. Recommended doses are in the range of 20 to 25 Gy, since higher doses failed to show improved efficacy.7 More important, in a recent analysis 5-year freedom from progression (FFP) was 74.1% for stage I but only 49.1% for stage II.8 These results may raise the question if radiotherapy alone reflects the best approach for all patients with limited disease. Furthermore, not all patients with early-stage FL receive radiotherapy, despite recommendation in international guidelines.9 The National Cancer Database documented a 13% decrease in the use of radiotherapy from 37% in 1999 to 24% in 2012.10 This is congruent with data from the National LymphoCare Study, a multicenter, prospective cohort trial, which reported radiotherapy as single treatment modality in 23% of patients, whereas 53% of patients received some form of systemic therapy, including 28% who received rituximab plus chemotherapy, 12% who received rituximab alone, and 13% who received radiation and chemotherapy.11,12 In a phase II trial combining IFRT with multiagent chemotherapy, the 10-year time to treatment failure (TTF) was 76% with an overall survival of 80%, which was superior to historical IFRT outcomes.13 In a recently published trial, 150 patients with stage I/II FL were randomized to either radiotherapy alone (30 Gy involved field) or to radiotherapy followed by six cycles of cyclophosphamide, vincristine, and prednisolone (CVP).14 A total of 31/75 patients received rituximab plus CVP. Progression-free survival (PFS) after 10 years was 59% with chemotherapy compared to 41% with radiotherapy alone. Adding rituximab to CVP, PFS further increased compared to radiotherapy alone (hazard ratio, 0.26; 95% CI, 0.07 to 0.97; p = 0.045). Fewer involved regions and PET staging were associated with better PFS. However, OS was around 90% at 10 years with no significant differences between both arms. Toxicities were higher with the use of chemotherapy.
In a multicenter study, two consecutive cohorts with a total of 94 patients with stage I/II FL, application of 4 cycles of rituximab before IFRT (group 2) was compared to IFRT alone (group 1).15 The 10-year PFS was significantly longer with the use of rituximab (64.4% vs 50.7%), but there was no effect on OS. In the German MIR study, 58 patients with early stage FL received 8 cycles of rituximab plus IFRT.16 PFS and OS at 5 years were 78% and 96% with a favorable toxicity profile.
It is not possible to draw a final conclusion on the best treatment approach in stage I/II follicular lymphoma. However, IFRT is well tolerated, and following adequate staging it achieves local lymphoma control and offers the possibility of cure for at least a portion of patients. Therefore, it is difficult to understand that a significant number of patients is deprived of IFRT. However, if patients will really benefit by adding rituximab or even chemotherapy to IFRT remains an open question.
Stage IIII/IV advanced disease
There is an overall agreement that a curative treatment in stage III/IV Fl is not established yet and that not all patients with stage III/IV FL will need immediate therapy. The decision to start or withhold treatment is mainly based on the occurrence of clinical symptoms and other signs of high tumor burden, as summarized in the GELF/BNLI cirteria.17,18 However, there is an increasing discussion about the role of so called “active surveillance” in asymptomatic patients. It is still unclear if transformation could be prevented by early treatment initiation. In this context, evidence suggests that baseline metabolic tumor volume (MTV) measured by positron emission tomography (PET) may predict PFS in patients with FL. In a very recent analysis, a correlation between baseline PET-derived MTV and outcome could not be demonstrated.19
In symptomatic patients with slow disease progression or in very old or comorbid patients, single agent rituximab might be a therapeutic option. Using rituximab alone, an event-free survival (EFS) of three to five years has been reported.20 However, in most patients requiring therapy, the combination of CD20 antibody and chemotherapy has become standard of care. Rituximab has been used for nearly two decades as CD20 antibody (and was initially approved for relapsed patients). Since 2017, Obinutuzumab is also approved in combination with chemotherapy in first-line FL. This compound is a type II CD20 antibody with a greater antibody-dependent cytotoxicity and direct apoptosis compared to rituximab.21,22 As chemotherapy backbone, most centers use bendamustine, CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), CVP, or, more and more rarely, FC(M) (fludarabine, cyclophosphamide, mitoxantrone). CHOP has been available since 1976 for the treatment of lymphomas, and its efficacy was impressively increased with the addition of rituximab (R-CHOP).23 In a randomized trial conducted by the Fondazione Italiana Linformi R-CHOP and R-FM were superior to R-CVP in terms of 8-year PFS (49% vs 52% vs 42%), but with no significant differences in OS.24 The authors observed a higher risk of dying as a result of causes unrelated to lymphoma progression with R-FM vs R-CVP. Furthermore, patients initially treated with R-CVP had a higher risk of lymphoma progression compared with those receiving R-CHOP, as well as a higher risk of requiring additional therapy. Based on these results, R-FM is used less frequently in FL, while R-CVP is often reserved to older or less fit patient populations.
The first time that R-CHOP was really challenged was in the STiL NHL 1-2003 trial which compared R-CHOP with bendamustine/rituximab (BR) without maintenance in a prospective, randomized fashion.25 In this study in a total of 535 patients, at a median follow-up of 45 months BR showed a significant better median PFS compared to R-CHOP (69.5 months vs 21.2 months; hazard ratio 0.58, 95% CI 0.44–0.74; p < 0.0001). The 10-year update was recently presented, which confirms a significant improvement regarding the time to next treatment for BR.26 Furthermore, BR was less toxic than R-CHOP. Similar results were reported in the BRIGHT study, which compared BR with either R-CHOP or R-CVP as first-line treatment of FL and mantle-cell lymphoma (MCL).27 At 5 years, PFS was 65.5% in the BR treatment group and 55.8% in the R-CHOP/R-CVP group (hazard ratio 0.61, 95% CI 0.45–0.85; p = 0.0025). There was no significant effect on OS between both groups. Safety profile was as expected with a higher number of secondary malignancies and infections in the BR treatment group. Overall, based on the better disease control using BR, the authors recommended BR as first-line treatment option in FL. In the GALLIUM trial in patients with untreated FL, obinutuzumab was combined with chemotherapy (CHOP, bendamustine, or CVP) and compared in a randomized, prospective fashion to rituximab plus chemotherapy, followed by maintenance in both arms.28 In this important study in 1202 patients, the estimated three-year rate of PFS was 80% for obinutuzumab plus chemotherapy and 73.3% for rituximab plus chemotherapy (hazard ratio 0.66, 95% CI 0.51–0.85; p = 0.0012). The same effect was also seen after a follow-up of almost 5 years.29 However, there was no effect on OS. The advantage of using Obinutuzumab compared to rituximab was independent of the selected chemotherapy, although the trial was not powered to show this difference.30 Furthermore, there was also a significant higher number of patients achieving minimal residual disease negativity with Obinutuzumab, which may explain the improved PFS.31 Adverse events, particular infusion-related events and neutropenia, were higher with obinutuzumab but manageable. According to chemotherapy, grade 3 to 5 adverse events, mainly cytopenias, were most frequent in patients treated with CHOP, whereas grade 3 to 5 infections and secondary malignancies were most frequent with bendamustine. These side effects of bendamustine have not been reported before and are explainable by the T cell suppression of bendamustine. Based on these data the use of bendamustine is discussed controversial, as part of induction therapy as well as maintenance.
In summary, at this time bendamustine or CHOP may be used as chemotherapy backbone in frontline FL. However, in rapidly growing FL or in patients with elevated LDH, CHOP may be the preferred treatment. Using bendamustine, investigors should be aware of possible side effects. Anti-infectious prophylaxis with cotrimoxazole should be considered. Obinutuzumab offers significant advantages compared to rituximab in terms of disease control.
Maintenance therapy
There is still an ongoing discussion about the role of maintenance in FL. In the PRIMA trial, a total of 1018 patients received rituximab maintenance vs observation following initial therapy with R-CHOP, R-CVP, or R-FCM.32 PFS was significantly prolonged with rituximab maintenance. After nine years of follow-up, median PFS was 10.5 years in the rituximab maintenance arm vs 4.1 years in the observation arm (hazard ratio 0.61, 95% CI 0.52–0.73; p < 0.001).33 However, there was no effect on OS. These results were confirmed by another study.35 The RESORT trial sought to determine how to maximize the long-term benefit of using rituximab. This study enrolled 289 patients with low-tumor FL. Re-treatment with rituximab at each disease progression was compared to scheduled maintenance rituximab after single-agent rituximab.36 With a median follow-up of 4.5 years, median TTF was 3.9 years for patients receiving rituximab re-treatment and 4.3 years for those receiving maintenance (p = 0.54). The median doses of rituximab were 4 for re-treatment vs 18 for maintenance and the authors concluded that there was no benefit to rituximab maintainance over re-treatment. The Swiss Group for Clinical Cancer Research (SAKK) performed a trial randomizing 207 patients after four doses of rituximab to receive 8 months of rituximab maintenance vs 5 years of rituximab maintenance.20 It could be demonstrated that long-term rituximab maintenance therapy did not improve EFS or OS but was associated with increased toxicity. In the GALIUM trial, patients with bendamustine had a higher risk of dying compared to CHOP or CVP, independently of the CD20 antibody.28 Many of these events occurred during the maintenance phase, which may be due to the T cell suppression by bendamustine, which has also been reported also by other investigators. Given the fact that maintenance therapy has not shown prolonged OS yet but may be associated with increased risk of side effects, especially after the use of bendamustine, the role of maintenance in FL is at least questionable and requires further evaluation.
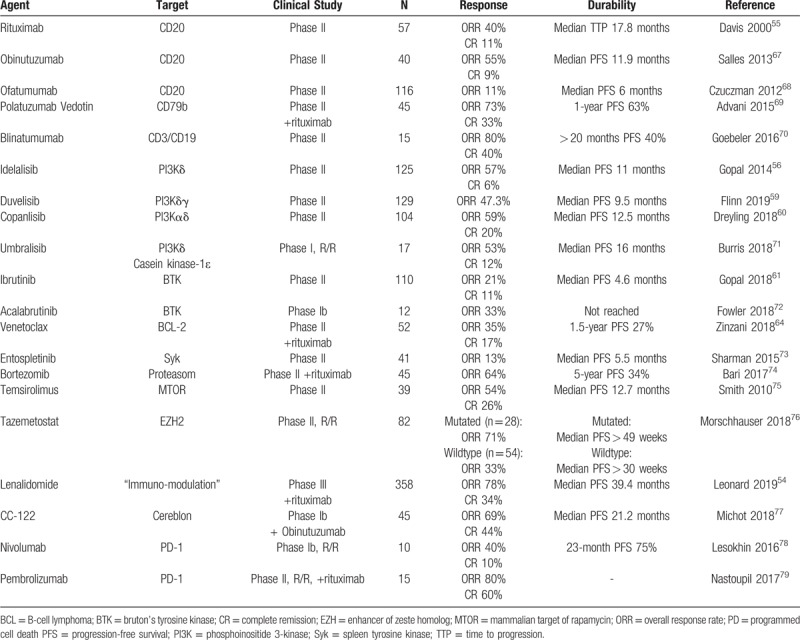
Since several years, investigators are confronted with a plethora of new compounds selectively targeting the cell surface, intracellular pathways and/or the microenvironment, which promise to be effective in FL (an overview is presented in Table 2). Most of these compounds are used in the relapsed or refractory situation, but there is also a discussion of the role of chemotherapy-free therapy in first line. The combination of rituximab and lenalidomide (R2) demonstrated promising efficacy in a phase II trial in untreated FL,37 and was consequently evaluated in a randomized phase III RELEVANCE trial.38 In this study, R2 was compared to rituximab plus chemotherapy, both followed by maintenance therapy, in untreated FL. A total of 1030 patients were enrolled. There was no difference in the response rate, 3-year PFS or 3-year OS between both arms. A higher percentage of patients receiving chemotherapy had grade 3 or 4 neutropenia and febrile neutropenia, whereas a higher percentage of patients receiving R2 had grade 3 or 4 cutaneous reactions. Although R2 failed to demonstrate superiority over standard immunochemotherapy and will not replace rituximab chemotherapy as first-line standard, this trial documents equivalence between immunochemotherapy and chemotherapy-free approach.
Table 2.
Selection of Chemotherapy-Free Treatment Approaches in Relapsed Follicular Lymphoma.
Relapsed follicular lymphoma
Early relapsed patients
There is an increasing understanding that the duration of response after first-line treatment greatly influenced overall prognosis. The National LymphoCare Study identified a group of 19% of patients who had early progression 2 years or less after initial immunochemotherapy (POD24 patients).5 Five-year OS was 50% in the POD24 group compared to 90% in patients without early relapse. In a detailed analysis the following risk factors were associated with increased risk of progression or death before 24 months: male gender, ECOG ≥2, high-risk Follicular Lymphoma International Prognostic Index, or baseline ß-2 microglobuline ≥ 3 mg/l.39 Factors associated with favorable outcome were achieving a CR and exposure to rituximab and/or anthracyclines. Furthermore, the regimen used as first-line therapy may influence the prognostic impact for OS of POD24. For example, the hazard ratio is 3.12 after rituximab or rituximab plus lenalidomide therapy but 6.44 following immunochemotherapy and antibody maintenance.5,40
Based on these results, treatment approaches have to be different for early relapsed patients compared to patients with late relapse, and several ongoing clinical trials are focusing on this point. Two essential questions are arising: What is the role of hematopoietic stem cell transplantation in this setting? And what is the best treatment approach for patients not fit enough for intensive regimens?
In a comprehensive analysis of two randomized trials from the German Low Grade Lymphoma Study Group, a total of 113 patients with POD24 received either salvage therapy including high-dose therapy followed by autologous stem cell transplantation (ASCT) or a transplant-free approach.41 The authors found a significant survival benefit associated with POD24 with a 5-year PFS for ASCT vs no transplant of 51% vs 19% (hazard ratio 0.38, 95% CI 0.24–0.62; p < 0.0001) and a 5-year OS of 77% vs 59% (hazard ratio 0.54, 95% CI 0.30–0.95; p = 0.031). In another analysis be the National LymphoCare Study and the Center for International Blood and Morrow Transplant Research (CIBMTR), 175 POD24 patients receiving ASCT were compared to 174 POD24-patients receiving non-ASCT.42 There was no difference in 5-year OS (ASCT, 67%; non-ASCT, 60%; p = 0.16). It is important to mention that among patients with ASCT, transplantation was not always performed at first relapse but after a median of 2 previous therapies. However, in a subgroup analysis in 123 patients receiving ASCT in the first year after treatment failure, 5-year OS with ASCT was 73% compared to 60% in patients without ASCT (p = 0.05). On multivariate analysis, early use of ASCT was associated with significantly reduced mortality (hazard ratio 0.63, 95% CI 0.42–0.94; p = 0.02). Therefore, salvage treatment with ASCT is strongly recommended in POD24 patients if possible.
The role of allogeneic stem cell transplantation (alloSCT) is much more controversial in FL, and there are only very limited data on alloSCT in POD24 patients. In 2013, the European Society for Blood and Marrow Transplantation (EBMT) published a consensus project summarizing indication for hematopoietic stem cell transplantation in patients with FL.43 Allo-SCT with preferable reduced conditioning should be considered at relapse after ASCT. However, the EBMT group did not reach a consensus in which situation an alloSCT should be preferred over ASCT. In an analysis of EBMT data and CIBMTR data, the 3-year OS following allogeneic transplantation was 66%; however, the transplant-related mortality remains high at 25% at three years.44 The CIBMTR retrospectively compared alloSCT with ASCT in POD24 patients.45 With a median follow-up of 69 to 73 months, the adjusted probability of 5-year OS was significantly higher after ASCT (70%; n = 240) or matched sibling donor alloSCT (73%; n = 105) vs matched unrelated donor alloSCT (49%; n = 95; p = 0.0008). The 5-year adjusted probability of non-relapsed mortality was significantly lower for ASCT (5%) vs matched sibling donor (17%) or matched unrelated donor alloSCT (33%; p < 0.0001). The role of alloSCT, given the early mortality, needs to be reevaluated in the light of newer treatment options. At this time, it is an option only for patients precluding use of ASCT (eg, with intensive bone marrow involvement) or after failed ASCT.
What will be the best approach to POD24 patients not suitable for any kind of transplantation? As reported by the LymphoCare study, standard immunochemotherapy is of limited efficacy. The phosphatidylinositol 3-kinase (PI3K)-inhibitor idelalisib has marked approval in multiple relapsed FL. Furthermore, idelalisib also showed antitumor activity in patients with high-risk FL relapsing within 24 months after initial immunochemotherapy.46 A total of 22/37 (59,4%) patients achieved a ≥50% decrease in the lymphoma mass. The median PFS was 11,1 months with no significant differences between “early-early” relapse patients (progressing in ≤12 months) and “late-early” relapse patients (progressing 12–24 months). These data clearly demonstrate the activity of idelalisib in POD24. Copanlisib, another PI3K-inhibitor, achieved a median PFS of 11.3 months in 93 POD24 patients.47 In a subgroup analysis of the AUGMENT trial (see below), 56 relapsed patients receiving R2 were identified as POD24.48 These patients had an ORR of 80%, with 30% CR and a median PFS of 30.4 months. In the MAGNIFY trial, R2 was also used in relapsed and refractory FL.49 The ORR of 370 patients was 74%, with 46% CR. Importantly, patients who were double refractory to both rituximab and alkylating agents achieved a median PFS of 15,5 months.50 POD24 patients had a median PFS of 23 months. PFS after one year was comparable for patients with less than two lines of previous therapies compared to patients with two or more lines of prior therapies.51 These data also demonstrate the efficacy of R2 in POD24 patients.
Since the vast majority of patients with POD24 are closely after rituximab treatment or even during rituximab maintenance, refractoriness to rituximab could be assumed in a subset of these. In the randomized GADOLIN trial, the anti-CD20 antibody obinutuzumab plus bendamustine followed by obinotuzumab maintenance was compared with bendamustine alone – without maintenance - in relapsed patients who were refractory to rituximab.52 Median PFS was significantly longer in the obinutuzumab/bendamustine arm (25.8 months) than in the bendamustine arm (14.1 months), with a hazard ratio for progression or death of 0.57 (95% CI 0.44–0.73; p < 0.001). A treatment benefit was also seen for OS (hazard ratio 0.67, 95% CI 0.47–0.96; p = 0.0269). Based on these results, obinutuzumab was approved for rituximab-refractory FL. In the GALLIUM trial, the use of obinutuzumab plus chemotherapy reduced the risk for POD24 compared to rituximab plus chemotherapy by 46%.53
Unfortunately, it is too early to give a final recommendation how to treat POD24 patients out of the transplant setting, but new compounds or chemotherapy-free combination could become an option and warrants further studies. In future, the treatment of patients with intermediate-early progresses (24–48 months) might also be challenging, since most of these patients will be rituximab refractory.
Late relapsed and multiple relapsed patients
The overall outcome for FL has impressively improved over the past few decades, and the majority of patients can anticipate a normal life expectancy, with an expected PFS after first-line therapy of up to 10 years.4,34 However, the relapsing nature of the disease necessitates serial treatment. ASCT is not recommended in first relapse outside POD24.43 In symptomatic patients with low tumor burden, rituximab monotherapy may be applied. For patients with low-risk profile and for those for whom conventional chemptherapies are not feasible, radioimmunotherapy offers an therapeutic option.9 In the vast majority of patients, investigators select immunochemotherapy as the appropriate treatment. However, new compounds are beginning to change the therapeutic landscape especially in multiple relapsed patients. Clinical trials in late relapsed should focus not only on efficacy but also on reducing toxicities.
The combination of lenalidomide and rituximab (R2) shows promising results both in first-line and early relapse patients as mentioned above but also in patients with late relapses and refractory FL. In the phase III, multicenter, randomized AUGMENT trail, R2 was compared to rituximab and placebo in indolent lymphoma patients.54 Of 358 patients enrolled, 82% had FL. The median number of prior therapies was one; however, 24% of patients had three or more systemic therapies. POD24 was seen in 33% of patients. PFS was significantly improved for R2 vs rituximab plus placebo, with a hazard ratio of 0.46 (95% CI 0.34–0.62; p < 0.001) and median duration of 39.4 months (95% CI 22.9 months to not reached) vs 14.1 months (95% CI 11.4–16.7 months), respectively. Estimated 2-year survival in the R2 arm was 93% (95% CI 87%–96%) vs 87% (95% CI 81%-92%) in the placebo plus rituximab arm. The safety profile was acceptable, though with significantly more infections, neutropenia, and cutaneous reactions using R2. Nevertheless, based on these results, market approval of R2 in relapsed FL has been granted in the US and is expected soon in Europe.
What can we learn from the AUGMENT trial? R2 is more effective compared to rituximab alone, what is not surprising. It is an excellent choice for patients who are refractory to chemotherapy or for patients with high tumor burden. However, rituximab monotherapy is also effective, as also shown by others,55 and remains a treatment options in comorbid patients or in patients with low tumor burden. Furthermore, since crossover was not allowed, we do not know if a R followed by R2 approach would be preferred with less toxicity than initial treatment with R2.
PI3K-inhibotors are a group of compounds with high activity in FL. Idelalisib is an inhibitor of the δ isoform of the PI3K. Approval was based on a phase II study, which showed a median PFS of 11 months and a median OS of 20.3 months in 125 patients with indolent lymphomas refractory to both alkylators and rituximab, 72 of them with FL.56 Patients had a median of four previous therapies. These results were recently approved by data from the British compassionate use program for idelalisib.57 However, investigators need to be aware of side effects. The most common adverse events reported were fatigue, diarrhea, nausea, rash, chills, and pyrexia, whereas the most frequent grade 3 and grade 4 adverse events were diarrhea, pneumonitis, and elevation of liver enzymes.58 Reports of deaths because of opportunistic infections with Pneumocystis jirovecii and CMV reactivation halted phase III studies with idelalisib,58 but with appropriate prophylaxis using cotrimoxazole and regularly CMV monitoring, idelalisib remains a clear treatment option.
There are further PI3K-Inhibitors that have been tested in phase II trials. Duvelisib, orally available, blocks the δ and γ isoforms of the PI3K. In the DYNAMO-trial, 129 patients with indolent lymphoma (83 of them with FL) refractory to rituximab and chemotherapy or radioimmunotherapy and with a median of three previous therapies achieved an ORR of 47.3%.59 The median PFS was 9.5 months and the median OS was 28.9 months. Duvelisib had a manageable safety profile. Most common grade III/IV adverse events were transient cytopenias and diarrhea.
Copanlisib is an intravenously available PI3K inhibitor blocking the α and δ isoforms of the PI3K. In the phase II CHRONOS-1 study of 142 patients (104 with FL), copanlisib showed an ORR of 61% with 17% CR.60 The median PFS was 12.5 months, the median OS was 42.6 months. Most frequent adverse events were transient hyperglycemia and hypertension with no evidence of worsening for patients treated long-term. Copanlisib has less severe toxicities compared to idelalisib, and received FDA approval for relapsed FL.
Ibrutinib is an orally available BTK-Inhibitor with high activity especially in chronic lymphocytic leukemia and mantle cell lymphoma. In the DAWN trial, ibrutinib was used as single agent in relapsed FL refractory to chemotherapy.61 A total of 110 patients had a median of 3 previous therapies. The ORR was 20.9% (CR 11%), with a median PFS of 4.6 months, and a 30-month OS of 61%. The most common adverse events were diarrhea, fatigue, cough, and muscle spasms. In a very recently published trial of 40 patients with recurrent FL, single agent ibrutinib achieved an ORR of 37.5%, with a median PFS of 14 months.62
Venetoclax inhibits BCL-2, normally overexpressed in FL. In a phase I trial, venetoclax was tested as single agent in various relapsed Non-Hodgkin lymphomas.63 In 29 patients with FL, the ORR was 38% (14% CR) with a median PFS of 11 months. Major toxicities were anemia, neutropenia, and fatigue. In the CONTRALTO trial, venetoclax was combined with rituximab or with BR and compared to BR in 154 FL patients with a median of three previous therapies.64 Addition of venetoclax to BR resulted in increased toxicity with consequent limitation of overall tolerability, but did not improve ORR compared to BR (venetoclax-BR, 84%; BR, 84%). Venetoclax plus rituximab achieved similar number of grade III/IV side effects compared to BR but lower ORR (venetoclax-R, 35%; BR, 84%).
At this time, the role of new compounds in FL is less promising than in other B cell lymphomas. PI3K-inhibitors are effective, but exhibit side effects which may preclude a wide application. Ibrutinib and venetoclax have only modest activity in relapsed FL. R2 will become a treatment option; however, the advantage over standard immunochemotherapy in the relapsed situation in uncertain. Table 2 summarizes clinical trials focusing on chemotherapy-free treatment in relapsed FL.
Chimeric antigen receptor (CAR) T cell therapy is a promising new class of cellular immunotherapy showing activity in several hematologic malignancies, especially in relapsed diffuse large B cell lymphoma and relapsed acute lymphocytic leukemia.65 In a recent trial involving 28 adult patients with relapsed or refractory lymphoma, 10 out of 14 with FL who received autologous CAR T cells achieved a CR, and at a median follow-up of 28.6 months, 89% of these maintained the response.66 In the entire cohort, 18% of patients developed a severe cytokine-release syndrome, and 11% developed serious encephalopathy. These data demonstrate the efficacy of CAR T cell therapy in FL, but much more patients have to be enrolled in clinical trials.
Conclusion
Although the majority of patients with FL have a life expectancy approaching that of healthy individuals, there are still significant controversies about the optimal treatment. Major challenges are the prevention and treatment of early relapses (POD24 patients), which is associated with a dismal prognosis. Furthermore, the implementation of new treatment options in present therapeutic algorithms in first-line and relapse /refractory patients will be a major requirement of future trials. These options include new compounds and chemotherapy-free regimens but also promising immunotherapy approaches as CAR T cell therapy.
Footnotes
Citation: Hübel K, Ghielmini M, Ladetto M, Gopal AK. Controversies in the Treatment of Follicular Lymphoma. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000317
KH has received honoraria from Celgene, Roche, Janssen, Hexal, Servier, Sanofi. MG received honoraries from Celgene, Roche, Mundipharma, Janssen, Abbvie, Gilead. ML reports honoraria from Abbvie, Acerta, Amgen, Celgene, ADC Therapeutics, IQVIA, Gilead, Pfizer, J&J, Roche, Roche Diagnostics, Sandoz, Takeda. AKG reports grants and nonfinancial research support from Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector; personal fees and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Compliment, Asana Bio, and Incyte.
The authors have no conflicts of interest to disclose.
References
- 1.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. [DOI] [PubMed] [Google Scholar]
- 2.Mounier M, Bossard N, Remontet L, et al. Changes in dynamics of excess mortality rates and net survival after diagnosis of follicular lymphoma or diffuse large B-cell lymphoma: comparison between European population-based data (EUROCARE-5). Lancet Haematol. 2015;2:e481–e491. [DOI] [PubMed] [Google Scholar]
- 3.Kuppers R, Stevenson FK. Critical influences on the pathogenesis of follicular lymphoma. Blood. 2018;131:2297–2306. [DOI] [PubMed] [Google Scholar]
- 4.Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national lymphocare study. J Clin Oncol. 2015;33:2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled Analysis of French and US cohorts. J Clin Oncol. 2019;37:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 8.Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by (18)F-FDG PET-CT: a collaborative study by ILROG. Blood. 2019;133:237–245. [DOI] [PubMed] [Google Scholar]
- 9.Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27 suppl 5:v83–v90. [DOI] [PubMed] [Google Scholar]
- 10.Vargo JA, Gill BS, Balasubramani GK, et al. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer. 2015;121:3325–3334. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg JW, Byrtek M, Link BK, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol. 2012;30:3368–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour JF, Pro B, Fuller LM, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:2115–2122. [DOI] [PubMed] [Google Scholar]
- 14.MacManus M, Fisher R, Roos D, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol. 2018;36:2918–2925. [DOI] [PubMed] [Google Scholar]
- 15.Ruella M, Filippi AR, Bruna R, et al. Addition of rituximab to involved-field radiation therapy prolongs progression-free survival in stage I-II follicular lymphoma: results of a multicenter study. Int J Radiat Oncol Biol Phys. 2016;94:783–791. [DOI] [PubMed] [Google Scholar]
- 16.Herfarth K, Borchmann P, Schnaidt S, et al. Rituximab with involved field irradiation for early-stage nodal follicular lymphoma. HemaSphere. 2018;(2):6.(e160). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15:1110–1117. [DOI] [PubMed] [Google Scholar]
- 18.Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362:516–522. [DOI] [PubMed] [Google Scholar]
- 19.Barrington SF, Trotman J, Sahin D, et al. Baseline PET-derived metabolic tumor volume metrics did not predict outcomes in follicular lymphoma patients treated with first-line immunochemotherapy and antibody maintenance in the phase III GALLIUM study. Blood. 2018;132 Suppl 1:2882. [Google Scholar]
- 20.Taverna C, Martinelli G, Hitz F, et al. Rituximab maintenance for a maximum of 5 years after single-agent rituximab induction in follicular lymphoma: results of the randomized controlled phase III Trial SAKK 35/03. J Clin Oncol. 2016;34:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinrich DA, Weinkauf M, Hutter G, et al. Differential regulation patterns of the anti-CD20 antibodies obinutuzumab and rituximab in mantle cell lymphoma. Br J Haematol. 2015;168:606–610. [DOI] [PubMed] [Google Scholar]
- 22.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. [DOI] [PubMed] [Google Scholar]
- 24.Luminari S, Ferrari A, Manni M, et al. Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the initial treatment of patients with advanced-stage symptomatic follicular lymphoma. J Clin Oncol. 2018;36:689–696. [DOI] [PubMed] [Google Scholar]
- 25.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 26.Rummel MJ, Maschmeyer G, Ganser A, et al. Bendamustin plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: nine-year updated results from the StiL NHL 1 study. J Clin Oncol. 2017;35 15 Suppl:7501. [Google Scholar]
- 27.Flinn IW, van der Jagt R, Kahl B, et al. First-line treatment of patients with indolent non-hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 29.Townsend W, Buske C, Cartron G, et al. Obinutuzumab-based immunochemotherapy prolongs progression-free survival and time to next anti-lymphoma treatment in patients with previously untreated follicular lymphoma: Four-year results from the Phase III GALLIUM study. Blood. 2018;132 Suppl 1:1597. [Google Scholar]
- 30.Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36:2395–2404. [DOI] [PubMed] [Google Scholar]
- 31.Pott C, Hoster E, Kehden B, et al. Minimal residual disease response at end of induction and during maintenance correlates with updated outcome in the phase III GALLIUM study of obinutuzumab- or rituximab- based immunochemotherapy in previously untreated follicular lymphoma partients. Blood. 2018;132 Suppl 1:396. [Google Scholar]
- 32.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. [DOI] [PubMed] [Google Scholar]
- 33.Bachy E, Seymour JF, Feugier P, et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients with Follicular Lymphoma: Long-Term Results of the PRIMA Study. J Clin Oncol. 2019;JCO1901073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salles GA, Seymour JF, Feugier P, et al. Long term follow-up of the PRIMA study: half of patients receiving rituximab maintenance remain progression free at 10 years. Blood. 2017;130 Suppl 1:486. [Google Scholar]
- 35.Hoster E, Unterhalt M, Hänel M, et al. Rituximab maintenance versus observation after immunochemotherapy (R-CHOP, R-MCP, R-FCM) in previously untreated follicular lymphoma: a randomized trial of GLSG and OSHO. Hematol Oncol. 2017;35 (S2):32. [Google Scholar]
- 36.Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol. 2014;32:3096–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casulo C, Le-Rademacher J, Dixon J, et al. Validation of POD24 as a robust early clinical endpoint of poor survival in follicular lymphoma: results from the follicular lymphoma analysis of surrogacy hypothesis (FLASH) investigation using individual data from 5,453 patients on 13 trials. Blood. 2017;130 Suppl 1:412. [Google Scholar]
- 40.Mocca A, Schär S, Hayoz S, et al. Predicitve value of POD24 validation in follicular lymphoma patients initially treated with chemotherapy-free regimens in a pooled analysis of three randomized trials of the Swiss Group for Clinical Cancer Research (SAKK). Hematol Oncol. 2019;37 Suppl 2:067. [DOI] [PubMed] [Google Scholar]
- 41.Jurinovic V, Metzner B, Pfreundschuh M, et al. Autologous stem cell transplantation for patients with early progression of follicular lymphoma: a follow-up study of 2 randomized trials from the german low grade lymphoma study group. Biol Blood Marrow Transplant. 2018;24:1172–1179. [DOI] [PubMed] [Google Scholar]
- 42.Casulo C, Friedberg JW, Ahn KW, et al. Autologous transplantation in follicular lymphoma with early therapy failure: a national lymphocare study and center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant. 2018;24:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montoto S, Corradini P, Dreyling M, et al. Indications for hematopoietic stem cell transplantation in patients with follicular lymphoma: a consensus project of the EBMT-Lymphoma Working Party. Haematologica. 2013;98:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sureda A, Zhang MJ, Dreger P, et al. Allogeneic hematopoietic stem cell transplantation for relapsed follicular lymphoma: a combined analysis on behalf of the Lymphoma Working Party of the EBMT and the Lymphoma Committee of the CIBMTR. Cancer. 2018;124:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SM, Godfrey J, Ahn KW, et al. Autologous transplantation versus allogeneic transplantation in patients with follicular lymphoma experiencing early treatment failure. Cancer. 2018;124:2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopal AK, Kahl BS, Flowers CR, et al. Idelalisib is effective in patients with high-risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood. 2017;129:3037–3039. [DOI] [PubMed] [Google Scholar]
- 47.Santoro A, Mollica L, Leppa S, et al. Outcomes for patients with high-risk relapsed or refractory indolent B-cell lymphoma treated with copanlisib in the CHRONOS-1 study. Blood. 2018;132 Suppl 1:395. [Google Scholar]
- 48.Leonard JP, Trneny M, Izutsu K, et al. Efficacy was improved with lenalidomide/rituximab (R2) vs rituximab/placebo in patients with follicular lymphoma irrespective of POD24 status in the phase III AUGMENT study. HemaSphere. 2019;3 Suppl 1:F483. [Google Scholar]
- 49.Andorsky DJ, Coleman M, Yacoub A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (Duppl):7513. [Google Scholar]
- 50.Sharman J, Coleman M, Yacoub A, et al. Interim analysis of phase IIIb MAGNIFY study of induction R2 followed by maintenance in patients with relapsed/refractory indolent non-Hodgkin lymphoma. Hematol Oncol. 2019;37 Suppl 2:070. [Google Scholar]
- 51.Andorsky D, Coleman M, Yacoub A, et al. Response rate to lenalidomide plus rituximab (R2) as independent of number of prior lines of therapy: interim analysis of initial phase of MAGNIFY phase IIIb study of R2 followed by maintenance in relapsed/refractory indolent NHL. J Clin Oncol. 2018;36 Suppl:7516. [Google Scholar]
- 52.Cheson BD, Chua N, Mayer J, et al. Overall survival benefit in patients with rituximab-refractory indolent non-hodgkin lymphoma who received obinutuzumab plus bendamustine induction and obinutuzumab maintenance in the GADOLIN Study. J Clin Oncol. 2018;36:2259–2266. [DOI] [PubMed] [Google Scholar]
- 53.Launonen A, Hiddemann W, Duenzinger U, et al. Early disease progression predicts poorer survival in patients with follicular lymphoma (FL) in the GALLIUM study. Blood. 2017;130 Suppl 1:1490. [Google Scholar]
- 54.Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III Study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. [DOI] [PubMed] [Google Scholar]
- 56.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyre TA, Osborne WL, Gallop-Evans E, et al. Results of a multicentre UK-wide compassionate use programme evaluating the efficacy of idelalisib monotherapy in relapsed, refractory follicular lymphoma. Br J Haematol. 2018;181:555–559. [DOI] [PubMed] [Google Scholar]
- 58.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in patients with refractory indolent non-hodgkin lymphoma. J Clin Oncol. 2019;37:912–922. [DOI] [PubMed] [Google Scholar]
- 60.Dreyling M, Santoro A, Mollica L, et al. Long-term efficacy and safety from the copanlisib CHRONOS-1 study in patients with relapsed or refractory indolent B-cell lymphoma. Blood. 2018;132 Suppl 1:1595. [Google Scholar]
- 61.Gopal AK, Schuster SJ, Fowler NH, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: results from the open-label, multicenter, Phase II DAWN Study. J Clin Oncol. 2018;36:2405–2412. [DOI] [PubMed] [Google Scholar]
- 62.Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood. 2018;131:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol. 2017;35:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinzani PL, Flinn IW, Yuen S, et al. Efficacy and safety of venetoclax (Ven) + rituximab (R) or Ven + bendamustine (B) + R randomized versus B + R in patients (pts) with relapsed/refractory (r/r) follicular lymphoma (FL): Final analysis of the phase II CONTRALTO study. Blood. 2018;132 Suppl 1:1614.30154115 [Google Scholar]
- 65.Buechner J, Kersten MJ, Fuchs M, et al. Chimeric antigen receptor- T cell therapy: practical considerations for implementation in Europe. Hemasphere. 2018;2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory b-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salles GA, Morschhauser F, Solal-Celigny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2920–2926. [DOI] [PubMed] [Google Scholar]
- 68.Czuczman MS, Fayad L, Delwail V, et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698–3704. [DOI] [PubMed] [Google Scholar]
- 69.Advani RH, Flinn I, Sharmann JP, et al. Two doses of polatuzumab vedotin (PoV, anti-CD79b antibody-drug conjugate) in patients (pts) with relapsed/refractory (RR) follicular lymphoma (FL): durable responses at lower dose level. J Clin Oncol. 2015;33 Suppl:8503. [Google Scholar]
- 70.Goebeler ME, Knop S, Viardot A, et al. Bispecific T-Cell Engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I Study. J Clin Oncol. 2016;34:1104–1111. [DOI] [PubMed] [Google Scholar]
- 71.Burris HA, 3rd, Flinn IW, et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19:486–496. [DOI] [PubMed] [Google Scholar]
- 72.Fowler NH, Coleman M, Stevens DA, et al. Acalabrutinib alone or in combination with rituximab (R) in follicular lymphoma (FL). J Clin Oncol. 2018;36 Suppl:7549. [Google Scholar]
- 73.Sharmann JP, Klein LM, Boxer M, et al. Phase 2 trial of entospletinib (GS-9973), a selective Syk inhibitor, in indoelent non-hodgkin's lymphoma (NHL). Blood. 2015;126:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bari A, Marcheselli R, Marcheselli L, et al. A multicenter phase ii study of twice-weekly bortezomib plus rituximab in patients with relapsed follicular lymphoma: long-term follow-up. Acta Haematol. 2017;137:7–14. [DOI] [PubMed] [Google Scholar]
- 75.Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin's lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol. 2010;28:4740–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morschhauser F, Tilly H, Chaidos A, et al. Interim update from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) follicular lymphoma (FL). Hemasphere. 2018;2 (S1):S100. [Google Scholar]
- 77.Michot JM, Bouabdallah R, Doorduijn JK, et al. Avadomide (CC-122), a novel cereblon modulating agent, in combination with obinutuzumab (GA101) in patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Blood. 2018;132 Suppl 1:449. [Google Scholar]
- 78.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase ib study. J Clin Oncol. 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nastoupil LJ, Westin JR, Fowler NH, et al. Response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: interim results of an open-label, phase II study. J Clin Oncol. 2017;35 15 Suppl:7519. [Google Scholar]


