At diagnosis most acute myeloid leukemia (AML) patients are older (>60 years) and outcomes in this group are generally inferior compared to younger patients. Mutations in the nucleophosmin 1 (NPM1) gene are common and associate with a more favorable prognosis in younger and older AML patients.1 The European LeukemiaNet (ELN) classifies AML patients with NPM1 mutations and absence of FLT3-ITD (NPM1mut/FLT3-ITDneg) into the favorable genetic risk group.2 Due to the favorable prognosis an allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR) is generally not recommended in these patients.3 However, in younger NPM1-mutated AML patients there is growing evidence that, depending on donor availability, HSCT in CR1 may be superior to consolidating chemotherapy or autologous HSCT.4,5 In older NPM1mut/FLT3-ITDneg AML patients the best consolidation treatment is still under debate.
We identified 157 AML patients 60 years or older who were not eligible for myeloablative HSCT or an autologous transplantation and who received NMA-HSCT in CR1 between 1998 and 2018 at our center. Thirty-one patients harbored a NPM1 mutation and 19 were NPM1mut/FLT3-ITDneg (Table 1 ). The median age at HSCT in these 19 patients was 67 (range 61–76) years. Twelve patients had de novo AML, while five patients had AML secondary to a myeloid neoplasm (4 patients after myelodysplastic syndrome, and 1 patient after chronic myelomonocytic leukemia), 2 patients had treatment-related AML (1 patient after chronic lymphocytic leukemia, and 1 patient after breast cancer). Seventeen patients had a normal karyotype, one had a trisomy 8 and trisomy 21, one karyotype was unknown. For nine patients next-generation targeted amplicon sequencing was performed at diagnosis. Additional mutations were detected in all but 1 patient (2 mutations in 2 patients, three mutations in 3 patients, and 4, 5, and 6 mutations in 1 patient each). The most commonly detected additional mutations were in DNMT3A (detected in 6 patients), NRAS (detected in 2 patients) and BCOR, IDH1, IDH2, PTPN11, SRSF2, and TET2 (detected in 2 patients each). NPM1-mutation based measurable residual disease (MRD) was available in 14 patients, 11 patients were NPM1 MRDneg and three patients were NPM1 MRDpos at HSCT.
Table 1.
Patients Characteristics (n = 19).
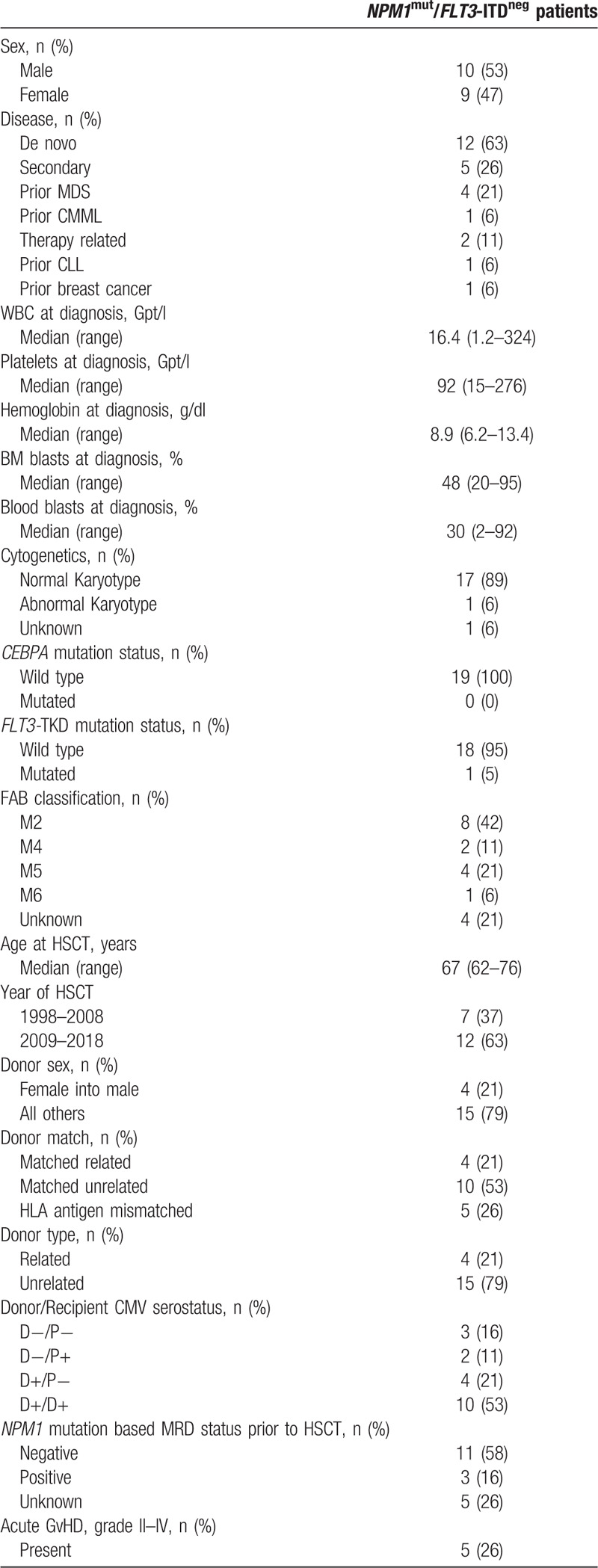
Table 1 (Continued).
Patients Characteristics (n = 19).
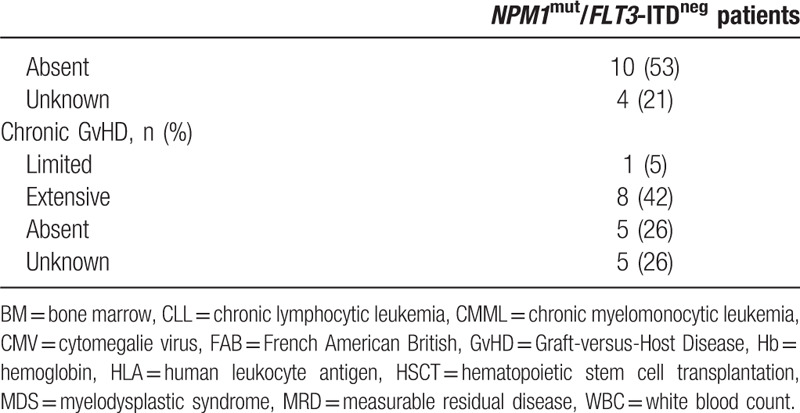
Donors were human leukocyte antigen matched related (n = 4), matched unrelated (n = 10) or had at least one antigen mismatch (n = 5). All patients received peripheral blood NMA-HSCT with 3 × 30 mg/m2 fludarabine and 2 Gy total body irradiation.6 Immunosuppression was cyclosporine A and mycophenolat mofetil (2 and 3 g per day after related or unrelated HSCT, respectively). Median follow up after HSCT was 5.2 (range 1.5–12.5) years.
Prior to HSCT, all but 1 patient (who received 2 cycles of azacitidine) received intensive induction chemotherapy before HSCT. One patient suffered graft rejection 37 days after HSCT and received a second HSCT. At 2 years patients in this NMA-HSCT treated cohort of older NPM1mut/FLT3-ITDneg AML had a relapse rate of 11% (95% Confidence Interval (CI) 2%–29%) and an OS of 68% (CI 50%–93%, Fig. 1). The 2-year NRM was 21% (CI 6%–42%). However, at 5 years NRM rose to 43% (CI 18%–67%) and OS dropped to 46% (CI 27%–80%), while the relapse rate remained at 11%. The 2 patients suffering relapse after HSCT had detectable NPM1-mutation based MRD prior to HSCT. Grade II-IV GvHD and III-IV acute GvHD occurred in 33% and 13%, respectively. Extensive chronic GvHD was observed in 57%. Causes of deaths were relapse (n = 2), GvHD (n = 3), infection (n = 3) and one fatal ventricular arrhythmia (for detailed patient information see Supplemental Table S1, Supplemental Digital Content).
Figure 1.
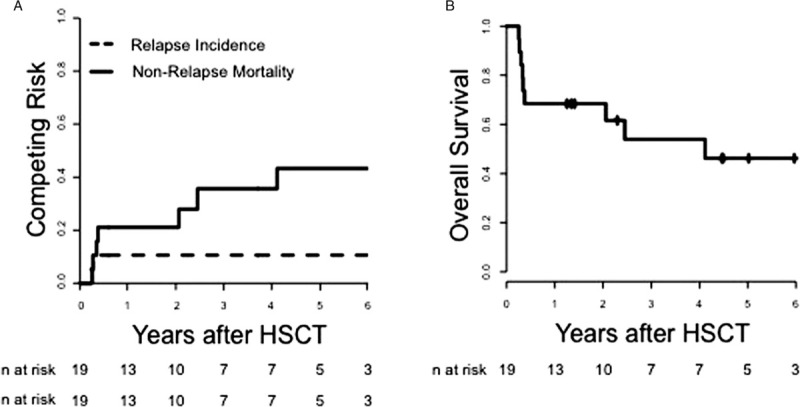
(A) Relapse Incidence, Non-Relapse Mortality, and (B) Overall Survival of older AML patients (>60 years) with NPM1 mutation and absence of FLT3-ITD receiving NMA-HSCT in first complete remission (n = 19).
Even with the low number of patients, our results also demonstrate that the long-term consequences of HSCT, including GvHD and prolonged immunosuppression and the impact on OS - especially in older AML patients - should not be underestimated.
In a recent report, Aldoss et al published a retrospective analysis of 17 older (>60 years) NPM1mut/FLT3-ITDneg AML patients who underwent HSCT in CR1.7 The median age in this study was 66 (range 61–73) years with the majority of patients harboring a normal karyotype. All but 1 patient, who received azacitidine alone, were treated with intensive induction chemotherapy followed by HSCT. Patients received reduced-intensity conditioning (RIC) with fludarabine and melphalan; 1 patient underwent non-myeloablative conditioning (NMA) with fludarabin, cyclophosphamid and total-body irradiation. Outcome results of this analysis were very favorable with a 2-year overall survival (OS) and disease free survival (DFS) of 88% and 81%, respectively, a 2-year non-relapse mortality (NRM) of 12% and a 2-year relapse rate of 7%. Acute grade II-IV graft-vs-host disease (GvHD) and III-IV acute GvHD occurred in 27% and 13%, respectively.
Considering results from historical non-transplant reports for this age group (≥608,9 or ≥6510 years) HSCT-based consolidation demonstrated improved outcomes.7–10 A possible biological background for these findings may be the presence of an allogeneic immune response mediated by CD4+ and CD8+ donor T lymphocytes against the mutated region of NPM1.11,12 These results may challenge the general perception of chemotherapy consolidation as an adequate approach in older favorable-risk AML and also affects the question about the best time-point of HSCT in these patients.
The patients included in our analysis had similar characteristics as the patients analyzed by Aldoss et al (Supplemental Table 1, Supplemental Digital Content).7 However, the main differences between the 2 studies are in the conditioning regimen applied (RIC vs NMA) and the GvHD prophylaxis. Limitations of our analysis are particularly the number of included patients and a potential selection bias towards HSCT.
Even though the outcomes observed in our NMA-HSCT treated group of older NPM1mut/FLT3-ITDneg AML patients are not as favorable as the group of patients analyzed by Aldoss et al treatment results still compare favorably to the outcomes observed in historical non-transplant patients consolidated with chemotherapy alone.7-10
Both studies demonstrated low relapse rates and a more favorable outcome of older NPM1mut/FLT3-ITDneg AML patients consolidated with HSCT, challenging the paradigm of a chemotherapy-based consolidation being sufficient in this older patient group. Certainly, the recent introduction of MRD analyses into the clinical practice may further refine the patient subpopulation that benefit from HSCT and emerging new therapies – for example, the combination of venetoclax and hypomethylating agents - may modulate the prognosis in this patient group. However, a randomized multi-center clinical trial – with adequate follow-up - would be desirable to identify those patients who benefit from an early HSCT in this group. Furthermore, the question of the best conditioning regimen for these patients (eg, NMA vs RIC) and whether a modification of conditioning regimens and/or GvHD prophylaxis might improve outcomes remains to be answered and should be addressed.
Supplementary Material
Acknowledgements
The authors thank Janet Bogardt, Annette Jilo, Ines Kovacs, Kathrin Wildenberger, Scarlett Schwabe, Christine Günther, Daniela Bretschneider, Evelin Hennig, and Christel Müller for their assistance.
Footnotes
Citation: Jentzsch M, Grimm J, Bill M, Goldmann K, Schulz J, Niederwieser D, Platzbecker U, Schwind S. Outcomes of Older Patients with NPM1 Mutated and FLT3-ITD Negative Acute Myeloid Leukemia Receiving Allogeneic Transplantation. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000326
This study was supported by Deutsche José Carreras Stiftung e.V. (04R/2016 [SS], PS15/15 [J. Grimm]), Verein Zusammen gegen den Krebs e.V. [SS].
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
References
- 1.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006;107:4011–4020. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. [DOI] [PubMed] [Google Scholar]
- 4.Rollig C, Bornhauser M, Kramer M, et al. Allogeneic stem-cell trans- plantation in patients with NPM1-mutated acute myeloid leukemia: results from a prospective donor versus no-donor analysis of patients after upfront HLA typing within the SAL-AML 2003 trial. J Clin Oncol. 2015;33:403–410. [DOI] [PubMed] [Google Scholar]
- 5.Poiré X, Labopin M, Polge E, et al. Hematopoietic stem cell transplantation for adult patients with isolated NPM1 mutated acute myeloid leukemia in first remission. Am J Hematol. 2019;94:231–239. [DOI] [PubMed] [Google Scholar]
- 6.Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–453. [DOI] [PubMed] [Google Scholar]
- 7.Aldoss I, Nakamura R, Yang D, et al. Favorable outcomes for allogeneic hematopoietic cell transplantation in elderly patients with NPM1-mutated and FLT3-ITD-negativ acute myeloid leukemia. Bone marrow transplant. 2019;doi: 10.1038/s41409-019-0553-x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholl S, Theuer C, Scheble V, et al. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplications in patients older than 60 yr with acute myeloid leukaemia. Eur J Haematol. 2008;80:208–215. [DOI] [PubMed] [Google Scholar]
- 10.Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UKNational Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015;33:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greiner J, Ono Y, Hofmann S, et al. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia Blood. 2012;120:1282–1289. [DOI] [PubMed] [Google Scholar]
- 12.Greiner J, Schneider V, Schmitt M, et al. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut) Blood. 2013;122:1087–1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


