Supplemental Digital Content is available in the text
Abstract
Fludarabine, cyclophosphamide and rituximab (FCR) was compared to bendamustine and rituximab (BR) in an international, randomized, open label, phase 3 trial in 561 previously untreated, fit patients with chronic lymphocytic leukemia (CLL) without del (17p). Primary endpoint was progression free survival (PFS). The final primary endpoint analysis after 37.1 months median follow up failed to show the non-inferiority of BR as compared with FCR. With extended median follow up of 58.2 months, median PFS was 42.3 months in BR-treated patients versus 57.6 months for FCR-treated patients (Hazard Ratio [HR] 1.593; 95% CI 1.271–1.996; p < 0.0001). For patients > 65 years, median PFS was 48.5 months with BR versus 57.9 months with FCR without reaching statistical significance (HR 1.352; 95% CI 0.912–2.006; p = 0.134). Median OS was not reached for both arms with 5-year OS rates of 80.1% vs 80.9%, respectively (HR 1.108; 95% CI 0.755–1.627; p = 0.599). No statistically significant difference was found in the time to secondary malignancy between the 2 groups (at 5 years, 86.6% free from secondary malignancies in the BR group vs 83.8% in the FCR group [HR 0.801; 95% CI 0.507–1.267; p = 0.344]). In patients >65 years secondary neoplasia occurred more frequently after FCR treatment [28 of 86 (32.6%) patients] as compared with BR [18 of 107 (16.8%) patients; p = 0.011]. Health-related quality of life was similar in both treatments. Despite the improved PFS for FCR, OS did not differ. These results also suggest an increase in secondary neoplasia associated with FCR in elderly fit CLL patients.
Introduction
In physically fit patients, chemoimmunotherapy is still a good choice as first line treatment of chronic lymphocytic leukemia (CLL) despite the approval of targeted drugs like ibrutinib, which has shown promising results alone or in combination in treatment-naive patients with respect to progression-free survival (PFS),1–3 but not with regard to overall survival (OS) when compared to bendamustine and rituximab (BR).2 Recently, data on improved PFS and OS were reported for ibrutinib in combination with rituximab compared to fludarabine, cyclophosphamide and rituximab (FCR) in younger CLL patients with good health status.3 However, it should be noted that the number of events remains small and median follow up is short. Furthermore, no significant difference in PFS between both arms was detected in the IGVH mutated subgroup in this trial.3
The combination of FCR has already demonstrated a prolonged survival and disease control in treatment naïve CLL, in young, fit untreated patients without del17p and with mutated IGHV.4–6 However, the preliminary data has shown that the combination of the alkylating agent bendamustine in combination with the CD20-antibody rituximab may have similar efficacy and less toxicity in this patient population.7 The CLL10 study of the German CLL study group (GCLLSG) was designed as an international phase 3 non-inferiority trial, comparing FCR to BR in patients with normal creatinine clearance and low comorbidity score and without del (17p). The final primary endpoint analysis of this trial after 37.1 months median observation time showed a longer PFS in the FCR treated patient group with median 55.2 months as compared to 41.7 in the BR arm and failed to show non-inferiority of BR as compared with FCR with a corresponding non-inferiority margin of 1.388. Of note, toxicity was higher after FCR, particularly in patients older than 65 years.6 Therefore, long-term follow up data is of interest in order to evaluate late toxicities of FCR in comparison to BR. Importantly, patient reported outcomes have gained increasing interest by clinical researchers especially in chronic diseases like CLL. Here we present the extended follow up data of the study with a median observation time of 58.2 months, including long-term safety data, as well as health-related quality of life data.
Subjects and methods
Study design and patients
The study design of the CLL10 trial has been reported previously.6 In summary, we conducted a randomized, open label phase 3 trial in previously untreated, fit patients with advanced CLL without del (17p). The study was performed according to the Declaration of Helsinki and each patient provided written informed consent before central screening. Six hundred eighty-eight patients were recruited by 158 sites (Supplemental Data Table S1) between October 2008 and July 2011, 561 were included after central screening. Within the 2-group, parallel design, the standard treatment consisted of six 28-day cycles of either intravenous FCR or BR. According to the protocol, there was no primary prophylaxis neither with antibiotics nor growth factors prescribed. In cases of severe neutropenia lasting more than 7 days, prophylaxis with cotrimoxazole against Pneumocystis jirovecii infection was recommended. G-CSF was only administered in case of severe neutropenia with fever ≥38.5°C or hypothermia, either with or without suspected or documented infection. After final restaging, patients were followed-up every 3 months during the first 2 years and every 6 months during the next 3 years unless the patient terminated the close follow up earlier due to progression, new CLL treatment or death. Patients whose disease progressed or who received new treatment were followed-up annually within the study protocol until 5 years after final restaging. Patients were followed up within the registry of the GCLLSG, if the patient was included by a center participating in the GCLLSG registry and the patient signed the agreement for the registry. All patients who received at least one dose of any study drug were included in the safety analysis on secondary malignancies (N = 557).
Study procedures and end points
Comprehensive assessment at baseline including confirmation of diagnosis by immunophenotyping, as well as central fluorescence in situ hybridization (FISH) and IGHV mutational status were previously described.6 Responses and disease progression were assessed according to the IWCLL response criteria8 by the study investigators and afterwards confirmed by a central, investigator-independent medical review. The primary objective of this study was the non-inferiority of BR compared to the standard treatment of FCR concerning the primary endpoint progression-free survival. Beyond already reported secondary endpoints, such as safety results, MRD rates, response in biologically defined subgroups, event-free survival and OS, health-related quality of life (HRQOL) according to the EORTC-C30 questionnaire including the fatigue scale were also analyzed. These questionnaires were sent to all patients at baseline and after 3 months at interim staging, at initial response assessment (1 month after beginning of the last cycle of treatment) and at final restaging (2 months after initial response assessment), then 6, 12, 24, 36, 48, and 60 months after final restaging and then annually, until the end of the study. This report presents the updated primary efficacy analysis, PFS and the main secondary endpoint OS, long-term safety results including secondary malignancies (including Richter's transformation, solid tumors, hematological neoplasia, and skin tumors) as well as HRQOL analyses. Additionally, collected data on subsequent treatments for CLL and the time to next CLL treatment (TTNT) were analyzed.
Statistical analysis
Time-to-event efficacy endpoints were analyzed using both Kaplan–Meier methodology. Hazard ratios (HRs) including 95% confidence intervals (CI) were calculated using proportional-hazards Cox regressions and tested using the likelihood ratio test. PFS was calculated from randomization to disease progression or death from any cause and OS from the date of randomization to death from any cause. TTNT was defined as the time between randomization and the start of the first subsequent treatment for CLL. Time to secondary malignancy referred to the time between randomization and date of first diagnosis of secondary malignancy. For sensitivity, a competing risk analysis was performed considering death without documented secondary malignancy as competing event. Independent prognostic factors for PFS and OS were identified by multivariable analyses using Cox proportional hazards regression models with forward stepwise selection and confirmed by using backward selection. All variables with significant association to PFS or OS in univariable analyses were included in the Cox models. As a pre-specified subgroup analysis, the interaction with study treatment was explored for the factors: age at baseline, IGHV mutational status and CLL-IPI risk group. A term for the interaction was included between the respective factor and the study treatment in a Cox regression model. HRQOL was evaluated using the EORTC C30 questionnaire. The global health status, functional and single item scales were calculated according to the standard procedure as given by the EORTC-QLQ-C30 manual. Age- and sex-matched scores of the general population were calculated according to Schwarz et al.9 The global health status as well as the fatigue scale were selected in advance as primary domains for detailed analyses due to their relevance at baseline. Significance was defined as p < 0.05 without adjustments for multiple testing. Reported p-values have an exploratory character. Analyses were performed using Statistical Package for the Social Sciences (SPSS) for Windows version 23 (SPSS, Chicago, IL).
Results
Five hundred sixty-one patients were randomized between October 2008 and July 2011. Two hundred eighty-two of these patients were allocated to the FCR arm and 279 to the BR arm. The results of the extended follow up data presented here comprise study data with a median observation time of 58.2 months for all patients being alive. Regarding the PFS analysis, 27 of 282 FCR patients (9.6%) died without disease progression, while the CLL of 106 (37.6%) progressed. Among the 279 patients in the BR arm, 18 died (6.5%) and in 159 (57.0%) patients, the disease progressed (Supplemental data, Fig. S1). As described previously,6 more patients in the BR arm exhibited an unmutated IGHV status (67.8% vs 55.3%) and were older than 70 years (18.3% vs. 9.9%). The CLL-IPI10 could be determined for 525 patients with all factors available except for TP53 mutational status. Considering the CLL-IPI risk groups, 22.7% were in the low CLL-IPI risk group, 41.5% in the intermediate risk group, 35.8% in the high-risk group, and 0.0% in the very high-risk group (because 17p was an exclusion criterion). Comparing the CLL-IPI in the two treatment arms, more low risk patients and fewer high-risk patients were distributed onto the FCR arm (low: FCR 28.7% vs BR 16.5%, intermediate FCR 43.0% vs BR 40.0%, high FCR 28.3% vs BR 43.5%; very high FCR 0.0% vs BR 0.0%).
Progression-free survival
Efficacy results of the long-term follow up confirmed previously published results.6 PFS was significantly shorter for BR-treated patients with a median of 42.3 months vs 57.6 months in FCR treated patients (HR 1.593; 95% CI 1.271–1.996; p < 0.0001) (Fig. 1 A). In a second step, PFS was evaluated according to treatment arm and age at study entry. There was no significant interaction between study treatment and age regarding PFS (p = 0.261) (Fig. 2A). Younger patients up to 65 years of age had a median PFS of 38.2 months in the BR arm whereas FCR-treated patients ≤65 years had a median PFS of 57.6 months (HR 1.771; 95% CI 1.344–2.335; p < 0.0001) (Fig. 1 B). Older patients over 65 years had a median PFS of 48.5 months in the BR treated population and 57.9 months in the FCR treated group (HR 1.352, 95% CI 0.912–2.006; p = 0.134) (Fig. 1 B). There was no statistical significant difference for PFS within the BR arm between both age groups [≤65: median PFS 38.2 months vs >65: median PFS 48.5 months (HR 0.767; 95% CI 0.562–1.046; p = 0.094)]. Similarly, no statistical difference for PFS in the FCR arm between both age groups was detected [≤65: median PFS 57.6 vs >65: median PFS 57.9 (HR 1.018; 95% CI 0.707–1.464; p = 0.925)]. Furthermore, no significant interaction between study treatment and IGHV mutational status (p = 0.627) was observed concerning PFS (Fig. 2A). As for patients with an unmutated IGHV status, BR treated patients had a significantly shorter PFS (median PFS 33.9 vs 43.0 months in FCR treated patients [HR 1.545; 95% CI 1.181–2.022; p = 0.0015]). The shorter PFS observed with BR in patients with mutated IGHV did not reach statistical significance in comparison to the FCR treated group (68.9 months vs not reached; HR 1.356; 95% CI 0.872–2.137; p = 0.173) (Fig. 1 C). Differences within each treatment arm between the IGHV mutated and IGHV unmutated subgroups were significant for BR [median PFS 68.9 months for IGHV mutated vs 33.9 months for IGHV unmutated (HR 2.860; 95% CI 1.971–4.148; p < 0.0001)] as well as for FCR [median PFS not reached for IGHV mutated and 43.0 months for IGHV unmutated (HR 2.431; 95% CI 1.674–3.530; p < 0.0001)].
Figure 1.
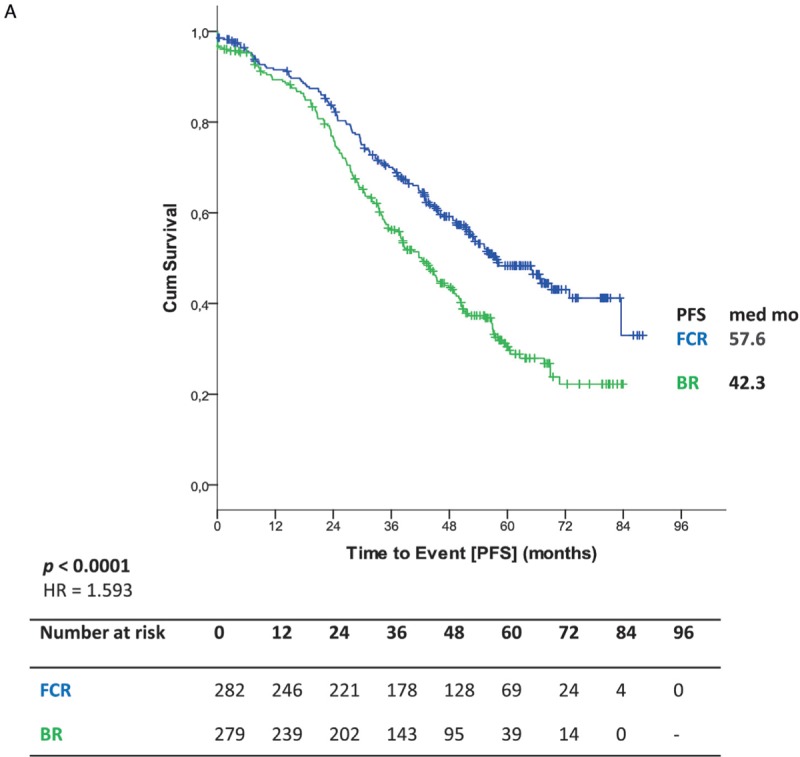
(A) Primary endpoint PFS (B) PFS according to treatment and age (C) PFS according to IGHV status (D) PFS according to IGHV status in patients ≤65.
Figure 1 (Continued).
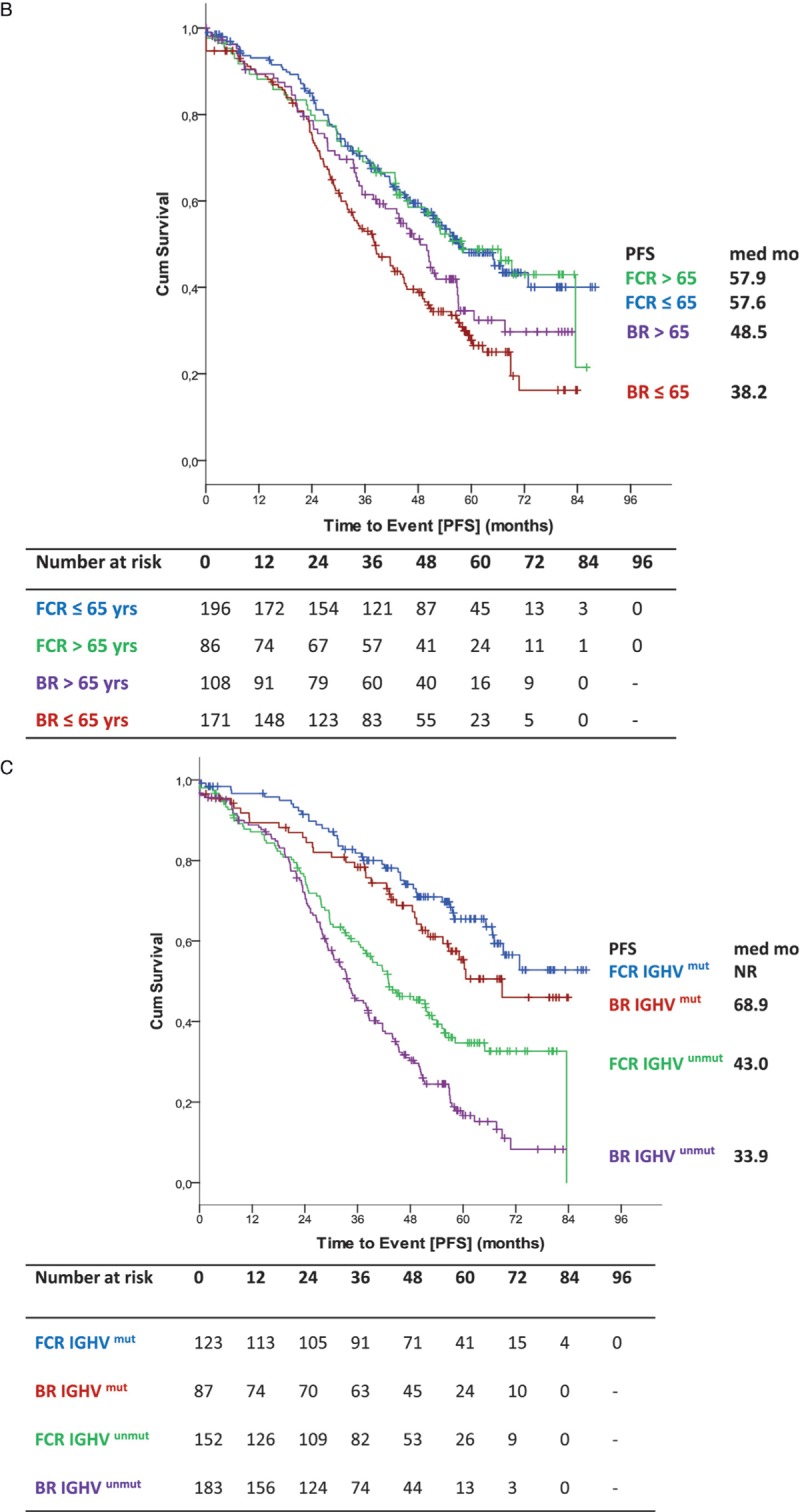
(A) Primary endpoint PFS (B) PFS according to treatment and age (C) PFS according to IGHV status (D) PFS according to IGHV status in patients ≤65.
In the subgroup of patients ≤65 years, BR patients with an unmutated IGHV status had a significant shorter median PFS than patients treated with FCR (median PFS 30.6 vs 43.1 months [HR 1.706; 95% CI 1.222–2.383; p = 0.002]), while the apparent difference between patients with a mutated IGHV status did not reach statistical significance (BR: 59.1 months vs FCR: not reached [HR 1.513; 95% CI 0.898–2.549; p = 0.120]) (Fig. 1 D). In patients older than 65 years, PFS according to different IGHV mutational status resulted in no significant difference between the single arms (Supplemental Data, Fig. S2a).
In patients with del11q, median PFS was prolonged after FCR with 39.9 months compared to 26.6 months after BR [HR 2.335; 95% CI 1.553–3.510; p < 0.001] (Supplemental Data, Fig. S2b).
There was a significant interaction between CLL-IPI risk groups and study treatment (p < 0.001). The hazard ratio was more strongly in favor of FCR in the high-risk group compared with the other 2 risk groups (Fig. 2A). In a multivariate analysis, variables that were independently associated with shorter PFS were elevated serum thymidine kinase level >10 U/L (vs ≤10.0; HR 1.402; 95% CI 1.047–1.876; p = 0.023), unmutated IGHV status (vs mutated, HR 1.916; 95% CI 1.436–2.556; p = 0.0001), deletion 11q (vs no, HR 1.825; 95% CI 1.406–2.370; p < 0.0001) and BR treatment (vs FCR; HR 1.634; 95% 1.291–2.069; p < 0.0001).
Overall survival
Median overall survival (OS) was not reached for either group. Five-year OS in all patients was 80.1% in the BR arm and 80.9% in the FCR arm (HR 1.108; 95% CI 0.755–1.627; p = 0.599) (Fig. 3 A). No significant interaction between study treatment and the different age groups could be shown for OS (p = 0.050) (Fig. 2B). According to the age subgroups, 5-year OS for patients ≤65 years was 81.1% for BR-treated patients and 85.6% for FCR-treated patients (HR 1.516; 95% CI 0.894–2.581; p = 0.122). In patients above 65 years 5-year OS was 78.8% for BR, but 70.9% for FCR (HR 0.712; 95% CI 0.403–1.256; p = 0.241) (Fig. 3 B). Also, no significant interaction between study treatment and IGHV mutational status (p = 0.171) was found for OS (Fig. 2B). OS according to IGHV status showed no statistically significant differences between both arms (unmutated IGHV: BR: 5-year OS 72.9% vs FCR 75.5% [HR 1.203; 95% CI 0.767–1.887; p = 0.420]; mutated IGHV: BR 93.3% vs FCR 87.6% [HR 0.573; 95% CI 0.236–1.393; p = 0.219]) (Fig. 3 C). Regarding OS according to IGHV status in the different age groups ≤65 (unmutated IGHV: BR: 5-year OS 72.9% vs FCR 81.2% [HR 1.691; 95% CI 0.897–3.185; p = 0.104]; mutated IGHV: BR 93.4% vs FCR 89.4% [HR 0.808; 95% CI 0.271–2.414; p = 0.703]) (Fig. 3 D) and >65 years (Supplemental data Fig. S3a), no significant difference could be seen.
Figure 1 (Continued).
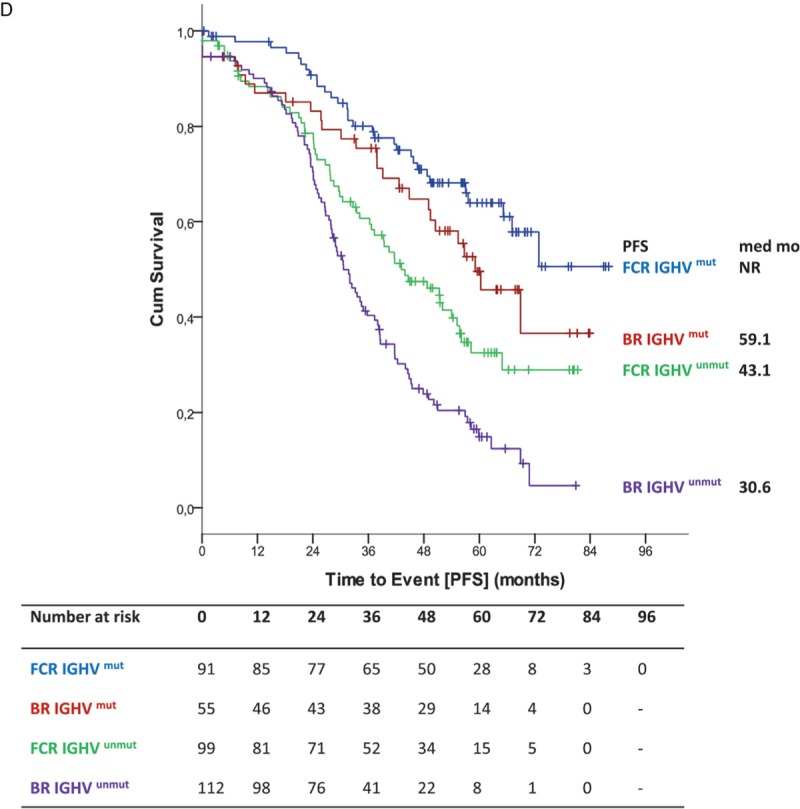
(A) Primary endpoint PFS (B) PFS according to treatment and age (C) PFS according to IGHV status (D) PFS according to IGHV status in patients ≤65.
Figure 3 (Continued).
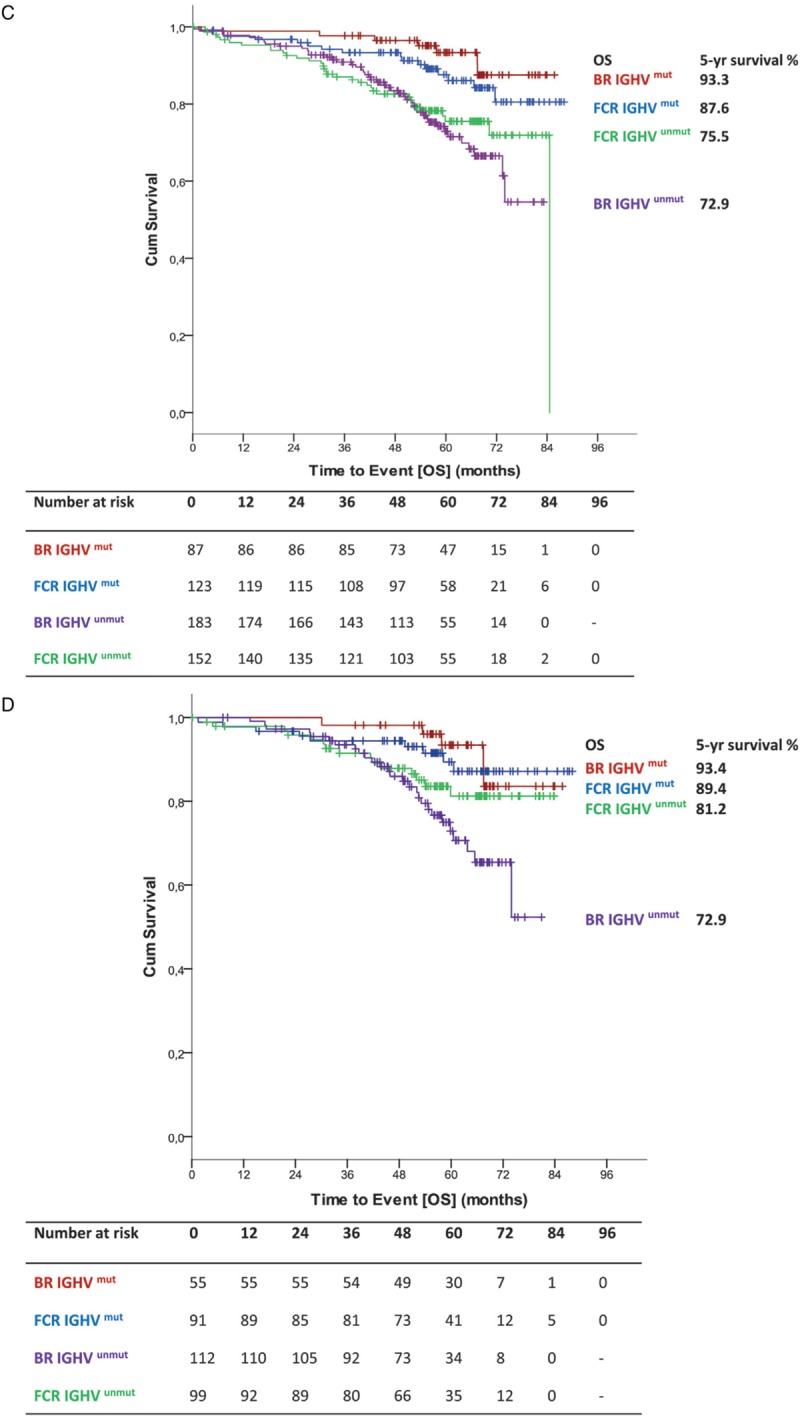
(A) OS for all patients (B) OS according to treatment and age (C) OS according to IGHV status (D) OS according to IGHV status in patients ≤65.
There was no significant interaction between study treatment and CLL-IPI risk group regarding OS (p = 0.806) (Fig. 2B). The 5-year OS in the different CLL-IPI risk groups10 was 91.7% for the low risk group, 80.6% for the intermediate risk group and 72.5% for the high risk group (Supplemental data Fig. S3b). Five-year OS for BR (Supplemental data Fig. S3c) in the low risk group was 92.3%, in the intermediate risk group 83.4% and in the high risk group 70.7%, whereas in the FCR arm (Supplemental data Fig. S3d), 5-year OS was 91.4% in the low risk group, 78.0% in the intermediate risk group and 74.7% in the high risk group.
In the multivariate analysis, factors significantly associated with OS were serum-TK > 10.0 U/l (vs ≤10.0; HR 2.446, 95% CI 1.347–4.441, p = 0.003) and unmutated IGHV mutational status (vs mutated; HR 2.144, 95% CI 1.330–3.457, p = 0.002).
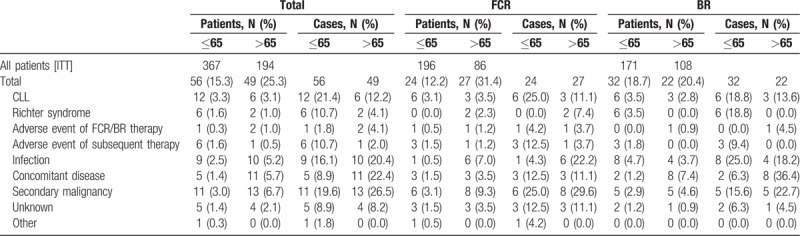
In total, 56 (15.3%) deaths occurred in the younger age group ≤65 years (Table 1). More patients younger than 65 died after BR (N = 32, 57.1% [18.7% of younger patients treated with BR]) than after FCR (N = 24, 42.1% [12.2% of all younger patients treated with FCR]). Only 4 (7.1%) of the death cases happened on or shortly after therapy (within 6 months after end of study treatment), 12 (21.4%) occurred in remission and 40 (71.4%) after relapse. Causes of death were mainly CLL (N = 12; 3.3% of all patients and 21.4% of all death cases; [6 in the FCR and 6 in the BR arm]), secondary malignancies (N = 11; 3.0% and 19.6%, respectively [6 in the FCR and 5 in the BR arm]) and infections (N = 9; 2.5% and 16.1%, respectively [1 in the FCR and 8 in the BR arm]) including 5 patients dying of sepsis and 2 patients dying of pneumonia. A total of 49 (25.3%) death cases happened in the group of elderly patients >65 years. Here, deaths were less observed after BR (N = 22, 44.9% [20.4% of all elderly patients treated with BR]) compared to FCR (N = 27, 45.1% [31.4% of all elderly patients treated with FCR]). Ten (20.4%) deaths occurred during or shortly after therapy, 18 (36.7%) in remission and 21 (42.9%) after relapse. In this age group, the most common causes of death were secondary malignancies (N = 13, 6.7% of all patients and 26.5% of all death cases [8 in the FCR and 5 in the BR arm]), concomitant diseases (N = 11, 5.7% and 22.44%, respectively [3 in the FCR and 8 in the BR arm]) and infections (N = 10; 5.2% and 20.4%, respectively [6 in the FCR and 4 in the BR arm]) including 4 cases with sepsis and 2 cases with pneumonia, followed by CLL (N = 6; 3,1% and 12.2%, respectively [3 in the FCR and 3 in the BR arm]) (Table 1).
Table 1.
Patients With Documented Death Cases and Documented Death Cases.
Subsequent treatments
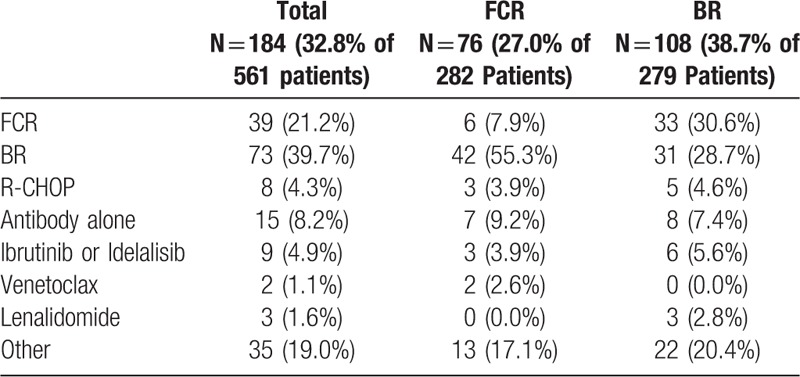
A total of 185 patients received between February 2009 and June 2016 at least one subsequent regimen, 108 (38.7%) in the BR arm and 77 (27.3%) in the FCR arm (one patient did not receive the study treatment; therefore, first line therapy was the subsequent therapy). The median number of subsequent treatments was one ranging from 1 to 4 after FCR and 1 to 6 after BR. Only one subsequent therapy was administered to 16.7% of the patients in the FCR arm and to 24.0% in the BR arm, and 7.4% respectively 7.5% received two subsequent therapies.
An overview on the most frequently used second line therapies is given in Table 2, showing that after FCR treatment a smaller number of patients were retreated (6; 7.9%) and more patients changed to BR (42; 55.3%), while after BR an equal number of patients either changed to FCR (33; 30.6%) or were re-exposed to BR (31; 28.7%). Novel agents (ibrutinib, idelalisib, venetoclax) were only used in a minority of patients (in 5 patients (6.6%) after FCR and in 6 (5.6%) after BR).
Table 2.
Second-line Therapies; Antibody Alone Subsumes Rituximab, Alemtuzumab, Obinutuzumab, Ofatumumab; Ibrutinib or Idelalisib Subsume Ibrutinib, BRI vs BR + Placebo, CLL2BIG Study, Idelalisib and Idelalisib + Rituximab; Lenalidomide Subsumes Lenalidomide and BRL
Time to next CLL treatment (TTNT) was significantly shorter for patients treated with BR as compared to FCR (median TTNT 71.7 months versus not reached, HR 1.536; 95% CI 1.146–2.058; p = 0.004) (Supplemental data Fig. S4a). This difference was statistically significant in younger patients (median 60.4 months in BR vs not reached in FCR; HR 1.790; 95% CI 1.269–2.525; p = 0.0009) (Supplemental data Fig. S4b), whereas in patients >65 years, the median was not reached for both arms (HR 1.122; 95% CI 0.642–1.963; p = 0.686) (Supplemental data Fig. S4c). There was no significant interaction between study treatment and age regarding TTNT (p = 0.178).
Secondary neoplasia
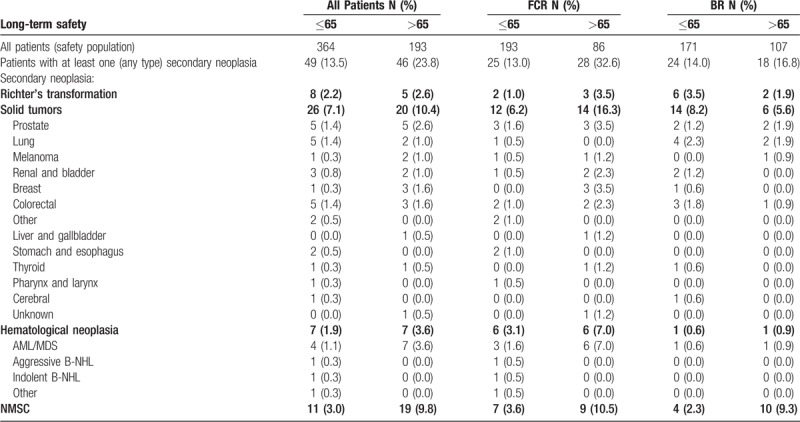
Altogether, 109 cases of secondary malignancies were observed in 95 patients. The maximum number of neoplasia reached from one to three in the FCR population (43 patients with one neoplasia (81.1%), 9 with 2 neoplasia (17.0%), 1 with 3 neoplasia (1.9%)), and from 1 to 2 in the BR arm (39 patients with 1 neoplasia (92.9%) and 3 with 2 neoplasia (7.1%)). In total 46 solid tumors including malignant melanomas, 14 hematological neoplasia and 13 Richter's transformations were reported. Numbers of patients with secondary malignancies were 53 of 279 (19.0%) after FCR and 42 of 278 (15.1%, p = 0.222) after BR. Solid tumors were 26 (9.3% of all treated patients) in the FCR arm and 20 (7.2%) in the BR arm (p = 0.389), non-melanoma skin cancer (NMSC) occurred in 16 patients in the FCR arm (5.7%) and 14 patients in the BR arm (5.0%; p = 0.706). Hematological neoplasia was more frequently reported in the FCR arm (12, 4.3% vs 2, 0.7%; p = 0.007) with AML/MDS being the most common entity [FCR: 9 patients (3.2%) vs BR 2 (0.7%) p = 0.034)]. Richter's transformations were found in 5 FCR treated (1.8%) and 8 (2.9%) BR treated patients (p = 0.396) (Table 3). For patients ≤65 years, the occurrence of secondary neoplasia did not differ (FCR: 25 of 193 patients, 13.0% vs BR: 24 of 171 patients, 14.0%; p = 0.763). Patients >65 years developed secondary neoplasia more frequently during or after FCR therapy (28 of 86 patients >65, 32.6%) vs during or after BR (18 of 107 patients >65, 16.8%; p = 0.011). Among them, particularly hematological neoplasia were increased after FCR (6 of 86 patients, 7.0%) in contrast to BR (1 of 107 patients, 0.9%; p = 0.046) including myelodysplastic syndromes (MDS) and secondary AML in six elderly patients receiving FCR (7.0%) compared to one receiving BR (0.9%; p = 0.046). A statistically significant difference was also observed for the incidence of solid tumors in elderly after FCR (14 of 86 patients, 16.3%) compared to BR (6 of 107 patients, 5.6%; p = 0.024). The time to secondary malignancy did not differ significantly between the 2 treatment arms. At 5 years, 86.6% of the BR treated patients were free of secondary malignancies versus 83.8% in the FCR arm (HR 0.801; 95% CI 0.507–1.267; p = 0.344). Median time to secondary malignancy was not reached for both treatment arms. This finding was confirmed by performing a competing risk analysis considering death without experiencing secondary malignancies as competing event.
Table 3.
Number of Patients with Various Categories of Secondary Malignancies and Percent of the Safety Population Within the Age/Treatment Group.
Health related quality of life (HRQOL)
Overall 540 (96.3%) of 561 patients completed at least one questionnaire including 272 (50.4%) patients from the FCR arm and 268 (49.6%) patients from the BR arm. Baseline characteristics are depicted in Table 4. A baseline questionnaire was available from 240 (44.4%) patients who were evaluable for HRQOL (123 (45.2%) from the FCR arm; 117 (43.7%) from the BR arm). The highest number of completed questionnaires was received at month 12, when 460 of 540 (85.2%) patients completed the questionnaire (FCR, 232 patients (85.3%); BR, 228 patients (85.1%)). In contrast, at follow up visits beyond 60 months, questionnaires were completed only by 105 (61.0%) of 172 patients who were still under observation (60 (63.2%) from the FCR arm and 45 (58.4%) from the BR arm) (Supplemental data, Fig. S5a and S5b).
Table 4.
Characteristics and Treatment Outcome of Patients With or Without HRQOL Questionnaires and With Baseline and Later Questionnaire or Just Baseline or Later Questionnaire.
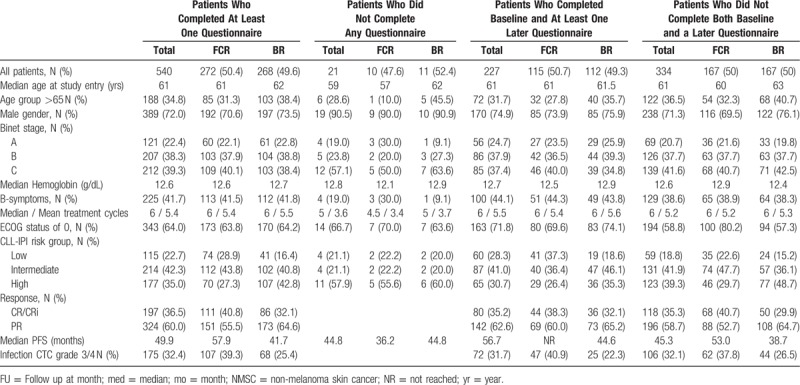
Baseline characteristics for patients with, versus without filled in questionnaires as well as patients with baseline and at least one later questionnaire, versus patients without both baseline and a later questionnaire are depicted in Table 4.
In comparison to age and sex-matched healthy controls of the general population, CLL patients had a significantly inferior outcome in physical functioning, pain, nausea/vomiting, appetite loss, constipation, diarrhea, and financial difficulties at all time points during treatment and follow up. Role functioning, emotional functioning, cognitive functioning, social functioning and insomnia were inferior only during the treatment phase. Global health status (Fig. 4 A) was decreased during the treatment phase but recovered even to superior grades compared to healthy controls. The symptom fatigue (Fig. 4 B) remained higher in CLL patients until 24 months after therapy and then converged towards healthy controls. There were no significant differences in all HRQOL scales between both treatment arms. Particularly with regards to global health status and fatigue, all patients benefited from treatment intervention (Fig. 4 C and D).
Figure 2.
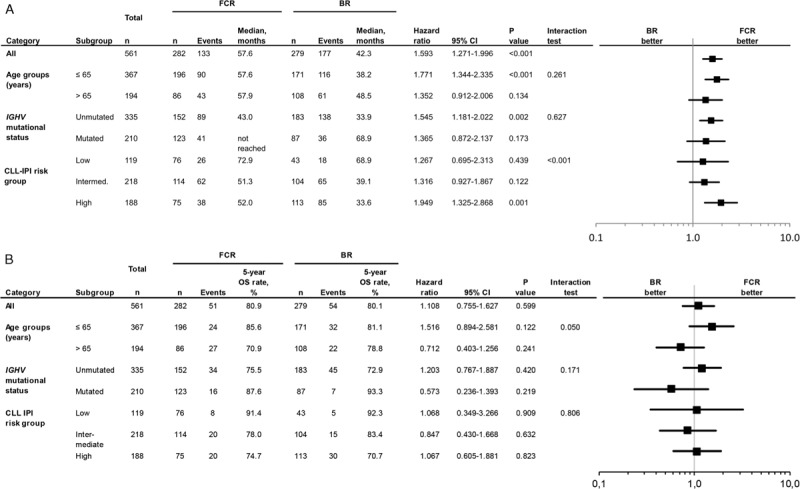
Forrest plot showing (A) progression-free survival of subgroups for FCR vs BR (B) overall survival of subgroups for FCR vs BR.
Figure 4.
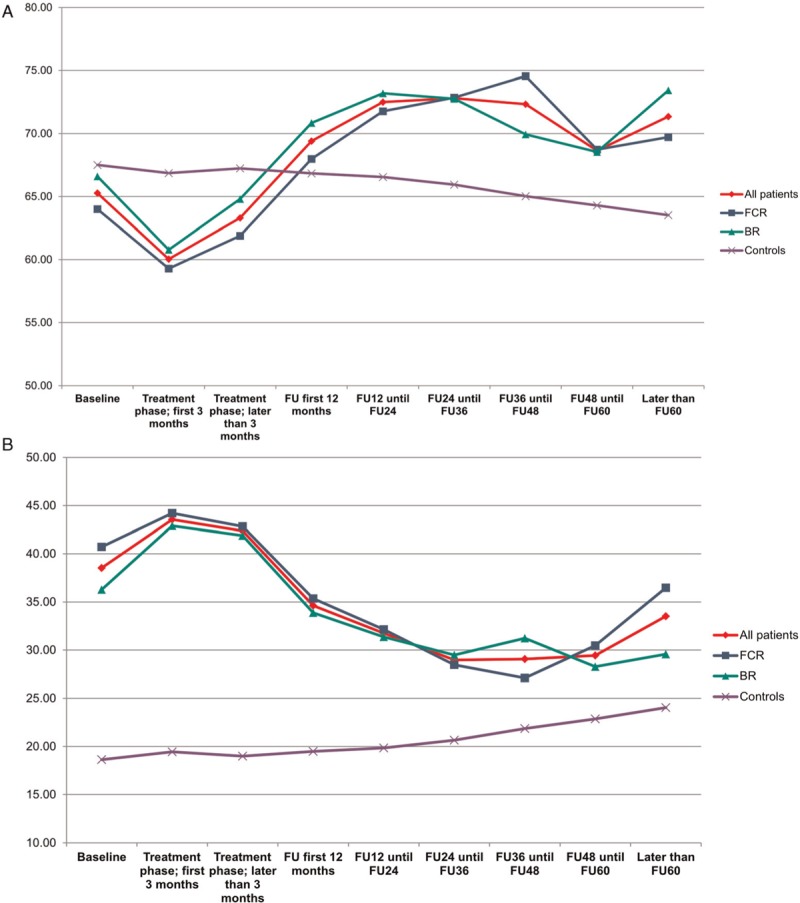
(A) Global health status in all patients vs healthy controls (B) fatigue in all patients vs healthy controls (C) global health status in both treatment arms and different age groups (D) fatigue in both treatment arms and different age groups.
Figure 4 (Continued).
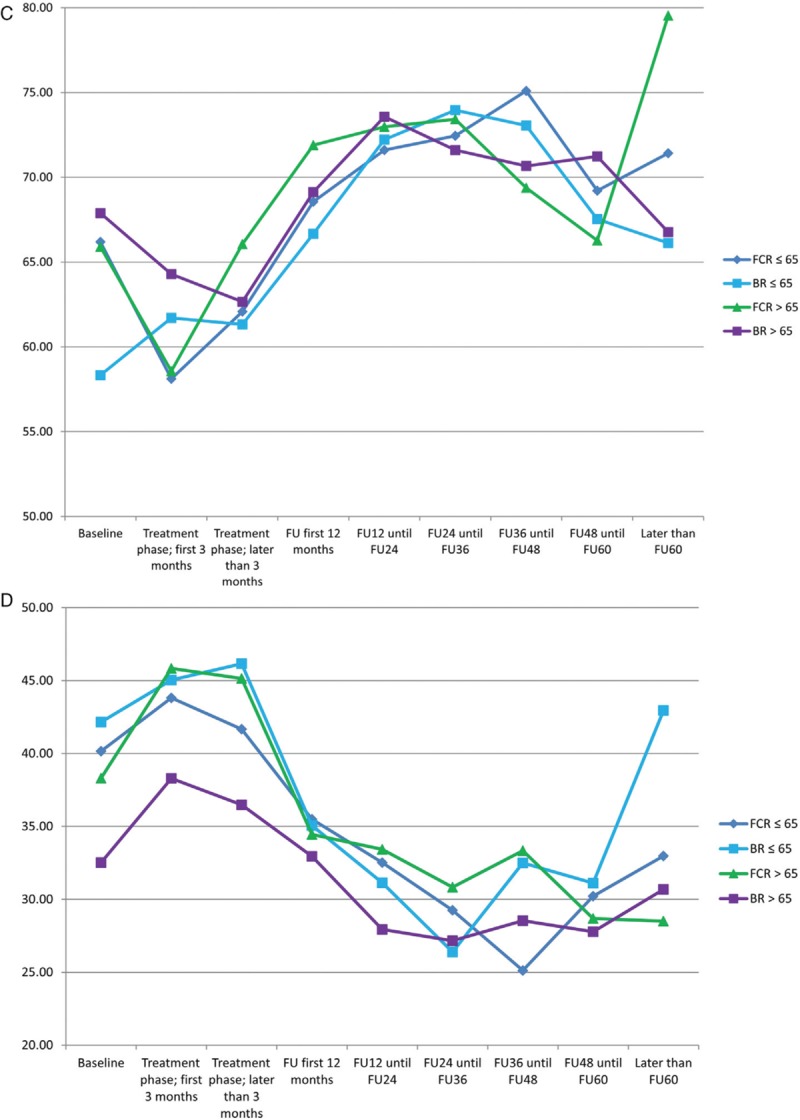
(A) Global health status in all patients vs healthy controls (B) fatigue in all patients vs healthy controls (C) global health status in both treatment arms and different age groups (D) fatigue in both treatment arms and different age groups.
Based on patients who completed the baseline as well as at least one later questionnaire we furthermore conducted a subgroup analysis in patients with severely impaired global health status (≤50) and very high fatigue scores (≥50) at baseline. Patients with low global health status as well as patients with severe fatigue were more likely to have a higher CIRS and ECOG and a lower hemoglobin level as well as B-symptoms. Within the subgroup of patients with a global health status of 50 and lower we found that the severely impaired patients converged towards the healthy controls during follow up (Fig. 5A). Patients with severe fatigue ≥50 had an improvement of their fatigue after treatment, but did not reach the levels of healthy controls (Fig. 5B). These effects were independent of the treatment arm.
Figure 3.
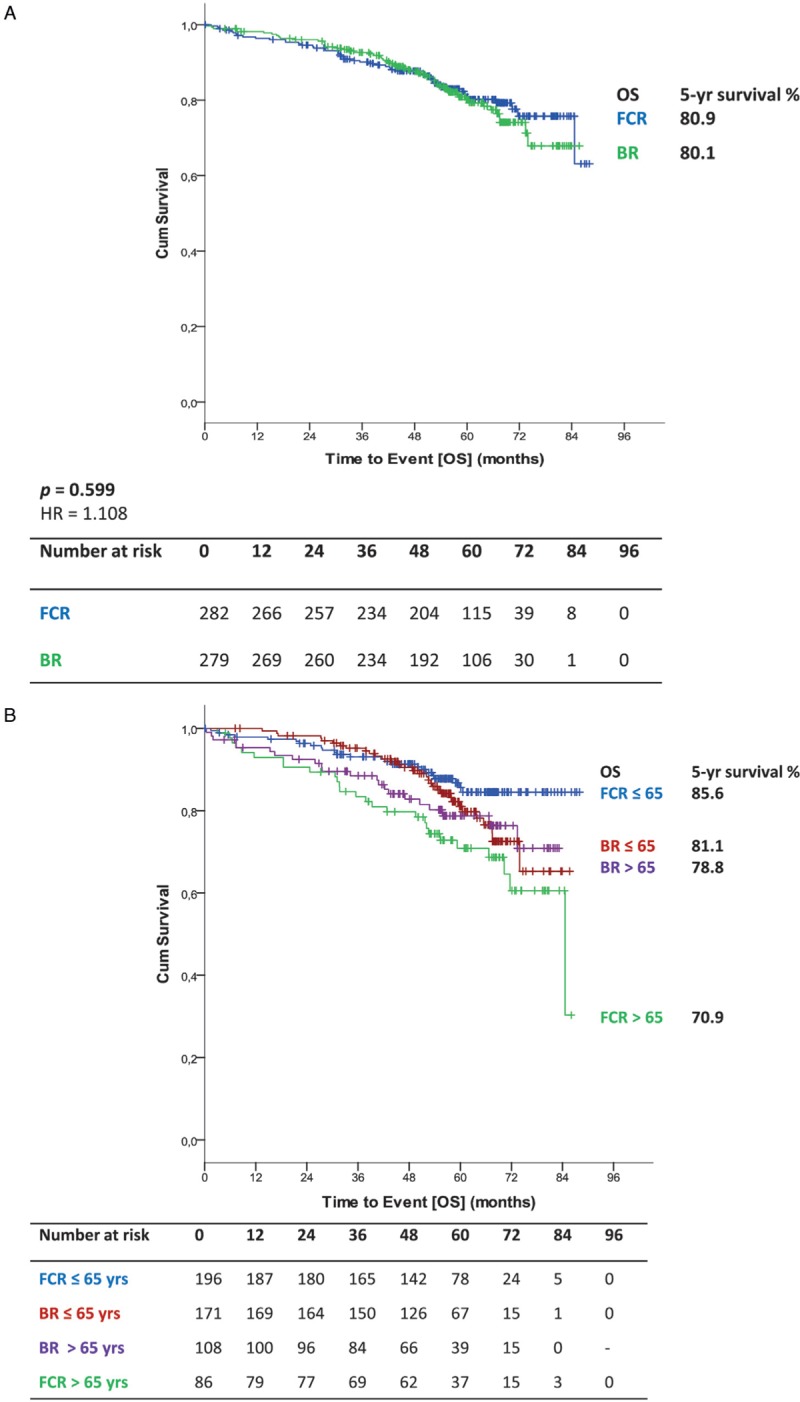
(A) OS for all patients (B) OS according to treatment and age (C) OS according to IGHV status (D) OS according to IGHV status in patients ≤65.
Figure 5.
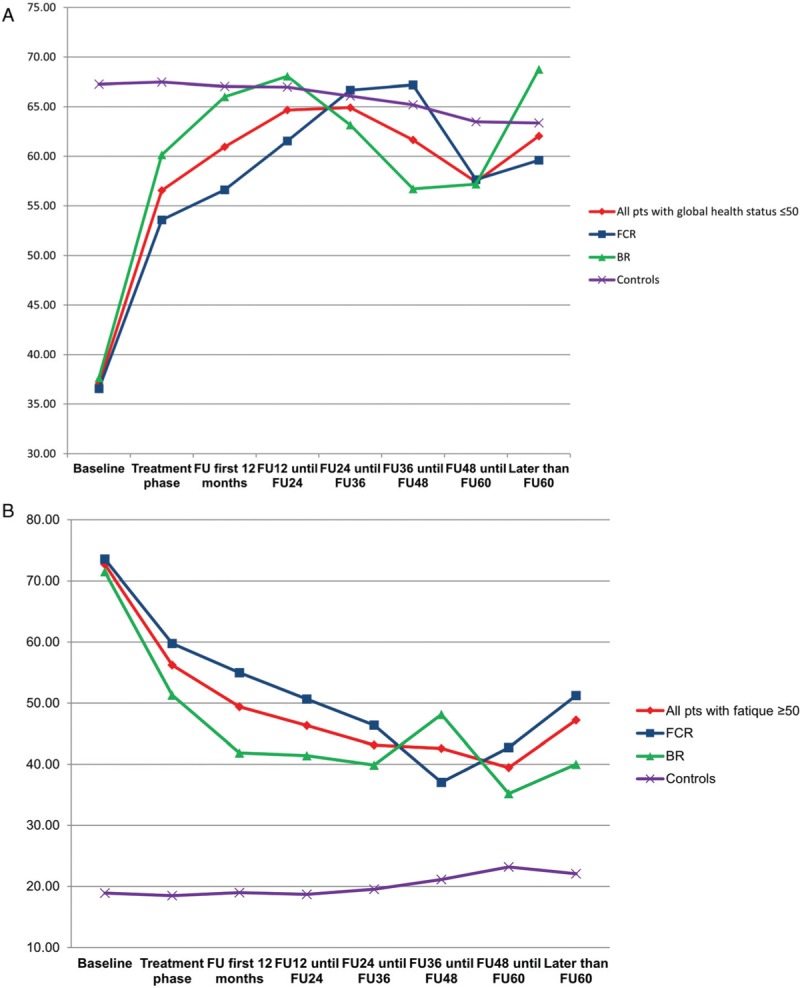
Subgroup analysis of (A) patients with severe impaired global health status vs healthy controls (B) patients with severe fatigue vs healthy controls.
Discussion
After an extended follow up of nearly 5 years FCR continues to show an excellent progression-free survival in line with the previously published data of this trial.6 Median PFS was 15.3 months longer with FCR therapy; in younger patients up to 65 years the PFS difference was even 19.4 months. This finding is consistent with the median PFS after FCR by long-term follow up.4,5 Because the PFS curve in IGVH mutated patients is at 12.8-years still at 53.9%,5 a curative potential of this regimen in the genetically favorable subgroups has been suggested.5,11 A trend to improved PFS with FCR has been observed in this trial in both subgroups, mutated and unmutated IGHV. The longer PFS within the BR arm for patients older than 65 compared with younger patients ≤65 might be misleading as it did not reach statistical difference and might be a fluctuation by chance. When contemplating survival curves of PFS according to age and IGHV status, younger patients ≤65 years seem to benefit from FCR treatment regardless of their IGHV status. However, even with longer follow up of almost five years, OS showed no statistical differences between the 2 treatment arms. In the subgroup of patients with mutated IGHV, there was a non-significant trend towards lower OS-rates with FCR. However, as there is no significant interaction, the different treatment effects between the 2 age groups could be due to chance. Considering the increasing use of novel agents for treatment of relapse, it is unlikely that an OS difference will be observed even with longer follow up.
Applying the CLL-IPI10 on the study population, most patients were in the intermediate and high-risk group. Remarkably, the high-risk group is clearly separated from the other risk groups although there were no 17p deleted patients included in the analysis. However, patients in the high-risk group seem to profit most from FCR.
The shorter OS of the high-risk group of patients identifies those patients who might be treated with novel agents or might be eligible for comparative trials because Shanafelt at al3 have recently reported that only IGHV unmutated but not IGHV mutated patients had an increased PFS with ibrutinib and rituximab when compared to FCR.
Among the older patients >65 years of age the number of deaths was higher in the FCR arm in comparison to the BR arm. The main reason for the higher mortality of elderly in the FCR arm is the higher rate secondary neoplasia, in particular solid tumors and hematologic malignancies with AML/MDS being the most common entity. Age12 as well as CLL itself13–16 have already been identified as risk factor for developing a second primary malignancy. There are no consistent data on the incidence of MDS or AML after different types of treatment.12,17–19 Previously it has been found that the risk of developing an MDS after BR exposure is low,20 while FCR has been shown to be associated with an increased incidence of secondary MDS and AML.21 These findings are confirmed by our updated study analysis in patients above the age of 65, therefore BR should be the preferred chemoimmunotherapy for this patient group.
The proportion of deaths due to CLL was slightly higher for younger patients as compared to older patients (12 of 56 [21.4%] vs 6 of 49 cases [12.21%]) which reflects a high rate of medical need in this patient group. With regard to infections, there were more patients younger than 65 years of age dying of infections in the BR arm than in the FCR arm and vice versa in the elderly patients. This also underlines the advantage of BR in the age group over 65 and of FCR in the younger patient group. A strict G-CSF prophylaxis in patients developing neutropenia CTC grade III-IV during treatment is meanwhile recommended.22 G-CSF use seems not to be associated with a higher risk of developing an AML/MDS because only 2 out of the 11 patients with AML/MDS received G-CSF.
Regarding HRQOL no difference was observed between the 2 treatment arms at any time point. The clinical benefit of therapy translated into an improved global health status even in patients with severely impaired global health status at baseline. A similar observation has been made in the CLL8 study where global health status converged towards healthy controls after the end of therapy.23 Patients with fatigue converged towards the fatigue level of healthy controls and even patients with severe fatigue improved after the course of treatment and follow up. The treatment benefit particularly for patients with low global health status or with severe fatigue indicates that patients with symptomatic disease profit from therapy, which has been previously shown in the GCLLSG CLL8 trial, the UK CLL4 trial and the GIBB study.23–25 It should be noted that the rate of questionnaires filled in at baseline and during follow up was low so that the results have to be interpreted cautiously given the incompleteness of the data and the danger of bias due to selective loss to follow up (eg, deaths, progressions, new treatments). Thus, formal statistical analysis for longitudinal data was also not possible.
Quality of life data on the new oral drugs for CLL are limited so far. Ibrutinib showed more clinically meaningful improvement in global health status and fatigue compared to ofatumumab in the RESONATE trial.26,27 When compared to chlorambucil within the RESONATE-2 trial, ibrutinib had significantly greater score improvement over time in fatigue measured with the FACIT score and also in global health status.28,29 Furthermore, ibrutinib in combination with BR improved severe fatigue within the HELIOS trial better than BR, and HRQOL improvement seemed to correlate with improvements in the hemoglobin level.30
FCR continues to be an effective first line therapy in younger, fit patients without del17p and with other favorable genetic markers like mutated IGHV status. Due to lower toxicity rates, including infections and secondary myeloid neoplasm as well as the smaller difference in median PFS between FCR and BR as well as no difference in OS, BR can be considered as a suitable alternative for older fit patients with mutated IGHV status. Furthermore, BR can be considered if there are contraindications to the BTK inhibitor ibrutinib. Although ibrutinib-based therapy has lately shown superiority to chemoimmunotherapy in previously untreated patients with respect to PFS,1–3 no difference in OS was observed in comparison to BR in patients of 65 years and older.2 In a subgroup of young patients with excellent health status, ibrutinib in combination with rituximab achieved also an OS benefit when compared with FCR,3 but the number of events is small and follow up still short. Furthermore, the superior PFS shown for ibrutinib and rituximab in this trial did not apply to the IGHV mutated subgroup.3 Notably, ibrutinib is so far given as indefinite treatment, resulting in high rates of treatment interruption outside clinical trials.31 Hence definite chemoimmunotherapy with FCR or BR remains a treatment alternative for low and intermediate risk CLL in fit patients, particularly when there is no access to frontline therapy with targeted agents.
Supplementary Material
Acknowledgments
We thank all patients and physicians for their participation in the study. For the GCLLSG Anne Westermann and Alana Hönig worked as project manager for the study, Sabine Frohs as safety manager and Annette Beer, Florian Drey, Carlo Eichendorf, Henrik Gerwin, Anna Wetzel, Rita Meister, Viktoria Monar and Irene Stodden as data manager. Petra Langerbeins, Natali Pflug and Gabor Kovacs did the medical review.
We acknowledge the data safety and monitoring board members: Eva Kimby (chair; Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden), Peter Hillmen (Leeds Teaching Hospital NHS Trust, Leeds, UK), Robin Foa (Clinica Ematologica dell’Universita di Roma “La Sapienza”, Rome, Italy), Albrecht Neiss (Institute of Medical Statistics and Epidemiology, Technical University, Munich, Germany).
Footnotes
Citation: Kutsch N, Bahlo J, Robrecht S, Franklin J, Zhang C, Maurer C, De Silva N, Lange E, Weide R, Kiehl MG, Sökler M, Schlag R, Vehling-Kaiser U, Köchling G, Plöger C, Gregor M, Plesner T, Herling M, Fischer K, Döhner H, Kneba M, Wendtner CM, Klapper W, Kreuzer KA, Böttcher S, Stilgenbauer S, Fink AM, Hallek M, Eichhorst B. Long Term Follow-up Data and Health-Related Quality of Life in Frontline Therapy of Fit Patients Treated With FCR Versus BR (CLL10 Trial of the GCLLSG). HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000336
The trial was performed with the help of the center for clinical trials (ZKS), Cologne and the competence net malignant lymphoma (KML), Germany. The study was funded by a restricted grant from the companies Roche Pharma AG and Mundipharma as well as by a grant from the German Federal Ministry of Education and Research (application number 01KI0771 and 01KI1017). Rituximab (MabThera®) study medication was provided by Roche Pharma AG.
NK: Research support: Gilead, Roche; travel support: Janssen.
ChM: travel support: Mundipharma, Amgen.
GKö: Royalties of payments for lectures/manuscript preparations/etc: Roche, Johnson/Johnson, MSD; Other, study documentation: Novartis, Clin. Assess, BMS, iOMedico.
MG: Royalties of payments for lectures/manuscript preparations/etc: Mundipharma, Roche, AbbVie, Amgen, Celgene, Janssen, Pfizer.
TP: Consultation: Janssen, Celgene, AbbVie, Takeda.
KF: Travel support: Roche; Honoraria (persona fees): Abbvie.
HD: Consultancy: AbbVie, Agios, Amgen, Astellas, Astex, Celgene, Janssen, Jazz, Novartis, Roche, Seattle Genetics; Research funding: Amgen, Celgene, Jazz, Novartis, Arog, Bristol Myers Squibb, Pfizer, Sunesis; Honoraria: AbbVie, Agios, Amgen, Astellas, Astex, Celgene, Janssen, Jazz, Novartis, Roche, Seattle Genetics.
MKn: Research grants and payment for lectures: Roche, Mundipharma, Travel support: Janssen.
C-MW: consultation and grants: Hoffmann-La Roche, Mundipharma, Janssen-Cilag, AbbVie, Gilead, Novartis, MorphoSys, AstraZeneca.
WK: Research Grants (paid to my institution): Amgen, Regeneron, Roche, Takeda; Travel support: Roche, Takeda.
K-AK: Royalties of payments for lectures/manuscript preparations/etc: Mundipharma, Roche; advisory board, consulting, speakers bureau, funding: AbbVie, Alexion, Amgen, Ariad, Baxalta, Bayer, Biotest, Bristol-Myers Squibb, Celgene, Chugai, CSL Behring, Daiichi Sankyo, Gilead, Grifols, Hexal, Janssen-Cilag, Jazz, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis, Shire, Takeda.
SB: Grants: Janssen; Honoraria: Janssen, Roche, AbbVie.
SS: Honoraria for consultancy, Advisory board member, Speaker honoraria, Research grants, Travel support: AbbVie, AstraZeneca, Celgene, Gilead, GSK, Hoffmann La-Roche, Janssen, Novartis.
A-MF: personal fees (advisory board): Janssen; research grants: Celgene.
MH: Research support/Speaker's bureau/Scientific Advisory Board: Roche, Gilead, Mundipharma, Janssen, Celgene, Pharmacyclics, AbbVie.
BE: Research support, honoraria for speaker's bureau and advisory boards: Roche, AbbVie, Janssen, Gilead, Novartis, Celgene, Beigene, ArQule.
References
- 1.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2018;20:43–56. [DOI] [PubMed] [Google Scholar]
- 2.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–1351. [DOI] [PubMed] [Google Scholar]
- 10.International CLLIPIwg. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–790. [DOI] [PubMed] [Google Scholar]
- 11.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–3254. [DOI] [PubMed] [Google Scholar]
- 12.Maurer C, Langerbeins P, Bahlo J, et al. Effect of first-line treatment on second primary malignancies and Richter's transformation in patients with CLL. Leukemia. 2016;30:2019–2025. [DOI] [PubMed] [Google Scholar]
- 13.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schollkopf C, Rosendahl D, Rostgaard K, et al. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121:151–156. [DOI] [PubMed] [Google Scholar]
- 15.Travis LB, Curtis RE, Hankey BF, et al. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422–1427. [DOI] [PubMed] [Google Scholar]
- 16.Hisada M, Biggar RJ, Greene MH, et al. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Vena DA, Barrett J, et al. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999;17:2454–2460. [DOI] [PubMed] [Google Scholar]
- 18.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. [DOI] [PubMed] [Google Scholar]
- 19.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. [DOI] [PubMed] [Google Scholar]
- 20.Burke JM, Bibeau K, Kahl B, et al. Evidence of low incidence of myelodysplastic syndrome (MDS) in patients exposed to bendamustine treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). J Clin Oncol. 2017;35 15_suppl:e19008. [Google Scholar]
- 21.Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber M, Fleiss K, Porpaczy E, et al. Prolonged progression-free survival in patients with chronic lymphocytic leukemia receiving granulocyte colony-stimulating factor during treatment with fludarabine, cyclophosphamide, and rituximab. Ann Hematol. 2011;90:1131–1136. [DOI] [PubMed] [Google Scholar]
- 23.Kutsch N, Busch R, Bahlo J, et al. FCR front-line therapy and quality of life in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2017;58:399–407. [DOI] [PubMed] [Google Scholar]
- 24.Else M, Cocks K, Crofts S, et al. Quality of life in chronic lymphocytic leukemia: 5-year results from the multicenter randomized LRF CLL4 trial. Leuk Lymphoma. 2012;53:1289–1298. [DOI] [PubMed] [Google Scholar]
- 25.Danilov A, Yimer HA, Boxer M, et al. Improvements in health-related quality of life and symptoms in patients with previously untreated chronic lymphocytic leukemia: results from the phase II GIBB study of the combination of obinutuzumab and bendamustine. Blood. 2019;134 Supplement_1:3491. [DOI] [PubMed] [Google Scholar]
- 26.Barrientos JC, O’Brien S, Brown JR, et al. Improvement in parameters of hematologic and immunologic function and patient well-being in the phase III RESONATE study of ibrutinib versus ofatumumab in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2019;doi:10.1038/s41375-019-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer P, Fraser G, Santucci-Silva R, et al. Improvement of fatigue, physical functioning, and well-being among patients with severe impairment at baseline receiving ibrutinib in combination with bendamustine and rituximab for relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma in the HELIOS study. Leuk Lymphoma. 2018;59:2075–2084. [DOI] [PubMed] [Google Scholar]
- 31.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


