Abstract
The objective of this guideline is to aid clinicians in making individual salvage treatment plans for pediatric and adolescent patients with first relapse or refractory (R/R) classical Hodgkin lymphoma (cHL). While salvage with standard dose chemotherapy followed by high dose chemotherapy and autologous stem cell transplant is often considered the standard of care in adult practice, pediatric practice adopts a more individualized risk stratified and response adapted approach to salvage treatment with greater use of non-transplant salvage. Here, we present on behalf of the EuroNet Pediatric Hodgkin Lymphoma group, evidence and consensus-based guidelines for standardized diagnostic, prognostic and response procedures to allocate children and adolescents with R/R cHL to stratified salvage treatments.
Introduction
The majority of children and young people with classical Hodgkin lymphoma (cHL) are cured with first line treatment and treatment failure rates with the most effective pediatric regimens are approximately 10% in low stage and 15% to 20% in advanced cHL.1–4 First line treatment strategies in children have evolved to reduce late toxicities while maintaining high cure rates and all patients receive multi-agent chemotherapy with pre-treatment prognostic factors defining chemotherapy burden (number of cycles or intensity of regimen) but radiotherapy (RT) now is often response adapted and restricted to patients with residual disease on early or late response assessment FDG-PET scans. The approaches to salvage described here therefore, address salvage after primary chemotherapy or combined modality therapy, as single modality radiotherapy is no longer used in first line treatment.
Cure may be achieved in the relapse/refractory (R/R) setting but the optimal salvage treatment has not been defined in children and adolescents as there are no randomized trials in children defining the “best” salvage chemotherapy regimen or comparing standard dose chemotherapy (SDCT) vs high dose chemotherapy and autologous stem cell transplant (HDCT/ASCT). In adults there have been 2 randomized trials of SDCT vs HDCT/ASCT salvage in first relapse, which showed superior progression free survival (PFS) for HDCT compared to SDCT leading to widespread adoption of HDCT/ASCT as the standard of care in adults in the relapse setting.5,6 These trials showed no overall survival (OS) benefit for HDCT/ASCT in first relapse, which was confirmed in a recent meta-analysis because HDCT/ASCT may be used to salvage patients in later relapse.7 The universal application of these trial data no longer reflects state of the art practice because both trials were conducted in the pre-FDG-PET era and, therefore, not FDG-PET response adapted and before availability of novel agents such as the targeted antibody drug conjugate Brentuximab vedotin or immunotherapy drugs such as Nivolumab which provide new therapeutic options in the relapse setting. Achieving a complete metabolic remission on FDG-PET prior to consolidation has subsequently been shown to be highly prognostic in the relapse setting8,9 and FDG-PET response is now universally assessed in the relapse setting to identify highly chemo-sensitive or chemo-resistant patients, which defines curative intent but may also be used to escalate or de-escalate treatment intensity between transplant and non-transplant salvage as described below.
Methodology
These guidelines have been written by a working group within the EuroNet (European Pediatric Hodgkin Lymphoma consortium), which is made up of a multi-disciplinary team of experts in the field including many of the National chairpersons of the EuroNet-PHL-C1 and C2 trials. The recommendations were generated based on a literature review to April 2019 from established databases (Medline, PubMed) using keywords paediatric, pediatric, Hodgkin, lymphoma, relapse, refractory, FDG-PET, transplantation, prognostic factors, response. Development of the key recommendations was based on the best evidence available from randomized studies, non-randomized trials, case series, literature review and expert consensus. Discussion and review of these recommendations was carried out by all members of the EuroNet clinical board at successive meetings of EuroNet in 2018–19 and approved in May 2019. The GRADE nomenclature is not used because levels of evidence are largely derived from non-randomized case series, phase II trials and standard of care.
A. SDCT vs HDCT/ASCT
The lack of any randomized trial comparing SDCT vs HDCT in children combined with the very limited evidence for a survival benefit of HDCT in first relapse has limited universal adoption of HDCT/ASCT in pediatric patients. In children non-transplant salvage with SDCT plus radiotherapy (RT) has been shown to be effective in a number of studies, which clearly show that subsets of patients do not require HDCT for cure (Table 1). If we consider the last 3 European pediatric national data sets published in first R/R HL the rate of transplantation in second line treatment ranges from 10% in the DAL ST-HD-86 trial, to 53% in the UK HD3 relapse trial, to 71% in the French SFCE study. The ST-HD-86 study is the largest prospective pediatric relapse trial published to date and reported 10-year disease free survival (DFS) of 86% with SDCT plus RT (no HDCT) in all patients with relapse over 12 months from primary treatment, including patients with intermediate risk and high risk disease in first line and the 10-year DFS was 96% in those with low stage disease in first line treated with only 2 cycles of SDCT.10 Of note, 102 of 132 surviving patients never received HDCT/ASCT in salvage in this trial. In other studies, subsets of patients have achieved good outcomes without HDCT, including 5-year DFS of 74% in the UK HD3 trial,11 10-year DFS of 80% in late relapse confined to original disease sites in a UK single center study,12 5-year DFS of 100% in low or intermediate risk relapse if patients had had an adequate response to first salvage chemotherapy in a US St Jude's study.13 All pediatric relapse data sets are small numbers but consistently show that subsets of patients may be salvaged without HDCT. There is a clear need to standardize selection of these subsets using risk stratified and response adapted models.
Table 1.
Outcomes with salvage SDCT plus RT only (no transplant)

Where HDCT/ASCT has been assessed in children most studies have failed to show a survival benefit with HDCT except in patients with primary progressive disease and multiply relapsed disease.14,15 The ST-HD-86 trial showed no significant DFS benefit for HDCT in a small group of early relapse and primary progression with 6-year DFS of 51% compared to 6-year DFS of 47% achieved with SDCT in first relapse, although there was a survival benefit in subsequent relapse. A retrospective British study compared OS in children with R/R HL treated with SDCT or with HDCT/ASCT and concluded that HDCT did not offer any survival advantage over SDCT plus RT salvage in relapsed HL, although there was a trend to improved survival in primary progressive HL.14 Another retrospective comparison of outcomes in first relapse found no difference in outcome for those receiving SDCT alone compared to those undergoing HDCT/ASCT concluding that the necessity for HDCT should be evaluated based on risk stratification.15
Most of these data sets are retrospective analyses, with limited patient numbers, and variation in risk and response criteria and variation in first line treatments with some data sets representing relapse after chemotherapy alone and others after combined modality treatment. Nevertheless, the fact that a number of studies have reported high DFS without HDCT/ASCT argues that there is the potential to avoid HDCT/ASCT and its consequent toxicities in selected patients. When SDCT has been used without HDCT/ASCT then RT consolidation has been given. The radiation burden in first line treatment in children within EuroNet studies has markedly reduced over the last 2 front line trials with approximately 50% of patients receiving RT in the EuroNet-PHL-C1 trial (EudraCT number: 2006–000995–33) and in the current EuroNet-PHL-C2 trial (EudraCT Number: 2012–004053–88) around 25% of patients will receive RT and in some patients the RT is targeted only to late FDG-PET positive residua which implies small fields. This means that most pediatric patients treated in accordance with EuroNet strategies are RT naïve at relapse and so SDCT plus RT may be an attractive option in relapse where RT was not given in first line treatment, or in rare situations where nodal relapse is exclusively outside of prior RT fields.
For the last decade salvage in pediatric patients in Europe has been based on the EuroNet consortium risk and response adapted relapse sub-study (R1) within the EuroNet-PHL-C1 trial. Three risk groups (RG) were defined at the point of relapse based on time to relapse and prior chemotherapy treatment group (TG) in first line. A low-risk group (RG1) of late relapse after 2 cycles of chemotherapy +/− RT in first line, were not eligible for HDCT/ASCT and had SDCT plus RT only. A high-risk group (RG3), which was all primary resistant cHL, were not eligible for non-transplant salvage and intensification with HDCT/ASCT consolidation was mandatory. All other patients had intermediate risk relapse (RG2), which included all early relapse and late relapse after 4 or 6 cycles of first line chemotherapy +/− RT and this was the largest group with approximately 70% of all patients in the study and salvage treatment was response adapted using early FDG-PET response after 2 cycles of chemotherapy with FDG-PET negative relapse de-escalating to the non-transplant strategy and FDG-PET positive relapse escalating to the HDCT/ASCT strategy. The results of this trial are awaited with interest but preliminary reports (Dirk Hasenclever, personal communication) do support a non-transplant strategy in selected patients.
This trial however no longer reflects state of the art practice for a number of reasons: (i) low risk patients did not have any FDG-PET assessment which is now considered standard of care, (ii) there was no goal for a negative pre-HDCT FDG-PET scan which is now known to be highly prognostic, (iii) the threshold for a positive FDG-PET was Deauville 3–5 when the consensus is now that Deauville 4–5 is positive, (iv) prior RT in first line did not influence the salvage strategy and patients could be re-irradiated if they relapsed in a prior RT site which we no longer recommend, (v) stage IV relapse was eligible for SDCT plus RT if the early response assessment FDG-PET was negative regardless of RT field volume or toxicity but RT toxicity is now an important factor in our salvage treatment stratification.
Panel recommendation:
In our proposed guidelines we seek to standardize recommendations for salvage of all R/R cHL using a risk stratified and response adapted approach and reflecting changes in practice since the inception of the R1 trial. Risk stratification at the point of relapse identifies 2 groups (low and standard risk) based on assessment of pre-salvage risk factors. A third group (high risk) emerges based on response and this group is defined by failure to achieve a negative FDG-PET after 2 lines of salvage SDCT. Specifically:
-
1.
The Low risk group is salvaged with SDCT plus RT consolidation only.
-
2.
The Standard risk group is salvaged with SDCT plus HDCT/ASCT consolidation.
-
3.
The High risk group is eligible for conventional HDCT/ASCT plus additional treatments pre and/or post HDCT/ASCT or experimental strategies.
In the following sections we discuss the justification for a risk and response adapted salvage strategy and define the most important pre-salvage prognostic factors and the time points and thresholds for adequate response assessment using FDG-PET for each group. After analysis of risk and response we define individual treatment pathways for each clinical scenario for low, standard and high risk patients. The overall approach follows a logical timeline with:
-
i.
complete disease assessment at point of relapse
-
ii.
analysis of pre-salvage prognostic factors which defines risk group
-
iii.
all patients commence treatment with 2 cycles of salvage re-induction SDCT
-
iv.
assessment of response with FDG-PET occurs in all after re-induction (PET2)
-
v.
further consolidation treatment is based on risk group and FDG-PET response
B. Diagnostic procedures at the point of relapse
Prior to starting salvage treatment, a full disease re-assessment is mandatory including biopsy and complete re-staging in all patients. Biopsy is considered mandatory to ensure histologic confirmation and ensures that the type of lymphoma has not changed which is occasionally seen, which is especially important both in unusually resistant patients and in later relapse to exclude second cancers and exclude false positives. Sufficient material must be obtained using either minimally invasive image guided techniques such as “trucut” biopsy or by conventional excision biopsy. Fine needle aspiration (FNA) is strictly not recommend, as this cannot provide sufficient material. Moreover, research on relapse samples is indispensable to gain more insight in the development of HL relapse. The implications and risks of salvage treatment merit confirmation of tissue diagnosis. Patients should also be fully re-staged to establish disease burden using anatomical (MRI, CT, U/S), and functional imaging (FI) modalities (specifically FDG-PET). If peripheral blood stem cell (PBSC) harvest is planned an assessment of bone marrow involvement is mandatory and although this may be done by bone marrow biopsy it is now more often done with FDG-PET scan with more sensitivity as demonstrated in first-line patients in both children and adults.16,17
Panel recommendation:
Histologic confirmation of recurrence by tissue biopsy and full re-staging is mandatory prior to embarking on salvage treatment.
C. Prognostic models and risk groups
There are currently no universally accepted prognostic criteria in children (or adults) defining individualized salvage treatment plans. At relapse individual differences between patients may include: (i) stage and prior treatment in first line, (ii) time to relapse, (iii) stage and disease burden at relapse. These individual differences between patients justify an individual approach to salvage treatment and this is supported by data from a number of pediatric studies. The ST-HD-86 trial is the largest (n = 176) prospective pediatric R/R cHL trial published to date and showed that salvage may be risk adapted because subgroups with markedly better or poorer prognosis than the average patients could be defined.10 While numerous prognostic factors have been identified the evidence for some is not consistent in the literature because studies are small, retrospective and patient groups are not comparable between studies due to variation in disease and treatment characteristics.18–20 Examples of some inconsistent risk factors, which are adverse in some studies but not others include B symptoms at relapse; relapse in original and distant new sites; bulk; extra-nodal relapse; raised erythrocyte sedimentation rate (ESR) and anaemia.19 Most of these published studies did not incorporate FDG-PET response assessment, which is now well recognized as an important, perhaps the most important prognostic factor, which may overcome the significance of some factors as is the case in first line treatment. In more recent studies FDG-PET response has overcome other prognostic factors with for example 3-year PFS of 78.3% in FDG-PET negative vs 40% in FDG-PET positive patients after initial salvage SDCT in a very recent report and the impact of all other risk factors on PFS was not significant.21
Panel recommendation:
A highly complex risk stratification incorporating every potential prognostic factor is neither justified nor pragmatic and we therefore propose an assessment of 3 pre-salvage prognostic factors to allocate patients to a low risk or a standard risk group because these are the most important factors and have the most influence on salvage treatment choice and these are: (i) time to relapse, (ii) prior treatment in first line and (iii) stage at relapse.
1. Time to relapse: It is defined from completion of first line treatment and is the most important prognostic factor at the point of relapse and highly significant for OS and EFS in pediatric studies dominating all other prognostic factors in multivariate analysis of the ST-HD-86 study (p 0.0001) for example. Three prognostic groups based on time to relapse are well described in both pediatric and adult practice: (i) primary refractory/progressive disease defined as progression during or within 3 months of primary treatment, (ii) early relapse between 3 and 12 months from primary treatment and (iii) late relapse over 12 months from primary treatment.
Primary refractory disease has the worst prognosis in pediatric HL, as in adult HL, and is adverse for disease free survival (DFS) and OS. Outcomes with non-transplant SDCT salvage in this group have reported very poor OS in adults.22,23 Two UK pediatric studies similarly showed poor outcomes in this group with SDCT salvage with no survivors in one study and primary refractory HL being the only significant factor associated with inferior OS in the UK HD3 study.24 In the ST-HD-86 trial the 10-year DFS was 41% and OS was 51% in primary refractory HL with SDCT plus RT salvage and 59% of all study deaths occurred in this group, which was only 29% of study patients, and deaths occurred within 12 months of initiating second salvage therapy. SDCT salvage is inadequate in primary refractory cHL and there is only a limited time in which intensified therapy can influence the course of primary refractory disease and so saving HDCT for later relapses is not appropriate. In the French SFCE study the outcome for refractory patients was also poor with EFS 35% and OS 48%.25 HL which relapses after a period of initial remission has a better outcome.
Early relapse has an intermediate prognosis between primary refractory and late relapse. The optimum treatment of early relapse HL is not clearly established but there is no doubt that SDCT plus RT is adequate in a large proportion of early relapse achieving a 10-year DFS of 55% and OS of 78% in the ST-HD-86 study. This is a rather poor DFS but most of these patients had already had RT in first line and the treatment was not response adapted and was in the pre-FDG-PET era. There was no DFS or OS advantage for HDCT (6-year DFS 51% and OS 66%) over SDCT plus RT (6-year DFS 47% and OS 65%) in early relapse in the ST-HD-86 trial. A retrospective UK study also concluded there was no OS advantage for HDCT salvage over SDCT plus RT at first relapse with 5-yr OS 67% for the SDCT salvage and 74% for HDCT/ASCT salvage in patients in whom duration of first remission was less than 12 months.14
Late relapse has the best outcome achieving a 10-year DFS of 86% and OS 90% in the ST-HD-86 study and all patients received SDCT plus RT salvage only including patients originally treated with up to 6 cycles of chemotherapy. Patients originally treated with only 2 cycles, had a 10-year DFS of 96% and HDCT/ASCT clearly cannot improve the outcome in the latter group. Another UK study showed that salvage with SDCT and RT in patients with late local relapse had a 5-year DFS of 90%.19
2. Prior treatment in first line: It is essential to consider when assessing salvage treatment options because prior chemotherapy and radiotherapy burden have both prognostic and pragmatic implications.
Prior chemotherapy should be assessed so that non-cross resistant drugs may be selected, and excessive cumulative doses of individual drugs avoided. Prior chemotherapy also however impacts risk stratification at relapse because it has prognostic implications. A higher first line chemotherapy burden is a poor prognostic factor in some studies where the DFS at relapse is inferior in patients that received more than 4 cycles compared to patients who received less than 4 cycles of chemotherapy in first line.10 In the EuroNet first line treatment strategies the number of cycles of first line chemotherapy given is risk stratified with 2 or 3, 4 and 6 cycles respectively for low, intermediate and advanced stage. Therefore, prior chemotherapy burden reflects the stage in first line (which is why stage in first line is not a separate prognostic factor in our guidelines).
Prior radiotherapy should be assessed because relapse within a prior RT site will generally exclude a low risk SDCT plus RT salvage because re-irradiation may result in unacceptable cumulative radiation doses and if combined modality treatment was ineffective in first line it may be risky to apply the same approach in salvage.
3. Stage at relapse: It is significant for DFS and OS in some studies with stage IV, extra-nodal and bulk disease at relapse as significant predictors for poor OS, EFS and PFS.26,27 These factors are not consistent across all relapse studies and FDG-PET response assessment may overcome the prognostic importance of some of these factors as in first line but stage at relapse affects the choice of salvage treatment because consolidation radiotherapy must be deliverable to all disease sites in low risk salvage, and the radiation doses and fields must not have unacceptable toxicity. Stage IV relapse is a systemic disease and so RT consolidation is either not feasible or potentially too toxic. This is also the case in extensive nodal relapse where RT consolidation is considered excessively toxic due to field volume or organ toxicity.
Panel recommendation:
Two salvage risk groups are defined at the point of relapse based on (i) time to relapse, (ii) prior treatment in first line and (iii) stage at relapse.
-
1.
Low Risk Group: The intention in this group is to select patients that may avoid the toxicity of HDCT/ASCT and in whom SDCT plus RT is an effective option without excessive RT toxicity.
The inclusion criteria for the Low risk group are:

-
2.
Standard risk Group: the intention in this group is to select patients for whom SDCT plus consolidation HDCT/ASCT is indicated and de-escalation to non-transplant salvage is not recommended.
The inclusion criteria for the Standard risk group are any of the following factors:

While relatively simple criteria may be written for risk stratification ultimately the clinical judgment of the treating team and their discussions with individual patients is important in reaching treatment decisions and clearly the risk factors in some patients may place them close to the borderlines between risk groups such as when time to relapse is around the primary resistant/early relapse or early/late relapse time cut off for example.
D. Response to salvage chemotherapy
This is the second step in defining the individual salvage treatment plan and guides whether the salvage strategy should be continued or altered. Response assessment previously used conventional imaging but functional imaging (FI) with FDG-PET response is more accurate as it may distinguish residual sclerotic tissue from viable tumor. Achieving a complete metabolic remission (CMR) or adequate response (AR) with SDCT on FDG-PET is highly prognostic and indeed may be the most important prognostic factor for long term disease control. Moscowitz et al reported a 5-year EFS of 31% vs 75% for FI positive and negative patients, respectively and concluded that the goal of salvage SDCT should be to achieve a negative FDG-PET pre-HDCT.28,29 Patients with residual FDG-PET positivity after first salvage SDCT who were switched to a non-cross resistant second line SDCT and achieved an FDG-PET negative status had a similar post-transplant outcome to those in CMR after first line. The poor prognostic impact of failure to achieve a CMR with first line SDCT can therefore be overcome by switching to alternative salvage regimens if a CMR is achieved with second line SDCT. Achieving a CMR on FDG-PET may also overcome the poor prognosis of primary refractory HL with 10-year EFS reported by Shah et al of 68% in FDG-PET-negative patients vs 33% for FDG-PET-positive patients.30 Another study in primary refractory HL or early relapse showed that if a CMR is reached after one line of salvage, patients have a good outcome after HDCT/ASCT.31 It is important to note however that failure to achieve an FDG-PET-negative state should not exclude patients from potentially life-saving treatments such as HDCT/ASCT. A meta-analysis of 11 studies with pre-HDCT FDG-PET status reported progression free survival (PFS) ranged between 0% and 52% in FDG-PET-positive patients compared to 55% to 85% in PET-negative patients and overall survival (OS) was 17% to 77% in PET-positive vs 78% to 100% in PET-negative patients and so curative options must be considered in all patients.32
A number of pediatric studies have confirmed the importance of response to salvage. A St. Jude study showed initial response to salvage chemotherapy was highly significant with 5-year OS of 17% in children with inadequate response, compared with 97% for good responders (P < .0001) and on multi-variate analysis initial response was the only significant factor for OS.13 A more recent pediatric series reported outcomes of pre-transplant FI in 49 consecutive children in a single institution and multiple lines of SDCT were given to achieve FI negativity pre-transplant.33 The 4-year PFS was 92% in the entire cohort and 95% in the FI negative group (n = 41) vs 75% in the FI positive group (n = 8). The excellent outcome was achieved despite 82% of the patients having primary refractory HL or early relapse and 29% extra-nodal disease at relapse. The prognostic impact of a positive pre-transplant FDG-PET was not seen but numbers were very low (n = 8) and post-transplant RT was administered in patients with residual PET positivity.
There is no published evidence defining the best time point for FDG-PET response assessment, but a general consensus is that this is done after 2 cycles of first line SDCT (PET2) and in those that switch to second line salvage a further FDG-PET scan is generally done after a further 2 cycles (PET4). The interpretation of FDG-PET response is generally based on the visual 5-point Deauville (D) scale with scores of 4 and 5 defined as positive.34 In the EuroNet group the semi-quantitative “qPET” method is widely used which is a quantitative extension of the Deauville scale; Deauville 4 and 5 are respectively equivalent to qPET values of 1.3 and 2.0 and a positive PET scan is a qPET value >1.3.35
Panel recommendation:
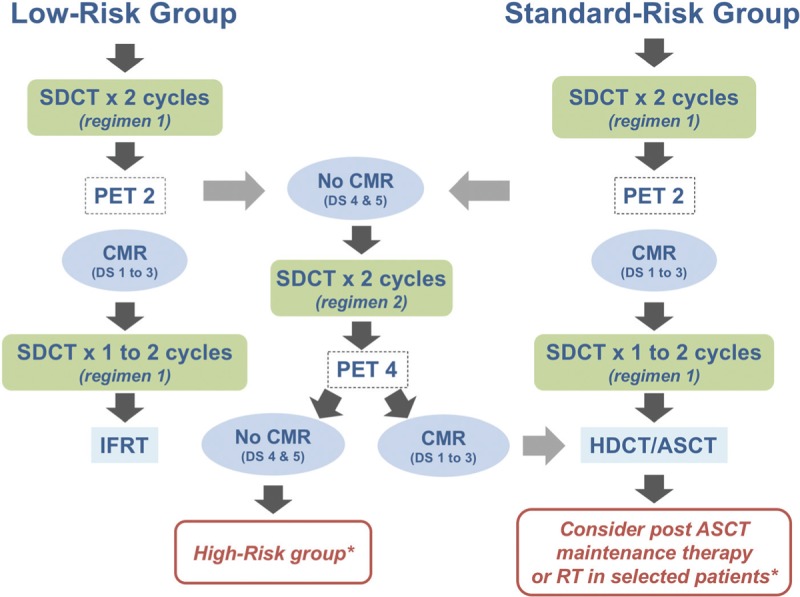
Achieving a CMR, defined as a Deauville score 1–3 or qPET <1.3, is the goal of salvage SDCT. FDG-PET response is critical in deciding whether the salvage within low and standard risk group is continued or altered (see Fig. 1):
-
1.
Low risk (LR) patients must meet all low risk criteria and must achieve a CMR after 2 cycles of first line salvage SDCT (PET2). If the PET2 is no CMR patients switch to second line SDCT and then follow the standard risk salvage detailed below.
-
2.
Standard risk (SR) patients includes patients identified at relapse with pre-salvage risk factors that make them ineligible for low risk salvage and former low risk patients with no CMR at PET2. This group must achieve a CMR after either first line SDCT (PET2) or second line chemotherapy (PET4) i.e. the pre-HDCT PET must be negative and if the PET4 is no CMR then patients switch to the high risk salvage strategy.
-
3.
High risk (HR) patients are those that fail to achieve a CMR after 2 lines of SDCT on PET4. Failure to achieve a CMR prior to HDCT is associated with an inferior prognosis compared to patients that achieve a negative FDG-PET scan pre-HDCT/ASCT. The best treatment in this group is undefined in children or adults but it is imperative that potentially curative options are not abandoned.
E. Summary of treatment strategies
We estimate based on previous European relapse data that relapse after a period of remission will be 80% of patients and 20% will have primary refractory disease. Overall approximately 35% to 40% will be low risk; 45% to 50% will be standard risk and 10% will be high risk.
1. Low Risk group Treatment Summary (Fig. 1):
Induction treatment is 2 cycles of first line salvage SDCT
Response assessment PET2 is CMR (D1–3 or qPET<1.3)
Consolidation treatment is 1–2 further cycles of SDCT followed by consolidation IFRT. Note by definition LR patients must be PET2 CMR. If PET2 is no CMR patients switch to SR detailed below in 2. (ii).
Figure 1.
Summary of Treatment strategies. ASCT = autologous stem cell transplant, CMR = complete metabolic remission, DS = Deauville score, HDCT = high dose chemotherapy, IFRT = involved filed radiotherapy, SDCT = standard dose chemotherapy. ∗see text for further discussion.
2. Standard Risk group Treatment Summary (Fig. 1):
-
i.Standard risk and PET2 CMR:
- Induction treatment is 2 cycles of first line salvage SDCT
- Response assessment PET2 is CMR (D1–3 or qPET<1.3)
- Consolidation treatment is HDCT/ASCT which should be pursued as soon as possible∗.
-
ii.Former Low risk and Standard risk and PET2 no CMR:
- Induction treatment is 2 cycles of first line salvage SDCT
- Response assessment PET2 is no CMR (D4–5 or qPET>1.3)
- Patients switch to 2 cycles of second line salvage SDCT
- Response assessment PET4 is CMR (D1–3 or qPET<1.3)
- Consolidation treatment is HDCT/ASCT which should be pursued as soon as possible∗.
3.∗High Risk group Treatment Summary:
Failure to achieve a CMR to 2 lines of SDCT on PET4 defines a small group of chemo-refractory patients and a number of studies discussed above have shown the outcome in patients with no CMR prior to HDCT is poor with conventional HDCT/ASCT consolidation alone. The intention in this group is to continue with curative treatment and it is critical that potentially curative treatment is not abandoned or withheld. At present there is no evidence-based recommendation that can be made in high risk patients. If conventional HDCT/ASCT is planned, we recommend consideration of additional treatment either before and/or after HDCT/ASCT to minimize the risk of progression post HDCT. Alternatively, experimental options may be considered in this group (which we do not recommend in low or standard risk patients) and these may include non-standard transplant procedures (such as allogeneic transplantation), early phase trials or immunotherapy. If PET4 is no CMR these patients are high risk and salvage options include:
-
I.
Proceed with HDCT/ASCT anyway in responding patients despite no CMR on pre-transplant PET and consider addition of further treatments after HDCT/ASCT such as RT and/or maintenance Brentuximab vedotin.
-
II.
Proceed with further lines of non-cross resistant SDCT or alternative novel agents to attempt to achieve a CMR on FDG-PET before HDCT/ASCT and consider addition of further treatments after HDCT/ASCT.
-
III.
Enrol patients in early phase clinical trials where available.
-
IV.
Consider non-standard transplant strategies such as allogeneic transplantation but at present there is very limited evidence for these and so they should usually be confined to trial settings and so beyond the scope of these guidelines.
F. Treatment modalities
F1. Conventional standard dose salvage chemotherapy (SDCT) regimens
There is no best salvage SDCT regimen because no randomized trials comparing salvage regimens have been done in children (or adults), which demonstrate the superiority of one regimen over another. Desirable qualities in salvage regimens include low toxicity and high efficacy, no impairment in the ability to mobilize and collect PBSCs and minimal late effects, including secondary myelodysplastic syndrome and myeloid leukemia. The overall response rates (ORR = CR and PR > 50%) of salvage regimens vary between 70% and 90% (Table 2). Salvage response rates come from single arm or retrospective studies, which are difficult to compare because prior treatment and prognostic factors are not consistent between case series. Hence the choice of salvage regimen is guided by factors including previous chemotherapy received to use non-cross-resistant drugs and avoid excess cumulative drug toxicities and individual patient factors (for example avoidance of cisplatin in renal impairment) as well as preference of the treating center for the regimen. For those patients that fail to achieve a CMR at PET2 a switch to second line salvage SDCT is recommended. Once again there are no randomized data to guide the choice of a particular regimen and a similar assessment of factors applies as in the choice of first line regimen. The standard pediatric salvage in the EuroNet-PHL-R1 trial was alternating IEP-ABVD but with changes in the front-line chemotherapy in the EuroNet-PHL-C2 trial the cumulative chemotherapy doses have changed, and so alternative choices of salvage are now recommended.
Table 2.
Response rates of conventional standard dose salvage chemotherapy regimens

Salvage regimens may be grouped into several categories:
-
(i)
Cisplatin based regimens include ESHAP36 (etoposide, methyl-prednisolone, high-dose cytarabine, cisplatin) and DHAP37 (cytarabine, cisplatin, dexamethasone) with similar response rates and both can result in high rates of successful peripheral blood stem cell collection. ASHAP38 (doxorubicin, methyl-prednisolone, high-dose cytarabine, cisplatin) is a modification of the above regimens that incorporates a continuous infusion of doxorubicin.
-
(ii)
Ifosfamide/etoposide based regimens include ICE39 (ifosfamide, carboplatin, etoposide) which was developed to reduce the non-hematologic toxicities observed with the cisplatin-containing regimens. Another regimen is IVE40 (ifosfamide, etoposide, epirubicin) has higher total doses of ifosfamide and etoposide than ICE (9gm/m2 vs 5 g/m2 and 600 mg/m2 vs 300 mg/m2, respectively) and is an effective regimen for mobilization of PBSCs but is associated with severe haematotoxicity and high rates of neutropenic sepsis.
-
(iii)
Gemcitabine based regimens. Single-agent response rates of 20% to 40% with Gemcitabine led to development of a number of combination regimens of which IGEV41 (ifosfamide, gemcitabine, vinorelbine, and prednisolone) is notable. The IGEV regimen has been used extensively in the last 5 years within the EuroNet group and has amongst the highest ORR (81.3%) with 54% CR and 28% PR reported and response assessment included FDG-PET, a low toxicity profile and very high stem cell mobilizing potential with non-cross reactive drugs compared to our first line EuroNet regimens. Other combinations include BeGEV,42 GDP (gemcitabine, dexamethasone, cisplatin)43 and GVD (gemcitabine, vinorelbine, liposomal doxorubicin).44
-
(iv)
Intensive conventional regimens include Dexa-BEAM and Mini-BEAM and these regimens use drugs commonly used in HDCT conditioning regimens, are intensive and associated with toxicity and prolonged cytopenia's with a measurable treatment-related mortality. In addition, they contain stem-cell toxic agents making stem cell collection difficult and so are not our recommended regimens.
Panel recommendation:
In first line salvage conventional SDCT regimens are recommended. There is no defined gold standard for choice of first line salvage regimen. The IGEV regimen has many desirable qualities required of a salvage SDCT regimen as already described and has been used increasingly within EuroNet in recent years and is a preferred first line SDCT regimen.
In second line salvage we generally recommend Brentuximab vedotin (BV) containing regimens and these are described below. Single agent BV achieves CR rates of around 33% to 34% in children and adults but combinations of BV with other agents show much higher CR rates. We therefore recommend BV in combination if possible and a preferred regimen in the EuroNet group in recent years is the BV plus Bendamustine - the BV-B regimen.
F2. Targeted agents
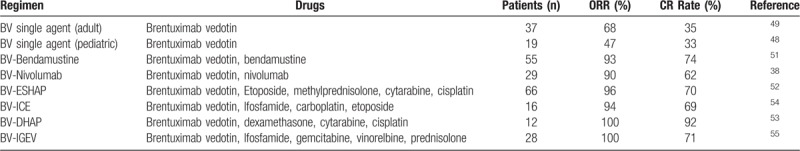
Novel agents have gained an increasing role in R/R HL in recent years, but this topic is not extensively reviewed in these guidelines, as the topic has been extensively reviewed by numerous investigators.45,46 Most of the evidence for these is extrapolated from small early phase studies in adult HL and pediatric data are extremely limited. For this reason, first line salvage remains conventional SDCT regimens as described above but novel agents are often incorporated into second or later lines of salvage. Outside of clinical trials Brentuximab vedotin (BV) is considered the first line novel agent in R/R HL. This is an anti-CD30 antibody conjugated to auristatin (MMAE), an antitubulin agent, and is highly effective in R/R cHL. Initial studies with BV were as a single agent and the pivotal Phase II study in adults reported an ORR of 75% (CR rate 34%).47 The data on single agent BV reported in the Ph I/II pediatric trial investigating single agent BV in CD30 positive R/R HL and anaplastic large cell lymphoma (ClinicalTrials.gov number NCT01492088) was ORR of 47% and CR rate 33% in HL patients with manageable toxicity at the recommended Ph II dose of 1.8 mg/kg48 and so is similar to outcomes reported in adults.49 More recently studies of BV in combination with either chemotherapy or immunotherapy drugs has shown more promising results and so single agent BV generally is no longer recommended as second line salvage. Bendamustine as single agent achieved CR rates of 33% in a Ph II study of R/R HL.50 There appears to be synergy between BV and Bendamustine in the BV-B combination which has reported excellent CR rates of 74% and ORR 94% in the Ph I/II single arm study in 45 patients with first relapse (58%) or refractory (42%) HL.51 The majority of CRs are achieved after 2 cycles of BV-B and stem cell mobilization and collection was adequate in all patients who underwent this procedure (n = 24). This outpatient-based regimen has a manageable safety profile, very high CR rate, durable response and successful stem cell mobilization and collection and represents a promising approach for maximizing response prior to ASCT at an earlier stage in salvage. Several other small studies reported CR rates approaching 80% with BV-B and in a UCLH series of 16 patients with R/R HL, BV-B achieved a CR rate of 87% on FDG PET defined as D1–3 in patients aged less than 30 years that had failed first salvage chemotherapy (S Daw personal communication). BV-B represents a very effective second line salvage as the path to achieving a CMR prior to HDCT. Data on other combinations of BV plus chemotherapy are limited but include BV in combination with ESHAP, DHAP, ICE and very recently encouraging results of BV in combination with IGEV are published.52–55 Brentuximab vedotin based salvage is summarized in Table 3.
Table 3.
Response rates of Brentuximab vedotin based salvage therapy
F3. Immunotherapy
The immune checkpoint inhibitors nivolumab and pembrolizumab are an option in patients’ refractory to 2 initial lines of salvage and so would be reserved for high risk patients. These agents target the Programmed Death 1 (PD1) pathway, which is a checkpoint to limit T-cell-mediated immune responses. There are 2 PD-1 ligands (PD-L1 and PD-L2), which engage the PD-1 receptor resulting in a reversible inhibition of T-cell activation and proliferation. Tumors, which express PD-1 ligands, can co-opt the PD-1 pathway to evade an immune response and this provides a potential therapeutic target in HL because Hodgkin Reed Sternberg (HRS) cells aberrantly overexpress PD-L1 and PD-L2, which can inhibit T cell activation.56 Antibodies that can block PD1/PD-L1/2 engagement may facilitate and enhance T cell activation and induction of T-cell mediated anti-tumor response. Single agent Nivolumab showed promising results in a small study (n = 23) in R/R HL with ORR of 87% (CR 17% and PR 70%).57 Of note in 20 patients with a CR or PR the rate of progression free survival was 86% (95% CI 62 to 95). These patients were very heavily pre-treated, 78% had prior ASCT and 78% had prior BV. Nivolumab has substantial therapeutic activity and an acceptable safety profile in heavily pre-treated HL however the CR rate of single agent Nivolumab was only 17% and so recent focus has been on combining Nivolumab with other agents in order to achieve better CR rates. There is an ongoing risk stratified and response adapted Phase II salvage trial in first R/R HL in children and young adults investigating immunotherapy as the first line salvage with induction BV plus Nivolumab followed by intensification with BV-B in poor initial responders (Checkmate 744 trial, AHOD1721; NCT02927769). The preliminary results of this study are recently presented showing 64% of patients achieved a CMR after BV plus Nivolumab58 which is similar to data reported in adult studies.59 Of note in the Checkmate 744 study those patients with an inadequate response to BV plus Nivolumab are switched to second line BV-B and the majority achieved a CMR after 2 cycles of this intensification. The overall CMR rate with either first or second salvage in this trial was 86% demonstrating that only a small number of patients cannot achieve a CMR pre-HDCT with these combinations. These data cannot be translated yet into routine clinical practice as only preliminary results of this study have been presented.
Panel recommendation:
Immunotherapy remains an experimental treatment in R/R cHL in children and young people and there is an ongoing Ph II trial. Single agent Nivolumab achieves a low CR rate and combination of BV plus Nivolumab looks more promising. Immunotherapy may be considered in high risk patients refractory to SDCT salvage regimens and Brentuximab vedotin based regimens as detailed in Tables 2 and 3.
F4. Radiotherapy
Radiotherapy (RT) is a highly effective treatment modality in HL and its role in enhancing local disease control in sites of R/R HL has been well established.60,61 Increasing numbers of patients are RT naïve at relapse as the use of RT is increasingly restricted in first line with approximately 50% avoiding RT in the EuroNet-PHl-C1 trial and predicted 75% avoiding RT in the EuroNet-PHL-C2 trial. RT fields are also becoming highly restricted in some first line trials to FDG-PET positive residua. Therefore, at relapse many patients have never received RT and some other patients may relapse only in prior disease sites that have never received RT because they received focal targeted RT only.
Panel recommendation:
Salvage with RT alone is not recommended but we propose integration of RT in salvage in 2 contexts:
-
1.
In all low risk group patients’ that are PET2 CMR, consolidation RT is given after salvage SDCT.
-
2.
In selected standard risk and/or high risk patients’ consolidation RT is given after HDCT/ASCT.
Radiotherapy in low risk salvage - consolidation after SDCT:
General principles: RT has been shown to be effective as part of non-transplant salvage in pediatric studies as already discussed and also in selected adult patients.62,63 A critical observation, which supports RT consolidation in the low risk group, is that patients frequently undergo further relapse in prior sites of disease and low risk patients by definition have disease limited to stage I-III. The rate of failure outside the irradiated volume increases with increasing stage as there is greater chance of undetected residual systemic disease beyond the treated RT volume. Radiotherapy volumes in the low risk cohort are expected to be modest because we do not advocate avoiding HDCT if the cost is excessive radiation toxicity. Hence very extensive nodal disease in which RT is not feasible is an exclusion from low risk salvage. If the RT fields are found to be excessive or radiation doses to critical organs unacceptable then patients may be treated with HDCT/ASCT instead, although the latter should not be considered routinely as a means of avoiding RT because RT may also be given after HDCT in some indications as discussed below. Prior RT will usually exclude SDCT plus RT salvage because if combined modality treatment failed in first line it is risky to employ this in salvage and secondly re-irradiation will result in high cumulative doses in re-irradiated sites with potentially unacceptable toxicity and in these circumstances, patients should receive HDCT/ASCT. A small group of patients who meet all other low risk criteria and had RT in first line could theoretically be eligible for SDCT plus RT salvage if they have limited stage relapse in sites exclusively outside previous radiotherapy fields. Delivery of RT should utilize modern radiation techniques including breath-hold techniques, IMRT, VMAT, and/or proton therapy when available.63-71
-
1.
Radiation fields and treatment planning: The International Lymphoma Radiation Oncology Group (ILROG) guidelines for consolidation RT recommend Involved Site RT (ISRT) which targets the pre-treatment sites of involvement with margin based on the fusion of the CT simulation with pre-treatment FDG-PET scan defined at re-staging at the point of relapse.64 For patients where the disease sites at relapse and first presentation are similar then clearly the radiation fields can incorporate all sites of disease. The difficulty is where there is a change in disease distribution at relapse compared to first presentation. A pragmatic approach is for patients who relapse within 12 months of first line treatment that RT includes unirradiated sites at first presentation and relapse as long as the RT fields and toxicity is considered acceptable by the treating team, but for patients who relapse more than 12 months after primary treatment RT includes only FDG avid sites at relapse.
-
2.
Radiation dose: R/R HL is more aggressive than de novo disease and therefore the recommended ISRT dose in salvage is a standard 3000 cGy (150 cGy per day) or 3060 cGy (180 cGy per day) to the ISRT volume. This higher dose of RT should be applied to all FDG-PET positive sites at relapse. In patients with early relapse (within 12 months) previously unirradiated FDG-PET positive sites that were involved at first presentation but negative at relapse may also be considered for irradiation and these sites may have lower RT dose as applied in first line which is 1980cGy (180cGY per day). This is a pragmatic approach for which there is international consensus within the EuroNet group and this strategy was also agreed with the US COG group for RT within the Checkmate 744 phase II trial (ClinicalTrials.gov Identifier: NCT02927769). Fractionation schedule is at the physician's discretion. The treatment is given over 5 days per week and total treatment time will be approximately 3.5 to 4 weeks depending on dose scheduling.
-
3.
Radiation timing: RT should begin as soon as possible after recovery from the last cycle of chemotherapy and ideally no later than 4 weeks from the end of the last chemotherapy cycle.
F5. HDCT conditioning regimens
There are no randomized trials to support one HDCT conditioning regimen vs another and the choice of conditioning regimen is often based on institutional preference or familiarity of the treating team. Currently, both in adult and pediatric patients in Europe the consensus is to not use radiotherapy as part of the conditioning regimen because there is no evidence of superiority of total body irradiation (TBI), total lymphoid irradiation (TLI) or sub-total lymph node irradiation (STLI)-containing regimens versus chemotherapy only conditioning and secondly because of the increased risk of side-effects including myelodysplastic syndromes and solid tumors associated with irradiation. Currently, the most commonly used conditioning regimens, in adult and pediatric patients in Europe, are the BEAM regimen (BCNU/carmustine, etoposide, cytarabine, melphalan) or the alternative LEAM regimen with lomustine replacing BCNU which appears to have equivalent toxicity and efficacy.72 The CBV regimen (cyclophosphamide, carmustine and etoposide) is used in the United States of America. Of note, a recent report suggests that, in adults, CBV, Bu-Cy and TBI-associated regimens are associated with higher treatment-related mortality than LEAM.73 Bendamustine, a very active drug in HL may also be used in a conditioning regimen and studies comparing BEAM and Benda-EAM (with bendamustine replacing BCNU) have recently been reviewed and a matched analysis comparing outcomes and toxicities between patients with R/R HL treated with high-dose Benda-EAM or BEAM followed by ASCT showed 4-year PFS and OS to be similar between patients receiving Benda-EAM or BEAM but acute non-hematological toxicity was more common in patients treated with Benda-EAM and long term toxicity is unknown and so at present there is no clear evidence that Benda-EAM is superior to BEAM.74 TBI-containing regimens have been largely abandoned in favor of chemotherapy only conditioning.
Panel recommendation:
-
1.
The standard pre-HDCT conditioning regimen is BEAM (or LEAM).
-
2.
Radiation based conditioning is not recommended.
F6 Consolidation treatments post HDCT/ASCT
There are 2 common post-HDCT treatment options and these are of particular relevance in high risk patients and selected standard risk patients:
I. Maintenance Brentuximab vedotin (BV)
Early maintenance with BV for up to 1 year improved the PFS vs placebo plus best supportive care alone with 5-year PFS of 59% (95% CI 51–66) with BV vs 41% (95% CI 33–49) with placebo in the Ph III Aethera trial.75 BV also showed good long-term tolerability but 67% of patients receiving BV experienced peripheral sensory neuropathy and most cases were managed with dose delays and reductions and were reversible in follow up but each BV infusion requires careful clinical assessment. Subgroup analysis seems to show that BV may be most beneficial in patients with 2 or more risk factors (defined below) but no PFS benefit in patients with only 1 risk factor although the numbers in this group were low; these findings have recently been confirmed in a 5-year AETHERA study analysis.76 There is interest in the use of other maintenance drugs after HDCT/ASCT such as the immunotherapy drug Pembrolizumab which is recently published but at present there is insufficient data on the use of this drug in children in this context to make any recommendation currently.87
Indications for BV maintenance after HDCT/ASCT: Patients with 2 or more risk factors as defined in the Aethera trial: (i) early relapse or primary progression, (ii) extra-nodal disease at relapse, (iii) B symptoms at relapse, (iv) no CMR to most recent SDCT, (v) patients who needed 2 or more lines of SDCT pre HDCT.
Panel recommendation:
BV maintenance is an option where available for post-HDCT treatment to reduce the risk of further progression in selected patients with 2 or more risk factors as described in the Aethera trial. This may be especially relevant in patients in whom RT consolidation post HDCT is not feasible because of disease spread as in for example Stage IV relapse.
II. Radiotherapy consolidation after HDCT/ASCT
There are no randomized studies, which demonstrate a benefit for RT in the peri-transplantation setting however multiple non-randomized studies support the addition of RT in the transplant setting by demonstrating significant benefit for local control, DFS/PFS and even overall survival and this topic has recently been comprehensively reviewed in updated guidelines form the International Lymphoma Radiation Oncology Group (ILROG).77 The addition of post-HDCT RT is increasingly relevant because the majority of first line patients in our EuroNet trials now receive chemotherapy only as already discussed and so are RT naïve at relapse. Evidence for a benefit for addition of RT in salvage largely comes from adult studies. RT was well tolerated when given post ASCT and associated with a decreased risk of local disease recurrence (hazard ratio, 0.3; p.02) in primary resistant HL and/or those patients with FDG-avid disease at the time of HDCT with 4-year local control rates of 81% with RT vs 49% without RT (p.03).78 Most patients in this study that received RT had high-risk localized disease leading to the conclusion that patients with targetable disease who are primary refractory or FDG-avid at the time of relapse may benefit from post HDCT consolidation RT. Another recent study showed that consolidative RT after HDCT/ASCT significantly improved the 2-year PFS (67% vs 42%, P < .02) and subgroup analysis showed consolidative RT improved 2-year PFS in patients with bulky disease (62% vs 39%, P = .02), B-symptoms (48% vs 28%, P = .05), primary refractory disease (47% vs 32%, P = .02) and those with a partial response on pre-transplant imaging (47% vs 32%, P = .02).79 The improvement seen on 2-year PFS remained significant on multivariate analysis and radiation was well tolerated with minimal toxicity. Another case-control study compared 46 patients with and 46 without IFRT within 2 months of SCT (either before or after ASCT) and found improved DFS (but not OS) in those that received IFRT and a benefit was demonstrated in non-bulky disease suggesting RT be considered not only in patients with bulky disease.80 A benefit associated with RT (given doses: 20 to 45 Gy) has also been evidenced in patients not in CR at the time of post-transplant evaluation.81 One study suggests a benefit associated with pre- or post-transplant RT for stages I-III patients; the median RT dose given was 30 Gy.82
While these studies varied in timing of RT (before or after HDCT), radiation dose and volume, and are susceptible to selection biases, which may favor RT (selecting low stage) or disadvantage RT (selecting patients with bulk or advanced stage or poor response) there is no doubt that RT has an important role to play in salvage even in the HDCT setting and while it may not be appropriate in every patient it is important to consider the risks and benefits in every patient in a systematic way. Concerns regarding toxicity have limited its widespread use in the HDCT setting but it must also be recognized that patients who suffer further relapse after HDCT have very limited additional options for cure and a significant risk of death from HL so toxicities are relatively less important in this group than for patients with primary HL. Indeed, re-irradiation of disease sites may be acceptable in this setting while we do not recommend this in low risk patients. Lastly, new RT approaches, especially proton beam radiotherapy are expected to reduce RT toxicity as discussed below.
Indications for RT consolidation after HDCT
There are no widely accepted indications for RT in the transplant setting. A detailed assessment is recommended in each patient taking account of previous treatment including prior RT, co-morbid medical conditions, organ toxicity / tolerance as well as the risk of further relapse and the stage or spread of disease at relapse as these factors help to determine the potential benefit as well as feasibility and potential toxicity of delivering RT. The ILROG guidelines suggest that RT should be considered in patients with localized limited volume R/R HL where RT has acceptable predicted toxicities. In patients with more disseminated disease, RT may be useful in targeting selected sites where local disease control may be a dominant problem. Specifically, patients that might benefit from RT include patients with primary refractory HL and patients with persistent FDG-avid disease after SDCT, which may include standard risk patients that required 2 lines of SDCT to achieve a CMR (PET2 positive/PET4 negative), or in our high risk group (PET4 positive) and this is a group where there is a particular focus on post-HDCT treatments. RT is particularly relevant in patients in whom post-HDCT BV maintenance is not possible for example due to intolerance or is not available for example due to lack of funding/authorization.
Panel recommendation:
Potential indications for RT in the HDCT setting are
-
1.
Primary refractory disease
-
2.
Persistent FDG-avid disease after salvage SDCT or after HDCT/ASCT
-
3.
Specific situations where RT is critical for local disease control as in compression of a vital structure such as spinal cord, nerve root, superior vena cava or airway
-
4.
Bulky disease (>5 cm) especially if not been previously irradiated
Note: When available consider new RT modalities including proton beam RT.
Timing of RT in standard risk patients:
RT is given after HDCT/ASCT as written in the recently published LYSA and American Society for Blood and Marrow transplantation guidelines for both adult and pediatric patients.83,84 We do not recommend RT to be delivered before ASCT as this is associated with additional toxicities including pulmonary toxicity, when RT fields include large part of the lungs, and neurological toxicity, e.g. myelitis, when RT fields include the spinal cord and cause modification of brain-blood barrier and busulfan-related toxicity.
Radiation field, dose and treatment planning:
Detailed guidance on RT technique, dose, volume and toxicity assessments is beyond the scope of these guidelines but has recently been comprehensively reviewed in the 2018 ILROG guidelines77 and similar principles apply as detailed in the RT section under low risk salvage above. FDG-PET imaging to assess response after SDCT or after HDCT in high risk patients is recommended since the results could affect the choice of RT dose and field volume. The RT dose, following a CMR to salvage chemotherapy and HDCT/ASCT, is 30 Gy. It is slightly increased up to 36 Gy in no-CMR sites.
Various techniques maybe employed to minimize toxicity. Some recent studies show a benefit of using proton beam therapy in reducing the avoidable dose to organs at risk in pediatric HL patients especially when protons are used in lesions located in the mediastinum, they are able to reduce the dose to the lungs, heart, and breast. The rate of local recurrence seems comparable to photon technique approaches.70,71 Therefore, this could be a good option in retreated patients or in patients with comorbidities, specifically in the lungs or the heart. But protons imply the necessity to be accurate about the margins of target. Due to the intrinsic characteristics of radiation, beyond the Bragg peak the dose falls almost to zero. The margins are never easy to determine with lymphoma lesions, which are frequently located in areas close to organs with intrinsic movements, like mediastinum. Delineation of target volume on a post-chemotherapy CT-scan is challenging.65,66 Another method to reduce the dose to the organs at risk is the deep inspiration breath hold. It can be combined with high conformal RT techniques, in which the patient takes a deep breath during treatment and holds this breath while the radiation is delivered. It potentially allows sparing of the RT dose to the heart and lungs and it is a promising method even in the pediatric setting, but reproducibility must be taken into account, especially in younger children, because it requires the full cooperation of the patient.
F7. Non-standard transplant procedures
Allogeneic transplant (AlloSCT) is a non-standard approach in children or adults with first R/R HL and it is generally reserved for patients that relapse post-HDCT/ASCT and this indication has recently been reviewed.85,86 There are no clear indications for AlloSCT in children in first salvage or in preference to autologous transplant and there is no prospective data in this context. Certainly in standard risk patients, which by definition in these guidelines have achieved a CMR by either PET2 or PET4, there would be no indication for allogeneic in preference to autologous transplant unless there were additional independent reasons why this would be indicated such as severe underlying immune deficiency which could also be cured by an allogeneic transplant. In high risk patients that remain FDG-PET positive after 2 or more lines of salvage, AlloSCT is often suggested as an option, but it must be borne in mind that failing to achieve a pretransplant CR/CMR is also highly prognostic in the allogeneic setting with lower PFS and OS compared to those transplanted in CR/CMR and so the additional benefit of AlloSCT procedures over ASCT is unknown in high risk patients that fail to achieve a complete remission with salvage SDCT regimens. Conventional HDCT/ASCT may still be curative in a proportion of patients with a positive pre-transplant FDG-PET as reported in the meta-analysis by Adams et al already discussed.32 A large recent retrospective series of AlloSCT outcomes reported that patients in CR pretransplant had a significantly lower rate of relapse compared to patients not in CR, 43% (95% CI 25–56) vs 59% (95% CI 41–69), P = .04.87 The non-relapse mortality (NRM) was 13% at day 100 and 24% at 2 years for the whole cohort, which far exceeds that of autologous transplantation, and the overall 2-year PFS was 27%. In this study many, but not all, had previous ASCT. The NRM in another recent study reporting outcomes only in reduced intensity transplant was 17% at 1 year.86 With the arrival of targeted therapies and immunotherapy and recent studies in which these have been combined to improve response rates as discussed above we now have expanded therapeutic options to achieve CMR pretransplant which predicts potentially good results for HDCT/ASCT thereby avoiding the risk of AlloSCT. Combined with the use of post-HDCT/ASCT consolidation strategies (RT and/or BV or pembrolizumab maintenance) the outlook is likely for less use of AlloSCT in future as other options become incorporated into standard practice. Tandem Transplantation is rarely used in children and there are no published series in pediatric patients to validate its use.
Panel recommendation:
AlloSCT and tandem SCT are not recommended in standard risk patients which by definition in these guidelines are FDG-PET negative after one or 2 lines of SDCT. In high risk patients without CR/CMR pretransplant these procedures are an option but the additional benefit over conventional HDCT/ASCT with addition of post-HDCT RT and/or maintenance BV is unknown.
Conclusions
These guidelines aim to guide clinicians in the difficult decisions to be made in salvage therapy for R/R classical HL. At the point of relapse, a full disease assessment is recommended after documenting R/R HL by histopathology. This is followed by analysis of pre-salvage prognostic factors, which allow allocation of patients to either a low risk or a standard risk group. All patients have a common starting point with 2 cycles of re-induction salvage chemotherapy followed by response assessment FDG-PET (PET2) and this response assessment is the second step, which determines whether the salvage strategy should continue or be changed. The aim of salvage chemotherapy is to achieve a CMR defined as Deauville 1–3 or qPET <1.3. Low risk patients with CMR on PET2 have non-transplant salvage with RT consolidation. All other patients have intensified consolidation with HDCT/ASCT and the goal in this group is to achieve a CMR after either PET2 or after second line salvage on PET4, which predicts a good outcome. A small group of patients fail to achieve a CMR after 2 lines of salvage SDCT and this defines a high risk group in whom additional treatment beyond HDCT should be considered. Options include further lines of salvage given before HDCT/ASCT and/or post-transplant RT and/or Brentuximab vedotin maintenance to reduce the risk of failure post HDCT/ASCT. With these approaches the majority of patients may be successfully salvaged although this comes with significant toxicities. As new options are continually emerging in new agents and early phase trials, the hope is that improvements will be made in reducing toxicities of salvage treatment further in the future.
Footnotes
Standard risk patients may proceed to HDCT/ASCT as soon as they achieve CMR on PET scan but for logistical reasons 1 to 2 further cycles of SDCT may be given to maintain dose intensity or facilitate stem cell collection if not yet done, but HDCT should not be delayed.
Citation: Daw S, Hasenclever D, Mascarin M, Fernández-Teijeiro A, Balwierz W, Beishuizen A, Burnelli R, Cepelova M, Claviez A, Dieckmann K, Landman-Parker J, Kluge R, Körholz D, Mauz-Körholz C, Wallace WH, Leblanc T. Risk and Response Adapted Treatment Guidelines for Managing First Relapsed and Refractory Classical Hodgkin Lymphoma in Children and Young People. Recommendations from the EuroNet Pediatric Hodgkin Lymphoma Group. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000329
The authors have no conflicts of interest to disclose.
References
- 1.Dörffel W, Luders H, Ruhl U, et al. Preliminary results of the multi-center trial GPOH-HD-95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klinische Padiatrie. 2003;215:139–145. [DOI] [PubMed] [Google Scholar]
- 2.Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28:3680–3686. [DOI] [PubMed] [Google Scholar]
- 3.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with ABMT in relapsed and resistant Hodgkin's disease, results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with ASCT for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;325:2065–2071. [DOI] [PubMed] [Google Scholar]
- 7.Rancea M, Monsef I, von Tresckow B, et al. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev. 2013;CD009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskowitz CH, Yahalom J, Zelenetz AD. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pretransplant functional imaging. Br J Haematol. 2010;148:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schellong G, Dorffel W, Claviez A, et al. Salvage therapy of progressive and recurrent Hodgkin's disease: results from a multicenter study of the Pediatric DAL/GPOH-HD Study Group. J Clin Oncol. 2005;23:6181–6189. [DOI] [PubMed] [Google Scholar]
- 11.Shankar A, Hayward J, Kirkwood A, et al. Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma - results of the UK HD3 relapse treatment strategy. Br J Haematol. 2014;165:534–544. [DOI] [PubMed] [Google Scholar]
- 12.James ND, Kingston JE, Plowman PN, et al. Outcome of children with resistant and relapsed Hodgkin's disease. Br J Cancer. 1992;66:1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzger ML, Hudson MM, Krasin MJ, et al. Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer. 2010;116:4376–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoneham S, Ashley S, Pinkerton R, et al. Outcome after autologous hemopoietic stem cell transplantation in relapsed or refractory childhood Hodgkin disease. J Pediatr Hematol Oncol. 2004;26:740–745. [DOI] [PubMed] [Google Scholar]
- 15.Belgaumi A, Al-Kofide AA, Jamil-Malik R, et al. Outcome of second line therapy for pediatric patients with hodgkin lymphoma who relapse following ABVD based therapy. Blood. 2009;114:Abstract 2691.(ASH Annual Meeting Abstracts). [Google Scholar]
- 16.Purz S, Mauz-Körholz C, Körholz D, et al. [18F]Fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin's lymphoma. J Clin Oncol. 2011;29:3523–3528. [DOI] [PubMed] [Google Scholar]
- 17.El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30:4508–4514. [DOI] [PubMed] [Google Scholar]
- 18.Daw S, Wynn R, Wallace H. Management of relapsed and refractory classical Hodgkin lymphoma in children and adolescents. Br J Haematol. 2011;152:249–260. [DOI] [PubMed] [Google Scholar]
- 19.Harker-Murray PD, Drachtman RA, Hodgson DC, et al. Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatr Blood Cancer. 2014;61:579–586. [DOI] [PubMed] [Google Scholar]
- 20.Prakash S, Kwang WA, Jeanette C, et al. A prognostic model predicting autologous transplantation outcomes in children, adolescents and young adults with hodgkin lymphoma. Bone Marrow Transplant. 2015;50:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdalla A, Hammad M, Hafez, et al. Outcome predictors of autologous hematopoietic stem cell transplantation in children with relapsed and refractory Hodgkin lymphoma: Single-center experience in a lower-middle-income country. Pediatr Transplant. 2019;23:e13531. [DOI] [PubMed] [Google Scholar]
- 22.Bonfante V, Santoro A, Viviani S, et al. Outcome of patients with Hodgkin's disease failing after primary MOPP- ABVD. J Clin Oncol. 1997;15:528–534. [DOI] [PubMed] [Google Scholar]
- 23.Longo DL, Duffey PL, Young RC, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin's disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10:210–218. [DOI] [PubMed] [Google Scholar]
- 24.Gorde-Grosjean S, Oberlin O, Leblanc T, et al. Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma, a study from the Société Française de Lutte contre le Cancer des Enfants et des Adolescents (SFCE). Br J Haematol. 2012;158:649–656. [DOI] [PubMed] [Google Scholar]
- 25.Lieskovsky YE, Donaldson SS, Torres MA, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin's disease: results and prognostic indices. J Clin Oncol. 2004;22:4532–4540. [DOI] [PubMed] [Google Scholar]
- 26.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and ASCT for patients with relapsing Hodgkin's disease: analysis of 280 patients from the French Registry. Bone Marrow Transplant. 1997;20:21–26. [DOI] [PubMed] [Google Scholar]
- 27.Gunjan L, Shah, Craig H, et al. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood. 2018;131:1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah GL, Yahalom J, Matasar MJ, et al. Risk factors predicting outcomes for primary refractory Hodgkin lymphoma patients treated with salvage chemotherapy and autologous stem cell transplantation. Br J Haematol. 2016;175:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson KJ, Kayani I, Ardeshna K, et al. A response-adjusted PET-based transplantation strategy in primary resistant and relapsed Hodgkin Lymphoma. Leukemia. 2013;27:1419–1422. [DOI] [PubMed] [Google Scholar]
- 30.Adams HJA, Kwee TC. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol. 2016;95:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozuah NW, Dahmoush HM, Grant FD, et al. Pretransplant functional imaging and outcome in pediatric patients with relapsed/refractory Hodgkin lymphoma undergoing autologous transplantation. Pediatr Blood Cancer. 2018;65: [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Fisher RI, Barrington SF, et al. United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasenclever D, Kurch L, Mauz-Körholz C, et al. qPET - a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1301–1308. [DOI] [PubMed] [Google Scholar]
- 34.Aparicio J, Segura A, Garcera S, et al. ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol. 1999;10:593–595. [DOI] [PubMed] [Google Scholar]
- 35.Josting A, Rudolph C, Reiser M, et al. Time- intensified dexamethasone /cisplatin /cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol. 2002;13:1628–1635. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez J, Rodriguez MA, Fayad L, et al. ASHAP: a regimen for cytoreduction of refractory or recurrent Hodgkin's disease. Blood. 1999;93:3632–3636. [PubMed] [Google Scholar]
- 37.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. [DOI] [PubMed] [Google Scholar]
- 38.Proctor SJ, Jackson GH, Lennard A, et al. Strategic approach to the management of Hodgkin's disease incorporating salvage therapy with high-dose ifosfamide, etoposide and epirubicin: a Northern Region Lymphoma Group study (UK). Ann Oncol. 2003;14 Supplement 1:i47–i50. [DOI] [PubMed] [Google Scholar]
- 39.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 2007;92:35–41. [DOI] [PubMed] [Google Scholar]
- 40.Santoro A, Mazza R, Pulsoni A, et al. Bendamustine in combination with gemcitabine and vinorelbine is an effective regimen as induction chemotherapy before autologous stem- cell transplantation for relapsed or refractory Hodgkin lymphoma: final results of a multicenter phase II study. J Clin Oncol. 2016;34:3293–3299. [DOI] [PubMed] [Google Scholar]
- 41.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762–1767. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. [DOI] [PubMed] [Google Scholar]
- 43.Younes A, Ansell SM. Novel agents in the treatment of Hodgkin lymphoma: biological basis and clinical results. Semin Hematol. 2016;53:186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borchmann S, von Tresckow B. Novel agents in classical Hodgkin lymphoma. Leuk Lymphoma. 2017;58:2275. [DOI] [PubMed] [Google Scholar]
- 45.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locatelli F, Mauz-Koerholz C, Neville K, et al. Brentuximab vedotin for paediatric relapsed or refractory Hodgkin's lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study. Lancet Haematol. 2018;5:450–461. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21:2136–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaCasce AS, Bociek G, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;126:3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Sanz R, Sureda A, Gonzalez AP, et al. Brentuximab vedotin plus ESHAP (BRESHAP) is a highly effective combination for inducing remission in refractory and relapsed Hodgkin lymphoma patients prior to autologous stem cell transplant: a trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO). Blood. 2016;128:1109. [Google Scholar]
- 51.Hagenbeek A, Mooij H, Zijlstra J, et al. Phase I dose-escalation study of brentuximab-vedotin combined with dexamethasone, high-dose cytarabine and cisplatin, as salvage treatment in relapsed/refractory classical Hodgkin lymphoma: The HOVON/LLPC Transplant BRaVE study. Haematologica. 2019;104:e151–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassaday RD, Fromm J, Cowan AJ, et al. Safety and activity of brentuximab vedotin (BV) plus ifosfamide, carboplatin, and etoposide (ICE) for relapsed/refractory (Rel/Ref) classical hodgkin lymphoma (cHL): initial results of a phase I/II trial. Blood. 2016;128:1834.27465916 [Google Scholar]
- 53.Abuelgasim KA, Alzahrani M, Alsharhan Y, et al. Chemoimmunotherapy with brentuximab vedotin combined with ifosfamide, gemcitabine, and vinorelbine is highly active in relapsed or refractory classical Hodgkin lymphoma. Bone Marrow Transplant. 2019;54:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armand P, Ansell SM, Lesokhin AM, et al. Nivolumab in patients with relapsed or refractory hodgkin lymphoma - preliminary safety, efficacy and biomarker results of a phase i study. Blood. 2014;124:289. [Google Scholar]
- 56.Harker-Murray P, Leblanc T, Mauz-Körholz C, et al. Response-adapted therapy with nivolumab and brentuximab-vedotin, followed by brentuximab-vedotin and bendamustine for suboptimal response in children, adolescents and young adults with standard-risk relapsed/refractory classical Hodgkin lymphoma. Blood. 2018;132 Supplement 1:927. [Google Scholar]
- 57.Herrera AF, Bartlett NL, Ramchandren R, et al. Preliminary results from a phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1105. [Google Scholar]
- 58.Wirth A, Corry J, Laidlaw C, et al. Salvage radiotherapy for Hodgkin's disease following chemotherapy failure. Int J Radiat Oncol Biol Phys. 1997;39:599–607. [DOI] [PubMed] [Google Scholar]
- 59.Josting A, Nogova L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin's lymphoma: A retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522–1529. [DOI] [PubMed] [Google Scholar]
- 60.Milgrom SA, Pinnix CC, Chuang H, et al. Early-stage Hodgkin lymphoma outcomes after combined modality therapy according to the post-chemotherapy 5-point score: can residual PET-positive disease be cured with radiotherapy alone? Br J Haematol. 2017;179:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89:854. [DOI] [PubMed] [Google Scholar]
- 62.Hodgson DC, Dieckmann K, Terezakis S, et al. International Lymphoma Radiation Oncology Group. Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol. 2015;5:85. [DOI] [PubMed] [Google Scholar]
- 63.Genovesi D, Cèfaro GA, Vinciguerra A, et al. Interobserver variability of clinical target volume delineation in supra-diaphragmatic Hodgkin's disease: a multi-institutional experience. Strahlenther Onkol. 2011;187:357. [DOI] [PubMed] [Google Scholar]
- 64.Aznar MC, Girinsky T, Berthelsen AK, et al. Interobserver delineation uncertainty in involved-node radiation therapy (INRT) for early-stage Hodgkin lymphoma: on behalf of the Radiotherapy Committee of the EORTC lymphoma group. Acta Oncol. 2017;56:608–613. 2017. [DOI] [PubMed] [Google Scholar]
- 65.Charpentier AM, Conrad T, Sykes J, et al. Active breathing control for patients receiving mediastinal radiation therapy for lymphoma: Impact on normal tissue dose. Pract Radiat Oncol. 2014;4:174. [DOI] [PubMed] [Google Scholar]
- 66.Fleckenstein J, Boda-Heggemann J, Siebenlist K, et al. Non-coplanar VMAT combined with non-uniform dose prescription markedly reduces lung dose in breath-hold lung SBRT. Strahlenther Onkol. 2018;194:815–823. [DOI] [PubMed] [Google Scholar]
- 67.Lundgaard AY, Hjalgrim LL, Rechner LA, et al. TEDDI: radiotherapy delivery in deep inspiration for pediatric patients - a NOPHO feasibility study. Radiat Oncol. 2018;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoppe BS, Hill-Kayser CE, Tseng YD, et al. Consolidative proton therapy after chemotherapy for patients with Hodgkin lymphoma. Ann Oncol. 2017;28:2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wray J, Flampouri S, Slayton W, et al. Proton therapy for pediatric hodgkin lymphoma. Pediatr Blood Cancer. 2016;63:1522–1526. [DOI] [PubMed] [Google Scholar]
- 70.Sharma A, Kayal S, Malik PS, et al. Comparison of BEAM vs. LEAM regimen in autologous transplant for lymphoma at AIIMS. SpringerPlus. 2013;2:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos KB, Luciano JMC, Gustavo B, et al. LEAM versus CBV for conditioning in autologous hematopoietic stem cell transplantation for lymphoma. Bone Marrow Transplant. 2019;54:625–628. [DOI] [PubMed] [Google Scholar]
- 72.Tsang ES, Villa D, Loscocco F, et al. High-dose Benda-EAM versus BEAM in patients with relapsed/refractory classical Hodgkin lymphoma undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2019;54:481–484. [DOI] [PubMed] [Google Scholar]
- 73.Moskowitz CH, Nademanee A, Masszi T, et al. AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–1862. [DOI] [PubMed] [Google Scholar]
- 74.Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132:2639. [DOI] [PubMed] [Google Scholar]
- 75.Constine LS, Yahalom J, Ng AK, et al. The role of radiation therapy in patients with relapsed or refractory Hodgkin lymphoma: guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;100:1100–1118. [DOI] [PubMed] [Google Scholar]
- 76.Milgrom SA, Jauhari S, Plastaras JP, et al. A multi-institutional analysis of peritransplantation radiotherapy in patients with relapsed/refractory Hodgkin lymphoma undergoing autologous stem cell transplantation. Cancer. 2017;123:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilke C, Cao Q, Dusenbery KE, et al. Role of consolidative radiation therapy after autologous hematopoietic cell transplantation for the treatment of relapsed or refractory hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2017;99:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kahn S, Flowers C, Xu Z, et al. Does the addition of involved field radiotherapy to high-dose chemotherapy and stem cell transplantation improve outcomes for patients with relapsed/refractory Hodgkin lymphoma? Int J Radiat Oncol Biol Phys. 2011;81(1):175. [DOI] [PubMed] [Google Scholar]
- 79.Mundt AJ, Sibley G, Williams S, et al. Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1995;33:261. [DOI] [PubMed] [Google Scholar]
- 80.Poen JC, Hoppe RT, Horning SJ. High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin's disease: the impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1996;36:3. [DOI] [PubMed] [Google Scholar]
- 81.Van Den Neste E, Casasnovas O, André M, et al. Classical Hodgkin's lymphoma: the Lymphoma Study Association guidelines for relapsed and refractory adult patients eligible for transplant. Haematologica. 2013;98:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashmi SK, Lee SJ, Savani BN, et al. ASBMT practice guidelines committee survey on long-term follow-up clinics for hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2018;24:1119. [DOI] [PubMed] [Google Scholar]
- 83.Mei M, Chen R. How to approach a hodgkin lymphoma patient with relapse after autologous SCT: allogeneic SCT clinical lymphoma, myeloma & leukemia. 2018;18:26–33. [DOI] [PubMed] [Google Scholar]
- 84.Jethava Y, Guru Murthy GS, Hamadani M. Relapse of Hodgkin lymphoma after autologous transplantation: Time to rethink treatment? Hematol Oncol Stem Cell Ther. 2017;10:47–56. [DOI] [PubMed] [Google Scholar]
- 85.Rivas MM, Berro M, Prates MV, et al. Allogeneic stem cell transplantation improves survival in relapsed Hodgkin lymphoma patients achieving complete remission after salvage treatment. Bone Marrow Transplantation. 2019. [DOI] [PubMed] [Google Scholar]
- 86.Spina F, Radice T, De Philippis, et al. Allogeneic transplantation for relapsed and refractory Hodgkin lymphoma: long-term outcomes and graft-versus-host disease-free/relapse-free survival. Leuk Lymphoma. 2019;60:101–109. [DOI] [PubMed] [Google Scholar]
- 87.Armand P, Chen YB, Redd R, et al. PD-1 blockade with pembrolizumab for classical hodgkin lymphoma after autologous stem cell transplantation. 2019;134:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


