Abstract
Cancer immunotherapies are promising treatments for many forms of cancer. Nevertheless, the response rates to, e.g., immune checkpoint inhibitors (ICI), are still in low double-digit percentage. This calls for further therapy optimization that should take into account combination of immunotherapies with classical tumor therapies such as radiotherapy. By designing multimodal approaches, immune modulatory properties of certain radiation schemes, additional immune modulation by immunotherapy with ICI and hyperthermia, as well as patient stratification based on genetic and immune constitutions have to be considered. In this context, both the tumor and its microenvironment including cells of the innate and adaptive immune system have to be viewed in synopsis. Knowledge of immune activation and immune suppression by radiation is the basis for well-elaborated addition of certain immunotherapies. In this review, the focus is set on additional immune stimulation by hyperthermia and restoration of an immune response by ICI. The impact of radiation dose and fractionation on immune modulation in multimodal settings has to be considered, as the dynamics of the immune response and the timing between radiotherapy and immunotherapy. Another big challenge is the patient stratification that should be based on matrices of biomarkers, taking into account genetics, proteomics, radiomics, and “immunomics”. One key aim is to turn immunological “cold” tumors into “hot” tumors, and to eliminate barriers of immune-suppressed or immune-excluded tumors. Comprehensive knowledge of immune alterations induced by radiation and immunotherapy when being applied together should be utilized for patient-adapted treatment planning and testing of innovative tumor therapies within clinical trials.
Keywords: Radioimmunotherapy, Hyperthermia, Immune checkpoint inhibitors, Tumor microenvironment, Personalized medicine, CITIM 2019
Introduction
Starting from antiquity until the 1980s, cancer was defined as a cellular disease caused by an invasion of abnormal cells into healthy tissue. Therefore, standard of care was to remove all cancer cells by surgical techniques, radiotherapy, and/or cytotoxic agents, referred to as “chemotherapeutics” [1]. As a result of ongoing research, findings about genetic mutations and epigenetic deviations from healthy cells improved the treatment concept. Cancer should be treated with customized drugs in an individualized manner [2]. Nevertheless, cancer was still seen as a local disease with the three consecutive steps of formation, progress, and finally uncontrolled growth [3]. Following, in 2002, the cancer immune editing concept has been worked out. This so-called 3E hypothesis is connecting initial elimination of malignant cells by innate and adaptive immune cells, equilibrium status in which tumor cells acquire further mutations in an immune-mediated tumor dormancy, and finally an escape of cancer cells from immune surveillance [4]. The escape phase is defined by essential characteristics, which belong also to the so-called hallmarks of cancer, i.e., cancer cells appear non-immunogenic during immunoediting phase or actively suppress the immune system [5]. Therefore, a regain of anti-cancer-immunosurveillance using additional immunotherapies such as anti-programmed death ligand 1 (PD-L1)/PD-1 and/or anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) [6] and immune stimulants such as hyperthermia [7, 8] in multimodal treatment settings is a further step forward to get the cancer under control and to cure it. However, immune biological rationales are very important for the design of multimodal therapies including radiotherapy and immunotherapy, which is content of this review.
Tumor microenvironment and its immune cells
The tumor microenvironment (TME) is a spatially organized, very complex and dynamic ecosystem with different cellular components. The latter include mainly the tumor, supporting cells, e.g., fibroblasts and endothelial cells, as well as cells of the immune system [9, 14]. Within the immune cell subpopulations, there can be found cells that are associated with acute inflammation, e.g., neutrophils, basophils, and eosinophils, cells of the innate immune response [e.g., natural killer (NK) cells and dendritic cells (DCs)], and cells from the adaptive immune response (e.g., T cells and B cells). Spatially regarded, T lymphocytes and macrophages are located both in the center and at the invasive margins of the tumor, whereas DCs as well as B lymphocytes primarily infiltrate tumor-adjacent lymphoid islets (TLSs) [10, 11, 14].
Sophisticated techniques in tumor microenvironment analysis
Within the last 2 decades, knowledge about the correlation between tumor microenvironment composition and the tumor tissue has increased significantly by a number of analytical techniques such as immunofluorescence (IF), immunohistochemistry (IHC), immunocytochemistry (ICC), as well as immunophenotyping (IPT). A new innovative technique is the so-called microenvironment cell populations (MCP) counter which gives very precise quantitative information about the cell content of immune and stromal cell populations in heterogeneous tissue samples from transcriptome data. Using MCP counter in 25 different cancers (n = 19,000), infiltration of CD8+ T cells could be correlated with favorable prognosis and the relative multifaceted cellular composition of the tumor microenvironment was shown in different cancers [12–15].
Function of different immune cells
DC In the 1970s, the Nobel Laureate Ralph Steinman and his colleague Zanvil Cohn discovered a cell type that they coined DCs. Under physiological conditions, the main function of DCs is to build a conjunction between the innate and adaptive immune response as they engulf antigens. As soon as they are exposed to danger signals or other activation signals, they mature and become activated and prime naïve T or B cells inside lymph nodes. DCs, as they have many phenotypes for an effective activation of the adaptive immune system, express a series of activatory and inhibitory receptors [16]. Furthermore, DCs can produce numerous pro-inflammatory or immunosuppressive cytokines. Interestingly, tumor cells can inhibit DC maturation and functionality. Nevertheless, high level of mDCs in the tumor and its microenvironment are associated with good clinical outcome in certain cancers [17].
T lymphocytes These cells of the adaptive immune system are responsible for the destruction of mutated cells as well as intracellular invaders, e.g., bacteria and viruses. Thus, T lymphocytes are essential for the cell-mediated immune response of adaptive immunity. According to their main surface (co-)receptors, a first T-cell subgroup classification into CD3+/CD4+ (T helper cells) and CD3+/CD8+ (cytotoxic T lymphocytes) is appropriate. T helper cells recognize a region of MHC class II protein, and cytotoxic T lymphocytes MHC class I proteins. Due to the fact that T lymphocytes are essential in adaptive immunity and tumor elimination, they can be considered as prognostic markers [9, 18]. For example in melanoma, head and neck, breast, bladder, urothelial, ovarian, colorectal, and lung cancer, a high density of CD3+ T cells, CD8+ cytotoxic T cells, and memory T cells (CD45RO+) was correlated with favorable disease-free survival (DFS) and overall survival (OS) [4, 12, 19], as well as a lower probability of metastatic spread and progression-free survival (PFS) [10, 20, 21] with a few exceptions, e.g., in clear cell renal cell carcinoma (ccRCC) [17, 22–24]. A first subgroup analysis of this ccRCC entity was reported by Giraldo et al. While patients with “normal” oligoclonal CD8+ T-cell texture had a good clinical outcome, patients with polyclonal CD8+ T-cell texture showed a limited cytotoxic capacity and presumably did not recognize any relevant tumor-associated antigens (TAAs) [24]. These results emphasize that both the tumor type and the TME including its immune cell (sub)populations have an impact on prognosis and clinical outcome.
NK cells They are large lymphocyte-like cells of the innate immune system whose primary function is the early defense against both allogenic (nonself) cells and autologous transformed cells, e.g., tumor cells and cells infected with viruses, bacteria, or parasites. This makes NK cells a good prognostic factor for clinical outcome, especially in the context of recurrences [12, 25, 26].
B cells These cells of the adaptive immune system are of central importance in human immunity as they produce immunoglobulins (antibodies). In a first step, antigens are encountered by a B cell receptor. This turns naïve mature B cells into activated B cells that can proliferate, differentiate into plasma cells, and finally produce antibodies. As for T lymphocytes, a summary by Vano et al. [14] shows that a high density of B cells within the TME can be correlated with a better prognosis including breast cancer [27], NSCLC [28] or head and neck cancer [29], whereas the database of B cells in the context of cancer is still scarce. However, some mechanistically explanations underline the positive role of B cells in the anti-tumor immune response as B cells can activate DCs or present antigens for an initial priming and expansion of CD4+ [30] and CD8+ T cells [31]. However, in this context, B cells may also play a negative role in anti-tumor immune response as they maintain a chronic inflammation [32] by the promotion of neo-angiogenesis [33], and/or by direct blocking of cytotoxic T-cell responses [34].
Macrophages These phagocytes exist in almost all tissues and develop both during embryonic phase as well as during the life span in the bone marrow. The mature forms of macrophages migrate into tissues, where a differentiation takes place. Macrophages engulf and destroy invading microorganisms as well as infected cells and pathogens. This makes them an important player in innate immune responses as they produce many cytokines and chemokines. They are central in initiation and termination of inflammation [35]. Macrophages release, e.g., the mostly immunosuppressive transforming growth factor (TGF)-β. The latter suppresses cytotoxic T cells (CD8+), pushes the differentiation of naïve CD4+ T cells to regulatory T cells (Tregs), and polarizes macrophages to a M2 phenotype. In general, inflammation has many positive effects that are described by Latin words calor, dolor, rubor, and tumor, meaning heat, pain, redness, and swelling. The phases of an inflammation are described by a recruitment of macrophages and neutrophils, followed by monocytes which rapidly differentiate into macrophages. Finally, if the inflammation continues, eosinophils migrate into the tissue. Within the tumor core and the invasive margin, the so-called tumor-associated macrophages (TAMs) are a major component. As already described for B and T cells, TAMs have tumor-specific positive or negative prognostic relevance. Positive prognosis was seen for example in prostate [36] or cervical cancer [37] in contrast to negative prognosis in breast [38], melanoma [39], and ovarian cancer [40]. An explanation for these discrepancies might be a switch in the phenotype from a more anti-tumoral one (M1) to a more pro-tumoral one (M2) and vice versa [48].
Immune modulatory effects of radiation
Targeted, non-targeted, and abscopal effects
Ionizing radiation (hereinafter: radiation) has both direct effects on DNA molecules and indirect effects via reactive oxygen or nitrogen species (RONS) that damage cell components like the DNA. Hence, until mid-2000s, radiation was considered to act only “targeted” on the cell, leading to cell-cycle arrest, and finally to cell death [41]. A paradigm shift has then taken place in the field of radiotherapy since “non-targeted” effects were described. These effects include not only the tumor, but also its microenvironment including bystander (5 mm), as well as nearby tissue (5 cm) [42], and the whole organism [43, 44]. “Non-targeted” effects are connected on one hand by communications between irradiated and non-irradiated cells close to each other and on the other hand by a release of signal molecules such as cytokines, chemokines, and damage-associated molecular patterns (DAMPs) into surrounding tissue [42, 45]. Besides “non-targeted” effects of radiation, also “off targeted” effects, which are better known as abscopal effects, do exist. Since abscopal effects occur out of the irradiation field, they are mostly related to systemic immune-mediated effects [46, 47].
Immune activatory effects by radiation
Historically, radiotherapy was considered to have only immunosuppressive effects. Prominent examples are the downregulation of the immune system for allogenic transplantation and the reduction of co-stimulatory surface markers (CD80 and CD86) on immature DCs, thus inhibiting T-cell activation [48]. Nowadays, it is well accepted that radiotherapy also induces immune activation. After radiotherapy, the expression of MHC molecules, stress ligands, adhesion molecules, death receptors, and ligands increase on tumor cells [45]. Furthermore, radiotherapy causes different cell-death modalities, such as apoptosis, necro(pto)sis, mitotic catastrophe, or senescence [49]. This leads to a spatiotemporal release of DAMPs that attract and activate cells of the innate and cells of the adaptive immune system. One prominent danger signal is heat shock protein 70 (HSP70). Furthermore, DAMPs are high-mobility group box 1 (HMGB1) protein, adenosine triphosphate (ATP), as well as DNA and RNA [50]. Interferons (IFNs) type I and II are induced by DAMPs. Type I interferons (INF-α and/or IFN-β) stimulate DCs to cross-present antigens, whereby clonal expansion of T cells is enhanced which finally results in tumor-specific T-cell responses [51]. Type II interferon (IFN-γ) is secreted by activated T cells and NK cells, and influences vasculature for immune cell trafficking and immune recognition [52]. Not fully explained remains the question, whether radiotherapy alone has the potential to act as a kind of in situ cancer vaccine [53].
Immunosuppressive effects by radiation
Each day, around 50–70 billion cells undergo programmed cell death (PCD), better known as apoptosis, which is necessary to self-renew, e.g., tissue or bone marrow. Thus, it is not surprising that this huge number of apoptotic cells is normally cleared by phagocytosis without inflammatory reactions or tissue scarring. Apoptotic tumor cells might, therefore, also foster immune suppression [54]. Tumor cells can further secrete TGF-β or increase the expression of immune suppressive checkpoint molecules such as PD-L1 to make their microenvironment immunosuppressive; radiation even augments these effects [55–57]. Furthermore, radiation can increase the level of chemokines like CCL2 that attracts monocytes into the tumor and its microenvironment. In the latter, differentiation of monocytes into TAMs occurs [58]. TAMs are of great importance in inflammation and immunosuppressive processes which are described above. Another mechanism to suppress immune responses is the upregulation of immune checkpoint molecules (ICM) on tumor cells and on tumor-infiltrating immune cells [59, 60]. The ICM expression is very dynamic and is influenced by tumor treatment modalities [57, 61]. Furthermore, radiation further induces immune suppression by locally and systemically killing of immune cells. Generally, lymphocytes are more radiosensitive than macrophages, DCs, or NK cells [62].
Fractionation scheme and the impact of radiation dose on immune modulation
If there is any perfect immunogenic radiation dose and fractionation scheme, it has not been found yet. This is amongst reasons like tumor size and/or genetic signature related to the dynamics of immune responses [45, 63]. With a radiation dose of 1.8–2.0 Gray (Gy) per day, classical normofractionation is applied five times a week over 3–7 weeks for treatment of solid tumors. Hypofractionation, in contrast, is any dose exceeding 2.0 Gray per day. This increases quality of life of the patients, since they do not have to come for irradiation every day and might even result in better outcome with equivalent or even less side effects, as already demonstrated for breast cancer [64]. Starting from very low single doses of 0.5–1.0 Gy and a total dose of 3.0–6.0 Gy, immunosuppressive effects of radiation, e.g., on macrophages can be found [65]. However, still low cumulative doses of 10 Gy (5 × 2 Gy) are capable of generating an immunostimulatory macrophage phenotype [66]. Single high radiation doses (e.g., 1 × 20 Gy) as they are applied in radiosurgery significantly boosted activation and maturation of DCs [67]. However, under distinct conditions, too high single doses may again dampen the immune response by triggering the expression of repair exonucleases such as TREX-1 that degrade radiation-induced immunogenic DNA [68, 69]. Nevertheless, Filatenkov et al. discovered in a murine model that a high single dose of 1 × 30 Gy induced a significantly higher infiltration of cytotoxic CD8+ T cells into tumors in comparison to hypofractionated irradiation with 10 × 3 Gy [70]. Another in vitro study considered the fractionation/dose correlation between supernatant from tumor cells and the secretion of immune activating cytokines and maturation markers of co-incubated DCs. DCs that had been incubated with supernatant from norm- (5 × 2 Gy) or hypofractionation (3 × 5 Gy) irradiated tumor cells expressed significantly higher levels of maturation markers such as CD80, CD83, and CD25, and secreted significantly higher levels of immune activating cytokines such as IL-12p70, IL-8, IL-6, and TNF-α in comparison to a single-dose irradiation of 1 × 15 Gy [71]. Other studies of dose-dependent immune-stimulatory effects have focused on HMGB1 [72] or on a combination of radiotherapy with checkpoint inhibitors such as anti-PD-L1 [73]. The most immunogenic radiation dose is most likely dependent of many factors (tumor, microenvironment, time of irradiation, and combination with immunotherapy) and alternating fractionations with higher and lower dose per fraction might be tested in future clinical trials, as already suggested in [74] based on immune biological considerations. The latter also include addition of hyperthermia to radiotherapy to improve anti-tumor immune responses [75].
Hyperthermia as immune modulator
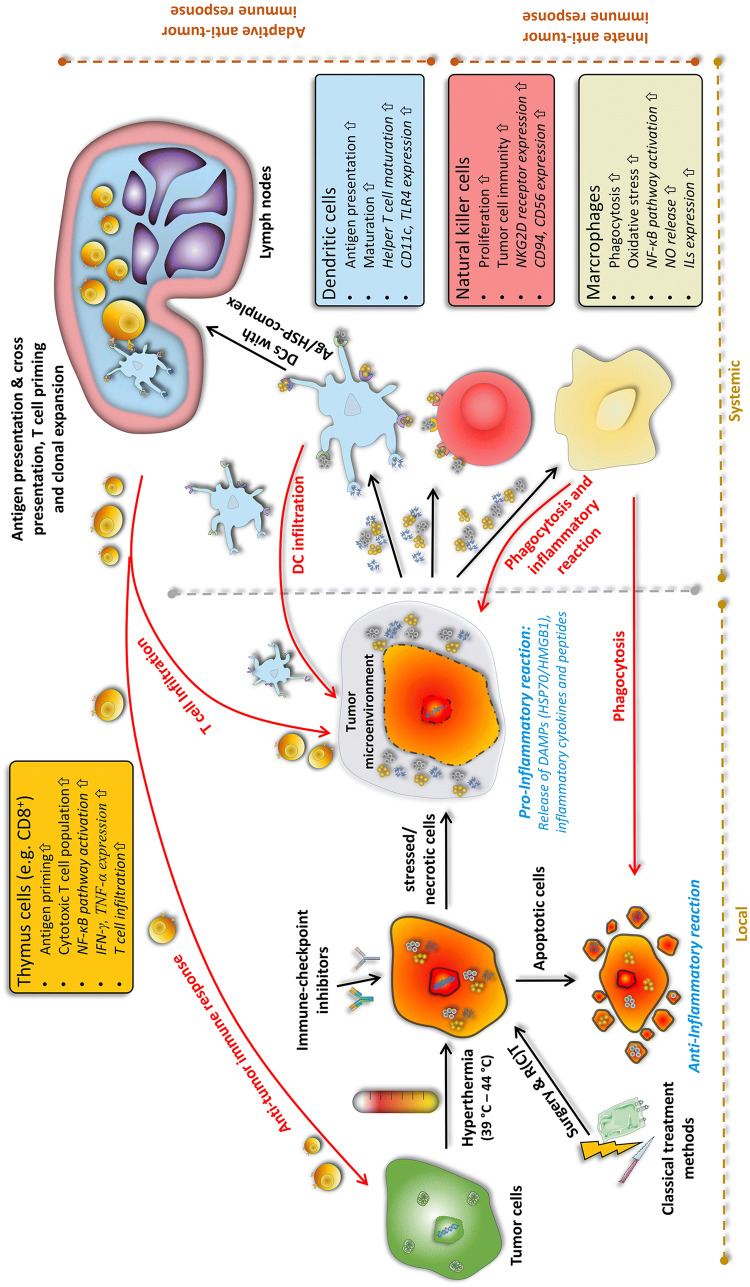
Findings of heat effects on our body date back to 5000 BC [76]. Almost 7000 years later, in the 18th and 19th century, correlations between shrinkage of tumors and febrile diseases like malaria were found. Consequently, William B. Coley initiated several studies on inducing heat by Streptococcus pyogenes extracts, later called “Coley’s toxin”, in tumor patients [77]. Since the 1980s, the positive effects of heat are utilized in the clinics under the name of hyperthermia. Via an exogenous energy source such as water-filtered IR-A, ultrasound, capacitive or radiative heating, the tumor area is heated up into supraphysiological range between 39 and 45 °C for typically 60 min per session [78]. Hyperthermia is, therefore, not to confuse with thermal ablation at temperatures above 60 °C, which leads to coagulative necrosis [79]. This led to the situation, in contrast to the accepted therapeutic benefit of radiation and/or chemotherapy, that hyperthermia still has a negative reputation caused by insufficient quality control and often non-standardized treatment methods [80]. In this context, it should be stressed that not each hyperthermia heating approach (local/regional/whole-body hyperthermia) is suitable for every tumor (superficial/deep-regional) and ensures the desired temperature profile within the tumor [81, 82]. However, since technical improvements like the development of radiative multi-antenna applicators with sensors for E-field monitoring, real-time temperature monitoring (particularly, online magnetic resonance tomography), and computer-based (online) treatment planning, hyperthermia is more and more accepted as an additive treatment modality in the clinics [78]. These technical improvements are converging with several phases I–III trials which have shown a significant beneficial effect of additive hyperthermia in terms of local control (e.g., malignant melanoma) and survival (e.g., soft-tissue sarcoma) [78, 83]. Besides improving hyperthermia devices and quality monitoring, the field of research is on the optimal (thermal) dose to achieve the maximum benefit with minimal side effects. This includes not only killing the tumor cells but also sensitizing the immune system (Fig. 1) [84]. Combination of radiotherapy with hyperthermia and immune checkpoint inhibitors should, therefore, be tested in the future. Furthermore, thermosensitive liposomes as targeted drug-delivery systems might add well to efficient stimulation of the immune system [85].
Fig. 1.
Induction of anti-tumor immune responses by multimodal treatment settings consisting of the classical tumor treatments (surgery and radio(chemo)therapy) combined with immune modulators such as immune checkpoint inhibitors and/or hyperthermia. Depending on the temperature, hyperthermia is capable of inducing both, apoptotic cells and necrotic cells, respectively. Primarily necrotic cells release danger-associated molecular patterns (DAMPs) and inflammatory cytokines into the tumor microenvironment. Dendritic cells (DCs) take up tumor antigens and tumor antigen–DAMP complexes and cross-present it to T cells in lymph nodes. This leads to T-cell priming, clonal expansion, and finally an adaptive anti-tumor immune response against the tumor. Additionally, DAMPs and cytokines can also directly activate natural killer (NK) cells or macrophages as parts of the adaptive immune system. Generally, hyperthermia has several stimulating mechanisms on immune cells, as shown in the colored boxes
Thermobiological rationale for hyperthermia
The “chief actors” in nearly every biological process are proteins. Heat can change their structural and mechanical function. Specifically, the secondary and tertiary protein structures (α-helix and β-sheet) change and become denaturized and aggregate non-specifically. This causes several intramolecular mismatches, finally resulting in, e.g., a decrease in the number of mitochondria and lysosomes, nucleoli swell, and a deposition of protein complexes. This results in cell death such as mitotic catastrophe [86]. Heat shock affects cell doubling as it also inhibits DNA replication, DNA transcription, mRNA processing, as well as the repair of damaged DNA. To sum up, heat can stop cell growth, silences (senescence) and kills cells [87]. But to be protected from these cell damaging effects, cells have intrinsic mechanism in forms of stress proteins, also called heat shock proteins (HSP). An upregulation of HSP is related to thermo-tolerance. HSP functions as a chaperone for the denaturation of heat-sensitive proteins [88].
It is further accepted that heat inhibits DNA repair by, e.g., inducing the degradation of DNA repair proteins [89, 90]. Since γH2AX, a predictive marker for DNA damage response, respectively, double-strand breaks (DSB), can be imaged by fluorescence staining, quantitative correlations of DNA damage and temperature and length of heat exposure were found [91, 92]. Heat induces DSBs mainly in S-phase of the cell cycle, while radiotherapy does it in G2/M-phase [75, 93].
One has always to be aware of that hyperthermia is applied in a temperature between 39 and 45 °C and different physiological effects happen in this temperature range. The most prominent one that is induced by heat, both in normal and cancerous tissue, is an increased blood flow by expanding the vessels to keep the temperature in a physiological range. Especially, within the chaotic and inefficient vascularized network of tumors, this leads to a re-oxygenation and enhanced infiltration of immune cells [94]. However, it was long controversially discussed, whether hyperthermia activates or suppresses the immune system by inducing tolerances [84]. Nevertheless, it has become clear that synergistic anti-tumor effects can be achieved by combining hyperthermia with other treatment methods, i.e., radio(chemo)therapy and/or ICI (Fig. 1).
We analyzed the additional effect of hyperthermia on tumor cell death forms in different in vitro models. Necrosis was found to be the prominent form of cell death, both under hyperthermia with 41.5 °C alone and significantly more in combination with radiotherapy [95, 96]. As already discussed, necrotic cells are generally pro-inflammatory and immune stimulatory as they lose their membrane integrity and release DAMPs. Higher concentrations of HSP70 and HMGB1, the most prominent danger signals, were found only after combination treatment of radiotherapy and hyperthermia [7, 97]. To mimic the clinical situation, B16-F10 tumor-bearing C57/BL6 mice were treated with combination of hyperthermia and radiotherapy or with the single modalities only. Adding hyperthermia to radiotherapy particularly fostered the infiltration of DCs into the solid tumors [97]. In addition, hyperthermia was demonstrated to increase NK cell-activating surface receptors such as NKG2D and MHC class I-related chain A, making NK cells more active against cancer cells [98]. Furthermore, NK cells seem to be important in the effector phase of tumor killing after treatment with radiotherapy plus hyperthermia [99]. Besides positive effects of adding hyperthermia to radiotherapy, certain chemotherapeutics also benefit from additional heating. Additive effects exist for doxorubicin, ifosfamide, and gemcitabine, while synergistic effects can be found, e.g., for mitomycin C, bleomycin, cisplatin, and carboplatin. Reasons for that are a better perfusion of the tumor tissue, a higher metabolic rate, and better membrane permeability of the cells [100]. We continuously have been focusing both on pre-clinical in vitro and in vivo model systems to examine immune modulations induced by combination of hyperthermia and radiotherapy. We additionally have started to perform longitudinal immunophenotyping of cancer patients who are treated within clinical trials, as, e.g., in the HYCAN-Trial (NCT02369939) for anal carcinoma. First data give hints that particularly cells of the innate immune system do recover faster in the peripheral blood when hyperthermia is added to radiochemotherapy.
Challenges of cancer immunotherapy
The already mentioned “Coley’s toxin” can be considered as one of the first cancer immunotherapies, as it activated an anti-tumor immune response with pro-inflammatory stimuli [77]. The current approaches of numerous monoclonal antibodies (mAbs) targeting the immune checkpoints PD-1/PD-L1 and CTLA-4 are different as they reactivate a pre-existing anti-tumor immunity. Therefore, immunological “hot” tumor entities with, e.g., a high degree of infiltrating CD8+ T cells, and/or high mutational burden like melanoma or non-small cell lung cancer (NSCLC) show good clinical response [101]. Nevertheless, most of the patients still do not respond adequately to immune checkpoint inhibitors. Some explanations are listed in the following.
“Cold” and “Hot” tumors The expression of PD-L1 and the density of CD8+ T cells are distinct biomarkers of response to PD-1/PD-L1 antagonists. While “hot” tumors are per definition “T cell infiltrated” and mostly respond to immunological treatment, “cold” tumors are a challenge, as no adequate adaptive immune response occurs [102]. Still under investigation is which step of the anti-cancer immune response is not functional. It could be the absence of T cells (e.g., CD8+) within the tumor, a deficit of antigen-presenting cells, i.e., DCs, not enough trafficking of T cells to and into the tumor mass or no adequate T-cell priming/activation [103]. Several reports confirm these thoughts, both in mice experiments [48, 103] and in humans, as, e.g., in melanoma patients [104, 105]. Currently, the CheckRad-CD8 study (NCT03426657) investigates the change of CD8+ tumor-infiltrating immune cell density for patient selection after initial chemo-immunotherapy. Well-responding patients with locally advanced HNSCC are consecutively treated with double checkpoint (durvalumab + tremelimumab) blockade and normofractionated radiotherapy. The aim is to replace toxic radiochemotherapy by combination of radiotherapy with immunotherapy in pre-selected patients.
The timing between radiotherapy/chemotherapy and immunotherapy In a pre-clinical study by Dovedi et al., tumor-bearing mice were locally irradiated with 10 Gy in five fractions simulating normofractionated radiotherapy. Anti-PD-L1 was given on the first or last day of irradiation, or 7 days after the last irradiation. While overall survival was not significantly prolonged by sequential treatment, simultaneous checkpoint-blockade revealed a significantly benefit in overall survival [60]. A comparable correlation between therapeutic outcome and the timing between radiotherapy and checkpoint-inhibitor donation (< or > 14 days) was observed in the PACIFIC-Trial by Antonia et al. in NSCLC. Patients who received the checkpoint-inhibitor close by to radiotherapy (< 14 days) profited the most [106]. Currently, a phase II study on small cell lung carcinoma (NCT02046733) is investigating whether additional immune checkpoint inhibitors (Nivolumab/Ipilimumab) improve clinical outcome, even if they are applied 6–8 weeks after the last of four sessions of chemotherapy. However, the perfect scheduling of radiotherapy/chemotherapy and immunotherapy is still a point of discussion, especially in terms of safety and efficacy [107].
The dose of radiation In a pre-clinical study by Grapin et al., CT26 cells in tumor-bearing mice were irradiated with same biologically effective dose of 18 × 2 Gy, 3 × 8 Gy, and 1 × 16.4 Gy, respectively. Additionally, anti-PD-L1 and anti-TIGIT (anti-T-cell immunoreceptor with Ig and ITIM domains) injection over 3 weeks was performed 3 times/week, starting on the first day of irradiation. First, without any antibody, tumor growth delay was higher at 18 × 2 Gy and 3 × 8 Gy in comparison to 1 × 16.4 Gy. Adding only anti-PD1, irradiation with 3 × 8 Gy was the most effective treatment (8/12 remissions). Using both anti-PD-L1 and anti-TIGIT resulted in 9/10 complete remissions with 3 × 8 Gy and 7/12 complete remissions with 18 × 2 Gy [108]. One has to stress that lymph nodes should be spared of irradiation, since tumor-specific CTL are primed there [109, 110].
The status of PD-L1 Still under investigation and controversially discussed is the correlation between PD-L1 status and clinical outcome of treatment with immune checkpoint inhibitors [111]. Normally, PD-L1 status is diagnosed by antibody staining of biopsies before radio(chemo)therapy and not during or afterwards. Many studies revealed significant benefit of combining radio(chemo)therapy with immunotherapy instead of monotherapy, but a lack of standardized antibody staining protocols, inhomogeneity in the biopsy material, and individual patient history and treatment processes, e.g., different drugs and irradiation schemes/doses, aggravate a clear statement [112, 113]. Positive treatment results of radiotherapy with inhibition of the PD-1/PD-L1 axis might even be influenced by initially PD-L1-negative patient subgroup [114]. However, again, PD-L1 status was just determined at the beginning and not during therapy. This calls for close meshed immune monitoring of patients who do receive multimodal tumor therapies.
Finally, to achieve higher response rates, a combination with immune-stimulatory methods such as radio(chemo)therapy and other vaccination techniques is under investigation with the encouraging results [115]. Going back to cancer development, whereas the 3E model consists of elimination, equilibrium and escape, immunotherapy could be described by the 3R model: reverse by, e.g., CTLA-4, rejuvenate by, e.g., CAR-T cells and restore by anti-PD-1/PD-L1 and further tumor-associated immune checkpoint inhibitors [116].
Multimodal tumor therapy setting: patient-adapted treatment design
Frey et al. demonstrated in a mouse model with syngeneic CT26 tumors that radiation-induced immune cell infiltration into tumors is time- and immune cell-dependent. While macrophages and DCs increasingly infiltrated 5 days after the first irradiation with 2 × 5 Gy, CD8+ T cells had a delayed infiltration with a maximum on day 8 until the first irradiation [63]. A comparable time effect on infiltration of CD8+ T cells was found by Hettich et al. in a B16 melanoma model with irradiation of the tumor with 24 Gy in 12 consecutive days, i.e., mimicking normofractionated radiotherapy [117]. To protect radiosensitive immune cells and to increase their infiltration, irradiation schemes could be optimized in the future based on such knowledge. It has, however, to be stressed that the function of remaining immune cells that were not killed by radiation often remains intact [118, 119] and that good anti-tumor immune responses can be achieved even by irradiation of tumors that show a high immune cell infiltration. It was hypothesized, as already mentioned above, that the regional lymph nodes are of high importance to assure sufficient supply tumor-specific T cells [120]. Future clinical studies with radiotherapy in combination with immune therapies should focus on such challenges to efficiently adapt dose and volume in radiotherapy with respect to radiation-induced immune alterations.
As meaningful for multimodal tumor therapies, “multimodal” biomarkers, matrices of biomarkers, taking into account genetics, proteomics, and “immunomics” should be considered, as already addressed in the cancer immunogram by Blank et al. [101]. By next-generation sequencing, e.g., a correlation between mismatch repair deficiency and the level of tumor mutational burden (TMB) can be deduced. The latter was the reason for the FDA to approve pembrolizumab (anti-PD-1) for solid tumor independently of the tumor entity [121]. However, the response to combination of radiotherapy and immunotherapy is not only a matter of genetic mutations; it is also connected to numerous immune cell actions. In a case study of a 33 year old melanoma patient who was treated with immunotherapy, immunophenotyping of peripheral blood revealed that HLA-DR expression was increased on monocytes upon additional radiotherapy, while the number of myeloid-derived suppressor cells (MDSCs) decreased [122]. The multicolor flow cytometry (MFC) is a very charming technique for immunophenotyping, because only a few milliliters of whole blood are sufficient to analyze immune cells and their subpopulations including the activation status of the cells [15]. High expression of HLA-DR on monocytes was shown to be connected to responses to anti-PD-1 immunotherapy of patients with melanoma. Here, responding patients additional showed a higher infiltration rate of CD8+ T cells as confirmed in biopsies [123]. Future translational research should strongly focus on complementary analyses of immune markers in the tumor in interconnection with the immune status in the peripheral blood. The latter can easily be monitored at multiple time points without any significant additional burden for the patients. This also applies for available diagnostic- and treatment planning-related imaging data sets generated by computed tomography or magnetic resonance imaging. With radiomic approach associations between qualitative and quantitative information extracted from clinical images, clinical data and immune status can be revealed. It was demonstrated just recently that such a radiomic approach is feasible to assess tumor-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy [124]. Currently, such data sets are also analyzed for patients who do receive immunotherapy in combination with radiotherapy.
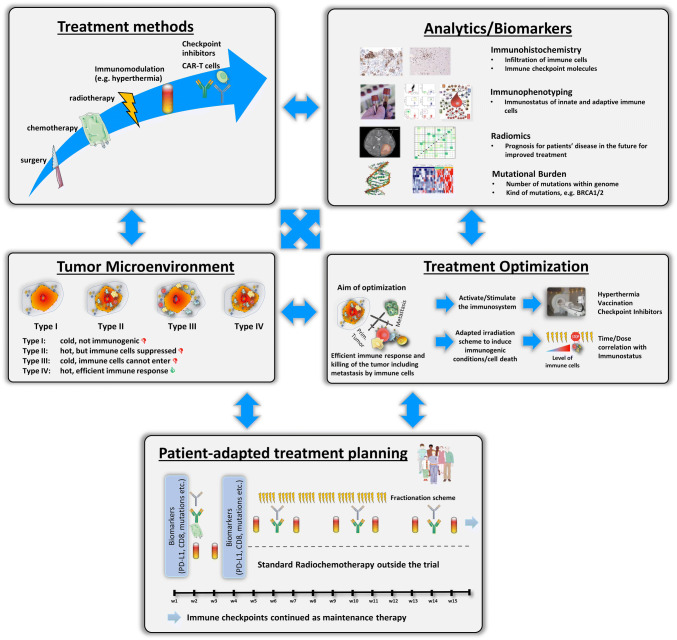
As shown by Multhoff et al. in pre-clinical models of glioblastoma and lung cancer, inhibition of PD-1 in combination with ex vivo HSP70-activated NK cells significantly prolonged overall survival by a factor of 2.3 in comparison to control animals or monotherapy [125]. A correlation between tumor size and HSP70 was also found for non-small cell lung cancer [126]. Therefore, additive methods such as hyperthermia and vaccination could further boost anti-tumor immune responses related to combined treatments with radiotherapy and/or chemotherapy and immune checkpoint inhibition [127]. Figure 2 summarizes the key and manifold challenges for optimized patient-adapted treatment planning with the aim to achieve both local and systemic tumor control and eradication. Besides biomarker-based improvement of anti-tumor responses, reduction of side effects should always be in the focus. This is already followed up in clinical trials as described above for the CheckRad-CD8 study and should be expanded to additional treatments as hypothetically suggested in Fig. 2.
Fig. 2.
Considerations for well-elaborated patient-adapted tumor treatment protocols. The outcome of conventional treatment methods, i.e., surgery and radio(chemo)therapy, can be improved by additional immunomodulation with, e.g., hyperthermia, CAR-T cells and immune checkpoint inhibitors. Furthermore, joint and innovative analytical techniques like immunohistochemistry, immunophenotyping, radiomics, and the detection of mutational burden might help to find out patient-specific properties and should be the basis for treatment optimization. One key aim in treatment optimization is to turn cold tumors (type I) into hot tumors (type IV) and to eliminate barriers of immune-suppressed or immune-excluded (type II and III) tumors. Finally, all parameters should be used for patient-adapted treatment planning and testing within clinical trials
Acknowledgements
Open Access funding provided by Projekt DEAL. The authors would like to thank the organizers and participants of CITIM 2019 for intensive and constructive scientific discussions. The joint ideas are partly integrated in the perspectives which are discussed in this manuscript.
Abbreviations
- ATP
Adenosine triphosphate
- ccRCC
Clear cell renal cell carcinoma
- CT
Chemotherapy
- CTLA-4
Cytotoxic T-lymphocyte associated protein 4
- DAMPs
Damage-associated molecular patterns
- DSB
Double-strand breaks
- FDA
U.S. Food and Drug Association
- HHP
High hydrostatic pressure
- HMGB1
High-mobility group box I protein
- HSP70
Heat shock protein 70
- ICC
Immunocytochemistry
- ICD
Immunogenic cell death
- ICI
Immune checkpoint inhibitors
- ICM
Immune checkpoint molecules
- IF
Immunofluorescence
- IPT
Immunophenotyping
- MCP
Microenvironment cell populations
- MFC
Multicolor flow cytometry
- NCT
National Center for Tumor Diseases
- NSCLC
Non-small cell lung cancer
- PCD
Programmed cell death
- RONS
Reactive oxygen or nitrogen species
- RT
Radiotherapy
- TAM
Tumor-associated macrophages
- TLS
Tumor-adjacent lymphoid islets
- TMB
Tumor mutational burden
Author contributions
The structure of the manuscript was compiled by all authors. The first draft of the manuscript was written by M. Hader and U. S. Gaipl. The figures were assembled by M. Hader and B. Frey. M. Hader, B. Frey, and U. S. Gaipl revised the paper. All authors reviewed and approved the final version of the manuscript.
Funding
This work was performed in the context of the Microthermia project (AZ-1261-17) that has been supported by the Bayerische Forschungsstiftung.
Compliance with ethical standards
Conflict of interest
M. Hader, U. S. Gaipl, and R. Fietkau received support for presentation activities from Dr. Sennewald Medizintechnik GmbH. R. Fietkau, U. S. Gaipl, B. Frey, and M. Hecht have received support for investigator initiated clinical studies (IITs) from Merck Sharp & Dohme and AstraZeneca. R. Fietkau, U. S. Gaipl, and M. Hecht also contributed at Advisory Boards Meetings of AstraZeneca and Bristol-Myers Squibb. M. Hecht further received travel support from Merck Serono, Merck Sharp & Dohme and Teva.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Sixth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2019), held in Tbilisi, Georgia, 29th April–2nd May 2019. It is part of a series of CITIM 2019 papers in Cancer Immunology, Immunotherapy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 2.Langreth R, Waldholz M. New era of personalized medicine: targeting drugs for each unique genetic profile. Oncologist. 1999;4(5):426–427. [PubMed] [Google Scholar]
- 3.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Victor CT-S, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, Fietkau R, Gaipl US. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol. 2011;101(1):109–115. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Datta NR, Stutz E, Gomez S, Bodis S. Efficacy and safety evaluation of the various therapeutic options in locally advanced cervix cancer: a systematic review and network meta-analysis of randomized clinical trials. Int J Radiat Oncol Biol Phys. 2019;103(2):411–437. doi: 10.1016/j.ijrobp.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 10.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pages F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Dieu-Nosjean M-C, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271(1):260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 12.Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, de Reyniès A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becht E, Giraldo NA, Beuselinck B, Job S, Marisa L, Vano Y, Oudard S, Zucman-Rossi J, Laurent-Puig P, Sautès-Fridman C, de Reyniès A, Fridman WH. Prognostic and theranostic impact of molecular subtypes and immune classifications in renal cell cancer (RCC) and colorectal cancer (CRC) Oncoimmunology. 2015;4(12):e1049804. doi: 10.1080/2162402X.2015.1049804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vano Y, Giraldo NA, Fridman WH, Sautès-Fridman C. The human tumor microenvironment. In: Zitvogel L, Kroemer G, editors. Oncoimmunology: a practical guide for cancer immunotherapy. Cham: Springer International Publishing; 2018. pp. 5–21. [Google Scholar]
- 15.Rühle PF, Fietkau R, Gaipl US, Frey B. Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int J Mol Sci. 2016;17(8):1316. doi: 10.3390/ijms17081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain MK, Wall KA. Use of dendritic cell receptors as targets for enhancing anti-cancer immune responses. Cancers. 2019;11(3):418. doi: 10.3390/cancers11030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, Lupo A, Alifano M, Damotte D, Cazes A, Triebel F, Freeman GJ, Dieu-Nosjean M-C, Oudard S, Fridman WH, Sautes-Fridman C. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21(13):3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 18.Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 19.Becht E, Giraldo NA, Germain C, de Reyniès A, Laurent-Puig P, Zucman-Rossi J, Dieu-Nosjean M-C, Sautès-Fridman C, Fridman WH. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 21.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 22.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Fléjou J-F, Gibault L, Verkarre V, Régnard J-F, Pagès O-N, Oudard S, Mlecnik B, Sautès-Fridman C, Fridman W-H, Damotte D. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19(15):4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 23.Tian C, Lu S, Fan Q, Zhang W, Jiao S, Zhao X, Wu Z, Sun L, Wang L. Prognostic significance of tumor-infiltrating CD8+ or CD3+ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J. 2015;128(1):105–110. doi: 10.4103/0366-6999.147828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giraldo NA, Becht E, Vano Y, Petitprez F, Lacroix L, Validire P, Sanchez-Salas R, Ingels A, Oudard S, Moatti A, Buttard B, Bourass S, Germain C, Cathelineau X, Fridman WH, Sautès-Fridman C. Tumor-infiltrating and peripheral blood t-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017;23(15):4416–4428. doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 25.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132(2):545–553. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 28.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud J-L, Fridman W-H, Sautès-Fridman C, Dieu-Nosjean M-C. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 29.Rothschild U, Muller L, Lechner A, Schlösser HA, Beutner D, Läubli H, Zippelius A, Rothschild SI. Immunotherapy in head and neck cancer—scientific rationale, current treatment options and future directions. Swiss Med Wkly. 2018;148:w14625. doi: 10.4414/smw.2018.14625. [DOI] [PubMed] [Google Scholar]
- 30.Yuseff M-I, Pierobon P, Reversat A, Lennon-Duménil A-M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol. 2013;13(7):475–486. doi: 10.1038/nri3469. [DOI] [PubMed] [Google Scholar]
- 31.Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, Bhattacharya N, Engleman EG. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521(7550):99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Barbera-Guillem E, May KF, Nyhus JK, Nelson MB. Promotion of tumor invasion by cooperation of granulocytes and macrophages activated by anti-tumor antibodies. Neoplasia. 1999;1(5):453–460. doi: 10.1038/sj.neo.7900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29(2):309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Röszer T. Understanding the biology of self-renewing macrophages. Cells. 2018;7(8):103. doi: 10.3390/cells7080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiao SL, Chu GC-Y, Chung LWK. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016;380(1):340–348. doi: 10.1016/j.canlet.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117:1583. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27(20):3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 40.Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi: 10.3389/fonc.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikitaki Z, Mavragani IV, Laskaratou DA, Gika V, Moskvin VP, Theofilatos K, Vougas K, Stewart RD, Georgakilas AG. Systemic mechanisms and effects of ionizing radiation: a new ‘old’ paradigm of how the bystanders and distant can become the players. Semin Cancer Biol. 2016;37–38:77–95. doi: 10.1016/j.semcancer.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Kadhim M, Salomaa S, Wright E, Hildebrandt G, Belyakov OV, Prise KM, Little MP. Non-targeted effects of ionising radiation–implications for low dose risk. Mutat Res. 2013;752(2):84–98. doi: 10.1016/j.mrrev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey B, Hehlgans S, Rödel F, Gaipl US. Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malign diseases. Cancer Lett. 2015;368(2):230–237. doi: 10.1016/j.canlet.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss E-M, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63(1):29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Weichselbaum RR, Liang H, Deng L, Fu Y-X. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 48.Cao M-D, Chen Z-D, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol Int. 2004;28(3):223–228. doi: 10.1016/j.cellbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 51.Burnette B, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu Y-X, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 53.Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016;6:141. doi: 10.3389/fonc.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willems JJLP, Arnold BP, Gregory CD. Sinister self-sacrifice: the contribution of apoptosis to malignancy. Front Immunol. 2014;5:299. doi: 10.3389/fimmu.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frey B, Rückert M, Deloch L, Rühle PF, Derer A, Fietkau R, Gaipl US. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol Rev. 2017;280(1):231–248. doi: 10.1111/imr.12572. [DOI] [PubMed] [Google Scholar]
- 56.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derer A, Spiljar M, Bäumler M, Hecht M, Fietkau R, Frey B, Gaipl US. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front Immunol. 2016;7:610. doi: 10.3389/fimmu.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, García-Martínez E, Rudqvist N-P, Formenti SC, Demaria S. Barriers to radiation-induced in situ tumor vaccination. Front Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 60.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 61.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu Y-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falcke SE, Rühle PF, Deloch L, Fietkau R, Frey B, Gaipl US. Clinically relevant radiation exposure differentially impacts forms of cell death in human cells of the innate and adaptive immune system. Int J Mol Sci. 2018;19(11):3574. doi: 10.3390/ijms19113574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frey B, Rückert M, Weber J, Mayr X, Derer A, Lotter M, Bert C, Rödel F, Fietkau R, Gaipl US. hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors. Front Immunol. 2017;8:231. doi: 10.3389/fimmu.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Simmons S, Sydenham MA, Venables K, Bliss JM, Yarnold JR. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 65.Seegenschmiedt MH, Micke O, Muecke R. Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br J Radiol. 2015;88(1051):20150080. doi: 10.1259/bjr.20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teresa Pinto A, Laranjeiro Pinto M, Patrícia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe Maia A, Castro P, Figueira R, Monteiro A, Marques M, Mareel M, Dos Santos SG, Seruca R, Adolfo Barbosa M, Rocha S, José Oliveira M. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765. doi: 10.1038/srep18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu Y-X. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackenzie KJ, Carroll P, Martin C-A, Murina O, Fluteau A, Simpson DJ, Olova N, Sutcliffe H, Rainger JK, Leitch A, Osborn RT, Wheeler AP, Nowotny M, Gilbert N, Chandra T, Reijns MAM, Jackson AP. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn G-O, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, Negrin RN, Engleman EG, Strober S. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kulzer L, Rubner Y, Deloch L, Allgäuer A, Frey B, Fietkau R, Dörrie J, Schaft N, Gaipl US. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol. 2014;11(4):328–336. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, He L, Bao G, He X, Fan S, Wang H. Ionizing radiation induces HMGB1 cytoplasmic translocation and extracellular release. Int J Radiat Med Nuclear Med. 2016;40(2):91–99. [PMC free article] [PubMed] [Google Scholar]
- 73.Kosinsky Y, Dovedi SJ, Peskov K, Voronova V, Chu L, Tomkinson H, Al-Huniti N, Stanski DR, Helmlinger G. Radiation and PD-(L)1 treatment combinations: immune response and dose optimization via a predictive systems model. J Immunother Cancer. 2018;6:17. doi: 10.1186/s40425-018-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frey B, Rubner Y, Wunderlich R, Weiss E-M, Pockley AG, Fietkau R, Gaipl US. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—implications for cancer therapies. Curr Med Chem. 2012;19(12):1751–1764. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 75.Schildkopf P, Ott OJ, Frey B, Wadepohl M, Sauer R, Fietkau R, Gaipl US. Biological rationales and clinical applications of temperature controlled hyperthermia—implications for multimodal cancer treatments. CMC. 2010;17(27):3045–3057. doi: 10.2174/092986710791959774. [DOI] [PubMed] [Google Scholar]
- 76.Kosack W. Der medizinische Papyrus Edwin Smith: the New York Academy of Medicine, Inv. Basel: Brunner; 2011. [Google Scholar]
- 77.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 78.Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E, Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753. doi: 10.1016/j.ctrv.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 80.Bakker A, Holman R, Rodrigues DB, Dobšíček Trefná H, Stauffer PR, van Tienhoven G, Rasch CRN, Crezee H. Analysis of clinical data to determine the minimum number of sensors required for adequate skin temperature monitoring of superficial hyperthermia treatments. Int J Hyperth. 2018;34(7):910–917. doi: 10.1080/02656736.2018.1466000. [DOI] [PubMed] [Google Scholar]
- 81.Kok HP, Crezee J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int J Hyperth. 2017;33(4):378–386. doi: 10.1080/02656736.2016.1268726. [DOI] [PubMed] [Google Scholar]
- 82.van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 83.Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, Ghadjar P, Hohenberger P, Angele M, Salat C, Vujaskovic Z, Daugaard S, Mella O, Mansmann U, Dürr HR, Knösel T, Abdel-Rahman S, Schmidt M, Hiddemann W, Jauch K-W, Belka C, Gronchi A. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018;4(4):483–492. doi: 10.1001/jamaoncol.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frey B, Weiss E-M, Rubner Y, Wunderlich R, Ott OJ, Sauer R, Fietkau R, Gaipl US. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperth. 2012;28(6):528–542. doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 85.Peller M, Willerding L, Limmer S, Hossann M, Dietrich O, Ingrisch M, Sroka R, Lindner LH. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J Controll Release. 2016;237:138–146. doi: 10.1016/j.jconrel.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 86.Nakahata K, Miyakoda M, Suzuki K, Kodama S, Watanabe M. Heat shock induces centrosomal dysfunction, and causes non-apoptotic mitotic catastrophe in human tumour cells. Int J Hyperth. 2002;18(4):332–343. doi: 10.1080/02656730210129736. [DOI] [PubMed] [Google Scholar]
- 87.Roti Roti JL, Kampinga HH, Malyapa RS, Wright WD, VanderWaal RP, Xu M. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones. 1998;3(4):245–255. doi: 10.1379/1466-1268(1998)003<0245:NMAATF>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burdon RH. Thermotolerance and the heat shock proteins. Symp Soc Exp Biol. 1987;41:269–283. [PubMed] [Google Scholar]
- 89.Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist MR, Soullié T, Rens J, Verhagen HJM, O’Connor MJ, Franken NAP, ten Hagen TLM, Kanaar R, Aten JA. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108(24):9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015;10(1):165. doi: 10.1186/s13014-015-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi A, Mori E, Nakagawa Y, Kajihara A, Kirita T, Pittman DL, Hasegawa M, Ohnishi T. Homologous recombination preferentially repairs heat-induced DNA double-strand breaks in mammalian cells. Int J Hyperth. 2017;33(3):336–342. doi: 10.1080/02656736.2016.1252989. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi A, Matsumoto H, Nagayama K, Kitano M, Hirose S, Tanaka H, Mori E, Yamakawa N, Yasumoto J-I, Yuki K, Ohnishi K, Ohnishi T. Evidence for the involvement of double-strand breaks in heat-induced cell killing. Cancer Res. 2004;64(24):8839–8845. doi: 10.1158/0008-5472.CAN-04-1876. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Yang L, Zhang J, Zhou M, Shen L, Deng W, Liang L, Hu R, Yang W, Yao Y, Zhang H, Zhang Z. Radiosensitization by irinotecan is attributed to G2/M phase arrest, followed by enhanced apoptosis, probably through the ATM/Chk/Cdc25C/Cdc2 pathway in p53-mutant colorectal cancer cells. Int J Oncol. 2018;53(4):1667–1680. doi: 10.3892/ijo.2018.4514. [DOI] [PubMed] [Google Scholar]
- 94.Song CW, Shakil A, Osborn JL, Iwata K (2009) Tumour oxygenation is increased by hyperthermia at mild temperatures. 1996. Int J Hyperth 25(2):91–95 [DOI] [PubMed]
- 95.Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, Lödermann B, Rödel F, Sauer R, Fietkau R, Gaipl US (2010) Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlentherapie Onkol Organ der Deutschen Rontgengesellschaft 186(11):587–599 [DOI] [PubMed]
- 96.Schildkopf P, Holmer R, Sieber R, Ott OJ, Janko C, Mantel F, Frey B, Fietkau R, Gaipl US. Hyperthermia in combination with X-irradiation induces inflammatory forms of cell death. Autoimmunity. 2009;42(4):311–313. doi: 10.1080/08916930902832041. [DOI] [PubMed] [Google Scholar]
- 97.Werthmöller N, Frey B, Rückert M, Lotter M, Fietkau R, Gaipl US. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperth. 2016;32(1):23–30. doi: 10.3109/02656736.2015.1106011. [DOI] [PubMed] [Google Scholar]
- 98.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 99.Finkel P, Frey B, Mayer F, Bösl K, Werthmöller N, Mackensen A, Gaipl US, Ullrich E. The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology. 2016;5(6):e1101206. doi: 10.1080/2162402X.2015.1101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rao W, Deng Z-S, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev Biomed Eng. 2010;38(1):101–116. doi: 10.1615/CritRevBiomedEng.v38.i1.80. [DOI] [PubMed] [Google Scholar]
- 101.Blank CU, Haanen JB, Ribas A, Schumacher TN (2016) Cancer immunology. The “cancer immunogram”. Science 352(6286):658–660 [DOI] [PubMed]
- 102.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 104.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, Alvarado MD, Wolf DM, Bogunovic D, Bhardwaj N, Daud AI, Ha PK, Ryan WR, Pollack JL, Samad B, Asthana S, Chan V, Krummel MF. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24(8):1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185. doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, Derangere V, Laurent P-A, Thibaudin M, Fumet JD, Crehange G, Ghiringhelli F, Mirjolet C. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J Immunother Cancer. 2019;7(1):160. doi: 10.1186/s40425-019-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70(7):2697–2706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 110.Marciscano AE, Nirschl TR, Francica BJ, Ghasemzadeh A, Theodros D, Velarde E, Wong JW, Thorek DL, DeWeese TL, Drake CG. Does prophylactic nodal irradiation inhibit potential synergy between radiation therapy and immunotherapy? Int J Radiat Oncol Biol Phys. 2016;96(2):S88. doi: 10.1016/j.ijrobp.2016.06.222. [DOI] [Google Scholar]
- 111.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, Faulkner E. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13(1):12. doi: 10.1186/s13000-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takamori S, Toyokawa G, Takada K, Shoji F, Okamoto T, Maehara Y. Combination therapy of radiotherapy and anti-PD-1/PD-L1 treatment in non-small-cell lung cancer: a mini-review. Clin Lung Cancer. 2018;19(1):12–16. doi: 10.1016/j.cllc.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 114.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer A-LN, de Langen AJ, Monkhorst K, Baas P. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, Haymaker C, Bernatchez C, Curran M, Zecchini Barrese T, Rodriguez Canales J, Wistuba I, Li L, Wang J, van der Burg SH, Melief CJ, Glisson B. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. J Hematol Oncol. 2018;11(1):142. doi: 10.1186/s13045-018-0684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hettich M, Lahoti J, Prasad S, Niedermann G. Checkpoint Antibodies but not T cell-recruiting diabodies effectively synergize with TIL-inducing γ-irradiation. Cancer Res. 2016;76(16):4673–4683. doi: 10.1158/0008-5472.CAN-15-3451. [DOI] [PubMed] [Google Scholar]
- 118.Basler L, Andratschke N, Ehrbar S, Guckenberger M, Tanadini-Lang S. Modelling the immunosuppressive effect of liver SBRT by simulating the dose to circulating lymphocytes: an in silico planning study. Radiat Oncol. 2018;13(1):10. doi: 10.1186/s13014-018-0952-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Belka C, Ottinger H, Kreuzfelder E, Weinmann M, Lindemann M, Lepple-Wienhues A, Budach W, Grosse-Wilde H, Bamberg M. Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol J Eur Soc Therap Radiol Oncol. 1999;50(2):199–204. doi: 10.1016/S0167-8140(98)00130-3. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X, Niedermann G. Abscopal effects with hypofractionated schedules extending into the effector phase of the tumor-specific T-cell response. Int J Radiat Oncol Biol Phys. 2018;101(1):63–73. doi: 10.1016/j.ijrobp.2018.01.094. [DOI] [PubMed] [Google Scholar]
- 121.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman R-A, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP, Becher B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24(2):144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 124.Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, Hollebecque A, Scoazec J-Y, Marabelle A, Massard C, Soria J-C, Robert C, Paragios N, Deutsch E, Ferté C. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 125.Shevtsov M, Pitkin E, Ischenko A, Stangl S, Khachatryan W, Galibin O, Edmond S, Lobinger D, Multhoff G. Ex vivo Hsp70-activated NK cells in combination with PD-1 inhibition significantly increase overall survival in preclinical models of glioblastoma and lung cancer. Front Immunol. 2019;10:454. doi: 10.3389/fimmu.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, Günther C, Gunther S, Habl G, Hautmann H, Hautmann M, Huber RM, Molls M, Offner R, Rödel C, Rödel F, Schütz M, Combs SE, Multhoff G. Heat shock protein 70 (Hsp70) peptide activated natural killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after radiochemotherapy (RCTx)—from preclinical studies to a clinical phase II trial. Front Immunol. 2015;6:162. doi: 10.3389/fimmu.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rückert M, Deloch L, Fietkau R, Frey B, Hecht M, Gaipl US. Immunmodulierende Eigenschaften von Radiotherapie als Basis für wohldurchdachte Radioimmuntherapien. Strahlentherapie Onkol Organ der Deutschen Röntgengesellschaft. 2018;194(6):509–519. doi: 10.1007/s00066-018-1287-1. [DOI] [PubMed] [Google Scholar]