Abstract
Human oral cavity is a complex habitat comprising about 700 microbial species and represents the most complex microbiota after gastrointestinal tract. In fact, oral microbiota directly influences health, metabolism and immune responses of the host. Metagenomic studies based on 16S rDNA profiling has reported the inhabitant bacteria mainly belonging to phyla Firmicutes, Proteobacteria, Actinobacteria, Fusobacteria, Spirochaetes and Bacteroidetes. Therefore, it is essential to isolate these strains and characterize in detail to understand their interaction. We have isolated strains from subgingival plaque from healthy to diseased individuals and the molecular characterization based on 16S rRNA gene sequence analysis showed predominance of Firmicutes, specifically members of the genus Streptococcus. Species of Lactobacillus and Veillonella were also found in significant number, which are considered as secondary colonizers. However, the population of Lactobacillus was decreased in diseased conditions with the increase in opportunistic pathogenic strains pertaining to genera like Campylobacter, Neisseria, Enterobacter, Pseudomonas and Morococcus. Further, we have also made an attempt to gain genomic insights on adaptation features and interactions of an isolate, Lactobacillus sp. strain DISK7 by performing whole genome sequencing and analysis, subsequently biochemical characterization to explore its functional and metabolic properties for the development as probiotic agent.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00828-8) contains supplementary material, which is available to authorized users.
Keywords: Dental plaque, Streptococcus, Lactobacillus, Biofilm, Genome sequence
Introduction
Oral cavity is the second complex ecosystem after the gut comprising over 700 diverse species of bacteria [1, 2]. Members of the genus Streptococcus such as Streptococcus salivarius, S. mitis, S. sanguinis, S. gordonii are the earliest colonizers of the tooth surface [3]. These species bind to tooth surface and gingiva to create a complex substrate environment with other species resulting in the formation of polymicrobial biofilms [4], which is known as dental plaque [5]. Formation of dental plaque is highly ordered sequence of events resulting in a structurally organized species rich polymicrobial community with defined functions [6]. The biofilm on the hard surface developed into spatially organized structure based upon the numerous cross feeding and competitive interactions amongst the dwelling microbes. It allows a close existence of diverse bacteria with complex growth requirements in the established climax community of a dental plaque and many of them remained uncultivable [7]. The two most prevalent oral diseases i.e. caries and periodontal disease have been linked to microbial shifts termed as oral dysbiosis, where a symbiotic microbial flora changes to a pathogenic flora due to either decrease in quantity of beneficial bacteria or increase in the opportunistic endogenous pathogens mainly Gram negative anaerobic flora [8]. Commonly, the pathogenic species do exist within the plaque biofilm, albeit in much lower proportions [9]. Biofilm grown cells displayed greater resistance than those grown in planktonic form, which is emerging as a serious concern for the management of periodontal disease [10]. Some of the early colonizers including Streptococcus, Lactobacillus and Veillonella interact with cariogenic species that have been well-documented and reported in literature [11]. However, members of the genus Lactobacillus are known to interact with other commensal bacteria that helps in maintaining oral health of the host, thus they were applied in probiotic formulations to prevent dental diseases [3]. Therefore, this study was carried out to identify the cultivable microbial populations in dental plaque associated with healthy and periodontal disease condition. Further, a representative Lactobacillus strain DISK7 isolated from plaque sample of a healthy individual is studied for its interaction with other strains and strategies for oral niche adaptation.
Materials and Methods
Sample Collection
Dental plaque samples of healthy and diseased individuals were collected from Dr. HS Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh. Sample extraction procedure and healthy subjects’ inclusion criteria was as mentioned earlier [12]. Systemically healthy subjects suffering from chronic periodontitis were enrolled with [age (> 20 years), > 20 teeth, > 8 teeth with pocket depth and/or attachment level > 4 mm] willingness and ability to sign informed consent. The pooled plaque samples were serially diluted up to 10−3 and 100 µl of each dilution was plated on nutrient agar (NA, Himedia, India), tryptone soya agar (TSA, Himedia, India), de man Rogosa and Sharpe agar (MRS, Himedia, India), reinforced clostridium agar (RCA, Himedia, India), brain heart infusion agar (BHA, Himedia, India) and anaerobic agar (AA, Himedia, India). All plates were incubated at 37 °C under aerobic and also anaerobic (N2:CO2:H2 in a ratio of 80:15:5) conditions using jars (Anoxomat, MART, the Netherlands).
Isolation, Grouping and Characterization of Isolates
Isolates were catalogued based on their differences in colony morphology and purified isolates were stored as glycerol stock at − 80 °C. Total protein profiling of isolates (for the final grouping) was determined by SDS-PAGE and isolates which appeared to be different on SDS-PAGE analysis were confirmed by 16S rRNA gene sequencing and blast analysis. Morphological features were determined by using phase contrast microscope (Axioplane, Germany). Other physiological and biochemical properties including carbon substrate utilization pattern was done as prescribed by the standard procedures [13].
Taxonomic Studies Using 16S rRNA Gene Phylogeny
Genomic DNA was obtained using ZR fungal/bacterial miniprep (Zymo Research, USA) following manufacturer’s instructions. Amplification of 16S rRNA gene (~ 1.5 Kb) was done using universal primers and the amplified PCR product was purified and sequenced. The 16S rRNA gene sequences obtained were used for identification through EzTaxon server (http://eztaxon-e.ezbiocloud.net) [14]. Phylogenetic analysis of gene sequences was performed using MEGA version 7.0 [15]. A neighbor-joining phylogenetic tree was constructed for the sequences (with > 500 nucleotide bases) along with their close relatives. Tamura-Nei method was used to compute evolutionary distances and expressed in units as base substitutions per site. Bootstrap values were expressed as percentage.
Exopolysaccharide and Biofilm Formation
Exopolysaccharides produced by a Lactobacillus sp. strain DISK7 was estimated by anthrone sulfuric acid method. Total polysaccharides were precipitated by adding 3 vol of CH3OH and 1 N HCl (in 95:5 ratio) to the cell pellet, extracted with 80% ethanol and dissolved in water. Quantification was done using glucose standard curve prepared with anthrone reagent. The biofilm formation ability of strain DISK7 was assessed as mono-species and in combination with other isolates from dental plaque to test even multispecies biofilm formation. All isolates were grown in MRS broth overnight at 37 °C and 100 µl of 1:100 diluted cell suspensions were transferred to 96 well-microtitre plate to asses biofilm formation in 24 h. Subsequently, the plates were processed for biofilm quantification using 0.1% crystal violet as described earlier [13, 16]. The quantification of biofilm was measured as OD595 and all experiments were performed in triplicate.
Resistance Determination
The antibiotic resistance property of a Lactobacillus sp. strain DISK7 was tested by disk diffusion agar method. MRS agar plate spread with strain DISK7 was placed with different antibiotic coated disks (Himedia, India) under aseptic conditions. Experiments to determine resistance to fluoride was carried out using MRS medium that was added with different concentrations of sodium fluoride. Plates were incubated in anaerobic jars at 37 °C and checked for growth up to 48 h.
Genome Sequencing, Assembly and Annotation
Genomic DNA of strain DISK7 was extracted in large quantities and libraries were prepared using Nextera XT sample preparation kit (Illumina, Inc., USA) with dual indexing adaptors, as per manufacturer’s instructions. Sequencing of libraries was done on an in-house Illumina Miseq sequencing platform (Illumina, Inc., USA), in 2 * 250 paired end sequencing kits. Adapter trimming was a default parameter by Miseq Control software. Quality of obtained reads was checked by using FastQC [17]. Reads obtained were assembled using CLC Genomics Workbench 7.5 (CLC bio, Aarhus, Denmark). DISK7 draft genome sequence has been deposited at NCBI with accession number MJET00000000. Identity of strain DISK7 with closely related strains was determined by average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values using the Genome-to-Genome Distance Calculator [14, 18]. Assembled genome of strain DISK7 was annotated by using Rapid Annotation Subsystem Technology (RAST) pipeline [19] to obtain genomic insights on adaptation strategies of the strain in oral environment.
Results and Discussion
Isolation and Grouping of Strains
Several strains were isolated from plaque samples incubated under both aerobic and anaerobic conditions based on their colony morphology. Since, most of the strains formed punctiform colonies, the isolates were further grouped based on their total cellular protein profiles by SDS-PAGE. Strains with identical protein patterns were considered as single group and a representative strain was taken for further characterization. Though most of the documented species are aerobic, they grew well under microaerophilic conditions. All isolates showed excellent growth when grown under atmosphere containing CO2. The BLAST analysis of 16S rRNA gene sequences of healthy individuals’ plaque samples were mostly belonged to different species of the genus Streptococcus. They were mainly assigned to species S. intermedius, S. salivarius, S vestibularis, S. sanguinis, S. mitis, S. constellatus and S. anginosus in their descending order of strain predominance. Other predominant genera included Lactobacillus and Veillonella as many isolates showed high identity with various species of these genera and the profile revealed minimal diversity (Table 1). Microbial profiles associated with the healthy periodontium represented commensal secondary colonizers in biofilm formation by co-adhesion [7] as samples have been obtained from established dental plaque biofilms. Though lot of strain variation was seen, major profile largely composed of Gram positive facultative anaerobic microorganisms only, which is very much in line with the previously documented literature [20]. Though members of Bacillus were known to reside in oral environment [21], Aneurinibacillus migulanus strain DISK15 isolated from subgingival plaque of the healthy subject has never been reported from this niche, but is again noted to be a Gram positive bacterium. In contrast, bacteria isolated from dental plaque of diseased individual showed presence of various species of genera like Neisseria, Pseudomonas, Lysinibacillus, Morococuus, Campylobacter, Bacillus and Staphylococcus (Table 1). Deemed caries pathogens S. mutans and V. rogosae were found in diseased individual plaques and only V. parvula was found in healthy individual plaques. These finding are consistent with the ecological plaque hypothesis [22]. Streptococci are the first bacteria to colonize oral surfaces of human mouth with a range of extracellular factors that enables them to form social networks with neighboring taxa such as Lactobacillus and Veillonella. Strains pertaining to primary and secondary colonizers initiate the formation of biofilms on tooth surfaces resulting in development of dental plaque [23]. Although Veillonella spp. are early colonizers of biofilm, they are incapable of utilizing carbohydrates as a carbon source. Thus, lactic acid produced by oral pathogens like S. mutans as a metabolic end product is consumed and detoxified by Veillonella spp., as a result of metabolic complementation they occur symbiotically in the form dual-species biofilms [24] that are complex, pathogenic and resistant to antimicrobial treatment [25]. Thus, oral cavity of individuals with periodontal disease, may acts as a reservoir for pathogenic bacteria [26]. Our study demonstrates that plaque communities from diseased individuals consist of distinctive bacterial profile including some pathogenic bacteria such as P. aeruginosa (strains SKVG5 and SKVG 7), which has been reported from the oral cavity of immune suppressed patients suffering from periodontal disease and poor oral hygiene [27]. M. cerebrosus (strain SKVGD5) was reported from a brain abscess (cerebellar) and also from subgingival plaques sample [28]. Various studies strongly propose that Campylobacter concisus (strain SKVGD9) primarily associated with oral cavity can be translocated to the intestinal tract [29] and cause inflammatory bowel disease (IBD). Similarly, Neisseria flavescens (SKVG2) and N. subflava (SKVGD8) isolated from oral cavity of diseased individual was associated with direct calcification [30]. In contrast, Lactobacillus spp. such as L. gasseri, L. fermentum, L. crispatus and L. gastricus were often found as dental flora of healthy individual that were involved in establishment of dental plaque. This genus constitutes a major part of oral microbiota and also have beneficial effects on oral health [31].
Table 1.
List of bacterial strains isolated from dental plaque obtained from healthy and diseased individuals and their identification based on 16S rRNA gene sequence analysis (strains identified as same taxon were differed in colony morphology)
| S. no. | Identification of isolates | Strains isolated from healthy plaque | Strains isolated from diseased plaque |
|---|---|---|---|
| 1 | Streptococcus salivarius | DISK1, DIGK 16 | SKVG1, SKVGD12 |
| 2 | Streptococcus mutans | – | SKVGD11 |
| 3 | Streptococcus vestibularis | DISK 4, DISK23 | SKVG3 |
| 4 | Streptococcus cristatus | DISK 19 | – |
| 5 | Streptococcus sanguinis | – | SKVG13 |
| 6 | Streptococcus mitis | – | SKVG16, SKVGD3, SKVGD13 |
| 7 | Streptococcus intermedius | DIGK 15, DIGK 20, DIGK 18, DIGK 27, DIGK 19.2 | SKVG15, SKVGD24, SKVGD25 |
| 8 | Streptococcus constellatus | – | SKVG21, SKVG37 |
| 9 | Streptococcus anginosus | DISK 29 | SKVG23, SKVG30, SKVG31 SKVG35, SKVG36, SKVG38 |
| 10 | Streptococcus cristatus | DISK 19 | – |
| 11 | Streptococcus gordonii | DISK 25, DISK 31 | – |
| 12 | Streptococcus oralis | DIGK 4B | – |
| 13 | Staphylococcus hominis | DIGK 7A, DIGK 7B | – |
| 14 | Streptococcus gallolyticus | – | SKVG29 |
| 15 | Streptococcus sp. | – | SKVG12 |
| 16 | Lactobacillus gasseri | DISK 7 | |
| 17 | Lactobacillus plantarum | – | SKVGD14 |
| 18 | Lactobacillus fermentum | DISK 10, DISK 6C | SKVG9, SKVG22 |
| 19 | Lactobacillus crispatus | DISK 12, DISK 17 | – |
| Lactobacillus paracasei | DISK 8, DISK 13, DISK 20 | – | |
| 20 | Lactobacillus gastricus | DISK 14, DISK 16 | – |
| 21 | Veillonella parvula | DISK 3.1, DISK24, DISK32, DISK 5A | – |
| 22 | Veillonella rogosae | – | SKVGD21 |
| 23 | Bacillus altitudinis | – | SKVG33 |
| 24 | Bacillus subtilis | – | SKVGD4, SKVGD10, SKVGD19 |
| 25 | Bacillus megaterium | – | SKVGD18 |
| 26 | Neisseria flavscens | SKVGD8 | |
| 27 | Neisseria subflava | – | SKVG2, SKVGD8 |
| 28 | Staphylococcus epidermidis | – | SKVGD17 |
| 29 | Staphylococcus sp. | – | SKVGD6 |
| 30 | Enterobacter cloacae | – | SKVG34 |
| 31 | Pseudomonas aeruginosa | – | SKVG5, SKVG7 |
| 32 | Aneurinibacillus migulanus | DISK 15 | – |
| 33 | Megasphaera paucivorans | DISK 30 | – |
| 34 | Lysinibacillus macroides | – | SKVGD1 |
| 35 | Morococcus cerebrosus | – | SKVGD5 |
| 36 | Campylobactor concisus | – | SKVGD9 |
| 32 | Lysinibacillus mcroides | – | SKVGD15 |
Phylogeny
Members of Firmicutes pertaining to genera Streptococcus, Lactobacillus and Veillonella were found common in both healthy and diseased plaque samples. Whereas, plaque from diseased individual encompassed various bacteria including opportunistic pathogens of genera like Campylobacter, Neisseria, Enterobacter, Bacillus, Lysinibacillus, Pseudomonas and Morococcus. Therefore, to find their relatedness, phylogenetic trees were constructed using 16S rRNA gene sequences of the isolates from healthy (Supplementary Fig. 1) and diseased (Supplementary Fig. 2) plaque samples. Phylogenetic analysis of these strains yielded most of the clusters according to the species from respective genera, but there were distinct clades contained strains pertaining to different genera, which might have presumed to be a result of unrooted tree. Nonetheless, using an outgroup was also showed similar clade formation. Further, the arbitrary clustering may be attributed to the presence of multiple copies of rrs genes reported in strains pertaining to genera like Streptococcus and Staphylococcus [32, 33]. Further, the phylogeny observed for isolates from diseased plaque followed tendency of commensal to pathogenic behavior. The most abundant genus Streptococcus, represented with a distinct cluster containing highest number of species. Strains of S. mutans involved in dental caries were found major flora only in diseased individual’s plaque. Since members of Lactobacillus distributed amongst various clusters, we have studied a Lactobacillus isolate designated as DISK7 for its ability to adaptation and interaction with other strains in oral environment.
Genomic Features and Subsystems Analysis of Lactobacillus sp.
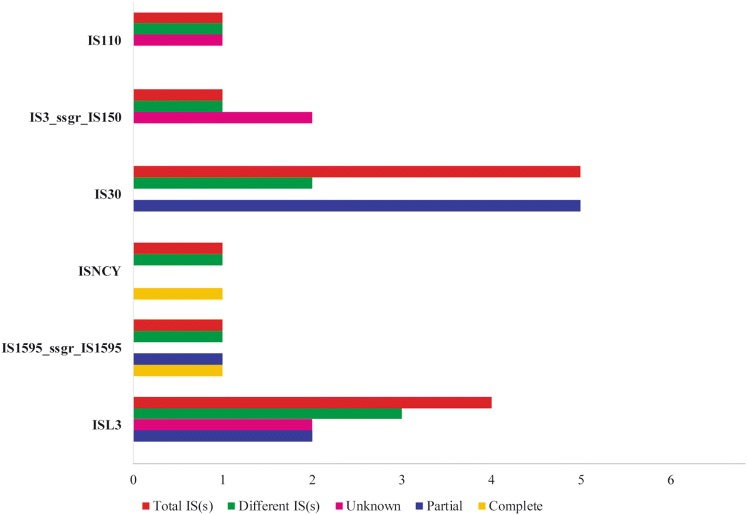
The identity of strain DISK7 was confirmed by rrs gene sequence analysis [34] and to gain more insights, strain DISK7 was subjected to whole genome sequencing. The draft genome has been submitted at NCBI with EMBL accession no. MJET00000000. High quality sequence data (86.7% passing filter with high N50 value) with coverage of 203X was obtained in 45 contigs. The genome size of strain DISK7 was ~ 2 Mb with 34.86 mol % G+C content. About 2000 coding sequences (CDS) were revealed in annotation of genome sequence. The whole genome sequence of strain DISK7 showed 99.6% and 97.5% identity in ANI and dDDH analysis, respectively, with type strain of L. paragasseri JCM 5343T. CDS annotated for whole genome sequence of strain DISK7 were allocated to a total 265 subsystems including primary and secondary metabolism. Nevertheless, only single gene was observed in subsystems pertaining to secondary metabolism, motility and aromatic compounds. There were 13 predicted IS(s) genes belonging to 6 different families including IS110, IS30, ISL3 and ISNCY (Fig. 1), but no CRISPR array was detected to defend it from foreign attacks. Most of the subsystems obtained in RAST analysis showed concurrent number of genes associated with adaptive features as they are common to other members surviving in oral cavity and possibly contributing in their colonization of oral habitat (Supplementary Table S1). Therefore, the genome sequence of strain DISK7 was studied in detail using in silico analysis to extract important genes involved in adaptive mechanism to the dynamic environment of oral cavity.
Fig. 1.
Genome analysis to identify insertion sequence elements IS(s) classified under different classes that are mapped in Lactobacillus sp. strain DISK7
Exopolysaccharide and Biofilm Estimation
Multiple genes encoding capsular polysaccharide biosynthesis protein coding genes were observed in strain DISK7 genome. These include rhamnose-containing cell wall polysaccharides known to produce exopolysaccharides and play an important role in formation of biofilms [35]. Moreover, homologues of genes viz., EpsA, EpsC and EpsD involved in exopolysaccharide biosynthesis were also found in annotation of strain DISK7 genome sequence. Lactobacillus spp. are known to produce exopolysaccharides (EPS) mostly in the form of glucans that act as binding site for microbial accumulation on tooth surface to facilitate establishment of biofilms [36]. Accordingly, experimental validation of extracted EPS estimation from strain DISK7 yielded about 120 mg/L of sugar as estimated by spectrophotometric method (Fig. 2a). Additionally, genes like PTS systems, PCs (PC1), cellobiose phosphotransferase system (celB) [37], YdjC-like protein encoding genes, glyceroltransferases (GftB) [38] and competing stimulating peptide (CSP)-mediated quorum sensing (ComCDE) [39] involved in biofilm formation were also found on genome of strain DISK7. Cconsidering the exopolysaccharide production, we have tested the biofilm formation ability of isolate DISK7 as monospecies and in combination with other strains of other species. For multispecies experiments, strain DISK7 was grown in co-culture with strains of genera Lactobacillus, Streptococcus and Veillonella. Results showed moderate biofilm formation by strain DISK7 as mono-species, but in co-culture experiments increased biofilm formation was observed with L. fermentum strain DISK26, S. salivarius strain DISK15, S. gordonii strain DISK25, S. sanguinis strain DISK 4B and S. mutans MTCC 960 (Fig. 2b). It is pertinent to mention that strain DISK7 showed low biofilm formation capability with species of the genus Veillonella that are considered to be opportunistic pathogens [23].
Fig. 2.
Exopolysaccharide (EPS) production and biofilm formation by strain DISK7. a EPS production by Lactobacillus sp. strain DISK7. Data expressed as mean value of three experiments. b Biofilm formation by Lactobacillus sp. strain DISK7 as mono-and dual-species
In Silico Resistance Profiling
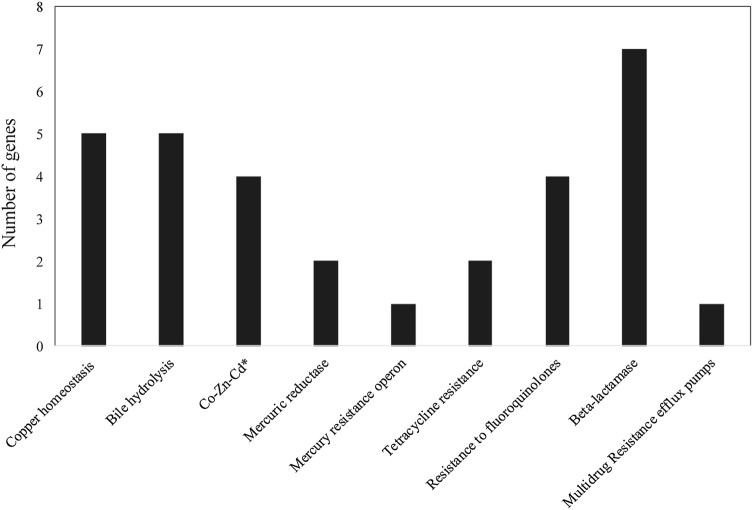
Most of the antibiotic resistance genes identified on strain DISK7 genome were involved in resistance to β-lactam antibiotics such as penicillin binding protein, multiple copies of β-lactamases (7 genes) with complete ORFs, class A and C proteins with Zn-dependent hydrolase, efflux system and a multidrug transport systems including permease (Fig. 3). Nevertheless, strain DISK7 was found to be sensitive to penicillin, tetracycline but showed resistance to kanamycin and gentamycin in antibiotic coated disk diffusion assay. Genome sequence of strain DISK7 also showed presence of genes involved in fluoride resistance (fluoroquinolones). Consequently, it displayed growth on media containing uptoc1500 µg/ml fluoride but did not grow at 2000 µg/ml concentration. Genetic machinery for resistance against quaternary ammonium compounds, fluorides etc., is also presence in the genome of strain DISK7, which are significant from the standpoint of oral cavity inhabitation. SugE gene responsible to resist the presence of quaternary ammonium disinfectants [40]. StraincDISK7 also showed the presence of enzyme choloylglycine hydrolasec (EC 3.5.1.24) that catalyzes the hydrolysis of glycine- and/or taurine-conjugated bile salts, which has been considered significant in terms of probiotic applications [41].
Fig. 3.
In-silico analysis of genome sequence using RAST annotation to determine putative gene analogues that are involved in resistance and adaptation mechanisms towards chemical stress of the environment
Stress Management and Response
There are 33 genes found to encode proteins involved in stress response such as uptake and biosynthesis of well-known osmo-regulators like choline and betaine. Several genes found to be associated with oxidative stress dealing (16 genes), heat shock management (11 genes), cold stress and detoxification (6 genes) were also annotated. Genome also enclosed genes involved in other defense mechanisms like bile hydrolysis, copper homeostasis, mercury resistance and efflux systems. These evidences suggest niche adaptation as a complex evolutionary process that involves extensive genomic alteration usually carried out by phages and transposable elements [42]. Indeed, presence of intact phages and IS(s)on genome of strain DISK7exposesthe vulnerable nature of DISK7 genome that might underwent genomic attacks in the past. This hypothesis is further supported by the fact of absence of CRISPR/CAS system that is used as a defense mechanism against horizontal gene transfers [43].
Carbohydrate Metabolism
Strain DISK7 was found to ferment diverse sugars inVITEK2 analysis, which is a significant feature in terms of inhabiting oral cavity [44] that is exposed to a variety of sugars in high concentrations. Interestingly, majority of CDS (191 genes) were found to encode enzymes associated with carbohydrate metabolism including few enzymes involved in Krebs cycle. Primarily, these genes included enzymes involved in glucose, fructose, rhamnose, mannose, galactose and maltose. However, genes encoding enzymes involved in cellobiose, xylulose, trehalose and sucrose metabolism were also observed. Consequently, the biochemical analysis of strain DISK7 showed ability to ferment sugars like cellobiose, galactose, glucose, mannose, maltose, maltotriose and sucrose. The organic acids metabolism analysis revealed multiple copies of D & L lactate dehydrogenases along with lactate permease, lactate monooxygenase and lactate decarboxylase. Additionally, fumarate reductase, malate permease and alcohol dehydrogenase multiple copies were also present amongst other genes encoding enzymes involved in lactate metabolism, which allowed strain DISK7 to produce enhanced amounts of lactic acid, a probiotic feature most commonly observed in Lactobacillus strains [45].
In this study, a far greater diversity in cultivable flora has been seen in disease associated plaque as compared to healthy individuals. The resident commensal flora performs many vital functions to maintain the oral microbial ecology in health, but microbes in dental plaque associated with oral disease may act as opportunistic pathogens. Our findings about the oral microbiome in health and disease may provide further directions to explore the functional and metabolic alterations associated with the diseased states and to identify potential probiotic strain for oral hygiene.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge Council of Scientific and Industrial Research (CSIR) and Department of Science and Technology (DST), Government of India, Chandigarh. We thank Ms. Richa Sharma, Mr. Sumit Mittal and Mr. Deepak Bhatt for their technical help.
Compliance with Ethical Standards
Conflict of interest
Authors declare that they have no conflicting interest associated with this research article.
Ethics, Consent and Permissions
The study was approved by the ethical committee of Panjab University, Chandigarh, India (PUIEC/2016/17/20/05 and PUIEC/218/113/A/9/01). Individuals were informed and consents were taken while collecting the samples. All samples were plated on diverse media to isolate bacterial strains.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Urvashi and Deepika Sharma have contributed equally to this work.
Contributor Information
Vishakha Grover, Email: vishakhagrover1976@gmail.com.
Suresh Korpole, Email: suresh@imtech.res.in.
References
- 1.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Xu T, Huang G, et al. Oral microbiomes more and more importance in oral cavity and whole body. Protein Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JL, Edlund A. Exploiting the oral microbiome to prevent tooth decay has evolution already provided the best tools? Front Microbiol. 2018 doi: 10.3389/fmicb.2018.03323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney C, Kean R, Short B, et al. Fungi at the scene of the crime: innocent bystanders or accomplices in oral infections? Curr Clin Microbiol Rep. 2018;5:190–200. doi: 10.1007/s40588-018-0100-3. [DOI] [Google Scholar]
- 5.GomezA Nelson KE. The oral microbiome of children: development, disease, and implications beyond oral health. Microb Ecol. 2017;73:492–503. doi: 10.1007/s00248-016-0854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 7.Marsh PD. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health. 2006;6:S14. doi: 10.1186/1472-6831-6-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang H, Lovell CR. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev. 2016;80:91–138. doi: 10.1128/MMBR.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriebel K, Hieke C, Müller-Hilke B, et al. Oral biofilms from symbiotic to pathogenic interactions and associated disease–connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol. 2018;9:53. doi: 10.3389/fmicb.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena P, Joshi Y, Rawat K, et al. Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J Microbiol. 2019;59:3–12. doi: 10.1007/s12088-018-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini R, Saini S, Sharma S. Biofilm: a dental microbial infection. J Nat Sci Biol Med. 2011;2:71. doi: 10.4103/0976-9668.82317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Browngardt CM, Jiang M, et al. Diversity in antagonistic interactions between commensal oral Streptococci and Streptococcus mutans. Caries Res. 2018;52:88–101. doi: 10.1159/000479091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayudu N, Patil PP, Pal VK, et al. Biochemical and genome sequence analyses of Megasphaera sp. strain DISK18 from dental plaque of a healthy individual reveals commensal lifestyle. Sci Rep. 2016;6:33665. doi: 10.1038/srep33665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz RK, Bartels D, Best AA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gafan GP, Lucas VS, Roberts GJ, et al. Prevalence of periodontal pathogens in dental plaque of children. J Clin Microbiol. 2004;42:4141–4146. doi: 10.1128/JCM.42.9.4141-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, Tsai LL, Wu BC, Hsin CH, Chuang CY, et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-16418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva ACBD, Cruz JDS, Sampaio FC, Araújo DAMD. Detection of oral streptococci in dental biofilm from caries-active and caries-free children. Braz J Microbiol. 2008;39:648–651. doi: 10.1590/S1517-83822008000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashima I, Nakazawa F. Interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stages of oral biofilm formation. J Bacteriol. 2015;197:2104–2111. doi: 10.1128/jb.02512-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44:S12–S22. doi: 10.1111/jcpe.12679. [DOI] [PubMed] [Google Scholar]
- 25.Kara D, Luppens SB, Cate JM. Differences between single- and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur J Oral Sci. 2006;114:58–63. doi: 10.1111/j.1600-0722.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 26.Souto R, Silva-Boghossian CM, Colombo APV. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45:495–501. doi: 10.1590/s1517-83822014000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavendran K, Mylotte JM, Scannapieco FA. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: the contribution of dental biofilms and periodontal inflammation. Periodontol 2000. 2007;44:164–177. doi: 10.1111/j.1600-0757.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS ONE. 2012;7:e34242. doi: 10.1371/journal.pone.0034242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Ma R, Wang Y, Zhang L. The clinical importance of Campylobacter concisus and other human hosted Campylobacter species. Front Cell Infect Microbiol. 2018;8:243. doi: 10.3389/fcimb.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baris O, Demir T, Gulluce M. Investigation of in vitro mineral forming bacterial isolates from supragingival calculus. Niger Clin Pract. 2017;20:1571–1575. doi: 10.4103/1119-3077.187316. [DOI] [PubMed] [Google Scholar]
- 31.Bizzini B, Pizzo G, Scapagnini G, et al. Probiotics and oral health. Curr Pharm Des. 2012;18:5522–5531. doi: 10.2174/138161212803307473. [DOI] [PubMed] [Google Scholar]
- 32.Kalia VC, Kumar R, Kumar P, Koul S. A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol. 2016;56:46–58. doi: 10.1007/s12088-015-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R, Koul S, Kumar P, Kalia VC. Searching biomarkers in the sequenced genomes of Staphylococcus for their rapid identification. Indian J Microbiol. 2016;56:64–71. doi: 10.1007/s12088-016-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koul S, Kalia VC. Comparative genomics reveals biomarkers to identify Lactobacillus species. Indian J Microbiol. 2016;56:253–263. doi: 10.1007/s12088-016-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52:3–12. doi: 10.1007/s12088-011-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes RA, Monteiro DR, Arias LS, et al. Virulence factors in Candida albicans and Streptococcus mutans biofilms mediated by farnesol. Indian J Microbiol. 2018;58:138–145. doi: 10.1007/s12088-018-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wua M, Chena Y, Lina T. Cellobiose-specific phosphotransferase system of Klebsiella pneumoniae and its importance in biofilm formation and virulence. Infect Immun. 2012;80:2464–2472. doi: 10.1128/IAI.06247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions and mechanisms. Annu Rev Biochem. 2008;77:1–2. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 39.Kaur G, Rajesh S, Princy SA. Plausible drug targets in the Streptococcus mutans quorum sensing pathways to combat dental biofilms and associated risks. Indian J Microbiol. 2015;55:349–356. doi: 10.1007/s12088-015-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung YJ, Saier MH., Jr Overexpression of the Escherichia coli sugE gene confers resistance to a narrow range of quaternary ammonium compounds. J Bacteriol. 2002;184:2543–2545. doi: 10.1128/jb.184.9.2543-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean M, Cervellati C, Casanova E, et al. Characterization of cholylglycinehydrolase from a bile-adapted strain of Xanthomonas maltophilia and is application for quantitative hydrolysis of conjugated bile salts. Appl Environ Microbiol. 2002;68:126–3128. doi: 10.1128/jb.184.9.2543-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelleher P, Bottacini F, Mahony J, et al. Comparative and functional genomics of the Lactococcus lactis taxon; insights into evolution and niche adaptation. BMC Genom. 2017;18:267. doi: 10.1186/s12864-017-3650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horvath R, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N. Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congress Series. 2005;1284:103–112. doi: 10.1016/j.ics.2005.06.071. [DOI] [Google Scholar]
- 45.Kumar R, Sood U, Gupta V, et al. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol. 2019 doi: 10.1007/s12088-019-00808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.