Abstract
Intrauterine devices (IUDs) are effective and safe long-acting reversible contraceptive methods for preventing unplanned pregnancies. While extensive studies were conducted to evaluate return to fertility after removal of IUDs, majority of them were focused on multiparous women using copper IUDs. Current trends indicate increased use of levonorgestrel (LNG) IUDs in nulliparous women for very long periods of time, with both nulliparity and long duration of LNG-IUD use being potentially associated with trends towards longer time to conception post removal. Understanding the effects that LNG-IUDs may have on endometrial morphology and gene expression has important implications to further understanding their mechanism of action. Studies examining endometrial gene expression show persistent changes in receptivity markers up to 1 year after removal of an inert IUD, and no similar studies have been performed after removal of LNG-IUDs. Given the current gap in the literature and trends in LNG-IUD use in nulliparous young women, studies are needed that specifically look at the interaction of nulliparity, long-term use of LNG-IUD, and return to normal fertility. Herein, we review the available literature on the mechanism of action of IUDs with a specific focus on the effect on endometrial gene expression profile changes associated with IUDs.
Keywords: Endometrial gene expression, Infertility, Intrauterine device, Levonorgestrel, LNG-IUD, Copper IUD
Introduction: endometrial receptivity and window of implantation
Successful implantation of an embryo into the endometrium requires a functionally normal embryo and a receptive endometrium. It is well established that embryonic factors play a pivotal role in successful implantation, of which the most commonly recognized embryonic defect is chromosomal abnormalities1. However, the majority of studies examining outcomes of euploid embryo transfer into uteri with appropriately developed and synchronized endometrium report that only 50–65% of euploid embryos result in live births, leading to the conclusion that a significant portion of these failures may be due to endometrial defect2, 3. Numerous studies within the last couple of decades have evaluated endometrial receptivity and window of implantation1–3. The window of implantation (WOI) is a limited period of time, typically occurring 6–8 days after ovulation, when implantation can occur. Endometrial receptivity is defined as “the temporally and spatially unique set of circumstances within the endometrium that facilitates successful embryonic implantation1.” Several studies have identified molecular markers that appear in the endometrium only during the specific timing of the WOI and that define endometrial receptivity to embryo implantation. These markers include integrins (ITGs), interleukin-1 (IL-1), calcitonin (CAL), amphiregulin (AREG), epidermal growth factor (EGF), HB (heparin binding)-EGF, colony stimulating factor (CSF), leukemia inhibitory factor (LIF), mucins (MUC), leptin (LEP), L-selectin ligands (SELL), Homeobox A (HOXA) , cyclooxygenase (COX), and others4. Failed implantation of morphologically and/or genetically normal embryo in the absence of structural uterine abnormality is suggestive of impaired endometrial receptivity.
Two hormones, estrogen and progesterone, are vital to the development of a receptive endometrium, with the WOI being determined by the time of exposure to progesterone after sufficient exposure to estrogen priming5. Progesterone is the main hormone that controls the luteal phase and the window of implantation. In most target tissues, including endometrium, estrogen upregulates progesterone receptors6, 7. After ovulation, progesterone levels rise and its physiologic effects on the endometrium are primarily mediated through the interaction with the progesterone receptors. It influences morphological changes by causing endometrial stromal decidualization and pinopode formation and indirectly influences the window of implantation by upregulation and downregulation of apposition and attachment molecules, cytokines, and growth factors and downregulation of estrogen receptors1, 3, 8. Specifically, progesterone is known to enhance expression of endometrial receptivity markers such as CSF, interleukins (ILs), vascular endothelial growth factor (VEGF), prostaglandins (PTGs), glycodelin, insulin-like growth factor II (IGF II), HB-EGF, fibronectin (FN), and L-selectin (SELL) and may indirectly influence expression of LIF and beta-3-integrin, all of which are important for implantation and thus for establishing a successful pregnancy1, 2.
These molecular changes correlate to histologic changes to the endometrium throughout the menstrual cycle as described by the Noyes et al. criteria.9 Additionally, changes in thickness and texture of the endometrium can be detected by ultrasonography throughout the cycle. After menses, the endometrium appears as a thin hyperechoic line. As estrogen levels rise, the basal layer proliferates and results in a triple-line appearance of the endometrium.9
Methods
During preparation of this review, an electronic literature review was conducted for published articles in English language, with full text available, pertaining to the levonorgestrel, copper, and inert intrauterine device; return to fertility after discontinuation; and endometrial gene expression and endometrial receptivity. The Pubmed online database was searched with the following keywords: “levonorgestrel IUD and return to fertility,” “endometrial receptivity and IUD,” “levonorgestrel IUD and endometrium,” “copper IUD,” “infertility,” and “endometrial gene expression.” After screening the abstracts for relevance of the content, the full texts of the select articles were reviewed. Additionally, references of articles selected for review were analyzed and used to find additional articles for inclusion.
Factors affecting endometrial function
There are several factors that can affect endometrial function, such as anatomic causes (submucosal fibroids, polyps, and intrauterine synechiae)10–12, infection13, 14, and endocrine disruptors15, 16. It is also well established in the IVF literature and clinical practice that endometrial thickness and pattern on ultrasound are linked to implantation rate17, 18. Persistent thin endometrium as a cause of infertility is rare but very difficult to treat. Several studies evaluated the effects of various hormonal agents on endometrium, including the levonorgestrel IUD (LNG-IUD)19–21, which is the primary focus of the current review. As endometrial suppression/atrophy is one of the mechanisms of action of LNG-IUDs22, 23, it is desirable that we understand the reversibility of this effect when recommending it for long-term use particularly in nulliparous women. To the best of our knowledge, there has yet to be a study that adequately assess long-term use of LNG-IUD in nulliparous compared with the multiparous population. The nulliparous population is of particular concern given their fertility has yet to be proven.
Overview of mechanism of contraceptive action of intrauterine devices with emphasis on endometrium
There are two types of IUDs available in the USA: the copper IUD (most commonly used Paragard IUD) and levonorgestrel (LNG) IUD (trade names Mirena, Liletta, Skyla, and Kyleena, varying in their LNG dose and approved length of use). The literature has described effects of IUDs on sperm motility, transport and function (outside of the scope of this review), follicular development, ovulation, fertilization, and endometrial receptivity (see below and Table 1). Additionally, while not available for use in the USA, inert IUDs were previously available in other countries and have been described in the literature as far as their effect on endometrial receptivity and gene expression.
Table 1.
Mechanisms of contraceptive actions of intrauterine devices
| Intrauterine device (IUD) mechanism of action | ||
|---|---|---|
| Copper IUD | Levonorgestrel IUD | |
| Cervical mucus | Copper ions penetrate cervical mucus and decrease sperm motility | Thickens cervical mucus |
| Spermatozoa and oocyte | Decreases sperm motility, viability, and fertilizing capability; damages oocyte prior to fertilization | Decreases sperm motility |
| Fertilization | Impairs fertilization | Impairs fertilization |
| Ovulation | No effect | Can cause anovulatory cycles within first year, but thereafter most cycles are ovulatory [27] |
| Endometrium | In vitro studies have shown copper affects endometrial gene expression [26] | Thins endometrial lining and causes some changes in endometrial gene expression [28] |
Copper IUD
The copper IUD was approved by the FDA for use in the USA in November 198424. The primary mechanism of action of the copper IUD is to impair fertilization25. Copper ions accumulate throughout the entire reproductive tract including the fallopian tubes and are toxic to spermatozoa and unfertilized and fertilized oocytes25. There is no effect on follicular development or ovulation25. While there are no in vivo studies specifically evaluating the effect of the copper IUD on the endometrium at the molecular level, Carrascosa et al. demonstrated that addition of copper to decidualized human endometrial stromal cells (dHESCs) in vitro disrupts the gene signature associated with endometrial receptivity26, thus suggesting that impairing endometrial receptivity can be part of the mechanism of action of the copper IUD.
In this study, dHESCs were decidualized over a period of 8 days; on days 6 and 7 of chemical decidualization, copper was added to the medium for the treatment group at the same concentration found in patients who used the copper IUD.26 Copper-treated dHESCs revealed dysregulation of 49 of 192 endometrial receptivity and immunological response genes, resulting in a characteristic gene signature. Copper decreased IGFBP1 levels to half the levels seen in the decidualized control group. Additionally, some well-known genes associated with the decidualization process were dysregulated including chemokine motif ligand 6 (CXCL6), ILs, and LIF.26 Interestingly, up to 19 of the 49 genes dysregulated during copper treatment are implicated in the endometriosis pathway.26 The authors hypothesized that the alteration in the gene signature induced by copper may explain one of the main side effects of the copper IUD such as increasing menstrual bleeding, through modification in the subendometrial microvascularization.26
Levonorgestrel IUD
The primary mechanism of action of the LNG-IUD is thickening of cervical mucus and decreasing motility of spermatozoa25. It has also been shown to affect ovulation to some degree within the first year of placement, but, thereafter, most cycles become ovulatory. One study showed the incidence of ovulatory cycles with the LNG-IUS 20 mg/24 h (Mirena) and the copper IUD to be the same (85%)27.
LNG-IUDs have a marked progesterone effect on endometrial morphology, as discussed below. Silverberg et al. analyzed endometrial morphological changes between 3 months and up to 7 years after insertion of a LNG-IUD28. Biopsies of the endometrium with the IUD in place exhibit glandular atrophy and decidualized stroma, and results did not differ based on the length of time that the IUD had been in place28 or the type of LNG-IUD (LNG-IUD 13.5mg, 19.5mg, or 52mg)28. Biopsies of the endometrium 1–3 months after LNG-IUD removal showed return to normal morphology of the endometrial tissue leading the authors to conclude that LNG-IUDs do not have long-term harmful effects on the endometrium as seen in pathology specimens28.
Thus, the initial studies demonstrated the morphologic effects of the LNG-IUD on the endometrium including decidualization and atrophy23, 28, 29. In subsequent years, with the use of genomic approach, studies demonstrated upregulation of decidualization markers IGFBP1 and prolactin after placement of the LNG-IUD20, 21. This coincided with histological evidence of stromal decidualization and atrophy of the endometrium in same samples20, 21. To this point, LNG-IUD was shown to induce endometrial decidualization, confirmed with histology and IGFBP1 expression, in postmenopausal women treated with selective estrogen receptor modulator tamoxifen.30 Another finding was the downregulation of both estrogen and progesterone receptors in the endometrium after insertion of the LNG-IUD, although expression gradually returned to baseline between 6 and 12 months21. Similar findings of decreased estrogen and progesterone receptor immunoexpression in eutopic and ectopic glandular and stromal endometrial compartments were reported after 6 months of LNG-IUD use in subjects with endometriosis.31
A more recent study, with the primary focus of evaluating the mechanisms of increased susceptibility to HIV in women using progestin-containing contraceptives, assessed effects of depot medroxyprogesterone acetate (DMPA) and LNG-IUDs on endometrial transcriptome19. Endometrial biopsies were obtained from DMPA and LNG-IUD users after at least 6 months of use and compared with mid-secretory phase endometrium in the control group19. The LNG-IUD group versus control had the greatest increase in expression of decidualization markers such as IGFBP1, MMP7, and prolactin, as expected, exhibiting significant gene dysregulation19.
Short term effects of LNG on the endometrium has been evaluated and demonstrated that a single oral post-ovulatory dose of LNG, as well as four oral or a single vaginal administration of LNG emergency contraception, did not have any significant effect on the endometrial gene expression during the receptivity period32, 33. The difference with the earlier described findings may indicate the importance of amount and length of exposure of the endometrium to LNG.
The inert IUD
The primary mechanism of action of the inert IUD is a local foreign body reaction that creates an unfavorable environment for spermatozoa and embryo implantation26, 34. While there is no data on the global gene expression profile in the setting of the copper IUD and LNG-IUD in situ as well as after removal in the same subjects, the effect of an inert IUD on the endometrial gene expression profile has been evaluated. That study specifically assessed the gene expression profile within the WOI (LH+7) in a natural cycle prior to IUD placement, in the month of IUD placement, and at 2 and 12 months after IUD removal34. The authors found that, in the presence of the inert IUD, 35% of dysregulated genes were established WOI genes. Comparison to the Goldfien et al. study shows that the inert IUD and the LNG-IUD have different effects on the gene expression profile of the endometrium19, 34. Some of them, such as glycodelin, LIF, alpha-catenin (CTNNA), and nuclear factor (NFK) I/B, remained dysregulated at 2 months after IUD removal. In fact, only five transcripts normalized at 2 months. At 1 year post IUD removal, only 80% of initially dysregulated genes recovered their normal expression, including glycodelin, LIF, CTNNA, and NFK I/B34, indicating not quite complete recovery of endometrial transcriptome even after inert IUD.
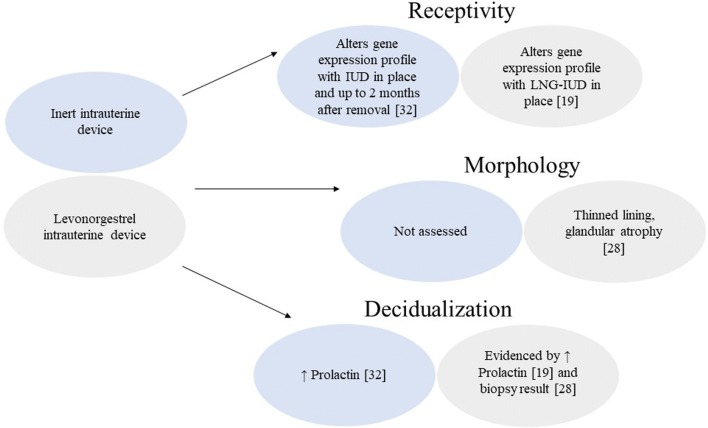
While the endometrium at the morphological level seems to normalize after just 1–3 months post LNG-IUD removal28, evidence that inert IUDs dysregulate endometrial gene expression profile for a longer period of time34 suggests that LNG-IUDs may have similar or even more profound effects on endometrial function and gene expression (Fig. 1). The gene expression profile changes seem to persist longer than the morphological changes. Moreover, it has been established that histological evaluation of endometrium is not a reliable measure of progesterone effect on endometrium35, 36. To our surprise, when working on this review, we discovered a significant breach in existing data on the evaluation of endometrium after LNG-IUD use.
Fig. 1.
Comparison of various effects on the endometrium between the inert intrauterine device and the levonorgestrel intrauterine device
Given the difference in the experimental design and subsequently in the conclusions of existing studies, it continues to remain a question whether long-term exposure of the endometrium to LNG, as occurs in LNG-IUD exposure, interferes with endometrial receptivity by dysregulating normal gene transcription patterns, while affecting endometrial morphology and growth. If it does, how long do the effects of the LNG-IUD persist after removal and do they have an effect on future fertility?
Return to fertility
Return to fertility after IUD removal has been well reported in the literature for decades; however, some controversy remains over how soon fertility returns and if various IUD formulations affect this differently. Most studies show that overall high rates of women desiring, both nulliparous and parous, are able to achieve pregnancy after removal of both the Cu-IUD and the LNG-IUD within a period of 12 months following discontinuation37–43. Of these studies, only two mention the specific indication for LNG-IUD placement being contraception; additionally, inclusion criteria for these two studies included history of regular menstrual cycles.40, 42 A large study assessing conception and pregnancy after removal of the LNG-IUD compared with the Nova-T copper-IUD found no statistically significant differences between conception and pregnancy rates following removal of the two devices43. A majority of women conceived within the first 12 months in the LNG-IUD group and the Nova-T group, 75.4% and 70.4%, respectively43. Although this conception rate may seem high and normal, it is lower than the widely accepted 85–92% conception rate after 12 months of trying in the general population44, 45. However, to the best of our knowledge, there is no study evaluating return to fertility in previous long-term LNG-IUD users compared with controls. Of note, both nulliparous and multiparous patients were included in the study, and the median duration of IUD use was 21 months for copper IUD (range 6–53 months) and 19 months for LNG-IUD (range 3–50 months)43. Gemzell-Danielsson et al. also showed in the cohort of 179 nulliparous and parous women (follow up data available for 163 out of the 179) that decided to discontinue use of LNG-IUD for desired pregnancy that 37.4% conceived within the first 3 months and 71.2% conceived within 12 months. In that study, only 59.9% and 37.9% of women completed 3 and 5 years of treatment, respectively42, indicating overall short-term LNG-IUD use in the study participants and pointing out once again lower than expected conception rate after 12 months of unprotected intercourse, see Table 2. This trial excluded participants with history of abnormal uterine bleeding and PID.42 Of note, the studies on return to fertility after LNG use have very few nulliparous women included and primarily look at return to fertility after shorter duration of use—mean use less than 5 years (Table 2)40, 42, 43. It is important to mention that the LNG-IUD has a small 5-year cumulative failure rate of 1.4%42. This indicates that it is possible to conceive despite changes to the endometrial receptivity; however, the failure rate is small, and it is not completely understood what factors lead to LNG-IUD failure other than device displacement. Overall, evaluating return to fertility is a challenging task given diversity of subjects, many potential confounders, and lack of proper controls.
Table 2.
Summary of fertility outcomes after IUD removal described in the literature
| Study | Sample size (n) | Mean age (range) | Nulliparous (%) | IUD type | Length of IUD use | Fertility outcome measures (%) |
|---|---|---|---|---|---|---|
| Andersson et al., 1992 [41] | 138 | 27 (18–36) | Information not available | LNG | 19 months (3–50) | CR (12 m)–79.1 |
| Doll et al., 2001 [45] | 162 | 27.7 (< 20–35+) | 100 | Copper |
Used for < 42 months Used for 42–78 months Used for > 78 months |
LBR (24 m)–76.5 By length of use: < 42 m - 88.1 42–78 m - 73.5 > 78 m - 68.3 |
| Zhu et al., 2013 [44] | 1770 | 37.3 (21–53) | 0 | Copper | 10.3 years (1–28) |
CR (12m)–70.96 By age: < 35 - 92.18 35–40 - 84.17 > 40 - 58.57 |
| Eisenberg et al., 2015 [38] | 68 | 27.3 (16–45)* | 57.7 | LNG | < 3 years | CR (12 m)–86.8 |
| Gemzell-Danielsson et al., 2017 [40] | 179 | 27.1 (18–35)* | 39.5 | LNG | < 5 years | CR (12 m)–71.2 |
IUD, intrauterine device, LNG = levonorgestrel, m = months, yrs = years, CR = conception rate, LBR = live birth rate
*Average age reported for entire LNG IUD study group, unavailable for subgroup of women who discontinued LNG IUD and were followed for time to conception
Several studies evaluating return to fertility after copper IUD demonstrated relatively high rates of conception upon discontinuation, though some decline was noted after long term use (> 6 years), which could in part be due to advanced maternal age (Table 2)46, 47.
Discussion
To summarize the data described above, the LNG-IUDs cause atrophy of endometrial glands and decidualization of endometrial stromal cells while in situ. Normalization of morphological changes appears months before normalization of endometrial gene expression in majority of, but not all, users at 12 months. The main challenge with these studies is that there is very limited data on endometrial parameters and function after removal of LNG-IUD, as well as fertility after prolonged IUD use (> 5 years) in both nulliparous and multiparous women. It is theoretically possible that, in some women, prolonged use of the LNG-IUD could lead to persistent endometrial atrophy or dysfunction due to diminished response to estradiol stimulation of endometrium because of chronic estrogen receptor downregulation or even progesterone resistance. With the current popularity of LNG-IUD among teenagers, nulliparous women, and their Ob/Gyn providers48–50, we may not see the effects of this trend for years until current teenagers are ready for procreation. In addition, endometrial dysfunction as a cause of infertility is rare and may not even be detected by overall statistics when analyzing fertility outcomes after LNG-IUD use, which does not necessarily mean it is not there. Hence, this is a call for basic and translational studies addressing endometrial morphology and gene expression and function after long-term LNG-IUD use to catch up with the evolving patient population. We would like to bring this issue to the attention of reproductive endocrinologists and infertility specialists, as well as general gynecologists, when evaluating infertile patients after LNG-IUD use. While we by no means aim to undermine the significance and utmost importance of LNG-IUD as a reliable long-acting reversible contraceptive device, it is our responsibility as a field to monitor the future fertility in these women and remain vigilant in our research and counseling.
While long-term effects of the LNG-IUD on endometrial function are unknown, they could be studied. A prospective study examining return to fertility after levonorgestrel IUD use in both nulliparous and multiparous women would be helpful in further elucidating this gap in the literature. With the high prevalence of IUD use, it would be possible to identify a large study population. Time of placement to time of removal could be followed stratifying total time of use into groups of less than 5 years, 5–10 years, and more than 10 years. Factors that influence fertility would need to be assessed such as age, prior reproductive performance (multiparous versus nulliparous), male factor infertility, prior pelvic inflammatory disease, and history of endometriosis/other gynecological disease to control for additional factors affecting baseline fertility and endometrial function. Age groups could be stratified as in prior studies46 to determine if trends are similar amongst various age groups. Sonographic measurement of endometrial thickness before and at various time points after LNG-IUD use would be performed. Additionally, molecular and/or microbial components could be assessed with endometrial biopsies before, a time point with levonorgestrel IUD in place, and a time point(s) after discontinuation.
Conclusions
Herein, we describe the state of the current literature on endometrial effects of different types of IUD use, with particular emphasis on LNG-IUD, and identify a clinical necessity to evaluate the long-lasting effects of the LNG-IUD on endometrial function and its potential impact on subsequent fertility. While the current literature on return to fertility after use of LNG-IUD shows that 70–75% of women conceive within 1 year of removal, it also indicates that the rate of infertility after IUD removal may be double the commonly quoted rate of infertility in the general population of 15%. Naturally, other factors such as age, semen parameters, history of PID, parity, and reproductive history play a significant role in fertility potential as well as initial indication for IUD insertion including pelvic pain/chronic gynecologic disease management, with some of these factors being controlled for in the studies mentioned in this review. Studies examining return to fertility after IUD removal do not adequately examine nulliparous women or women with prolonged use of the LNG-IUD to reflect the current growing patient population. While it is of paramount importance to provide our patients with reliable and safe long-acting and easy to use contraception, we have to be aware of any possible long-lasting side effects, such as persistent endometrial atrophy and dysfunction or lack thereof. This review highlights the state of the literature, in which studies examining return to normal endometrial function after removal of IUDs are currently insufficient or lacking.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoozemans DA, Schats R, Lambalk CB, Homburg R, Hompes PGA. Human embryo implantation: current knowledge and clinical implications in assisted reproductive technology. Reprod Biomed Online. 2004;9(6):692–715. doi: 10.1016/S1472-6483(10)61781-6. [DOI] [PubMed] [Google Scholar]
- 2.Miravet-Valenciano JA, Rincon-Bertolin A, Vilella F, Simon C. Understanding and improving endometrial receptivity. Curr Opin Obstet Gynecol. 2015;27(3):187–192. doi: 10.1097/GCO.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 3.Valbuena D, Valdes CT, Simon C. Introduction: endometrial function: facts, urban legends and an eye to the future. Fertil Steril. 2017;108(1):4–8. doi: 10.1016/j.fertnstert.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19(2):204–211. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrenz B, Fatemi H. Effect of progesterone elevation in follicular phase of IVF-cycles on the endometrial receptivity. Reprod Biomed Online. 2017;34(4):422–428. doi: 10.1016/j.rbmo.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard P. Progesterone and the progesterone receptor. J Reprod Med. 1999;44(2 Suppl):153–157. [PubMed] [Google Scholar]
- 7.Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56(5):1205–1215. doi: 10.1095/biolreprod56.5.1205. [DOI] [PubMed] [Google Scholar]
- 8.Snijders MP, de Goeij AF, Debets-Te Baerts MJ, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil. 1992;94(2):363–371. doi: 10.1530/jrf.0.0940363. [DOI] [PubMed] [Google Scholar]
- 9.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1(1):3–25. doi: 10.1097/00006254-195008000-00044. [DOI] [PubMed] [Google Scholar]
- 10.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111(4):629–640. doi: 10.1016/j.fertnstert.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez H, Al-Najjar F, Chauveaud-Lambling A, Frydman R, Gervaise A. Fertility after treatment of Asherman’s syndrome stage 3 and 4. J Minim Invasive Gynecol. 2006;13(5):398–402. doi: 10.1016/j.jmig.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Chen X, Huang J, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109(5):832–839. doi: 10.1016/j.fertnstert.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 14.McQueen DB, Perfetto CO, Hazard FK, Lathi RB. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. 2015;104(4):927–931. doi: 10.1016/j.fertnstert.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Aghajanova L, Giudice LC. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod Biomed Online. 2011;22(3):249–256. doi: 10.1016/j.rbmo.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan M, Hu M, Lou Y, et al. Environmentally relevant levels of bisphenol A affect uterine decidualization and embryo implantation through the estrogen receptor/serum and glucocorticoid-regulated kinase 1/epithelial sodium ion channel α-subunit pathway in a mouse model. Fertil Steril. 2018;109(4):735–744. doi: 10.1016/j.fertnstert.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Abdalla HI, Brooks AA, Johnson MR, Kirkland A, Thomas A, Studd JW. Endometrial thickness: a predictor of implantation in ovum recipients? Hum Reprod. 1994;9(2):363–365. doi: 10.1093/oxfordjournals.humrep.a138509. [DOI] [PubMed] [Google Scholar]
- 18.Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 19.Goldfien GA, Barragan F, Chen J, et al. Progestin-containing contraceptives alter expression of host defense-related genes of the endometrium and cervix. Reprod Sci. 2015;22(7):814–828. doi: 10.1177/1933719114565035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RL, Critchley HO. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum Reprod. 2000;15(Suppl 3):162–172. doi: 10.1093/humrep/15.suppl_3.162. [DOI] [PubMed] [Google Scholar]
- 21.Rutanen E.-M. Insulin-like growth factors and insulin-like growth factor binding proteins in the endometrium. Effect of intrauterine levonorgestrel delivery. Human Reproduction. 2000;15(suppl 3):173–181. doi: 10.1093/humrep/15.suppl_3.173. [DOI] [PubMed] [Google Scholar]
- 22.Pakarinen PI, Lähteenmäki P, Lehtonen E, Reima I. The ultrastructure of human endometrium is altered by administration of intrauterine levonorgestrel. Hum Reprod. 1998;13(7):1846–1853. doi: 10.1093/humrep/13.7.1846. [DOI] [PubMed] [Google Scholar]
- 23.Critchley HO, Wang H, Jones RL, Kelly RW, Drudy TA, Gebbie AE, Buckley CH, McNeilly A, Glasier AF. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13(5):1218–1224. doi: 10.1093/humrep/13.5.1218. [DOI] [PubMed] [Google Scholar]
- 24.Elaine C. Esber. ParaGard Copper T Model TCU 380A Intrauterine Contraceptive. 1984. [Google Scholar]
- 25.Gemzell-Danielsson K, Berger C. P.G.L. L. Emergency contraception—mechanisms of action. Contraception. 2013;87(3):300–308. doi: 10.1016/j.contraception.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Carrascosa JP, Cotán D, Jurado I, Oropesa-Ávila M, Sánchez-Martín P, Savaris RF, Tan J, Sánchez-Alcázar JA, Tan SL, Horcajadas JA. The effect of copper on endometrial receptivity and induction of apoptosis on decidualized human endometrial stromal cells. Reprod Sci. 2018;25(7):985–999. doi: 10.1177/1933719117732165. [DOI] [PubMed] [Google Scholar]
- 27.Apter D, Gemzell-Danielsson K, Hauck B, Rosen K, Zurth C. Pharmacokinetics of two low-dose levonorgestrel-releasing intrauterine systems and effects on ovulation rate and cervical function: pooled analyses of phase II and III studies. Fertil Steril. 2014;101(6):1656–1662. doi: 10.1016/j.fertnstert.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. Int J Gynecol Pathol. 1986;5(3):235–241. doi: 10.1097/00004347-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard BL. Endometrial morphological changes in IUD users: a review. Contraception. 1987;36(1):1–10. doi: 10.1016/0010-7824(87)90057-6. [DOI] [PubMed] [Google Scholar]
- 30.Philip Sarah, Taylor Anthony H., Konje Justin C., Habiba Marwan. The levonorgestrel-releasing intrauterine device induces endometrial decidualisation in women on tamoxifen. Journal of Obstetrics and Gynaecology. 2019;39(8):1117–1122. doi: 10.1080/01443615.2019.1587600. [DOI] [PubMed] [Google Scholar]
- 31.Engemise SL, Willets JM, Taylor AH, Emembolu JO, Konje JC. Changes in glandular and stromal estrogen and progesterone receptor isoform expression in eutopic and ectopic endometrium following treatment with the levonorgestrel-releasing intrauterine system. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):101–106. doi: 10.1016/j.ejogrb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Meng C-X, Marions L, Bystrom B, Gemzell-Danielsson K. Effects of oral and vaginal administration of levonorgestrel emergency contraception on markers of endometrial receptivity. Hum Reprod. 2010;25(4):874–883. doi: 10.1093/humrep/deq007. [DOI] [PubMed] [Google Scholar]
- 33.Vargas MF. Tapia–Pizarro AA, Henríquez SP, et al. Effect of single post-ovulatory administration of levonorgestrel on gene expression profile during the receptive period of the human endometrium. J Mol Endocrinol. 2012;48(1):25–36. doi: 10.1530/JME-11-0094. [DOI] [PubMed] [Google Scholar]
- 34.Horcajadas JA, Sharkey AM, Catalano RD, Sherwin JR, Domínguez F, Burgos LA, Castro A, Peraza MR, Pellicer A, Simón C. Effect of an intrauterine device on the gene expression profile of the endometrium. J Clin Endocrinol Metab. 2006;91(8):3199–3207. doi: 10.1210/jc.2006-0430. [DOI] [PubMed] [Google Scholar]
- 35.Murray MJ, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003. [DOI] [PubMed] [Google Scholar]
- 36.Young SL, Savaris RF, Lessey BA, Sharkey AM, Balthazar U, Zaino RJ, Sherwin RA, Fritz MA. Effect of randomized serum progesterone concentration on secretory endometrial histologic development and gene expression. Hum Reprod. 2017;32(9):1903–1914. doi: 10.1093/humrep/dex252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skjeldestad FE. The impact of intrauterine devices on subsequent fertility. Curr Opin Obstet Gynecol. 2008;20(3):275–280. doi: 10.1097/GCO.0b013e3282fe7427. [DOI] [PubMed] [Google Scholar]
- 38.Lohr PA, Lyus R, Prager S. Use of intrauterine devices in nulliparous women. Contraception. 2017;95(6):529–537. doi: 10.1016/j.contraception.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Andolsek L, Teeter RA, Kozuh-Novak M, Wheeler R, Fortney JA, Rosenberg MJ. Time to conception after IUD removal: importance of duration of use, IUD type, pelvic inflammatory disease and age. Int J Gynaecol Obstet. 1986;24(3):217–223. doi: 10.1016/0020-7292(86)90100-1. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16. doi: 10.1016/j.contraception.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Randic L, Vlasic S, Matrljan I, Waszak CS. Return to fertility after IUD removal for planned pregnancy. Contraception. 1985;32(3):253–259. doi: 10.1016/0010-7824(85)90048-4. [DOI] [PubMed] [Google Scholar]
- 42.Gemzell-Danielsson K, Apter D, Dermout S, et al. Evaluation of a new, low-dose levonorgestrel intrauterine contraceptive system over 5 years of use. Eur J Obstet Gynecol Reprod Biol. 2017;210:22–28. doi: 10.1016/j.ejogrb.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrel-releasing intrauterine device and Nova-T. Contraception. 1992;46(6):575–584. doi: 10.1016/0010-7824(92)90122-A. [DOI] [PubMed] [Google Scholar]
- 44.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503–509. doi: 10.1016/S0015-0282(16)58144-8. [DOI] [PubMed] [Google Scholar]
- 45.Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18(9):1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Lei H, Huang W, et al. Fertility in older women following removal of long-term intrauterine devices in the wake of a natural disaster. Contraception. 2013;87(4):416–420. doi: 10.1016/j.contraception.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Doll H, Vessey M, Painter R. Return of fertility in nulliparous women after discontinuation of the intrauterine device: comparison with women discontinuing other methods of contraception. BJOG. 2001;108(3):304–314. doi: 10.1111/j.1471-0528.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 48.Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol. 2015;126(5):917–927. doi: 10.1097/AOG.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashlyn H. Savage, MD and Sarah F. Lindsay M. ACOG Committee Opinion No. 735: Adolescents and long-acting reversible contraception implants and intrauterine devices. Obstet Gynecol. 2018;131(5):e130–e139. doi: 10.1097/AOG.0000000000002632. [DOI] [PubMed] [Google Scholar]
- 50.Steiner RJ, Liddon N, Swartzendruber AL, Rasberry CN, Sales JM. Long-acting reversible contraception and condom use among female US high school students. JAMA Pediatr. 2016;170(5):428–434. doi: 10.1001/jamapediatrics.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]