Abstract
Purpose
To evaluate the association between anti-Müllerian hormone (AMH) and follicle density in infertile women with diminished ovarian reserve (DOR) versus women with normal ovarian reserve?
Methods
Case–control study comparing follicle densities in ovarian cortex from 20 infertile women with DOR (AMH ≤ 5 pmol/L) and 100 controls with presumed normal ovarian reserve.
Results
For all women > 25 years, the follicle densities correlated positively with AMH levels. For each single picomole per liter increase in AMH the follicle density increased by 6% (95% CI 3.3–8.5%) when adjusted for age. This was similar for women with DOR and controls. The follicle density was 1.8 follicles/mm3 cortical tissue in women with DOR versus 7.0 in age-paired controls (p = 0.04). The women with DOR had a median AMH of 1.8 pmol/L versus 14.4 pmol/L in the age-paired control group (p < 0.001). The ratio of AMH/follicle density was 1:1 (1.8/1.8) in women with DOR and 2:1 (14.4/7.0) in the age-paired controls. Analyses for gonadotropin receptor polymorphisms could not explain the characteristics of women with DOR. The proportion of secondary follicles was higher in women with DOR compared with controls (4.6% versus 1.4%, p = 0.0003). Pooling all patients, the follicle density decreased significantly by 7.7% for every year added (p < 0.0001). The women with DOR had lower follicle densities than the controls, but the slopes were equal in the two cohorts.
Conclusions
Follicle density and AMH concentrations correlate also when AMH is low. However, AMH is only a reliable marker for the true ovarian reserve when age is included in the estimation and women with DOR may have more follicles than their AMH levels imply.
Keywords: AMH, Follicle density, Diminished ovarian reserve, Ovarian biopsy
Introduction
Clinically, women identified with diminished ovarian reserve (DOR) are characterized by low anti-Müllerian hormone (AMH), low antral follicle counts determined by ultrasound, as well as increased FSH levels [1]. These parameters are considered to reflect the ovarian reserve, defined as the pool of remaining follicles in the ovaries. AMH is secreted to the circulation by granulosa cells and it is estimated the around 60% of the AMH serum levels originate from 5 to 8 mm sized follicles [2]. Several studies have shown that both serum AMH (reviewed in [3]) and the number of remaining follicles decrease with increasing age (reviewed in [4]). The AMH concentration decreases from an age of 24.5 years [5], suggesting that from the mid-twenties, AMH is a proxy for the true ovarian reserve. Thus, AMH is considered to reflect a woman’s reproductive range. Currently, no in vivo techniques exist for assessing follicle density and only a limited number of studies [5–8] have analyzed the association between serum AMH levels and the true number of remaining follicles in the ovarian cortex determined by histological examination. However, these previous studies used older AMH assays. Hence, with the introduction of the highly sensitive and improved Elecsys AMH assay, an assessment of the ovarian follicle density in women with low AMH serum levels is sought to clarify whether low AMH serum levels are reflected by a proportionally low follicle density in women diagnosed with DOR.
In this study, we investigated the association between AMH serum levels and the follicle density in pieces of ovarian cortical tissue from infertile women with DOR to explore to what extent very low serum AMH levels are associated with a very low ovarian follicle density. Additionally, we assessed follicle densities in women with a presumed normal ovarian reserve, in order to analyze if women with DOR differed from these in term of associations between their AMH concentrations and the follicle densities as well as the frequency of follicles at different developmental stages.
Materials and methods
Patients
Infertile patients with low AMH (cases)
A total of 20 infertile patients identified with DOR were recruited from April 2016 to December 2018 at the Fertility Clinic at the University hospital of Copenhagen Rigshospitalet, Denmark. Inclusion criteria were infertility with an indication for IVF/ICSI, repeated serum AMH measurements < 5 pmol/L (0.7 ng/mL) (at least two independent measurements within 1 year prior to screening), and age 25–39 years. The exclusion criteria were any ovarian pathology (endometriosis, cysts, malignancy), chromosomal abnormalities, autoimmune diseases (except TPO antibodies), and contraindications for laparoscopy at inclusion. Screening for plasma–ovary–antibodies and plasma–adrenal–antibodies was done prior to inclusion.
These patients were part of a clinical trial investigating the potential of ovarian cortical fragmentation and autotransplantation for follicle activation [9]. Median age in the case group was based on the age at study inclusion.
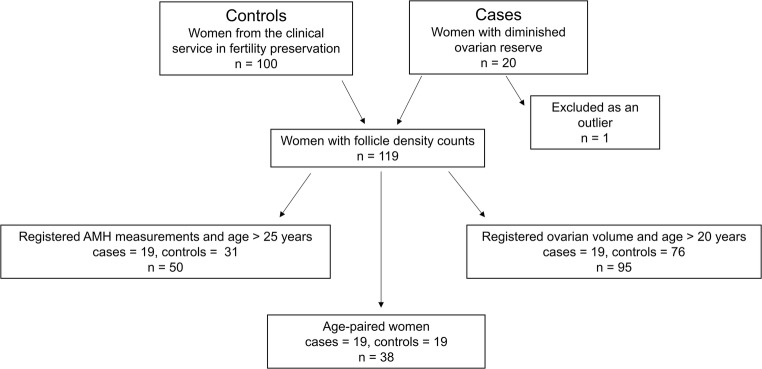
One out of the 20 patients with DOR displayed a high follicle density of 93.7 follicles/mm3. This patient was defined as an outlier and excluded from the study analyses, apart from the median follicle density as shown in Table 1 and the receptor polymorphisms analyses (also see Fig. 1).
Table 1.
Clinical characteristics of women with DOR (cases) and a control group (controls)
| Cases | Controls | |
|---|---|---|
| Cohort,n | 20 | 100 |
| Age (years) | ||
| Median (range) | 38.3 (30.8–39.8) | 29.9 (2.8–42.7) |
| Diagnoses (%) | ||
| Breast cancer | – | 39 |
| Cervical cancer | – | 6 |
| Diminished ovarian reserve | 100 | – |
| Hodgkin/non-hodgkin disease | – | 20 |
| Sarcoma | – | 13 |
| Others | – | 22 |
| Follicle density (follicles per mm3 tissue) | ||
| Median (range) | 1.9 (0–93.7) | 16.3 (0–1578) |
| Cohort,n | 20 | 35 |
| AMH (pmol/L) | ||
| Median (range) | 1.9 (0.21–5.2*) | 14.4 (2.7–50.4) |
| FSH (IU/L) | ||
| Median (range) | 10.4 (4.8–25.9) | NA |
| AFC | ||
| Median (range) | 5 (2–9) | NA |
| Cohort,n | 20 | 78 |
| Ovarian volume (mL) (n) | ||
| Median (range) | 3.96 (1.77–10.22) | 5.87 (0.5–15.10) |
DOR diminished ovarian reserve, AMH anti-Müllerian hormone, FSH follicle-stimulating hormone, AFC antral follicle count
*One patient with DOR had a single AMH level of 5.2 pmol/L before the laparoscopy
Fig. 1.
A patient diagram of the included women. In total, 20 women with diminished ovarian reserve (cases) and 100 women from the clinical service in fertility preservation by cryopreservation of ovarian tissue (controls) were included. To perform an age-paired comparison of follicle densities in cases versus controls, we conducted a new control group that included 38 women. For analysis of the correlation between follicle density and anti-Müllerian hormone (AMH), only women above 25 years were included. For analysis of the correlation between follicle density and ovarian volume, only women older than 20 years were included
Fertility preservation patients (controls)
The control group included a total of 100 patients who had fertility preservation by cryopreservation of ovarian tissue. All women underwent unilateral ovariectomy for fertility preservation and had presumably healthy ovaries (Table 1). None of the patients from the control group received gonadotoxic treatment prior to cryopreservation of their ovarian tissue. Median age in the control group was based on the age at the time of cryopreservation.
For the regression analysis including AMH measurements, only women older than 25 years were included, since earlier studies have found that the AMH serum levels peak on average at 24.5 years [5, 10]. For the regression analysis including ovarian volume, only women older than 20 years were included, since earlier studies have found that the ovarian volume peaks on average at 20 years [11].
An overview of the included women and subgroups made for statistical analyses is depicted in Fig. 1.
Ovarian cortical tissue
As part of a clinical trial [9], patients with DOR had four biopsies removed laparoscopically from one of their ovaries. Each biopsy had a volume of approximately 5 × 5 × 3 mm. According to the protocol, one of the four biopsies was donated for the histological examination.
In the fertility preservation group, one biopsy of the cortical tissue measuring approximately 5 × 5 × 2 mm was donated for histological examination as part of the research program on cryopreservation of ovarian tissue.
Histology and density estimation
In all cases and controls, the cortex piece was mechanically isolated from the medulla, fixed in Bouin’s solution overnight (12–18 h), embedded in paraffin, and sectioned into 25–30-μm-thick sections. The sections were mounted on glass slides and stained with Periodic acid–Schiff (PAS). All follicles of a normal appearance were counted on a Zeiss Axiophot upright microscope (× 16 magnification). Normal appearing follicles were defined as round follicles; follicle structures that had collapsed were not counted. The follicle density was estimated by the mathematical model described by Schmidt el al. [12] with a correction factor (α) in order to account for the probability of counting the same follicle several times. In addition, the model accounts for the actual thickness of the sections. Follicles were counted in every second section, and the area of the sections was measured in every fourth section on an Olympus BH-2 microscope with VIS version 4.6.1779 software (Visiopharm, Hoersholm, Denmark).
The follicles were classified as follows: primordial follicle, an oocyte surrounded by a single layer of flattened granulosa cells; primary follicle, single layer of cuboidal granulosa cells; and secondary follicle, more than one layer of cuboidal granulosa cells.
Eight of the biopsies from the women with DOR were cryopreserved and thawed prior to fixation and histological examination.
AMH
AMH serum levels were analyzed using the Elecsys AMH Plus assay (Roche Diagnostics GmbH, Mannheim, Germany) with a detection limit of 0.21 pmol/L (0.03 ng/mL). The intra- and inter-assay coefficients of variation were 0.5–1.4% and 0.7–1.9% [13]. In women with DOR, median AMH level was based on the AMH measurement taken before the surgery. One patient had a single AMH measurement of 5.2 pmol/L before the surgery; however, she was included as her previous AMH levels ranged from 3.4 to 3.9 pmol/L within 1 year prior to the surgery.
In the control group, a single AMH measurement was available in 35 out of the 100 women before surgery for cryopreservation. Ten of the 35 women had serum AMH levels analyzed by the ELISA assay (Immunotech; Beckman Coulter, Marseilles, France). These values have been multiplied with a conversion factor of 0.80 to be equivalent to the Elecsys AMH Plus assay values [14, 15].
Ovarian volume and antral follicle count
The patients with DOR had ovarian volume and number of antral follicles evaluated sonographically prior to laparoscopy at cycle days 2–5. In the control group, ovarian volume was estimated ex situ when the ovary was retrieved; antral follicle count was not evaluated. To be able to compare the sonographically estimated ovarian volume with the ex situ estimated ovarian reserve, the ovarian volume estimated in women with DOR was multiplied with 1.27 [16].
Gonadotropin receptor polymorphisms analysis
The 20 women with DOR were genotyped using DNA extracted from blood samples collected at study inclusion [9] to analyze for single nucleotide polymorphisms (SNPs). Based on literature implying an association between SNPs and ovarian function, three SNPs of interest were chosen (see Electronic Supplementary Material 1). The FSHR -29G>A and FSHR 307 polymorphism genotyping were done using the CADMA-based genotyping as previously published by Borgbo et al. [17]. Genotyping for the LHCGR N312S was performed by using the protocol by Lindgren et al. [18].
Statistical analyses
Clinical characteristics from the two cohorts are presented as median (range min–max) since variables were not symmetrically distributed with similar variance between the two groups. Hence, comparisons were performed using Wilcoxon rank sum test for non-parametric variables.
To analyze if the follicle density and age correlated, log10(follicle density + 1) was used as outcome to account for a right skewed distribution. An adjustment for whether the individual was a woman with DOR or from the control group allowed us to depict a fitted line for each group with an exponential development.
The correlation between the follicle density and AMH or ovarian volume was done using the same log10 transformation for follicle density with the regression analysis adjusted for age. Statistical tests of interaction between slopes for women with DOR and healthy controls were performed. By stepwise elimination, the best fitted model was determined.
AMH serum levels below the detection limit were given the value of the detection limit (= 0.21 pmol/L). The ggplot2 package was used for plotting the fitted models.
Comparison of the distribution of follicle classes (primordial, primary, and secondary) was done by Pearson’s X2 test.
To assess whether there was a correlation between the follicle density and genotypic variants of receptor polymorphisms, we used a Kruskal–Wallis test, since the residuals were not optimally distributed.
Statistical analyses were carried out using R version 3.4.4. P values of less than 0.05 were considered statistically significant.
Ethical approval
The patients recruited for the clinical study [9] all signed a written informed consent in which it was specified that one biopsy would be used for histological examination. The clinical study was approved by the Scientific Ethical Committee of the Capital Region of Denmark (H-15011975) and The Danish Data Protection Agency (2012-58-0004).
The control group was recruited from the research program on cryopreservation of ovarian tissue prior to treatment of malignant disease and was approved by the Ethical Committee of Copenhagen and Frederiksberg (H-2-2001-044). Retrospective follow-up was approved by the Danish patient safety authority (3-3013-2790/1).
Results
Clinical characteristics
Overall, infertile patients with DOR had a median age of 38.3 years (30.8–39.8 years), whereas the median age in the control group (n = 100) was 29.9 years (2.8–42.7 years). Table 1 shows that the median AMH level was 1.9 pmol/L (0.21–5.2 pmol/L) and 14.4 pmol/L (2.7–50.4 pmol/L) in patients with DOR and the control group (n = 35), respectively. Histological assessment showed that the median follicle density was 1.9 follicles/mm3 tissue (0–93.7 follicles/mm3) in patients with DOR compared with 16.3 follicles/mm3 tissue (0–1578 follicles/mm3) in the control group (n = 100) (Table 1). The median volume of histologically evaluated tissue was similar in patients with DOR and in the control group (5.6 mm3 vs. 6.9 mm3, p = 0.69).
Additionally, we did an analysis for three known receptor polymorphisms (FSHR G-29A, FSH 307, and LHCGR N312S) and found that the distribution of the genotypes in women with DOR was similar to the distribution in a control population based on previously published data (see Electronic Supplementary Material 1).
Follicle density and AMH
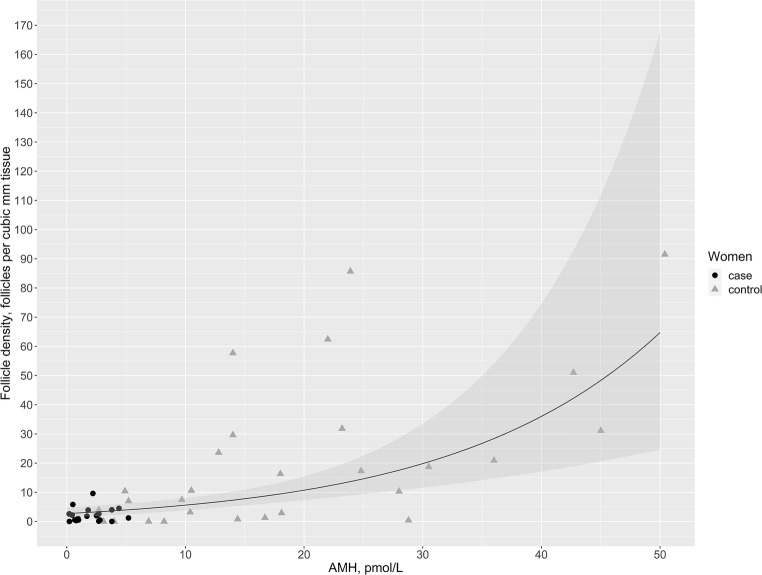
Based on the log-linear regression model adjusted for age in the study population where AMH measurements were available (DOR (n = 19) and controls (n = 31) > 25 years; in total n = 50), follicle density in the cortical tissue increased by 6% (95% CI 3.3–8.5%) for each single picomole per liter increase in AMH. Using this analysis, Fig. 2 shows the association between follicle density and serum AMH levels at age 35. There was no statistically significant difference between the trajectories representing patients with DOR and controls (p = 0.97). When the regression model was not adjusted for age, the follicle density increased by 8% (95% CI 5.5–11%) for each single picomole per liter increase in AMH.
Fig. 2.
The correlation between follicle density and anti-Müllerian hormone (AMH) serum levels in both cases and controls. The black line represents the best fitted line for the estimated follicle density according to serum levels of AMH. The gray area represents the 95% confidence interval. Follicle densities from the cases (women with diminished ovarian reserve) are shown with black dots while densities from the controls are shown with gray triangles
Table 2 shows the estimated follicle density in five age groups from 25 to 45 years in relation to AMH groups with serum levels from 0 to 50 pmol/L. It is seen that for equal AMH levels the density remains highly dependent on age. For example, in two women aged 25 and 35 years, but with similar AMH, e.g., 16–20 pmol/L, the follicle density declined around 7-fold from 63.0 to 9.5 follicles per mm3 tissue with increasing age.
Table 2.
Estimated follicle density depending on age and serum levels of anti-Mullerian hormone
| Follicle density (follicles per mm3 tissue) | |||||
|---|---|---|---|---|---|
| AMH (pmol/L) | 25 years | 30 years | 35 years | 40 years | 45 years |
| 0–5 | 25.3 | 9.6 | 3.3 | 0.7 | – |
| 6–0 | 35.0 | 13.6 | 4.9 | 1.4 | – |
| 11–15 | 47.0 | 18.4 | 6.9 | 2.2 | 0.3 |
| 16–20 | 63.0 | 24.9 | 9.5 | 3.2 | 0.7 |
| 21–25 | 84.3 | 33.5 | 13.0 | 4.7 | 1.3 |
| 26–30 | 112.6 | 45.0 | 17.6 | 6.5 | 2.0 |
| 31–35 | 150.5 | 60.3 | 23.8 | 9.0 | 3.1 |
| 36–40 | 200.9 | 80.7 | 32.1 | 12.4 | 4.4 |
| 41–45 | 268.0 | 107.9 | 43.1 | 16.9 | 6.2 |
| 46–50 | 357.6 | 144.2 | 57.8 | 22.8 | 8.6 |
AMH anti-Müllerian hormone
Age-paired comparison
To perform an age-paired comparison of follicle densities in women with DOR versus controls, we conducted a new control group that included the 19 women with the highest age from the 100 controls. No perfect age match was possible. The women with DOR were on average 2 years older than the attempted age-paired controls.
In this age-paired comparison, the median AMH in patients with DOR was 1.8 pmol/L vs. 14.4 pmol/L in the controls (p = 2.3 × 10−8) (Table 3). In the patients with DOR, the ratio of AMH/density was 1:1 since the follicle density was 1.8 follicles/mm3 (0–9.6 follicles/mm3). In the control group, the ratio of AMH/density was 2:1, since the follicle density was 7.0 follicles/mm3 (0–416.4 follicles/mm3). The difference between the follicle density medians was statistically significant (p = 0.04).
Table 3.
Clinical and histological comparison of women with DOR (cases) and age-paired controls
| Cases (n = 19) | Controls (n = 19) | p value | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 38.4 (30.8–39.8) | 36.3 (34.4–42.7) | 0.01 |
| AMH (pmol/L) | |||
| Median (range) | 1.8 (0.21–5.2*) | 14.4 (2.7–45.6) | < 0.0001 |
| Ovarian volume (mL) | |||
| Median (range) | 4.0 (2.3–10.2) | 6.4 (2.5–15.1) | 0.007 |
| Follicle density (follicle per mm3 tissue) | |||
| Median (range) | 1.8 (0–9.6) | 7.0 (0–416.4) | 0.04 |
| Follicle classification distribution | |||
| All follicles, n | 306 (100%) | 1978 (100%) | |
| Primordial, n | 266 (86.9%) | 1830 (92.5%) | |
| Primary, n | 26 (8.5%) | 120 (6.1%) | |
| Secondary, n | 14 (4.6%) | 28 (1.4%) | 0.0003 |
DOR diminished ovarian reserve, AMH anti-Müllerian hormone
*One patient with DOR had a single AMH level of 5.2 pmol/L before the laparoscopy
Table 3 also shows the distribution of cortical follicles. In patients with DOR, 86.9% of the follicles were primordial follicles, 8.5% were primary and 4.6% were secondary follicles. In the control group, the distribution was skewed towards more primordial follicles and fewer later stage follicles, as only 1.4% were secondary follicles while 92.5% follicles were primordial. The follicle developmental stage distribution in the two groups differed significantly (p = 0.0003).
Follicle density associated with age
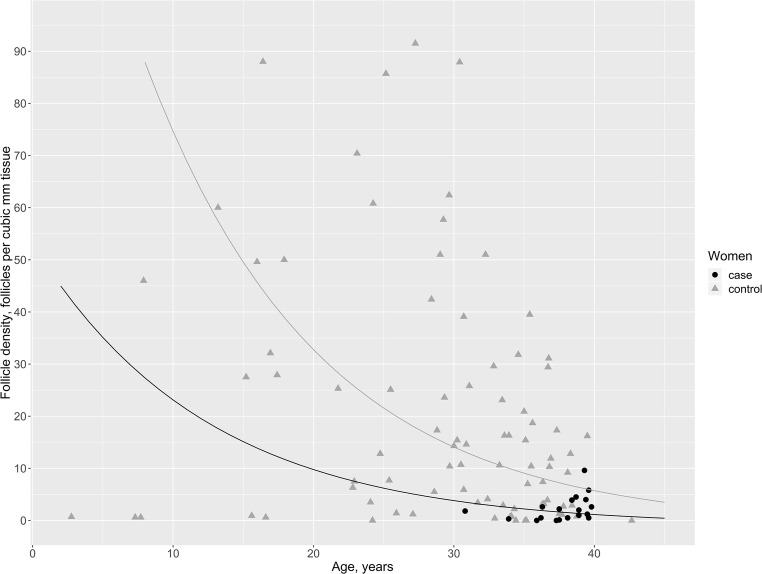
Figure 3 shows that the follicle density decreased with increasing age in an exponential manner. When the fit was anti-logged, the slope was a percentage decrease. The density decreased significantly by 7.7% (95% CI 4.8–10.6%, p = 1.07 × 10−6) for every year added. To find the best fit to our data, we compared three models: one in which we allowed the trajectory of women with DOR to have a unique slope (equation with an interaction segment), one where the trajectories were parallel but with individual intercepts (equation with an adjustment segment), and the last where the trajectories were overlapping (pooled data). These three comparisons revealed that the equation with an adjustment segment was the best fit, since the two groups had significantly different intercepts (p = 0.006). However, there was no difference in the slope of the decline in the number of follicles in the two groups (p = 0.2). The women with DOR had a follicle density that was 68% lower than the follicle density in the control group (95% CI 28–86%, p = 0.006). We did not adjust for AMH, since previous studies have shown that AMH correlates negatively with age until around 25 years and then shifts to a positive correlation [5, 10].
Fig. 3.
The correlation between follicle density and age. The black line represents the best fitted line for the estimated follicle density according to age in women with diminished ovarian reserve (cases) while the gray line represents the best fitted line for the estimated follicle density according to age in controls. The black dots represent the follicle densities in cases and the gray triangles represent the follicle densities in controls
Follicle density associated with ovarian volume
As seen in Table 1, the ovarian volume in patients with DOR was significantly lower than in the control group (3.96 mL vs. 5.87 mL, p = 0.01). For each milliliter increase in ovarian volume, the follicle density increased by 13% (95% CI 2–25.5%) when adjusted for age alone. When the model was adjusted for case/control, the effect of ovarian volume was no longer significant (p = 0.09).
Follicle density and genotyping in women with DOR
Based on the Kruskal–Wallis test, there was no association between the median follicle densities and the different genotypes of the three receptor polymorphisms in the 20 women with DOR (all three p values > 0.05; see Electronic Supplementary Material 1). However, comparison with a Student’s t test of the log-transformed density from the GG genotype with the AA genotype of the LHCGR N312S polymorphism showed that the mean follicle density was significantly higher in the AA genotype group (1.1 vs. 4.8 follicles per mm3 tissue, p = 0.01). This could suggest a poorer recruitment of follicles in the AA genotype group. In fact, corresponding with the literature, 5 out of 7 patients with DOR with the GG genotype became pregnant (Electronic Supplementary Material 1).
Discussion
Our data showed that patients diagnosed with DOR had a 4-fold lower follicle density (1.8 vs. 7.0 follicles per mm3 tissue) but a median AMH concentration 8-fold lower than the level in the age-paired control group (1.8 vs. 14.4 pmol/L). Therefore, infertile women diagnosed with DOR by low AMH levels may have relatively more follicles in their cortex than reflected by their AMH levels. In this study, all follicles in the cortex biopsies were counted, thereby including the pool of follicles that remains in the ovaries after menopause, which may never start to grow [19]. This may explain the discrepancy between follicle density and AMH in women with DOR. The lack of a direct association between AMH and follicle density may also be related to the fact that women with few follicles in their ovaries also have fewer follicles en route towards maturation and ovulation per cycle than the average woman [20]. These follicles are exactly those contributing with 60% of the circulating AMH [2]. However, we cannot rule out that the discrepancy in some way could be due to undeliberated inclusion of degenerated follicles in the density estimation, since only collapsed follicle structures were excluded from the counting.
By pooling data on cases and controls with available AMH measurements (> 25 years), we found that AMH and the follicle density correlated positively (Fig. 2). Based on a regression analysis adjusted for age the follicle density increased by 6% for each picomole per liter, the concentration of AMH increased; this correlation did not differ between cases and controls. This positive association is in line with results from the few other studies investigating histologically counted follicles and AMH serum levels [5–8]. Kelsey et al. found that the recruitment rate of remaining follicles correlated with both AMH levels and the size of the residual pool. They suggested that a low rate of follicle recruitment corresponded to low AMH levels [5]. In agreement, we found that the best fit to our data was exponential, giving a slope of percentage, suggesting that the follicle depletion decreases with decreasing AMH levels. This stands in contrast with the earlier hypothesis that follicle depletion accelerates with advanced age [19].
Nevertheless, follicle density and AMH intervals stratified by age showed that in women of a similar AMH, age remains an important indicator of cortical follicle density (Table 2). As exemplified here, a 25-year-old woman with an AMH of 16–20 pmol/L is estimated to have a follicle density of 63 per mm3 tissue, whereas a 10-year older woman of 35 years with a similar AMH of 16–20 pmol/L is estimated to have 9.5 follicles per mm3 tissue—this is almost a 7-fold reduction. This illustrates the limitations of AMH as a sole marker of the ovarian reserve, that is, the density of follicles in the ovarian cortex. Underpinning this, Hansen et al. found that the effect of age on the follicle density was greater than the effect of AMH and Wallace et al. found that 81% of the variation in the number of remaining follicles was due to age [7, 20].
The infertile women with DOR also differed from the women in the control group by a different distribution of primordial, primary, and secondary follicles. In patients with DOR, developing follicles (primary and secondary) constituted around 13% of the counted follicles while they made up only 7.5% in the control group. These findings may suggest that patients with a low AMH concentration (DOR) recruit a relatively larger proportion of their primordial follicles for growth in terms of percentage, however, not in absolute numbers. This is likely due to higher FSH levels and reduced atresia rates, which could be a physiological mechanism to maintain the ovarian function. Indeed, this corresponds to the increased proportion of larger antral follicles concomitantly with decreased AFC and AMH concentration observed clinically by ultrasound, as previously found in women of advanced age [21].
It is an important question whether women with idiopathic diminished ovarian reserve constitute a different class of patient with, perhaps, different kinetics of follicle loss or are they just at the end of the normal spectrum of follicle numbers. Whether early menopause, defined as prior to the average age of 51 years at menopause, is due to an accelerated depletion of the ovarian reserve or a congenitally lower ovarian reserve is debated [22]. Our study could not determine the true rate of follicle depletion. However, based on our log-linear regression model of age and follicle density, we cautiously propose that women with DOR have a congenitally lower ovarian reserve than do reproductively healthy women, as we saw two parallel curves with similar slopes but different intercepts for the follicle density as a function of age (Fig. 2). Thus, a woman with DOR has a smaller remaining follicle pool compared with an age-paired reproductively healthy woman, suggesting that ovaries of women with DOR are physiologically older than the chronological age, at least in relation to follicle quantity. Interestingly, previous studies have described a reproductive range with fixed timepoints, in which subfertility starts about 10 years before end of fertility, which again occurs 10 years before menopause, regardless of menopausal age [23, 24]. When taking only this three-stage reproductive timeframe (i.e., subfertility–infertility–menopause) into consideration, women diagnosed with DOR are thought to have a poor ovarian reserve quantitatively as well as qualitatively in terms of fertility potential. Fortunately for these women recent studies imply that the oocyte itself is comparable to age-paired controls in relation to fertilization rates as well as live birth rates [25], reviewed in [26]. In addition, a new epidemiological study reporting pregnancies 5 and 10 years prior to menopause according to age at menopause found that 24% of the women with menopause before the age of 45 years gave birth within 5 years before menopause [27]. This reinforces the perception that the three-stage reproductive timeframe is not applicable for all women. Even in relation to perinatal outcomes, chronological age was found to overrule the physiologically age of the ovaries [28].
We also investigated whether the clinical and histological characteristics of the women with DOR could be explained by gonadotrophin receptor polymorphisms (FSHR G-29A, FSHR 307, and LHCGR N312S). This was not the case. Even the patient defined as an outlier had receptor SNPs similar to the rest of the women with DOR. One could speculate if the markedly high follicle density with accumulation of residual follicles could be due to a dysfunction in some of the pathways regulating activation of primordial follicles, e.g., the EIF2 signaling or the mTOR signaling [29]. Lastly, we found no differences in the distribution of genotypes in the SNPs compared with controls from the literature nor did we find a correlation between the genotypes and the follicle density in women with DOR.
Our study adds pertinent information to the relatively scanty knowledge of the true ovarian reserve and its correlation to AMH levels. Especially, in women diagnosed with DOR, where the true ovarian reserve is particularly important, this relationship has not been investigated before. However, this study has some limitations. The use of a random ovarian biopsy as a proxy for the whole ovary has been questioned. In fact, studies have shown that ovarian follicles are unevenly distributed in the cortex and seems to be organized in clusters [12]. The randomness of a single biopsy’s follicle load may explain some of the variation observed in the follicle densities. Nevertheless, it is still the best feasible tool to estimate the true ovarian reserve. In addition, with the new and more sensitive AMH assays, it has been suggested that AMH has intra- and inter-cyclic variations [30, 31] which may well play a small part in the variability in follicle density within the same AMH serum level, especially in our control group where AMH was taken on a random cycle day and a few of the concentrations analyzed with an older AMH assay, although a correlation factor was used. Finally, women in the control group had various indications for cryopreservation of their ovarian tissue. We cannot rule out that these diagnoses may have influenced their ovarian reserve and follicular growth pattern.
In conclusion, we found that AMH serum levels and follicle density correlated well and that women with DOR had a correspondingly low follicle density. Thus, we confirm that AMH is an indirect marker for the ovarian reserve. Nevertheless, this should be translated cautiously, and the woman’s age should be included in the estimation of the true ovarian reserve.
Acknowledgments
The authors would like to thank all personnel from the clinical service in fertility preservation.
Funding information
This study is part of ReproUnion collaborative study, co-financed by the European Union, Interreg V ÖKS (grant number 20200407).
Compliance with ethical standards
The patients recruited for the clinical study [9] all signed a written informed consent in which it was specified that one biopsy would be used for histological examination. The clinical study was approved by the Scientific Ethical Committee of the Capital Region of Denmark (H-15011975) and The Danish Data Protection Agency (2012-58-0004).
The control group was recruited from the research program on cryopreservation of ovarian tissue prior to treatment of malignant disease and was approved by the Ethical Committee of Copenhagen and Frederiksberg (H-2-2001-044). Retrospective follow-up was approved by the Danish patient safety authority (3-3013-2790/1).
Disclaimer
The funders had no role in the study design, data collection and interpretation, or decision to submit the work for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35:17–23. doi: 10.1007/s10815-017-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeppesen J. V., Anderson R. A., Kelsey T. W., Christiansen S. L., Kristensen S. G., Jayaprakasan K., Raine-Fenning N., Campbell B. K., Yding Andersen C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Molecular Human Reproduction. 2013;19(8):519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 3.Depmann Martine, Broer Simone L., van der Schouw Yvonne T., Tehrani Fahimeh R., Eijkemans Marinus J., Mol Ben W., Broekmans Frank J. Can we predict age at natural menopause using ovarian reserve tests or motherʼs age at menopause? A systematic literature review. Menopause. 2016;23(2):224–232. doi: 10.1097/GME.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 4.Depmann M., Faddy M. J., van der Schouw Y. T., Peeters P. H. M., Broer S. L., Kelsey T. W., Nelson S. M., Broekmans F. J. M. The Relationship Between Variation in Size of the Primordial Follicle Pool and Age at Natural Menopause. The Journal of Clinical Endocrinology & Metabolism. 2015;100(6):E845–E851. doi: 10.1210/jc.2015-1298. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WHB. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 6.Garavaglia E, Sala C, Taccagni G, Traglia M, Barbieri C, Ferrari S, et al. Fertility preservation in endometriosis patients: anti-Müllerian hormone is a reliable marker of the ovarian follicle density. Front Surg [Internet] 2017;4:1–6. doi: 10.3389/fsurg.2017.00040/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen Karl R., Hodnett George M., Knowlton Nicholas, Craig LaTasha B. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertility and Sterility. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Sermondade N, Sonigo C, Sifer C, Valtat S, Ziol M, Eustache F, et al. Serum antimullerian hormone is associated with the number of oocytes matured in vitro and with primordial follicle density in candidates for fertility preservation. Fertil Steril [Internet] 2019;111:357–362. doi: 10.1016/j.fertnstert.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Lunding Stine Aagaard, Pors Susanne Elisabeth, Kristensen Stine Gry, Landersoe Selma Kloeve, Jeppesen Janni Vikkelsø, Flachs Esben Meulengracht, Pinborg Anja, Macklon Kirsten Tryde, Pedersen Anette Tønnes, Andersen Claus Yding, Andersen Anders Nyboe. Biopsying, fragmentation and autotransplantation of fresh ovarian cortical tissue in infertile women with diminished ovarian reserve. Human Reproduction. 2019;34(10):1924–1936. doi: 10.1093/humrep/dez152. [DOI] [PubMed] [Google Scholar]
- 10.Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients J Clin Endocrinol Metab [Internet]. 2010 [cited 2014 Mar 10];95:5003–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20719830 [DOI] [PubMed]
- 11.Kelsey Thomas W., Dodwell Sarah K., Wilkinson A. Graham, Greve Tine, Andersen Claus Y., Anderson Richard A., Wallace W. Hamish B. Ovarian Volume throughout Life: A Validated Normative Model. PLoS ONE. 2013;8(9):e71465. doi: 10.1371/journal.pone.0071465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt KLT, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod [Internet]. 2003 [cited 2016 Mar 9];18:1158–64. Available from: http://humrep.oxfordjournals.org.ep.fjernadgang.kb.dk/content/18/6/1158 [DOI] [PubMed]
- 13.Li HWR, Wong BPC, Ip WK, Yeung WSB, Ho PC, Ng EHY. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod [Internet] 2016;31:2796–2802. doi: 10.1093/humrep/dew248. [DOI] [PubMed] [Google Scholar]
- 14.Tadros T, Tarasconi B, Nassar J, Benhaim JL, Taieb J, Fanchin R. New automated antimüllerian hormone assays are more reliable than the manual assay in patients with reduced antral follicle count. Fertil Steril. 2016;106:1800–1806. doi: 10.1016/j.fertnstert.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Friis Petersen J, Løkkegaard E, Andersen LF, Torp K, Egeberg A, Hedegaard L, et al. A randomized controlled trial of AMH-based individualized FSH dosing in a GnRH antagonist protocol for IVF. Hum Reprod Open. 2019;2019:1–11. doi: 10.1093/hropen/hoz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosendahl M, Ernst E, Rasmussen PE, Andersen CY. True ovarian volume is underestimated by two-dimensional transvaginal ultrasound measurement. Fertil Steril [Internet] Elsevier Ltd. 2010;93:995–998. doi: 10.1016/j.fertnstert.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Borgbo T, Sommer Kristensen L, Lindgren I, Yding Andersen C, Hansen LL. Genotyping common FSHR polymorphisms based on competitive amplification of differentially melting amplicons (CADMA) J Assist Reprod Genet. 2014;31:1427–1436. doi: 10.1007/s10815-014-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindgren I, Baath M, Uvebrant K, Dejmek A, Kjaer L, Henic E, et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod. 2016;31:672–683. doi: 10.1093/humrep/dev342. [DOI] [PubMed] [Google Scholar]
- 19.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 20.Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:1–9. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-Müllerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98:1602–1611. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 22.Richardson MC, Guo M, Fauser BCJM, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20:353–369. doi: 10.1093/humupd/dmt057. [DOI] [PubMed] [Google Scholar]
- 23.te Velde E, Scheffer G, Dorland M, Broekmans F, Fauser BCJ. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol [Internet] 1998;145:67–73. doi: 10.1016/S0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 24.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 25.Abrahami N, Izhaki I, Younis JS. Do young women with unexplained infertility show manifestations of decreased ovarian reserve ? J Assist Reprod Genet. J Assist Reprod Genet. 2019;36:1143–1152. doi: 10.1007/s10815-019-01467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ata B, Seyhan A, Seli E. Diminished ovarian reserve versus ovarian aging : overlaps and differences. Curr Opin Obstet Gynecol. 2019. [DOI] [PubMed]
- 27.Gottschalk Marthe Sørli, Eskild Anne, Tanbo Tom Gunnar, Bjelland Elisabeth Krefting. Childbirth close to natural menopause: does age at menopause matter? Reproductive BioMedicine Online. 2019;39(1):169–175. doi: 10.1016/j.rbmo.2019.03.209. [DOI] [PubMed] [Google Scholar]
- 28.Richardson A, Mascarenhas M, Balen A. Is a woman’s chronological age or ‘ovarian age’ more important in determining perinatal outcome after assisted reproductive treatment ? Hum Fertil [Internet] Taylor & Francis; 2019;0:1–7. Available from: 10.1080/14647273.2019.1597987 [DOI] [PubMed]
- 29.Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L, et al. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation Hum Reprod [Internet]. 2017 [cited 2018 Feb 22];32:1684–700. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28854595 [DOI] [PubMed]
- 30.Bungum L, Tagevi J, Jokubkiene L, Bungum M, Giwercman A, Macklon N, et al. The impact of the biological variability or assay performance on AMH measurements: a prospective cohort study with AMH tested on three analytical assay-platforms. Front Endocrinol (Lausanne) 2018;9:1–10. doi: 10.3389/fendo.2018.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melado L, Lawrenz B, Sibal J, Abu E, Coughlan C, Navarro AT, et al. Anti-müllerian hormone during natural cycle presents significant intra and intercycle variations when measured with fully automated assay. Front Endocrinol (Lausanne) 2018;9:1–8. doi: 10.3389/fendo.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]