Abstract
The present study was aimed to get an insight into the bacterial biota of ready-to-eat small crickets (Acheta domesticus) already marketed in the European Union. 16S rRNA gene of the DNAs extracted from thirty-two samples of ready-to-eat crickets commercialized by 4 European Union producers located in Austria, Belgium, France and the Netherlands (2 batches per producer) was analyzed by Polymerase Chain Reaction–Denaturing Gradient Gel Electrophoresis (PCR–DGGE). The species belonging to the genera Hespellia, Ruminococcus and Clostridium were detected in samples from Austria, while those from genera Lysobacter, Staphylococcus and Clostridium were detected in samples from Belgium. Moreover, samples from France were characterized by Staphylococcus, Pseudomonas, and Hydrogenophilus genera. Finally, the genera Staphylococcus, Hydrogenophilus, Clostridium and Ruminococcus were identified in the samples produced in the Netherlands. When insects are intended for commercialization, rearing, processing and handling could affect the presence of the occurring microbial species. Hence, to assure a safe product, the need for a full standardization of production technologies, including feed supply as well as rearing and processing practices, is recommended.
Keywords: Edible insects, Crickets, PCR–DGGE, Batches
Edible insects are a novel source of high value proteins [1]. Notwithstanding, their introduction in the European food market has only recently been authorized with Regulation (EU) No. 2015/2283. To assure health protection and disease prevention, in 2015 the European Food Safety Authority (EFSA) delivered a Scientific Opinion on the risk profile associated with the use of insects as food for humans and animals [2]. The opinion also highlighted the lack of scientific studies on the potential chemical and microbiological hazards in edible insects whether they are used as food or feed. Our study was aimed to get an insight into the bacterial biota of ready-to-eat small crickets (Acheta domesticus) already marketed in the European Union (EU). DNAs were extracted from 32 samples of ready-to-eat crickets commercialized by 4 European Union producers (2 batches per producer) and 16S rRNA genes were subsequently analyzed by Polymerase Chain Reaction–Denaturing Gradient Gel Electrophoresis (PCR–DGGE). In more detail, (boiled and dried) ready-to-eat crickets were purchased from producers each located in a European country, being Austria, Belgium, France and the Netherlands. From each producer, 2 different batches of the crickets were bought in October 2017 (Batch 1) and April 2018 (Batch 2), respectively, each including 4 samples (replicates). EU countries for collection of the crickets were selected among those that are most active in insect rearing, counting the highest number of start-ups and companies producing insects for human consumption.
Bacterial DNA extraction was performed as previously described by Osimani et al. [3]. Equal portions of DNA extracts (standardized to 25 ng/μL) obtained from the 4 cricket samples derived from the same producer and production batch were pooled together and vortexed vigorously. Eight final pooled DNA samples were obtained and labeled as follows: AU-B1 and AU-B2 (DNA pools of the samples from the two batches from Austria); BL-B1 and BL-B2 (DNA pools of the samples from the two batches from Belgium); FR-B1 and FR-B2 (DNA pools of the samples from the two batches from France); NL-B1 and NL-B2 (DNA pools of the samples from the two batches from the Netherlands). PCR–DGGE analysis was performed following the protocol reported by Osimani et al. [3].
The PCR products were then sequenced and identified at the genus or species level. In more detail, the obtained sequences were analyzed by BLAST (Basic Local Alignment Search Tool), available from NCBI (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov). The sequences were submitted to the NCBI GeneBank under the accession numbers MH891553–MH891537.
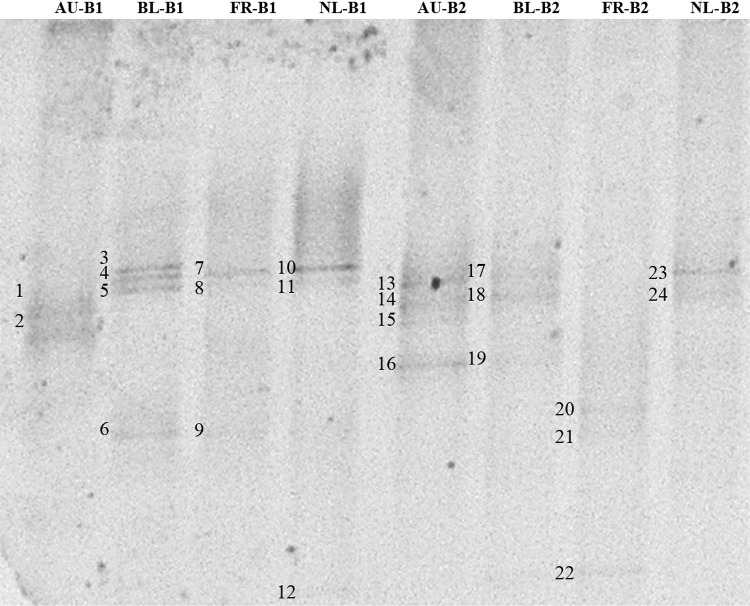
PCR–DGGE has been extensively applied in food and environmental microbiology representing a valid molecular tool for the exploration of the food microbial communities [4]. The results of PCR–DGGE analysis are shown in Table 1 and Fig. 1.
Table 1.
The results of the sequencing of the bands excised from the DGGE gel
| Sample | Banda | Closest relative | % Identityb | Acc. noc | Acc. nod |
|---|---|---|---|---|---|
| AU B1 | 1 | Hespellia porcina | 100 | NR_025206 | MH891537 |
| 2 | Hespellia porcina | 96 | NR_025206 | MH891538 | |
| BL B1 | 3 | Staphylococcus capitis | 99 | MH158289 | MH891539 |
| 4 | Staphylococcus sp. | 99 | MF948900 | MH891542 | |
| 5 | Staphylococcus capitis | 99 | MH158289 | MH891540 | |
| 6 | Lysobacter sp. | 98 | MG198704 | MH891541 | |
| FR B1 | 7 | Staphylococcus sp. | 99 | MF948900 | MH891542 |
| 8 | Failed | – | – | – | |
| 9 | Pseudomonas sp. | 100 | HM626451 | MH891543 | |
| NL B1 | 10 | Staphylococcus sp. | 97 | MH817399 | MH891544 |
| 11 | Failed | – | – | – | |
| 12 | Hydrogenophilus hirschii | 100 | NR_104788 | MH891545 | |
| AU B2 | 13 | Ruminococcus gauvreauii | 100 | NR_044265 | MH891546 |
| 14 | Clostridium sp. | 97 | AF443594 | MH891547 | |
| 15 | Failed | – | – | – | |
| 16 | Clostridium sp. | 95 | AF443594 | MH891548 | |
| BL B2 | 17 | Clostridium sp. | 97 | LC341577 | n.d. |
| 18 | Clostridium sp. | 97 | AF443594 | MH891549 | |
| 19 | Failed | – | – | – | |
| FR B2 | 20 | Failed | – | – | – |
| 21 | Pseudomonas sp. | 100 | HM626451 | MH891550 | |
| 22 | Hydrogenophilus hirschii | 99 | MF595849 | MH891551 | |
| NL B2 | 23 | Ruminococcus gauvreauii | 98 | NR_044265 | MH891552 |
| 24 | Clostridium sp. | 98 | AF443594 | MH891553 |
n.d., sequences not deposited in the GenBank database (< 200 bp); AU, Austria; BL, Belgium; FR, France, NL, the Netherlands; B1, Batch 1; B2, Batch 2
aBands are numbered as specified in Fig. 1
bPercent of similarity between the sequences obtained from the PCR–DGGE analysis and the sequences deposited in the GenBank database
cAccession number of the sequences found by a BLAST search
dGenBank accession number of the deposited sequences
Fig. 1.
DGGE profiles of the bacterial DNA obtained from insect samples and amplified with primer pair U968GC-L1401R. The labeled DGGE bands were sequenced. The identification results are reported in Table 1. AU, Austria; BL, Belgium; FR, France; NL, the Netherlands; B1, Batch 1; B2, Batch 2
Samples from Austria were found to be most closely related to Hespellia (in pooled samples from batch 1), Ruminococcus and Clostridium (both in pooled samples from batch 2), with a sequence identity from 97 to 100%. By contrast, the samples from Belgium were found to be most closely related to Lysobacter, Staphylococcus (both in pooled samples from batch 1) and Clostridium (in pooled samples from batch 2), with sequence identities between 97 and 99%.
Moreover, pooled samples from Batch 1 of the crickets produced in France were found to be most closely related to Staphylococcus and Pseudomonas, whereas pooled samples from Batch 2 were characterized by Pseudomonas and Hydrogenophilus, with sequence identities from 97 to 100%.
Finally, regarding the pooled samples produced in the Netherlands, batch 1 was characterized by the presence of the Staphylococcus and Hydrogenophilus, whereas batch 2 by the presence of the Clostridium and Ruminococcus, all with a sequence identities between 97 and 100%.
Regarding the species detected in the pooled samples, some considerations can be made. Clostridium was detected in the Austrian, Belgian and the Dutch samples. This spore forming bacterial genus has previously been reported in ready-to-eat small crickets and cricket powder analyzed by both PCR–DGGE and metagenomic sequencing, as well as in other edible insects such as giant water bugs, mealworms, desert locusts, and black soldier flies [1, 5, 6]. In foodstuffs, pathogenic Clostridium species like Clostridium botulinum, Clostridium difficile and Clostridium perfringens, represent a health threat for consumers because they are able to resist heat treatments and produce toxins [7].
The detection of Staphylococcus at both the species and genus levels in the batch 1 pooled samples from Belgium, France and the Netherlands agrees well with its previous detection in a number of edible insects including cockroaches, ants, rhino beetles, beetles, butterflies, silkworms, mealworms and flies [1]. The genus Staphylococcus encompasses both saprophytic species and species with pathogenic potential. Pseudomonas was detected only in the pooled samples from France. To date, this bacterial genus has been found in cricket-based products through metagenomic sequencing, as well as in different insect species, such as grasshoppers, cockroaches, flies, rhino beetles, lygaeid bugs, butterflies, and mealworms [1]. Interestingly, Osimani et al. [3] has recently detected the DNA of Pseudomonas aeruginosa in the frass of laboratory-reared mealworms, thus suggesting the possible occurrence of this pathogenic species in edible insects.
Concerning Hespellia porcina, to the authors’ knowledge, this is its first detection of this organism in insects and more specifically, in crickets produced in Austria. First isolated from swine manure storage pits, it has been more recently detected in the cecal microbiota of chicken as a commensal microorganism, suggesting the possible occurrence of this microorganism in the insect gut [8, 9]. No reports on the occurrence of H. porcina in foodstuff are currently available in the scientific literature for a further comparison of the data.
To the authors’ knowledge, the presence of Ruminococcus gauvreauii, detected in both the pooled samples from Austria (batch 2) and the Netherlands (batch 2), has never been reported in edible insects, although Garofalo et al. [4] highlighted the presence of Ruminococcaceae in samples of ready-to-eat small crickets and cricket powder by using Next Generation Sequencing (NGS) [4]. Very recently, the detection of Ruminococcus in the gut microbiota of chicken that were fed edible insect-based feed was also observed [10].
It is noteworthy that, for biological threats, the specific production procedures, the type of the substrate, the harvest period, the type of the insect and their developmental phase could exert an impact on the occurrence of microbial species. Furthermore, the vertical transmission of the microorganisms can be due to the evacuation of the bacteria during egg laying or by the contamination of the egg with symbionts defaecated from the mother’s gut to offspring, thus affecting the microbial community of the insects [3]. When insects are intended for commercialization, further processing could also alter the presence of the occurring microbial species. Hence, to assure a safe product, the need for a full standardization of production technologies, including feed supply as well as rearing and processing practices, is recommended.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lucia Aquilanti, Email: l.aquilanti@univpm.it.
Andrea Osimani, Email: a.osimani@univpm.it.
References
- 1.Garofalo C, Milanović V, Cardinali F, Aquilanti L, Clementi F, Osimani A. Current knowledge on the microbiota of edible insects intended for human consumption: a state-of-the-art review. Food Res Int. 2019 doi: 10.1016/j.foodres.2019.108527. [DOI] [PubMed] [Google Scholar]
- 2.European Food Safety Authority (EFSA) Scientific Committee Scientific opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015;13:4257. doi: 10.2903/j.efsa.2015.4257. [DOI] [Google Scholar]
- 3.Osimani A, Milanović V, Cardinali F, Garofalo C, Clementi F, Pasquini M, Riolo P, Ruschioni S, Isidoro N, Loreto N, Franciosi E, Tuohy K, Petruzzelli A, Foglini M, Gabucci C, Tonucci F, Aquilanti L. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): from feed to frass. Int J Food Microbiol. 2018;272:49–60. doi: 10.1016/j.ijfoodmicro.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Garofalo C, Bancalari E, Milanović V, Cardinali F, Osimani A, Sardaro MLS, Bottari B, Bernini V, Aquilanti L, Clementi F, Neviani E, Gatti M. Study of the bacterial diversity of foods: PCR-DGGE versus LH-PCR. Int J Food Microbiol. 2017;242:24–36. doi: 10.1016/j.ijfoodmicro.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Schlüter O, Rumpold B, Holzhauser T, Roth A, Vogel RF, Quasigroch W, Vogel S, Heinz V, Jäger H, Bandick N, Kulling S, Knorr D, Steinberg P, Engel KH. Safety aspects of the production of foods and food ingredients from insects. Mol Nutr Food Res. 2017;61:1600520. doi: 10.1002/mnfr.201600520. [DOI] [PubMed] [Google Scholar]
- 6.Wynants E, Crauwels S, Verreth C, Gianotten N, Lievens B, Claes J, Van Campenhout L. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiol. 2018;70:181–191. doi: 10.1016/j.fm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Popoff MR. Clostridium difficile and Clostridium sordellii toxins, proinflammatory versus anti-inflammatory response. Toxicon. 2017 doi: 10.1016/j.toxicon.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead TR, Cotta MA, Collins MD, Lawson PA. Hespellia stercorisuis gen. nov., sp. nov. and Hespellia porcina sp. nov., isolated from swine manure storage pits. Int J Syst Evol Microbiol. 2004;54:241–245. doi: 10.1099/ijs.0.02719-0. [DOI] [PubMed] [Google Scholar]
- 9.Stanley D, Geier MS, Denman SE, Haring VR, Crowley TM, Hughes RJ, Moore RJ. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Biasato I, Ferrocino I, Biasibetti E, Grego E, Schiavone A, Gasco L, Gai F, Cocolin LS, Capucchio MT. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. J Comp Path. 2018;158:93–149. doi: 10.1186/s12917-018-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]