Abstract
Purpose
In this review, the current knowledge on anti-Müllerian hormone (AMH) is presented, concerning its value in disease and IVF treatment as well as in terms of its prospective clinical use.
Methods
AMH is becoming the most appropriate biomarker for the ovarian reserve measured predominantly for assisted reproductive treatment (ART) patients in comparison to the currently used antral follicle count (AFC). However, this is not the only way AMH measurements can be used in the clinics. Because of this, we reviewed the current literature for the use of AMH in current or prospective clinical practice.
Results
We found that AMH has a high predictive value in assessing the ovarian reserve, which can lead to a better efficiency of in vitro fertilization (IVF) procedures. It has a high potential to be developed as a staple diagnostic marker of ovarian disease, especially for ovarian cancers and even as a possible treatment tool for certain cancers. It could potentially be used to prevent oocyte loss due to chemo- or radiotherapy.
Conclusion
AMH is an important hormone especially in women reproductive organs and is currently seen as the best biomarker for a multitude of uses in reproductive medicine. Currently, the biggest issue lies in the lack of international standardization of AMH. However, it is encouraging to see that there is interest in AMH in the form of research on its action and use in reproductive medicine.
Keywords: Anti-Müllerian hormone, Assisted reproductive technology, Fertility, Hormone receptor, In vitro fertilization, Oocyte
Introduction
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein belonging to the transforming growth factor β (TGF-β) superfamily, even though there is only one domain that is homologous to other members of TGF-β superfamily—it is the 3′ part of the fifth exon which encodes the bioactive part of the AMH molecule (a GC rich region) [1]. Among the many other TGF-β family proteins are activins, inhibins, bone morphogenic proteins (BMPs), and growth and differentiation factors (GDFs) [2]. AMH was discovered in the 1940s by Alfred Jost, who described the role of the hormone in the differentiation of gender in the embryo [3]. It has also been proven that it has a strong influence on the function of ovaries, especially on the growth of follicles [4, 5]. This discovery opened a completely new spectrum of AMH use in gynaecology, from in vitro fertilization (IVF) to diagnostics of different ovarian diseases and cancers as well as its future use.
The basics of AMH expression and action
AMH gene
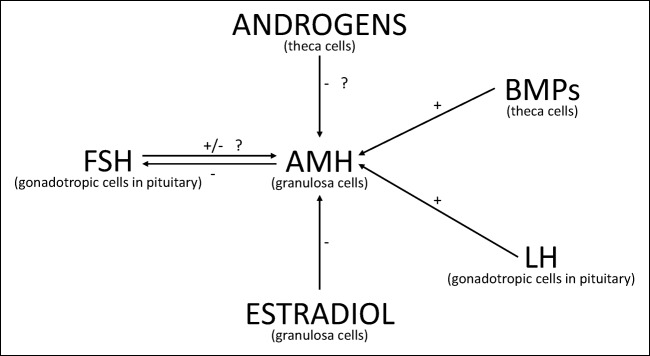
The AMH gene was discovered and sequenced 32 years ago [6] and resides on the short arm of chromosome 19, coded between the regions p13.2 and p13.3. It is divided among five exons with 275 base pairs (bp) [7]. It has a molecular weight of approximately 140 kDa. The expression of the AMH gene is transcribed by a 180-bp sequence which is contiguous to the protein Sap62. It has three binding sites for the transcription—one is a conserved motif of 20 bp that binds the orphan nuclear receptor SF-1, the other is 50 bp upstream from the SF-1 binding site which promotes the binding of the SOX9, a high-mobility group protein. The last binding site can be found downstream of the SF-1 binding site, which binds the GATA-4 from the GATA family of transcription factors. The transcription of AMH starts with the action of SOX9 on the HMG box. The transcription is then upregulated when SF-1 is bound to the promoter and if it comes to the interaction of the SOX9, WT1, and GATA-4 [1]. It has been observed that BMPs from ovarian theca cells and LH from gonadotropic endocrine (Fig. 1) cells from anterior pituitary upregulate AMH expression [8], while FSH and estradiol may downregulate the AMH gene and expression of anti-Müllerian hormone receptor II (AMHRII) in adult rat ovaries [9], although it is still not entirely clear, if FSH has a positive or negative effect on AMH expression [8]. There are three factors that regulate the expression of AMH gene by testicular Sertoli cells—androgens (in their absence the level of serum AMH stays high), gonadotropins (they increase the expression of AMH gene in the absence of androgens), and the maturation of germ cells (when they enter the process of meiosis, the AMH gene expression stops) [1]. Promoter of the AMH gene does not include any androgene and cyclic AMH response elements. That means that androgens and FSH act on the AMH promoter indirectly in two possible ways. The first one is a cooperation between cell-specific factors, which bind to the AMH promoter. The second option is binding to the protein, which works as a coactivator or corepressor for transcription of AMH [10, 11]. The result of the AMH gene expression is a precursor protein, which is then cleaved by a proteolytic enzyme to form the N- and C-terminus. The N- terminus end is important for the folding of the AMH protein, forming disulfide bonds and the secretion of AMH, while the C-terminus is essential for its biological activity and binding to AMH receptors [12].
Fig. 1.
The effect of other hormones and proteins on expression of AMH in the ovarian granulosa cells (GC). It has been proven that BMP9 and LH have a positive effect on expression of AMH in GC cells, as well as that estradiol has an adverse effect on the expression of AMH at the stage of antral follicle development. However, the direct effect of androgens on AMH expression is not known. There are conflicting results on effect of FSH on AMH and it is not concluded, whether FSH has a positive or negative effect on AMH expression [8]
AMH receptors
The signal pathway of AMH is controlled by two heteromeric serine/threonine kinase transmembrane receptors. The receptors are divided into type I and type II [13]. Free AMH can bind to the AMHRII, which in turn phosphorylates the anti-Müllerian hormone receptor I (AMHRI). This phosphorylation results in a downstream signalling through the activation of the cytoplasmic Smad proteins. AMH activates the signal transduction of the Smad1 protein but not the Smad2 [1]. When the phosphorylated Smad proteins are translocated into the nucleus, they can activate or inhibit the transcription of specific genes [1]. AMH is related to the bone morphogenetic protein—like pathway for its signal transduction [2]. The AMH type II receptor is the only type II receptor for the AMH hormone. It becomes to be expressed in the ovary immediately after birth and continues to be expressed in it throughout life [14]. The AMHRII receptor is present in the Sertoli and Leydig cells in the testes and on the theca and granulosa cells in the ovaries [15], as well as in the prostate [16], endometrium [17], and in the ductal epithelium of the mammary gland [18]. The receptor AMHRII has been also found in several cancer cell lines such as cervical, endometrial, ovarian epithelium, and breast [7, 13]. The gene for the receptor AMHRII was discovered in 1995 and can be found on chromosome 12, divided among 8 exons with 8000 bp. Many of the AMH-target organs express AMHRII, for example, the Müllerian ducts and the gonads. In mice and rats, the AMH is localized in the mesenchyme around the Müllerian ducts and the urogenital ridge in both males and females [13]. The loss of function of AMHRII (gene mutation) or even just the AMH ligand causes the persistent Müllerian duct syndrome in humans. This syndrome causes normal male reproductive organ development; however, with the non-activity of AMHRII receptor, the Müllerian ducts are not broken down which lead to the additional development of the uterus and fallopian tubes of affected males.
In comparison to AMHRII, the AMHRI receptor is still largely unknown, primarily because of less extensive studies on it in comparison to the AMHRII, especially in the gonads. There are three AMHRI receptors that have been studied excessively—the Alk2, Alk3, and Alk6 (activin receptor-like protein kinases). Alk2 and Alk6 mediate the AMH action on other cells [19, 20], while the Alk3 mediates the action of AMH on the Müllerian ducts [21].
Mutations on AMH and AMHRII genes can lead to a multitude of illnesses of the reproductive tract in women. Yoshida et al. [22] performed a study on the frequency and distribution of single nucleotide polymorphisms (SNPs) on the AMH and AMHRII genes in infertile patients and how these polymorphisms affect the infertility treatment outcome. There are already at least 38 different mutations of AMH gene which may cause the persistent Müllerian duct syndrome. However, the mutations did not show any apparent effect on assisted reproductive technology (ART) outcomes. On the other hand, the same could not be said for a SNP on the AMHRII receptor—the result of the study suggests that SNP on AMHRII receptor could cause poor follicular development in all affected patients. In can be concluded that abnormalities of AMH receptors may be related to gonadal abnormalities and infertility. It is important to better understand the abnormalities of AMH receptors to improve the efficiency of treatment in infertile patients.
AMH and gender dimorphism
AMH received its name from its action on the Müllerian ducts during the embryonic period of life. It is the first molecule that is synthesized by Sertoli cells in the developing male gonads. After the period of indifferent gonads, the dimorphism of genders happens at 6th–8th week of gestation. During this developmental phase, AMH functions as an inducer of degeneration of the Müllerian ducts and, with co-action of testosterone, development of the Wolffian ducts into the epididymis, vas deferens, and seminal vesicles [7]. Expression of AMH is tightly regulated and it is active at the exact time when the Müllerian ducts are susceptible to it [1]. Nothing is left from the Müllerian ducts by the 10th week of gestation, although the secretion of AMH is still active until puberty in men. In this period, its function is still unknown [23]. In females, due to the absence of Sertoli cells, the Müllerian ducts persist and later develop in the tubes, uterus, and the upper part of the vagina.
AMH expression in the ovaries: folliculogenesis
Expression in the ovarian follicles
Although AMH is expressed in endometrial and endometriotic cells [24], its measurement in serum is reflected by the secretion of AMH in the gonads. For example, in serum of women who have their ovaries removed, the AMH appears at an untraceable amount. In women, AMH is secreted by the ovarian granulosa cells of pre-antral and antral follicles starting at the 36th week of gestation [25]. The ovarian granulosa cells are constantly maintaining expression of AMH during the reproductive period of life, even though this cannot be reflected in the serum concentration—it increases up to the age of 24.5 years and afterwards decreases until menopause. After this, the expression of AMH is significantly lowered and the serum concentration of AMH is no longer measurable [1]. The granulosa cells in antral follicles secrete the AMH into the follicular fluid and the bloodstream. It is agreed that ovarian follicles secrete AMH; however, there is no consensus to the size—some mention to the diameter of 4 mm [26, 27], others to 6 mm [28], 8 mm [29], or even 9 mm [30]. Also, growing follicles release AMH until the size and the developmental stage at which they are selected as dominant follicles by action of exogenous FSH [13]. When the follicles are further developed and are big enough to be selected for a dominant follicle, the transcription of AMH gene stops. In mice, this happens at the early antral stage of the small growing follicles [13]. However, some studies have shown that the expression of AMH is not completely stopped but only declines in the ovarian granulosa cells after selection of a dominant follicle [31]. In the AMH-knockout mice, it was found that their ovarian follicles are recruited faster than in normal mice and are more sensitive to FSH [22, 32]. The maximal expression of AMH is held in the pre-antral and small antral follicles, while the expression in the primordial, dominant, and atretic follicles declines with age [33–35].
In larger follicles, the AMH is produced in the follicular cells around the oocyte and the antrum [7]. When the oocytes are in the primordial follicle, they are at the dormant stage of meiosis I until puberty. Until then the granulosa cells do not secrete AMH. The AMH secretion begins again with the recruitment of follicles with the maximal concentration of AMH at the average age of 24.5 years. The plateau phase usually begins around the age of 25 years, and then the concentration of AMH gradually decreases until menopause [1, 36, 37].
Regulation of the number of ovarian follicles
AMH is supposed to regulate the number of growing follicles and their selection for ovulation [7] and is a negative regulator of early stages of the follicular development [13]. AMH inhibits the recruitment and growth of follicles by restricting growth factors and the effect of gonadotropins (Fig. 1), especially FSH [32]. In mice, it has even been proven that there was a 40–50% decrease in the number of growing follicles, if their ovaries were cultured with added AMH [38]. In this sense, AMH is supposed to inhibit the beginning of follicle growth and FSH-dependant follicle growth. The lack of AMH results in a faster depletion of the follicular pool in the ovaries [39]. In AMH null mice, it has been shown that in older females (13 months old), the primordial follicle pool had diminished for around threefold in comparison with wild-type mice. This finding leads to the discovery that AMH knockout female mice stopped ovulating at 16–17 months of age (56%), while the older wild-type female mice were still cycling normally (82%) [14]. AMH inhibits the activity of aromatase bringing about a decrease in oestrogen biosynthesis [40]. The concentration of AMH in the follicular fluid, retrieved in the in vitro fertilization programme, is in correlation with the concentration of estradiol, which can mean that AMH is a co-regulator of the steroidogenesis [7]. It also appears that AMH has an autocrine role in the maturation of normal follicles, as studies on rats showed that AMH inhibits the first meiotic division of diplotene oocytes [41]. In humans, it was discovered that AMH blocks the proliferation of granulosa-luteal cells in vitro [42]. Another hypothesis is that oocytes in the pool of growing follicles may control the pool of primordial follicles by modulation of the expression of AMH [13]. In the testicles, AMH inhibits the differentiation of the precursor cells into mature Leydig cells and expression of the steroidogenic enzymes into primary Leydig cells [1].
AMH, ovarian reserve, and infertility
In the modern age, social and behavioural life has changed dramatically. There is a trend that women delay their pregnancy and childbirth until their thirties or even forties. Unfortunately, this trend has brought several problems, especially in the way of the lower probability of conceiving [43]. This is particularly the problem of reproductive aging, which is more and more leading to the subfertility stage or even menopause transition [43]. The increasing female age has become a social and also biological indication of infertility. A good solution to this problem would be an accurate ovarian reserve test available to reproductive medicine practitioners and their patients. This test could motivate a proportion of women to have their children earlier or reassure others that they still have time for conception [44]. It is worth noting that individual women move through the stages of the reproductive age differently. Women of the same age can greatly differ in their potential of reproduction as is the case of the age at which menopause occurs. Even though this knowledge exists, there are still no validated biomarkers that would enable the characterization of each woman’s fertility individually [43], and AMH is an interesting candidate.
Ovarian aging in elder women
Ovarian aging can be defined as a period in which there is a measurable decline in the levels of circulating AMH and inhibin B as well as the rise of FSH and estradiol levels in the blood [43]. It has been proven that female age has a high effect on the AMH concentration in the blood [45]. They have found that AMH levels in the blood drop yearly by approximately 0.384 μg/L.
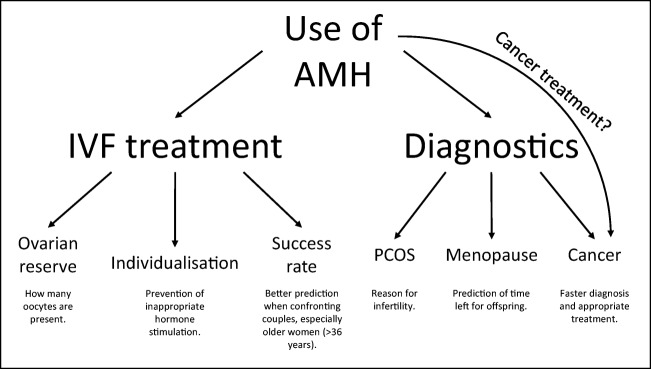
The levels of these hormones are related to the number of available oocytes in a patient. Despite the physiological association of the ovarian function with these hormonal markers, their potential for predicting fertility in the general population has never been thoroughly tested, especially in women without reproductive problems. Steiner et al. [43] evaluated the levels of these markers in a group of women without fertility problems and looked for an association between the endocrine profile, ovarian aging, and fecundability with measurements of FSH, estradiol, AMH, and inhibin B in serum and FSH and estrone 3-glucuronide in urine of women. This study included a group of women aged between 30 and 44 years, which had a higher risk of developing ovarian aging. They have found that among all markers tested, only AMH was significantly associated with natural fertility, as measured daily for the probability of pregnancy in women. All other markers were not significantly associated with female fertility. It was concluded that AMH is a good predictor of age-related reduction of fecundability in women (Fig. 2).
Fig. 2.
The current and future application of serum AMH measurements and therapy in patients
Ovarian diseases
AMH could also be used as a diagnostic tool to estimate secondary oligo-amenorrhea, the level of damage of the ovaries after surgery or cancer treatment (chemotherapy), and the condition of the granulosa cells after tumour removal [7]. In this sense, it has the potential to be a good biomarker for certain ovarian diseases such as those described below.
Polycystic ovary syndrome
Women with polycystic ovary syndrome (PCOS) usually have oligo/anovulation, hyperandrogenism, and characteristic ultrasound ovarian features. The dysfunction in these women is characterized by the arrest of follicle maturation and the disturbed selection of the dominant follicle, and an increased number of early antral follicles [44]. PCOS is often associated with obesity (38–66% PCOS patients), insulin resistance (IR), and decreased pregnancy rates in natural and assisted conception cycles [46]. However, it is debatable if IR is the consequence of PCOS or obesity itself [46]. Ovarian aging and age at menopause could be delayed in women with PCOS [44].
Among women in the reproductive age, PCOS is the most common endocrine disorder, affecting around 5–10% of the female population [47]. There are three diagnostic criteria used to confirm PCOS in a patient, and each differs in the prevalence of PCOS. The criteria from the National Institutes of Health/National Institute of Child Health (NIH/NICHD) reports a prevalence of 6.1–8.7%, the prevalence by the Rotterdam criteria (ESHRE/ASRM) is between 15.2–19.9% and 12–15.3% by the androgen excess and PCOS society criteria [46, 48]. PCOS is supposed to be a genetic-related condition; however, the cause for it is still unknown [49]. PCOS is associated with high levels of AMH that are secreted by numerous small growing follicles (Fig. 2). There is also an existing hypothesis that high concentrations of AMH which are present in a pregnant woman could heighten the offspring’s chance to develop PCOS in adulthood via the impact of AMH on the neuroendocrine and ovarian function of the offspring [50]. The density of pre-antral and small antral follicles in the PCOS is as much as six times larger than in normal ovaries. This is why the serum AMH is higher in patients with PCOS [51] and strongly correlated to AFC [52]. The cutoff values of AMH in the blood serum for PCOS patients are usually between 3.14 and 4.45 ng/mL or even higher [46], while normal AMH levels are commonly around 1–2 ng/mL and the value declines with age. The higher expression of AMH in PCOS patients results in a slower follicle growth which in turn causes follicle accumulation at every growing stage, a so-called stockpiling effect [53]. In patients with a normal ovarian reserve who undergo the ovarian stimulation (OS), the levels of AMH in the serum fluctuate during the cycle. The connection between the ovarian reserve and fluctuation of the AMH concentration during the OS is still unclear [54].
Primary ovarian insufficiency
Primary ovarian insufficiency (POI) is a reproductive endocrine disorder in women that is the cause of ovarian follicle dysfunction. Its symptoms are usually amenorrhea for more than 4 months and overproduction of FSH (> 40 IU/L) [55] in women before 40 years of age. This hormonal deficiency increases the chance of cardiovascular disease, metabolic syndrome, and osteoporosis as well as impaired reproductive capacity [56]. The POI condition is usually present in women with Turner syndrome [44] or in some other conditions such as genetic abnormalities, ovarian autoimmunity [57], infections, or even chemotherapy and radiotherapy [58], but in most cases, it cannot be explained. In women with POI, the AMH concentration is undetectable [59]. This is why AMH levels are considered a predictor of POI among women, especially those which have autoimmune thyroiditis [55].
Menopause
There is a large variability in the process of ovarian aging in women. It is believed that it is due to the different pace of follicle pool depletion. This is also a reason why the age range for menopause is so large—between 40 and 60 years [44]. However, some women at a younger age have more advanced ovarian aging than other women from the same age group [60]. Low levels of AMH and antral follicle count (AFC) and high levels of FSH in the serum are predictors of the onset of menopause (Fig. 2). It is still unknown which of these measures or their combination is the best predictor of menopause. It is interesting that natural menopause occurs because of the mutation of the gene AMHRII. It has been proposed that in patients with POI and at an early age of menopause, the mutation of this gene is the same as in normal patients but it happens earlier [39]. It is interesting to speculate that AMH could be used as a predictor of diseases connected to menopause (i.e., osteopenia and atherosclerosis) in the future [61]. As a marker for predicting menopause, it could have an added benefit of preventing menopause-associated problems and with female health in general. For example, women with a high-risk profile such as low AMH at a younger age could be included in prevention programs to improve their health [44].
Although AMH is an interesting and perspective marker of different ovarian diseases, it is mostly not used in daily clinical practice and should be further considered in the future.
Diagnostics and prediction of clinical outcome in IVF patients
Estimation of ovarian reserve
A multitude of authors [7, 26, 29, 30, 56, 57, 62–65] suggest the use of AMH as the best biomarker for ovarian reserve evaluation in patients, although, it is possible that AMH gene expression and serum levels of AMH can be altered by some genetic and environmental factors. The better knowledge of the effect of these factors on the levels of AMH in the serum of patients could lead to better clinical practice and optimization of infertility treatments. Known environmental factors that may influence the AMH expression are vitamin D deficiency, obesity, and smoking, while among the genetic factors, there are mutations of genes BRCA1, FMR1, and MTHFR C677T genotype [66]. It is important to note that studies on all of these factors and their effect on AMH levels show conflicting results at present and the need for further research.
The ESHRE consensus criteria consider the concentrations of AMH of < 0.5–1.1 ng/mL or 3.57–7.85 pmol/L as a risk factor for poor ovarian reserve although others suggest the cutoff value for poor ovarian response at 12 pmol/L [67]. This means that the use and interpretation of the results regarding AMH levels need to be taken cautiously.
Response to controlled ovarian hormonal stimulation
It has been found that in ART, the serum AMH level is a better endocrine indicator (Fig. 2) of the follicular response to OS in comparison to other commonly measured markers such as FSH, estradiol, inhibin B, and the age of the patient by itself [68]. In the study of Daney de Marcillac et al. [69], it was found that women with normal AMH levels in their serum have a better response to OS, lower cancellation rate, higher oocyte count after ultrasound-guided aspiration and an increased pregnancy rate per stimulation cycle, irrelevant of FSH levels. Additionally, in women with low AMH levels, their effects were exactly opposite with a lower number of oocytes retrieved, irrelevant of FSH levels (Fig. 2). The AMH test could not completely replace the FSH assay but they need to be used in cohesion [69]. There is a higher risk of IVF cycle cancellation in women with low AMH and higher FSH values, especially in women with a higher prevalence of ovarian endometriosis or after surgery due to ovarian endometriosis [70]. It has been noted that women less than 36 years of age, regardless of AMH concentration, have approximately the same pregnancy rate [45]. Also, if a patient has a high concentration of AMH in the serum before OS, there is a high risk of developing ovarian hyperstimulation syndrome [71]. Some researchers suggest that high AMH values in the serum of patients can indicate oocytes of better quality including their maturity and eventual positive reproductive outcome [45], while others [69] assume that AMH is not a good predictor of embryo quality or pregnancy, which suggest that AMH is more likely a quantitative not qualitative marker of oocytes.
Healthcare providers and even national guidelines suggest the individualization of starting doses of gonadotropin for OS. They should be administered depending on the patients’ characteristics and markers of their ovarian reserve including AMH (Fig. 2). Currently, clinicians apply hormonal treatments based on their own experiences, usually using subjective preferences for certain parameters [72]. The main reason is that there is no given standard respecting different factors when determining the dose of gonadotropin for OS that should be used for each individual patient. Because of more developed assays for measuring AMH serum levels, it has been proposed that AMH should be able to predict the ovarian response to OS better than the currently established measures [72]. It is encouraged to measure the serum AMH levels in women who have PCOS or have a higher risk of cycle cancellation [70]. Nyboe Andersen et al. [72] conducted a study which included 1329 women, where they compared individualized versus conventional ovarian stimulation protocol for OS in patients included in the in vitro fertilization programme. They found that individualized OS resulted in a higher proportion of women with 8–14 oocytes per cycle, fewer clinically relevant cases of poor or excessive ovarian response, and a lower need for ovarian hyperstimulation syndrome (OHSS) preventative measures. The individualized FSH doses to start OS were calculated based on the serum AMH level and body weight. They even assert that it would be possible to develop a biomarker-driven dosing strategy for other gonadotropins to stimulate the ovaries of patients included in the in vitro fertilization programme.
AMH is a good biomarker of the ovarian reserve in infertile patients undergoing ART procedures due to minimal fluctuations of serum AMH during the whole menstrual cycle in contrast to other markers such as FSH, estradiol, and inhibin B [54]. Some experts even suggest that AMH is a better biomarker for the prediction of ovarian response to hormonal stimulation in ART patients than the currently used AFC [73]. Even though many studies report that the levels of AMH are stable during the menstrual cycle, during the OS, the serum levels of AMH mildly fluctuate [36, 67]. Because of its low fluctuation, it may be used for the epidemiologic studies of stored blood samples of women [13, 57]. The serum AMH is supposed to decrease in the second and third trimester of pregnancy thus reflecting ovarian inactivity; in the first trimester of pregnancy, the concentration of AMH stays similar as in the time of preconception [74]. After birth, the levels of serum AMH return to normal [75].
Outcome of IVF
In IVF, the studies on the role of AMH and its ability to predict pregnancy and live birth rates show varying results [76–79]. However, Goswami and Nikolaou [80] found that AMH levels in patients’ serum can predict live births in older women following the IVF procedure (Fig. 2). Kavoussi et al. [81] found that low serum AMH levels (< 1 ng/mL) significantly lower the chances of blastocyst cryopreservation after IVF than in those patients with higher AMH levels (1–4 ng/mL), regardless of patient age. There is still little known about the connection between the variations in the serum AMH level and the outcomes of IVF in patients. Melado Vidales et al. [36] found that AMH concentrations which were measured during the IVF stimulation predicted the ovarian response in patients during the days of follicular growth. This is an important advantage of AMH compared to other ovarian reserve biomarkers. This finding could lead to better individualization of the IVF treatment in terms of more efficient ovarian stimulation that is independent on the status of the ovarian reserve in a patient.
Maturation of oocytes in vitro
Along with other beneficial aspects, the recombinant AMH could be used as a compound added to the in vitro maturation (IVM) medium, which is used for maturation of immature oocytes collected from patients in the IVF programme. The immature oocytes represent around 15% of all oocytes retrieved by ultrasound-guided aspiration of ovarian follicles after OS in patients, included in the in vitro fertilization programme. Immature oocytes cannot be fertilized unless matured in vitro. The procedure of IVM is currently suboptimal and has a low maturation rate, but some groups have been able to use IVM as a successful supplement to IVF in the natural cycle [82–85]. Further, Zhang et al. [86] found that the addition of 100 ng/mL of recombinant AMH to the IVM medium improves the quality of matured mouse oocytes resulting in a better quality of embryos after in vitro fertilization. They suggest that the added AMH upregulates the expression of genes GDF9 and BMP15 during the IVM procedure, which improves the quality of matured oocytes and embryos. It is an important task to repeat this work in human oocytes and maybe to improve the outcome of the oocyte in vitro maturation procedure in humans. Successful in vitro maturation of human oocytes would be beneficial for infertile patients and young cancer patients who cryopreserve their oocytes before chemo- and radiotherapy to retain their reproductive potential.
Contraception
Administration of recombinant AMH in human ovarian tissue in vitro has been shown to suppress the initiation of primordial follicle recruitment [87], although another study claims oppositely [88]. This knowledge has led to the hypothesis that AMH could be used as a way of reversible contraception to halt primordial follicular recruitment and with this delay the onset of menopause [2]. Hayes et al. [89] have even demonstrated in an in vivo mouse model that the administration of recombinant AMH decreased follicular development and inhibited ovulation, which resulted in an interrupted estrous cycle. A similar study showed that when the administration of AMH was cancelled, the folliculogenesis returned to the normal stage, which suggests that contraception with AMH is reversible [90]. Even certain mathematical models on the potential application of AMH as a contraceptive have been made, which calculated that the reproductive lifespan during AMH-induced contraception should be prolonged for as long as AMH is applied [91]. As a contraceptive, AMH could prove invaluable in preserving the fertility of women with lower ovarian reserve at a younger age as well as becoming a more natural and non-invasive means of contraception in comparison to currently available substances.
AMH and cancer
Gynaecological tumours and cancer
AMH is associated with different and known gynaecological tumours such as granulosa cell tumours of the ovary (GCT), ovarian cancer with epithelial ovarian tumours, and cervical cancer. However, AMH can also affect endometrial, breast, and prostate cancers [16, 92, 93]. GCT represents around 70% of all sex cord-stromal tumours. It is uniquely characterized by the ability to secrete oestrogen and can evolve in all age groups of women [94]. AMH has been confirmed as a good tumour marker (in addition to inhibin B) for the primary and recurrent GCT occurrence, especially because of its stability during the menstrual cycle [95]. The serum AMH in these patients is higher than in patients without tumours; however, it can only be efficiently applied to patients younger than 65 years of age [96]. In older women, the serum AMH is too low to measure regardless of their disease. The higher concentration of AMH in tumours is due to enlarged granulosa cells which release larger amounts of AMH into the bloodstream [95]. The same positive association of high circulating AMH with higher risk for breast cancer has been found in breast cancer patients [97].
Though it is suggested that the majority of ovarian cancers within the Müllerian tract arise from the fimbriated end of the fallopian tube, there are certain types of tumours which derive from the secondary Müllerian system [98]. Knowing that AMH acts in the regression of Müllerian ducts during the male gender differentiation in the embryo, scientists have proposed the possibility of using AMH as a potential treatment agent in epithelial ovarian cancers [99]. It has been proven, that recombinant AMH affects certain ovarian cancer cell lines (OVCAR 8 and IGROV 1) and inhibits their growth [100].
One of the most common cancers in women is cervical cancer. Because the cervical epithelium originates from the same Müllerian ducts as the ovarian epithelium, the same principle of using AMH as a tumour suppressor has been debated [30]. There were some studies on human endometrial, breast, and prostate cancer cells or tissues which have shown that AMHRII receptors are present on them and that the binding of AMH to these receptors causes the inhibition of cancer cell growth [16, 92, 93].
The levels of AMH in healthy and cancer patients vary depending on the type of cancer. It has been found that in younger breast cancer patients (28–44 years old), the level of AMH was the same as in healthy women (30–44 years old) [101]. This means that the ovarian reserve in breast cancer patients is not impacted by cancer disease. Oppositely, it was found that patients with Hodgkin lymphoma had lower AMH levels and a lower ovarian reserve than healthy women [102]. Moreover, the serum AMH levels can predict the function of ovaries after cancer treatment, even the possibility of ovarian recovery; women with a higher AMH level have a faster recovery of their ovarian function and fertility. The measurement of AMH in patients’ serum could represent a more robust and reliable method of ovarian reserve than the measurements of FSH or inhibin B levels [103]. We can conclude that different types of cancer may affect the ovarian reserve and consequently the serum AMH as its marker.
The main problem of cancer treatment, especially chemo- and radiotherapy, is the induction of amenorrhea or premature menopause in young patients of reproductive age. Usually, the levels of AMH and estradiol in the serum of post-menopausal women decrease, while the levels of FSH increase. It is thought that AMH could play an important role in the prediction of chemotherapy-induced menopause (CIM) in young patients with cancer [104]. However, Passildas et al. [104] discovered that younger breast cancer patients are more likely to recover menstruation after chemotherapy and have a lower risk of premature menopause after treatment. AMH could also be used as an addition to chemotherapy and applied to cancer patients to preserve their growing ovarian follicles in a dormant state [105].
Cancer treatment
As mentioned above, there is a high prospect of using AMH for several aspects of cancer treatment and outcomes (Fig. 2). One of them could be the forecasting of the reproductive outcome in women who have undergone cancer treatment. There is also a possibility of transplanting ovarian tissue that was frozen before cancer treatment to restore fertility. The AMH values in the serum of these patients may be able to predict the success of transplantation [106]. In some breast cancer patients, the AMH levels were still very low 2 years after treatment and can show the long-term post-chemotherapy loss of ovarian function. Because of this, determination of the levels of AMH before cancer treatment may aid in the making of decisions regarding efficient treatment options and fertility preservation after chemotherapy treatment [107]. AMH could potentially be used as a contraceptive without losing the existing pool of follicles and with a reversible contraceptive effect to prevent the preterm activation of growing follicles, especially in the case of chemotherapy [2, 90]. Although the idea is of high clinical interest, there is a lack of pure and safe recombinant AMH on the market to apply it to the patients.
Measurement of AMH levels in biological samples
The measurement of AMH levels in serum is possible with several different AMH assays which are provided by many manufacturers worldwide (gen. II assays). At present, there are three fully automated immunoassays available on the market that have high specificity and sensitivity: The Elecsys AMH from Roche, AMH ELISA from Ansh Labs, and Access AMH from Beckman Coulter. The latter was also used in a study using AMH levels as a predictor for AFC in patients [108]. A cutoff value of 1.77 ng/mL was found for classification of women with more than 15 follicles in their ovaries on a sample of 856 patients. The use and development of relevant immunoassays is an important tool for the easier prediction of follicle count as well as early menopause or an alarm for decreasing fecundability of women. The difficulty in measuring AMH serum levels is that there is still no international, generally accepted standard for sample preparation, storage, and AMH assay [44]. This poses a major difficulty in the application of AMH measurements to any clinical use. Ferguson et al. [109] are currently developing an international standard for AMH measurements in biological samples (serum) on the basis of recombinant human AMH as reference.
Conclusions
AMH is a hormone that has a lot of potential to be clinically used, especially in the field of IVF treatment. At some clinics, it is used to help the clinicians with the individualization of OS in patients attending IVF procedures. However, the more pronounced use of AMH is mainly obstructed by unreliable diagnostics, which is caused by the lack of international standardization of AMH assay, which lowers the specificity of the measurements and clinical use. Thus, international standardization is the most important issue that should be tackled in the near future. Also, there is still no human recombinant AMH available that is safe for clinical use. It is encouraging that research of cancer treatment using AMH is rapidly progressing in terms of AMH measurements or even the application of AMH to avoid the preterm activation of follicles in the ovaries during chemotherapy and radiotherapy and consequent infertility. An interesting consideration would be to elucidate, if AMH can act directly on oocytes and the molecular mechanisms of this action. This new knowledge could be the foundation of new hormonal therapies and improve the suboptimal procedure of human oocyte maturation in vitro. It is certainly an interesting time for the research of new aspects of AMH action and its use in reproductive medicine.
Funding information
This work was supported by the Slovenian Research Agency (ARRS): research programme P3-0124 and grant offered to the young researcher Jure Bedenk.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Josso N, di Clemente N, Gouédard L. Anti-Müllerian hormone and its receptors. Mol Cell Endocrinol. 2001;179:25–32. doi: 10.1016/s0303-7207(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 2.Kushnir VA, Seifer DB, Barad DH, Sen A, Gleicher N. Potential therapeutic applications of human anti-Müllerian hormone (AMH) analogues in reproductive medicine. J Assist Reprod Genet. 2017;34:1105–1113. doi: 10.1007/s10815-017-0977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Müllerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev PER. 2006;3:347–358. [PubMed] [Google Scholar]
- 4.Rey R, Sabourin J-C, Venara M, Long W-Q, Jaubert F, Zeller WP, et al. Anti-Müllerian hormone is a specific marker of Sertoli- and granulosa-cell origin in gonadal tumors. Hum Pathol. 2000;31:1202–1208. doi: 10.1053/hupa.2000.18498. [DOI] [PubMed] [Google Scholar]
- 5.Visser JA, de Jong FH, Laven JSE, Themmen APN. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 6.Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 7.Peluso C, Fonseca FLA, Rodart IF, Cavalcanti V, Gastaldo G, Christofolini DM, et al. AMH: an ovarian reserve biomarker in assisted reproduction. Clin Chim Acta. 2014;437:175–182. doi: 10.1016/j.cca.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22:709–724. doi: 10.1093/humupd/dmw027. [DOI] [PubMed] [Google Scholar]
- 9.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 10.Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Yu RN, Jameson JL. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol. 1998;12:290–301. doi: 10.1210/mend.12.2.0059. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Zhang H. Association of genetic polymorphisms of anti-Müllerian hormone (AMH) and its type II receptor with ovarian hyperstimulation syndrome. J Reprod Contracept. 2013;24:30–37. [Google Scholar]
- 13.La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64:603–610. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 14.Visser JA, Themmen APN. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Pfennig F, Standke A, Gutzeit HO. The role of Amh signaling in teleost fish – multiple functions not restricted to the gonads. Gen Comp Endocrinol. 2015;223:87–107. doi: 10.1016/j.ygcen.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Segev DL, Hoshiya Y, Hoshiya M, Tran TT, Carey JL, Stephen AE, et al. Mullerian-inhibiting substance regulates NF–κB signaling in the prostate in vitro and in vivo. Proc Natl Acad Sci. 2002;99:239–244. doi: 10.1073/pnas.221599298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Dicken C, Lustbader JW, Tortoriello DV. Evidence for a Müllerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil Steril. 2009;91:1195–1203. doi: 10.1016/j.fertnstert.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Segev DL, Hoshiya Y, Stephen AE, Hoshiya M, Tran TT, MacLaughlin DT, et al. Müllerian inhibiting substance regulates NFκB signaling and growth of mammary epithelial cells in vivo. J Biol Chem. 2001;276:26799–26806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 19.Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Müllerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces Smad6 expression. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 20.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen APN, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Müllerian inhibiting substance signaling. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 21.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y, Yamashita Y, Saito N, Ono Y, Yamamoto H, Nakamura Y, et al. Analyzing the possible involvement of anti-Müllerian hormone and anti-Müllerian hormone receptor II single nucleotide polymorphism in infertility. J Assist Reprod Genet. 2014;31:163–168. doi: 10.1007/s10815-013-0134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhide P, Homburg R. Anti-Müllerian hormone and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:38–45. doi: 10.1016/j.bpobgyn.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Carrarelli P, Rocha ALL, Belmonte G, Zupi E, Abrão MS, Arcuri F, et al. Increased expression of antimüllerian hormone and its receptor in endometriosis. Fertil Steril. 2014;101:1353–1358. doi: 10.1016/j.fertnstert.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 25.Rajpert-De Meyts E, Jørgensen N, Græm N, Müller J, Cate RL, Skakkebæk NE. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 26.AbdelHafez FF, Tang Y, Hassan MH, Saleem TH. Assessment of anti-Mullerian hormone (AMH) levels in a pilot cohort of peripubertal females: correlation with sex maturity rating (SMR) Middle East Fertil Soc J. 2018;23:278–280. [Google Scholar]
- 27.Feyereisen E, Lozano DHM, Taieb J, Hesters L, Frydman R, Fanchin R. Anti-Müllerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod BioMed Online. 2006;12:695–703. doi: 10.1016/s1472-6483(10)61081-4. [DOI] [PubMed] [Google Scholar]
- 28.Alanazi H, Bushaqer N, Ayyoub H, Dayoub N, Hassan S. Antimullerian hormone (AMH) level and IVF/ICSI cycle outcome in expected poor responders. Middle East Fertil Soc J. 2018;23:246–250. [Google Scholar]
- 29.Anderson RA. What does anti-Müllerian hormone tell you about ovarian function? Clin Endocrinol. 2012;77:652–655. doi: 10.1111/j.1365-2265.2012.04451.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Mao H, Cai J, Zhao Y. Research progress on anti-mullerian hormone clinical applications and immunoassay development. Front Lab Med. 2018;2:14–18. [Google Scholar]
- 31.Ilha GF, Rovani MT, Gasperin BG, Ferreira R, de Macedo M, Neto OA, et al. Regulation of anti-Müllerian hormone and its receptor expression around follicle deviation in cattle. Reprod Domest Anim. 2016;51:188–194. doi: 10.1111/rda.12662. [DOI] [PubMed] [Google Scholar]
- 32.Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- 33.Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–1287. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- 34.Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. MHR Basic Sci Reprod Med. 2013;19:519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 35.Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. MHR Basic Sci Reprod Med. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 36.Melado Vidales L, Fernández-Nistal A, Martínez Fernández V, Verdú Merino V, Bruna Catalán I, Bajo Arenas JM. Anti-Müllerian hormone dynamics during GNRH-antagonist short protocol for IVF/ICSI in women with varying ovarian reserve levels. Minerva Ginecol. 2017;69:128–134. doi: 10.23736/S0026-4784.16.03951-4. [DOI] [PubMed] [Google Scholar]
- 37.Schenk M, Kröpfl JM, Obermayer-Pietsch B, Feldmeier E, Weiss G. Anti-Mullerian hormone concentrations in individual follicular fluids within one stimulated IVF cycle resemble blood serum values. J Assist Reprod Genet. 2017;34:1115–1120. doi: 10.1007/s10815-017-0908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 39.Qin C, Yuan Z, Yao J, Zhu W, Wu W, Xie J. AMH and AMHR2 genetic variants in Chinese women with primary ovarian insufficiency and normal age at natural menopause. Reprod BioMed Online. 2014;29:311–318. doi: 10.1016/j.rbmo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19:828–837. doi: 10.1093/molehr/gat065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueno S, Manganaro TF, Donahoe PK. Human recombinant Mullerian inhibiting substance inhibition of rat oocyte meiosis is reversed by epidermal growth factor in vitro. Endocrinology. 1988;123:1652–1659. doi: 10.1210/endo-123-3-1652. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Seibel MM, MacLaughlin DT, Donahoe PK, Ransil BJ, Hametz PA, et al. The inhibitory effects of Müllerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab. 1992;75:911–917. doi: 10.1210/jcem.75.3.1517385. [DOI] [PubMed] [Google Scholar]
- 43.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimüllerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 45.Gomez R, Schorsch M, Hahn T, Henke A, Hoffmann I, Seufert R, et al. The influence of AMH on IVF success. Arch Gynecol Obstet. 2016;293:667–673. doi: 10.1007/s00404-015-3901-0. [DOI] [PubMed] [Google Scholar]
- 46.Jahromi BN, Dabbaghmanesh MH, Bakhshaie P, Parsanezhad ME, Anvar Z, Alborzi M, et al. Assessment of oxytocin level, glucose metabolism components and cutoff values for oxytocin and anti-mullerian hormone in infertile PCOS women. Taiwan J Obstet Gynecol. 2018;57:555–559. doi: 10.1016/j.tjog.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 48.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:29–38. doi: 10.1016/j.mce.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. doi: 10.1038/ncomms10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webber L, Stubbs S, Stark J, Trew G, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 52.Alebić MŠ, Stojanović N, Dewailly D. Discordance between serum anti-Müllerian hormone concentrations and antral follicle counts: not only technical issues. Hum Reprod. 2018;33:1141–1148. doi: 10.1093/humrep/dey098. [DOI] [PubMed] [Google Scholar]
- 53.Maciel GAR, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 54.Melado Vidales L, Fernández-Nistal A, Martínez Fernández V, Verdú Merino V, Bruna Catalán I, Bajo Arenas JM. Anti-Müllerian hormone levels to predict oocyte maturity and embryo quality during controlled ovarian hyperstimulation. Minerva Ginecol. 2017;69:225–232. doi: 10.23736/S0026-4784.16.03958-7. [DOI] [PubMed] [Google Scholar]
- 55.Rashad NM, Moafy H, Saleh HS, Amin AI, Gomaa AF. Anti-Müllerian hormone: predictor of premature ovarian insufficiency in Egyptian women with autoimmune thyroiditis. Middle East Fertil Soc J. 2018;23:286–291. [Google Scholar]
- 56.Nyström A, Mörse H, Nordlöf H, Wiebe K, Artman M, Øra I, et al. Anti-müllerian hormone compared with other ovarian markers after childhood cancer treatment. Acta Oncol. 2019;58:218–224. doi: 10.1080/0284186X.2018.1529423. [DOI] [PubMed] [Google Scholar]
- 57.Nair S, Slaughter JC, Terry JG, Appiah D, Ebong I, Wang E, et al. Anti-mullerian hormone (AMH) is associated with natural menopause in a population-based sample: the CARDIA Women’s Study. Maturitas. 2015;81:493–498. doi: 10.1016/j.maturitas.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol. 2019;55:2–13. doi: 10.1016/j.bpobgyn.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Knauff EAH, Eijkemans MJC, Lambalk CB, ten Kate-Booij MJ, Hoek A, Beerendonk CCM, et al. Anti-Müllerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab. 2009;94:786–792. doi: 10.1210/jc.2008-1818. [DOI] [PubMed] [Google Scholar]
- 60.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim C, Slaughter JC, Wang ET, Appiah D, Schreiner P, Leader B, et al. Anti-Müllerian hormone, follicle stimulating hormone, antral follicle count, and risk of menopause within 5 years. Maturitas. 2017;102:18–25. doi: 10.1016/j.maturitas.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolat SE, Ozdemirci S, Kasapoglu T, Duran B, Goktas L, Karahanoglu E. The effect of serum and follicular fluid anti-Mullerian hormone level on the number of oocytes retrieved and rate of fertilization and clinical pregnancy. North Clin Istanb. 2016;3:90–96. doi: 10.14744/nci.2016.02418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumont A, Robin G, Jonard S, Dewailly D. Role of anti-Müllerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol 2015;13. [DOI] [PMC free article] [PubMed]
- 64.Islam Y, Aboulghar MM, AlEbrashy AE-D, Abdel-Aziz O. The value of different ovarian reserve tests in the prediction of ovarian response in patients with unexplained infertility. Middle East Fertil Soc J. 2016;21:69–74. [Google Scholar]
- 65.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJC, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–4655. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahrokhi SZ, Kazerouni F, Ghaffari F. Anti-Müllerian hormone: genetic and environmental effects. Clin Chim Acta. 2018;476:123–129. doi: 10.1016/j.cca.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 67.Alson SSE, Bungum LJ, Giwercman A, Henic E. Anti-müllerian hormone levels are associated with live birth rates in ART, but the predictive ability of anti-müllerian hormone is modest. Eur J Obstet Gynecol Reprod Biol. 2018;225:199–204. doi: 10.1016/j.ejogrb.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 68.Arce J-C, Nyboe Andersen A, Fernández-Sánchez M, Visnova H, Bosch E, García-Velasco JA, et al. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimüllerian hormone–stratified, dose–response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102:1633–1640.e5. doi: 10.1016/j.fertnstert.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Daney de Marcillac F, Pinton A, Guillaume A, Sagot P, Pirrello O, Rongieres C. What are the likely IVF/ICSI outcomes if there is a discrepancy between serum AMH and FSH levels? A multicenter retrospective study. J Gynecol Obstet Hum Reprod. 2017;46:629–635. doi: 10.1016/j.jogoh.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Revelli A, Biasoni V, Gennarelli G, Canosa S, Dalmasso P, Benedetto C. IVF results in patients with very low serum AMH are significantly affected by chronological age. J Assist Reprod Genet. 2016;33:603–609. doi: 10.1007/s10815-016-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arce J-C, La Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril. 2013;99:1644–1653.e1. doi: 10.1016/j.fertnstert.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 72.Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce J-C, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107:387–396.e4. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 73.Nelson SM, Klein BM, Arce J-C. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil Steril. 2015;103:923–930.e1. doi: 10.1016/j.fertnstert.2014.12.114. [DOI] [PubMed] [Google Scholar]
- 74.Köninger A, Kauth A, Schmidt B, Schmidt M, Yerlikaya G, Kasimir-Bauer S, et al. Anti-Mullerian-hormone levels during pregnancy and postpartum. Reprod Biol Endocrinol RBE. 2013;11:60. doi: 10.1186/1477-7827-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.La Marca A, Giulini S, Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod Oxf Engl. 2005;20:1569–1572. doi: 10.1093/humrep/deh819. [DOI] [PubMed] [Google Scholar]
- 76.Fiçicioǧlu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–596. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 77.McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod. 2007;22:778–785. doi: 10.1093/humrep/del435. [DOI] [PubMed] [Google Scholar]
- 78.Sahmay S, Oncul M, Tuten A, Tok A, Acıkgoz AS, Cepni I. Anti-Müllerian hormone levels as a predictor of the pregnancy rate in women of advanced reproductive age. J Assist Reprod Genet. 2014;31:1469–1474. doi: 10.1007/s10815-014-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang JG, Douglas NC, Nakhuda GS, Choi JM, Park SJ, Thornton MH, et al. The association between anti-Müllerian hormone and IVF pregnancy outcomes is influenced by age. Reprod BioMed Online. 2010;21:757–761. doi: 10.1016/j.rbmo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 80.Goswami M, Nikolaou D. Is AMH level, independent of age, a predictor of live birth in IVF? J Hum Reprod Sci. 2017;10:24–30. doi: 10.4103/jhrs.JHRS_86_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kavoussi SK, Odenwald KC, Boehnlein LM, Summers-Colquitt RB, Pool TB, Swain JE, et al. Antimüllerian hormone as a predictor of good-quality supernumerary blastocyst cryopreservation among women with levels <1 ng/mL versus 1–4 ng/mL. Fertil Steril. 2015;104:633–636. doi: 10.1016/j.fertnstert.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Teramoto S, Osada H, Sato Y, Shozu M. Nondominant small follicles are a promising source of mature oocytes in modified natural cycle in vitro fertilization and embryo transfer. Fertil Steril. 2016;106:113–118. doi: 10.1016/j.fertnstert.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Lim J, Park SG, Yoon S, Yang S-H, Chian RC. Combination of natural cycle IVF with IVM as infertility treatment. In: Tan SL, Chian RC, Buckett WM, editors. In-vitro maturation of human oocytes: basic science to clinical application. London: Informa Healthcare Press; 2007. pp. 353–360. [Google Scholar]
- 84.Tang-Pedersen M, Westergaard LG, Erb K, Mikkelsen AL. Combination of IVF and IVM in naturally cycling women. Reprod BioMed Online. 2012;24:47–53. doi: 10.1016/j.rbmo.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Lim J-H, Yang S-H, Xu Y, Yoon S-H, Chian R-C. Selection of patients for natural cycle in vitro fertilization combined with in vitro maturation of immature oocytes. Fertil Steril. 2009;91:1050–1055. doi: 10.1016/j.fertnstert.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Shao L, Xu Y, Cui Y, Liu J, Chian R-C. Effect of Anti-Mullerian hormone in culture medium on quality of mouse oocytes matured in vitro. PLoS One. 2014;9:e99393. doi: 10.1371/journal.pone.0099393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen APN, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt KLT, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Müllerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol. 2005;234:87–93. doi: 10.1016/j.mce.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, et al. Intra-cellular mechanism of Anti-Müllerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016;433:56–65. doi: 10.1016/j.mce.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A. 2017;114:E1688–E1697. doi: 10.1073/pnas.1620729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Margolskee A, Selgrade JF. A lifelong model for the female reproductive cycle with an antimüllerian hormone treatment to delay menopause. J Theor Biol. 2013;326:21–35. doi: 10.1016/j.jtbi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 92.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc Natl Acad Sci. 2005;102:111–116. doi: 10.1073/pnas.0407772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, et al. Müllerian inhibiting substance inhibits breast cancer cell growth through an NFκB-mediated pathway. J Biol Chem. 2000;275:28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 94.Levin G, Zigron R, Haj-Yahya R, Matan LS, Rottenstreich A. Granulosa cell tumor of ovary: a systematic review of recent evidence. Eur J Obstet Gynecol Reprod Biol. 2018;225:57–61. doi: 10.1016/j.ejogrb.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Färkkilä A, Koskela S, Bryk S, Alfthan H, Bützow R, Leminen A, et al. The clinical utility of serum anti-Müllerian hormone in the follow-up of ovarian adult-type granulosa cell tumors—a comparative study with inhibin B. Int J Cancer. 2015;137:1661–1671. doi: 10.1002/ijc.29532. [DOI] [PubMed] [Google Scholar]
- 96.Chong YH, Campbell AJ, Farrand S, McLennan IS. Anti-Müllerian hormone level in older women: detection of granulosa cell tumor recurrence. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2012;22:1497–1499. doi: 10.1097/IGC.0b013e318270ac69. [DOI] [PubMed] [Google Scholar]
- 97.Ge W, Clendenen TV, Afanasyeva Y, Koenig KL, Agnoli C, Brinton LA, et al. Circulating anti-Müllerian hormone and breast cancer risk: a study in ten prospective cohorts. Int J Cancer. 2018;142:2215–2226. doi: 10.1002/ijc.31249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Basal E, Ayeni T, Zhang Q, Langstraat C, Donahoe PK, Pepin D, et al. Patterns of Müllerian inhibiting substance type II and candidate type I receptors in epithelial ovarian cancer. Curr Mol Med. 2016;16:222–231. doi: 10.2174/1566524016666160225151131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified Müllerian inhibiting substance inhibits human ovarian cancer in vivo. Clin Cancer Res. 2002;8:2640–2646. [PubMed] [Google Scholar]
- 101.Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-mullerian hormone levels in young women. Breast Cancer Res Treat. 2013;137:571–577. doi: 10.1007/s10549-012-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98:141–144. doi: 10.1016/j.fertnstert.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 104.Passildas J, Collard O, Savoye A-M, Dohou J, Ginzac A, Thivat E, et al. Impact of chemotherapy-induced menopause in women of childbearing age with non-metastatic breast cancer – preliminary results from the MENOCOR study. Clin Breast Cancer. 2019;19:e74–e84. doi: 10.1016/j.clbc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, et al. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci. 2017;114:3186–3191. doi: 10.1073/pnas.1617233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donnez J, Dolmans M-M, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 107.Anderson RA, Wallace WHB. Antimüllerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil Steril. 2013;99:1469–1475. doi: 10.1016/j.fertnstert.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Jacobs MH, Reuter LM, Baker VL, Craig LB, Sakkas D, Surrey E, et al. A multicentre evaluation of the Elecsys® anti-Müllerian hormone immunoassay for prediction of antral follicle count. Reprod BioMed Online. 2019;38:845–852. doi: 10.1016/j.rbmo.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 109.Ferguson JM, Pépin D, Duru C, Matejtschuk P, Donahoe PK, Burns CJ. Towards international standardization of immunoassays for Müllerian inhibiting substance/anti-Müllerian hormone. Reprod BioMed Online. 2018;37:631–640. doi: 10.1016/j.rbmo.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]