Abstract
A newly marine Halomonas pacifica strain Cnaph3 was isolated, as a naphthalene degrader and biosurfactant producer, from contaminated seawater collected in Ataya’s fishing harbor, located in Kerkennah Islands, Tunisia. Chromatography flame ionization detector analysis revealed that 98.8% of naphthalene (200 mg/L) was degraded after 7 days of incubation, at 30 g/L NaCl and 37 °C. Strain Cnaph3 showed also a noticeable capacity to grow on a wide range of aliphatic, aromatic, and complex hydrocarbons. Interestingly, strain Cnaph3 showed a significant potential to produce biosurfactants in the presence of all tested substrates, particularly on glycerol (1%, v/v). Electrospray ionization analysis of the biosurfactant, designated Bios-Cnaph3, suggested a lipopeptide composition. The critical micelle concentration of Bios-Cnaph3 was about 500 mg/L. At this concentration, the surface tension of the water was reduced to 27.6 mN/m. Furthermore, Bios-Cnaph3 displayed interesting stabilities over a wide range of temperatures (4–105 °C), salinities (0–100 g/L NaCl), and pH (2.2–12.5). In addition, it showed promising capacities to remove used motor oil from contaminated soils. The biodegradation and biosurfactant-production potential of the Halomonas sp. strain Cnaph3 would present this strain as a favorite agent for bioremediation of hydrocarbon-contaminated sites under saline conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2085-x) contains supplementary material, which is available to authorized users.
Keywords: Naphthalene, Biodegradation, Lipopeptides, Halomonas pacifica, Hydrocarbons remobilization
Introduction
Petroleum extraction and industrial and boating activities are serious sources of seawater contamination because they release huge amounts of hydrocarbons into the marine environment (Tornero and Hanke 2016). They contribute enormously to the rise of toxicity induced by polycyclic aromatic hydrocarbons (PAHs). These latter are common environmental contaminants which are highly toxic due to their carcinogenic and mutagenic effects (Ojha et al. 2019). The United States Environmental Protection Agency (US-EPA) classified many PAHs such as pyrene, fluoranthene, phenanthrene, anthracene, and naphthalene as priority pollutants (Cerniglia 1992). Their widespread presence in the environment and bioaccumulation potential can cause massive damage to the marine environment (Tornero and Hanke 2016). Hence, research has never ceased attempting to find solutions to reduce or eliminate PAHs (Tornero and Hanke 2016). Several physical and chemical methods have been implemented for this purpose (Dave and Ghaly 2011). Unfortunately, most of the designed physiochemical processes proved to be inefficient, costly, or not environment-friendly. Consequently, researches turned to biotech-based approaches as an alternative or complement to the physico-chemical techniques (Azubuike et al. 2016). Bioremediation was defined as a process that uses microorganisms or their enzymes to transform hydrocarbons to less or non-hazardous compounds. Bioremediation processes by microorganisms including bacteria, fungi, and algae were adopted as promising methods by which most xenobiotic pollutants are eliminated from the marine environment (Azubuike et al. 2016). Nevertheless, despite the great advances of this field and the advantages of this process, it seemed that bioremediation still suffered from some limitations. Indeed, Fathepure (2014) observed that high salt concentrations affected the performance of conventional microbiological treatment methods. In fact, high salinity affects the microbial life by disturbing the integrity of cell membrane and by denaturing enzymes (Fathepure 2014). In this context, several studies suggest that halophilic bacteria may have greater potential to degrade hydrocarbons. These microorganisms develop different mechanisms for their adaptation to the osmotic pressure exerted by the high salinity of the medium (Fathepure 2014; Singh et al. 2019).
Furthermore, due to the recalcitrant nature of hydrocarbons, the use of surfactant compounds has also been suggested for numerous industrial and environmental applications. In fact, they increase the hydrophobicity of degrading microorganisms and the accessibility of cells to hydrophobic substrates (Peele et al. 2016). Most commercially available surfactants are from chemical origin and are petroleum products. They cause a risk to the environment because of their high toxicity and lack of biodegradability (Banat et al. 2014). Therefore, researchers have become more interested in the application of biosurfactants known by their high biodegradability, high selectivity, low toxicity, low environmental impact, and production in the presence of renewable resources (Banat et al. 2014; Peele et al. 2016). Because of these advantages, biosurfactants were extensively used in various applications such as bioremediation, enhanced oil recovery, food processing, and pharmaceutics (Santos et al. 2016).
For these reasons, this study purported to fulfill, firstly, on the screening of a newly halotolerant strain, named Cnaph3, from contaminated seawater taken from Ataya fishing harbor located in Kerkennah Islands. Secondly, the capacity of strain Cnaph3, isolated on naphthalene, to metabolize hydrocarbons and to produce biosurfactant, accelerating the biodegradation rate, would be studied. Finally, the production of biosurfactants by strain Cnaph3, using glycerol as carbon source, and its use in the remediation of hydrocarbons contaminated environments, would be also investigated. Consequently, the use of a polluting by-product (glycerol) reduces the cost of the process and rid the environment of a serious source of pollution.
Materials and methods
Sampling
Seawater samples contaminated with hydrocarbons were collected in January 2014 from Ataya fishing harbor (34° 43′ 35.1″ N. 11° 17′ 53.8″ E) located in Kerkennah islands, Tunisia. The samples were transferred in sterile bottles and were stored at 4 °C and away of light for further studies, such as, the physico-chemical characterization and the isolation of marine hydrocarbonoclastic bacteria.
Culture media and chemicals
The modified Luria–Bertani (LB) medium was prepared by dissolving 10 g peptone, 5 g yeast extract, and 30 g NaCl in one liter of distilled water. Nutrient broth medium (NB) consisted of (g/L): 15 peptone, 3 yeast extract, 1 glucose, and 6 NaCl. The basal medium (BM) contained 0.3 g KH2PO4, 0.4 g NH4Cl, 0.33 g MgCl2·6H2O, 0.05 g CaCl2·2H2O, 30 g NaCl and 1 mL trace-element solution (Widdel and Pfennig 1981) dissolved in one liter of distilled water. The desired pH (7–7.2) was adjusted with 10 M NaOH solution. The media were autoclaved at 121 °C for 20 min to get sterilized. Aliphatic hydrocarbons, octane, decane; monoaromatic compounds, benzene, toluene, ethylbenzene, o-, m-, and p-xylenes (BTEX), gentisic, vanillic, syringic, gallic, caffeic and ferulic acids, phenol, and tyrosol; and polycyclic aromatic hydrocarbons (PAHs) (naphthalene, phenanthrene, fluoranthene, and pyrene), were obtained from Sigma-Aldrich Company (98–99% purity). Aliphatics and monoaromatics were filtrated (pore size 0.45 μm; Millipore) while PAHs were autoclaved, to get sterilized. Shell Company (Sfax, Tunisia) provided the complex hydrocarbons needed such as: motor oil and diesel oil which were filtrated (pore size 0.45 μm; Millipore) to get sterilized. The necessary crude oil was obtained from “Thyna Petroleum Services” (Sfax, Tunisia). It was sterilized through autoclaving. Olive oil and corn oil were purchased from local commercial centers. They were sterilized by filtration (pore size 0.45 μm; Millipore). Glycerol and chemical surfactants (Titon X-100, Tween 80 and sodium dodecyl sulfonate (SDS)) were bought from Sigma Aldrich Company (98–99% purity). Glycerol was sterilized by autoclaving. Chemical surfactants, dissolved at concentrations corresponding to their CMCs, were filtrated (pore size 0.45 μm; Millipore).
Physico-chemical analyses, microscopy, and analytical techniques
pH, electrical conductivity (EC), chemical oxygen demand (COD), 5-day biological oxygen demand (BOD5), total Kjeldahl nitrogen (TKN) and total organic carbon (TOC) were performed as described by Chebbi et al. (2016). Mineral and heavy metal concentrations were determined using a flame atomic absorption spectrometry (Analyst 200 Atomic Absorption Spectrometer, PerkinElmer). An OLYMPUS BX51 phase contrast microscope equipped with an OLYMPUS DP70 digital camera was used to examine the purity, shape, and motility of bacteria. Bacterial growth was assessed, using a Shimadzu model UV-1800 spectrophotometer, by measuring the absorbance at 600 nm.
Seawater samples (50 mL) were extracted with dichloromethane (DCM, v/v) (2 times), evaporated, dissolved in equal volume of DCM, and then analyzed by gas chromatography-mass spectrometry (GC–MS) as explained previously (Hentati et al. 2016), to identify hydrocarbons present in the samples. To evaluate the ability of the isolated strain to degrade hydrocarbon, samples of culture Cnaph3 containing the hydrocarbon and an abiotic control were extracted with dichloromethane (DCM, v/v) (2 times), evaporated, dissolved in equal volume of DCM and then analyzed by chromatography flame ionization detector (GC-FID). This latter was performed with an Agilent technology model 6890 N chromatograph apparatus equipped with a capillary HP-5 column (length, 30 m; internal diameter, 0.25 μm). The carrier gas was helium used at a flow rate of 1 mL/min. The temperature was first set at 50 °C for 10 min and was increased to 250 °C at 15 °C/min, then to 300 °C, and finally set at 300 °C for 5 min.
A digital Gibertini Tensiometer (TSD132389, Milan, Italy) was utilized to measure the surface tension according to the method described previously (Chebbi et al. 2016). Fourier transform infrared (FTIR) and electrospray ionization (ESI) (LC/MSD-TOF, Agilent Technologies, Palo Alto, CA) analyses were carried out as reported by Chebbi et al. (2017) and Coronel-León et al. (2015), respectively.
Enrichment cultures and isolation of naphthalene-degrading bacteria
The seawater collected from Ataya fishing harbor was used for the screening of aerobic hydrocarbon degrading bacterial strains at 37 °C, 30 g/L NaCl and in the presence of a PAH (naphthalene, phenanthrene, fluoranthene, or pyrene) (200 mg/L), as described by Hentati et al. (2016). After several enrichments, a stable microbial growth on naphthalene (200 mg/L), obtained after 4 times of subculturing during 1–2 weeks, was then used to isolate pure bacteria. Twelve single colonies were chosen and a pure strain named Cnaph3 was selected, for further experimental assays, based on its high capacity to degrade naphthalene (200 mg/L), in solid and liquid media, at 37 °C, 30 g/L NaCl, and without addition of yeast extract.
Characterization and biodegradation studies
Flask cultures containing 50 mL BM with 200 mg/L naphthalene were used to perform growth studies under the following conditions: 30 g/L NaCl, 180 rpm, and at 37 °C. All experiments were duplicated with an inoculum proportion of 3% (v/v), which was sub-cultured at least once under the same conditions. Similar flasks, without bacterial inoculation, were used as abiotic controls to confirm that there were no losses of naphthalene by evaporation. Experiments were performed in 250 mL-Erlenmeyer flasks which were closed with rubber stoppers to prevent naphthalene losses. Moreover, only 50 mL of basal medium were poured in these Erlenmeyer flasks to leave approximately 200 mL-air-headspace to maintain aerobic conditions. Growth of strain Cnaph3 on naphthalene was confirmed in liquid medium by measuring the OD at 600 nm, by determination of Colony Forming Units (CFU) and by GC-FID analyses at different times of culture. Phenotypic and phylogenetic analyses were performed as reported by Hentati et al. (2016).
The capacity of strain Cnaph3 (3%, v/v) to grow on several hydrocarbons was assessed by measuring the OD 600 nm of different cultures containing BM supplemented with these substrates: PAHs, phenanthrene, fluoranthene, and pyrene (200 mg/L), benzoic and cinnamic derivatives (gentisic, vanillic, syringic, gallic, caffeic, and ferulic acids), phenol and tyrosol (5 mM), aliphatic hydrocarbons (octane and decane) and BTEX (benzene, toluene, ethylbenzene and o-, m-, and p-xylenes) (0.5% (v/v)), complex hydrocarbons: diesel, motor oil and crude oil (1% (v/v)), and Olive oil (1% (v/v)).
Production of biosurfactants by strain Cnaph3 using various carbon sources
The potential of strain Cnaph3 to produce biosurfactants was studied on a rich medium (NB) and by adding various substrates including: olive oil, corn oil, glycerol, diesel, motor oil, and crude oil, at a concentration of 1% (v/v) into culture flasks containing BM, during 10 days at 37 °C and under agitation of 180 rpm. The capacity of strain Cnaph3 (4%, v/v) to produce biosurfactants was determined by measuring the surface tension and the oil displacement, as described by Hentati et al. (2019).
Biosurfactant extraction and purification
The recuperation of biosurfactant was realized as reported by Chebbi et al. (2017), after 3 days of incubation for NB, olive oil, corn oil, and glycerol and after 4 days for hydrocarbons. The crude biosurfactant, produced on NB medium, was purified using a silica gel column (60 Mesh) (Merck, Darmstadt, Germany) and a mixture of ethyl acetate/methanol (20:80, v/v), as eluent. Fractions of 1 mL were analyzed by thin layer chromatography (TLC) on silica gel plates 60 G (Machery-Nagel, duren, Germany) with the same eluent. The resulting spots on the TLC were detected under UV light by spraying with ninhydrin and Molisch reagents, specific for free amino groups, and carbohydrates compounds, respectively. Fractions having the same polarity were pooled, while, samples showing the positive reaction with ninhydrin and Molisch reagents were analyzed by FTIR and ESI–MS.
Biosurfactant characterization
The critical micelle concentration (CMC) of the crude biosurfactant Bios-Cnaph3, produced on glycerol (1%, v/v), was estimated by measuring the surface tension of different concentrations (0–2 g/L) (Chebbi et al. 2017). The effect of temperature (4, 30, 37, 55, 70 °C and 105 °C), salinity (0, 20, 30, 40, 60, 80, 100, 120, and 150) and pH (2.2, 4.1, 7.2, 9.1, 11.1, and 12.5) on Bios-Cnaph3 stability was also evaluated using cell-free supernatants of strain Cnaph3 growing on glycerol (1%, v/v) as reported by Hentati et al. (2019).
Bios-Cnaph3 application in remobilization of hydrocarbons-polluted soil
To evaluate the capacity of Bios-Cnaph3 biosurfactant in hydrocarbons remobilization, 20 mL of used motor oil were used to impregnate 100 g of soil collected from a field in Sfax, Tunisia. Specimens (10 g) placed into 250-mL Erlenmeyer flasks were subject to these treatments: addition of 20 mL Milli-Q water (control) or 20 mL of crude biosurfactant Bios-Cnaph3 solution at the CMC (0.05%, w/v) or 20 mL of the cell-free supernatant of strain Cnaph3 grown on glycerol or 20 mL of the aqueous solutions of synthetic surfactants prepared at their CMCs: Triton X-100 (0.0155%, w/v), Tween 80 (0.0016%, w/v) and SDS (0.2304%, w/v). The specimens were maintained for 24 h at 30 °C and 180 rpm, then centrifuged 20 min at 6000 rpm to part the soil from the supernatant. This latter was extracted (twice, v/v) using hexane. The amount of hydrocarbons removal from the soil after each treatment was gravimetrically measured and expressed as stated by Hentati et al. (2019).
Statistical analysis
Values given were represented as standard deviation (means ± SD) of three independent replicates. All data were statistically analyzed by one-way ANOVA. Tukey's multiple comparisons test with a significance level of p < 0.05 was applied.
Results and discussions
Physico-chemical characterization
Seawater pH
Table 1 shows the physico-chemical characteristics of the seawater collected from the fishing harbor of Ataya, Kerkennah Islands, Tunisia, used to assess the level of pollution in the studied environment. The first observation would be related to the 8.2 neutral pH value of the seawater under investigation. This would imply that this environment was adequate for the life of aquatic animals. Indeed, as reported by Faragallah et al. (2009), aquatic animals prefer pH values ranging between 6.5 and 8.5 to survive. Any pH values outside this range would be stressful for the physiological organ systems of organisms. Similar result was found for seawater collected from the fishing harbor of Sfax, Tunisia, which was also characterized by a neutral pH (pH 7.4) (Hentati et al. 2016).
Table 1.
Physico-chemical characteristics of the seawater
taken from the fishing harbor of Ataya, Kerkennah Islands
| Characteristics | Values |
|---|---|
| pH | 8.20 |
| Temperature (°C) | 17.5 |
| Electrical conductivity (mS/cm) | 31.5 |
| Salinity (g/L) | 26.7 |
| Total carbon (TC) (mg/L) | 253 |
| Total organic carbon (TOC) (mg/L) | 236 |
| Inorganic carbon (IC) (mg/L) | 17 |
| COD (mg O2/L) | 771 |
| BOD5 (mg O2/L) | 150 |
| COD/ BOD5 | 5.14 |
| Total nitrogen (mg/L) | 60.2 |
| Total hydrocarbons (mg/L) | 0.22 |
| Minerals (mg/L) | |
| Na | 936 |
| Ca | 170 |
| Mg | 204 |
| K | 150 |
| Metals (μg/L) | |
| Fe | 405 |
| Cu | 0 |
| Mn | 4 |
| Ni | 58 |
| Zn | 201 |
| Cr | 138 |
| Cd | 4 |
Seawater temperature
As can be seen in Table 1, the seawater temperature was 17.5 °C. This was in total agreement with Hentati et al. (2016) who reported that the temperature of the Sfax fishing harbor, recorded in January 2014, was about 17.4 °C. Moreover, this result corroborates with the Global Climate Report (2015) estimating that as a semiarid climate area, Sfax seawater would have 16 °C as a mean temperature in January. Indeed, Faragallah et al. (2009) rightly argued that seawater temperature was directly dependent on different factors such as weather and stormwater.
Minerals concentration
Table 1 exhibits the concentrations of Na, Ca, Mg, and K. These minerals had concentrations of 936, 170, 204, and 150 mg/L, respectively. The mineral concentration values recorded in this study were lower than the mineral concentrations measured in the Mediterranean seawater (11600, 416, 1290, and 390 mg/L for Na, Ca, Mg and K, respectively (Klein et al. 1999). This finding about the low mineral concentrations was supported by the low conductivity. Indeed, as can be seen in Table 1, Ataya fishing harbor had an EC of only 31.5 mS/cm.
Seawater salinity
The salinity of the Ataya fishing harbor is shown in Table 1. This value was 26.7 g/L. Such salinity was lower than the 35 g/L salinity of seawater reported by Huber et al. (2000). More importantly, it was much lower than the 38 g/L salinity of the Mediterranean Sea reported by the same scholars. More surprisingly, this low salinity value was much lower than the 36.2 g/L salinity of the seawater collected from the fishing harbor of Sfax, Tunisia (Hentati et al. 2016). However, this can be explained by the fact that salinity changes with the evaporation rate and the amount of precipitations (Huber et al. 2000).
Heavy metals concentrations
As can be seen in Table 1, the concentrations of heavy metals reached 405, 4, 58, 201, 138, and 4 μg/L for Fe, Mn, Ni, Zn, Cr, and Cd, respectively. In addition, there was a total absence of Cu in the studied sample. The criteria of maximum concentration of heavy metals for water quality reported by USA United States Environmental Protection Agency (US EPA 2011) were 1000, 100, 470, 170, 586, 2, and 35.3 µg/L for Fe, Mn, Ni, Zn, Cr, Cd, and Cu, respectively. Therefore, the data provided by this study revealed that the heavy metals concentrations (Fe, Mn, Ni and Cr) in Ataya fishing harbor seawater were lower than the standard concentrations. However, the 201 μg/L concentration of Zn and the 4 μg/L concentration of Cd were above the standard concentrations, 170 μg/L and 2 μg/L, respectively, reported for the quality of water by the US EPA (2011). Moreover, the concentrations of heavy metals recorded in Ataya fishing harbor were also lower than those recorded in the Sfax fishing harbor reported by Hentati et al. (2016). Similarly, these heavy metals concentrations were lower than those reported by Zaghden et al. (2016) for the Sfax coasts. Indeed, these latter scholars found that the mean concentrations of Ni, Zn, Cr, Cd, and Cu were 12290, 135160, 71270, 11770, and 19750 μg/L, respectively. Zaghden et al. (2016) rightly explained these results by the fact that Sfax city was exposed to high anthropogenic and industrial effluents. Our findings would confirm previous results reported by Morley et al. (1997) who investigated this subject in the Western Mediterranean seawater and concluded that the concentrations of some heavy metals present in Western Mediterranean seawater away of pollution were 173.7, 92.1, 118.3, 6.1, and 79.6 ng/L for Fe, Mn, Ni, Cd, and Cu, respectively, since heavy metals naturally occurring in marine environment would have very small amounts. When above the critical levels, heavy metals would become toxic. They would damage marine life, by reducing cell division rates, and human health by their accumulation in several food chains (Zaghden et al. 2016).
Total organic carbon (TOC)
Table 1 shows that the organic carbon in Ataya harbor seawater was 236 mg/L. This concentration was much higher than the 17 mg/L concentration of inorganic carbon. Similar results were reported for seawater collected from fishing harbor of Sfax (organic carbon = 292.6 mg/L; inorganic carbon = 37.4 mg/L) (Hentati et al. 2016). Zaghden et al. (2016) rightly argued that contamination by organic compounds in coastal areas and harbors contributed to high levels of TOC in seawaters and marine sediments.
COD and BOD5
The results exhibited in Table 1 show that the COD was equal to 771 mg/L and BOD5 was equal to 150 mg/L. These findings were very close to the COD and the BOD5 values in seawater collected from the fishing harbor of Sfax, Tunisia (COD = 827 mg/L and BOD5 = 256 mg/L) (Hentati et al. 2016). The latter scholars rightly observed that COD and BOD5 were indicators of organic matters in marine water. Nevertheless, Zaghden et al. (2016) argued that if the ratio COD/BOD5 was higher than 3, then there would be a chemical pollution that should be treated biologically. The ratio calculated in this study was equal to 5.14. Therefore, it could be induced that there was a strong presence of a chemical pollution which was difficult to eliminate naturally.
Nitrogen concentration
As can be seen in Table 1, nitrogen concentrations were 60.2 mg/L. These values were much lower than those recorded in the fishing harbor of Sfax city reaching 103 mg/L (Hentati et al. 2016). Zaghden et al. (2016) explained the high values of nitrogen compounds by the discharge of industrial wastes, the domestic sewage, and the solid wastes in the coastal area of Sfax city.
Hydrocarbons concentrations
Table 1 reveals that the seawater sample from Ataya fishing harbor contained concentrations of hydrocarbons equal to 220 μg/L. Such concentrations were much higher than the maximum of 100 μg/L tolerated by US EPA (1986). Nevertheless, these values were much lower than the hydrocarbons concentrations recorded in the neighboring fishing harbor of Sfax. Indeed, Hentati et al. (2016) reported values of hydrocarbons as high as 600 μg/L in the latter harbor. This contamination was rightly explained by Zaghden et al. (2016) as a result of intensive industrial and boating activities in Sfax. The GC–MS analysis shown in Table 2 confirms these results. Indeed, aliphatic hydrocarbons were detected. The presence of n-alkanes, from C13 to C28, in the studied seawater would suggest a petroleum contamination. This observation would also confirm Hentati et al.'s (2016) finding about the presence of a variety of hydrocarbons such as n-alkanes from C12 to C25. Hexadecane was also present in the studied seawater. Gifford et al. (2006) explained its presence by the development of urbanization, industrialization, and boating activities. Table 2 also reveals the presence of eicosane in the sample. This would indicate that diesel was the source of contamination in the fishing harbor of Ataya. In line with Güven et al. (1997), who detected eicosane in seawater collected from Izmit Bay, Turkey, it could be induced that industrial discharges and ship traffic were the causes of petroleum pollution in this area.
Table 2.
GC–MS analysis of hydrocarbons present in seawater samples collected from the fishing harbor of Ataya, Kerkennah Islands, Tunisia
| Compounds | Molecular weight (MW) (g/mol) | Retention time (Rt) (min) | m/z (M+) | % of identity in database |
|---|---|---|---|---|
| Tridecane (C13H28) | 184.3 | 6.92 | 184 | 97 |
| Tetradecane (C14H30) | 198.3 | 7.62 | 198.3 | 97 |
| Pentadecane (C15H32) | 212.4 | 8.27 | 212 | 98 |
| Hexadecane (C16H34) | 226.4 | 8.89 | 227 | 98 |
| Heptadecane (C17H36) | 240.48 | 9.47 | 241 | 97 |
| Octadecane (C18H38) | 254.5 | 10.02 | 255 | 98 |
| Nonadecane (C19H40) | 268.52 | 10.52 | 269 | 97 |
| Eicosane (C20H42) | 282.5 | 10.93 | 282 | 98 |
| Heneicosane (C20H42) | 296.5 | 11.31 | 296 | 98 |
| Docosane (C22H46) | 310.6 | 11.64 | 310.4 | 99 |
| Tricosane (C23H48) | 324.6 | 11.94 | 324.4 | 99 |
| Tetracosane (C24H50) | 338.6 | 12.23 | 338.4 | 99 |
| Hexacosane (C26H54) | 366.7 | 12.84 | 366.4 | 98 |
| Octacosane (C28H58) | 394.7 | 13.71 | 394 | 95 |
In conclusion, the physical and chemical characterization of the studied seawater showed that the seawater in Sfax coasts and harbor was much more polluted than the seawater in the harbor of Ataya, Kerkennah despite the proximity of the two locations. In line with Zaghden et al. (2016), this difference could be plausibly explained by the more intensive urbanization, industrial, and boating activities in Sfax. However, there was a particularly concerning presence of hydrocarbons pollution in Ataya harbor. This would be due to the recent petroleum exploration and exploitation activities in the area. If not rapidly addressed, the issue of pollution would be very dangerous for the ecosystem.
Characterization of strain Cnaph3
Strain Cnaph3 was an aerobic, Gram-negative, rod-shaped, motile, and non-spore-forming bacterium that occurred individually. Oxidase and catalase activities were positive. Agar colonies, formed after overnight culture, were generally circular, slightly convex, smooth, opaque, cream and with 1–2 mm diameters. As can be seen in Table 3, the strain Cnaph3 was able to grow at temperatures ranging between 30 and 45 °C. At 37 °C, the strain showed an optimal growth. It was also observed that at 55 °C the strain Cnaph3 was unable to grow. In addition, Table 3 shows that concentrations of NaCl between 0 and 200 g/L were adequate for growth. However, the optimum growth could be achieved at 30 g/L. Furthermore, the study revealed that whereas the strain Cnaph3 could grow within pH values ranging between 6.5 and 10, the optimal pH value for the growth was 7. Yet, the strain failed to grow at pH 4.2 or 12.1. Finally, Table 3 and Figure S1 exhibit the results related to the phylogeny and the taxonomy of the strain Cnaph3 and its closest neighbors in the genus Halomonas. This strain could be closely related to members of the genus Halomonas, in particular to the species of Halomonas pacifica (Type strain: ATCC 27840T) described by Baumann et al. (1983), with a similarity of 98.7%. Its 16S rRNA gene sequence, comprising 1403 nucleotides, was deposited in the nucleotide database GenBank under accession number KX946967.
Table 3.
The differential phenotypic characteristics of the strain Cnaph3 and other related strains of the genus Halomonas
| Characteristics | Strain Cnaph3 | Halomonas pacifica DSM 7474T (Baumann et al. 1983) | Halomonas salifodinae JCM 14803T (Wang et al. 2008) | Halomonas elongata ATCC33173T (Vreeland et al. 1980) | Halomonas eurihalina ATCC 49336T (Quesada et al. 1990) |
|---|---|---|---|---|---|
| 16S RNA similarity (%) | 100 | 98.7 | 98.4 | 94.5 | 93.5 |
| Colony pigmentation | Cream | Cream | Brown-yellow | White | Cream |
| Morphology | Rods | Rods | Long rods | Rods | Rods |
| Cell size (μm) | 0.5–0.7*1.5–2.0 | 0.6–0.8*1.6–1.9 | 0.8–1.2*4.0–6.0 | nd | 0.8–1.0*2.0–2.5 |
| Motility | + | + | + | + | + |
| Gram stain | − | − | − | − | − |
| Oxidase | + | + | + | + | − |
| Catalase | + | + | + | + | nd |
| Growth NaCl range (%, w/v) | 0–20 | 0–20 | 0.5–20 | 0–20 | 0.5–25 |
| Optimum NaCl (%, w/v) | 3 | 0.5–3 | 3 | 3–8 | 7.5 |
| Growth temperature range (°C) | 30–45 | 4–45 | 4–48 | 4–45 | 4–45 |
| Optimum temperature (°C) | 37 | 27 | 30 | 30 | 32 |
| Growth pH range | 6.5–10 | 5–10 | 6–9 | 5–10 | 5–10 |
| Optimum pH | 7 | nd | 7 | 7.5 | 7.2 |
| Growth on | |||||
| l-Arabinose | − | − | − | + | − |
| d-Galactose | nd | + | nd | − | − |
| d-Glucose | + | + | + | + | − |
| Maltose | − | − | + | + | − |
| d-Mannitol | + | + | + | + | − |
| d-Mannose | + | + | nd | − | − |
| Origin | Seawater taken from the Ataya’s fishing harbor, Kerkennah islands, Tunisia | Seawater, Hawaii | Saline soil sample collected from a salt mine at Baichengin Xinjiang Province, China | Solarsalt Co. facility located on Bonaire, Netherlands Antilles | Hyprsaline soil located near Alicante in southern Spain |
+ positive, − negative, nd not determined, T type strain
DSM Deutsche Sammlung von Mikroorganismen, JCM Japan Collection of Microorganisms, ATCC American Type Culture Collection
Biodegradation of naphthalene by strain Cnaph3
The biodegradation of naphthalene by strain Cnaph3 was studied at 200 mg/L in basal liquid and solid media. Figure 1 presents the result of the ability assessment of the strain Cnaph3 to degrade naphthalene. This assessment was conducted by determining the OD600 nm and the number of viable microbial cells at different culture times. Figure S2 d3 and d7 show the GC–FID profiles of 200 mg/L naphthalene remaining in basal medium after aerobic incubation, without yeast extract addition, at 37 °C, 30 g/L NaCl and 180 rpm after 3 and 7 days. The graphs analysis revealed that Cnaph3 was able to remove 60.4% and 98.8% of the 200 mg/L naphthalene after 3 and 7 days of incubation, respectively.
Fig. 1.
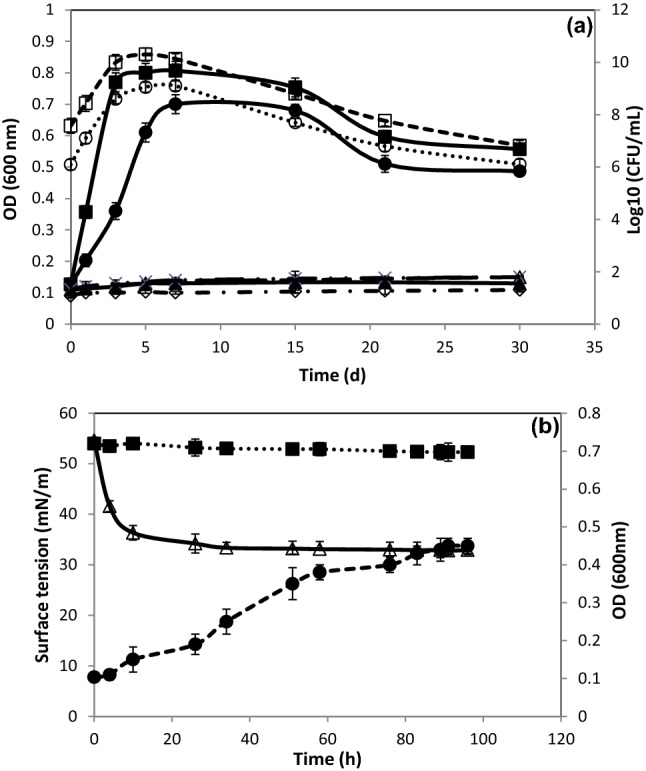
Growth of Halomonas sp. strain Cnaph3 on (filled circle) naphthalene (200 mg/L), (filled sqaure) naphthalene (200 mg/L) + Tween 80 (0.05%, v/v) and (filled triangle) Tween 80 (0.05%, v/v), in the presence of 30 g/L NaCl, at 37 °C and 180 rpm, monitored by measuring OD600nm and by determination of bacterial cell counts ((open circle) naphthalene (200 mg/L), (open square) naphthalene (200 mg/L) + Tween 80 (0.05%, v/v)). ( ×) abiotic control of naphthalene (200 mg/L), (open lozenge) abiotic control of naphthalene (200 mg/L) + Tween 80 (0.05%, v/v), (open triangle) abiotic control of Tween 80 (0.05%, v/v) (a). Surface tension detection of culture Cnaph3 on basal medium containing naphthalene (200 mg/L) at 180 rpm and 37 °C. (open triangle) Surface tension of the cell-free culture supernatant Cnaph3, (filled square) Surface tension of the abiotic control, and (filled circle) OD600 nm of the culture Cnaph3 (b)
Hence, firstly, it can be deduced that this bacterial strain showed promising capability for the biodegradation of naphthalene. Secondly, when compared to previous studies on the same subject, the strain Cnaph3 would prove more efficient that other strains in the biodegradation of hydrocarbons. Indeed, Bacillus fusiformis strain BFN, isolated from an oil refining wastewater sludge, was capable of degrading 99% of naphthalene at only a concentration of 50 mg/L after 4 days of incubation (Lin et al. 2010). Similarly, Ochrobactrum sp. strain VA1, isolated from contaminated seawater collected from the harbor of Chennai, India, degraded almost 89% of naphthalene, at a concentration of 3 mg/L, after 4 days of incubation (Arulazhagan and Vasudevan 2011).
Furthermore, as can be seen in Fig. 1a, the rate of naphthalene degradation by strain Cnaph3 was accelerated when adding synthetic surfactant, the Tween 80 (0.05%, v/v). Indeed, it was clearly observed that 99.2% of naphthalene was degraded after only 5 days of incubation. This finding confirms previous results reported by Mnif et al. (2009) and Chamkha et al. (2011) who discovered that the addition of Tween 80 (0.05%, v/v) accelerated the growth kinetics and crude oil biodegradation by Halomonas lutea strain C2SS100 and Klebsiella oxytoca strain BSC5, respectively. This phenomenon was plausibly explained by Peele et al. (2016), who argued that surfactants, known by the presence of polar and apolar regions, increase the solubility of hydrocarbons, thereby decreasing the interfacial tension.
Finally, Fig. 1b shows that naphthalene biodegradation (200 mg/L) was accompanied with surface tension reduction of the cell-free medium from 54.6 to 33.3 mN/m, after 34 h of incubation. This observation could be explained by the production of biosurfactants by the strain Cnaph3.
Growth on other hydrocarbons
Table 4 exhibits the growth pattern of the strain Cnaph3 on various hydrocarbons and olive oil as the sole carbon and energy sources. The strain Cnaph3 seemed to grow on most of the tested hydrocarbons including monoaromatics, PAHs, complex hydrocarbons, aliphatic hydrocarbons as well as olive oil. However, it was unable to grow on syringic acid. As regards the BTEX family, the strain Cnaph3 seemed to survive only on benzene. This would imply that the strain Cnaph3 possesses a broad spectrum catabolic machinery needed to grow on various hydrocarbons. In this context, Garcia et al. (2004) found that the Halomonas organivorans strain G16.1 degraded different aromatic pollutants at 100 g/L NaCl. Similarly, Alva and Peyton (2003) observed that the Halomonas campisalis strain 4A was capable of degrading both phenol and catechol at 150 and 100 g/L NaCl, respectively. Finally, Abdelkafi et al. (2006) revealed that the Halomonas sp. strain IMPC was capable of degrading p-coumaric acid and other aromatics under saline conditions with 80 g/L NaCl.
Table 4.
Growth pattern of the strain Cnaph3 on various hydrocarbons and olive oil as the sole carbon and energy sources at 30 g/L NaCl and without yeast extract added
| Substrates | ODmax (600 nm) | Growth of strain Cnaph3 | |
|---|---|---|---|
| Monoaromatics | Benzene | 0.32 (3 d) | + |
| Toluene | 0.12 (7 d) | − | |
| Ethylbenzene | 0.11 (7 d) | − | |
| p-xylene | 0.11 (7 d) | − | |
| o-xylene | 0.11 (7 d) | − | |
| m-xylene | 0.11 (7 d) | − | |
| Gentisic acid | 0.32 (4 d) | + | |
| Vanillic acid | 0.30 (4 d) | + | |
| Syringic acid | 0.18 (7 d) | − | |
| Gallic acid | 0.68 (3 d) | ++ | |
| Tyrosol | 0.23 (5 d) | + | |
| Phenol | 0.30 (5 d) | + | |
| Caffeic acid | 0.28 (5 d) | + | |
| Ferulic acid | 0.30 (5 d) | + | |
| Polyaromatics | Fluoranthene | 0.43 (4 d) | + |
| Phenanthrene | 0.45 (4 d) | + | |
| Pyrene | 0.39 (4 d) | + | |
| Alcanes | Octane | 0.28 (2 d) | + |
| Decane | 0.31 (2 d) | + | |
| Complex hydrocarbons | Diesel oil | 0.27 (3 d) | + |
| Motor oil | 0.22 (3 d) | + | |
| Crude oil | 0.32 (4 d) | + | |
| Olive oil | 0.84 (2 d) | ++ | |
d days, OD600 nm (Biological control) = 0.11 (7 d)
− OD600 nm max < 0.2 (no growth), +: OD600 nm max: 0.2–0.5 (growth), ++ OD600 nm max > 0.5 (important growth)
Biosurfactant Bios-Cnaph3 studies
On the one hand, Table 5 shows the evaluation of biosurfactant production by Halomonas pacifica strain Cnaph3 growing on various carbon sources at 37 °C and 180 rpm during 10 days. The strain Cnaph3 showed a better growth on NB, olive oil, corn oil, and glycerol than on hydrocarbons (p < 0.05). On the other hand, Fig. 2 shows the evaluation of surface tension and oil displacement test of the strain Cnaph3 growing on basal medium containing glycerol (1%, v/v) as the sole carbon and energy source, at 180 rpm and 37 °C during 10 days.
Table 5.
Evaluation of biosurfactant production by Halomonas Pacifica strain Cnaph3 growing on various carbon sources at 37 °C and 180 rpm during 10 days
| Carbon sources | ODmax (600 nm) | STI (mN/m) | STF (mN/m) | RST (mN/m) | ODT (cm) | Biosurfactant yield (g/L) |
|---|---|---|---|---|---|---|
| Nutrient Broth (NB) | 1.78 ± 0.1**** (2d) | 59.3 ± 0.4 | 27 ± 0.06**** | 32.3 ± 0.53**** | 7.8 ± 0.24**** | 1.21 ± 0.08**** |
| Olive oil (1%, v/v) | 1.66 ± 0.1**** (2d) | 56.2 ± 0.7*** | 31.3 ± 0.64**** | 24.9 ± 1.42**** | 7.9 ± 0.35**** | 0.85 ± 0.03**** |
| Corn oil (1%, v/v) | 1.35 ± 0.08**** (2d) | 57.5 ± 0.5** | 32.2 ± 0.57**** | 25.2 ± 0.08**** | 7.8 ± 0.15**** | 0.81 ± 0.06**** |
| Glycerol (1%, v/v) | 1.26 ± 0.15**** (5d) | 54.5 ± 0.4**** | 27.2 ± 0.17**** | 27.3 ± 0.26**** | 8.5 ± 0.06**** | 0.75 ± 0.03**** |
| Diesel oil (1%, v/v) | 0.31 ± 0.07 (3d) | 57 ± 0.7** | 42.6 ± 0.46**** | 14.4 ± 1.17**** | 4.1 ± 0.13**** | 0.12 ± 0.006 |
| Motor oil (1%, v/v) | 0.23 ± 0.07 (3d) | 55.1 ± 0.5**** | 43.9 ± 0.62**** | 11.2 ± 0.8**** | 3.7 ± 0.51**** | 0.11 ± 0.004 |
| Crude oil (1%, v/v) | 0.31 ± 0.05 (4d) | 55.4 ± 0.5**** | 42.9 ± 1.46**** | 12.5 ± 1.68**** | 3.6 ± 0.5**** | 0.13 ± 0.01 |
| Biotic control | 0.14 ± 0.02 (2d) | 61.4 ± 1.6 | 59.2 ± 0.17 | 2.16 ± 1.44 | 0.6 ± 0.06 | 0 |
Values given represent the mean of three replicates ± standard deviation
ODmax maximum optical density, d days, STI initial surface tension, STF final surface tension, RST reduction of surface tension, ODT oil displacement test
*, **, ***, ****Significant differences (p < 0.05) versus biotic control (**** < 0.0001; *** < 0.001; ** < 0.01 and * < 0.05)
Fig. 2.
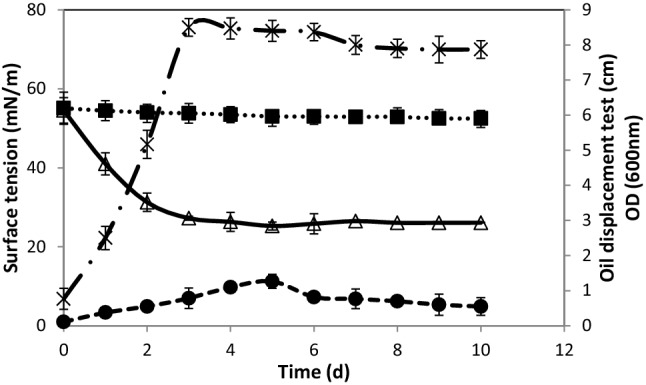
Evaluation of surface tension and oil displacement test of strain Cnaph3 growing on basal medium containing glycerol (1%, v/v) as the sole carbon and energy source, at 180 rpm and 37 °C during 10 days. (filled circle) OD600nm (open triangle) Surface tension of the cell-free culture supernatant Cnaph3, (filled square) Surface tension of the abiotic control, and ( ×) Oil displacement test determined by measuring the diameter of clear zone formed
It seemed that NB, vegetable oils, and glycerol allowed an interesting ability of the strain Cnaph3 to reduce surface tension (RST = 32.3, 24.9, 25.2 and 27.3 mN/m, respectively) (p < 0.05) (Table 5). Moreover, oil displacement test indicated the creation of different halo zones with varying diameters from 3.6 to 8.5 cm, suggesting a biosurfactant synthesis (p < 0.05). The largest clear zones were noticed in the cases of glycerol (ODT = 8.5 cm), vegetable oils (olive oil, corn oil with ODT = 7.9 and 7.8 cm, respectively), and NB (ODT = 7.8 cm), as substrates (Table 5). In contrast, in the presence of hydrocarbons, strain Cnaph3 showed less ability to produce surface active agents, during 10 days of incubation (Table 5). The highest biosurfactant production (1.21 g/L) was noticed when using NB medium. Besides, the quantities of biosurfactants produced were 0.85, 0.81 and 0.75 g/L when strain Cnaph3 was grown on olive oil, corn oil, and glycerol, respectively (p < 0.05). When growing on hydrocarbons only little production of biosurfactant (0.11–0.13 g/L) was noticed (Table 5). The highest production in nutrient broth (1.21 g/L) may be explicated by the high content of protein present in this medium (Hentati et al. 2019). In contrast, the synthesis of biosurfactants using diesel, motor and crude oils, as carbon sources, was low (0.11–0.13 g/L), because of the less biodegradability of these hydrocarbons (Chebbi et al. 2017). Hence, it can be concluded that the biosurfactant yield was dependent on the type of the used carbon source. Consequently, this study would recommend the selection of NB and glycerol as the most adequate media for growing the strain Cnaph3 that can yield satisfactory amounts of biosurfactants with a low surface tension in a relatively short time.
From an environmental perspective, the principal by-product of the biodiesel production (glycerol) can be effectively used as a carbon source to synthetize biosurfactants. Therefore, the more biodiesel is consumed the more glycerol becomes available which would represent a serious problem for biodiesel companies because its removal is costly. Fortunately, da Silva et al. (2009) reported that glycerol could be effectively used for different microbial production as carbon source thanks to its solubility in water. Hence, the findings of this study would support previous works since any excess of glycerol would be welcome as an efficient nutrient for the strain Cnaph3 of Halomonas pacifica. Thus, strain Cnaph3 of Halomonas pacifica would serve a double objective. It would rid the environment of glycerol as a pollutant and would alter it into a high value product. This valorization of glycerol would have beneficial economic returns. In this context, Ndlovu et al. (2017) reported that using water miscible substrates, such as glucose and glycerol, allows to obtain high yields of surfactins, biosurfactants from Bacillus amyloliquefaciens ST34, and rhamnolipids, biosurfactants from Pseudomonas aeruginosa ST5. Likewise, Zhang et al. (2016) revealed that Bacillus atrophaeus strain 5-2a, growing on glycerol, produced an amount of 0.72 g/L of lipopeptides. In addition, two oil-degrading strains of Pseudomonas aeruginosa produced a yield around 1.7 g/L of rhamnolipids biosurfactants when growing on glycerol (Rahman et al. 2002).
Among different biosurfactants groups, the best-studied compounds are lipopeptides, from Bacillus species, and glycolipids, from Pseudomonas species (Chebbi et al. 2017; Hentati et al. 2019). Furthermore, there are few reports describing Halomonas species as biosurfactant producers. Indeed, Halomonas sp. strain MB-30 was isolated as a glycolipid producer (Dhasayan et al. 2014). Likewise, Halomonas sp. strain BS4 was able to produce glycolipid biosurfactant (Donio et al. 2013). In addition, Halomonas meridiana strain BK-AB4 produces a fatty-acid biosurfactant in the presence of palm oil (Sari et al. 2018). Finally, Halomonas elongata strain BK-AG18 was able to bioconvert glycerol into a glycolipid biosurfactant (Alvonita and Hertadi 2018).
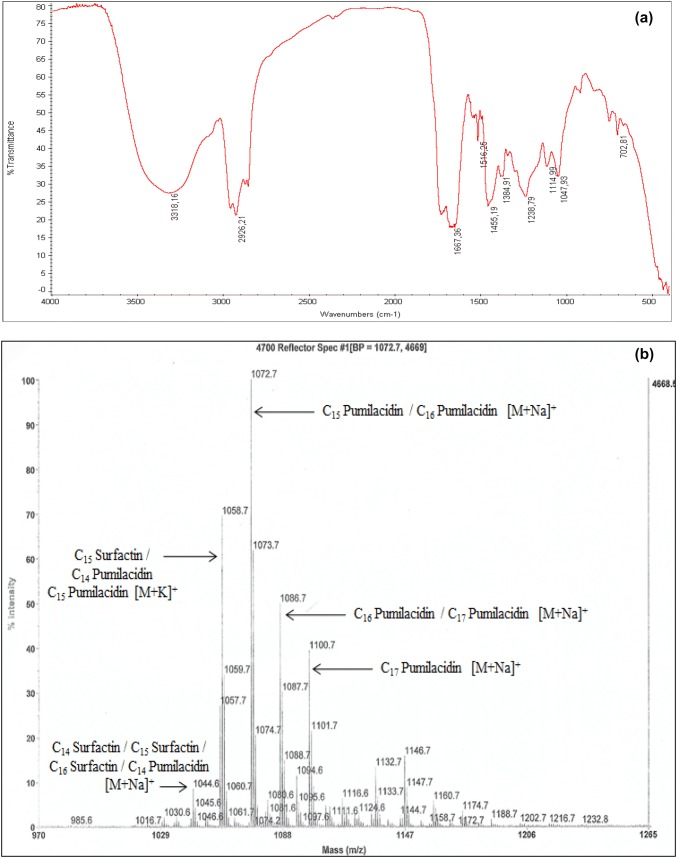
The primarily characterization of the crude biosurfactant Bios-Cnaph3, produced on NB, was conducted by TLC analysis. After spraying with ninhydrin reagent, a spot with a pink color appeared showing the presence of amine groups. Results of Molisch test revealed that no reaction was detected suggesting the absence of carbohydrates compounds. These results confirm the lipopeptide nature of the biosurfactant Bios-Cnaph3. Additionally, the crude biosurfactant was separated on silica gel column. Twenty six fractions were collected and examined using TLC. Based on their molecular weight, 4 big fractions were pooled and the sample (Fraction 3) showing positive response with ninhydrin was analyzed by FTIR and ESI–MS. Figure 3a shows the chemical characterization of the biosurfactant Bios-Cnaph3 through the FTIR analysis. In line with Zhang et al. (2016), it can be induced that Bios-Cnaph3 belonged to the class of lipopeptides. Furthermore, as shown in Fig. 3b, the ESI–MS analysis of Bios-Cnaph3 revealed the presence of one well-resolved cluster of peaks at m/z values between 1044.6 and 1100.7 Da. According to the mass numbers reported in literature (Pecci et al. 2010; Hentati et al. 2019), the peaks at 1044.6; 1058.7; 1072.7; 1086.7, and 1100.7 Da approve the affiliation of Bios-Cnaph3 to lipopeptides, particularly to surfactin and pumilacidin families (Fig. 3b). As far as we know, no previous studies have been reported on the production of lipopeptide by Halomonas species.
Fig. 3.
Chemical characterization of the biosurfactant Bios-Cnaph3; Fourier transform infrared spectrum (FTIR) (a); ESI–MS spectrum of molecular mass biosurfactants produced by Halomonas pacifica strain Cnaph3 (b)
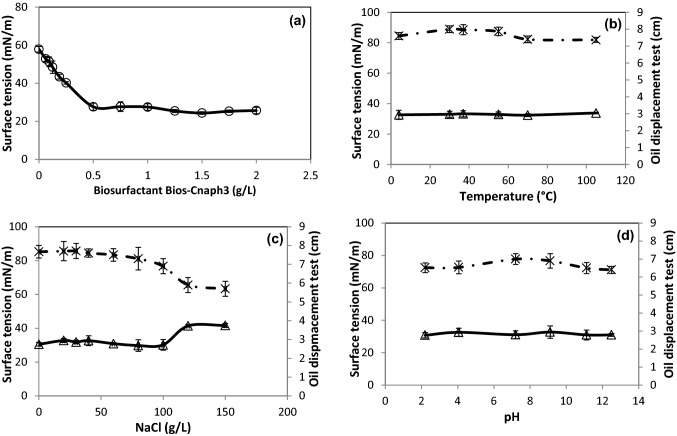
Critical micelle concentration (CMC) is defined as the concentration of Bios-Cnaph3 beyond which the surface tension does not change (Chebbi et al. 2017). Figure 4a exhibits the determination of CMC of the crude Bios-Cnaph3. The surface tension decreased up to 27.6 mN/m at a Bios-Cnaph3 concentration of 500 mg/L. It was shown previously that the CMC of lipopeptides from Bacillus subtilis strain TK-1 was about 512 mg/L (Cao et al. 2009). Moreover, Al-Wahaibi et al. (2014) reported that Bacillus subtilis strain B30 produced a lipopeptide with a CMC of 300 mg/L. In addition, Bacillus stratosphericus strain FLU5 produced, on residual frying oil, a lipopeptide biosurfactant with CMCs of 250 and 50 mg/L for crude and purified biosurfactant, respectively (Hentati et al. 2019). The CMCs values decreased when the degree of purity of biosurfactant increased (Hentati et al. 2019).
Fig. 4.
Determination of critical micelle concentration of the crude Bios-Cnaph3 (a) and stability of Bios-Cnaph3 at various temperatures (b), NaCl concentrations (c) and pH (d). (open triangle) Surface tension and ( ×) Oil displacement test
The use of biosurfactant in numerous fields such as environmental, food, and biomedical depend on their stability at various temperatures, salinities, and pH (Sharma et al. 2018). The effects of these environmental parameters on surface tension and oil displacement of Bios-Cnaph3 were investigated (Fig. 4b, c, d). Figure 4b shows that at temperatures between 4 and 105 °C, Bios-Cnaph3 remained stable and its performance was not affected (At 4 °C, ST = 32.6 mN/m and ODT = 7.6 cm; At 105 °C, ST = 33.8 mN/m and ODT = 7.3 cm). Moreover, Fig. 4c reveals that during the increase of NaCl concentration from 0 to 100 g/L Bios-Cnaph3 maintained its stability (At 100 g/L NaCl, ST = 30.2 mN/m and ODT = 6.9 cm). However, above 100 g/L NaCl significant changes were detected (At 120 g/L NaCl, ST = 41.4 mN/m and ODT = 5.9 cm). de França et al. (2015) explained this by the fact that the modification of biosurfactants properties were due to the reduction of the size and shape of the micelle caused by the increase of NaCl concentrations. Finally, Fig. 4d shows that no significant effect was observed on the properties of Bios-Cnaph3 when varying the pH between 2.2 (ST = 30.7 mN/m; ODT = 6.5 cm) and 12.5 (ST = 30.9 mN/m; ODT = 6.4 cm). These findings would confirm previous results by Khopade et al. (2012) who observed that lipopeptides are stable at neutral pHs. However, the surface activity was less affected at highly alkaline conditions than at highly acidic pH. In this respect, Hentati et al. (2019) assessed the stability of a lipopeptide from Bacillus stratosphericus strain FLU5 under various temperatures (4–121 °C), salinities (0–120 g/L) and pHs (2.1–12). In addition, the lipopeptide from Pseudomonas aeruginosa strain MR01 was stable during exposure to temperatures (0–120 °C), salinity (0–80 g/L) and pH (6–12) (Lotfabad et al. 2009). These results would further prove that Bios-Cnap3 can be operational even under extreme conditions of temperature, salinity, and pH.
Hydrocarbon removal by biosurfactants is one of the most promising remediation methods. Figure 5 represents the assessment of the potential of biosurfactants produced by strain Cnaph3 to remove hydrocarbons from contaminated soils with used motor oil (20%, w/v), compared with synthetic surfactants (Triton X-100, Tween 80 and SDS). To begin with, when compared to water, crude Bios-Cnaph3 was 3.5 times more efficient than water (p < 0.05) to solubilise motor oil. Likewise, cell-free supernatant of culture Cnaph3 on glycerol proved to be 4.7 times more efficient than water to remove the same contaminant. Secondly, the crude biosurfactant Bios-Cnaph3 was more effective than the chemical surfactants (Tween80 and SDS) in removing motor oil (p < 0.05). These results were in total agreement with Chebbi et al. (2017) and Hentati et al. (2019) who reported that crude biosurfactant BSW10 and crude lipopeptide BS-FLU5, respectively, were highly effective in removing motor oil.
Fig. 5.
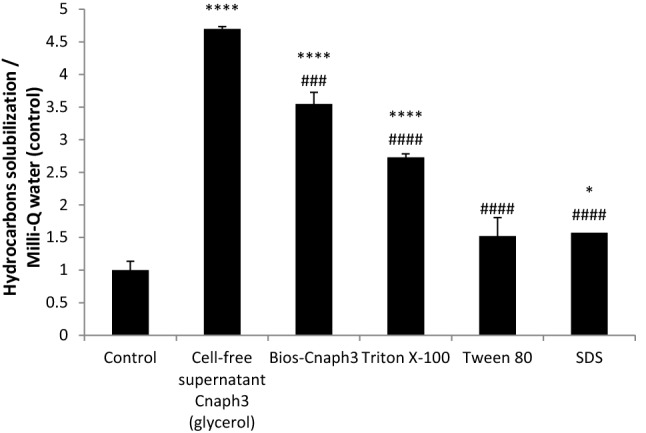
Assessment of the potential of biosurfactants produced by strain Cnaph3 to remove hydrocarbons from contaminated soils with used motor oil (20%, w/v), compared with synthetic surfactants (Triton X-100, Tween 80 and SDS). Values given represent the mean of three replicates ± standard deviation. The asterisk indicates p < 0.05 versus control. The sharp indicates p < 0.05 versus cell-free supernatant treatment. (****, ####p < 0.0001; ###p < 0.001; ##p < 0.01 and *p < 0.05)
Therefore, in line with Dhasayan et al. (2014), this study would recommend the biosurfactant Bios-Cnaph3 as an efficient and cost effective biosurfactant for enhancing oil recovery, cleaning up oil spills, and removing oil residues from storage tanks. The possibility of using the cell-free supernatant of the culture of Cnaph3 could be a good choice in hydrocarbon removal because it avoids the steps of purification.
Conclusion
A hydrocarbon contamination was recorded in the fishing harbor of Ataya located in Kerkennah Islands, Tunisia. A marine halotolerant strain Cnaph3, affiliated to Halomonas pacifica, showed an interesting naphthalene degradation at 30 g/L NaCl, and it was also capable of growing on various complex, aromatic, and aliphatic hydrocarbons. Moreover, the strain Cnaph3 revealed a significant potential to produce biosurfactants on various carbon sources. This biosurfactants, Bios-Cnaph3, was able to grow on glycerol, a polluting by-product, thus ridding the environment of a source of contamination. It was also characterized as a highly stable product under extreme conditions such as temperature, NaCl concentration, and pH. Furthermore, the lipopeptides Bios-Cnaph3 showed excellent efficiency in the remobilization of used motor oil from contaminated soil compared with the tested synthetic surfactants. To our knowledge, there was no previous study stating the efficiency of a Halomonas pacifica strain in naphthalene degradation and lipopeptides production under saline conditions. Overall, our findings suggested that strain Cnaph3 as well as the biosurfactant Bios-Cnaph3 would be efficiently used for the bioremediation of hydrocarbons contaminated sites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by a grant provided by the Tunisian Ministry of Higher Education and Scientific Research and the Laboratory of Environmental Bioprocesses, Centre of Biotechnology of Sfax, Tunisia. This work was also partially financed by the Ministerio de Economia y Competitividad, Spain. Authors would like to thank Mrs. Fatma Rezgui for her technical assistance in GC–MS analyses, Mr. Nidhal Baccar for his technical assistance in GC-FID and FTIR analyses and Mr. Slim Loukil for his help with chemical analyses. Finally, the authors would like to thank Dr. Ayadi Hajji for proofreading, correcting and improving the English of the manuscript.
Author contributions
MC performed most of the experiments, data analysis and drafted the manuscript, DH helped in the manuscript interpretation, AC helped in the Phylogenetic analysis, NM performed the molecular analysis, Professor SS assisted with financial support, Professor AMM performed the ESI–MS analysis and Professor MC designed and directed the study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this manuscript.
References
- Abdelkafi S, Labat M, Casalot L, et al. Isolation and characterization of Halomonas sp. strain IMPC, a p-coumaric acid-metabolizing bacterium that decarboxylates other cinnamic acids under hypersaline conditions. FEMS Microbiol Lett. 2006;255:108–114. doi: 10.1111/j.1574-6968.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Alva VA, Peyton BM. Phenol and catechol biodegradation by the Haloalkaliphile Halomonas campisalis: influence of pH and salinity. Environ Sci Technol. 2003;37:4397–4402. doi: 10.1021/es0341844. [DOI] [PubMed] [Google Scholar]
- Alvonita M, Hertadi R. Bioconversion of glycerol to biosurfactant by halophilic bacteria Halomonas elongata BK-AG18. Indones J Chem. 2018 doi: 10.22146/ijc.26737. [DOI] [Google Scholar]
- Al-Wahaibi Y, Joshi S, Al-Bahry S, et al. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf B. 2014;114:324–333. doi: 10.1016/j.colsurfb.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Arulazhagan P, Vasudevan N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar Pollut Bull. 2011;62:388–394. doi: 10.1016/j.marpolbul.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol. 2016;32:180. doi: 10.1007/s11274-016-2137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banat IM, Satpute SK, Cameotra SS, et al. Cost effective technologies and renewable substrates for biosurfactants production. Front Microbiol. 2014 doi: 10.3389/fmicb.2014.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L, Bowditch RD, Baumann P. Description of Deleya gen. nov. created to accommodate the marine species Alcaligenes aestus, A. pacijicus, A. cupidus, A. venustus, and Pseudomonas marina. Int J Syst Bacteriol. 1983;33:793–802. doi: 10.1099/00207713-33-4-793. [DOI] [Google Scholar]
- Cao X-H, Liao Z-Y, Wang C-L, et al. Evaluation of a lipopeptide biosurfactant from Bacillus natto TK-1 as a potential source of anti-adhesive, antimicrobial and antitumor activities. Braz J Microbiol. 2009;40:373–379. doi: 10.1590/S1517-838220090002000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. In: Rosenberg E, editor. Microorganisms to combat pollution. Netherlands, Dordrecht: Springer; 1992. pp. 227–244. [Google Scholar]
- Chamkha M, Trabelsi Y, Mnif S, Sayadi S. Isolation and characterization of Klebsiella oxytoca strain degrading crude oil from a Tunisian off-shore oil field. J Basic Microbiol. 2011;51:580–589. doi: 10.1002/jobm.201100073. [DOI] [PubMed] [Google Scholar]
- Chebbi A, Jaoua H, Loukil S, et al. Biodegradation of malodorous mercaptans by a novel Staphylococcus capitis strain isolated from gas-washing wastewaters of the Tunisian Chemical Group. Int J Environ Sci Technol. 2016;13:571–580. doi: 10.1007/s13762-015-0897-8. [DOI] [Google Scholar]
- Chebbi A, Hentati D, Zaghden H, et al. Polycyclic aromatic hydrocarbon degradation and biosurfactant production by a newly isolated Pseudomonas sp. strain from used motor oil-contaminated soil. Int Biodeterior Biodegrad. 2017;122:128–140. doi: 10.1016/j.ibiod.2017.05.006. [DOI] [Google Scholar]
- Coronel-León J, de Grau G, Grau-Campistany A, et al. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: production, chemical characterization and properties. Ann Microbiol. 2015;65:2065–2078. doi: 10.1007/s13213-015-1045-x. [DOI] [Google Scholar]
- da Silva GP, Mack M, Contiero J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv. 2009;27:30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Dave D, Ghaly AE. Remediation technologies for marine oil spills: a critical review and comparative analysis. J Environ Sci. 2011;7(5):423. doi: 10.3844/ajessp.2011.423.440. [DOI] [Google Scholar]
- de França ÍWL, Lima AP, Lemos JAM, et al. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal Today. 2015;255:10–15. doi: 10.1016/j.cattod.2015.01.046. [DOI] [Google Scholar]
- Dhasayan A, Kiran GS, Selvin J. Production and characterisation of glycolipid biosurfactant by Halomonas sp. MB-30 for potential application in enhanced oil recovery. Appl Biochem Biotechnol. 2014;174:2571–2584. doi: 10.1007/s12010-014-1209-3. [DOI] [PubMed] [Google Scholar]
- Donio MBS, Ronica FA, Viji VT, et al. Halomonas sp. BS4, a biosurfactant producing halophilic bacterium isolated from solar salt works in India and their biomedical importance. SpringerPlus. 2013 doi: 10.1186/2193-1801-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragallah HM, Askar AI, Okbah MA, Moustafa HM. Physico-chemical characteristics of the open Mediterranean sea water far about 60 Km from Damietta harbor. Egypt J Ecol Nat Enivorn. 2009;1(5):106–119. [Google Scholar]
- Fathepure BZ. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front Microbiol. 2014 doi: 10.3389/fmicb.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MT, Mellado E, Ostos JC, Ventosa A. Halomonas organivorans sp. nov., a moderate halophile able to degrade aromatic compounds. Int J Syst Evol Microbiol. 2004;54:1723–1728. doi: 10.1099/ijs.0.63114-0. [DOI] [PubMed] [Google Scholar]
- Gifford SP, Macfarlane GR, O’Connor WA, Dunstan RH. Effect of the pollutants lead, zinc, hexadecane and octocosane on total growth and shell growth in the akoya pearl oyster, pinctada imbricata. J Shellfish Res. 2006;25:159–165. doi: 10.2983/0730-8000(2006)25[159:EOTPLZ]2.0.CO;2. [DOI] [Google Scholar]
- Global Climate Report (2015) Global Climate Report—Annual 2015 | State of the Climate | National Centers for Environmental Information (NCEI). https://www.ncdc.noaa.gov/sotc/global/201513. Accessed 15 May 2019
- Güven KC, Yazici Z, Ünlü S, et al. Oil pollution on sea water and sediments of Istanbul Strait caused by Nassia Tanker accident. Turk J Marit Mar Sci. 1997;2:65–85. [Google Scholar]
- Hentati D, Chebbi A, Loukil S, et al. Biodegradation of fluoranthene by a newly isolated strain of Bacillus stratosphericus from Mediterranean seawater of the Sfax fishing harbour, Tunisia. Environ Sci Pollut Res. 2016;23:15088–15100. doi: 10.1007/s11356-016-6648-7. [DOI] [PubMed] [Google Scholar]
- Hentati D, Chebbi A, Hadrich F, et al. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol Environ Saf. 2019;167:441–449. doi: 10.1016/j.ecoenv.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Huber C, Klimant I, Krause C, et al. Optical sensor for seawater salinity. Fresenius J Anal Chem. 2000;368:196–202. doi: 10.1007/s002160000493. [DOI] [PubMed] [Google Scholar]
- Khopade A, Biao R, Liu X, et al. Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination. 2012;285:198–204. doi: 10.1016/j.desal.2011.10.002. [DOI] [Google Scholar]
- Klein B, Roether W, Manca BB, et al. The large deep water transient in the Eastern Mediterranean. Deep Sea Res I. 1999;46:371–414. doi: 10.1016/S0967-0637(98)00075-2. [DOI] [Google Scholar]
- Lin C, Gan L, Chen Z-L. Biodegradation of naphthalene by strain Bacillus fusiformis (BFN) J Hazard Mater. 2010;182:771–777. doi: 10.1016/j.jhazmat.2010.06.101. [DOI] [PubMed] [Google Scholar]
- Lotfabad TB, Shourian M, Roostaazad R, et al. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf B. 2009;69:183–193. doi: 10.1016/j.colsurfb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Mnif S, Chamkha M, Sayadi S. Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions. J Appl Microbiol. 2009;107:785–794. doi: 10.1111/j.1365-2672.2009.04251.x. [DOI] [PubMed] [Google Scholar]
- Morley NH, Burton JD, Tankere SPC, Martin J-M. Distribution and behaviour of some dissolved trace metals in the western Mediterranean Sea. Deep Sea Res II. 1997;44:675–691. doi: 10.1016/S0967-0645(96)00098-7. [DOI] [Google Scholar]
- Ndlovu T, Rautenbach M, Khan S, Khan W. Variants of lipopeptides and glycolipids produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa cultured in different carbon substrates. AMB Express. 2017 doi: 10.1186/s13568-017-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha N, Mandal SK, Das N. Enhanced degradation of indeno (1,2,3-cd) pyrene using Candida tropicalis NN4 in presence of iron nanoparticles and produced biosurfactant: a statistical approach. 3 Biotech. 2019;9:86. doi: 10.1007/s13205-019-1623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecci Y, Rivardo F, Martinotti MG, Allegrone G. LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J Mass Spectrom. 2010;45:772–778. doi: 10.1002/jms.1767. [DOI] [PubMed] [Google Scholar]
- Peele KA, Ravi Teja Ch V, Kodali VP. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech. 2016;6:177. doi: 10.1007/s13205-016-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada E, Valderrama MJ, Bejar V, et al. Volcaniella eurihalina nov sp. nov. a moderately halophilic nonmotile gram-negative rod. Int J Syst Bacteriol. 1990;40:261–267. doi: 10.1099/00207713-40-3-261. [DOI] [Google Scholar]
- Rahman KSM, Rahman TJ, McClean S, et al. Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog. 2002;18:1277–1281. doi: 10.1021/bp020071x. [DOI] [PubMed] [Google Scholar]
- Santos D, Rufino R, Luna J, et al. Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci. 2016;17:401. doi: 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari IP, Basyiruddin MI, Hertadi R. Bioconversion of palm oil into biosurfactant by Halomonas meridiana BK-AB4 for the application of corrosion inhibitor. Indones J Chem. 2018;18:718. doi: 10.22146/ijc.27040. [DOI] [Google Scholar]
- Sharma R, Singh J, Verma N. Optimization of rhamnolipid production from Pseudomonas aeruginosa PBS towards application for microbial enhanced oil recovery. 3 Biotech. 2018;8:20. doi: 10.1007/s13205-017-1022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Patil Y, Rale V. Biosurfactant production: emerging trends and promising strategies. J Appl Microbiol. 2019;126:2–13. doi: 10.1111/jam.14057. [DOI] [PubMed] [Google Scholar]
- Tornero V, Hanke G. Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull. 2016;112:17–38. doi: 10.1016/j.marpolbul.2016.06.091. [DOI] [PubMed] [Google Scholar]
- US EPA (1986) Quality criteria for water. United States Environmental Protection Agency. Washington, D.C. EPA 440/5-86-001. Accessed 6 Apr 2019
- US EPA (2011) Criteria revisions published in 2002 National Recommended Water Quality Criteria, EPA-822-R-02-047. Accessed 15 May 2019
- Vreeland RH, Litchfield CD, Martin EL, Elliot E. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol. 1980;30:485–495. doi: 10.1099/00207713-30-2-485. [DOI] [Google Scholar]
- Wang Y, Wu Y-H, Wang C-S, et al. Halomonas salifodinae sp. nov., a halophilic bacterium isolated from a salt mine in China. Int J Syst Evol Microbiol. 2008;58:2855–2858. doi: 10.1099/ijs.0.2008/000729-0. [DOI] [PubMed] [Google Scholar]
- Widdel F, Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch Microbiol. 1981;129:395–400. doi: 10.1007/bf00406470. [DOI] [PubMed] [Google Scholar]
- Zaghden H, Serbaji MM, Saliot A, Sayadi S. The Tunisian Mediterranean coastline: potential threats from urban discharges Sfax-Tunisian Mediterranean coasts. Desalination Water Treat. 2016;57:24765–24777. doi: 10.1080/19443994.2016.1149107. [DOI] [Google Scholar]
- Zhang J, Xue Q, Gao H, et al. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5–2a and their potential use in microbial enhanced oil recovery. Microb Cell Factories. 2016 doi: 10.1186/s12934-016-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.