Abstract
Microbial biofilms act as reservoirs for pathogenic sessile microbes which reside inside the three dimensional matrix of the biofilm, and are thus protected against anti-microbial drugs. Most of the anti-microbial drugs fail to completely abolish the biofilm associated infections. In the present study, we provide evidence of Hibiscus sabdariffa (Hs) extract having possible anti-microbial activity, with emphasis on Candida albicans biofilm. The Hs extract was shown to be effective against C. albicans pre-formed biofilm at 3.125 mg/ml and was able to inhibit the hyphae initiation and adherence of cells. Furthermore, Hs extract was able to reduce the C. albicans load in C. elegans by effectively killing the Candida cells thereby reducing the viable colony count and effectively increasing the lifespan of worms. The percentage of viable hatched progeny of worms exposed to Hs extract (both at conc. 1.5 mg/ml and 6.25 mg/ml), was also comparable to that of the control untreated eggs. The Hs extract was also found to be significantly effective against fluconazole resistant C. albicans isolated from patients. Thus, we, for the first time, propose Hs extract as a prospective drug candidate and substitute for eradicating pre-formed biofilm and inhibiting the growth of C. albicans.
Keywords: Candida albicans, Bio-film, Hibiscus sabdariffa, Natural products, Caenorhabditis elegans
Introduction
Candida albicans is both a member of the healthy human microbiome, and a major pathogen in immuno-compromised individuals. It is the fourth most prevalent pathogen implicated in nosocomial infections. The misuse of antibiotics and/or sub-optimal drug dosing, plays a pivotal role in drug resistance. In addition, environmental factors, horizontal gene transfer among microflora, and the evolution of several mutant strains with increased/altered virulence factors, has contributed towards the development of resistant strains. One of the most important virulence factors of microbes is their capacity for biofilm formation.
Biofilms are a structured population of microorganisms, encapsulated within a self-developed three-dimensional matrix adherent to a living or inanimate surface. A biofilm may compose of a single microbial species, but naturally biofilms generally consist of mixtures of microbial species, debris and corrosion products. Candida biofilms are multi-layered, three dimensional components, essential for pathogenicity, and protected from anti-fungal agents in the extra-cellular matrix [1, 2]. They act as reservoirs to persistent fungal infections and it is estimated that approximately 80% of candidal infections are associated with biofilm formation [3]. Thus, biofilms play an important role in generating anti-fungal resistant pathogenic cells.
The screening of bio-active natural products (NP), obtained from marine organisms, microbial fermentation or extracts of plant tissues, has provided invaluable antibiotics, therapeutics and life-saving drugs to mankind. NP and their derivatives are used to treat various infectious diseases caused by micro-organisms. Till date, approximately 80% of all available antibiotics are derived from NP directly or indirectly [4]. However, antibiotic resistant infectious diseases and multi drug resistant (MDR) microorganisms have created a dire need for novel drugs. The discovery and screening of new beneficial NPs from bacteria, fungi and plants, that are capable of circumventing MDR with minimal adverse effects, is the need of the hour. Among all NPs, the plant-derived potential drugs have gained more attention, due to their versatile applications, easy availability and less toxicity.
One of the approaches to identify a novel drug is by screening herbal/botanical/phyto medicine derived from the seeds, roots, bark, leaves, flowers or berries of plants which are being used for medicinal purposes since ancient times. Herbs have been used as medicinal plants, from time immemorial, and have been associated with certain cultural traditions all over the world, especially in India. Antimicrobial properties of different plant extracts have been reported under laboratory trials, further demonstrating that plant extracts are effective antimicrobial agents [5–7]. Thus, selection of crude extracts in screening for antimicrobial properties has higher possibility of success than screening of pure compounds directly [8].
One such plant known for its various medicinal properties is Hibiscus sabdariffa (Hs). This plant has traditionally been used as a food, herbal drink (both as cold and hot beverages), flavoring agent, fiber source and as a herbal medicine for its anti-bacterial, anti-oxidant, diuretic, anti-cholesterol, anti-diabetic, anti-hypertensive and anti-cancer activities [9, 10]. The methanolic and aqueous extract of Hibiscus calyces have shown anti-bacterial activity against cariogenic Streptomyces mutants [11] and the aqueous-methanolic extract showed favourable effect against various pathogenic bacteria [12].
In the present study, the crude extracts of Hs calyces in water and in DMSO were screened against different pathogenic microbes such as Bacillus atrophaeus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Candida albicans, to determine their anti-microbial activity. Further, the mechanism of Hs crude extract on growth of C. albicans and on biofilm formation was also elucidated. The study attempted to throw some light on the potential of Hs extract in removing the pre-formed Candida biofilm as well as its ability to inhibit biofilm initiation and adherence of Candida cells.
Materials and Methods
Preparation of Plant Extract
The plant material used in this study was red calyces of Hibiscus sabdariffa flowers devoid of other floral parts. The material was purchased from a local vendor as dried calyx of Hibiscus flowers. The botanical identification of its species was performed by National Institute of Science Communication and Information Resource (NISCAIR), CSIR, Delhi (Reference No. NISCAIR/RHMD/Consult/2015/2884/77). The dried calyces were powdered using mortal-pestle and suspended at a concentration of 200 mg/ml in both DMSO and in water. The suspension was kept at 37 °C for at least 48 h with continuous shaking, filter sterilized and stored at room temperature for further use. Various concentrations of Hs extract ranging from 0.0976 to 50 mg/ml, were prepared in RPMI 1640 medium with l-glutamine w/o sodium bicarbonate, adjusted to pH 7.0 with 165 mM 3-[N-morpholine] propane sulphonic acid (MOPS) buffer, and used for all the assays described below, unless otherwise stated [13]. Wells without test compounds served as full-growth controls. Amphotericin B was used as the standard antifungal agent.
Test Microorganisms
Gram-positive bacteria used in the study were Bacillus atrophaeus and Staphylococcus aureus, gram-negative bacteria included Escherichia coli and Pseudomonas aeruginosa. All the tested strains were maintained on nutrient agar (BD Difco) at 37 °C and grown in nutrient broth (BD Difco) for experimental purposes. The fungal strain used was Candida albicans which was maintained routinely on Yeast Peptone Dextrose (YPD, BD Difco) agar plates at 30 °C and grown in YPD broth (BD Difco) for experimental procedures unless otherwise stated.
Antimicrobial Assay
Antimicrobial activity of Hs against various pathogenic organisms was determined according to Kirby–Bauer disc diffusion method recommended by Clinical and Laboratory Standards Institute (CLSI) guidelines [13]. Different concentrations of Hs crude extracts, prepared either in water or DMSO, were tested for antimicrobial activity as described previously [14]. DMSO or water was used as solvent control, along with Ampicillin (100 mg/ml) for bacteria and Amphotericin B (250 µg/ml) for C. albicans, as a standard drug control. The diameter of inhibition zone was measured and the total area of the inhibition was calculated according to the following equation:
where r is radius of the zone of inhibition, π = 3.14.
C. albicans Isolates from Patients
The Candida spp. was isolated from patients diagnosed with vulvo-vaginal candidiasis (VVC) by vaginal swab, examined microscopically by Gram stain, KOH preparation and further processed for fungal culture on Sabouraud’s dextrose agar (without cycloheximide). A germ tube test was performed on all Candida isolates to separate the Candida albicans species from non albicans species. Further identification of colonies was done by growing cultures on CHROMagar Candida media (HiMedia, Mumbai, India) and studying the morphology on corn-meal agar media (CMA). A positive germ tube test, bright green colored colonies on CHROMagar Candida media and chlamydospore formation on corn meal agar were some of the parameters used in identification of the isolate as C. albicans. The identified Candida albicans isolates were further used for in vitro susceptibility testing.
In-Vitro Anti-fungal Susceptibility Test
The antifungal activity of Hs extract was tested by the broth micro-dilution method according to CLSI, M27-A3 [15]. The test was carried out in RPMI 1640 medium in 96-well round-bottomed plates. The overnight grown culture of Candida cells was used throughout the study, finally adjusted to a final inoculum of 0.5 × 103 cells/ml in RPMI 1640 [15]. The plates were incubated at 35 °C for 48 h after addition of Hs extract with appropriate controls. Minimum inhibitory concentrations (MICs) were determined by visual reading after agitating the cell suspension and measuring the optical density at 600 nm with the microplate reader (Tecan infinite M200). The background optical densities were subtracted and the MIC50 and MIC80 were determined.
In addition, the Minimum Inhibitory Concentration (MIC) strips were also used for performing the antifungal susceptibility test (E-test strips, Biomerieux) of the C. albicans isolated from patients against fluconazole, an antifungal agent used most commonly. The culture suspension of turbidity matching 0.5 Mc Farland, was made and spreaded on Petri dish with RPMI supplemented with 2% glucose agar for 48 h at 35 °C. The reading was observed by assessing the point of intersection between the halo formed and the E-test strip. The profile of the antifungal drug sensitivity was classified as sensitive (S), dose-dependent sensitivity (S-DD), and/or intermediate (I), and resistant (R) as defined by CLSI standard M27-A3 [15]. The MIC values of ≤ 8 μg/ml for fluconazole were considered susceptible (S), 16–32 μg/ml was considered as susceptible dose-dependent (SDD), and ≥ 64 μg/ml as resistant (R). For Amphotericin B (AMB), MICs ≤ 1 μg/ml were considered to be S and ≥ 1 μg/ml as R.
Filamentation Assay
For assessing effect of Hs extract on hyphae formation in C. albicans, spider media agar plates with or without Hs extract (MIC50) was used. Overnight grown Candida in YPD broth was streaked onto the plates and were then incubated at 37 °C for 7–14 days. Images of colony edges were obtained using a stereo-zoom microscope (Nikon, SMZ1000).
For assessing the hyphae/germ-tube formation in liquid media, Candida was grown in RPMI 1640 medium with or without Hs extract. The 6-well flat bottom plates were incubated at 37 °C for 4 h, and the individual budding cells and the number of cells with hyphae were counted and results were expressed as percentage of germ tube formation using the following formula:
Adherence Assay on the Well Surface of Microtiter Plates
Cell suspension of Candida cells in RPMI 1640 was seeded in flat bottom 96-well plate with different concentrations of Hs extract and incubated at 37 °C for 4 h. Following the initial incubation, the medium was aspirated and non-adherent cells were removed by thoroughly washing the wells with sterile PBS thrice. A semiquantitative measurement of adhered cells was done using 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay as described previously [16, 17]. The colorimetric change at 490 nm was measured with a microplate reader (Tecan infinite M200) and MIC50 and MIC80 values were calculated.
Biofilm Assay
Cell suspension (100 μl each) of C. albicans with inoculums of 1 × 106 cells/ml was inoculated into 96-well flat bottom polystyrene plates at 37 °C for 4 h to score the effect of Hs extract on adhered cells and for 20–24 h for formation of mature biofilm. Non-adhered cells were removed by washing three times with sterile PBS and then 200 μl of desired concentration of Hs extract in RPMI 1640 medium was added to each well. The plates were incubated at 37 °C for 24 h to allow the effect of Hs extract on formation of biofilm and pre-formed biofilm respectively. Biofilm growth was analyzed using XTT as described above.
In-Vivo Toxicity, Antifungal Activity and Evaluation of Fungal Burden in C. elegans
In-vivo toxicity was assessed by measuring Caenorhabditis elegans lifespan as previously described in literature [18]. Briefly, L4-stage synchronized worms (75–100 per experimental group) were exposed to C. albicans culture mixed with E. coli OP50 (2:1) along with 12.5 mM of streptomycin. Plates lacking C. albicans were used as negative controls (uninfected). Kaplan–Meier survival analysis was used to compare the mean lifespan of different treatments and P values were calculated using the log rank (Mantel–Cox method).
Another assay that was performed to evaluate the toxicity of Hs extract is the percent hatching of worms in the presence of Hs extract and then scoring their viability. Gravid adult worms were collected from the plates using M9 buffer, and centrifuged at 1000 g for 3 min. The pellet was resuspended in 5 ml of bleaching solution (0.7 M NaOH with 2% Na-hypochlorite) to break the cuticle. The suspension was mixed thoroughly, centrifuged again and the pellet consisting of worm debris was then washed three times with M9 buffer. The pellet was resuspended to a final concentration of 8–10 eggs/μl; 10 μl of this solution containing eggs were added to M9 buffer in 6-well plates with or without Hs extract. The plates were incubated at 20 °C for 24 h to obtain hatched progeny; the hatched larvae were counted and compared with no-treatment control.
The colony-forming units (CFU) of C. albicans from C. elegans were quantified based on the protocol described previously [19] to determine the fungal burden on worms after exposure to Hs extract. Twenty worms per group were analyzed and colonies were counted to determine CFU per nematode.
All the microscopic examinations were performed in 6-well microtiter plates at MIC50 of Hs extract with similar procedure and a quantitative assessment of cellular morphology was performed with an inverted light microscope. All the experiments throughout the study were repeated at least thrice with each experiment having three replicates.
Results and Discussion
The use of ethano-medicinal plants is common worldwide, but the supporting scientific evidence proving their efficacy for the said use is lacking for many of these traditional medicinal plants. Experimental evaluation of their pharmacological use can provide evidence based alternative therapies for herbal drug and/or discovery of novel drug targets. Hibiscus is often taken as a beverage in many countries owing to its medicinal properties [9, 10, 20]. In this study, we provide evidence for the anti-microbial property of Hs extract with special emphasis on its effect on C. albicans growth and biofilm formation.
Anti-microbial Effect of Hs Crude Extracts
Previous studies by various groups have unanimously reported that Hs calyces collected from different varieties and different localities around the world have effective antimicrobial properties [14, 21, 22]. However, the bioactivity of the extract from same plant species may also vary depending on the extraction process which includes solvent, method of extraction, temperature, fresh or dried calyces, season of harvesting and age of plant [23, 24]. Keeping this in mind, we prepared the extract in two biologically compatible solvents; DMSO and water; and studied the antimicrobial effects of these extracts on different pathogenic microbes using disc diffusion assay. The results of antimicrobial assay showed that both the extracts are effective as anti-bacterial agents against both Gram positive and Gram negative bacteria showing significant area of inhibition ranging from 0.79 to 1.77 cm2 (Table 1). The sensitivity against the tested bacteria differed, with B.atrophaeus being the most sensitive with inhibition zone of approx 1.5 cm in DMSO based extract and 1.2 cm in aqueous extract, while the least sensitive was E. coli with a zone of approximately 1.0 cm in both the extracts. Previous studies have shown the anti-bacterial effect of Hs calyces extract using different solvents such as methanol or ethanol [10, 25].
Table 1.
In vitro antimicrobial activity of Hibiscus sabdariffa (Hs) calyces extract
| S. no. | Microorganism | Diameter of inhibition (cm) | Area of inhibition (cm2) | ||
|---|---|---|---|---|---|
| DMSO | Water | DMSO | Water | ||
| 1 | Candida albicans | 0.8 ± 0.2 | – | 0.5 ± 0.031 | – |
| 2 | Pseudomonas aeruginosa | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.13 ± 0.071 | 0.79 ± 0.031 |
| 3 | Escherichia coli | 1.0 ± 0.3 | 1.0 ± 0.1 | 0.79 ± 0.071 | 0.79 ± 0.007 |
| 4 | Bacillus atrophaeus | 1.5 ± 0.2 | 1.2 ± 0.5 | 1.77 ± 0.031 | 1.13 ± 0.2 |
| 5 | Staphylococcus aureus | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.95 ± 0.071 | 1.13 ± 0.13 |
In addition, when the efficacy was checked against the eukaryotic microbe C. albicans, we observed that the extract prepared in DMSO was effective, with the area of inhibition as 0.5 cm2, whereas the extract prepared in water failed to show any inhibition (Table 1). The difference in anti-candida activity, of DMSO based extract, may be attributed to the presence of various secondary metabolites such as alkaloids, flavonoids, glycosides, phenols, saponins, sterols which are preferentially extracted in DMSO rather than in aqueous phase.
To further establish the action of Hs extract on C. albicans we determined the minimum concentration required to inhibit the growth of planktonic yeast cells to 50% (MIC50) and 80% (MIC80). We found MIC50 value to be 1.5 mg/ml and MIC80 value as 6.25 mg/ml against planktonic cells. However, when we assessed the effect of Hs extract on C. albicans and its ability of adhering to the substrate to form biofilm, biofilm maturation or effect on preformed biofilm, we found increased MIC50 and MIC80 values as shown in Table 2. The Hs crude extract prepared in DMSO (1.5 mg/ml) was effective against formation or initiation of biofilm as approximately 50% of Candida cells were not able to adhere to the plates for further biofilm formation and at 3.125 mg/ml the pre-formed biofilm was effectively abolished resulting in the cell death. We also observed that both for planktonic as well as preformed biofilm of C. albicans, the MIC80 value was four times higher than that of MIC50 value (Table 2). To the best of our knowledge for the first time, we are reporting the effect of Hs extract on pre-formed biofilms of C. albicans.
Table 2.
MIC50, MIC80 and MIC80/MIC50 ratios of DMSO extract of H. sabdariffa calyces against C. albicans ATCC 90028 strain
| S. no. | Inhibition of | DMSO extract of H. sabdariffa (mg/ml) | ||
|---|---|---|---|---|
| MIC50 | MIC80 | MIC80/MIC50 | ||
| 1. | Planktonic cells | 1.5 ± 0.2 | 6.25 ± 0.4 | 4.17 |
| 2. | Biofilm adherence | 1.5 ± 0.3 | 6.25 ± 0.75 | 4.17 |
| 3. | Biofilm maturation | 2.5 ± 0.5 | 10.0 ± 1.0 | 4.0 |
| 4. | Pre-formed biofilm | 3.125 ± 0.75 | 12.5 ± 0.75 | 4 |
Hs Extract Affect the Yeast to Hyphae Transition in C. albicans
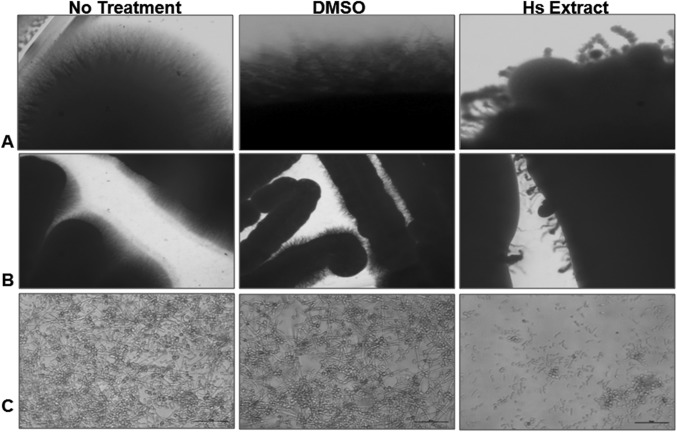
The important virulence-mediating attribute of C. albicans is transition from yeast cells to hyphae. This transition helps C. albicans to penetrate the host tissues and this invasive growth leads to the establishment of systemic infection. Since, multiple stimuli can induce hyphal morphogenesis [26], the study was initiated to observe the transition of yeast cells to hyphae formation in the presence of Hs extract on solid agar Spider’s medium and liquid RPMI medium. The plate containing DMSO was taken as the solvent control and cells without any additive was the positive control for the experiment. The hyphae formation on the plates with Hs extract was significantly less as compared to the control plates after 7 days and 14 days of incubation (Fig. 1). Whatever limited hyphae were observed in the treated plates looked shrunken and curly in nature. This observation was further validated by microscopic observation of hyphae formation in liquid media, where the hyphae were completely inhibited (Fig. 1c). Thus, we propose that Hs extract not only inhibits the hyphae production, but also brings about significant morphological changes in the hyphae. There are various reports which concretely suggest that formation of hyphae is dependent on the pH, type of media (whether solid or liquid), temperature and other factors [27]. Thus, the retarded formation of hyphae on solid media and complete absence of hyphae formation in liquid media may be attributed to the fact that in solid media, hyphal growth recorded is from a colony [27] instead of individual cell. So, based on our results we suggest that Hs extract have the potential to inhibit yeast to hyphae transition of C. albicans cells. Equivalent concentration of DMSO used as solvent control had no effect on cell viability and hyphae formation of C. albicans.
Fig. 1.
Inhibition of C. albicans filamentation by Hs extract in different hyphal-inducing media. Colonies were grown on spider media agar plates containing either Hs extract or DMSO (solvent control) for 7 days (a) and 14 days (b). C. albicans with Hs extract was grown in (c) RPMI1640 medium at 37 °C for 6 h. Images of colony edges were obtained using a stereozoom microscope and Candida cells with hyphae using inverted microscope at 40 ×
Hs Extract Inhibits Adhesion of C. albicans Cells
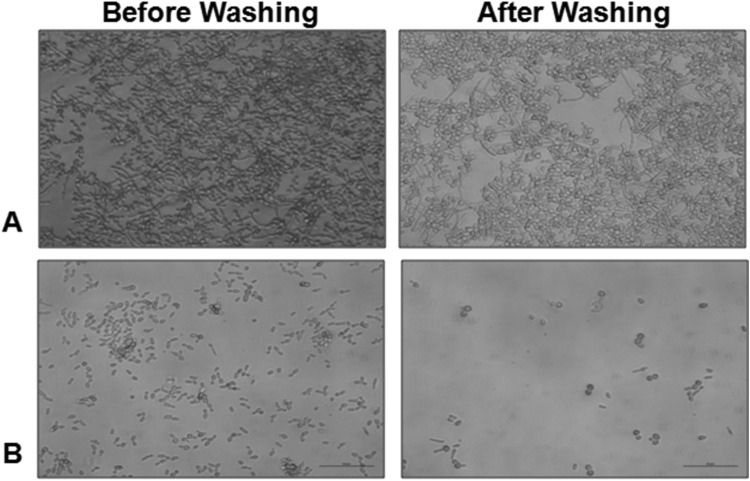
Biofilm formation is a multicellular, complex process, involving various important steps such as cell adhesion, growth, morphogenic switching between yeast and filamentous states, and quorum sensing [1]. The pre-requisite for biofilm formation is the adhesion of C. albicans cells to materials or host cells that subsequently lead to cell–cell interactions in the hierarchical organization of cells within the formed biofilm. Since, this study results suggest that Hs extract can inhibit the biofilm formation (Table 2), the probable efficacy in circumventing the adherence of C. albicans cells was then checked. The cells were grown in the presence and absence of Hs extract for 4 h, followed by washing, and then cells attached to the substrate were analyzed. It was found that cells grown in the presence of Hs extract lost their attachment and were easily washed away during the washing step (Fig. 2).
Fig. 2.
Loss of adherence property of C. albicans in the presence of Hs extract. C. albicans ATCC 90028 cells were grown in the RPMI 1640 medium without any additive (a) and with Hs extract (b) at 37 °C for 4 h. The cells before and after washing with PBS were visualized by microscopy
Hs Extract Inhibits the Biofilm Formation and Disrupts the Pre-formed Biofilm
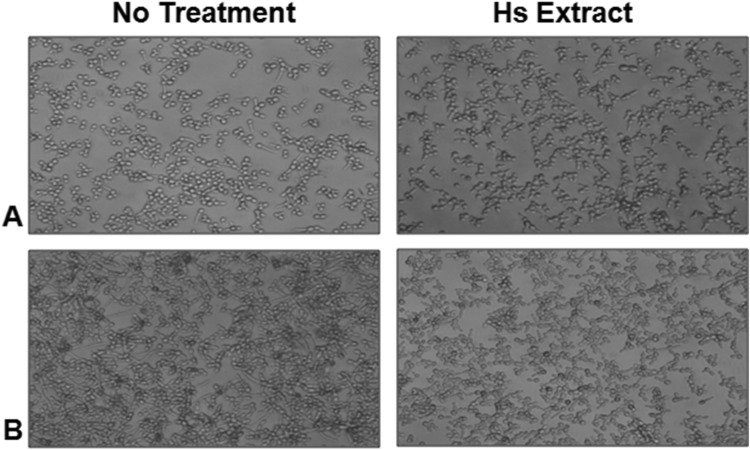
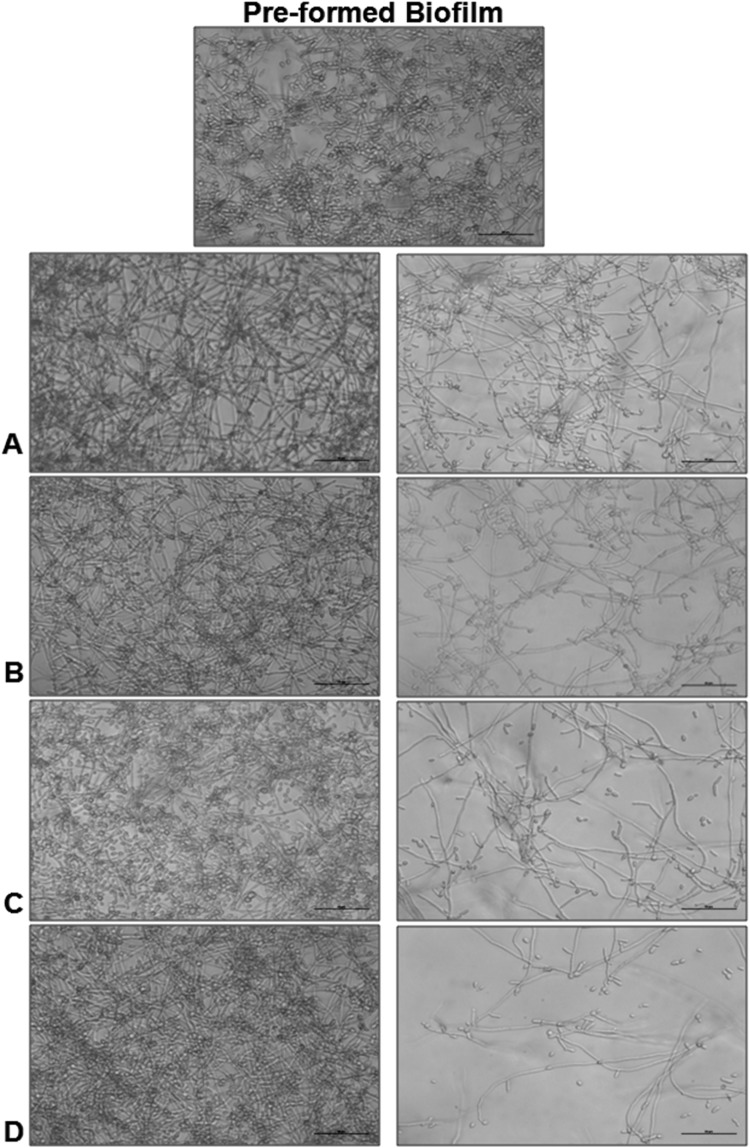
The conversion of yeast cells to filamentous forms is a critical step in biofilm biogenesis, which provides strength and support to the developing heterogeneous biofilm structure, also defined as the three dimensional matrix of hyphae. In the present study, we checked for the effect of Hs extract on the formation of filamentous forms in C. albicans cells. The percentage of germ tube observed in Hs treated C. albicans cells was 2.85% at 2 h and 4.65% at 4 h. The microscopic observations of the untreated Candida cells start showing the formation of germ tube from 2 h onwards (Fig. 3a) which further elongated to characteristic hyphae within 4 h. Almost all the cells showed hyphae formation after 4 h (Fig. 3b). On the other hand, cells incubated with Hs extract (MIC50) failed to initiate germ tube formation within two h and after 4 h the cells were arrested with a few budding or pseudo-hyphae formation (Fig. 3b). Based on these observations, it was concluded that presence of Hs extract effectively inhibited the initiation of hyphae, which subsequently lead to the formation of biofilm. Next, we wanted to check whether Hs extract could disrupt the pre-formed biofilm. The incubation of the pre-formed biofilm with Hs extract showed significant reduction with time. The pre-formed biofilm of control untreated cells kept on increasing with time (Fig. 4), while that of Hs extract treated cells started dislodging and decreasing from 4 h onwards (Fig. 4a–d, right panel) and was completely abolished by 24 h (Fig. 4d). These observations fall in line with the assumption that Hs extract is effective against pre-formed biofilm, and also has the ability to inhibit the formation of new biofilm. Rukayadi et al. [28] suggested the anti-fungal role of Hs using the disc diffusion assay in terms of inhibition zone but provided no observation on biofilm formation. Another study by Alshami and Alharbi [29], performed the MIC assay in broth dilution and also suggested the role of Hs in inhibiting the biofilm formation. Our study, for the first time, provides evidence not only for the inhibition of biofilm formation but also loss of adherence property and most importantly its effectiveness against pre-formed biofilm, which is the landmark of established Candida infection. Another major difference between previous two studies and the present study, is the solvent used to prepare Hs extract; DMSO was used in the present study, while alcohol/methanol were used to prepare the extracts in other studies.
Fig. 3.
Inhibition of C. albicans biofilm formation/hyphae initiation by Hs extract. C. albicans ATCC 90028 cells were grown continuously in the presence of extract at 37 °C for a 2 h and b 4 h
Fig. 4.
Disruption of C. albicans pre-formed biofilm by Hs extract. C. albicans ATCC 90028 cells were grown in RPMI 1640 at 37 °C for 24 h to allow the formation of mature biofilm. These biofilms were then observed microscopically after addition of Hs extract at 4 h (a), 6 h (b), 12 h (c) and 24 h (d)
Hs Extract Exhibited Anti-Candida Activity in the In Vivo C. elegans System
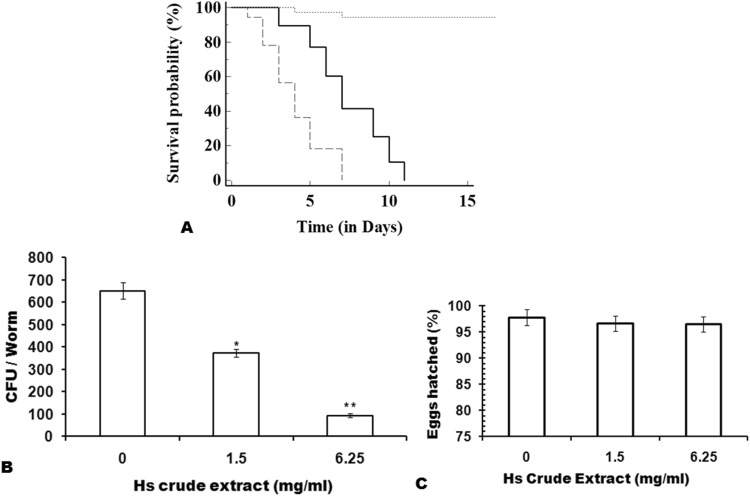
The next important evidence for any compound to be projected as a probable antimicrobial compound, is to determine its toxicity and anti-microbial nature under in vivo conditions. The toxicity test using cell lines is a cellular phenomenon, whereas use of C. elegans for the toxicity and anti-microbial assays provide information about the response from a whole animal. The data so obtained can be used indirectly for predicting mammalian host responses. The worms with their intact and metabolically active digestive, endocrine, reproductive, sensory and neuromuscular systems have been shown to be as predictive of rat LD50 ranking as mouse LD50 ranking [30]. In the present study, the C. elegans, infected with C. albicans showed a significant decrease in lifespan with mean and maximum lifespan as 4 and 7 days, respectively, as compared to the uninfected nematodes (Fig. 5a, P < 0.0001). Exposing the infected worms to 1.5 mg/ml (MIC50) of Hs extract increased both the mean and maximum lifespan of worms to 7 and 11 days, respectively. Moreover, it was found that treatment with 1.5 mg/ml (MIC50) of Hs extract significantly reduced the CFU of C. albicans isolated from the infected worms (Fig. 5b). Thus, the Hs extract significantly reduced the colonization of C. albicans in the worms, and thereby increased the lifespan of infected nematodes. To further ascertain the feasibility to use Hs extract in Candida infections, it was necessary to evaluate the toxicity of Hs extract in the in vivo system. For this, the percentage of worm’s eggs that hatched, and were alive in the media containing Hs extract were checked. It was observed that at both the MIC50 (1.5 mg/ml) and MIC80 (6.25 mg/ml), the difference in egg hatching was insignificant and the larvae were alive till 12 h of observation. This again supports the hypothesis that Hs extract, being a natural plant extract, is safe and effective against C. albicans biofilm. The significant anti-candida biofilm activity and less toxicity of Hs extract further motivated us to test the efficacy of Hs extract on C. albicans isolated from patients.
Fig. 5.
Anti-Candida activity of Hs extract in C. elegans. a Kaplan–Meier survival curve showing the lifespan of uninfected worms (dotted line), infected with C. albicans (dashed lines) and infected worms exposed to Hs extract (solid lines). b Fungal Burden of C. albicans in nematodes. The infected nematodes were grown with Hs extract at concentration of 0, 1.5 and 6.25 mg/ml and CFU of C. albicans was quantified. Bars represent mean ± S.E.M.; P < 0.001
In-Vitro Susceptibility of Clinical Isolates of Candida
In the present study, 30 C. albicans, isolates obtained from patients visiting gynae-department at VMMC and Safdarjung Hospital, New-Delhi were checked for in vitro anti-fungal susceptibility by different methods. They were then tested for antifungal susceptibility and effect of Hs extract on mature biofilm. In the Fluconazole susceptibility test performed using the E-test method, C. albicans isolates were grouped as 13% resistant, 53% with intermediate sensitivity and 33% sensitive. For the isolates that were grouped as sensitive, the sensitivity to Amphotericin B and Hs extract was similar to that of control C. albicans strain, when assessed for antifungal sensitivity at planktonic and biofilm stage. However, the isolates showing intermediate sensitivity and resistance towards fluconazole were still sensitive to Hs extract at slightly increased dose from 1.5 to 2.15 mg/ml in planktonic cells (Table 3). Similarly, we observed that Hs extract was effective in inhibiting preformed biofilms in these isolates at higher concentration (from 3.125 to 4.25 mg/ml). Thus, based on the study results and clinical samples, it was deduced that DMSO extract of Hibiscus sabdariffa is also effective on the fluconazole resistant C. albicans strains.
Table 3.
Antifungal susceptibility testing of 30 C. albicans isolates from patients visiting OBG Department, VMMC and Safdarjung Hospital, New-Delhi
| E-test (μg/ml) | Planktonic cells (MIC50) | Pre-formed biofilm (MIC50) | |||||
|---|---|---|---|---|---|---|---|
| Fluconazole (μg/ml) | Amp B (μg/ml) | Hs extract (mg/ml) | Fluconazole (μg/ml) | Amp B (μg/ml) | Hs extract (mg/ml) | ||
| C. albicans sensitive strain (10) | 1.5–8 | 2.0–8.0 | 0.5 | 1.5 | > 64 | 2 | 3.125 |
| C. albicans intermediate sensitivity (16) | 12.0–32.0 | 14.0–32.0 | 0.5 | 1.72–1.82 | > 64 | 2 | 3.25–3.9 |
| C. albicans resistant strain (4) | 48–> 256 | > 64 | 0.5 | 1.9–2.15 | > 64 | 2 | 3.8–4.25 |
Conclusion
One of the most important virulence traits of C. albicans is its ability to switch reversibly between its filamentous forms (hyphae) to budding yeast forms. This transition is the key factor for C. albicans infections. These hyphae ultimately form biofilms which protect sessile yeast cells from anti-fungal agents and are the source of new infections. Therefore, new anti-fungal agents should have the ability to impair hyphal development and, in addition, should have the ability to abolish the pre-formed biofilm, thereby targeting the sessile yeast cells.
Our results for the first time indicate that DMSO extract of Hibiscus sabdariffa has the potential to alleviate C. albicans pathogenesis, which is in line with the current anti-fungal paradigm to target virulence trait of microbial cells [31]. The Hs extract not only prevents adhesion, biofilm initiation and formation, but also destroys the preformed biofilm. Our results also point towards differences in antimicrobial activity of different extracts of Hs, which highlights the importance of choosing the correct combination of solvent and chemical compound, for testing antimicrobial activity. A detailed pharmacological analysis is needed to identify the active compounds that can be used as drugs against Candida.
Acknowledgements
The authors are thankful to the DBT-BIF bioinformatics facility of ACBR for all the logistic support. Strains used in this study were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Abbreviations
- CFU
Colony-forming units
- DMSO
Dimethyl sulfoxide
- Hs
Hibiscus sabdariffa
- MDR
Multi drug resistant
- MIC
Minimum inhibitory concentration
- NP
Natural products
- XTT
2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide
Funding
This work was supported by the scheme of Post Doctoral Fellowship for Women funded by University Grants Commission, India (Grant No. PDFWM-2014-15-GE-DEL-22743) to MD and SAPII by UGC and DST-Purse Grant-II to DS funded by Department of Science and Technology, India.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was as per the Guidelines and Standards for Research. The study was approved by the Ethics Committee of ACBR (ACBR/IHEC/DS-02/09-18), University of Delhi and VMMC and Safdarjung Hospital (IEC/VMMC/SJH/Project/November/2018-1104).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandra J, Mukherjee PK. Candida biofilms: development, architecture, and resistance. Microbiol Spectr. 2015 doi: 10.1128/microbiolspec.MB-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M. Candida species biofilms’ antifungal resistance. J Fungi. 2017;3:8. doi: 10.3390/jof3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dongari-Bagtzoglou A. Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti Infect Ther. 2008;6:201–208. doi: 10.1586/14787210.6.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 5.Elisha IL, Botha FS, McGaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. 2017;17:133. doi: 10.1186/s12906-017-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vashist H, Jindal A. Antimicrobial activities of medicinal plants—review. Int J Res Pharm Biomed Sci. 2012;3:222–230. [Google Scholar]
- 7.Silva N, Fernandes Júnior A. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 2010;16:402–413. doi: 10.1590/S1678-91992010000300006. [DOI] [Google Scholar]
- 8.Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, Shimotohno K. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phyther Res. 1995;9:180–184. doi: 10.1002/ptr.2650090305. [DOI] [Google Scholar]
- 9.Hassan STS, Berchová K, Šudomová M. Antimicrobial, antiparasitic and anticancer properties of Hibiscus sabdariffa (L.) and its phytochemicals: in vitro and in vivo studies. Ceska Slov Farm. 2016;65:10–14. [PubMed] [Google Scholar]
- 10.Panaitescu M, Lengyel E. Monitoring the antibacterial activity of Hibiscus sabdariffa extracts. Manag Sustain Dev. 2017;9:31–34. doi: 10.1515/msd-2017-0011. [DOI] [Google Scholar]
- 11.Afolabi OC, Ogunsola FT, Coker AO. Susceptibility of cariogenic Streptococcus mutans to extracts of Garcinia kola, Hibiscus sabdariffa, and Solanum americanum. West Afr J Med. 2008;27:230–233. [PubMed] [Google Scholar]
- 12.Olaleye TM. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J Med Plants Res. 2007;1:009–013. [Google Scholar]
- 13.Wayne P. Performance standards for antimicrobial disk susceptibility tests; approved standard. 9. Wayne: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 14.Borrás-Linares I, Fernández-Arroyo S, Arráez-Roman D, Palmeros-Suárez PA, Del Val-Díaz R, Andrade-Gonzáles I, Fernandez-Gutierrez A, Gomez-Leyva JF, Segura-Carretero A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa) Ind Crops Prod. 2015;69:385–394. doi: 10.1016/j.indcrop.2015.02.053. [DOI] [Google Scholar]
- 15.Rex JH, Alexander BD, Andes D, Arthington-Skaggs B, Brown SD, Chaturvedi V, Ghannoum MA, Espinel-Ingroff A, Knapp CC, Ostrosky-Zeichner L, Pfaller MA, Sheehan DJ, Walsh TJ. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. 3. Wayne: Clinical and Laboratory Standards Institute; 2008. pp. 1–25. [Google Scholar]
- 16.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Mowat E, Ramage G, Lopez-Ribot JL. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3:1909–1924. doi: 10.1038/nprot.2008.192. [DOI] [PubMed] [Google Scholar]
- 18.Fazly A, Jain C, Dehner AC, Issi L, Lilly EA, Ali A, Cao H, Fidel PL, Jr, Kaufman PD. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci. 2013;110:13594–13599. doi: 10.1073/pnas.1305982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother. 2018;102:575–586. doi: 10.1016/j.biopha.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Abdallah EM. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J Acute Dis. 2016;5:512–516. doi: 10.1016/j.joad.2016.08.024. [DOI] [Google Scholar]
- 22.Abdallah EM. Antibacterial efficiency of the Sudanese Roselle (Hibiscus sabdariffa L.), a famous beverage from Sudanese folk medicine. J Intercult Ethnopharmacol. 2016;5:186–190. doi: 10.5455/jice.20160320022623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez-Rodrigues MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci. 2011;76:429–435. doi: 10.1111/j.1750-3841.2011.02091.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Rodrigues MM, Balaban MO, Marshall MR, Rouseff RL. Hot and cold water infusion aroma profiles of Hibiscus sabdariffa: fresh compared with dried. J Food Sci. 2011;76:212–217. doi: 10.1111/j.1750-3841.2010.01989.x. [DOI] [PubMed] [Google Scholar]
- 25.Fullerton M, Khatiwada J, Johnson JU, Davis S, Williams LL. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Esherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J Med Food. 2011;14:950–956. doi: 10.1089/jmf.2010.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst JF. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology. 2000;146:1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 28.Rukayadi Y, Shim J-S, Hwang J-K. Screening of Thai medicinal plants for anticandidal activity. Mycoses. 2008;51:308–312. doi: 10.1111/j.1439-0507.2008.01497.x. [DOI] [PubMed] [Google Scholar]
- 29.Alshami I, Alharbi AE. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac J Trop Biomed. 2014;4:104–108. doi: 10.1016/S2221-1691(14)60217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt PR. The C. elegans model in toxicity testing. J Appl Toxicol. 2017;37:50–59. doi: 10.1002/jat.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today. 2009;14:214–222. doi: 10.1016/j.drudis.2008.11.013. [DOI] [PubMed] [Google Scholar]