Abstract
Purpose
The objective of this study was to examine the effect of tauroursodeoxycholic acid (TUDCA) on intracytoplasmic sperm injection (ICSI) embryos by evaluating endoplasmic reticulum (ER) stress, apoptosis, and embryo developmental competence in vitro and in vivo.
Methods
ER stress–associated genes and apoptosis-associated genes were measured and apoptosis index was analyzed. Embryo developmental competence was assessed in vitro and in vivo via the inner cell mass (ICM)/trophectoderm (TE) index, pregnancy and implantation rates, and birth rate.
Results
The relative mRNA and protein expression of binding immunoglobulin protein (BIP) was significantly higher in the ICSI embryo group without TUDCA treatment (ICSI-C) than in the in vitro fertilization (IVF) group and in the ICSI embryo group with TUDCA treatment (200 μM) (ICSI-T), while TUDCA ameliorated ER stress in ICSI embryos. Embryos in the ICSI-C group showed a higher apoptosis index than those in the IVF group and ICSI-T group, and there was no significant difference between the IVF group and ICSI-T group. TUDCA can significantly improve ICSI embryo developmental competence in vitro and in vivo based on the ICM/TE index, pregnancy and implantation rates, and birth rate.
Conclusion
ICSI embryos manifested high ER stress and high apoptosis, while TUDCA ameliorated ER stress and reduced apoptosis in ICSI embryos. TUDCA can significantly improve the developmental competence of ICSI embryos in vitro and in vivo. This study provides a new idea for improving the efficiency of ICSI, and it will also have a positive effect on the development of assisted reproduction technologies for humans and other animals.
Keywords: ICSI, Tauroursodeoxycholic acid, ER stress, Apoptosis
Introduction
With the increasing occurrence of human reproductive system diseases, the number of people conceived by assisted reproductive technology has grown much faster than expected, reaching several millions today and rapidly approaching 0.1% of the total world population [1]. Infertile couples account for 10–15% of couples worldwide, and approximately half of the infertility experienced by couple is caused by male factors [2]. The emergence of intracytoplasmic sperm injection (ICSI) has great significance in the treatment of male infertility [2]. ICSI has been widely used in patients with IVF failure, ejaculation disorders, high levels of antisperm antibodies, cancer requiring chemical and radiotherapy, spinal injuries, azoospermia, or oligozoospermia [3]. Studies have shown that ICSI embryos have abnormal imprinting, which may be related to the damage of eggs and embryos caused by ICSI operation [4–7]. Epidemiological studies have reported that ICSI offspring are at high risk for abnormalities related to the cardiovascular, neuromuscular, and endocrine systems [8–10].
ICSI is also widely used in genetically modified animal production and in animal sex control [11] and involves the treatment of sperm with surfactant or the repeated freezing and thawing of sperm and incubation with exogenous genes. Incubated sperm is injected into metaphase II (MII) oocytes by the ICSI method, from which transgenic embryos may be obtained. Transgenic animals could also be obtained after embryo transfer. This method is simple and efficient, and the proportion of homozygous offspring obtained by the direct injection of exogenous DNA into oocytes is significantly increased [12]. ICSI is also a very promising method for the production of sexed embryos, which can reduce damage by biopsy and avoid the decline in sperm fertilization caused by flow cytometry [13]. In addition, ICSI is an important method to study fertilization, reproduction, development, embryonic stem cells, and other fields [14].
Since the first mouse offspring was born in 1995 due to ICSI using Piezo micromanipulation, this procedure has been widely used in ICSI, somatic cell nuclear transfer (SCNT), blastocyst injection with ES cells, and other embryo engineering techniques [15, 16]. Although this method can greatly improve efficiency, it can still cause stress and damage to oocytes, induce DNA damage in embryos, induce apoptosis, reduce blastocyst numbers, and decrease the rate of development to term [17]. The endoplasmic reticulum (ER) is an important organelle for several biological macromolecules, such as proteins, lipids, and carbohydrates. Chemical drugs, environmental stress, and other stimuli affect the normal folding of proteins, accelerating the accumulation of unfolded and misfolded proteins in the endoplasmic reticulum, thus affecting the homeostasis of the endoplasmic reticulum and leading to ER stress [18]. It has been recognized that high ER stress induces abnormal epigenetic modification, apoptosis, and death [19, 20]. In this study, to ameliorate the stress caused by ICSI and improve ICSI efficiency, mice were used as the model, and tauroursodeoxycholic acid (TUDCA), an effective ER stress inhibitor, was supplemented in ICSI embryo culture medium. The expression of crucial genes, the apoptosis index, the inner cell mass (ICM)/trophectoderm (TE) index, and the birth rate were analyzed to examine the effect of TUDCA on ICSI embryo development.
Materials
All mice were housed under the following conditions: temperature of 24 ± 1 °C, relative humidity of 55 ± 5%, and photoperiod of 12 L:12 D. The animals were given free access to water and food. The Animal Care and Use Committee of Zhengzhou University approved all procedures related to the use of and experimentation on animals.
ICSI
The B6D2F1 (C57BL/6×DBA/2) hybrid strain were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Females 8–10 weeks of age (B6D2F1) were superovulated by serial intraperitoneal injection of 5 IU PMSG followed 45–54 h later by 5 IU hCG. At 12–15 h after hCG injection, the mice were sacrificed, and their oviducts were collected and placed in CZB (Sigma-Aldrich, St. Louis, MO, USA). Working under the stereomicroscope, the oviduct wall was held with one pair of forceps and torn with another pair of forceps where the oocyte-cumulus complex makes a bulge. The oocyte-cumulus complex was carefully removed from the oviduct as a single mass. The oviductal remnant was discarded, the oocyte-cumulus complex was treated with a hyaluronidase (300 μg/mL) droplet for 5 min, and the cumulus cells were removed. The oocytes were washed with fresh CZB 3 times to remove hyaluronidase. Then, the oocytes were transferred to a drop of equilibrated culture medium under mineral oil (Sigma-Aldrich) in a 3.5-cm dish. The dish containing the oocytes was placed in an incubator with 5% (v/v) CO2 at 37.5 °C for at least 15 min until required.
Sperm was collected from the cauda epididymides of males that were at least 10 weeks old, the sperm suspensions were mixed with polyvinylpyrrolidone (PVP) solution (12%, w/v), and MII oocytes were removed from the CO2 incubator and placed into a microinjection droplet. A sperm was brought into sharp focus, and the head was drawn into a microinjection pipette. The oocyte was oriented such that its MII plate was located straight up or down along the y-axis. The fine z-axis control was used to move the pipettes up or down so that their ends were in focus. The oocyte zona and plasma membrane were pushed to engage the holding pipette aperture, to anchor the oocyte and to facilitate injection. The microinjection pipette was pushed toward the holding pipette to make the pipette rapidly pass through the zona, stopping when the tip advanced through 95% of the oocyte diameter. Sustained positive pressure was maintained until the final sperm was 10–50 mm from the tip of the microinjection pipette, and the piezo preset channel was changed to a gentler setting. The sperm head was deposited in the cytoplasm with the application of low positive pressure in the microinjection pipette and then the needle was smoothly withdrawn from the oocyte. The injected oocyte was released by gently applying positive pressure within the holding pipette, and dead eggs were removed from the surviving eggs during transfer to the CO2 incubator.
In vitro fertilization
Sperm and oocytes were collected as in the ICSI procedure, and the collected sperm were placed in HTF medium (Aibeier, Nangjing, China) and incubated at 37 °C for 1 h. The sperm and oocytes were incubated together in HTF medium for 6 h. Fertilized eggs were selected, washed with KSOM medium (Sigma-Aldrich) 3 times, and then transferred to KSOM medium for further culture.
Culture and treatment of embryos
TUDCA (Sigma-Aldrich) was dissolved in DMSO (Sigma-Aldrich) and then added different quantities into embryo medium. TUDCA at different concentrations was used to define the optimal concentration to reduce ER stress. The medium used for culturing embryos was KSOM medium supplemented with amino acids, covered with mineral oil, and cultured with 5% (v/v) CO2 at 37.5 °C.
Embryo transfer
Embryo transfer was performed as previously described [21]. The recipients were anaesthetized, and the oviduct was accessed by making a small incision on the recipient’s back. A small cavity was made in the oviduct using a thin needle, and embryos at the 2-cell stage were inserted using a pipette. The incision was then closed. This ET procedure was performed unilateral, and the mice were placed in a warm environment.
Real-time PCR
Total RNA was extracted from blastocysts (20 from each group) using a Cells-to-Signal Kit (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using a PrimeScript™ RT Reagent Kit (TaKaRa, Tokyo, Japan) as previously reported [22]. Real-time PCR was performed using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using SYBR Premix Ex Taq™ II (TaKaRa, Tokyo, Japan). The detailed procedure was as follows: thermal cycling at 95 °C for 1 min; 40 PCR cycles of 5 s at 95 °C for DNA denaturation, 30 s at 60 °C for primer annealing and extension; followed by 65 to 95 °C for melting (increment, 0.5 °C/5 s). The primer sequences are listed in Table 1. All examined genes were quantified in triplicate.
Table 1.
List of primers used in real-time PCR
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| BIP | GTGTGTGAGACCAGAACCGT | TAGGTGGTCCCCAAGTCGAT |
| BAX | TACAGGGTTTCATCCAGG | GTCAGCAATCATCCTCTG |
| BCL2 | GAGTTAGTTCGTCTGAGTAG | ATAGGTCAAGAGGGAGTG |
| PPIA | GAGCTCTGAGCACTGGAGAGA | CCACCCTGGCACATGAAT |
Western blotting
Protein from embryos (90 from each group) was extracted using RIPA buffer. The concentration of the proteins derived from exosomes was determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The proteins were separated by SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat milk powder/Tris-buffered saline containing 0.05% Tween 20 (TBST), incubated with primary antibody. Then, the membranes were washed three times in TBST and incubated in TBST/0.2% BSA containing secondary antibody. The immunoblots were visualized using an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA).
Immunofluorescence
Immunofluorescence was performed as previously described [23]. Immunodetection was based on the detection of CDX2, a specific marker of trophectoderm (TE). Embryos were fixed using 4% (v/v) paraformaldehyde (at RT for 2 h), permeabilized using 0.2% (v/v) Triton X-100 (at RT for 20 min), and blocked in a blocking solution (at 4 °C for 12 h). The embryos were then incubated overnight with a primary antibody against CDX2 (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C followed by incubation with Alexa Fluor 488 secondary antibody (Beyotime, Shanghai, China) for 2 h at RT. After incubation with primary or secondary antibodies, the embryos were washed three times in 0.1% PBS-polyvinyl alcohol (PVA) (at RT for 15 min) and treated with 4,6-diamidino-2-phenylindole hydrochloride (DAPI) for 3 min. After washing and mounting, the slides were examined by epifluorescence using a Nikon Eclipse Ti-S microscope, and images were captured using a digital sight DS-U3 camera (Nikon, Tokyo, Japan). The ICM/TE index was calculated by dividing the cell number in the ICM by the cell number in the TE. The cell number in the ICM was the total cell number minus the CDX2-positive cell number, and the cell number in the TE was the CDX2-positive cell number.
Apoptosis detection
Apoptosis detection was performed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) as previously described [24]. Briefly, blastocysts were fixed in 4% paraformaldehyde (at RT for 2 h), permeabilized in 0.5% Triton X-100 (at RT for 5 min), and incubated with FITC-conjugated dUTP and terminal deoxynucleotidyl transferase in the dark (37 °C for 1 h). The tailing reaction was terminated using 2× SSC (provided by the manufacturer) in the dark (RT for 15 min). Embryos were then incubated with PBS containing 25 μg/mL RNase A in the dark (RT for 30 min). After staining with DAPI and washing with PBS in the dark, the slides were examined by epifluorescence using a Nikon Eclipse Ti-S microscope, and images were captured using the digital camera. Apoptosis index is the number of TUNEL-positive cell divided by the number of total cell of blastocyst.
Statistical analysis
Eight days after embryo transfer, mice were injected with 0.1 mL of Chicago blue (1%) via the tail vein. Three minutes later, the mice were sacrificed, and then, we remove the uterus and counted implantation sites. The implantation rate was defined as a ratio of the number of implantation sites and the total number of transferred embryos. The pregnancy rate was defined as a ratio of the number of recipients who became pregnant and the total number of recipients. The birth rate was determined as a ratio of the number of pups and the number of transferred embryos. Cleavage rate, blastocyst rate, pregnancy rate, implantation rate, and birth rate were analyzed with χ2 test. The relative expression of gene transcripts, the apoptotic index, the ICM/TE index, and the weight of pups were analyzed using ANOVA. Statistical analyses were conducted using the SPSS software package (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± SEM. P < 0.05 was considered statistically significant.
Results
TUDCA increased blastocyst rate of mouse ICSI embryos in vitro
To determine the optimal concentration of TUDCA, mouse ICSI embryos were treated with different concentrations (0, 100, 200, and 500 μM) of TUDCA and subjected to further culture. The results showed that there was no significant difference in the cleavage rates among the groups. The blastocyst rate was significantly higher in the 200 μM TUDCA group than in the other groups (Table 2).
Table 2.
The effect of TUDCA on the cleavage rate and blastocyst rate of ICSI embryo in mice
| TUDCA (μM) | Numbers of embryo | Cleavage rate (%) | Blastocyst rate (%) |
|---|---|---|---|
| IVF(0) | 189 | 95.2a | 91.0a |
| ICSI (0) | 185 | 94.0a | 73.5b |
| ICSI (100) | 168 | 92.9a | 76.8b |
| ICSI (200) | 180 | 93.8a | 91.6b |
| ICSI (500) | 175 | 89.7a | 81.7a |
Values in the same row with different superscripts differ significantly (P < 0.05)
TUDCA ameliorated ER stress and decreased apoptosis in mouse ICSI embryos in vitro
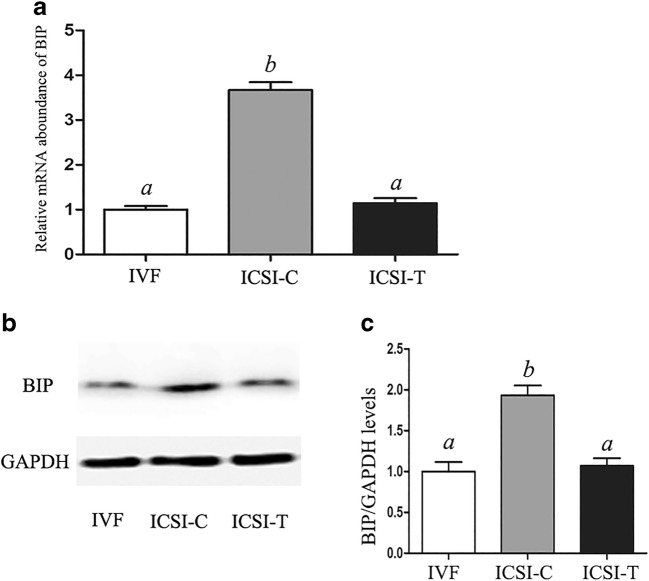
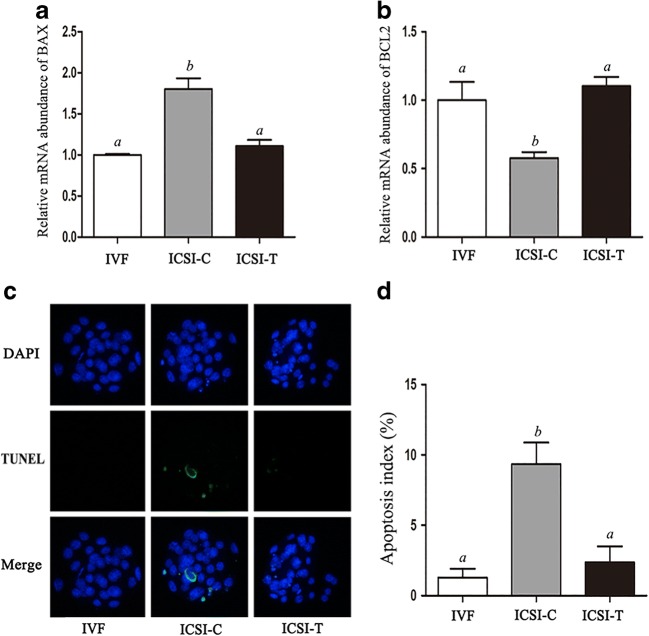
The level of the ER stress–associated transcript, binding immunoglobulin protein (BIP), was detected at the blastocyst stages in the in vitro fertilization (IVF) group, the ICSI embryo group without TUDCA treatment (ICSI-C), and the ICSI embryo group with TUDCA (200 μM) treatment (ICSI-T) using real-time PCR (Fig. 1). The relative mRNA expression of BIP was significantly higher in the ICSI-C group than in the IVF group, while treatment with TUDCA (ICSI-T) significantly decreased the expression of BIP. Western blotting was used to further determine the protein expression level of BIP (Fig. 1). The protein expression level of BIP was significantly higher in the ICSI-C group than in the IVF and ICSI-T groups. The levels of BAX and BCL2 were detected at the blastocyst stages using real-time PCR (Fig. 2). The results showed that the level of BAX was significantly higher in the ICSI-C group than in the IVF and ICSI-T groups, while the level of BCL2 was significantly lower in the ICSI-C group than in the IVF and ICSI-T groups. The apoptosis index of blastocysts in each group was analyzed using the DeadEnd Fluorometric TUNEL System (Fig. 2). The results showed that embryos in the ICSI-C group showed a higher apoptosis index than those in the IVF group and ICSI-T group, and there was no significant difference between the IVF group and ICSI-T group.
Fig. 1.
Relative expression levels of BIP mRNA and protein in blastocysts. (A) Relative expression of BIP mRNA in blastocysts in each group. (B) BIP protein levels in blastocysts in each group. (C) Quantification of BIP protein levels in blastocysts in each group. Data are shown as the mean ± SEM. Values with different superscripts differ significantly (P < 0.05)
Fig. 2.
Relative mRNA expression levels of BAX and BCL2 and the apoptosis index in blastocysts. (A) Relative expression of BAX mRNA in blastocysts in each group. (B) Relative expression of BCL2 mRNA in blastocysts in each group. (C) Apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay in blastocysts. Apoptotic blastomeres were detected by TUNEL (green). DNA was stained by DAPI (blue) to visualize all blastomeres. (D) Quantification of the apoptosis index of blastocysts in each group. Data are shown as the mean ± SEM. Values with different superscripts differ significantly (P < 0.05)
Effect of TUDCA on ICSI blastocyst quality and subsequent in vivo development to term
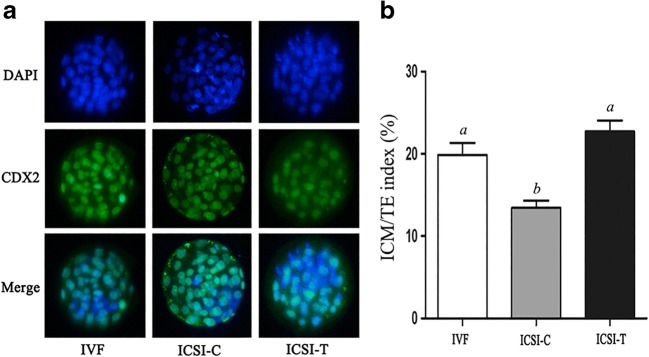
The ICM/TE index of blastocysts was assessed by immunofluorescence. The results showed that compared with the IVF group, the ICSI-C group showed a decreased ICM/TE index, while TUDCA (200 μM) treatment significantly increased the ICM/TE index (Fig. 3). Embryos in each group at the 2-cell stage were transferred to the oviduct, and pregnancy rate and implantation rate were analyzed. There was no significant difference in the pregnancy rate among the groups (Table 3). The implantation rate of the ICSI-C group was significantly lower than that of the IVF and ICSI-T groups. The birth rate of the ICSI-C group was significantly lower than that of the IVF group, while the TUDCA treatment significantly increased the birth rate (Table 4). There was no significant difference in the weight of pups among the groups (Table 4).
Fig. 3.
Index of inner cell mass (ICM):trophectoderm (TE) in blastocysts. Staining was performed by immunostaining for CDX2, a specific marker of TE (green), and DAPI, to stain the nuclei of all blastomeres (blue). (B) Quantification of the ICM/TE index of blastocysts in the IVF, ICSI-C, and ICSI-T groups. Data are shown as the mean ± SEM. Values with different superscripts differ significantly (P < 0.05)
Table 3.
The effect of TUDCA on the pregnancy and implantation rate
| Groups | Number of transferred embryo | Number of recipients | Pregnancy rate (%) | Implantation rate (%) |
|---|---|---|---|---|
| IVF | 96 | 12 | 58.3 (7/12)a | 57.8 (55/96)a |
| ICSI-C | 98 | 12 | 58.3 (7/12)a | 39.8 (39/98)b |
| ICSI-T | 96 | 12 | 58.3 (7/12)a | 56.2 (54/96)a |
Values in the same row with different superscripts differ significantly (P < 0.05)
Table 4.
The effect of TUDCA on the birth rate and weight of pups
| Groups | Number of transferred embryo | Number of recipients | Birth rate (%) | Weight of pups (mg) |
|---|---|---|---|---|
| IVF | 102 | 12 | 55.9 (57/102)a | 768.1 ± 9.1a |
| ICSI-C | 98 | 12 | 35.7 (35/98)b | 771.6 ± 7.8a |
| ICSI-T | 106 | 13 | 53.8 (57/106)a | 770.2 ± 6.5a |
Values in the same row with different superscripts differ significantly (P < 0.05)
Discussion
In vitro cultured embryos suffer changes in temperature, pH and osmotic pressure, and oxidative stress, which might cause ER stress, induce abnormal gene expression and epigenetic modification, and result in metabolic disturbance and low developmental competence [25]. In the mouse cumulus oocyte complex, excessive fatty acid secretion, protein synthesis disorders, and changes in mitochondrial membrane channels cause the ER stress response, which impairs oocyte maturation [26, 27]. It has been reported that the mechanisms responsible for controlling ER stress affect preimplantation embryo development via the following three major sensors: double-stranded RNA-activated protein kinase–like ER kinase (PERK), activating transcription factor6 (ATF6), and inositol-requiring enzyme 1 (IRE1) [28]. BIP is essential for embryonic cell growth and pluripotent cell survival and can also be a reliable marker for ER stress [29]. BIP−/− embryos lead to peri-implantation lethality, with embryos that do not hatch from the zona pellucida in vitro, fail to grow in culture, and exhibit proliferation defects and markedly increased apoptosis in the ICM [29]. Embryos under high ER stress induced by tunicamycin (TM) exhibited high expression of BIP [30]. Mouse embryos treated with more than 5 μg/mL TM were completely blocked at the 2-cell stage and failed to develop into blastocysts, and treatment with 0.5 μg/mL TM significantly decreased the blastocyst formation rate [31, 32]. In this study, the mRNA and protein expression level of BIP in embryos of ICSI-C group was significantly higher than that in vitro fertilized embryos, suggesting that ICSI embryos performed higher ER stress than in vitro fertilized embryos.
Embryo pipetting often produces shear stress, which can damage oocytes and negatively impact embryo development [33]. SCNT embryos exposed to micromanipulation and electrofusion-mediated activation are subject to an increased ER stress response, and decreased ER stress during SCNT embryo production can improve SCNT embryo development [30, 34]. During the ICSI procedure, the cytoskeleton and organelles of oocytes may be injured when sperm are injected into the oocyte cytoplasm with a microinjection needle. Additionally, the collection process and subsequent manipulations of oocytes may also induce stress. We hypothesized that ICSI might induce ER stress. The downstream signaling pathway of ER stress mediates the activation of apoptotic pathways, such as the upregulation of BAX and the downregulation of BCL2 [35]. ER stress–induced apoptosis has been reported in mammalian embryos. A high level of ER stress was reported to significantly increase the mRNA expression of BAX, decrease the mRNA expression of BCL, and induce apoptosis in blastocysts [30, 34]. In this study, the expression of BCL2 significantly decreased, and the expression of BAX and the apoptosis index of ICSI embryos significantly increased in the ICSI-C group, suggesting that ICSI embryos have high apoptosis rates, which may be related to ER stress.
TUDCA can effectively enhance oocyte maturation and improve the developmental competence of parthenogenetic embryos, fertilized embryos, and SCNT embryos in vitro by inhibiting endoplasmic reticulum stress and attenuating apoptosis [30, 36, 37]. TUDCA was reported to significantly increase both blastocyst formation and offspring production rates following IVF in mice [37]. TUDCA improved the viability of vitrified-warmed oocytes and their subsequent embryo competence [38]. Treatment of embryos with TUDCA decreased genome damage and cleavage kinetics to rescue the developmental competence of poorly developing embryos in porcine [39]. The addition of TUDCA during micromanipulation can inhibit cellular damage and enhance the in vitro development of SCNT embryos by reducing ER stress [30]. The addition of TUDCA improved the developmental competency of embryos derived from in vitro matured pig oocytes injected intracytoplasmically with boar evaporative drying spermatozoa by reducing reactive oxygen species production and DNA degradation and fragmentation [40]. In the present study, we found that TUDCA downregulated BIP and BAX expression, upregulated BCL2 expression, and decreased the apoptosis index of mouse embryos in the ICSI-C group, suggesting that TUDCA can be a potential treatment option for improving ICSI embryo development by reducing ER stress.
To evaluate the effect of TUDCA on embryonic development competence, the inner cell mass/trophectoderm (ICM/TE) index of blastocysts was analyzed. The ICM/TE ratio offers important information to evaluate the quality of individual embryos [22, 41]. An embryo with a high ICM/TE index is considered to have better quality and developmental potential [21, 42]. In this study, we found that ICSI embryos in the ICSI-T group had a higher ICM/TE index than those in the ICSI-C group, indicating that TUDCA improved the developmental competence of ICSI embryos. To further evaluate the effect of TUDCA on embryo development competence, the efficiency of in vivo embryo transfer was analyzed. The results showed that the implantation rate and birth rate of the ICSI-T group were significantly higher than those of the ICSI-C group, indicating that TUDCA enhances ICSI embryo development in vivo.
In conclusion, our results demonstrate that TUDCA can ameliorate ER stress and inhibit apoptosis in ICSI embryos in mice. Furthermore, TUDCA can enhance the developmental competence of ICSI embryos in vitro and in vivo. Moreover, the present study provides a new sight for improving the efficiency of ICSI, which positively affect the development of assisted reproduction technology.
Funding information
This work was supported in part by the Natural Science Foundation of China (Grant Number 81571407).
Compliance with ethical standards
The Animal Care and Use Committee of Zhengzhou University approved all procedures related to the use of and experimentation on animals.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deng Tengfei and Xie Juanke contributed equally to this study and should be considered as co-first authors.
References
- 1.Faddy MJ, Gosden MD, Gosden RG. A demographic projection of the contribution of assisted reproductive technologies to world population growth. Reprod Biomed Online. 2018;36:455–458. doi: 10.1016/j.rbmo.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Singh K, Jaiswal D. Human male infertility: a complex multifactorial phenotype. Reprod Sci. 2011;18:418–425. doi: 10.1177/1933719111398148. [DOI] [PubMed] [Google Scholar]
- 3.Rubino P, Vigano P, Luddi A, Piomboni P. The ICSI procedure from past to future: a systematic review of the more controversial aspects. Hum Reprod Update. 2016;22:194–227. doi: 10.1093/humupd/dmv050. [DOI] [PubMed] [Google Scholar]
- 4.Khoueiry R, Ibala-Romdhane S, Al-Khtib M, Blachere T, Lornage J, Guerin JF, Lefevre A. Abnormal methylation of KCNQ1OT1 and differential methylation of H19 imprinting control regions in human ICSI embryos. Zygote. 2013;21:129–138. doi: 10.1017/S0967199411000694. [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Liu Z, Zhang R, Su C, Yang W, Yao Y, Zhao S. Imprinting alterations in sperm may not significantly influence ART outcomes and imprinting patterns in the cord blood of offspring. PLoS One. 2017;12:e0187869. doi: 10.1371/journal.pone.0187869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choux C, Binquet C, Carmignac V, Bruno C, Chapusot C, Barberet J, Lamotte M, Sagot P, Bourc’his D, Fauque P. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum Reprod. 2018;33:331–340. doi: 10.1093/humrep/dex366. [DOI] [PubMed] [Google Scholar]
- 7.Marchesi DE, Qiao J, Feng HL. Embryo manipulation and imprinting. Semin Reprod Med. 2012;30:323–334. doi: 10.1055/s-0032-1320013. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Heilbronn LK. The health outcomes of human offspring conceived by assisted reproductive technologies (ART) J Dev Orig Health Dis. 2017;8:388–402. doi: 10.1017/S2040174417000228. [DOI] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Kosteria I, Sakka S, Gkourogianni A, Terentes-Printzios D, Koutagiar I, Skoumas I, Miliou A, Papassotiriou I, Gardikioti V, Loutradis D, Chrousos G, Kanaka-Gantenbein C, Tousoulis D. PCSK9 and Lp(a) levels of children born after assisted reproduction technologies. J Assist Reprod Genet. 2019;36:1091–1099. doi: 10.1007/s10815-019-01474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cram DS, Song B, McLachlan RI, Trounson AO. CAG trinucleotide repeats in the androgen receptor gene of infertile men exhibit stable inheritance in female offspring conceived after ICSI. Mol Hum Reprod. 2000;6:861–866. doi: 10.1093/molehr/6.9.861. [DOI] [PubMed] [Google Scholar]
- 11.Salamone DF, Canel NG, Rodriguez MB. Intracytoplasmic sperm injection in domestic and wild mammals. Reproduction. 2017;154:F111–F124. doi: 10.1530/REP-17-0357. [DOI] [PubMed] [Google Scholar]
- 12.Arias ME, Sanchez-Villalba E, Delgado A, Felmer R. Effect of transfection and co-incubation of bovine sperm with exogenous DNA on sperm quality and functional parameters for its use in sperm-mediated gene transfer. Zygote. 2017;25:85–97. doi: 10.1017/S096719941600037X. [DOI] [PubMed] [Google Scholar]
- 13.Jo HT, Bang JI, Kim SS, Choi BH, Jin JI, Kim HL, Jung IS, Suh TK, Ghanem N, Wang Z, et al. Production of female bovine embryos with sex-sorted sperm using intracytoplasmic sperm injection: efficiency and in vitro developmental competence. Theriogenology. 2014;81(675-82):e1. doi: 10.1016/j.theriogenology.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill CL, Chow S, Rosenwaks Z, Palermo GD. Development of ICSI. Reproduction. 2018;156:F51–F58. doi: 10.1530/REP-18-0011. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 16.Salgado RM, Brom-de-Luna JG, Resende HL, Canesin HS, Hinrichs K. Lower blastocyst quality after conventional vs. Piezo ICSI in the horse reflects delayed sperm component remodeling and oocyte activation. J Assist Reprod Genet. 2018;35:825–840. doi: 10.1007/s10815-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Ding C, Wang E, Chen X, Li X, Zhao C, Fan Y, Wang L, Beaujean N, Zhou Q, Jouneau A, Ji W. Piezo-assisted nuclear transfer affects cloning efficiency and may cause apoptosis. Reproduction. 2007;133:947–954. doi: 10.1530/REP-06-0358. [DOI] [PubMed] [Google Scholar]
- 18.Sampieri L, Di Giusto P, Alvarez C. CREB3 transcription factors: ER-Golgi stress transducers as hubs for cellular homeostasis. Front Cell Dev Biol. 2019;7:123. doi: 10.3389/fcell.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Komarya SK, Jena G. Phenylbutyrate and beta-cell function: contribution of histone deacetylases and ER stress inhibition. Epigenomics. 2017;9:711–720. doi: 10.2217/epi-2016-0160. [DOI] [PubMed] [Google Scholar]
- 20.Lee WS, Yoo WH, Chae HJ. ER stress and autophagy. Curr Mol Med. 2015;15:735–745. doi: 10.2174/1566524015666150921105453. [DOI] [PubMed] [Google Scholar]
- 21.Qu P, Zhao Y, Wang R, Zhang Y, Li L, Fan J, Liu E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod Fertil Dev. 2019;31:324–332. doi: 10.1071/RD18203. [DOI] [PubMed] [Google Scholar]
- 22.Qu P, Qing S, Liu R, Qin H, Wang W, Qiao F, Ge H, Liu J, Zhang Y, Cui W, et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One. 2017;12:e0174535. doi: 10.1371/journal.pone.0174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu P, Zuo Z, Liu Z, Niu Z, Zhang Y, Du Y, et al. Sperm-borne small RNA regulate alpha-tubulin acetylation and epigenetic modification of early bovine somatic cell nuclear transfer embryos. Mol Hum Reprod. 2019. [DOI] [PubMed]
- 24.Jia L, Ding B, Shen C, Luo S, Zhang Y, Zhou L, Ding R, Qu P, Liu E. Use of oocytes selected by brilliant cresyl blue staining enhances rabbit cloned embryo development in vitro. Zygote. 2019;27:166–172. doi: 10.1017/S0967199419000200. [DOI] [PubMed] [Google Scholar]
- 25.Kelley RL, Gardner DK. In vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned media. Reprod Biomed Online. 2017;34:441–454. doi: 10.1016/j.rbmo.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Bradley J, Swann K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int J Dev Biol. 2019;63:93–103. doi: 10.1387/ijdb.180355ks. [DOI] [PubMed] [Google Scholar]
- 27.Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol. 2012;26:562–573. doi: 10.1210/me.2011-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res. 2016;363:159–167. doi: 10.1007/s00441-015-2124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin T, Lee JE, Oqani RK, Kim SY, Cho ES, Jeong YD, Baek JJ, Jin DI. Tauroursodeoxycholic acid improves pre-implantation development of porcine SCNT embryo by endoplasmic reticulum stress inhibition. Reprod Biol. 2016;16:269–278. doi: 10.1016/j.repbio.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Ullah O, Li Z, Ali I, Xu L, Liu H, Jin HZ, Fang YY, Jin QG, Fang N. Pterostilbene exerts a protective effect via regulating tunicamycin-induced endoplasmic reticulum stress in mouse preimplantation embryos. In Vitro Cell Dev Biol Anim. 2019;55:82–93. doi: 10.1007/s11626-018-0308-9. [DOI] [PubMed] [Google Scholar]
- 32.Ali I, Liu HX, Zhong-Shu L, Dong-Xue M, Xu L, Shah SZA, Ullah O, Nan-Zhu F. Reduced glutathione alleviates tunicamycin-induced endoplasmic reticulum stress in mouse preimplantation embryos. J Reprod Dev. 2018;64:15–24. doi: 10.1262/jrd.2017-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74:1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- 34.Park YR, Park HB, Kim MJ, Jung BD, Lee S, Park CK, Cheong HT. Effects of endoplasmic reticulum stress inhibitor treatment during the micromanipulation of somatic cell nuclear transfer in porcine oocytes. Dev Reprod. 2019;23:43–54. doi: 10.12717/DR.2019.23.1.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T, Diao YF, Kang JW, Lee JE, Kim DK, Jin DI. Tauroursodeoxycholic acid improves the implantation and live-birth rates of mouse embryos. Reprod Biol. 2015;15:101–105. doi: 10.1016/j.repbio.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Mochizuki M, Miyagi K, Kishigami S. Optimizing treatment of tauroursodeoxycholic acid to improve embryonic development after in vitro maturation of cumulus-free oocytes in mice. PLoS One. 2018;13:e0202962. doi: 10.1371/journal.pone.0202962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao N, Liu XJ, Li JT, Zhang L, Fu Y, Zhang YJ, Chen RX, Wei XQ, Wang R, Wang Y, Zhang JM. Endoplasmic reticulum stress inhibition is a valid therapeutic strategy in vitrifying oocytes. Cryobiology. 2015;70:48–52. doi: 10.1016/j.cryobiol.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Dicks N, Bohrer RC, Gutierrez K, Michalak M, Agellon LB, Bordignon V. Relief of endoplasmic reticulum stress enhances DNA damage repair and improves development of pre-implantation embryos. PLoS One. 2017;12:e0187717. doi: 10.1371/journal.pone.0187717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li XX, Diao YF, Wei HJ, Wang SY, Cao XY, Zhang YF, Chang T, Li DL, Kim MK, Xu B. Tauroursodeoxycholic acid enhances the development of porcine embryos derived from in vitro-matured oocytes and evaporatively dried spermatozoa. Sci Rep. 2017;7:6773. doi: 10.1038/s41598-017-07185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wydooghe E, Vandaele L, Beek J, Favoreel H, Heindryckx B, De Sutter P, Van Soom A. Differential apoptotic staining of mammalian blastocysts based on double immunofluorescent CDX2 and active caspase-3 staining. Anal Biochem. 2011;416:228–230. doi: 10.1016/j.ab.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 42.Ilina IV, Khramova YV, Filatov MA, Sitnikov DS. Femtosecond laser is effective tool for zona pellucida engraving and tagging of preimplantation mammalian embryos. J Assist Reprod Genet. 2019;36:1251–1261. doi: 10.1007/s10815-019-01424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]