Abstract
Endophytic fungi provide rich reservoir for novel antimicrobial compounds. An endophytic fungus, from Carica papaya plant identified as Phomopsis tersa, was investigated for attenuating the quorum sensing mediated pathogenicity of Pseudomonas aeruginosa PAO1. Crude extract of P. tersa was found to reduce the production of redox-active pigments—pyocyanin and pyoverdine in P. aeruginosa PAO1 by 92.46% and 71.55%, respectively at sub-MIC concentration of 900 μg/mL. In addition, the crude extract was also able to inhibit the expression of virulence factors involved in biofilm formation: exopolysaccharide (72.21%) and alginate (72.50%). Secretion of cell-lytic enzymes was also found to be reduced: chitinase by 79.73% and elastase by 74.30%. 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione identified from GC-MS analysis, displayed favorable molecular interactions with P. aeruginosa transcriptional regulators, LasR and RhlR with good docking scores of − 6.873 kJ/mol and − 6.257 kJ/mol, respectively. The study thus reveals the potential use of P. tersa for discovering drugs against infectious pathogens.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00840-y) contains supplementary material, which is available to authorized users.
Keywords: Pseudomonas aeruginosa, Quorum sensing, Phomopsis tersa, Biofilm formation, Secondary metabolites
Introduction
The chronic bacterial infections are major cause of solicitude for healthcare sectors. Biofilm establishing bacteria contributes to around 65% bacterial infection of the world [1]. Bacterial biofilm forming ability and expression of virulence factors are mediated by a unique communication system termed as quorum sensing (QS) [2]. Pseudomonas aeruginosa is a nosocomial pathogen causing severe chronic infection in immunocompromised individuals with lung disease, pneumonia and severe burns. QSS, designated as LasI/R and RhlI/R operon, regulates bacterial pathogenicity by controlling the expression of various pathogenic determinants and has positive influence on motility factors and biofilm mediated chronic infections [2–4]. The P. aeruginosa QS cascade involves two major signaling molecules, N-3-Oxo-Dodecanoyl-L-Homoserine lactone (C12HSL) and N-Butyryl-L-Homoserine lactone (C4HSL). These signaling molecules with respect to bacterial density interacts with their cognate receptors and initiate bacterial infection [5].
With increased risk of emergence of antibiotic resistance among infection causing bacteria, conventional antibiotics have been shown low effectiveness towards infections. The major reason for the antibiotic resistance is the limited permeability of cell membrane which contributes to slow down the penetration of effective dose of the anti-microbial compounds and increases the pathogenicity in the bacterial cell [6]. QS inhibition (QSI) can be achieved via different mechanisms such as inhibition of signaling molecule production, blocking the binding of AHL molecule to the receptors, alteration in the structure of target molecule by enzymes and interrupting the transport facility of the signaling molecule through the membrane channel. Molecules interfering with the QSS have gained importance to develop anti-infective drugs [7–9]. Natural compounds from plants as well as microbial sources are highly important to combat microbial pathogenicity especially drug resistant bacteria [10–12]. Plants are rich source of compounds that can act as QSIs by decreasing the expression level of virulence factor gene cluster without affecting bacterial growth. Microbial secondary metabolites are chemically diverse natural compound that can be utilized as QSIs and anti-biofilm agents.
Microorganisms associated with plants in symbiotic and non-symbiotic relationship generate a wide range of QSI molecules. Microorganisms are also a rich source of bioactive molecules. Endophytic fungi are beneficial microorganism that colonizes different plant parts in symbiotic manner [13]. Endophytic fungal metabolites are biologically active with high antimicrobial, antiviral, antifungal and insecticidal activities. Endophytic fungus, Alternaria alternata isolated from Carica papaya displayed QSI and altered the biofilm architecture against P. aeruginosa PAO1. A. alternata also showed inhibition of various pathogenic traits such as pyocyanin pigment, elastase, protease, chitinase and minimized biofilm formation via hindering alginate, EPS production and eDNA secretion [14]. Similarly, a marine fungi also significantly inhibited the biofilm dynamics of S. aureus, Listeria monocytogenes, E. coli and Salmonella typhi [15].
In the present work, the QSI activity of P. tersa isolated from C. papaya leaves was determined. Additionally, the identified bioactive compounds from GC-MS were subjected for their interaction with QS receptors using molecular docking analysis.
Materials and Methods
Bacterial culture, P. aeruginosa PAO1 and biosensor strain Chromobacterium violaceum (ATCC 12472) were grown overnight and anti-quorum sensing assays were conducted using crude extract of P. tersa based on available literature. Isolation, identification and phylogenetic analysis of fungal species were performed. Bioactive metabolites present in the fungal crude extract were determined using Gas chromatography-mass spectrometry (GC-MS) technique [14]. The MIC of P. tersa on P. aeruginosa was determined as per CLSI guidelines [16]. Anti-quorum sensing activities including inhibition of pigment production i.e. violacein [16], pyocyanin [14] and pyoverdine [17], virulence factors such as hydrogen peroxide [18], secretion of cell lytic enzymes; staphylolytic protease, protease, chitinase [14], elastase [19], biofilm promoting factors; exopolysaccharide [14], cell surface hydrophobicity, alginate [20], bacterial motility [16] and biofilm growth determination [20] using confocal laser scanning microscopic analysis were determined. Molecular docking was used as a tool for analyzing the molecular interaction between identified metabolites and QS receptors, LasR and RhlR [19, 21].
Results and Discussion
Identification of Endophytic Fungi
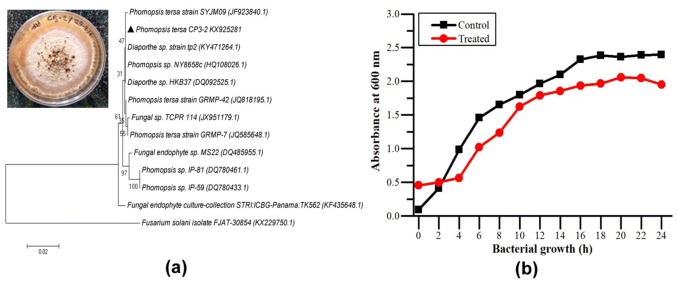
Twenty-four endo-symbiotic fungal strains were isolated from C. papaya leaves. Based on their antimicrobial activities against P. aeruginosa, the most active endophytic fungus was selected and identified using ITS region sequencing. BLAST analysis revealed it has maximum similarity with endophytic fungal strain P. tersa. The ITS sequencing data and BLAST analysis confirmed the selected strain as P. tersa based on 99% similarity among reported strains. The gene sequence was submitted to NCBI GenBank with an accession number KX925281. P. tersa was cultivated in large scale to isolate the crude extract for further studies (Fig. 1).
Fig. 1.
a Morphological and molecular characterization of endophytic fungus, Phomopsis tersa CP3 b growth pattern of P. aeruginosa at sub-MIC concentration 900 μg/mL of fungal extract with control
Determination of MIC
The MIC value was determined based on growth inhibition at varying concentrations ranging from 250 to 1500 μg/mL and found to be 1250 μg/mL. All subsequent assays including growth curve analysis were performed at sub MIC value of 900 μg/mL (Fig. 1).
Anti-quorum Sensing Activities
Inhibition of Pigment Production in P. aeruginosa PAO1 and C. violaceum
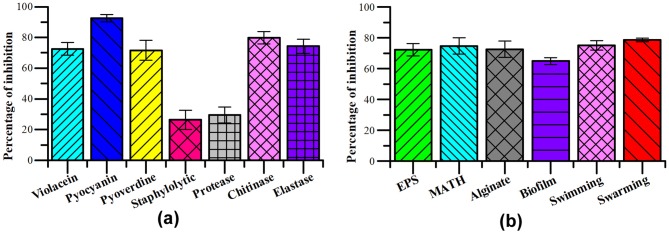
C. violaceum, a known biomarker strain for determining QSI activity when subjected to 900 μg/mL of P. tersa crude extract, resulted in 72.54 ± 4.15% reduction in violacein production (Fig. 2a). P. aeruginosa treated with fungal extract showed 92.46 ± 2.34% of reduction in pyocyanin production and 71.55 ± 6.49% reduction in pyoverdine production compared to the control (Fig. 2a). Streptomyces sp. TOHO-Y209 and TOHO-O348 secreted bioactive compounds, Piericidin A1, 3′-rhamnopiericidin A1 and piericidin E and showed QSI activity against C. violaceum and significantly decreased the production of violacein pigment in a dose dependent manner [22]. The present results were as per the earlier report depicting the potential of Andrographis paniculata extract in inhibiting the production of pyocyanin pigment [23].
Fig. 2.
a Inhibitory effect of P. tersa crude extract (900 μg/mL) on quorum sensing regulated processes: a production of pigments, in C. violaceum and P. aeruginosa PAO1, lysis of Staphylococcus aureus cell, degradation of Azocasein, production of chitinase and elastase. b Inhibition of virulent factors: exopolysaccharide (EPS), microbial adhesion to hydrocarbon (MATH), alginate, biofilm formation, swimming and swarming motility
Inhibition of Hydrogen Cyanide (HCN) Production
P. aeruginosa PAO1 treated with P. tersa extract demonstrated noticeable difference in HCN production via color changing properties of picric acid soaked filter paper. The filter paper placed with sample exhibited discoloration of filter paper from pale yellow to orange color. The color change observed in control sample was more compared to the treated sample (Fig. 3a).
Fig. 3.
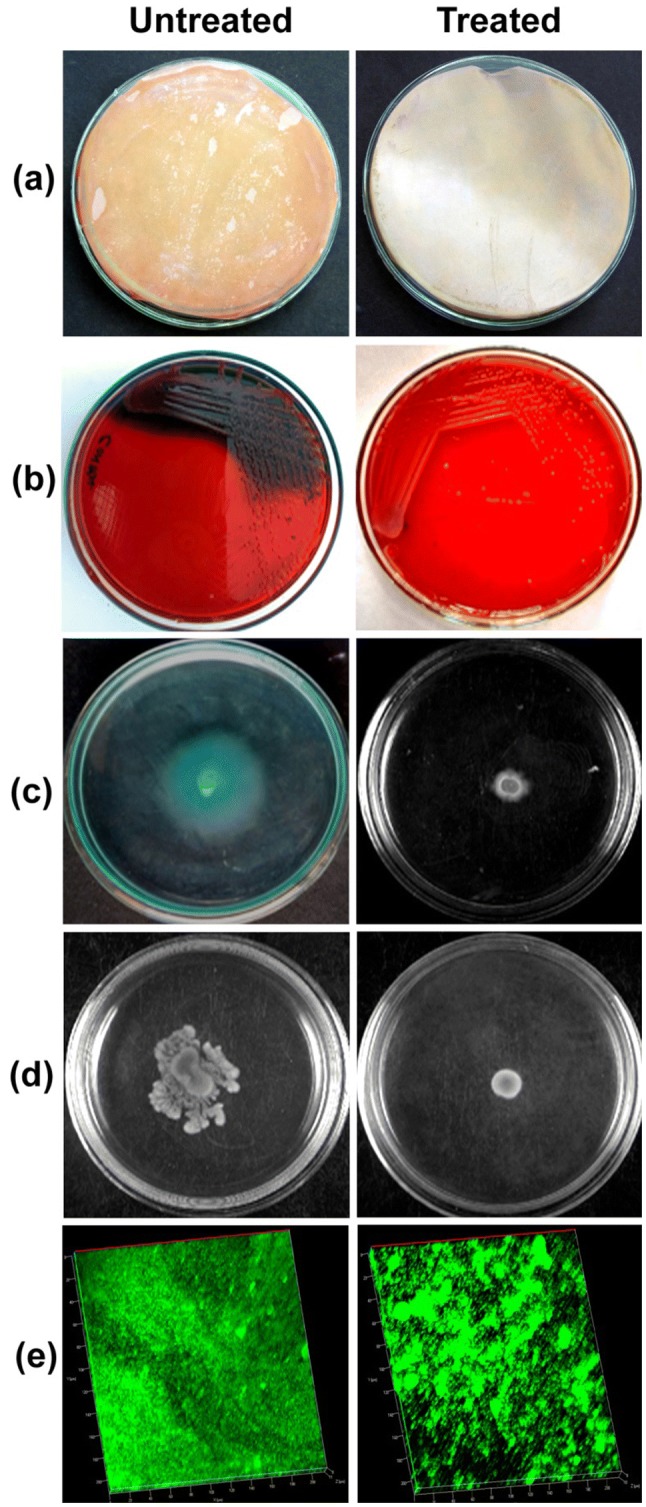
Inhibitory effect of fungal crude extract, P. tersa on quorum sensing mediated characteristics of P. aeruginosa PAO1: a Hydrogen cyanide (HCN) production, b Exopolysaccharide (EPS) production, c Swimming, d Swarming motility, and e Biofilm formation observed using confocal laser scanning microscopy (CLSM)
Las A Staphylolytic Activity
Staphylolytic enzyme production was significantly altered when treated with sub-MIC of P. tersa with an inhibition of 26.41 ± 6.21% (Fig. 2a).
Las A Protease Activity
Based on the azocasein assay it was observed that QS regulated protease production was inhibited by 29.56 ± 5.24% compared to control (Fig. 2a). Anti-proteolytic activity of Rhizophora spp. against the protease produced by PAO1, CI-I and CI-II was recorded with the inhibition of 39%, 93% and 92%, respectively [24].
Las B Chitinase Activity
The production of Las B chitinase enzyme was reduced by 79.73 ± 3.96% compared to the untreated control (Fig. 2a). The methanolic extract of Mangifera indica L. at 800 μg/mL concentration showed tremendous reduction in chitinase enzyme production by 55.3% [25].
Las B Elastase Activity
Reduction in elastase production was found to be 74.30 ± 4.55% in P. tersa crude extract treated P. aeruginosa at sub MIC (900 μg/mL) (Fig. 2a).
Exopolysaccharide (EPS) Inhibition
The EPS producing capacity of P. aeruginosa PAO1 was greatly affected when treated with P. tersa with an inhibition of 72.21 ± 3.96% compared to the untreated bacterial sample. Fungal crude extract treated agar plate showed pink color colonies with less EPS production compared to the untreated cells (Figs. 2a and 3b).
Microbial Adhesion to Hydrocarbons (MATH)
Cell surface hydrophobicity (CSH) index was measured quantitatively with MATH assay using toluene as substrate. After treatment with fungal crude extract, CSH index of treated P. aeruginosa PAO1 was found to be 74.65 ± 5.32% less compared to the control (Fig. 2b). Ability of bacterial cells to attach to hydrophobic solvent after treatment with Diaporthe phaseolorum SSP12 crude extract was inhibited in a study by 58.01%, 50.59% and 30.03% at 250, 500 and 750 μg/mL concentrations, respectively [20].
Alginate Inhibition
A significant reduction in alginate production (72.50 ± 5.29%) was observed upon treatment with fungal crude extract when compared with untreated sample (Fig. 2b). P. cucumerina, isolated from a Chinese medicinal plant, Orychophragmus violaceus reduced alginate production by 52% at a concentration of 1 mg/mL [26].
Swimming and Swarming Motility
The result showed decrease in diameter of bacterial halo zone that confirms the inhibition of swimming and swarming motility upon treatment with P. tersa crude extract at sub MIC concentration. The bacterial cell movement from the point of inoculation to petri dish edge was less than the control in both swimming (75.06 ± 2.95%) and swarming assays (78.52 ± 1.20%) (Fig. 3c, d).
Biofilm Inhibition
The cell growth rate after treatment with P. tersa crude extract was inhibited by 64.83 ± 2.13% at a sub-MIC concentration of 900 μg/mL compared to the control (Fig. 2b).
Confocal Laser Scanning Microscopy
Microscopic analysis results represent a remarkable decrease in bacterial growth rate and EPS production in biofilm matrix in treated cells. The CLSM results showed highly thick and dense biofilm in untreated sample compared to the treated sample and reduction in bacterial growth was observed in fungal crude extract treated sample (Fig. 3e).
GC-MS Analysis
GC-MS analysis provided the availability of bioactive compounds with diversified chemical structures. A cyclo dipeptide, 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione was present with retention time (20.815 min) and peak area % (38.86). It has been previously reported to have biological properties such as antibacterial, antifungal and anticancer activities. Presence of several bioactive compounds was observed with different retention times and peak area % such as Sulfurous acid, 2-propyl tridecyl ester, Ethanethioic acid, S-(2-methylbutyl) ester, 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester which could be contributed anti QS activity (Table 1).
Table 1.
List of identified fungal metabolites in ethyl acetate crude extract of endophytic fungus, Phomopsis tersa using GC-MS analysis
| S. No. | Fungal metabolites | Molecular formula | Molecular weight (g/mol) | Retention time | Peak area % |
|---|---|---|---|---|---|
| 1 | 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 278.348 | 17.029 | 39.962 |
| 2 | Ethanethioic acid, S-(2-methylbutyl) ester | C7H14OS | 146.250 | 17.164 | 4.992 |
| 3 | 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione | C11H18N2O2 | 210.2728 |
18.170 18.215 |
4.822 15.528 |
| 4 | Heneicosane | C21H44 | 296.583 | 20.931 | 3.736 |
| 5 | Sulfurous acid, 2-propyl tridecyl ester | C16H34O3S | 306.505 | 24.802 | 3.595 |
| 6 | Octacosane | C28H58 | 394.772 | 21.751, 22.551 |
9.242 7.864 |
| 7 | Heptacosane | C27H56 | 380.74 | 23.322 | 6.412 |
| 8 | Tetratetracontane | C44H90 | 619.204 | 24.067 | 3.846 |
Molecular Docking Studies
LasR and RhlR, transcriptional regulators have a vital role in the expression of QSS in P. aeruginosa. Docking studies were conducted to use these proteins as potential drug targets to determine the specific interaction and affinity of fungal metabolites. Natural QS signaling molecules in P. aeruginosa, C12HSL and C4HSL were docked against LasR and RhlR receptors, respectively. Molecular docking studies displayed that C12HSL signal molecule exhibited high binding affinity for the receptor protein, LasR with docking score -6.658 kJ/mol. Hydrophobic interaction (Van der Waal force) also contributes to the increased efficacy of signal molecule and cognate receptors binding affinity. Hydrophobic interactions were found to be with amino acids, LEU36, TYR47, ILE52, TYR64, VAL76, PHE101, LEU110, PRO74 (Fig. 4a) and confirmed by formation of hydrogen bond with TYR56, SER129 amino acids. The signal molecule, C4HSL (Fig. 5a) showed interaction with RhlR receptor protein with the docking score − 5.081 kJ/mol. Fungal bioactive compound, 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione showed docking score of − 6.873 kcal/mol (Fig. 4b) and -6.257 kcal/mol (Fig. 5b) with LasR and RhlR receptor, respectively. This ligand has two hydrogen bonds with residue TRP60 and SER129 of LasR and additionally possesses hydrophobic interaction with the active site residues of LasR (LEU36, TYR64, TRP60, TYR56, PRO74, ALA105, TYR93, PHE102, PHE101, TRP88, ALA127, VAL76, LEU110). Fungal compound owned hydrogen bond at TRP68 and TYR72 with non-polar interaction includes ALA83, ALA44, TYR64,TYR72, SER135 and ILE84 that shows high binding efficacy of fungal metabolite towards RhlR receptor protein. Fungal metabolites, 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (Figs. 4c and 5c), Sulfurous acid, 2-propyl tridecyl ester (Figs. 4d and Fig. 5d) and Ethanethioic acid, S-(2-methylbutyl) ester shares docking score of (− 7.372, − 5.985, − 4.803 kJ/mol) and (− 4.329, − 5.779, − 4.726 kJ/mol), for LasR and RhlR, respectively. Docking studies confirmed the possibility of binding of fungal metabolites at the active binding socket to inhibit the QS cascade in P. aeruginosa PAO1 (Table 2). Study conducted to understand the molecular interaction between the bioactive compounds secreted from A. alternata and LasR receptor of P. aeruginosa PAO1 revealed that sulfurous acid-2-propyl tridecyl ester had the highest docking score of − 7.525 kJ/mol with residual interaction involving TYR56 and TRP60. Bioactive compounds, 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester also showed a good docking score of − 6.384 kJ/mol [14]. Our study shows a promising approach to combat P. aeruginosa pathogenicity by interfering with the QSS and biofilm formation by the treatment of P. tersa crude extract.
Fig. 4.

Molecular docking of ligands with LasR receptor protein from Pseudomonas aeruginosa PAO1 showing the key hydrophobic and polar interactions of a 3-oxo-C12—HSL b 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione c 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester d Sulfurous acid, 2-propyl tridecyl ester
Fig. 5.
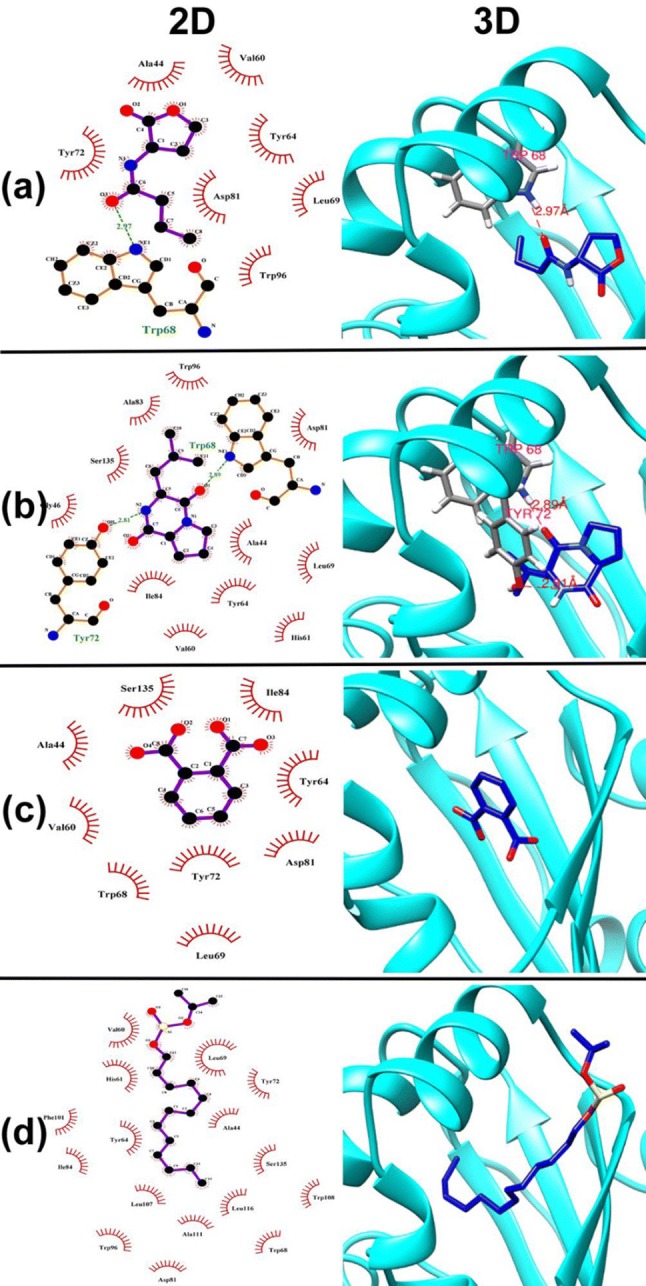
Molecular docking of ligands with RhlR receptor protein from P. aeruginosa PAO1 showing the key hydrophobic and polar interactions of a C4—HSL b 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione c 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester d Sulfurous acid, 2-propyl tridecyl ester
Table 2.
Molecular docking analysis data represent the electrostatic interaction between signal molecules, N-(3-Oxooctanoyl)-L homoserine and N-butyryl-homoserine lactone with transcriptional regulators, LasR and RhlR of P. aeruginosa PAO1. Fungal metabolites displayed propitious interaction involving hydrogen bond formation and hydrophobic interaction with LasR and RhlR receptors
| S.No | Compound interacting with Receptor protein | Glide Docking Score | Glide Emodel | Hydrogen Bonds with Residue names | Hydrophobic interaction residues name |
|---|---|---|---|---|---|
| LasR receptor | |||||
| 1 | N-(3-Oxooctanoyl)-L homoserine | − 6.658 | − 52.888 | TYR56, SER129 | LEU36, TYR47, ILE52, TYR64, VAL76, PHE101, LEU110, PRO74 |
| 2 | 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione | − 6.873 | − 34.797 | TRP60, SER129 | LEU36, TYR64, TRP60, TYR56, PRO74, ALA105, TYR93, PHE102, PHE101, TRP88, ALA127, VAL76, LEU110 |
| 3 | 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester | − 5.985 | − 43.424 | TYR56, ARG61, TRP60, SER129, TYR56, | TYR64, ASP73, PHE101, LEU36, ILE52, ALA127, VAL76, LEU110, TRP88, TYR56 |
| 4 | Sulfurous acid, 2-propyl tridecyl ester | − 7.372 | − 67.983 | TYR56 | LEU40, ASP73, PHE101, ALA105, PHE102, TRP88, TYR93, TYR56, LEU36, ILE52, TYR64, TYR93, TYR56, LEU36, ILE52, TYR64, TYR47, LEU39 |
| 5 | Ethanethioic acid, S-(2-methylbutyl) ester | − 4.803 | − 31.031 | TYR56, SER129 | LEU36, ASP73, THR75, VAL76, TYR64, TRP60, TRP88, LEU110, TRP88, ALA105, PRO74 |
| RhlR receptor | |||||
| 1 | N-Butyryl homoserine lactone | − 5.081 | − 36.629 | TRP68,ASP81 | ALA44,TYR72,LEU107,LEU116,TYR64 |
| 2 | 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione | − 6.257 | − 15.992 | TRP68, TYR72 | ALA83, ALA44, TYR64,TYR72, SER135, ILE84 |
| 3 | 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester | − 4.329 | − 7.548 | – | ALA44, VAL60, TYR64, TYR72, ILE84 |
| 4 | Sulfurous acid, 2-propyl tridecyl ester | − 5.779 | − 27.202 | – | ALA44, TRP68, LEU107, HIS61, TYR64, LEU69, VAL60, TYR45 |
| 5 | Ethanethioic acid, S-(2-methylbutyl) ester | − 4.726 | − 28.495 | TRP68 | LEU116, TYR64, LEU69, VAL60, ALA44, TYR64, ASP81, ALA83, TRP96, ALA111 |
In summary, development of antibiotic resistance under the process of selective pressure is one of the major challenges of the present era for the treatment of infectious diseases. The present study explains endophytic fungus P. tersa and its efficacy for producing secondary metabolites as QSI molecules against P. aeruginosa. The crude extract suppressed QS mediated virulence factors. Bioactive compounds reported in the study can further be utilized as new drug candidates in the fight against chronic infections.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Fungal Biotechnology Laboratory, Department of Biotechnology, Pondicherry University for isolating the fungi from Carica papaya. Dr. Busi Siddhardha is thankful to the start-up research grant from the Department of Science and Technology – The Science & Engineering Research Board [SB/YS/LS-32/2014] for funding the project work. This work was supported by Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2019H1D3A2A01060226) to work at Konkuk University (VCK). This research was supported by Basic Science Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2013M3A6A8073184) (JKL).
Compliance with Ethical Standards
Conflict of interest
The authors hereby declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vipin Chandra Kalia, Email: vckaliaku@gmail.com.
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
Busi Siddhardha, Email: siddhardha.busi@gmail.com.
References
- 1.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2014;68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Kalia VC. In search of versatile organisms for quorum-sensing inhibitors: acyl homoserine lactones (AHL)-acylase and AHL-lactonase. FEMS Microbiol Lett. 2014;359:143. doi: 10.1111/1574-6968.12585. [DOI] [PubMed] [Google Scholar]
- 4.Kalia VC. Microbes, antimicrobials and resistance: the battle goes on. Indian J Microbiol. 2014;54:1–2. doi: 10.1007/s12088-013-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van’t Wout EFA, van Schadewijk A, van Boxtel R, Dalton LE, Clarke HJ, Tommassen J, Marciniak SJ, Hiemstra PS. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog. 2015;11:e1004946. doi: 10.1371/journal.ppat.1004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-Homoserine lactone-Acylase and -Lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC. Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 8.Kim AL, Park SY, Lee CH, Lee CH, Lee JK. Quorum quenching bacteria isolated from the sludge of a wastewater treatment plant and their application for controlling biofilm formation. J Microbiol Biotechnol. 2014;24:1574–1582. doi: 10.4014/jmb.1407.07009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Guo Z, Gao H, Peng X, Li Y, Sun S, Lee JK, Lin W. Interaction of Pseudostellaria heterophylla with quorum sensing and quorum quenching bacteria mediated by root exudates in a consecutive monoculture system. J Microbiol Biotechnol. 2016;26:2159–2170. doi: 10.4014/jmb.1607.07073. [DOI] [PubMed] [Google Scholar]
- 10.Guo Z, Zhao X, He Y, Yang T, Gao H, Li G, Chen F, Sun M, Lee J-K, Zhang L. Efficient (3R)-acetoin production from meso-2,3-butanediol using a new whole-cell biocatalyst with co-expression of meso-2,3-butanediol dehydrogenase, NADH oxidase, and Vitreoscilla hemoglobin. J Microbiol Biotechnol. 2017;27:92–100. doi: 10.4014/jmb.1608.08063. [DOI] [PubMed] [Google Scholar]
- 11.Otari SV, Pawar SH, Patel SKS, Singh RK, Kim S-Y, Lee JH, Zhang L, Lee JK. Canna edulis leaf extract mediated preparation of stabilized silver nanoparticles: characterization, antimicrobial activity, and toxicity studies. J Microbiol Biotechnol. 2017;27:731–738. doi: 10.4014/jmb.1610.10019. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong S-H, Kim T, Haw JR, Kim S-Y, Kim IW, Lee JK. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 13.Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 2016;7:906. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashmi M, Meena H, Meena C, Kushveer JS, Siddhardha B, Murali A, Sarma VV. Anti-quorum sensing and antibiofilm potential of Alternaria alternata, a foliar endophyte of Carica papaya, evidenced by QS assays and in silico analysis. Fungal Biol. 2018;122:998–1012. doi: 10.1016/j.funbio.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Mahyudin NA, Daud NIHM, Rashid NKMA, Muhialdin BJ, Saari N, Noordin WN. Bacterial attachment and biofilm formation on stainless steel surface and their in vitro inhibition by marine fungal extracts. J Food Saf. 2018;38:e12456. doi: 10.1111/jfs.12456. [DOI] [Google Scholar]
- 16.Noumi E, Snoussi M, Merghni A, Nazzaro F, Quindos G, Akdamar G, Mastouri M, Al-Sieni A, Ceylan O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb Pathog. 2017;109:169–176. doi: 10.1016/j.micpath.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Orazi G, O’Toole GA. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio. 2017;8:e00873-17. doi: 10.1128/mBio.00873-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reetha AK, Pavani SL, Mohan S. Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina phaseolina (Tassi.) Goid. Int J Curr Microbiol App Sci. 2014;3:172–178. [Google Scholar]
- 19.Hnamte S, Parasuraman P, Ranganathan S, Ampasala DR, Reddy D, Kumavath RN, Suchiang K, Mohanty SK, Busi S. Mosloflavone attenuates the quorum sensing controlled virulence phenotypes and biofilm formation in Pseudomonas aeruginosa PAO1: in vitro, in vivo and in silico approach. Microb Pathog. 2019;131:128–134. doi: 10.1016/j.micpath.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Pattnaik SS, Ranganathan S, Ampasala DR, Syed A, Ameen F, Busi S. Attenuation of quorum sensing regulated virulence and biofilm development in Pseudomonas aeruginosa PAO1 by Diaporthe phaseolorum SSP12. Microb Pathog. 2018;118:177–189. doi: 10.1016/j.micpath.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Parasuraman P, Devadatha B, Sarma VV, Ranganathan S, Ampasala DR, Siddhardha B. Anti-quorum sensing and antibiofilm activities of Blastobotrys parvus PPR3 against Pseudomonas aeruginosa PAO1. Microb Pathog. 2019;138:103811. doi: 10.1016/j.micpath.2019.103811. [DOI] [PubMed] [Google Scholar]
- 22.Ooka K, Fukumoto A, Yamanaka T, Shimada K, Ishihara R, Anzai Y, Kato F. Piericidins, novel quorum-sensing inhibitors against Chromobacterium violaceum CV026, from Streptomyces sp. TOHO-Y209 and TOHO-O348. J Med Chem. 2013;3:93–99. doi: 10.4236/ojmc.2013.34012. [DOI] [Google Scholar]
- 23.Banerjee M, Moulick S, Bhattacharya KK, Parai D, Chattopadhyay S, Mukherjee SK. Attenuation of Pseudomonas aeruginosa quorum sensing, virulence and biofilm formation by extracts of Andrographis paniculata. Microb Pathog. 2017;113:85–93. doi: 10.1016/j.micpath.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Annapoorani A, Kalpana B, Musthafa KS, Pandian SK, Ravi AV. Antipathogenic potential of Rhizophora spp. against the quorum sensing mediated virulence factors production in drug resistant Pseudomonas aeruginosa. Phytomedicine. 2013;20:956–963. doi: 10.1016/j.phymed.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Husain FM, Ahmad I, Al-Thubiani AS, Abulreesh HH, AlHazza IM, Aqil F. Leaf extracts of Mangifera indica L. inhibit quorum sensing-regulated production of virulence factors and biofilm in test bacteria. Front Microbiol. 2017;8:727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Bi S, Chen H, Chen T, Yang R, Li M, Fu Y, Jia AQ. Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Front Microbiol. 2017;8:769. doi: 10.3389/fmicb.2017.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.