Abstract
Vitamin B12 (cobalamin) is a cobalt-containing compound that acts as an essential co-factor for various enzymes involved in the metabolic processes of the living cells. The constructed FRET Sensor for Vitamin Anemia Linked (SenVitAL) displayed marginal FRET efficiency. Here, we report the development of a molecular SenVitAL containing enhanced cyan fluorescent protein (ECFP) and venus as FRET pair to improve the FRET efficiency for optical imaging and screening of already developed sensor by our group. The sensor is the improved version of previously reported SenVitAL and consists of ECFP/venus as FRET pair instead of the originally used pair CFP/YFP. To increase the physiological range of vitamin B12 measurement, affinity mutants were created. Compared to the wild type, SenVitAL-5 with W44Q mutation has higher affinity and displayed large dynamic detection range (0.10–480 µM) in response to vitamin B12 binding. For cell-based monitoring and dynamic measurement of vitamin B12 flux rates, SenVitAL-5 was successfully expressed in cytosol of yeast and mammalian cells. Changes in the emission intensities of the two fluorophores were detected using confocal microscopy in both cell types in response to vitamin B12. With the addition of 50 µM extracellular vitamin B12 to the cells, the emission intensity of venus increased and that of ECFP decreased over the time. Furthermore, the results show that the variant SenVitAL-5 measures the vitamin B12 in a concentration-dependent manner, showing the resulting increase in the FRET ratio and thus confirming its utility as an ideal fluorescent indicator for the detection of vitamin B12 in eukaryotic systems in real time.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2073-1) contains supplementary material, which is available to authorized users.
Keywords: Affinity mutants, SenVitAL, Vitamin B12, Genetically encoded, FRET, Nanosensor
Introduction
Vitamins, a group of highly versatile organic compounds occur as trace amounts in daily human diet. Being essential for normal metabolism, absence of vitamins cause different disorders, which can be cured by resupply of these nutrients (Marshall 1986). Vitamins are relatively diverse in comparison to fats, carbohydrates and proteins. As our body is unable to synthesize the vitamins, so their intake through human diet is indispensable for life. Chemically, vitamins are complex compounds and have significant role in growth and development of the human body. In humans, deficiency of vitamins can cause serious or even fatal diseases (McDowell 2000).
Like other members of vitamins, vitamin B12 (cobalamin) also plays an important role in energy metabolism and other vital biological processes. However, unlike other vitamins, vitamin B12 has some unique features: it can be stored in human body for several years and has a much larger and complex molecular structure. Besides these distinctions, this vitamin is crucial in maintaining cardiovascular health and production of red blood cells in humans. Vitamin B12 deficiency causes a special type of anemia as it prevents the increase in level of homocysteine in human blood. Pertinently, higher level of blood homocysteine is usually associated with numerous diseases, such as peripheral vascular disease, stroke and coronary heart disease. Vitamin B12 is a crucial co-factor for DNA synthesis. Its deficiency is associated with atrophic gastritis, pernicious anemia, fatigue, neuropathy, tinnitus, migraine, asthma, depression, memory loss, shingles, muscular degeneration, Alzheimer’s disease, kidney disease and multiple sclerosis (Gueant and Alpers 2013).
Contemporary research in mass spectrometry and nuclear magnetic resonance-based measurement of metabolic flux has opened ways to survey and screen production strains (Bennett et al. 2009; Sreekumar et al. 2009; Frendt et al. 2010). So far various methods has been developed for the detection of Vitamin B12 namely biological assay that include microbiological and immunoassay, chemical assay include high-performance liquid chromatography (HPLC), and optical detection techniques like fluorescence spectroscopy, Raman spectroscopy and surface plasmon resonance (SPR) effects (Carey 1978; Liao et al. 2006; Lee and Griffiths 1985; Bigio and Mourant 1997; Karmi et al. 2011). Microbiological detection is a traditional method for measurement of vitamin B12, which is based on growth curve assays (Capps et al. 1949; Hoffmann and Stokstad 1949; Ford 1952). Stefova et al. (1997) first time used HPLC for identification of vitamin B12 and later on this technique has been used for detection of Vitamin B12 in food and dietary supplements (Baik and Russell 1999). Generally, vitamin B12 do not emit light under optical excitation. Therefore, chemiluminescence is applied for detection of vitamin. Vitamin B12 catalyst cobalt (II) ion was detected using chemiluminescence with dodecylbenzene sulonate (DBS)-layered double hydroxides (LDHs) as reported by Zhang et al. (2014). In aqueous solution vitamin B12 and its derivatives identifies by the Raman Spectroscopy (Tsai and Morris 1975; Nestor et al. 1976; Rajoria and Nath 1977). It is a unique detection technique for direct molecule identification via cobalt ion linked to an organic corrin ring, which is found in vitamin B12 (Andruniow et al. 2002). SPR is useful for indirect determination of vitamin B12 by monitoring interaction between vitamin B12 and its binding protein. It has been used for determination of vitamin B12 level in dietary supplement. These methods and techniques have limited temporal and spatial resolution due to disrupting sample preparation and the required sample amount, respectively. Hence the techniques developed so far invasive in nature and do not allow in vivo monitoring (Cannon et al. 2002).
On the other hand, FRET-based techniques allowing real time measurement of metabolite changes and variations in local metabolite concentration at the single cell level are valuable to study flux of metabolites. Genetically encoded FRET-based nanosensor offer the possibility to detect/quantify the change in such a small and specific metabolite into an optical production. These sensors can sense and respond to dynamic levels of metabolites within the host cell, thus allowing investigators to monitor and optimize natural and introduced metabolic pathways (Okumoto et al. 2005; Mohsin et al. 2013). FRET is a multipurpose and profound tool for qualitative and quantitative examination of biological interactions and developments. The exclusive capability of FRET to probe nanoscale inter- and intramolecular separation distances, has led to a fast developing field of fundamental FRET studies of biomolecules and biological complexes. Sensors based on a conformational change represent the vast majority of FRET-based sensors. These sensors operate the ability of proteins to change their conformation upon a biological process. Importance of such probes is their flexibility when it comes to sense specific factors, therefore making such type of sensors appropriate for an extensive variety of biological developments. FRET-based sensors are compatible tools for advance analysis of numerous signaling pathways. Since dynamic and subtle spatiotemporal changes are continuously taking place in cellular networks, to capture such minor and significant variations as accurately as imaginable FRET-based sensors are used (Vandame et al. 2014). We can also use such type of sensors to detect and determine natural processes like cell cycle progression and division, on the other hand also to examine a variability of immunological events. Oxygen as one of the basic building blocks of life, proteins not only require it in their molecular structure, but also depend on a certain concentration of cellular oxygen during maturation to function correctly (Shaner et al. 2015). Many fluorescent proteins, which has led to the development of hypoxia-sensitive sensors by linking an oxygen-insensitive fluorophore as the donor with an oxygen sensitive fluorophore as the acceptor. The lower the oxygen level is the less energy is absorbed by the acceptor, leading to a fading of FRET signal that is directly relevant to the state of hypoxia in a cell. FRET sensors are tools that make use of the FRET effect so as to make biological variables effortlessly and non-invasively quantifiable by converting them into a shift in FRET efficiency. The quantity of such variables, especially in living tissues will become more significant for an improved understanding of the multifaceted signaling cascades and setups that control sophisticated biological systems. The development of the concept to the measurement of FRET-sensors at the same time in a single sample will facilitate scientists to measure a variety of biological processes or parameters simultaneously allowing for multi-factorial analyses at high temporal and spatial resolution. We are persuaded by these tactics, they lead to a better understanding of signaling networks and their pathways providing an origin for other specific targeting of significant compulsive signaling centers in the future. (Hochreiter et al. 2015).
Previously reported genetically encoded fluorescent SenVitAL (Ahmad et al. 2018) has relatively low FRET efficiency and was not suitable for quantitative experiments due to their poor dynamic range. To visualize vitamin B12 dynamics in real time with higher optical efficiency, faster fluorescent indicators are required. Here, we report the development of SenVitAL variants with comparable brightness but altered affinity binding constant (Kd) for real time monitoring of vitamin B12 in living cells. The constructed nanosensor allowed in vitro and cell-based determination of FRET ratio changes in a concentration-dependent manner. A set of affinity sensors were generated by site-directed mutagenesis of the amino acid residues involved in vitamin B12 binding. Developing the mutants altered the dynamic detection range of the sensors and was useful to measure cobalamin level at different physiological scales. These sensors were found to be specific to vitamin B12 and binds the vitamin with high affinity. The variant SenVitAL-5 was further targeted, like previous genetically encoded SenVitAL to Saccharomyces cerevisiae (S. cerevisiae) to carry out cell-based monitoring and quantification of vitamin B12 in single living yeast cell. Furthermore, SenVitAL-5 was also expressed in human embryonic kidney (HEK)-293T cell line to visualize real time dynamics of vitamin B12 flux rates in mammalian cells. The ultimate goal of any technology, such as the one developed in present study, is its application in biologically relatable enquiries. This study also demonstrated the ability of these sensors to monitor target concentrations as a means to investigate the processes of vitamin B12 import and metabolism.
Materials and methods
Materials
Cells and media
Bacterial strains used in entire work were Escherichia coli (E. coli) DH5α, K12, and BL21. E. coli K12 was used for isolation of genomic DNA and as model strain for transformation and other purposes. BL-21(Rosetta) was used for protein expression. Both LB Broth and LB Agar were purchased from HiMedia laboratories, India. For yeast expression, S. cerevisiae/URA3 strain BY4742 was used as eukaryotic host model. The strain was maintained on synthetic defined (SD) agar and grown in liquid yeast extract peptone dextrose (YEPD) medium at 30 °C with aeration on a shaker (Metrex). SD Agar and YEPD were purchased from HIMedia. For transfection, HEK-293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM). DMEM and fetal bovine serum (FBS) were obtained from Sigma, USA.
Vectors, enzymes, and primers
Vectors used in entire work were pRSET-B, pENTR-4.0, pYES-DEST, pCDNA3.1 (-) and all were purchased from Invitrogen, USA. For PCR and other reactions, restriction enzymes like KpnI, BamHI, HindIII, and LR-clonase-II enzymes were purchased from ThermoFisher, USA. Primers were designed by oligoAnalyzer tool (software) and synthesized from integrated DNA technologies (IDT) with the sequences of restriction enzymes.
Kits used
To increase the physiological range and affinity of the sensor, site-directed mutagenesis was carried out by QuikChange II mutagenesis kit, Agilent Technologies, USA. For PCR and other gel extraction purposes, sure extract PCR clean-up/Gel Extraction Kit were purchased from Genetix, USA. For plasmid isolation, Plasmid Mini Kit was purchased from Qiagen, USA.
Molecular biology reagents used
Agarose, chloramphenicol, isopropyl-β-D-1-thiogalactopyranoside (IPTG), N,N,N',N'-tetramethylethylenediamine (TEMED), ammonium persulphate (APS), galactose, sucrose, poly L-Lysine, sodium dodecylsulphate (SDS) were procured from HiMedia Laboratories, India. For protein purification, nickel-nitrilotriacetic acid (Ni-NTA) His-tag affinity columns from Merck, USA and Ni-NTA resin from Qiagen, USA were purchased.
Methods
Cloning of vitamin B12 binding protein into bacterial expression vector
Following the concept developed for the genetically encoded SenVitAL (Ahmad et al. 2018), periplasmic binding protein (BtuF) of E. coli K12 was attached with ECFP and venus at N-terminus and C-terminus, respectively. We have replaced CFP/YFP pair with ECFP/venus in the SenVitAL construct. BtuF (PDB ID: 1N4A), a vitamin B12 binding protein belongs to the ATP-binding cassette (ABC) type transporter superfamily. BtuF crystal structure and sequence were retrieved from RCSB protein data bank (PDB) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, respectively. The gene encoding BtuF was amplified by PCR from genomic DNA of E. coli K-12 after removing first 23 amino acids (signal peptides) using SignalP4.1 server (CBS, Denmark). The forward primer used was 5ʹ-CGGggtaccCCGCGCGTCATCACGCTTTCTCC-3ʹ and the reverse primer was 5ʹ-CGGggtaccTACCTGTGAAAGCGCATTACAGA-3ʹ. The lowercase sequence shows the restriction site KpnI. STOP codon was removed from the 3ʹ end. Amplified BtuF fragment was inserted into the ECFP_venus cassette yielding the ECFP_BtuF_venus sensor construct in pRSET-B bacterial expression vector.
Molecular docking
The X-ray crystal structure and atomic coordinates of the wild type E coli BtuF were retrieved from RCSB PDB (www.rcsb.org; PDB ID: 14NA) to study the binding of vitamin B12 with BtuF. Vitamin B12 details were retrieved from the Pubchem database, and the coordinate file was generated using online SMILES Translator and STRUCTURE File Generator program. Autodock 4.2 version was used for the docking analysis. PDB files of BtuF and vitamin B12 were imported into Auto dock tools (ADT) and PDBQT files were generated. Many separate docking runs were performed using these PDBQT files in AutoDock Vina to test the feasibility of vitamin B12 binding with BtuF.
Computational studies of SenVitAL, previously reported, showed that vitamin B12 interacted with BtuF through hydrophobic interactions with Trp44, Pro144, Tyr28, Trp63, Ala225, Pro9, Ala10, Phe140, Trp174 and polar interaction with Thr43, Ser219, Ser8, Gln154. Three interacting residues (Trp44, Pro144, Ala225) were selected and mutated to Gln, Gly and Pro, respectively to achieve sensor variants with a better binding affinity and a wide range of detection of physiological vitamin B12. Mutagenesis wizard of PyMOL was used to generate PDB file formats of all the three variants of BtuF and molecular docking was carried out using vitamin B12 to determine the residues which interact. PyMOL and LigPlot v.2.2.25 visualization programs were used to image vitamin B12 bound to wild type BtuF (and sensor variants).
Affinity variants of SenVitAL by site-directed mutagenesis
A series of PCR-based DNA point mutations were performed on pRSET-B_ECFP_BtuF_venus to increase the physiological range and affinity of the sensor. Based on the molecular docking studies (Ahmad et al. 2018), site-directed mutagenesis was carried out by QuikChange II mutagenesis kit using the following primers (5′ to 3′):
W44Q, ATTGAGCAGGTTTCCACCCAGCAGGGGATGAATCTGGAA
P144G, CTGCAATTCGGCATTAAATGGGCCATTTACCAGTGGAAAA
A225P, AGTGACTGGTTTGAACGTCCTAGCCCACGTATTATCCTC
Three mutants were created by substituting tryptophan with glutamine (Trp44Gln), proline with glycine (Pro144Gly) and alanine with proline (Ala225Pro), respectively. Mutations were confirmed by DNA sequencing (Sanger).
Expression and purification of the construct
Wild type (WT) pRSET-B_ECFP_BtuF_venus and variant proteins were expressed in E. coli BL21 (Rosetta) cells. The cells were grown at 20 °C supplemented with 100 μg/ml ampicillin and 20 μg/ml chloramphenicol till the optical density (O.D.) reaches to 0.6. Protein expression was induced by adding 1 mM IPTG and grown for additional 22 h at 16 °C under dark conditions. The expressed cells were harvested by centrifugation at 4500 rpm for 20 min and the pellet was suspended in ice chilled 20 mM Tris–Cl (pH 7.8). The cells were lysed by ultra-sonication (Sonics, USA) for 5 min at 40% aptitude and the cell free extract was obtained by centrifugation at 4500 rpm for 30 min. Supernatant of the cell lysates were collected using 0.45 μm filter paper. Purification of WT and mutant sensor proteins was done by Ni-NTA His-tag affinity chromatography. The lysates were mixed with Ni-NTA resin and kept at 4 °C for 1 h for binding of the His-tagged sensor proteins. Washing of the column was done by 20 mM Tris–Cl and 10 mM imidazole (pH 8.0), and proteins were eluted with ice-chilled buffer mix of 20 mM Tris–Cl and 300 mM imidazole (pH 8.0). Purified sensor proteins were stored at 4 °C overnight for proper folding to its native conformation. Integrity of the sensor protein was assessed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
In vitro characterization and binding titration of the sensor proteins
Affinity assays of the sensor proteins were performed using a monochromatic microplate reader (Biotek, USA). 180 μl of the sensor protein was mixed with the 20 μl of increasing concentration of vitamin B12 on a 96 well plate. Excitation filter was set as 420/20 nm while the emission filter used was 485/20 nm for ECFP and 540/20 nm for venus. All readings were taken in triplicates to obtain a ligand saturation curve. Affinity constant (Kd) values were obtained by fitting the data to the binding isotherm equation: S = (r − rapo)/(rsat − rapo) = [L]/(Kd + [L]), where S is saturation; [L] is ligand concentration; r is the ratio; rapo is the ratio in the absence of a ligand and rsat is the ratio at saturation with a ligand using GraphPad Prism 7 software.
Measurement of vitamin B12 uptake in yeast cells
The variant SenVitAL-5 was selected for carrying out cell-based analysis of the vitamin B12 dynamics in the eukaryotes. SenVitAL-5 sequence was shuttled into pENTR 4.0 dual selection vector to generate an entry clone. The sequence was then transferred to pYES-DEST52 destination vector through Gateway technology using LR-clonase-II enzyme according to the manufacturer protocol for expression in the yeast cells. The yeast strain was grown in YEPD medium at 30 °C with aeration on a shaker. The cells were further induced by 3% galactose and 2% sucrose which was used as a carbon source for expression of the nanosensor in yeast cells. For real time imaging, cells were fixed on a poly L-lysine coated cover slide with a total volume of 10 μl using a medical adhesive. Vitamin B12 (50 µM) was added to the cells positioned on the glass slide. The cells were incubated for one minute before fluorescence measurements were taken. Images were acquired using confocal microscope DMRE equipped with a confocal head (TCS SPE, Leica, Germany) and a 63× oil immersion objective. For measurement of dynamic flux rates of vitamin B12 in a single yeast cell, venus/ECFP emission intensity ratio was recorded using 420 nm excitation filter and two emission filters (485 nm for ECFP and 540 nm for venus) and LAS-AF software (Leica, Germany) for 600 s. Images were acquired by selecting the region of interest (ROI) to avoid any interference in the FRET ratio. FRET sensitized emission tool of LAS-AF software without background subtractions was used to display the desired data.
Monitoring and quantification of vitamin B12 flux dynamics in mammalian cells
The construct SenVitAL-5 was sub-cloned by restriction-ligation into pcDNA3.1(-) vector for mammalian cell expression at BamHI and HindIII restriction sites. HEK-293T cells were cultured in DMEM containing 10% FBS and 50 μg/ml ampicillin at 37 °C with 5% CO2 in a humidifier chamber. HEK-293T cells were seeded in 6-well culture plates onto cover slide. These cells were transiently transfected with SenVitAL-5 by calcium phosphate method and cultured for two days for expression of the sensor protein. Before the fluorescence measurements, HEK-293T cells were washed with a buffer-mix containing 50 mM NaCl, 5 mM KCl, 1 mM MgCl2, 25 mM HEPES and 5 mM D-glucose (pH 7.2–7.4). Ratiometric imaging of the nanosensor expressed in the HEK-293T cell line was performed after two days of transfection on a confocal microscope (TCS-SPE, Leica, Germany) with 1.53N.A., 63× oil immersion objective and cooled charge coupled camera. The vitamin B12 (50 µM) was added to the cells onto a cover slide just after the first laser scans. Dual emission intensity ratios were recorded using the same laser settings as described in case of imaging of yeast. Images were acquired at an interval of 50 s and an exposure time of 300 ms.
Result and discussion
Designing and generation of nanosensor
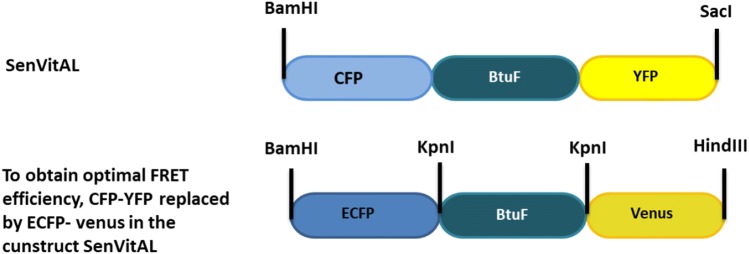
In general, genetically encoded FRET-based sensors make use of periplasmic binding proteins (PBPs) of gram-negative bacteria as sensing domains in their design. BtuF, a PBP binds vitamin B12 and delivers it to the periplasmic surface of the ABC transporter BtuCD. BtuF is composed of two alpha/beta domains linked by a rigid alpha-helix and exhibit considerable conformational changes during ligand binding process (Karpowich et al. 2003). Based on the molecular dynamics, it is suggested that when vitamin B12 binds in the wide acidic cleft located between these domains, BtuF-vitamin B12 complex shows opening-closing and twisting motion tendencies, thereby entrapping the ligand, hence the name “Venus flytrap” (Felder et al. 1999; Liu et al. 2008). Probably, Trp44-Gln45 pair, which is situated at the mouth of the binding pocket of BtuF act as a gate in the binding and unbinding process of vitamin B12 (Liu et al. 2008). Based on this open and close conformational dynamics of BtuF upon vitamin B12 binding, it was considered suitable for the development of the SenVitAL. For the construction of nanosensor, donor fluorophore ECFP and acceptor fluorophore venus is attached to the N- and C- terminus of the BtuF domain, respectively. FRET pair CFP/YFP in the design of SenVitAL was replaced by the enhanced version of fluorophores ECFP/venus. Larger portions of the ECFP emission and venus excitation spectrum overlap, making them an ideal pair for measuring FRET to detect varying ranges of separation and meeting one of the key FRET requirements (Fig. S1). Arrangement of the three genes in designing the sensor construct (ECFP_BtuF_venus) is depicted in a linear diagram in Fig. S2a while Fig. S2b represents the circular map of SenVitAL construct in pRSET-B expression vector. Fidelity of the BtuF gene was verified by sequencing (Fig. S3). ECFP and venus is a commonly used FRET pair that comes in close vicinity upon ligand binding so that the energy from an excited donor fluorophore is non radiatively transferred to an acceptor fluorophore (Kremers et al. 2006). Figure 1 representing the schematic design of the genetically encoded vitamin B12 fluorescent indicator. The constructed new version of SenVitAL was further characterized in vitro and in cells to establish its efficacy as a nanosensor that could successfully monitor vitamin B12 levels in living cells.
Fig. 1.
Designing of newly genetically encoded FRET-based vitamin B12 nanosensor construct. Here FRET pair CFP and YFP of older SenVitAL was replaced by ECFP and venus
Docking studies, mutagenesis and in vitro characterization
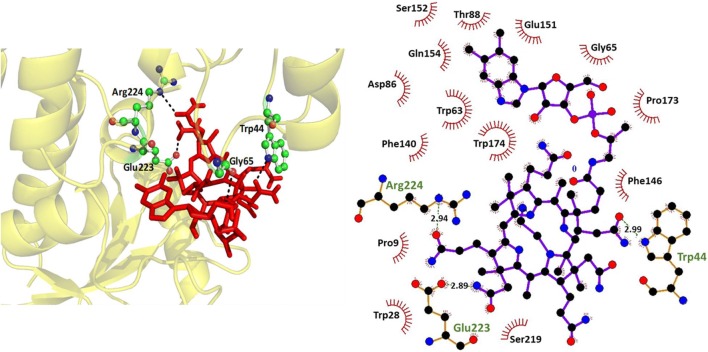
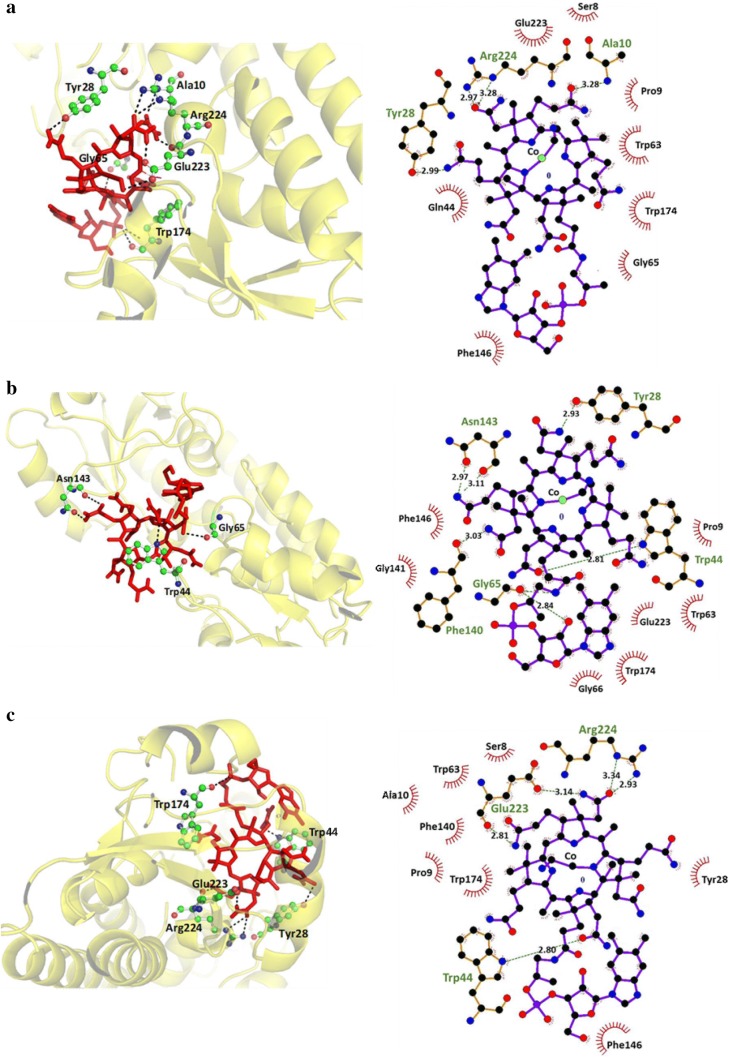
Autodock software was used to dock vitamin B12 against BtuF (WT and sensor variants) for finding the docked model of protein and ligand. The molecular docking studies revealed that vitamin B12 interacts with BtuF (WT and sensor variants) through non-covalent amino acid residues. Minimum binding energies are estimated from the docking runs and listed in Table S1. The docked pose and 2D plot of vitamin B12 complexed with BtuF (WT and sensor variants) are shown in Figs. 2, 3. The docking results also showed the binding affinity of vitamin B12 to WT BtuF and its sensor variants.
Fig. 2.
In silico binding studies of WT BtuF. Left, Docking pose of WT BtuF with vitamin B12. The ligand (vitamin B12) is shown in red as stick and the interacting residues are shown in ball and stick form. Right, 2D plot of vitamin B12 complexed with WT BtuF which is shown in ball and stick form
Fig. 3.
In silico binding studies of affinity mutants of BtuF. a Left, Docking pose of W44Q mutant with vitamin B12. Right, 2D plot of vitamin B12 complexed with W44Q mutant which is shown in ball and stick form. b Left, Docking pose of P144G mutant with vitamin B12. Right, 2D plot of vitamin B12 complexed with P144G. c Left, Docking pose of A225P mutant with vitamin B12. Right, 2D plot of vitamin B12 complexed with A225P mutant
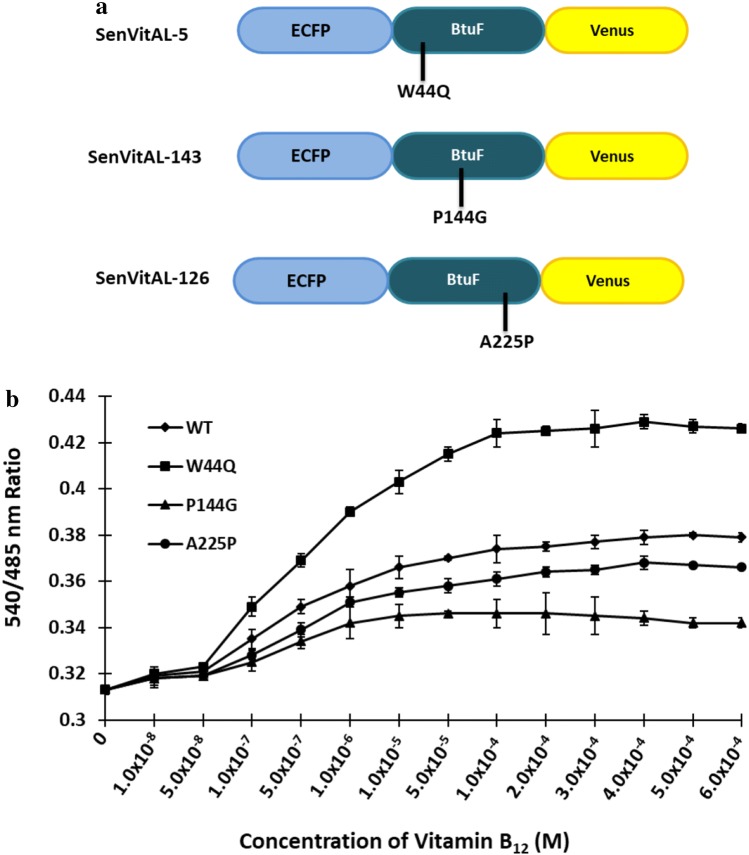
Three variants of SenVitAL were created by mutating amino acid residues coordinating vitamin B12 or in the vicinity of the binding site. Mutations were confirmed by sequence analysis (Fig. S4). The WT and mutants were transformed in E. coli BL21 (Rosetta) through calcium chloride method. Cells were grown at 20 °C in dark condition to avoid damage to the fluorophores since they are light sensitive (Ameen et al. 2016). Sensor proteins were purified by affinity chromatography using Ni-NTA agarose resin and size was confirmed with SDS-PAGE. The chimeric proteins were characterized by FRET method as change in the emission intensity ratio of acceptor to the donor fluorophores and ligand binding saturation response curve was obtained by titrating the sensor proteins with varying concentrations of vitamin B12 on a monochromator microplate reader. It was observed that with the addition of vitamin B12, there was enough change in venus/ECFP emission ratio which is in accordance with the strategy adapted to design the developed SenVitAL. By introducing PCR-based point mutations, variants W44Q (SenVitAL-5), P144G (SenVitAL-143) and A225P (SenVitAL-126) were created with affinity constants 5.04 μM, 143.40 μM and 126.27 μM, respectively (Fig. 4a–b). Contrast to the WT sensor which has an affinity constant 93.36 µM, one mutant W44Q lowered, and two (P144G and A225P) increased the Kd for vitamin B12. SenVitAL developed in this study has a Kd value of 93.36 μM which is significantly lower than the originally designed SenVitAL (Kd value: 157 μM) constructed using CFP and YFP as a FRET pair, thus showing that this improved version has a better binding efficiency for vitamin B12 and can therefore act as a better sensor than the previous version. Developing the mutants also enhanced the detection range of vitamin B12 measurement at different physiological scales (Table 1). Based on the values of affinity constants, we selected SenVitAL-5 to carry out cell-based studies of vitamin B12 in living yeast and mammalian cells.
Fig. 4.
Ligand binding saturation curve of WT and affinity mutants of SenVitAL. a Design of SenVitAL variants. b Emission intensity ratio (540/485 nm) alteration was recorded in presence of varying concentrations of vitamin B12. Affinity mutants W44Q, P144G and A225P compared with the WT nanosensor. Data are means of three independent values (n = 3). Vertical bars indicate standard error
Table 1.
Binding constants and dynamic range of SenVitAL-WT and mutants
| Sensor namea | Sequences | Kd (M) (± SD) | Dynamic rangeb (µM) |
|---|---|---|---|
| SenVitAL-93 | WT | 93.36 × 10–6 (± 0.02) | 0.53–360 |
| SenVitAL-5 | W44Q | 5.04 × 10–6 (± 0.03) | 0.10–480 |
| SenVitAL-143 | P144G | 143.40 × 10–6 (± 0.02) | 5.00–300 |
| SenVitAL-126 | A225P | 126.27 × 10–6 (± 0.01) | 0.78–400 |
Binding constants was determined in vitro
SD standard deviation
aNumber next to the mutant name stands for the Kd of the sensor variants
bEffective quantification range between 10 and 90% saturation of the nanosensor
There are several methods established in literature for the construction of biosensors that vary primarily in the type of affinity probe used, the type of metal nanoparticale, the sensing mechanism, specificity, selectivity, reproducibility, assay time and the cost involved. A thermally-reduced carbon dot (t-CD) optical FRET detector reported showed a fivefold increase in quantum yield and measured levels of vitamin B12 in aqueous solution up to a concentration range of 1 to 12 mg/ml (Wang et al. 2015). A reported electrochemical sensor used electropolymerization of pyrrole (Py) for vitamin detection in the presence of ferromagnetic nanoparticle-incorporated triazine dendrimer (FMNPs@TD) on a gold electrode (Au). The detector had a linear detection range of vitamin B12 (2.50 nM–0.5 μM) (Parvin et al. 2018). A colorimetric detector based on RNA aptamer that uses gold nanoparticles (AuNPs) to detect vitamin B12 was proposed. The 35-mer aptamer that binds to vitamin B12 was structurally modified by altering the 2′-hydroxyl group of ribose to 2′-flouro in all aptamer pyrimidines. The vitamin B12 binding RNA aptamer gets desorbed from the surface of AuNPs as a result of the aptamer-target interaction, resulting in the aggregation of AuNPs and color change from red to purple. The sensor detection limit for vitamin B12 was reported to be 0.1 μg/ml (Selvakumar and Thakur 2012).
Nonetheless, before considering these vitamin B12 detection methods, there are several issues to be addressed. Carbon quantum dots (CQDs) are cytotoxic nanocolloids and their wide excitation spectra further limit their use in quantification studies as FRET acceptors. It is also difficult to introduce QD-fused protein sensors in living cells as they are complex due to their large size and ability to switch randomly between emitting and non-emitting states (Resch-Genger et al. 2008; Ma et al. 2014; Rowland et al. 2015). On the other hand, metal nanoparticles (MNPs)-based sensors are highly questionable and unreliable in their sensitivity, selectivity and efficiency. Biosensors based on MNPs are complex, costly, show unsatisfactory detection limits and result in the loss of non-negligible amounts of detectable molecular species when diffusing to the electrode (Holzinger et al. 2014; Malekzad et al. 2017). The SenVitAL developed overcomes all of these limitations and has the potential to non-invasively determine the level of vitamin B12 in real time.
Cell-based studies of vitamin B12 uptake in yeast cells
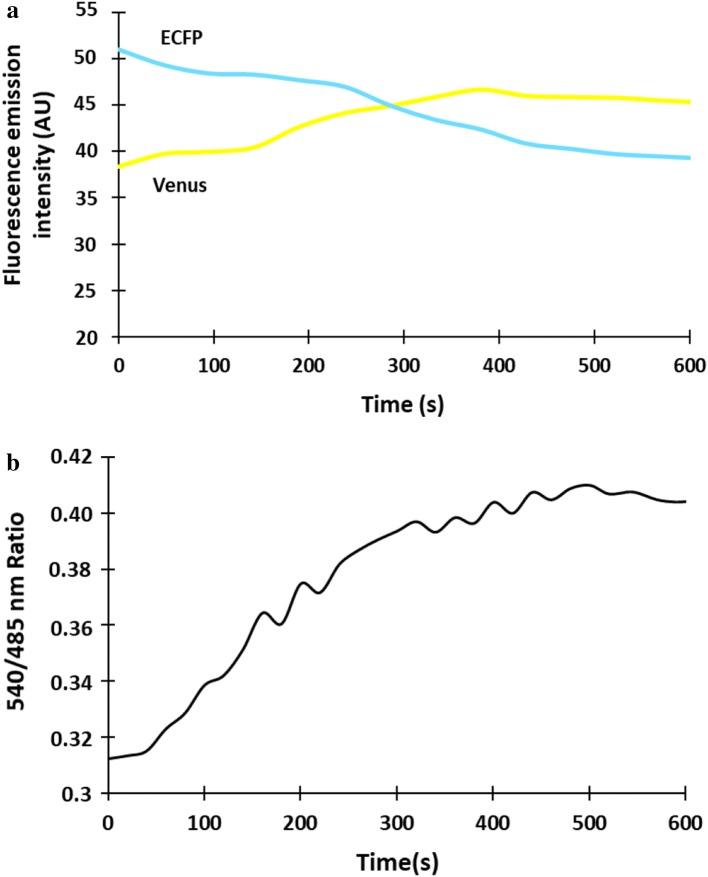
Function of SenVitAL-5 was monitored in living yeast cells to evaluate the flux of vitamin B12 in real time. The sequence of SenVitAL-5 was sub-cloned in pYES-DEST52 vector and transformed in S. cerevisiae strain BY4742 (taken as eukaryotic host organism). The cells were further grown in YEPD media along with 3% glucose to obtain good expression of the nanosensor and optimal FRET ratio. Yeast cells imaging was performed using the confocal microscope with 63 × immersion oil objective. Real time imaging showed successful expression of the nanosensor in the cytosol of the yeast cell as it was highly stained whereas major portion of the cell remains unstained with no fluorescent signal representing the vacuolar region (Fig. 5). Constructed nanosensor shows FRET ratio (Em540/Em485 nm) change with the added 50 µM of vitamin B12. Addition of vitamin B12 to the SenVitAL-5 transformed yeast cell showed clear change in the fluorescence emission intensity of ECFP and venus (Fig. 6a). Emission intensity (venus/ECFP) change was recorded upto 10 min with addition of vitamin B12 that shows ratiometric increase from 0.317 to 0.410 till 450 s then gets saturated (Fig. 6b). The results clearly indicate that after vitamin B12 uptake, it is transported into the cytosol, where it is recognized by SenVitAL-5. It was also observed that FRET ratio started to saturate after a sharp increase at 450 s showing that vitamin B12 is consumed completely and the binding sites of the protein BtuF gets totally occupied by vitamin B12. Live cell imaging of the yeast cells shows that the developed nanosensor is working effectively and specifically respond towards the changing concentration of vitamin B12. Similar approach has been used in case of leucine (FLIP-Leu) (Mohsin et al. 2013), methionine (FLIPM) (Mohsin and Ahmad 2014) and maltose (Fehr et al. 2002) for detection and quantification of these metabolites non-invasively in real time in a single cell.
Fig. 5.
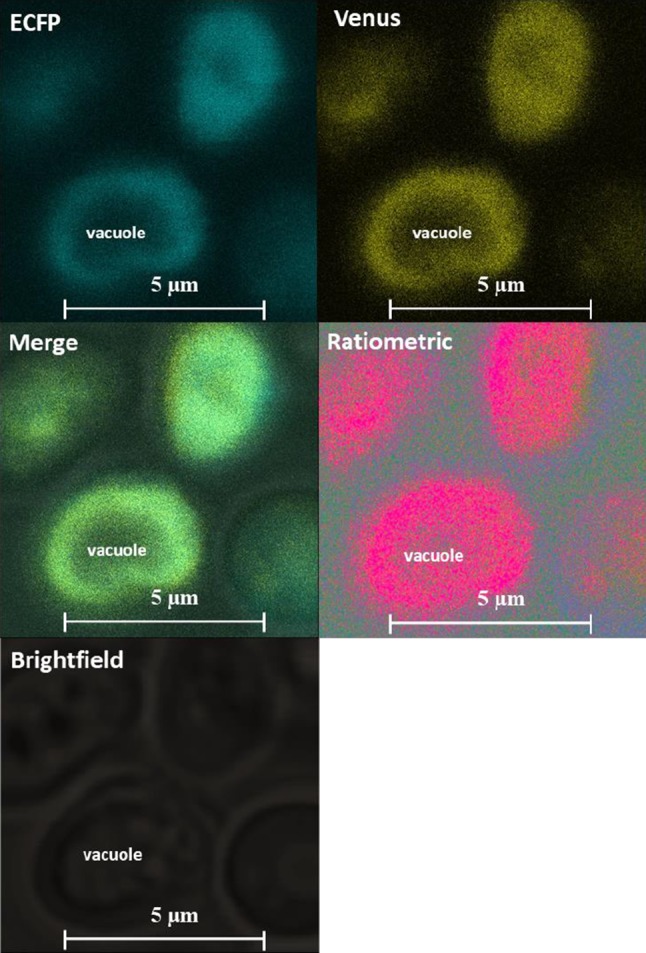
Expression of SenVitAL-5 in a single yeast cell. The confocal fluorescence images show successful expression of SenVitAL-5 (scalebar-5 μm)
Fig. 6.
Monitoring the flux rate changes of vitamin B12 in yeast. a Fluorescence emission intensity of ECFP decreases with a concomitant increase in the venus emission intensity after addition of vitamin B12 indicating energy transfer from the donor to the acceptor fluorophore. b Time-dependent FRET ratio change in the cytosol of single yeast cell for 10 min in presence of 50 µM vitamin B12
Monitoring of vitamin B12 dynamics in mammalian HEK-293T cells
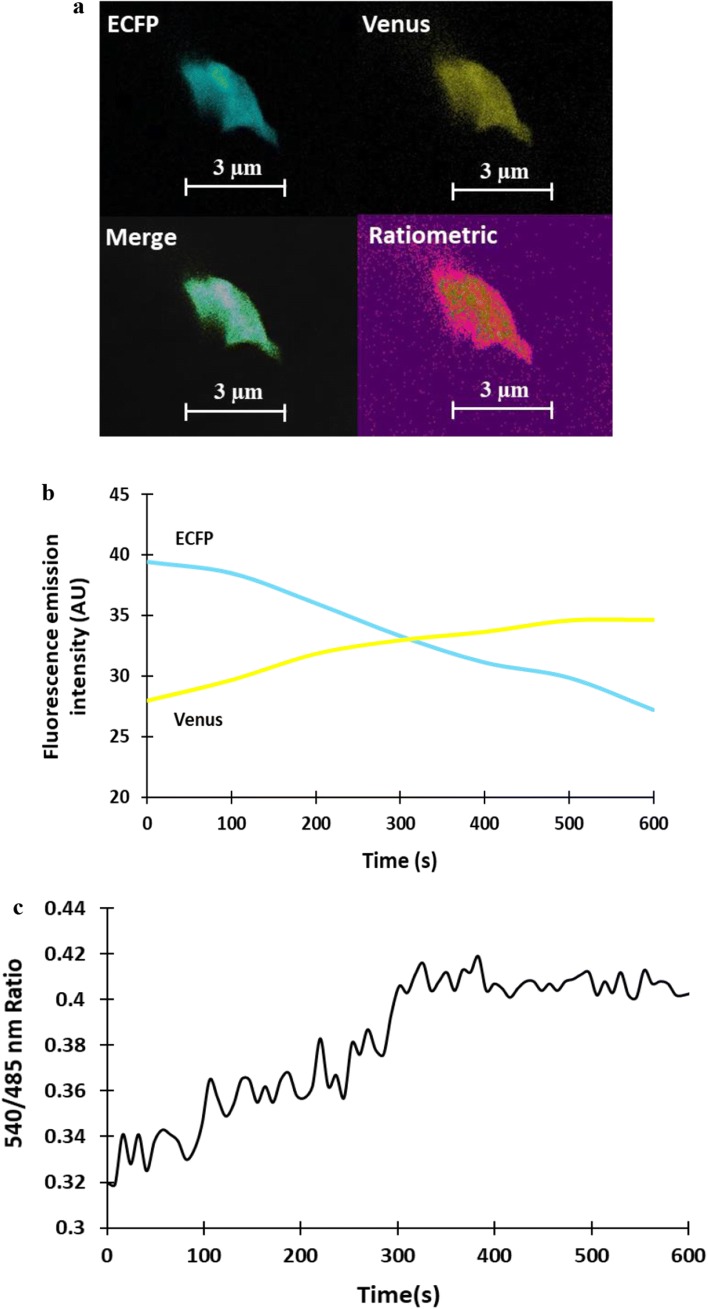
SenVitAL-5 was transfected in mammalian model (HEK-293T cells) to monitor the level of vitamin B12 non-invasively in cell-based studies. Confocal imaging of HEK-293T cell showed that SenVitAL-5 is getting expressed in cytoplasm (Fig. 7a) and with the addition of 50 µM vitamin B12, emission intensity of ECFP decreases and venus increases (Fig. 7b). Change in fluorescence intensity of venus/ ECFP emission ratio was recorded for 10 min that is showing constant increase in the FRET ratio from 0.320 to 0.417 upto 400 s after that saturation is obtained suggesting fully occupied binding site of the protein BtuF with vitamin B12 (Fig. 7c). The outcome is clearly showing that small amount of vitamin B12 is detected in short spam by SenVitAL-5 using this advanced monitoring tool. Using such FRET-based nanosensor it is possible to detect the level of vitamin B12 in different localized spaces and sub-cellular regions like previous genetically encoded sensors as in case of ATP, Imamura et al. 2009 developed a probe (ATeam) to monitor the level of ATP using different polypeptides in mitochondrial space and nucleolar region. Genetically encoded SenVitAL-5 has the potential to monitor vitamin B12 level in real time and non-invasively as Hessels et al. (2015) monitored the level of Zn2+ in both cytosol and ER using eCALWY-4.
Fig. 7.
Real time measurement of SenVitAL-5 in mammalian cells. a Confocal image of HEK-293T cell expressing the nanosensor showing the channels: ECFP, venus, merge and ratiometric (Scalebar–3 μm). b Change in the emission intensities of the fluorophores ECFP and venus with addition of vitamin B12. c Time-dependent FRET ratio change in the presence of externally supplied 50 µM vitamin B12. The graph indicating the venus/ECFP ratio change for 10 min
Conclusions
In the present work, the protein BtuF is successfully converted into a high efficiency genetically encoded FRET-based sensor to measure steady-state concentration and to monitor and quantify the flux of vitamin B12 in living cells. In contrast to the dye-based probes, ECFP/venus, which is used as a FRET pair to construct this sensor overcomes the limitation of potential toxicity, encountered during cellular imaging. The enhanced fluorophores ECFP/venus pair shows the improved and qualitative version SenVitaL. Site-directed mutagenesis led to the generation of a set of affinity mutants covering a wide range of physiologically relevant vitamin B12 concentrations very much than the previously developed SenVitAL where only wild type sensor was used. W44Q mutation in the variant SenVitAL-5 lowered the Kd value for vitamin B12 and shows an increase in the emission intensity ratio after exposure to vitamin B12 in living cells. This newly developed enhanced efficiency sensor SenVitAL with other variants can be utilized for the purpose to quantify the level of vitamin B12 at various concentrations. Moreover, these sensors can be targeted to cellular and sub-cellular compartments of specific organelles and analysis of vitamin B12 can be carried out as many times as required in living cells. The sensor can serve as an excellent tool to comprehend the patho-physiological conditions of complex human biology system in response to the changes in the concentration of vitamin B12 with high spatial and temporal resolution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Financial Assistance in the form of a startup research grant (file no. YSS/ 2014/000393/LS) from the SERB, Department of Science and Technology, Govt. of India for conducting this research work is gratefully acknowledged.
Author contributions
NS and MM designed the study and prepared the original manuscript. NS, RN & MM conducted all in vitro and in vivo experiments and analyzed the data. NA and MA did the live cell imaging of yeast and HEK cells and analyzed the data. NS and MM revised the manuscript. All authors were engaged in commenting on the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they no conflict of interest.
References
- Ahmad M, Mohsin M, Iqrar S, Manzoor O, Siddiqi TO, Ahmad A. Live cell imaging of vitamin B12 dynamics by genetically encoded fluorescent nanosensor. Sens Actuators B. 2018;257:866–874. doi: 10.1016/j.snb.2017.11.030. [DOI] [Google Scholar]
- Ameen S, Ahmad M, Mohsin M, Qureshi MI, Ibrahim MM, Abdin MZ, Ahmad A. Designing, construction and characterization of genetically encoded FRET-based nanosensor for real time monitoring of lysine flux in living cells. J Nanobiotechnol. 2016;14:49. doi: 10.1186/s12951-016-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andruniow T, Zgierski MZ, Kozlowski PM. Vibrational analysis of methylcobalamin. J Phys Chem A. 2002;106(7):1365–1373. doi: 10.1021/jp013271k. [DOI] [Google Scholar]
- Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–377. doi: 10.1146/annurev.nutr.19.1.357. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5(8):593–599. doi: 10.1038/nchembio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio IJ, Mourant JR. Ultraviolet and visible spectroscopies fort issue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy. Phys Med Biol. 1997;42(5):803–814. doi: 10.1088/0031-9155/42/5/005. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Myszka DG, Bagnato JD, Alpers DH, West FG, Grissom CB. Equilibrium and kinetic analyses of the interactions between vitamin B12 binding proteins and cobalamins by surface plasmon resonance. Anal Biochem. 2002;305(1):1–9. doi: 10.1006/abio.2002.5647. [DOI] [PubMed] [Google Scholar]
- Capps BF, Hobbs NL, Fox SH. A method for the microbiological assay of vitamin B12. J Biol Chem. 1949;178(1):517. [PubMed] [Google Scholar]
- Carey PR. Resonance Raman spectroscopy in biochemistry and biology. Q Rev Biophys. 1978;11(03):309–370. doi: 10.1017/s0033583500002298. [DOI] [PubMed] [Google Scholar]
- Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc Natl Acad Sci USA. 2002;99(15):9846–98451. doi: 10.1073/pnas.142089199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CB, Graul RC, Lee AY, Merkle HP, Sadee W. The venus Flytrap of periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS PharmSci. 1999;1(2):E2. doi: 10.1208/ps010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JE. The microbiological assay of vitamin B12. Br J Nutr. 1952;6(3):324–330. doi: 10.1079/BJN19520034. [DOI] [PubMed] [Google Scholar]
- Frendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, Sauer U. Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Mol Syst Biol. 2010;6:356. doi: 10.1038/msb.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueant JL, Alpers DH. Vitamin B12, a fascinating micronutrient, which influences human health in the very early and later stages of life. Biochimie. 2013;95(5):967–969. doi: 10.1016/j.biochi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Hessels AM, Taylor KM, Merkx M. Monitoring cytosolic and ER Zn2+ in stimulated breast cancer cells using genetically encoded FRET sensors. Metallomics. 2015;8(2):211–217. doi: 10.1039/c5mt00257e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter B, Garcia AP, Schmid JA. Fluorescent proteins as genetically encoded FRET biosensors in life sciences. Sensors (Basel) 2015;15(10):26281–26314. doi: 10.3390/s151026281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann CE, Stokstad EL. The microbiological assay of vitamin B12 with Lactobacillus leichmannii. J Biol Chem. 1949;181(2):635–644. [PubMed] [Google Scholar]
- Holzinger M, Le Goff A, Cosnier S. Nanomaterials for biosensing applications: a review. Front Chem. 2014;2:63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Yamada YK, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA. 2009;106(37):15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmi O, Zayed A, Baraghethi S, Qadi M, Ghanem R. Measurement of vitamin B12 concentration: a review on available methods. IIOAB J. 2011;2(2):23–32. [Google Scholar]
- Karpowich NK, Huang HH, Smith PC, Hunt JF. Crystal structures of the BtuF periplasmic-binding protein for vitamin B12 suggest a functionally important reduction in protein mobility upon ligand binding. J Biol Chem. 2003;278(10):8429–8434. doi: 10.1074/jbc.M212239200. [DOI] [PubMed] [Google Scholar]
- Kremers GJ, Goedhart J, van Munster EB, Gadella TWJ. Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET förster radius. Biochemistry. 2006;45(21):6570–6580. doi: 10.1021/bi0516273. [DOI] [PubMed] [Google Scholar]
- Lee DS, Griffiths BW. Human serum vitamin B12 assay methods—A review. Clin Biochem. 1985;18(5):261–266. doi: 10.1016/S0009-9120(85)80028-X. [DOI] [PubMed] [Google Scholar]
- Liao H, Nehl CL, Hafner JH. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine. 2006;1(2):201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- Liu M, Sun T, Hu J, Chen W, Wang C. Study on the mechanism of the BtuF periplasmic-binding protein for vitamin B12. Biophys Chem. 2008;135(1–3):19–24. doi: 10.1016/j.bpc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Ma L, Yang F, Zheng J. Application of fluorescence resonance energy transfer in protein studies. J Mol Struct. 2014;1077:87–100. doi: 10.1016/j.molstruc.2013.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekzad H, Sahandi Zangabad P, Mirshekari H, Karimi M, Hamblin MR. Noble metal nanoparticles in biosensors: recent studies and applications. Nanotechnol Rev. 2017;6(3):301–329. doi: 10.1515/ntrev-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CW. Vitamins and Minerals: Help or Harm? Philadelphia: George F Stickley Company; 1986. [Google Scholar]
- McDowell LR. Vitamins in animal and human nutrition. 2. Florida, USA: Iowa State University Press/Ames; 2000. [Google Scholar]
- Mohsin M, Ahmad A. Genetically-encoded nanosensor for quantitative monitoring of methionine in bacterial and yeast cells. Biosens Bioelectron. 2014;59:358–364. doi: 10.1016/j.bios.2014.03.066. [DOI] [PubMed] [Google Scholar]
- Mohsin M, Abdin MZ, Nischal L, Kardam H, Ahmad A. Genetically encoded FRET-based nanosensor for in vivo measurement of leucine. Biosens Bioelectron. 2013;50:72–77. doi: 10.1016/j.bios.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Nestor J, Spiro TG, Klauminzer G. Coherent anti-Stokes Raman scattering (CARS) spectra, with resonance enhancement, of cytochrome c and vitamin B12 in dilute aqueous solution. Proc National Acad Sci. 1976;73(10):3329–3332. doi: 10.1073/pnas.73.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci USA. 2005;102(24):8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin MH, Azizi E, Arjomandi J, Lee JY. Highly sensitive and selective electrochemical sensor for detection of vitamin B12 using an Au/PPy/FMNPs@TD-modified electrode. Sens Actuators B. 2018;261:335–344. doi: 10.1016/j.snb.2018.01.168. [DOI] [Google Scholar]
- Rajoria DS, Nath A. IR spectroscopic studies of some cobalamins. J Inorg Nucl Chem. 1977;39(7):1291–1294. doi: 10.1016/0022-1902(77)80372-2. [DOI] [Google Scholar]
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5(9):763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- Rowland CE, Brown CW, III, Medintz IL, Delehanty JB. Intracellular FRET-based probes: a review. Methods Appl Fluoresc. 2015;3(4):042006. doi: 10.1088/2050-6120/3/4/042006. [DOI] [PubMed] [Google Scholar]
- Selvakumar LS, Thakur MS. Nano RNA aptamer wire for analysis of vitamin B12. Anal Biochem. 2012;427(2):151–157. doi: 10.1016/j.ab.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2015;2(12):905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Stefova M, Stafilov T, Stojanoski K, Cepreganova-Krstic B. Determination of vitamin B12 in multivitamin tablets by high performance liquid chromatography. Anal Lett. 1997;30(15):2723–2731. doi: 10.1080/00032719708001816. [DOI] [Google Scholar]
- Tsai CW, Morris MD. Application of resonance Raman spectrometry to the determination of vitamin b12. Anal Chim Acta. 1975;76(1):193–198. doi: 10.1016/s0003-2670(01)82001-8. [DOI] [PubMed] [Google Scholar]
- Vandame P, Spriet C, Trinel D, Gelaude A, Caillau K, Bompard C, Biondi E, Bodart JF. The spatio-temporal dynamics of PKA activity profile during mitosis and its correlation to chromosome segregation. Cell Cycle. 2014;13(20):3232–3240. doi: 10.4161/15384101.2014.950907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei J, Su S, Qiu J. Novel fluorescence resonance energy transfer optical sensors for vitamin B12 detection using thermally reduced carbon dots. New J Chem. 2015;39(1):501–507. doi: 10.1039/c4nj00538d. [DOI] [Google Scholar]
- Zhang L, Rong W, Lu C, Zhao L. Organo-modified layered double hydroxide-catalyzed Fenton-like ultra-weak chemiluminescence for specific sensing of vitamin B12 in egg yolks. Talanta. 2014;129:126–131. doi: 10.1016/j.talanta.2014.05.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.