Abstract
Purpose
To detect putative differences in the miRNomic profile of follicular fluids collected after follicular-phase-stimulation (FPS-FFs) and paired luteal-phase-stimulation (LPS-FFs) in the same ovarian cycles (DuoStim).
Methods
Exploratory study at a private IVF center and University involving FPS-FFs and paired-LPS-FFs collected from 15 reduced ovarian reserve and advanced maternal age women undergoing DuoStim (n = 30 paired samples). The samples were combined in 6 paired pools (5 samples each) and balanced according to maternal age and number of cumulus-oocyte-complexes. Micro-RNAs were isolated and sequenced. Four miRNAs were then selected for further validation on 6 single pairs of FPS-FFs and LPS-FFs by qPCR.
Results
Forty-three miRNAs were detected in both FPS-FFs and paired-LPS-FFs after sequencing and no statistically significant differences were reported. Thirty-three KEGG pathways were identified as regulated from the detected miRNAs. Four miRNAs (miR-146b, miR-191, miR-320a, and miR-483) were selected for qPCR validation since consistently expressed in our samples and possibly involved in the regulation/establishment of a healthy follicular environment. Again, no significant differences were reported between FPS-FFs and paired-LPS-FFs, also when the analysis was corrected for maternal age and number of cumulus-oocyte-complexes in generalized linear models.
Conclusions
These data complement the embryological, chromosomal, and clinical evidence of equivalence between FPS and LPS published to date.
Keywords: Follicular fluid, DuoStim, Double stimulation, Luteal phase stimulation, miRNA
Introduction
Controlled ovarian stimulation (COS) has been complemented with novel strategies in the last decades. Massin in 2017 has comprehensively reviewed the plethora of novel COS protocols that might be adopted in modern IVF [1] to better suit the characteristics of each woman in view of a personalized and more efficient treatment. Unconventional COS encompasses random start, luteal phase stimulation-only (LPS-only), as well as follicular and luteal phase stimulation (FPS plus LPS) in the same ovarian cycle (i.e., DuoStim) approaches. The clinical efficiency of these protocols has been investigated from several studies [2–12]. Similarly, reassuring obstetrical and neonatal data of safety have been also preliminarily outlined [13, 14]. Yet, the most fascinating aspect of these COS protocols is that they highlighted how the ovarian stimulation can be disconnected from the menstrual cycle in an IVF setting to retrieve competent oocytes from follicular waves otherwise anovulatory. Indeed, in a recent study we demonstrated that the oocytes retrieved after FPS and paired-LPS in the same ovarian cycle from 188 patients show similar competence, defined as blastulation and euploidy rates [15]. Such an outcome directly translates into more couples obtaining at least 1 euploid blastocyst with respect to the FPS-only approach in a single ovarian cycle [11]. This evidence has been also reported reproducible across 4 centers that implemented DuoStim for the treatment of poor prognosis women [12], nevertheless further analyses are required to keep building the evidence of LPS safety.
In this regard, here we aimed at investigating the follicular environment where the oocytes matured during COS to outline whether any difference exists between FPS and paired-LPS. Therefore, we focused on the analysis of the follicular fluid (FF), as a valuable source of such information. Several groups have suggested that this abundant and easily collectable biological fluid, which represents a by-product of IVF, might unveil captivating information on oocyte and embryo quality. To this end, FFs were screened for their content in growth factors, proteins, metabolites, reactive oxygen species, and nucleic acids [16–20]. More recently, microRNAs (miRNAs) have been investigated from human FFs collected after oocyte retrieval [21–33]. These small (~ 20–22 nucleotides) single strand non-coding RNA molecules figure among the main transcriptional/post-transcriptional regulators of gene expression, especially due to their pleiotropy. Yet, one of most important features of miRNAs is their role as carrier of a message between different cells. In fact, these molecules might be secreted from donor cells and travel through various human body fluids [34] towards recipient cells, where they finally exert their regulatory function. An evidence already reported also for crucial processes in human reproduction such as oocyte maturation, fertilization, and implantation [35]. Intriguingly, miRNAs function as ideal biomarkers in medicine showing crucial characteristics such as resistance to many biological, mechanical, and chemical insults as thoroughly described by Chen and colleagues in 2008 [36]. Specific miRNA signatures have been, in fact, already associated with pathological conditions like cancer, infarction, and liver injury [36–40].
Here, we focused on the comparison of miRNAs profiling between 3 pools of FPS-FFs and 3 paired LPS-FFs from women undergone DuoStim within the same ovarian cycle. Total miRNAs from both groups were isolated and processed for sequencing. Sequencing data were further analyzed to define a specific miRNomic signature specifically associated either with FPS-FFs or with LPS-FFs or shared by both tested groups. Four selected miRNA candidates were finally validated by qPCR in 6 pairs of samples obtained from single patients.
Material and methods
Study design, population of patients, and duration
This paired exploratory study was performed between October 2016 and May 2017 in a routine infertility program of a private IVF clinic (GENERA center for reproductive medicine) in collaboration with the Department of Experimental and Clinical Medicine of the University “Magna Graecia” of Catanzaro.
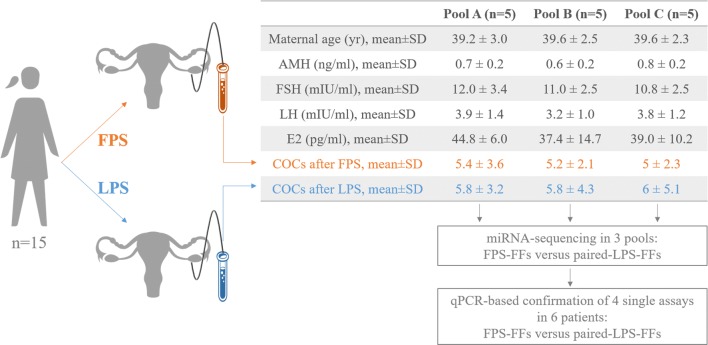
The FFs from the cohorts of oocytes retrieved after FPS and paired-LPS of 15 women undergoing a DuoStim protocol (n = 30 samples) were collected and combined in 6 pools (i.e., 3 FPS and 3 paired-LPS). The composition of the 6 pools was similar in terms of maternal age and number of cumulus oocyte complexes (COCs) retrieved to reduce the inter-sample variability. The women included in the study were all characterized by a reduced ovarian reserve (mean antral follicle count (AFC) 5.1 ± 1.9 and mean anti-Müllerian (AMH) hormone 0.7 ± 0.2 ng/ml), idiopathic infertility and advanced maternal age (mean age 39.5 ± 2.4 year). The mean basal follicle stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) levels were 11.3 ± 2.7 mIU/ml, 3.6 ± 1.2 mIU/ml and 40.4 ± 10.7 pg/ml, respectively. None of them had a history of polycystic ovarian syndrome (PCOS), endometriosis, obesity, or other conditions that might impact per se the composition of the FFs. The 6 pools were submitted to miRNA sequencing to outline putative differences between FPS and paired-LPS. The data were confirmed on 6 further single pairs of FPS-FFs and paired LPS-FFs through the qPCR analysis of 4 selected assays. Figure 1 shows the study design.
Fig. 1.
Flowchart of the study. FPS, follicular phase stimulation; LPS, luteal phase stimulation; AMH, Anti-Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; COCs, cumulus oocyte complexes
The Institutional Review Board of the clinic approved the study.
DuoStim protocol
In the menstrual cycle before FPS, luteal estradiol priming was performed (4 mg/die of estradiol valerate, Progynova, Bayer, Germany). After scan and basal assessment of the ovaries, FPS was conducted with a fixed dose of rec-FSH and rec-LH (300 IU/die of Gonal-F, Merck KGaA, Germany or Puregon, MSD, USA plus 150 IU/die of Luveris, Merck KGaA). GnRH antagonist (cetrorelix, Cetrotide, Merck KGaA; ganirelix, Orgalutran, MSD) was administered daily after the identification of the leading follicle up to the day of ovulation trigger. When at least 2 follicles > 17–18 mm of diameter were shown, final maturation was triggered with a single subcutaneous bolus of buserelin at the dose of 0.5 mL (Suprefact 5.5 mL; Hoechst Marion Roussel). Oocyte retrieval was performed after 35 hours, according to standard procedure. All follicles > 12–13 mm were aspirated. The duration of FPS was on average 10.0 ± 0.9 days. Five days after FPS, LPS was started with same protocol and daily dose. Induction of oocyte maturation was performed as for FPS. The duration of LPS was on average 11.3 ± 2.7 days.
IVF procedures
After oocyte retrieval, the COCs were incubated for 2–4 h until denudation and intracytoplasmatic sperm injection (ICSI), as previously described [41]. Fertilization was defined as the presence of two pronuclei at 16–18 h post-insemination. Blastocyst culture was conducted in a continuous single step culture media (CSCM, Irvine Scientific, USA) at 37 °C in a humidified atmosphere with 6% CO2 and 5% O2 up to 7 days post-insemination. All blastocysts underwent trophectoderm biopsy and vitrification, as previously described [42, 43].
Follicular fluid collection and RNA extraction
After oocyte retrieval, FFs were collected in 15-ml conical tubes and centrifuged at 1500g for 15 min to remove any cell residue and blood trace. The supernatant was slightly mixed without re-suspending the pellet to obtain a homogenous sample and then immediately transferred into polypropylene tubes (~ 5 ml) and stored in liquid nitrogen at − 196 °C. RNA extraction and miRNA enrichment were performed using the Plasma/Serum RNA Purification Kit (Norgen Biotek, Canada) according to manufacturer’s instructions. miRNA concentration and quality were assessed through the Bioanalyzer 2100 using Agilent Small RNA Chips (Agilent Technologies, USA).
miRNA sequencing
A total of 50 ng of total RNA per sample was mixed to obtain three pools for FPS and three for paired-LPS (five samples per pool). To obtain a concentration/volume ratio requested for the preparation of the libraries, the pools were concentrated using Speed Vac (Thermo Fisher Scientific, USA) and further measured with Bioanalyzer 2100. The pooled samples were used for indexed libraries preparation with TruSeq Small RNA Library Prep Kit (Illumina, USA), according to the manufacturer’s instructions. Libraries quality was checked using the Agilent Tape Station 2200 (Agilent Technologies) and samples were subjected to cluster generation and sequencing using an Illumina HiSeq 2500 (Illumina) in a 1 × 36 single-end format at a final concentration of 10 pmol. The reads were filtered for low-quality ones, contaminating 5′ adapters, homopolymers and trimmed for 3′ adapters. Quality control analysis was further investigated using FastQC software (v 0.11.2) (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), trimming of Small 3′ RNA adapter sequences, using Trimmomatic with default parameters. Quality checked reads were then aligned to the human genome (hg19 assembly) by Bowtie2 using the following parameters: --local -p 8 -q --phred33 -D 20 -R 3 -N 0 -L 8 -i S,1,0.50. Gene annotation was obtained for all known genes in the human genome, as provided by Ensembl (GRCh37) (https://support.illumina.com/sequencing/sequencing_software/igenome.ilmn). Using the reads mapped to the genome, the number of reads mapping to each transcript was calculated with HTSeq-count (http://wwwhuber.embl.de/users/anders/HTSeq/doc/overview.html). These raw read counts were then used as input to DESeq2 v 1.20.0 for calculation of size factor and scaling factor of normalized signal to bring the count values across all the samples to a common scale for each transcript. A miRNA was considered valid when detected in ≥ 2 out of 3 pools with a read count ≥ 10. Differential expression was reported as Fold Change (FC) along with associated adjusted p values (computed according to Benjamini-Hochberg).
Target and pathway prediction analyses
Target and pathway prediction analyses were performed with DIANA miRPath software v3.0 (http://snf-515788.vm.okeanos.grnet.gr/). This software exploits the miRNAs and pathway information provided by miRBase (http://www.mirbase.org) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg) databases. The experimentally validated miRNA-gene interactions were retrieved from the DIANA-TarBase v7.0 database. The “pathways union” merging algorithm was chosen, which identifies all significantly targeted pathways by the miRNAs consistently detected in the FFs. At first, the server performs an enrichment analysis and calculates the p values between miRNAs and pathways. Then, a merged p value is obtained by combining all the previous significance levels using a Fisher’s combined probability method. The false discovery rate was determined according to the Benjamini and Hochberg method before displaying the results.
Quantitative polymerase chain reaction
Four miRNAs were selected based on their consistency and reproducibility across the pools, as well as because of a thorough literature search outlining a putative role for them in the regulation of stress response, follicular metabolism, hormone responsiveness and/or oocyte maturation and quality.
A total of 20 ng of total RNA from 6 FPS-FFs and paired-LPS-FFs was used for retro-transcription with the miRCURY LNA RT Kit (Qiagen, Germany). UniSp6 and cel-miR-39 were added as exogenous controls in the RT reaction. The obtained cDNA was diluted 1:30 and 3 μl were used for qPCR using the miRCURY LNA miRNA PCR Assays (hsa-miR-483, catalogue number YP00205693; hsa-miR-320a, catalogue number YP00206042; hsa-miR-191, catalogue number YP00204306; hsa-miR-146b, catalogue number YP00204553; cel-miR-39, catalogue number YP00203952) and the miRCURY LNA SYBR Green PCR Kit (Qiagen). The reaction was run on a Step One Plus real-time PCR system (Thermo Fisher Scientific). The whole protocol was performed following manufacturer’s instructions. Gene expression levels were normalized to the Ct mean of UniSp6 and cel-miR-39 exogenous controls.
Statistical analysis
The software SPSS was used for statistics. Kolmogorov-Smirnov and Shapiro-Wilk tests were conducted to assess a normal distribution of the data. Paired t test or Wilcoxon signed rank test were conducted to determine statistically significant differences (p ≤ 0.05) between FPS-FFs and paired-LPS-FFs. The correlation coefficient between different pools/samples was assessed by the Pearson’s method. The differential expression analysis in the pools was presented through a Volcano plot showing the log2 of the FC in LPS-FFs versus FPS-FFs on the x-axis and the log10 of the adjusted p value on the y-axis. The differential expression analysis in the single assays was performed through the 2−ΔΔCt method and presented as ΔCt in FPS-FFs and paired LPS-FFs. The single assay results were also investigated through generalized linear models corrected for putative confounders, such as maternal age and number of COCs retrieved.
Results
The miRNomic profile of FPS-FFs and paired LPS-FFs was similar
Overall, 43 miRNAs from the FFs passed the quality control. All of them were found in both FPS-FFs and paired LPS-FFs pools. The Pearson’s correlation between the 3 FPS-FFs pools was 0.93 and between the 3 LPS-FFs pools was 0.95. The mean Pearson’s correlation instead between each FPS-FFs and its paired-LPS-FFs pool was 0.99 ± 0.1. In fact, the volcano plot outlined the absence of significant differences in the two miRNA signatures (Fig. 2a). The list of valid miRNAs, the FC in LPS-FFs versus FPS-FFS, and their relative adjusted p values are reported in Fig. 2b.
Fig. 2.
a Volcano plot displaying the differential miRNA expression analysis conducted in the pools of follicular fluids (FFs) obtained after follicular phase stimulation (FPS, control) and paired-luteal phase stimulation (LPS) in the same ovarian cycle. Each miRNA is represented as a black dot plotted according to the log2 of the fold change (FC) and the log10 of the p value; no statistically significant difference was reported (the threshold of significance is represented from dotted red line, p = 0.05). b List of the miRNAs identified with related FC in LPS-FFs versus FPS-FFs and p value. No miRNA was found either solely after the FPS or solely after the LPS
The target and pathway prediction analysis suggested a role for these miRNAs in the follicular environment
Thirty-three KEGG pathways were predicted as significantly (p < 0.01) regulated from the 43 miRNAs detected in the FFs of 15 poor prognosis women undergoing DuoStim. On average the pathways involved 13.8 ± 5.3 (range 6–24) miRNAs and 71.5 ± 52.4 (range 6–259) experimentally validated targets (Fig. 3a). Among these pathways, the Hippo signaling, the adherens junction, the ECM-receptor interaction, the TGF-beta signaling, the endocytosis, the thyroid hormone signaling, the fatty acid biosynthesis and metabolism, the steroid biosynthesis and the oocyte meiosis ones suggest an involvement of the identified miRNAs in the regulation of follicular environment and a direct/indirect effect on the reproductive outcomes. Three main macro-areas were outlined to cluster the 33 pathways identified: cell cycle, cell growth and cancer (n = 14/33, 42%), cell signaling and communication (n = 9/33, 27%), and metabolism (n = 7/33, 21%) (Fig. 3b).
Fig. 3.
a KEGG pathways significantly predicted to be regulated by the miRNAs identified in the pools of follicular fluids after follicular phase stimulation and paired-luteal phase stimulation conducted in the same ovarian cycle. b Macro-areas of biological functions clustering the KEGG pathways identified
Selection of 4 miRNAs for the qPCR experiments on single assays
Four miRNAs were selected for qPCR validation based on the following criteria: consistently high read number across all pools and biological function suggestive of a role in the regulation/establishment of a healthy follicular environment (e.g., stress response, cell growth and differentiation, cell communication, hormone response, oogenesis). These characteristics were matched by miR-146b, miR-191, miR-320a, and miR-483.
miR-146b was the most abundant miRNA in all the FFs pools analyzed. It is a tumor suppressor miRNA found also in several body fluids, which showed anti-inflammatory properties in several diseases [44–47], as well as a role in the regulation of metalloproteases [48].
miR-191 was the fifth most abundant miRNA found from this dataset. It is an onco-miR, reported also in body fluids, with an important emerging role in several diseases [49]. Also, this miRNA might be involved in the regulation of metalloproteases [50, 51]. Moreover, it was shown to be estrogen-responsive and upregulated in gynecological conditions like endometriosis and ovarian cancer [52–54].
miR-320a sets among the 10 least abundant miRNAs from our FFs pools. Nonetheless, it showed a good consistency across them and is one the most studied assays in the literature, also in previous papers investigating human FFs. It is an anti-oncogenic miRNA, reported to regulate estradiol concentrations and significantly associated with PCOS from the analysis of both cumulus cells and FFs [22, 55]. An association with a higher concentration of miR-320a in the FFs was also reported for increasing body mass indexes in non-PCOS women [33]. Moreover, an important role for this miRNA has been outlined in the regulation of (i) metalloproteases [56], (ii) oxidative stress-response by acting on the glycolytic pathway [57], and (iii) X chromosome inactivation [58, 59]. Yet, the most interesting evidence for our purpose comes from its analysis in the FFs of women undergoing IVF: miR-320a is associated with a larger number of mature oocytes after ovarian stimulation [28], as well as a better developmental potential and embryo quality [21].
miR-483 also sets among the 10 least abundant miRNAs from our FFs pools. However, as for miR-320a, there is a substantial amount of data highlighting its importance for follicular physiology and oocyte maturation. Specifically, it has been reported (i) to negatively regulate the production of the extracellular matrix [60], possibly by acting on metalloproteases [61], (ii) downregulated in the cumulus cells of women affected from PCOS [62], but upregulated in the FFs of women showing increased body mass indexes [33], and (iii) significantly associated with progesterone level [22]. Lastly, it showed lower concentrations in the FFs surrounding metaphase II compared to germinal vesicle oocytes [25].
qPCR validation of the sequencing data
The main data of the 6 patients whose FPS-FFs and paired LPS-FFs were collected for the qPCR validation experiments are shown in Fig. 4a. The Pearson’s correlation between the 6 FPS-FFs and their 6 paired-LPS-FFs was 0.9 for miR-146b, 0.88 for miR-191, 0.65 for miR-320a, and 0.6 for miR-483. The ΔCts for each miRNA in each FF sample analyzed are displayed in Fig. 4b, and the dotted red lines represent the average ΔCts. No difference was shown between FPS-FFs and paired LPS-FFs, thereby confirming the data from the pools. The ΔCt of each miRNA were also investigated through generalized linear models corrected for maternal age and number of COCs retrieved: no association was reported also from this analysis between the miRNA level and the phase of the cycle COS was started at (i.e., follicular or luteal). The only significant association reported from the generalized linear models, corrected for maternal age and number of COCs, was between miR-320a ΔCt and oocyte maturation rate (partial η2 = 0.45, power = 0.75, and p = 0.03). Specifically, higher miR-320a concentration in the FFs (i.e., lower ΔCt values) corresponded to higher maturation rates after both FPS and LPS.
Fig. 4.
qPCR analysis of four selected miRNAs in the follicular fluids (FFs) retrieved from 6 single patients after follicular phase stimulation (FPS, control) and paired-luteal phase stimulation (LPS) in the same ovarian cycle. a Embryological outcomes of the patients included. COC, cumulus oocyte complexes; MII, metaphase II oocytes; Mat., maturation; 2PN, 2 pronuclei zygotes; Fert., fertilization; Blast., blastulation; Eup. Blast., euploid blastocyst. b ΔCt in the FFs after FPS and paired-LPS. Each patient is shown in a different shade of blue corresponding to the table above, while the dotted red line shows the mean ΔCt values. No statistically significant differences were reported. AMH, Anti-Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol
Discussion
LPS figures among a series of unconventional COS protocols theorized, implemented, and clinically-applied in the last decades, and it is crucial when a high number of MII oocytes must be obtained in a short timeframe (e.g., poor prognosis and advanced maternal age women or oncological patients) [1, 7, 63]. To date, the LPS-based strategies showed various evidence of safety from embryological, clinical, and preliminarily also obstetrical and neonatal perspectives [2, 8, 11–15, 64]. Nevertheless, several studies are still needed to shed light on the physiology on which LPS is grounded. In fact, after Baerwald and colleagues codified the existence of multiple waves of follicular growth in humans [65, 66], limited effort has been invested worldwide to outline the biological principles supporting an equal competence and safety for the oocytes retrieved from the anovulatory phase of the ovarian cycle. This study sets in this scenario: the finding of similar miRNomic signatures from the FFs retrieved after FPS and paired-LPS in the same ovarian cycle in poor prognosis women undergoing IVF represents a further little element to support the latter, when required.
The DuoStim protocol allowed us to adopt a paired design and compare FPS-FFs and LPS-FFs conducted with the same protocol in the absence of bias. The only difference between the two FFs samples collected from each patient was in fact the phase of the ovarian cycle COS was started and the duration of LPS, on average 1 day longer than FPS. MiRNAs investigation in the human FFs after COS has been previously described by other studies, but none of them collected samples after LPS. Therefore, to the best of our knowledge, our study is the first that investigates and compares the miRNAs profile of FFs collected after FPS and LPS within the same ovarian cycle. Our data fit well in an international scenario where several centers started implementing LPS-based stimulation protocols in IVF.
The evidence highlighted from this exploratory study suggests that the follicular environment where the oocytes mature during LPS is similar at least for what concerns the 43 miRNAs consistently identified in the FFs. Of note, these miRNAs are potentially involved in key biological processes orchestrating crucial functions in the ovary, like hormonal response, communication between granulosa/cumulus cells and the oocytes, meiosis completion, ovulation, as well as signaling pathways involved in folliculogenesis, but also in pre-/post-implantation embryo development, like the TGF-beta and the Hippo pathways [67–69].
All the previous groups focusing their research on the detection of miRNAs in human FFs shared a wish for future studies to confirm the reproducibility of their evidence. Indeed, such a feature is crucial to testify the technical reliability of a method and of the results produced through it, in turn supporting any biological and/or clinical speculation based on the latter. In this view, we compared the results produced in our dataset to the existing literature in this field. Sang and colleagues identified several miRNAs either free in the FFs or embedded in micro-vesicles [22] and 80% (n = 34/43) of the miRNAs reliably detected from our samples were found in their study. Of note, the 4 miRNAs chosen for the qPCR experiments were among the ones showing the highest concentrations in the FFs of Sang’s analysis. Sørensen and colleagues in 2016 reported that only 183 miRNAs could be identified in the FFs of 12 women affected from PCOS and 4 controls [29]. Nevertheless, the spectrum of consistently detected miRNAs was even narrower (n = 79). Then, they investigated a sub-group of 12 miRNAs as potential biomarkers of PCOS condition, which were selected on the basis of their putative role in folliculogenesis and ovarian steroidogenesis, as well as of their reliability in their analysis. Interestingly, miR-let-7 g and miR-151, miR-193b, miR-320a, and miR-518f were included in this cluster and confirmed also in our dataset. Feng and colleagues recently suggested that the concentration of miR-320 in the FFs is significantly associated with embryo morphological quality. Moreover, by knocking down this miRNA in murine oocytes, they reported a severe impairment in their developmental potential beyond the 2-cell stage [21]. MiR-30d and miR-10b were among the most prevalent miRNAs identified by Machtinger and colleagues in the FFs of women undergoing IVF, while miR-16 was reported among the miRNAs significantly associated with fertilization success [23]. The same group in a more recent study then reported also miR-92a and miR-130b among the miRNAs encapsulated within extracellular vesicles most significantly associated with fertilization failure [24]. Such evidence was then corroborated with a multivariate logistic regression analysis that pinpointed also miR-let-7a, miR-10b-3p, miR-30d, and miR-181a as predictors of the same outcome, while miR-148a associated with embryo morphological quality at the cleavage stage. The latest paper from this group highlighted also that miR-193b, miR-320a, miR-483 are higher in the FFs of women showing an increased body mass index [33]. Moreno and colleagues also reported the latter of these miRNAs (miR-483) as significantly over-expressed in the FFs of germinal vesicle oocytes [25]. In 2014, Santonocito and colleagues characterized the population of miRNAs both free in the FFs and encapsulated in micro-vesicles. To this end, they selected 15 healthy young women undergoing IVF because of a severe male factor. Five of the 37 miRNAs they found upregulated with respect to paired plasma samples were identified also in our dataset: miR-100, miR-193b, miR-125b, miR-10b, and miR-483. Of note, former three were highlighted also in Santonocito’s study for a pivotal role in granulosa and cumulus cells, by targeting mRNAs involved in ovarian physiology [27]. Lastly, Scalici and colleagues selected and analyzed 5 circulating miRNAs in the FFs of women affected from PCOS versus women with normal ovarian reserve. Among them, miR-30a, miR-140, and miR-320a were identified also in our dataset. These authors reported the former 2 as highly associated with PCOS condition, while miR-320a was found associated with a larger number of mature oocytes retrieved after COS [28]. Overall, this thorough search of the existing literature based on the miRNomic analysis of human FFs supports the trustworthiness of our data, as well the related speculations.
The miRNAs selected (with the related reasons) for the qPCR validation of the sequencing data have been thoroughly detailed in the results section although it is interesting to highlight that miR-320a, while not different in the FFs after FPS and paired-LPS, showed instead a significant association with higher maturation rates from an overall analysis corrected for maternal age and number of COCs. A result, which supports the evidence previously reported by both Scalici and Feng, who claimed that this miRNA might be a predictor of the size of the cohort of mature oocytes and of embryo development beyond the very first cellular divisions, respectively [21, 28].
In conclusion, the main limitation of this study is its sample size. Future single follicle rather than cohort analyses are suggested to further increase the depth of the investigation. Similarly, studies specifically targeting the exosomal component of the FFs are warranted along with transcriptomic and/or proteomic profiling to corroborate our miRNomic evidence. Nevertheless, these data provide a further piece of evidence to support that LPS, if required, might be similar to FPS.
Authors’ contribution
DC, LR, and GC designed the study. AV, RV, FZ, and FMU recruited the patients. RC and EA collected the samples. EIP, SS, GSan, MTDA, and GSar processed the samples and performed the experiments. DC and RC analyzed the data and drafted the manuscript. All authors contributed to the comprehensive discussion of the data.
Funding information
The University “Magna Graecia” of Catanzaro and GENERA financed this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Filippo Maria Ubaldi and Giovanni Cuda contributed equally to this work.
References
- 1.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–220. doi: 10.1093/humupd/dmw047. [DOI] [PubMed] [Google Scholar]
- 2.von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92(4):1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95(6):2125 e9-11. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril. 2011;96(1):e51–e54. doi: 10.1016/j.fertnstert.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 5.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 6.Buendgen NK, Schultze-Mosgau A, Cordes T, Diedrich K, Griesinger G. Initiation of ovarian stimulation independent of the menstrual cycle: a case-control study. Arch Gynecol Obstet. 2013;288(4):901–904. doi: 10.1007/s00404-013-2794-z. [DOI] [PubMed] [Google Scholar]
- 7.Martinez F, Clua E, Devesa M, Rodriguez I, Arroyo G, Gonzalez C, et al. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril. 2014;102(5):1307–1311. doi: 10.1016/j.fertnstert.2014.07.741. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, Ai A, Lyu Q, Kuang Y. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol. 2016;84(5):720–728. doi: 10.1111/cen.12983. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Yang W, Chen X, Li L, Zhang Q, Yang D. Comparison between follicular stimulation and luteal stimulation protocols with clomiphene and HMG in women with poor ovarian response. Gynecol Endocrinol. 2016;32(1):74–77. doi: 10.3109/09513590.2015.1081683. [DOI] [PubMed] [Google Scholar]
- 10.Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod BioMed Online. 2014;29(6):684–691. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–95 e1. doi: 10.1016/j.fertnstert.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Vaiarelli A, Cimadomo D, Trabucco E, Vallefuoco R, Buffo L, Dusi L, et al. Double stimulation in the same ovarian cycle (duostim) to maximize the number of oocytes retrieved from poor prognosis patients: a multicenter experience and swot analysis. Front Endocrinol (Lausanne) 2018;9:317. doi: 10.3389/fendo.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaiarelli A, Cimadomo D, Colamaria S, Ferrero S, Giuliani M, Trabucco E, et al. No evidences that implantation of vitrified euploid blastocysts is influenced by ovarian stimulation conducted in luteal versus follicular phase: interim analysis of a prospective multicentre study. Hum Reprod. 2018;33(Suppl July 2018):i138–i1i9. [Google Scholar]
- 14.Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103(5):1194–201 e2. doi: 10.1016/j.fertnstert.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Cimadomo Danilo, Vaiarelli Alberto, Colamaria Silvia, Trabucco Elisabetta, Alviggi Carlo, Venturella Roberta, Alviggi Erminia, Carmelo Ramona, Rienzi Laura, Ubaldi Filippo Maria. Luteal phase anovulatory follicles result in the production of competent oocytes: intra-patient paired case-control study comparing follicular versus luteal phase stimulations in the same ovarian cycle. Human Reproduction. 2018;33(8):1442–1448. doi: 10.1093/humrep/dey217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod. 2008;23(9):2001–2009. doi: 10.1093/humrep/den192. [DOI] [PubMed] [Google Scholar]
- 17.Barroso G, Barrionuevo M, Rao P, Graham L, Danforth D, Huey S, Abuhamad A, Oehninger S. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil Steril. 1999;72(6):1024–1026. doi: 10.1016/S0015-0282(99)00442-2. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, et al. Reactive oxygen species level in follicular fluid--embryo quality marker in IVF? Hum Reprod. 2006;21(9):2403–2407. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 19.O'Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction. 2013;146(4):389–395. doi: 10.1530/REP-13-0184. [DOI] [PubMed] [Google Scholar]
- 20.Wu YT, Wu Y, Zhang JY, Hou NN, Liu AX, Pan JX, et al. Preliminary proteomic analysis on the alterations in follicular fluid proteins from women undergoing natural cycles or controlled ovarian hyperstimulation. J Assist Reprod Genet. 2015;32(3):417–427. doi: 10.1007/s10815-014-0419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y, et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci Rep. 2015;5:8689. doi: 10.1038/srep08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 23.Machtinger R, Rodosthenous RS, Adir M, Mansour A, Racowsky C, Baccarelli AA, et al. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4):525–533. doi: 10.1007/s10815-017-0876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez RM, Liang L, Racowsky C, Dioni L, Mansur A, Adir M, et al. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci Rep. 2018;8(1):17036. doi: 10.1038/s41598-018-35379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno JM, Nunez MJ, Quinonero A, Martinez S, de la Orden M, Simon C, et al. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil Steril. 2015;104(4):1037–46 e1. doi: 10.1016/j.fertnstert.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(3):355–362. doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzì P, Rizzari S, Maugeri M, Scollo P, Tatone C, Valadi H, Purrello M, di Pietro C. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102(6):1751–61 e1. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Scalici E, Traver S, Mullet T, Molinari N, Ferrieres A, Brunet C, et al. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci Rep. 2016;6:24976. doi: 10.1038/srep24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen AE, Wissing ML, Englund AL, Dalgaard LT. MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. J Clin Endocrinol Metab. 2016;101(4):1579–1589. doi: 10.1210/jc.2015-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu J, Qu RG, Zhang YJ, Gu RH, Li X, Sun YJ, Wang L, Sang Q, Sun XX. Screening of miRNAs in human follicular fluid reveals an inverse relationship between microRNA-663b expression and blastocyst formation. Reprod BioMed Online. 2018;37(1):25–32. doi: 10.1016/j.rbmo.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Xue Y, Lv J, Xu P, Gu L, Cao J, Xu L, Xue K, Li Q. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem. 2018;119(5):3913–3921. doi: 10.1002/jcb.26531. [DOI] [PubMed] [Google Scholar]
- 32.Naji M, Nekoonam S, Aleyasin A, Arefian E, Mahdian R, Azizi E, Shabani Nashtaei M, Amidi F. Expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells, follicular fluid, and serum of women with polycystic ovary syndrome (PCOS) Arch Gynecol Obstet. 2018;297(1):221–231. doi: 10.1007/s00404-017-4570-y. [DOI] [PubMed] [Google Scholar]
- 33.Martinez Rosie M., Baccarelli Andrea A., Liang Liming, Dioni Laura, Mansur Abdallah, Adir Michal, Bollati Valentina, Racowsky Catherine, Hauser Russ, Machtinger Ronit. Body mass index in relation to extracellular vesicle–linked microRNAs in human follicular fluid. Fertility and Sterility. 2019;112(2):387-396.e3. doi: 10.1016/j.fertnstert.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22(2):182–193. doi: 10.1093/humupd/dmv055.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 37.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31(6):659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 38.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56(7):1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rienzi L, Ubaldi F, Anniballo R, Cerulo G, Greco E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13(4):1014–1019. doi: 10.1093/humrep/13.4.1014. [DOI] [PubMed] [Google Scholar]
- 42.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–1181. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 43.Cimadomo Danilo, Capalbo Antonio, Levi-Setti Paolo Emanuele, Soscia Daria, Orlando Giovanna, Albani Elena, Parini Valentina, Stoppa Marta, Dovere Lisa, Tacconi Luisa, Ievoli Elena, Maggiulli Roberta, Ubaldi Filippo Maria, Rienzi Laura. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Human Reproduction. 2018;33(11):1992–2001. doi: 10.1093/humrep/dey291. [DOI] [PubMed] [Google Scholar]
- 44.Chen BB, Li ZH, Gao S. Circulating miR-146a/b correlates with inflammatory cytokines in COPD and could predict the risk of acute exacerbation COPD. Medicine (Baltimore) 2018;97(7):e9820. doi: 10.1097/MD.0000000000009820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffer D, Rossmanith E, Lang I, Falkenhagen D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an In vitro sepsis model. PLoS One. 2017;12(6):e0179850. doi: 10.1371/journal.pone.0179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5(7):1017–1034. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A. 2013;110(28):11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S, Wang Q, Yan Y, Kang C, Jin S, An T, Shi C, Xu J, Wei C, Liu J, Sun J, Wen Y, Zhao S, Kong Y. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013;339(2):260–269. doi: 10.1016/j.canlet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Nagpal N, Kulshreshtha R. miR-191: an emerging player in disease biology. Front Genet. 2014;5:99. doi: 10.3389/fgene.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin S, Zhu Y, Ai F, Li Y, Bai B, Yao W, Dong L. MicroRNA-191 correlates with poor prognosis of colorectal carcinoma and plays multiple roles by targeting tissue inhibitor of metalloprotease 3. Neoplasma. 2014;61(1):27–34. doi: 10.4149/neo_2014_005. [DOI] [PubMed] [Google Scholar]
- 51.Gu Y, Ampofo E, Menger MD, Laschke MW. miR-191 suppresses angiogenesis by activation of NF-kappaB signaling. FASEB J. 2017;31(8):3321–3333. doi: 10.1096/fj.201601263R. [DOI] [PubMed] [Google Scholar]
- 52.Nagpal N, Ahmad HM, Molparia B, Kulshreshtha R. MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis. 2013;34(8):1889–1899. doi: 10.1093/carcin/bgt107. [DOI] [PubMed] [Google Scholar]
- 53.Dong M, Yang P, Hua F. MiR-191 modulates malignant transformation of endometriosis through regulating TIMP3. Med Sci Monit. 2015;21:915–920. doi: 10.12659/MSM.893872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen J, DiCioccio R, Odunsi K, Lele SB, Zhao H. Novel genetic variants in miR-191 gene and familial ovarian cancer. BMC Cancer. 2010;10:47. doi: 10.1186/1471-2407-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang CL, Wang H, Yan CY, Gao XF, Ling XJ. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem Biophys Res Commun. 2017;482(4):1469–1476. doi: 10.1016/j.bbrc.2016.12.059. [DOI] [PubMed] [Google Scholar]
- 56.Meng F, Zhang Z, Chen W, Huang G, He A, Hou C, Long Y, Yang Z, Zhang Z, Liao W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1beta-induced chondrocyte responses. Osteoarthr Cartil. 2016;24(5):932–941. doi: 10.1016/j.joca.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Tang H, Lee M, Sharpe O, Salamone L, Noonan EJ, Hoang CD, et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26(11):4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun YX, Zhang YX, Zhang D, Xu CM, Chen SC, Zhang JY, Ruan YC, Chen F, Zhang RJ, Qian YQ, Liu YF, Jin LY, Yu TT, Xu HY, Luo YQ, Liu XM, Sun F, Sheng JZ, Huang HF. XCI-escaping gene KDM5C contributes to ovarian development via downregulating miR-320a. Hum Genet. 2017;136(2):227–239. doi: 10.1007/s00439-016-1752-9. [DOI] [PubMed] [Google Scholar]
- 59.Xu X, Ma C, Liu C, Duan Z, Zhang L. Knockdown of long noncoding RNA XIST alleviates oxidative low-density lipoprotein-mediated endothelial cells injury through modulation of miR-320/NOD2 axis. Biochem Biophys Res Commun. 2018;503(2):586–592. doi: 10.1016/j.bbrc.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Liu L, Deng X, Li D, Cai H, Ma Y, Jia C, Wu B, Fan Y, Lv Z. MicroRNA 483-3p targets Pard3 to potentiate TGF-beta1-induced cell migration, invasion, and epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Oncogene. 2019;38(5):699–715. doi: 10.1038/s41388-018-0447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Zhang H, Sun Q, Wang Y, Yang J, Yang J, et al. Intra-articular delivery of antago-mir-483-5p inhibits osteoarthritis by modulating matrilin 3 and tissue inhibitor of metalloproteinase 2. Mol Ther. 2017;25(3):715–727. doi: 10.1016/j.ymthe.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Shi L, Liu S, Zhao W, Shi J. miR-483-5p and miR-486-5p are down-regulated in cumulus cells of metaphase II oocytes from women with polycystic ovary syndrome. Reprod BioMed Online. 2015;31(4):565–572. doi: 10.1016/j.rbmo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta-analysis. J Assist Reprod Genet. 2016;33(8):971–980. doi: 10.1007/s10815-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, Shoham Z. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–111. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80(1):116–122. doi: 10.1016/S0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- 66.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- 67.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8(8):613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 68.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27(1):14–23. doi: 10.1055/s-0028-1108006... [DOI] [PMC free article] [PubMed] [Google Scholar]