Abstract
Purpose
Our aim was to elucidate the mechanisms involved in follicle activation of the ovarian reserve after human ovarian tissue transplantation, with specific focus on the role of the effectors of the PI3K (mTOR and FOXO1) and Hippo (YAP) signaling pathways and whether they are somehow altered.
Methods
Frozen-thawed ovarian tissue was collected from six women (age 25–35 years) undergoing surgery for non-ovarian pathologies and divided into 4 fragments in each case: one for non-grafted controls and three for grafting to immunodeficient mice for 3, 7 and 21 days. The tissue was processed for hematoxylin and eosin staining, immunohistochemistry and immunofluorescence at different timepoints before and after grafting. Activation of the PI3K and Hippo signaling pathways was investigated by analysis of mTOR phosphorylation, FOXO1 cytoplasmic localization and YAP nuclear localization.
Results
No change in mTOR levels was observed in primordial follicles post-transplantation, but a significant upturn was recorded in growing follicles compared with primordial follicles, irrespective of grafting time. A higher percentage of primordial follicles was also found with FOXO1 in the cytoplasm after 3 days of transplantation than in non-grafted controls. Finally, a greater proportion of primordial follicles was detected with YAP in the nucleus at all timepoints after grafting.
Conclusions
This study supports the hypothesis that follicle activation may occur as an early event after transplantation, with follicle growth and death both contributing to the burnout phenomenon. This is the first time that the effectors of the PI3K and Hippo pathways have been investigated in grafted human ovarian tissue and their role in burnout documented.
Keywords: Follicle activation, Ovarian tissue transplantation, Burnout effect, PI3K pathway, Hippo pathway
Introduction
Cancer survival rates have increased progressively over recent decades, but chemotherapy and radiotherapy are highly gonadotoxic treatments, often leading to premature ovarian insufficiency (POI) and infertility in affected women [1, 2]. Among currently available options that can be offered to these patients to preserve and subsequently restore their fertility, ovarian tissue cryopreservation and transplantation is the only alternative for young women who need to undergo immediate treatment, and also for prepubertal patients [2]. Although more than 130 live births have been achieved worldwide with this technique [2], there are still unresolved issues related to the procedure, as massive loss of primordial follicles constituting the ovarian reserve is known to occur after transplantation. This event appears to be the consequence of early hypoxia that characterizes the immediate post-grafting period [3, 4]. However, this loss of dormant follicles is accompanied by an increase in the growing follicle population [5, 6], suggesting a double mechanism at play: follicle death and follicle activation [7].
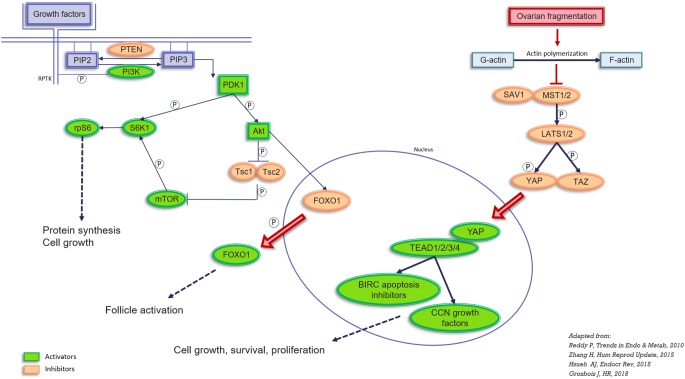
The phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/phosphoinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling cascade is fundamental to a wide range of cellular processes, including nutrient metabolism, cell survival, cell growth and apoptosis [8] (Fig. 1). Akt activation is regulated by the balance between PTEN and PI3K, which ultimately results in varying degrees of Akt phosphorylation and consequently activation of a number of pathways, including forkhead box O (FOXO) and the mechanistic target of rapamycin complex 1 (mTORC1). Animal studies have proved that stimulation of the Akt/FOXO and mTOR pathways does indeed lead to primordial follicle activation and growth [9–16]. The Hippo pathway also appears to be involved in follicle activation and growth, through the action of yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) downstream effectors [17]. Nevertheless, there is no conclusive proof of the role of these pathways in the activation of human primordial follicles, despite animal studies suggesting a clear involvement of these factors in primordial follicle activation in rodents. In 2013, Kawamura et al [18] documented disruption of the Hippo pathway in human ovarian biopsies from POI patients after vitrification, incubation with Akt stimulators and xenografting for 4 weeks. We decided to apply this current knowledge and compare the main effectors of the PI3K and Hippo pathways at different timepoints after short-term grafting. We recently demonstrated that follicle density decreases after ovarian tissue transplantation after only 3 days of grafting and that Akt phosphorylation increases in primordial follicles over the same 3-day period, indicating that Akt could be responsible for early follicle activation [7]. Advancing further along the pathway involved in follicle activation in human ovarian tissue and comparing the main effectors of the PI3K and Hippo pathways at different timepoints after grafting, could well shed more light on follicle dynamics in the early days after transplantation of frozen-thawed human ovarian tissue.
Fig. 1.
Representation of the PI3K and Hippo pathways involved in follicle activation. PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PTEN: phosphatase and tensin homolog deleted on chromosome 10; PI3K: phosphoinositol-3-kinase; PDK1: pyruvate dehydrogenase lipoamide kinase isozyme 1; Akt: protein kinase B; FOXO: forkhead box O; TSC1 and TSC2: tuberous sclerosis proteins 1 and 2; mTOR: mechanistic target of rapamycin; S6K1: ribosomal protein S6 kinase beta-1; RPS6: ribosomal protein S6. MST1/2: mammalian Ste20-like 1/2; SAV1: protein salvador homolog 1; LATS1/2: large tumor suppressor kinase 1/2; YAP: yes-associated protein, TAZ: transcriptional coactivator with PDZ-binding motif; TEAD 1/2/3/4: TEA domain family members 1/2/3/4; BIRC: baculoviral inhibitors of apoptosis repeat containing
Material and methods
Experimental design
Frozen-thawed human ovarian tissue from six different patients (age 25–35 years, mean 29.8 years) was used for the study. All six women underwent surgery for non-ovarian pathologies (breast cancer n = 4, Hodgkin’s lymphoma n = 1 and sarcoma n = 1) and provided written informed consent to donate their cryopreserved ovarian tissue for research purposes. There was no significant difference in follicular density between patients. Use of human ovarian tissue was approved by the Institutional Review Board of the Université Catholique de Louvain (Reference 2012/23MAR/125).
Two or 3 cryovials containing ovarian tissue were thawed per patient in order to obtain 4 fragments, each measuring approximately 5 × 8 × 1 mm3, one to be used for non-grafted controls and three to be grafted. Three grafting durations were evaluated: 3, 7 and 21 days. Follicle activation was analyzed by immunohistochemistry (IHC) for phospho-mTOR (p-mTOR) and FOXO1, and by immunofluorescence for YAP.
Ovarian tissue freezing and thawing
Cryopreservation of ovarian tissue was undertaken according to the slow-freezing protocol, as described elsewhere [19, 20]. Frozen ovarian tissue was thawed at room temperature for 2 min, immersed in a water bath at 37 °C for another 2 min, and then washed three times in fresh HEPES-MEM medium (Gibco, ThermoFisher, Belgium) to remove the cryoprotectant [21].
Transplantation to immunodeficient mice
Guidelines for animal welfare were fully respected and the study was granted approval from the Committee on Animal Research of the Université Catholique de Louvain (Reference 2018/UCL/MD/40). Nine female severe combined immunodeficient (SCID) mice (Charles River, L’Arbresle, France) were used for the transplantation experiments. A median incision was made in the abdomen and peritoneum of the animals, and human ovarian tissue fragments were fixed to the inner sides of the peritoneum with 1 or 2 stitches of 6-0 Prolene (Ethicon; Johnson & Johnson International, USA). The abdominal wall and skin were then closed with 6-0 Prolene (Ethicon; Johnson & Johnson International, USA). The mice were kept in sterile conditions for the entire duration of the grafting period and subsequently euthanized by cervical dislocation. The ovarian grafts were left in place for 3, 7 and 21 days. Upon retrieval, they were subjected to histological analysis (formol).
Histology
For each patient, non-grafted ovarian tissue and grafts from the three timepoints were fixed in 4% formaldehyde, embedded in paraffin and serially sectioned (5-μm-thick sections). Every fourth slide was stained with hematoxylin and eosin (H&E) (Merck, Darmstadt, Germany) for histological evaluation.
Follicle activation
Follicle activation was evaluated by immunostaining for p-mTOR, FOXO1 and YAP. Sections were first deparaffinized with Histosafe (Yvsolab SA, Beerse, Belgium) and rehydrated in 2-propanol (Merck, Darmstadt, Germany). After blocking endogenous peroxidase activity by incubation with 3% H2O2 (Merck, Darmstadt, Germany) for 30 min at room temperature, and heat epitope retrieval in citrate buffer for 75 min at 98 °C, nonspecific staining was blocked by incubation with goat serum for 30 min. The slides were incubated overnight at 4 °C with primary antibodies, namely rabbit anti-human p-mTOR (Ser2448) (dilution 1/200, #2976, Cell Signaling, USA), rabbit anti-human FOXO1 (dilution 1/200, #2880, Cell Signaling, USA) and mouse anti-human YAP (dilution 1/200, #12395, Cell Signaling, USA), followed by a further 60 min at room temperature with EnVision anti-rabbit and anti-mouse secondary antibodies (Dako, NC, USA). For p-mTOR and FOXO1, diaminobenzidine (DAB vector 4100, Dako, NC, USA) was used as a chromogen and nuclei were counterstained with hematoxylin, while for YAP, tyramide 488 (dilution 1/200, ThermoFisher, Brussels, Belgium) was applied and nuclei were counterstained with Hoechst 33342 (dilution 1/1000, ThermoFisher, Brussels, Belgium). Negative controls were conducted in line with current guidelines using rabbit-specific and mouse-specific FLEX polyclonal antibodies (ready to use, IS600, Dako, NC, USA) to substitute the primary antibody [22]. Comprehensive images of p-mTOR sections were obtained with the Leica SCN400 slide scanner at 20X magnification (Leica Biosystems, Dublin, Ireland). This analysis was conducted by two observers, one of whom was blinded, and only follicles with a visible oocyte in the central area were evaluated. After delineation and annotation, computer-assisted quantification of staining concentrations was achieved with Leica’s proprietary artificial intelligence (TissueIA, Wetzlar, Germany) by applying the following formula: (where IA=average intensity annotation A; Imean=mean of average staining intensities of all annotations; SposA=total tissue area of annotation A; SposTot=sum of total tissue area of all annotations) [23–26]. FOXO1 and YAP slides were digitized by automated whole-slide capture using the Pannoramic P250 Flash III scanner (3DHISTECH, Hungary) in brightfield and the green (SR-FITC-Zero) filter respectively. Image files were analyzed with CaseViewer (3DHISTECH) software.
Statistics
GraphPad Prism, version 7.00 for Windows (GraphPad Software, USA), was applied for statistical analyses. The paired t test was used to evaluate p-mTOR, and Fisher’s exact test to assess FOXO1 and YAP. A p value < 0.05 was considered statistically significant.
Results
Follicle activation
p-mTOR analysis
Staining for p-mTOR was observed in oocytes, granulosa cells (GCs) of primordial follicles and follicles at every growth stage, and stromal cells (Fig. 2a–h). Only follicles with a visible oocyte in the central area were investigated using the appropriate software (TissueIA, Wetzlar, Germany). A total of 478 follicles were analyzed after classification into primordial or growing stages (Table 1), and the paired t test was carried out. Quantification of p-mTOR in primordial follicles, based on the intensity and extent of the signal, revealed no increase after 3 days of grafting compared with primordial follicles in non-grafted tissue (mean staining concentration: 1172 in non-grafted tissue vs 1226 on day 3, p = 0.8) (Fig. 2i). A significant increase in mTOR phosphorylation was nevertheless observed in growing follicles compared with primordial follicles in the non-grafted group (mean staining concentration: 1172 in primordial follicles vs 2330 in growing follicles, p = 0.0016) (Fig. 2i).
Fig. 2.
Phospho-mTOR immunostaining in primordial (a) and growing (b) follicles in non-grafted ovarian tissue, in primordial (c) and growing (d) follicles in ovarian tissue grafted for 3 days, in primordial (e) and growing (f) follicles in ovarian tissue grafted for 7 days, and in primordial (g) and growing (h) follicles in ovarian tissue grafted for 21 days. i mTOR phosphorylation in primordial (left) and growing (right) follicles in the non-grafted group (D0), and on D3, D7 and D21 post-grafting (mean + SEM), scale bar: 10 μm. a significantly different from b (p < 0.001)
Table 1.
Number of follicles analyzed for mTOR (left), FOXO1 (center) and YAP (right)
| mTOR | Primordial follicles (n) | Growing follicles (n) | FOXO1 | Primordial follicles with cytoplasmic staining (n) | Total number of primordial follicles (n, %) | YAP | Primordial follicles with nuclear staining (n) | Total number of primordial follicles (n, %) |
|---|---|---|---|---|---|---|---|---|
| D0 | 101 | 32 | D0 | 23 | 60 (38,3%) | D0 | 9 | 40 (22.5%) |
| D3 | 49 | 24 | D3 | 20 | 22 (90,9%) | D3 | 16 | 23 (69.5) |
| D7 | 45 | 130 | D7 | 37 | 46 (80,4%) | D7 | 17 | 26 (65.3%) |
| D21 | 46 | 51 | D21 | 13 | 27 (48,1%) | D21 | 18 | 35 (51.4%) |
| Total | 241 | 237 | Total | 93 | 155 | Total | 60 | 124 |
FOXO1 analysis
FOXO1 staining was detected in both oocytes and GCs of primordial and growing follicles (Fig. 3a–e). Cytoplasmic FOXO1 was observed in oocytes of growing follicles at all timepoints after grafting (Fig. 3e). Consistent with the aim of this study, namely elucidating the dynamics of early follicle activation in resting follicles, we focused on localizing FOXO1 in oocytes of primordial follicles. A total of 155 primordial follicles were counted (Table 1). They were considered activated when FOXO1 was identified in the cytoplasm (Fig. 3b–d), and results are reported as the percentage of primordial follicles with cytoplasmic FOXO1. The paired t test was used for verification. Although there was an increase in primordial follicles expressing FOXO1 in their cytoplasm at all timepoints after grafting, it was only significant on day 3 (80%, p = 0.01) compared with non-grafted controls (14.6%) (Fig. 3f).
Fig. 3.
FOXO1 immunostaining in a primordial follicle in non-grafted ovarian tissue (a) and in ovarian tissue grafted for 3 (b), 7 (c) and 21 (d) days. Follicles were considered activated when FOXO1 was identified in the cytoplasm. Cytoplasmic staining in the oocyte is visible at all time-points after grafting (arrow). FOXO1 immunostaining in a growing (primary) follicle in non-grafted ovarian tissue (e). f FOXO1 staining in primordial follicles in non-grafted ovarian tissue (D0), and on D3, D7 and D21 post-grafting (mean + SEM), p = 0.01, scale bar: 10 μm
YAP analysis
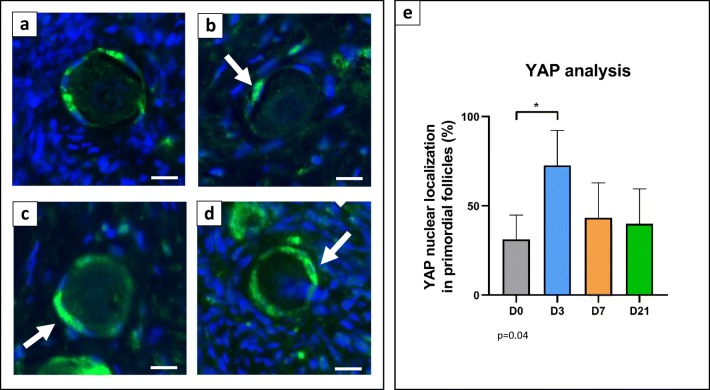
YAP staining was detected in GCs of primordial and growing follicles (Fig. 4a–d). In this case too, we focused on localizing YAP in either the nucleus or cytoplasm of GCs of primordial follicles. A total of 124 primordial follicles were counted (Table 1). They were considered activated when YAP was identified in the nucleus of at least one GC. The paired t test was used for statistical analysis. An increase in primordial follicles expressing YAP in the nucleus was detected after 3 days of grafting (43.8%, p = 0.04) compared with non-grafted controls (26%) (Fig. 4e).
Fig. 4.
YAP immunofluorescent staining in a primordial follicle in non-grafted ovarian tissue (a) and in ovarian tissue grafted for 3 (b), 7 (c) and 21 (d) days. Follicles were considered activated when YAP was identified in the nucleus of at least one GC. Nuclear staining in GCs is visible at all time-points after grafting (arrow). e YAP staining in primordial follicles in non-grafted ovarian tissue (D0), and on D3, D7 and D21 post-grafting (mean + SEM), p = 0.04, bar scale: 10 μm
Discussion
While a number of studies have addressed the molecular mechanisms involved in primordial follicle activation in rodents [9–16], knowledge with respect to human ovarian tissue remains limited. Our objective was to describe the phenomenon of follicle activation after transplantation of human ovarian tissue and the role that signaling pathways play.
In a previous study [7], we demonstrated a significant increase in growing follicles, leading to a significant decrease in the primordial follicle population 3, 7 and 21 days post-transplantation. Significant follicle death was also encountered after grafting, with around half the follicle population lost after 3 days and a further 50% in the subsequent 4 days [7]. This indicated that the decline in the primordial follicle pool is due to two events, follicle activation and growth on the one hand, and follicle death on the other, both concurring and contributing to the burnout effect. In that study, we also found enhanced Akt phosphorylation in human primordial follicles 3 days after ovarian tissue transplantation, and can therefore infer that this could be a direct consequence of the hypoxic window that characterizes the immediate post-grafting period [3, 4, 23–26].
In the current study, we were aiming at further elucidate the mechanisms involved in follicle activation and determine whether the effectors of the PI3K (mTOR and FOXO1) and Hippo (YAP) pathways are somehow altered after grafting, which is why we decided to investigate these three specific effectors. mTOR is a conserved serine/threonine kinase, negatively regulated by tuberous sclerosis complex 1 and tuberous sclerosis complex 2, and suppression of mTOR activity appears to be a prerequisite for maintaining dormancy of primordial follicles [12–14, 16]. FOXO is a downstream effector of the Akt pathway and acts as a transcriptional factor in the nucleus of primordial follicles, where it suppresses follicle growth. Upon phosphorylation, it is translocated to the cytoplasm and relieved of its inhibitory role, giving rise to follicle activation [9, 11, 27].
In this study, we did not observe any upturn in mTOR phosphorylation levels in primordial follicles after grafting, but detected significantly higher levels in growing follicles in non-grafted tissue and after 3 days of grafting. This may be due to its physiological role, since mTOR plays a fundamental part in regulating cell growth and metabolism in response to growth factors and nutrients [28, 29], so increased activity of this factor was expected in growing follicles. Our hypothesis is that the lack of change seen in levels of mTOR phosphorylation in primordial follicles after grafting could be the reason of mTOR phosphorylation being a late event in the signaling cascade, occurring when follicles have already started to grow and need to promote further growth. We therefore hypothesize that mTOR phosphorylation is not a consequence of transplantation-induced follicle activation, but a physiological event that happens when follicles start to develop to more advanced stages. Concerning FOXO1, an increase was observed in the cytoplasm of primordial follicles after grafting. This translocation of FOXO1 from the nucleus to the cytoplasm can be directly attributed to Akt phosphorylation [10], and this latter result confirms that transplantation of human ovarian tissue may promote follicle activation via the Akt-FOXO pathway.
PI3K pathway manipulation was recently attempted in an effort to improve primordial follicle activation and growth to more advanced stages [15, 14, 18, 30, 31], with three live births reported in the literature [31, 32]. However, recent data from our laboratory demonstrate that there is no difference in terms of follicle density or follicle growth after grafting human ovarian tissue with or without prior in vitro culture with Akt stimulators [33]. These findings corroborate our results and indicate that the procedure of transplantation itself is enough to cause significant follicle activation. The lifespan of ovarian tissue grafts is related to the ovarian reserve present in the graft itself, so we believe that future studies should focus on finding ways to preserve the primordial follicle population by preventing its activation and death. Efforts should concentrate on improving the early post-transplantation period by enhancing oxygenation and promoting revascularization. Indeed, favorable results were recently obtained by Manavella et al [3, 4] after preparing the transplantation site with adipose tissue-derived stem cells prior to grafting.
We also decided to evaluate a key component of the Hippo pathway. This pathway is fundamental to maintaining optimal organ size [34, 35] and includes a number of negative growth regulators acting in a kinase cascade that eventually phosphorylates and inactivates the key Hippo signaling effectors YAP/TAZ. When Hippo signaling is impaired, upon ovarian fragmentation for example, nuclear levels of YAP increase as a consequence of decreases in YAP phosphorylation, ultimately leading to stimulation of cell growth, survival, and proliferation [34, 36, 37]. We detected elevated expression of YAP in the nucleus of primordial follicles after 3 days of grafting, confirming that the Hippo pathway is involved in follicle activation.
The present study adds relevant new information to the field of follicle activation and burnout. By comparing the effectors of the PI3K and Hippo pathways, we evidenced a probable synchrony in Akt/FOXO1 and YAP responses to transplantation, as levels of both rose significantly after 3 days of grafting. Our findings may indeed suggest a link between the PI3K and Hippo pathways, with both contributing to the phenomenon of follicle burnout [38].
Nevertheless, the study has some limitations. First, we opted to use immunostaining in order to distinguish changes in phosphorylation levels of mTOR and the shifting localization of FOXO1 and YAP, as it is known that the function of both these proteins depends on their site. Determining their cellular localization gives us an indication of the state of activation of follicles. Additional techniques and analyses, such as western blotting, should now be applied to corroborate our results. Moreover, it is known that mTOR is involved in two different complexes (mTORC1 and mTORC2), with mTORC1 implicated in follicle activation through the PI3K/Akt signaling pathway and mTORC2 acting at the upper level of the Akt pathway and also possessing different cellular functions [39]. A second limitation is that analysis of mTOR alone might not be sufficient to identify which complex is involved, so other effectors of the pathway, such as S6K1, RPS6 and 4EBP1, should be investigated in the future.
In conclusion, this study supports the hypothesis that activation of human primordial follicles and growth to later developmental stages is an early event after transplantation, and that significant follicle death contributes to burnout too, eventually culminating in early depletion of the ovarian reserve. We also corroborate the premise that Akt plays a central role in follicle activation in human ovarian tissue and is potentially involved in both follicle growth and death. Its effect on follicle growth is explained by FOXO1 translocation. Transplantation of ovarian tissue has no direct impact on mTOR, which may itself participate in further promoting follicle growth. Finally, our results clearly demonstrate that the Hippo pathway also has a role in transplantation-induced follicle activation and could contribute to the phenomenon of burnout. Indeed, activation of both the PI3K and Hippo pathways could trigger parallel survival and cell growth signaling pathways, ultimately resulting in highly significant follicle depletion. This is the first time that the effectors of the PI3K and Hippo pathways have been analyzed and compared in grafted human ovarian tissue, and their role in follicle burnout elucidated and documented.
Acknowledgments
The authors thank Mira Hryniuk, B.A., for reviewing the English language of the article, and Dolores Gonzalez and Olivier Van Kerk for their technical assistance.
Author contribution statement
R.M.: conception and design of the study, experimental procedures, analysis of results, statistical analysis and article preparation; C.H.: experimental procedures; M.C.C.: analysis of results and discussion contribution; D.D.M.: experimental procedures, analysis of results and discussion contribution; C.A.A.: discussion contribution; J.D.: data evaluation, discussion contribution and article revision; M.M.D.: conception of the study, data evaluation, discussion contribution and article revision.
Funding information
This study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (FNRS-PDR Convention T.0077.14, Télévie grant 7.4590.16 awarded to Rossella Masciangelo, EOS grant 30443682 to Maria Costanza Chiti, Télévie grant no. 7.6515.16 F to Diego Daniel Manavella and 5/4/150/5 grant to Marie-Madeleine Dolmans; CAA is an FSR-FNRS research associate), the Fonds Speciaux de Recherche, and the Foundation Against Cancer.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377:1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 3.Manavella DD, Cacciottola L, Desmet CM, Jordan BF, Donnez J, Amorim CA, Dolmans J. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: a potential way to improve ovarian tissue transplantation. Hum Reprod. 2018;33:270–279. doi: 10.1093/humrep/dex374. [DOI] [PubMed] [Google Scholar]
- 4.Manavella DD, Cacciottola L, Pomme S, Desmet CM, Jordan BF, Donnez J, Amorim CA, Dolmans MM. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum Reprod. 2018;33:1107–1116. doi: 10.1093/humrep/dey080. [DOI] [PubMed] [Google Scholar]
- 5.Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, Camboni A, Van Langendonckt A, Donnez J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–262. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- 6.Gavish Z, Spector I, Peer G, Schlatt S, Wistuba J, Roness H, Meirow D. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J Assist Reprod Genet. 2018;35:61–69. doi: 10.1007/s10815-017-1079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masciangelo R, Hossay C, Donnez J, Dolmans MM. Does the Akt pathway play a role in follicle activation after grafting of human ovarian tissue? Reprod BioMed Online. 2019;39(2):196–198. doi: 10.1016/j.rbmo.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 10.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 12.Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari D, Gorre N, Risal S, Zhao Z, Zhang H, Shen Y, Liu K. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS One. 2012;7:e39034. doi: 10.1371/journal.pone.0039034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21:779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 20.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 21.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, Donnez J, Van Langendonckt A. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–1298. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62:693–697. doi: 10.1369/0022155414545224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouzin C, Saini ML, Khaing KK, Ambroise J, Marbaix E, Gregoire V, Bol V. Digital pathology: elementary, rapid and reliable automated image analysis. Histopathology. 2016;68:888–896. doi: 10.1111/his.12867. [DOI] [PubMed] [Google Scholar]
- 24.Courtoy Guillaume E., Donnez Jacques, Marbaix Etienne, Barreira Matilde, Luyckx Mathieu, Dolmans Marie-Madeleine. Progesterone Receptor Isoforms, Nuclear Corepressor-1 and Steroid Receptor Coactivator-1 and B-Cell Lymphoma 2 and Akt and Akt Phosphorylation Status in Uterine Myomas after Ulipristal Acetate Treatment: A Systematic Immunohistochemical Evaluation. Gynecologic and Obstetric Investigation. 2017;83(5):443–454. doi: 10.1159/000480011. [DOI] [PubMed] [Google Scholar]
- 25.Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, Dolmans MM. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Ting AY, Zelinski MB. Characterization of FOXO1, 3 and 4 trascription factors in ovaruies of fetal, prepubertal and adult rhesus macaques. Biol Reprod. 2017;96:1052–1059. doi: 10.1093/biolre/iox034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante M., Sabatini D. M. mTOR Signaling. Cold Spring Harbor Perspectives in Biology. 2011;4(2):a011593–a011593. doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novella-Maestre E, Herraiz S, Rodriguez-Iglesias B, Diaz-Garcia C, Pellicer A. Short-term PTEN inhibition improves in vitro activation of primordial follicles, preserves follicular viability, and restores amh levels in cryopreserved ovarian tissue from cancer patients. PLoS One. 2015;10:e0127786. doi: 10.1371/journal.pone.0127786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 32.Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, Hu L, Zhang Y, Wang J, Dai S, Li J, Sun J, Hsueh AJ, Kawamura K, Sun Y. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101:4405–4412. doi: 10.1210/jc.2016-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolmans MM, Cordier F, Amorim CA, Donnez J, Vander LC. In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? Front Endocrinol (Lausanne) 2019;10:520. doi: 10.3389/fendo.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 35.Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40:124–128. doi: 10.1042/BST20110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013;12:3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]