Abstract
To investigate the potential applications and the molecular mechanisms of transcranial direct current stimulation (tDCS) on cognitive impairment in a vascular dementia (VD) animal model. Sprague-Dawley rats were used in this study. VD rat model was induced by modified permanent bilateral common carotid artery occlusion (2-VO) approach. Anodal tDCS was applied to the animals. Morris water maze was used to analyze spatial memory and navigation ability. The pathological changes in the hippocampal CA1 region and cerebral cortex were examined via Hematoxylin-Eosin staining. The rats were sacrificed for the measurement of the level of superoxide (SOD), glutathione (GSH), reactive oxidative species (ROS), malondialdehyd (MDA), Interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α level in the hippocampus. Western blot was carried out to measure the hippocampal expression of microtubule-associated protein 1 light chain 3 (LC-3) and p62. Rats with VD have decreased number of neurons in the hippocampus and cerebral cortex, as well as worse cognitive impairment. The proliferation of activated microglia and astroglia, accompanied with attenuation of myelination were observed in the white matter about 1 month after 2-VO operation. These abnormalities were significantly ameliorated by tDCS treatment. Further study revealed that anodal tDCS could suppress the MDA and ROS level, while enhance the SOD and GSH level to reduce the oxidative stress. Anodal tDCS could inhibit hypoperfusion-induced IL-1β, IL-6, and TNF-α expression to attenuate inflammatory response in hippocampus. Moreover, anodal tDCS treatment could alleviate autophagy level. The study has demonstrated a possible therapeutic role of tDCS in the treatment of cognitive impairment in VD.
Keywords: vascular dementia, tDCS, oxidative stress, inflammation, autophagy
Introduction
Vascular dementia (VD) is the second most common cause of dementia after Alzheimer’s disease, accounting for around 15% of cases (O’Brien and Thomas, 2015). Rates of VD rise with age. There have been no effective approved pharmacological treatments available for VD up till now. Chronic cerebral hypoperfusion played a causative role in VD (Du et al., 2017). Previous studies demonstrated that cerebral hypoperfusion could lead to oxidative stress, neuroinflammation, neurotransmitter system dysfunction, mitochondrial dysfunction, disturbance of lipid metabolism, and alterations of growth factor (Du et al., 2017). Oxidative stress plays an important role in cognitive deficits induced by the chronic cerebral hypoperfusion (Chunjiea et al., 2016). Neuroinflammation characterized by Interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α plays an important role in VD (Belkhelfa et al., 2018). Autophagy, a lysosome-mediated catabolic pathway, contributes to the maintenance of cellular homeostasis (Moloudizargari et al., 2017). Decreasing autophagic activity may contribute to cognitive improvement in rats with VD (Liu et al., 2018; Venkat et al., 2018).
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulation technique that has been used to modulate brain function (Paulus, 2011). Anodal tDCS could induce long-lasting alterations of cortical excitability and enhance cerebral plasticity, both in experimental animals and humans (Datta et al., 2010). Anodal tDCS could affect synaptic plasticity, modulate the level of oxidative stress, neuroinflammation, and autophagy (Scelzo et al., 2011; Laste et al., 2012; Lu et al., 2015; Lee et al., 2018). Indeed, anodal tDCS is a promising approach for brain diseases associated with impaired neuroplasticity, which is simple to be used and is beneficial for brain function (Podda et al., 2016). It’s speculated that anodal tDCS might exert similar roles in alleviate cognitive impairment in VD. Anodal tDCS has been proven to be beneficial for cognitive function in patients with VD (André et al., 2016). However, the studies of tDCS on VD animal model are limited.
In the current study, we have tried to investigate the possible roles and mechanisms of tDCS on improvement of cognitive impairment of VD rat model. The study could provide some evidences for using anodal tDCS as a potential non-pharmacological treatment for VD.
Materials and Methods
Animal Model
Animal use protocols were approved by the Institutional Animal Care and Use Committee of Central South University in compliance with National Institutes of Health guidelines. Sixty male Sprague-Dawley rats, weighting 250–280 g (6–8 weeks old), were provided by department of laboratory animals in Central South University. The rats were maintained under controlled temperature and humidity (22 ± 3°C and 50%, respectively) with a 12 h light–dark cycle. All efforts were made to limit the number of rats used and to minimize animal suffering. All food and water were provided ad libitum throughout the trial. To generate a rat model of VD, permanent bilateral common carotid artery occlusion (2-VO) approach was applied as previously reported (Zhu et al., 2011). Briefly, rats were anesthetized with chloral hydrate; a neck ventral midline incision was made. The common carotid arteries were exposed and then gently separated from the vagus nerve. Carotids were occluded with a 1-week interval between interventions, the right common carotid being the first to be processed and the left one being occluded 1 week later (Cechetti et al., 2010; Mirzapour et al., 2015). The sham operated rats underwent the same procedures without carotid artery ligation. After surgery, rats were left to recover for a period of 1 week. Sixty SD rats were randomly divided into three groups: (1) Sham group: Sham operation group treated with sham stimulation, (2) VD group: VD rat models treated with sham stimulation, (3) tDCS group: VD rat models treated with anodal tDCS. Figure 1 summarized the temporal evolution of the study protocols. Rats that exhibited abnormal behavioral effects during the study, such as seizures, were excluded to avoid any potential impact on the final results.
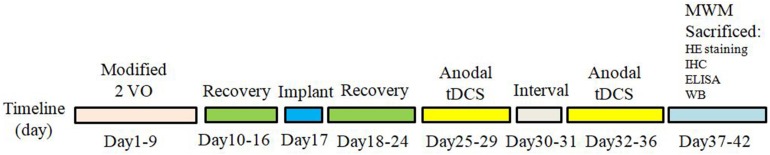
FIGURE 1.
The time schedule of protocols in the present study.
tDCS Treatment
One week after surgery, rats were placed in a stereotactic frame after anethetized with chloral hydrate (350 mg/kg). A sagittal incision was made in the scalp. A scalp and the underlying tissues were removed, then the skull was dried with cotton swabs. A custom-made polycarbonate tubes with the inner diameter of 1 mm and the contact area on the skull of 3.14 mm2 were stereotactically placed on the sagittal suture with the center of the electrode resting on 2.5 mm posterior to bregma, then the tubes were subsequently attached to the bone surface with a thin layer of non-toxic dental cement (super-bond C&B, Sun Medical, Japan) and a second layer of two-component luting resin (Ketac Cem Plus, 3MESPE AG, Germany) (Pikhovych et al., 2016; Yu et al., 2019). To ensure current flow during stimulation, the hollow implant was kept free of cement. After surgery, the rats were transferred back to their home cages and were allowed to recover for 1 week before undergoing tDCS. After at least 1 week of post-surgical recovery, rats were randomized into two groups receiving anodal tDCS or sham stimulation, respectively. All procedures of tDCS and sham stimulation had identical duration of current fade-in (10 s), fade-out (10 s) and current strength (200 μA), except the stimulation duration (tDCS 30 min, sham 10 s) (Yang et al., 2019; Yu et al., 2019). Anodal tDCS was repeated daily for 5 consecutive days, followed by a 2-day pause, then subjected to another set of 5 stimulation days, resulting in a total of 10 days of tDCS stimulation (Rueger et al., 2012; Pikhovych et al., 2016; Yang et al., 2019). The stimulation was conducted by the same researcher at the same time every day. The anodal electrode was inserted into the polycarbonate tube, which was filled with saturated saline. To avoid debris accumulating in the polycarbonate tube, a cotton ball was placed to seal the tube when not in use (Pikhovych et al., 2016). The cathode electrode was a conventional rubber-plate electrode wrapped by a wet cotton sheath (11 cm2) applied over the ventral thorax of the rat by an elastic bandage (Podda et al., 2016). Both anodal and cathodal electrodes were connected to a direct current stimulator (Ruihaikanglian, Jiangsu, China) for electric current stimulation.
Morris Water Maze Task
The cognitive function was analyzed by the Morris water maze (MWM) test 24 h after the last tDCS stimulation according to previously described protocols (Guo et al., 2017; Schoenfeld et al., 2017). Briefly, a circular water tank with 150 cm diameter and 50 cm depth was filled with 25°C water to a depth of 21 cm. A circular platform (diameter: 10 cm; height: 20 cm) was located in the center of the target quadrant. The navigation trials were conducted for 5 consecutive days. The time taken for the rats to find the platform was recorded as the escape latency. When the rats reached the platform within 90 s, they remained on the platform for 20 s. If the rats failed to find the platform within 90 s, they were gently guided to the platform and left on it for 20 s. The escape latency was recorded as 90 s in such cases. One day after the navigation trial, the platform was removed for a probe trial. The time spent in the target quandrant and the numbers of swimming across the platform site for up to 90 s were recorded using a computer-based image analyzer automatically.
Tissue Preparation
The brain tissues from rats were collected after completion of the tDCS treatment. All rats were deeply anesthetized using 10% chloral hydrate (400 mg/kg). A subset of rats were perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS). The brains of these rats were removed and post-fixed in the same fixative at 4°C overnight. Then they were immersed consecutively in 20 and 30% sucrose at 4°C until they sank. The remaining post-fixed brains from 2.15 to 5.76 mm behind bregma were embedded in paraffin and then cut into 10 μm thick coronal sections. Hematoxylin and eosin (HE) staining were used to observe any histological changes. Fresh brain tissues were quickly taken and then was fixed in 4% paraformaldehyde for immunocytochemistry or stored at −80°C for enzyme-linked immunoabsorbent assay (ELISA) and western blot.
HE Staining
Hematoxylin and eosin staining was used to evaluate the morphological changes of the hippocampal region CA1 and cerebral cortex. Sections were then sequentially immersed in hematoxylin for 10 min and in eosin for 1 min (Fan et al., 2018). Four random visual fields from each brain slice were analyzed. Morphological changes of neurons in the hippocampus and cerebral cortex were observed under a microscope at 100 and 400× magnification respectively. The number of surviving neurons in the hippocampal CA1 area and cerebral cortex was quantified under a light microscope at 400× magnification through Image-Pro Plus software.
Immunohistochemistry
The coronal sections were incubated overnight with anti-glial fibrillary acidic protein (GFAP) antibody, anti-ionized calcium binding adaptor molecule 1 (Iba1) antibody, and anti-myelin basic protein (MBP) antibody. After they were washed, the sections were treated with appropriate biotinylated secondary antibodies. To stain cell nuclei, the sections were incubated with 4′-6-diamidino-2-phenylindole (DAPI) in PBST for 30 min. These sections were visualized by the diaminobenzidine tetrahydrochloride (DAB) and H2O2. The white matter lesions, astroglia and microglia activations were analyzed in corpus callosum and internal capsule. Quantitative analysis of GFAP, Iba1 or MBP-positive cells present in the sections was carried out under 400 × microscopic magnification by Image-pro Plus software respectively. At least three random microscopic fields from each section (three sections per rat) were calculated. The results were presented in the tiled images. All counts were performed in a blinded fashion.
Measurement of ROS
The intracellular levels of reactive oxidative species (ROS) were assessed by the probe of DCFH-DA. The hippocampus was separated, rinsed twice with PBS, made into cell suspensions, then incubated in trypsin at 37°C for 25 min. Add ice-cold PBS to stop the reaction. Cells were collected and centrifugated. The sediment was incubated with DCFH-DA containing PBS at 37°C for 30 min. The fluorescene-oxidized deprivation of DCFH-DA was minitored with fluorescene microplate and ROS levels were measured (Jiang et al., 2017).
Enzyme-Linked Immunoabsorbent Assay
The hippocampus was taken out and put into the homogenate tube after being washed by cold normal saline. Appropriate amount of normal saline at 4°C was added into the tube. Homogenized the tissue, centrifugated at 4000 × g for 15 min, then collected the supernatant. The oxidative stress markers in hippocampus were measured: superoxide dismutase (SOD), malonic dialdehyde (MDA), and glutathione (GSH) (Jiang et al., 2017; Al-Amin et al., 2018). The protein levels of interleukin (IL)-1β, IL-6 and TNF-α were evaluated by specific ELISA kits following manufacturer’s instructions (Akang et al., 2019). The commercial kits for examining these parameters were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Western Blot
The expression levels of autophagy markers in the hippocampus were analyzed by Western blot. In brief, hippocampuses were washed with ice-cold PBS and homogenized in ice-cold RIPA lysis buffer. Total protein concentration was quantified by BCA kit. About 20–40 μg protein samples were loaded onto SDS-PAGE gel for electrophoresis separation and were transferred to PVDF membrane. After blocking, the membranes were incubated overnight at 4°C with primary antibodies. The membrane was then developed by ECL substrate, and images were captured by a computerized system. Quantification was performed using Image J software. The following primary antibodies were used: microtubule-associated protein 1 light chain 3 (LC3) and p62. Insoluble p62 cannot be extracted by conventional methods, so soluble p62 was studied in the present study.
Statistical Analysis
All statistical analysis was performed using SPSS 22.0 software. The graphs were prepared by GraphPad prism (version 6.0, GraphPad Software, Inc.). Multiple comparisons were analyzed by one-way ANOVA with post hoc Bonferroni test. All data were presented as mean ± standard deviation (SD). P < 0.05 was considered as statistically significant.
Results
Effects of tDCS on Hypoperfusion-Induced Cognitive Impairment
In this study, 60 male SD rats were used. Two rats died during the 2-VO surgery. The remaining rats completed all the experiments without any abnormal behavioral effects, such as seizures. All rats successfully finished the task with decreasing latencies to reach the platform. The latencies to find the hidden platform and the swimming distances of the VD rats were significantly longer than those of the rats in the sham group (P < 0.05). Furthermore, tDCS induced a significant decrease in escape latencies and swimming distances across 5 days’ MWM training compared with the rats in the VD group (P < 0.05) (Figures 2A,B). In the spatial probe trials, rats in the VD model group made significantly fewer crossings of the platform area compared to those in the sham group (P < 0.05), whereas rats in the tDCS group crossed the platform area significantly more times than those in the VD group (P < 0.05) (Figure 2C). Rats in the VD group spent less time in the target quadrant compared with the rats in the sham group, while tDCS improved the reduction (P < 0.05) (Figure 2D). The typical swimming paths recorded in the spatial probe trial of each group were displayed in Figure 2E.
FIGURE 2.
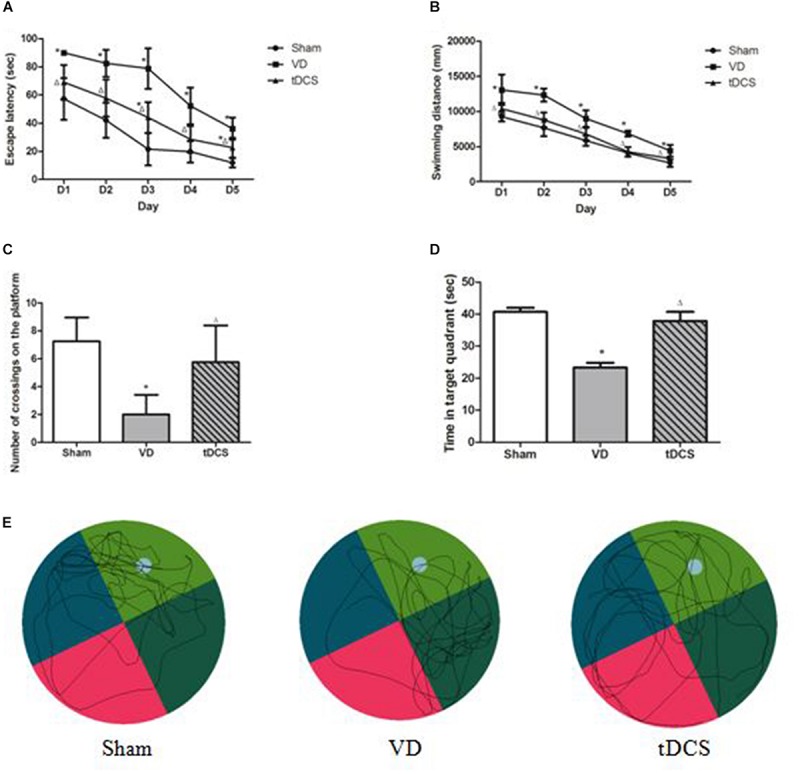
Transcranial direct current stimulation (tDCS) attenuated learning and memory deficits induced by chronic hypoperfusion. (A) Escape latency. (B) Swimming distance. (C) Number of crossings on the platform. (D) The time spent in the target quadrant in the spatial probe trials. (E) Typical swimming traces of all groups in the spatial probe trials (compared to Sham group, *P < 0.05; compared to VD group, △P < 0.05).
Effects of tDCS on Neurons in Hippocampus and Cerebral Cortex
The representative photomicrographs of neurons in hippocampal CA1 region and cerebral cortex were shown in Figure 3. Neurons exhibited regular and compact arrangement in the hippocampal CA1 region and cerebral cortex of sham group rats, with the cytoplasm stained and well-distributed. In the VD group, neuron loss, shrinkage and loose arrangement were observed in the hippocampal CA1 region and cerebral cortex. While in the tDCS group, the neurons demonstrated a nearly normal appearance in the hippocampal CA1 region and cerebral cortex, comparable to those in the sham group. The number of live neurons significantly decreased in the hippocampal CA1 region and cerebral cortex of the VD group than the sham group, while a higher count of live neurons was found in the hippocampal CA1 region and cerebral cortex of tDCS group than the VD group (P < 0.05) (Figure 3).
FIGURE 3.
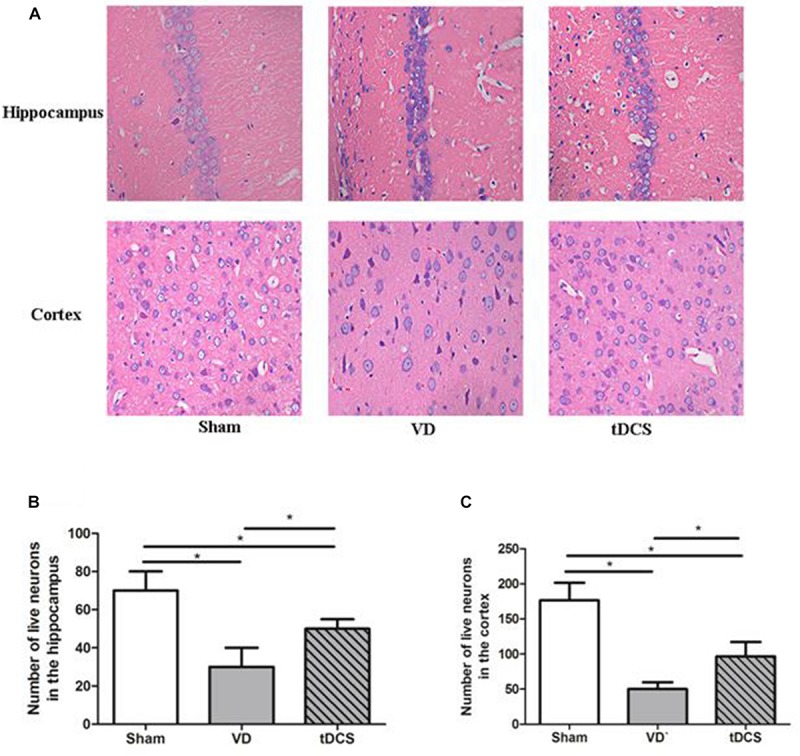
(A) Morphological changes in the hippocampal CA1 area and cerebral cortex of rats in each group (HE staining) (×400); Quantification analysis of the numbers of live neurons in the hippocampus (B) and the cortex (C) of rats from each group (*P < 0.05).
Effects of tDCS on Demyelination and Glial Activation
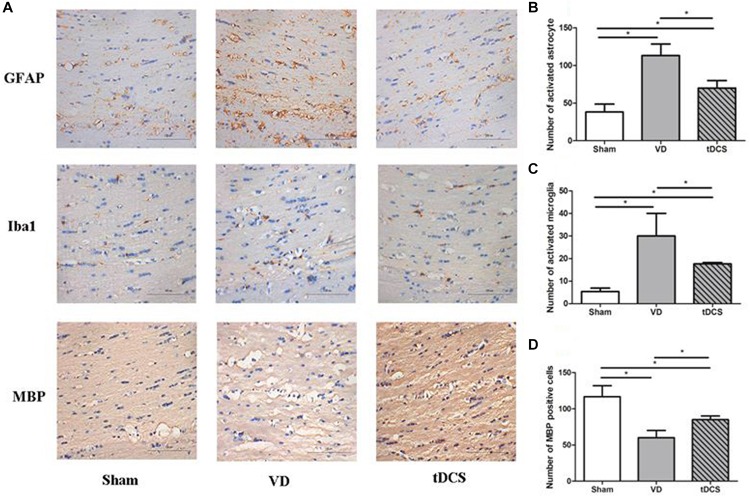
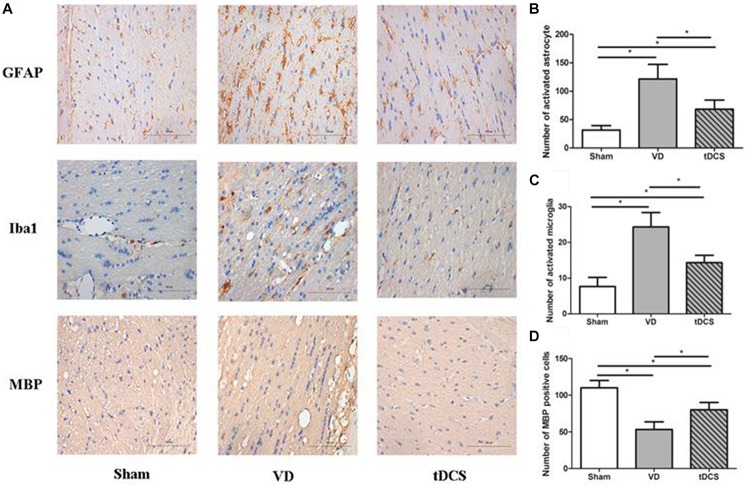
In the corpus callosum and internal capsule, a significant increase in microglia and astroglia was observed in rats in the VD group compared with rats in the sham group, accompanied by a greater loss of white matter myelin in rats in the VD group. Anodal tDCS attenuated demyelination and glial activation in rats with VD (Figures 4, 5).
FIGURE 4.
(A) Photomicrographs of immunohistochemistry staining for GFAP, Iba1, and MBP in the corpus callosum of rats in each group (×400), scale bar: 100 μm. Quantification analysis of the number of GFAP positive (B), Iba1 positive (C), and MBP (D) positive neurons in the corpus callosum of rats from each group (n = 6 per group, three sections per rat) (*P < 0.05).
FIGURE 5.
(A) Photomicrographs of immunohistochemistry staining for GFAP, Iba1, and MBP in the internal capsule of rats in each group (×400), scale bar: 100 μm. Quantification analysis of the number of GFAP positive (B), Iba1 positive (C), and MBP positive (D) neurons in the internal capsule of rats from each group (n = 6 per group, three sections per rat) (*P < 0.05).
Effects of tDCS on Oxidative Stress Level
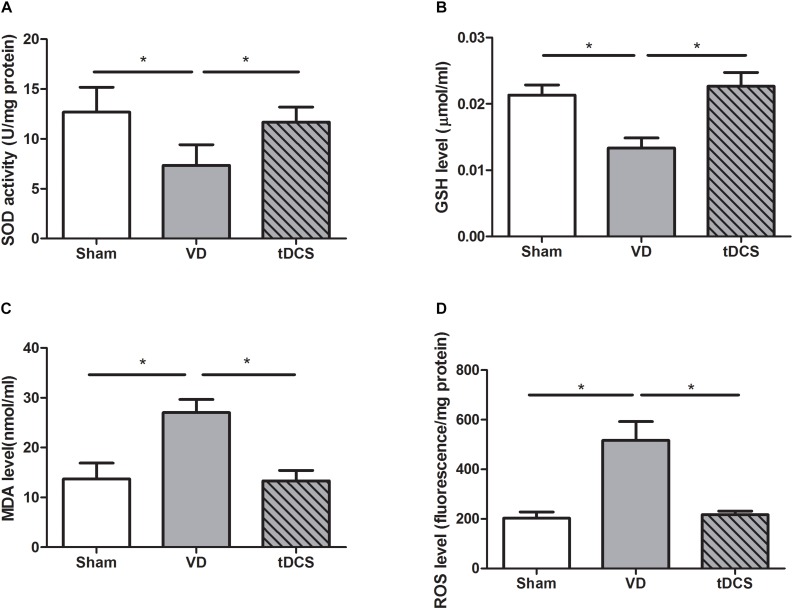
By using commercial kits, we found that SOD (P < 0.05) and GSH (P < 0.05) reduced significantly, while the levels of ROS (P < 0.05) and MDA (P < 0.05) increased significantly in the hippocampus of rats with VD. Treatment with tDCS significantly increased the SOD and GSH content. After tDCS treatment, the level of ROS and MDA were suppressed. Thus, tDCS could effectively inhibit the oxidative stress in rats with VD (Figure 6).
FIGURE 6.
Effects of tDCS on the levels of SOD (A), GSH (B), MDA (C), and ROS (D) in the hippocampus of rats from each group (*P < 0.05).
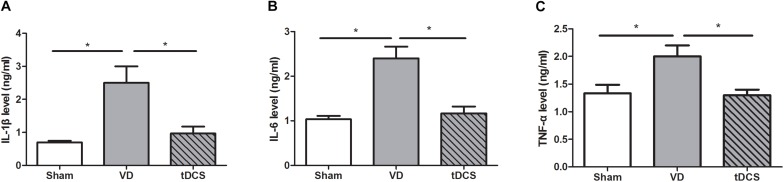
Effects of tDCS on Inflammatory Parameters
By ELISA quantification, we found that the VD rats had remarkably increased levels of inflammatory factors such as IL-1β, IL-6, and TNF-α (P < 0.05). Anodal tDCS significantly alleviated hippocampal protein levels of IL-1β, IL-6, and TNF-α (P < 0.05) in the rat model of VD (Figure 7).
FIGURE 7.
Effects of tDCS on the levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the hippocampus of rats from each group (*P < 0.05).
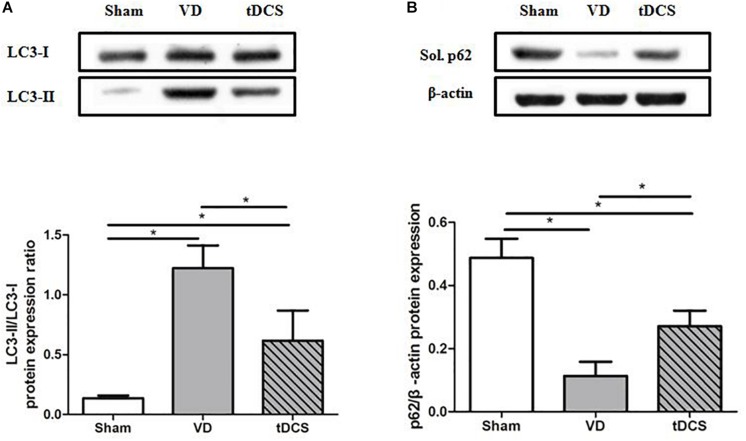
Effects of tDCS on Autophagy Level
To determine the effects of tDCS on autophagy, the ratio of LC3-II/LC3-I and the protein expression level of soluble p62 in the hippocampus were analyzed. The ratio of LC3-II/LC3-I was significantly greater in the VD group than that in the sham group (P < 0.05). In addition, the expression level of the soluble p62 protein was markedly decreased in the VD group compared with that in the sham group (P < 0.05). Anodal tDCS attenuated these changes (Figure 8).
FIGURE 8.
Effects of tDCS on the levels of LC3-II/LC3-I (A) and soluble p62 (B) in the hippocampus of rats from each group (*P < 0.05).
Discussion
Anodal tDCS has promising potential therapeutic effects for symptoms associated with dementia (Elder and Taylor, 2014). Anodal tDCS exerts positive effects on cognition and brain functions in mild cognitive impairment and major dementias, including Alzheimer’s disease, Pakinson’s disease, however, available studies on VD were limited (Meinzer et al., 2015; Adenzato et al., 2019; Gomes et al., 2019).
Chronic cerebral hypoperfusion is closely related to progressive cognitive impairment in rats (Lin et al., 2011). The most widely used experimental model of VD and chronic cerebral hypoperfusion is 2-VO rats (Du et al., 2017). It’s reported that the modified 2-VO protocol may be more applicable, with similar cognitive impairment and lower mortality rates, compared to the standard 2-VO procedure (Cechetti et al., 2010). The present study established a rat model to reproduce chronic cerebral hypoperfusion by modified 2-VO. Cerebrovascular white matter lesions are caused by chronic cerebral hypoperfusion in VD. The neuropathological changes in VD rat models were characterized by diffuse demyelination and gliosis in the white matter, accompanied with neurodegeneration in the hippocampus and cerebral cortex, which were in accordance with previous reports (Shibata et al., 2004). Hippocampus was sensitive to ischemia, especially the hippocampal CA1 region (Ohtaki et al., 2003).
Brain stimulation techniques can attenuate cognitive impairment in many neuropsychiatric diseases (Chang et al., 2018). The anodal tDCS has been conducted in healthy subjects, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, depression, and attention disorders, etc. (Lefaucheur, 2016; Chang et al., 2018; Charvet et al., 2018; Manenti et al., 2018). However, studies investigating the effect of tDCS on VD were limited. It’s reported that anodal tDCS of the left dorsolateral prefrontal cortex could improve visual short-term memory in patients with VD (André et al., 2016). The present study demonstrated the beneficial effects of anodal tDCS on the cognitive deficit in a VD rat model. Our study is new in the field of tDCS treatment by demonstrating its anti-autophagy, anti-inflammatory, and anti-oxidant effects in VD.
Autophagy is a self-degradative process that is important for balancing sources of energy. It involves lysosomal-dependent recycling, synthesis, and degradation of intracellular components, thus maintaining the stability of the internal environment. It’s reported that decreasing activity of autophagy may contribute to cognitive improvement in rats with VD (Venkat et al., 2018; Wang et al., 2018). Inhibition of autophagy was beneficial to the hippocampal synaptic plasticity of the VD rat model (Bin et al., 2019). In the present study, the autophagy-lysosomal pathway was activated in the hippocampus of rats with VD. Anodal tDCS could restore the excessive activation of autophagy and help to partly recover the lost learning and memory in VD rats. It’s reported that BDNF expression was enhanced by anodal tDCS, and BDNF could modulate autophagy through the PI3K/Akt/mTOR pathway (Chen et al., 2013). At present, role of autophagy activation in ischemic stroke remain controversial. Autophagy is a double-edged sword. Excessive autophagy can induce autophagic cell death. On the other hand, moderate activation may play a protective role in cell damage.
Increased production of ROS or decreased capacity to clear them could result in oxidative stress. Our data revealed a decrease in the activities of antioxidant enzyme (SOD and GSH) in VD rats, which was attenuated by tDCS. The level of MDA and ROS was reduced by tDCS. In this study, anodal tDCS suppressed oxidative stress induced by chronic cerebral hypoperfusion and protected the hippocampal neurons from further damage induced by overload of ROS. It’s reported that tDCS could reduce oxidative stress in a mouse model of Parkinson’s disease as well (Chengbiao et al., 2015). However, how the altered oxidative status caused by tDCS remained to be determined in the future study.
Neuroinflammation plays an important role in VD. Proinflammatory cytokines IL-1β, IL-6, and TNF-α independently resulted in cognitive impairment (Dugan et al., 2009; Kitazawa et al., 2011; Belarbi et al., 2012). Our data demonstrated that anodal tDCS could significantly restore hippocampus-dependent cognitive deficit induced by neuroinflammation. Previous studies have revealed a decrease in IL-1β and TNF-α after a treatment with tDCS (Leffa et al., 2018; Oliveira et al., 2019). However, how tDCS modulate neuroinflammatory pathways is still not completely understood.
There were some limitations in the present study. First, rats were sacrificed immediately after the last tDCS stimulation. Longer time interval and different time points should be adopted to evaluate the persistence of the effects of tDCS. Second, VD is reported clinically in both males and females. Only male rats were used in the study, therefore a further study is needed to include both male and female rats. What’s more, 2-VO rat model is a VD rat model induced by chronic cerebral hypoperfusion. Rats performed MWM test only about 1 month after 2-VO surgery in the study. Longer time intervals should be adopted to reveal whether the improvements were permanent or not, such as 2 or 3 months after 2-VO surgery.
In summary, our data confirmed that anodal tDCS exerted a neuroprotective effect in the 2-VO rat model. The mechanisms by which anodal tDCS exerted its neuroprotective effects likely involved the modulation of oxidative stress, neuroinflammation, and autophagy. The present study suggested a theoretical basis for the use of anodal tDCS as a potential neuroprotective therapy to improve cognitive impairment of VD.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
All animal procedures were approved by the Ethics Committee of the Central South University. This study was carried out in strict accordance with the Institutional Animal care and Use Committee of Central South University in compliance with NIH guidelines.
Author Contributions
JF conceived and designed the experiments. JF, ZT, and SH performed the experiments. JF, TG, ZT, SH, and YL analyzed the data, contributed data, materials, and analysis tools. JF and TG wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by Natural Science Foundation of Hunan Province (No. 2018JJ3726).
References
- Adenzato M., Manenti R., Enrici I., Gobbi E., Brambilla M., Alberici A., et al. (2019). Transcranial direct current stimulation enhances theory of mind in Parkinson’s disease patients with mild cognitive impairment: a randomized, double-blind, sham-controlled study. Transl. Neurodegener. 8:1. 10.1186/s40035-40018-40141-40039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akang E. N., Dosumu O. O., Olasunmbo O., Afolayana A., Fagoroye D. M., Osiagwu D. D., et al. (2019). Combination antiretroviral therapy (cART)-induced hippocampal disorders: highlights on therapeutic potential of Naringenin and Quercetin. IBRO Rep. 6 137–146. 10.1016/j.ibror.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin M. M., Choudhury M. F. R., Chowdhury A. S., Chowdhury T. R., Jain P., Kazi M., et al. (2018). Pretreatment with risperidone ameliorates systemic LPS-induced oxidative stress in the cortex and hippocampus. Front. Neurosci. 12:384. 10.3389/fnins.2018.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André S., Heinrich S., Kayser F., Menzler K., Kesselring J., Khader P., et al. (2016). At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J. Neurol. Sci. 369 185–190. 10.1016/j.jns.2016.07.065 [DOI] [PubMed] [Google Scholar]
- Belarbi K., Jopson T., Tweedie D., Arellano C., Luo W., Greig N. H., et al. (2012). TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflam. 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhelfa M., Beder N., Mouhoub D., Amri M., Hayet R., Tighilt N., et al. (2018). The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J. Neuroimmunol. 320 48–57. 10.1016/j.jneuroim.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Bin L., Liu J., Jinxia Z., Wenjing M., Shiying L. (2019). Effects of autophagy on synaptic-plasticity-related protein expression in the hippocampus CA1 of a rat model of vascular dementia. Neurosci. Lett. 707:134312. 10.1016/j.neulet.2019.134312 [DOI] [PubMed] [Google Scholar]
- Cechetti F., Worm P. V., Pereira L. O., Siqueira I. R., Netto C. A. (2010). The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz. J. Med. Biol. Res. 43 1178–1183. 10.1590/s0100-879x2010007500124 [DOI] [PubMed] [Google Scholar]
- Chang C.-H., Lane H.-Y., Lin C.-H. (2018). Brain stimulation in Alzheimer’s disease. Front. Psychiatry 9:201. 10.3389/fpsyt.2018.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet L. E., Dobbs B., Shaw M. T., Bikson M., Datta A., Krupp L. B. (2018). Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult. Scler. 24 1760–1769. 10.1177/1352458517732842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Xiong L., Tong Y., Mao M. (2013). Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 8 1011–1016. 10.3892/mmr.2013.1628 [DOI] [PubMed] [Google Scholar]
- Chengbiao L., Yun W., Rui H., Yong W., Kun L., Xiaoli L. (2015). Transcranial direct current stimulation ameliorates behavioral deficits and reduces oxidative stress in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced mouse model of Parkinson’s disease. Neuromodulation 18 442–447. 10.1111/ner.12302 [DOI] [PubMed] [Google Scholar]
- Chunjiea N., Huijuan N., Zhao Y., Jianzhao W., Xiaojian Z. (2016). Betaine reverses the memory impairments in a chronic cerebral hypoperfusion rat model. Neurosci. Lett. 615 9–14. 10.1016/j.neulet.2015.11.019 [DOI] [PubMed] [Google Scholar]
- Datta A., Bikson M., Fregni F. (2010). Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. Neuroimage 52 1268–1278. 10.1016/j.neuroimage.2010.04.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S.-Q., Wang X.-R., Xiao L.-Y., Tu J.-F., Zhu W., He T., et al. (2017). Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol. Neurobiol. 54 3670–3682. 10.1007/s12035-016-9915-1 [DOI] [PubMed] [Google Scholar]
- Dugan L. L., Ali S. S., Shekhtman G., Roberts A. J., Lucero J., Quick K. L., et al. (2009). IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 4:e5518. 10.1371/journal.pone.0005518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G., Taylor J. (2014). Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 6:74. 10.1186/s13195-014-0074-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Du L., Fu Q., Zhou Z., Zhang J., Li G., et al. (2018). Inhibiting the NLRP3 inflammasome with MCC950 ameliorates isoflurane-induced pyroptosis and cognitive impairment in aged mice. Front. Cell. Neurosci. 12:426. 10.3389/fncel.2018.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M., Akiba H., Gomes J., Trevizol A., Lacerda A. D., Dias Á. (2019). Transcranial direct current stimulation (tDCS) in elderly with mild cognitive impairment: a pilot study. Dement. Neuropsychol. 13 187–195. 10.1590/1980-57642018dn13-020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Lou J., Han X., Deng Y., Huang X. (2017). Repetitive transcranial magnetic stimulation ameliorates cognitive impairment by enhancing neurogenesis and suppressing apoptosis in the hippocampus in rats with ischemic stroke. Front. Physiol. 8:559. 10.3389/fphys.2017.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Li B., Chen Y., Gao S. I. (2017). The toxic influence of dibromoacetic acid on the hippocampus and pre-frontal cortex of rat: involvement of neuroinflammation response and oxidative stress. Metab. Brain Dis. 32 2009–2019. 10.1007/s11011-017-0095-0 [DOI] [PubMed] [Google Scholar]
- Kitazawa M., Cheng D., Tsukamoto M., Koike M., Wes P., Vasilevko V., et al. (2011). Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 187 6539–6549. 10.4049/jimmunol.1100620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laste G., Caumo W., Adachi L. N. S., Rozisky J. R., Macedo I. C. D., Filho P. R. M., et al. (2012). After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp. Brain Res. 221 75–83. 10.1007/s00221-012-3149-x [DOI] [PubMed] [Google Scholar]
- Lee S. B., Kim H. T., Yang H. O., Jang W. (2018). Anodal transcranial direct current stimulation prevents methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity by modulating autophagy in an in vivo mouse model of Parkinson’s disease. Sci. Rep. 8:15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J.-P. (2016). A comprehensive database of published tDCS clinical trials (2005—2016). Neurophysiol. Clin. 46 319–398. 10.1016/j.neucli.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Leffa D. T., Bellaver B., Salvi A. A., Oliveira C. D., Caumo W., Grevet E. H., et al. (2018). Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimul. 11 743–751. 10.1016/j.brs.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Lin M. S., Chiu M. J., Wu Y. W., Huang C. C., Chao C. C., Chen Y. H., et al. (2011). Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion and cerebral ischemia. Stroke 42 2850–2854. 10.1161/STROKEAHA.111.613133 [DOI] [PubMed] [Google Scholar]
- Liu B., Gao J. M., Li F., Gong Q. H., Shi J. S. (2018). Gastrodin Attenuates Bilateral Common Carotid Artery Occlusion-Induced Cognitive Deficits Via Regulating Aβ-related proteins and reducing autophagy and apoptosis in rats. Front. Pharmacol. 9:405 10.3389/fphar.2018.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Wei Y., Hu R., Wang Y., Li K., Li X. (2015). Transcranial direct current stimulation ameliorates behavioral deficits and reduces oxidative stress in 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neuromodulation 18 442–446. 10.1111/ner.12302 [DOI] [PubMed] [Google Scholar]
- Manenti R., Cotelli M., Cobelli C., Gobbi E., Brambilla M., Rusich D., et al. (2018). Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: a randomized, placebo-controlled study. Brain Stimul. 11 1251–1262. 10.1016/j.brs.2018.07.046 [DOI] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Phan M., Ulm L., Volk C., Flöel A. (2015). Transcranial direct current stimulation in mild cognitive impairment: behavioral effects and neural mechanisms. Alzheimers Dement. 11 1032–1040. 10.1016/j.jalz.2014.07.159 [DOI] [PubMed] [Google Scholar]
- Mirzapour S., Rafieirad M., Rouhi L. (2015). Hydroalcoholic extract of ferulago angulata improves memory and pain in brain hypoperfusion ischemia in rats. Jundishapur. J. Nat. Pharm. Prod. 10:e17451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloudizargari M., Asghari M., Ghobadi E., Fallah M., Rasouli S., Abdollahi M. (2017). Autophagy, its mechanisms and regulation: implications in neurodegenerative diseases. Ageing Res. Rev. 40 64–74. 10.1016/j.arr.2017.09.005 [DOI] [PubMed] [Google Scholar]
- O’Brien J. T., Thomas A. (2015). Vascular dementia. Lancet 386 1698–1706. 10.1016/S0140-6736(15)00463-8 [DOI] [PubMed] [Google Scholar]
- Ohtaki H., Mori S., Nakamachi T., Dohi K., Yin L., Endo S., et al. (2003). Evaluation of neuronal cell death after a new global ischemia model in infant mice. Acta Neurochir. Suppl. 86 97–100. 10.1007/978-3-7091-0651-8_22 [DOI] [PubMed] [Google Scholar]
- Oliveira C. D., Freitas J. S. D., Macedo I. C., Scarabelot V. L., Ströher R., Santos D. S., et al. (2019). Transcranial direct current stimulation (tDCS) modulates biometric and inflammatory parameters and anxiety-like behavior in obese rats. Neuropeptides 73 1–10. 10.1016/j.npep.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Paulus W. (2011). Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21 602–617. 10.1080/09602011.2011.557292 [DOI] [PubMed] [Google Scholar]
- Pikhovych A., Stolberg N. P., Flitsch L. J., LuiseWalter H., Graf R., Fink G. R., et al. (2016). Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells Int. 2016:2715196. 10.1155/2016/2715196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda M. V., Cocco S., Mastrodonato A., Fusco S., Leone L., Barbati S. A., et al. (2016). Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci. Rep. 6:22180. 10.1038/srep22180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger M. A., Keuters M. H., Walberer M., Braun R., Klein R., Sparing R., et al. (2012). Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One 7:e43776. 10.1371/journal.pone.0043776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scelzo E., Giannicola G., Rosa M., Ciocca M., Ardolino G., Cogiamanian F., et al. (2011). Increased short latency afferent inhibition after anodal transcranial direct current stimulation. Neurosci. Lett. 498 167–170. 10.1016/j.neulet.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Schoenfeld R., Schiffelholz T., Beyer C., Leplow B., Foreman N. (2017). Variants of the Morris water maze task to comparatively assess human and rodent place navigation. Neurobiol. Learn. Mem. 139 117–127. 10.1016/j.nlm.2016.12.022 [DOI] [PubMed] [Google Scholar]
- Shibata M., Ohtani R., Ihara M., Tomimoto H. (2004). White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 35 2598–2603. 10.1161/01.str.0000143725.19053.60 [DOI] [PubMed] [Google Scholar]
- Venkat P., Chopp M., Zacharek A., Cui C., Landschoot-Ward J., Qian Y., et al. (2018). Sildenafil treatment of vascular dementia in aged rats. Neurochem. Int. 127 103–112. 10.1016/j.neuint.2018.1012.1015 [DOI] [PubMed] [Google Scholar]
- Wang D. P., Yin H., Kang K., Lin Q., Su S. H., Hai J. (2018). The potential protective effects of cannabinoid receptor agonist WIN55,212-2 on cognitive dysfunction is associated with the suppression of autophagy and inflammation in an experimental model of vascular dementia. Psychiatry Res. 267 281–288. 10.1016/j.psychres.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Yang W.-J., Wen H.-Z., Zhou L.-X., Luo Y.-P., Hou W.-S., Wang X., et al. (2019). After-effects of repetitive anodal transcranial direct current stimulation on learning and memory in a rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 161 37–45. 10.1016/j.nlm.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Yu T.-H., Wu Y.-J., Chien M.-E., Hsu K.-S. (2019). Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor. Neuropharmacology 144 358–367. 10.1016/j.neuropharm.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Zhu H., Zhang J., Sun H., Zhang L., Liu H., Zeng X., et al. (2011). An enriched environment reverses the synaptic plasticity deficit induced by chronic cerebral hypoperfusion. Neurosci. Lett. 502 71–75. 10.1016/j.neulet.2011.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.