Abstract
The purpose of this study was to compare differences of energy expenditure and substrate metabolism between motorized-treadmill and overground running in three different velocities in Chinese middle-aged women. In total, 74 healthy middle-aged women (age, 48 ± 4 years; height, 159.4 ± 4.9 cm; weight, 58.6 ± 6.7 kg; and body-mass index (BMI), 23.1 ± 2.7 kg/m2) volunteered to participate in this study. Bioelectrical-impedance analysis was used to measure body composition. Energy expenditure, carbohydrates (CHO), and fat oxidation were calculated with indirect calorimetry during motorized-treadmill and overground running. Running speed from slow to fast was 7.0, 8.0, and 9.0 km/h. The duration of each velocity was 6 min, separated by 5–15 min rest. There was no significant difference in energy expenditure between overground and treadmill running at the speed of 7 km/h (8.10 ± 1.25 vs. 7.75 ± 1.13 kcal/min, p > 0.05). Energy expenditure of overground running at 8 and 9 km/h was higher than that of treadmill running (9.36 ± 1.40 vs. 8.54 ± 1.21 kcal/min; 10.33 ± 1.55 vs. 9.54 ± 1.36 kcal/min; both p < 0.01). Fat contribution to energy consumption was significantly higher during treadmill running than during overground running (both p < 0.01) at speeds of 8 and 9 km/h. Overground running at high intensity incurred greater energy consumption than treadmill running did. However, results showed greater fat utilization during treadmill running than during overground running at high intensity. It is critical that these differences are taken into account when we prescribe training modes and intensities for middle-aged women.
Subject terms: Biological techniques, Biophysical methods
Introduction
Multiple studies showed that physical inactivity is a major risk factor for morbidity and mortality1,2. A series of studies published on the Lancet from 2012 to 2016 indicated that physical inactivity is a global epidemic, and we should increase physical activity levels to prevent it3–5. Energy expenditure and substrate metabolism are important elements when considering physical activities. Choosing optimal activity modes and intensities according to the characteristics of energy expenditure and substrate metabolism may help prescriptions for improving quality of life.
Overground and treadmill running are two types of widely available movement patterns since they do not require special exercise skills. Treadmill running is commonly used in daily life, and a large number of runners regularly train on motorized treadmills, but whether physiological demands in treadmill running can be a substitute for overground running is unclear. Bidder6 suggested that outdoor track running (tarmac, grass) demands greater energy expenditure compared with a motorized treadmill at a same level. Aubry7 also found that a higher rate of oxygen uptake was needed in overground running than in treadmill running. However, several studies found the opposite results. Some studies8,9 found that there was no significant difference in oxygen uptake between the two modes, while others10,11 found that the oxygen uptake of motorized-treadmill running was higher than that of overground running at the same speed. Concerning the differences in subjects, test conditions, and methods, there is no consensus in previous researches on whether the energy demands of treadmill and overground running are similar. Carbohydrates (CHO) and free fatty acids are two main fuel sources that are oxidized during exercise, the contributions of which are influenced by physical activity intensity12, duration13, and exercise modes14. It should also be clear what the CHO and fat oxidation characteristics in overground and treadmill running are.
The majority of studies on the energy consumption of overground and treadmill running have been performed in young adults or athletes6,7,15. There is much evidence suggesting that findings on young subjects may not apply to other populations16–18. Bartolomeu et al.16 found that metabolic variables (heart rate, blood lactate concentration, oxygen uptake, energy expenditure) were significantly lower for older women at maximal intensity. Identical outcomes were reported by Campbell18 when comparing both age groups in similar conditions. Important reasons for energy expenditure changes were changes in age-related lactate production, stroke volume, arteriovenous oxygen difference, maximum heart rate (HRmax), and musculoskeletal changes19–21. Reports in middle-aged and older adults are limited22,23, and all of which are comparisons between overground and treadmill walking. To date, there are no data on the energy-expenditure comparison of overground and treadmill running in middle-aged and older adults.
Given the above, the primary purpose of the present study was to compare the energy expenditure and substrate metabolism of overground and motorized-treadmill running at three different speeds in middle-aged women. We hypothesized that energy consumption and substrate utilization would be different during treadmill and overground running at the same speed.
Materials and Methods
Subjects
In total, 74 healthy middle-aged women were enrolled in this study. The eligibility criteria were for subjects to be healthy middle-aged women between 40 and 55 years with no recent experience of dieting or losing weight. Subjects were excluded if they were taking medication with known significant metabolism effects or if they were diagnosed with cardiovascular, respiratory, digestive-system, metabolic, bone and joint, thyroid, blood-system, and urinary-system diseases, or any condition that limited mobility. Participant characteristics are presented in Table 1. Subjects were told to wear comfortable clothes and shoes for all tests, and were asked to abstain from strenuous exercise, caffeine, and alcohol the day before the tests. They were also asked to arrive at the laboratory in a fasted state to eliminate the thermic effect of food (TEF).
Table 1.
Characteristics of Subjects (n = 74, mean ± SD).
| Anthropometrical data (Mean ± SD) | |
|---|---|
| Age (years) | 48 ± 4 |
| Height (cm) | 159.4 ± 4.9 |
| Weight (kg) | 58.6 ± 6.7 |
| Body mass index (kg/m2) | 23.1 ± 2.7 |
| FFM (kg) | 41.1 ± 3.2 |
| Body fat (%) | 28.9 ± 6.9 |
| Bust circumstance (cm) | 86.4 ± 5.9 |
| Waist circumstance (cm) | 74.9 ± 7.8 |
| Hipline circumstance (cm) | 92.3 ± 5.9 |
| WHR | 0.81 ± 0.06 |
| SBP (mmHg) | 111 ± 10 |
| DBP (mmHg) | 72 ± 9 |
FFM Fat Free Mass, WHR waist-to-hip ratio, SBP systolic blood pressure, DBP diastolic blood pressure.
All subjects were asked to sign informed consent prior to the study. All procedures in this study were in accordance with the guidelines in the Declaration of Helsinki and were approved by the China Institute of Sport Science Committee (ethical code: CISSIRD-201604).
Anthropometric measurements
The same trained tester performed all anthropometric measurements on subjects, namely, height, weight, body composition, and chest, waist, and hip circumference. Height was measured with the Su Heng Health Scale (RGZ-120, China). Weight and body-fat content was measured with a Body Composition Analyzer (INBODY 770, South Korea). Chest, waist, and hip circumference were measured by tape (SECA, Hamburg, Germany). We used the BMI = weight (kg)/height (m) squared formula to estimate the subject BMI.
Energy-expenditure tests
Energy expenditure was measured by using indirect calorimetry (Metamax 3B-R2 metabolic measurement system, Germany). A standard mixture of known oxygen and carbon dioxide gas concentrations was used to calibrate the portable gas metabolism system to ensure precise sensor operation. A 3000 ml syringe was used to calibrate flow-sensor calibration. The idea behind the equipment is to use the method of every breath measurement to acquire real-time data of expired ventilation (VE), respiratory rhythm, oxygen consumption (VO2), carbon dioxide production (VCO2), and other parameters in the process of locomotion24. Room temperature and humidity were controlled to 22–25 °C and 40%–50%, respectively.
The energy-expenditure tests of treadmill and overground running were completed at an indoor gymnasium in Beijing. Before tests, the subjects were allowed to walk or run overground and on the treadmill for about 6 min to familiarize themselves with the test environment and conditions25, including running overground with a metronome and according to voice prompts (“fast, slow”, etc). A rest break of about 5 min was given to allow participants’ heart rates to recover to a level ± 5% of their resting heart rates26. After habituation with the test conditions, formal data collection was conducted. Treadmill and overground running speeds from low to high were 7, 8, and 9 km/h (commonly used speeds in regular populations). The duration of each speed level was 6 min whatever the running mode. The overground running field was a rectangular field with a 40 m perimeter (15 m long and 5 m wide). Pylons were placed every 5 m on the track to control subjects’ actual running speed. A sound signal was produced every 2.6, 2.2, and 2 s at 7, 8, and 9 km/h, respectively, and subjects had to adjust their speed to reach a pylon every time they heard a sound signal. A metronome to control speed was widely used by several studies27–30. Furthermore, every subject was accompanied by a pacesetter of our research team during their overground running. Treadmill running (Rodby RL3500E, Sweden) was completed in the same stadium to minimize environmental influences on performance. Stable-state data of the last 2 min of each speed level were used to calculate the energy consumption of overground and treadmill running. The definition of a steady state usually calls for 3–5 min where VO2 and VCO2 vary by <10%–15%31,32. Achievement of a steady-state period during exercise testing reduces error in the assessment of energy expenditure. The next speed level of the running test was started after the subject had recovered to a level ± 5% of their resting heart rates (at least 5 min)26. Subject heart rate was monitored during all running tests.
Calculations
Energy expenditure was calculated using the following equation, assuming a negligible contribution of protein oxidation. Energy expenditure was calculated assuming that 1 g carbohydrate = 4 kcal, and 1 g fat = 9 kcal33. Fat and carbohydrate oxidation were calculated from respiratory measurements (VO2, VCO2) according to the table of nonprotein respiratory quotient34. CHO and fat oxidation contributions were calculated using the Dumortier formula35. All formulas are shown in Table 2.
Table 2.
Formulas of energy expenditure and substrate metabolism.
| Variables | Formulas |
|---|---|
| Energy expenditure (kcal/min) | fat oxidation (g/min) × 9 + carbohydrate oxidation (g/min) × 4 |
| Fat oxidation (g/min) | 1.695 × VO2 (l/min) − 1.701 × VCO2 (l/min) |
| Carbohydrate oxidation (g/min) | 4.585 × VCO2 (l/min) − 3.226 × VO2 (l/min) |
| % Fat | ((1 − RER) / 0.29) × 100 |
| % CHO | ((RER − 0.71) / 0.29) × 100 |
Statistics
Two-way ANOVA 3 (speed: 7, 8, and 9 km/h) × 2 (mode: overground and treadmill running) with repeated measures was used to examine differences in energy expenditure, VO2, heart rate, CHO and fat oxidation, and fat contribution (%). Simple-effect analysis was applied to examine differences between treadmill and overground conditions. Results were analyzed using SPSS23.0. Significance level was set at p < 0.05. Effect size was evaluated with η2 (Eta partial squared), where 0.01 < η2 < 0.06 represented a small effect, 0.06 < η2 < 0.14 a medium effect, and η2 > 0.14 a large effect36.
Results
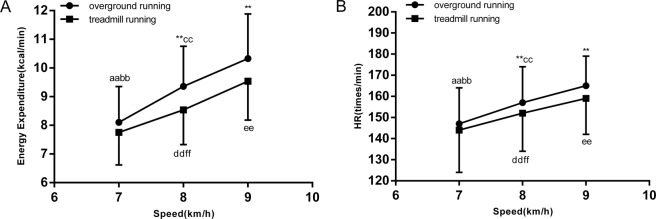
A significant effect of increasing the running speed from 7 to 9 km/h was found on energy expenditure (F = 229.7, p < 0.01, η2 = 0.76; Fig. 1A), VO2 (F = 294.3, p < 0.01, η2 = 0.80; Table 3), CHO oxidation (F = 119.4, p < 0.01, η2 = 0.62; Fig. 2), fat oxidation (F = 15.4, p < 0.01, η2 = 0.18; Fig. 2), fat contribution (%) (F = 63.3, p < 0.01, η2 = 0.47; Fig. 3), heart rate (F = 257.3, p < 0.01, η2 = 0.78; Fig. 1B). Simple-effect analysis showed that, with increasing running speed from 7 to 9 km/h on overground and treadmill running, energy expenditure, VO2, HR, CHO oxidation increased; however, fat oxidation and contribution (%) decreased.
Figure 1.
(A) Mean and standard deviations of energy expenditure for three different running speeds. (B) Mean and standard deviations of heart rate (HR) for three different running speeds. **Compared with same speed on treadmill running p < 0.01; aap < 0.01 overground running of 7 vs. 8 km/h; bbp < 0.01 overground running of 7 vs. 9 km/h; ccp < 0.01 overground running of 8 vs. 9 km/h; ddp < 0.01 treadmill running of 7 vs. 8 km/h; eep < 0.01 treadmill running of 7 vs. 9 km/h; ffp < 0.01 treadmill running of 8 vs. 9 km/h.
Table 3.
Energy expenditure and substrate metabolism during overground and treadmill running at same speed for middle-aged women (mean ± SD).
| Speed | 7(km/h) | 8(km/h) | 9(km/h) | |||
|---|---|---|---|---|---|---|
| Variable | OG | TM | OG | TM | OG | TM |
| CHO oxidation (g/min) | 1.31 ± 0.53aabb | 1.16 ± 0.44ddee | 1.74 ± 0.65**cc | 1.36 ± 0.49ff | 2.03 ± 0.69** | 1.73 ± 0.64 |
| Fat oxidation (g/min) | 0.32 ± 0.17aabb | 0.35 ± 0.16ddee | 0.27 ± 0.19**cc | 0.34 ± 0.18ff | 0.25 ± 0.19** | 0.29 ± 0.20 |
| Contribution of fat (%) | 42.68 ± 20.16aabb | 46.13 ± 20.13ddee | 32.62 ± 22.41**cc | 41.98 ± 19.59ff | 27.59 ± 20.38** | 33.41 ± 21.32 |
| EE (kcal/min) | 8.10 ± 1.25aabb | 7.75 ± 1.13ddee | 9.36 ± 1.40**cc | 8.54 ± 1.21ff | 10.33 ± 1.55** | 9.54 ± 1.36 |
| VO2 (ml/min/kg) | 27.36 ± 2.64aabb | 26.77 ± 2.99ddee | 30.83 ± 2.99**cc | 29.28 ± 2.86ff | 33.78 ± 3.59** | 31.74 ± 2.87 |
| HR (beats/min) | 147 ± 17aabb | 144 ± 20ddee | 157 ± 17**cc | 152 ± 18ff | 165 ± 14** | 159 ± 17 |
**p < 0.01 overground running vs. treadmill running with speed of 7, 8, and 9 km/h. aap < 0.01 overground running of 7 vs. 8 km/h. bbp < 0.01 overground running of 7 vs. 9 km/h. ccp < 0.01 overground running of 8 vs. 9 km/h. ddp < 0.01 treadmill running of 7 vs. 8 km/h. eep < 0.01 treadmill running of 7 vs. 9 km/h. ffp < 0.01 treadmill running of 8 vs. 9 km/h.
Figure 2.
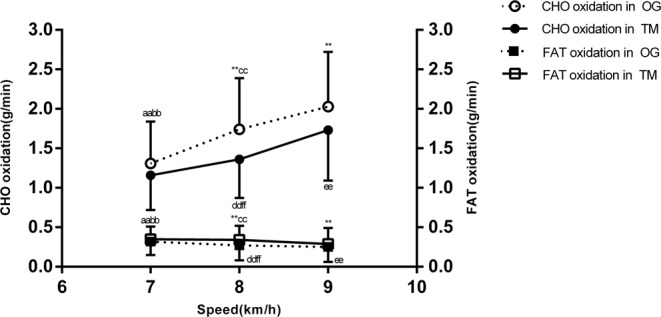
Mean of all participants’ carbohydrate (CHO) and fat oxidation for three different running speeds. **Compared with same speed on treadmill running p < 0.01; aap < 0.01 overground running of 7 vs. 8 km/h; bbp < 0.01 overground running 7 vs. 9 km/h; ccp < 0.01 overground running 8 vs. 9 km/h; ddp < 0.01 treadmill running 7 vs. 8 km/h; eep < 0.01 treadmill running of 7 vs. 9 km/h; ffp < 0.01 treadmill running of 8 vs. 9 km/h.
Figure 3.

Mean values of contribution of CHO (%) and fat (%).
A significant effect of the modes was found on energy expenditure (F = 31.8, p < 0.01, η2 = 0.13; Fig. 1A), VO2 (F = 44.1, p < 0.01, η2 = 0.17, Table 3), CHO oxidation (F = 35.2, p < 0.01, η2 =0.14; Fig. 2), fat oxidation (F = 15.1, p < 0.01, η2 = 0.06; Fig. 2), fat contribution (%) (F = 38.9, p < 0.01, η2 = 0.15; Fig. 3), heart rate (F = 17.9, p < 0.01, η2 = 0.08; Fig. 1B). Simple-effect analysis showed that all variables during overground running at speeds of 8 and 9 km/h were higher than those during treadmill running at the same speed, but fat oxidation and contribution (%) were lower than those of treadmill running. However, there were no significant differences found between overground and treadmill running at 7 km/h (p > 0.05).
Discussion
This study was aimed to compare the energy expenditure and substrate metabolism between overground and treadmill running for middle-aged women. The principal finding of the present study was that physiological variables (energy expenditure, oxygen uptake, heart rate) during overground running were significantly higher than those during motorized-treadmill running at speeds of 8 and 9 km/h. No variable was significantly different between overground and treadmill running at the speed of 7 km/h. As speed increased, the difference in VO2 and heart rate (HR) between overground and treadmill running increased. Moreover, regardless of overground and treadmill running, with increased exercise intensity, the relative contribution of fat oxidation to total energy expenditure decreased, whereas the contribution of carbohydrate oxidation increased.
Niemeyer37 compared the energy and carbohydrate demand for interval training on a track and treadmill, and found that the track demand was higher than that of the treadmill. Aubry7 also found metabolic demand on an outdoor track was significantly higher compared with that of a treadmill. Consistent with previous studies, our results showed that the energy expenditure of overground running was, on average, 9.6% and 8.3% higher than that of treadmill running at speeds of 8 and 9 km/h, respectively (9.36 ± 1.40 vs. 8.54 ± 1.21 kcal/min; 10.33 ± 1.55 vs. 9.54 ± 1.36 kcal/min, both p < 0.01). It is not very clear why higher energy expenditure was observed in overground running in comparison to that of treadmill running. One possible explanation is that the running mechanics of overground running is different from that of treadmill running. Kinematic and kinetic characteristics were reported between the two types; speed and contact style can affect the kinematics and kinetics of running38,39. The kinematics and kinetics of running were also influenced by the shoes of the subjects, which varied in style and condition40,41. It was shown that increasing and decreasing stride length and frequency resulted in increased metabolic cost42–44, and energy expenditure is lower at freely chosen stride frequency compared to running with other stride frequencies45. In our study, overground energy expenditure was probably increased since it might not have been the freely chosen stride frequency due to the turning of overground running and the speed control. In addition, one important possible factor was the surface-stiffness difference between the overground track and the treadmill, which affected running mechanics and induced the energy-cost difference. Several studies showed that stiffer surfaces need more aerobic demand46–48. We speculated that the surface of our treadmill was softer than the overground surface. Besides surface-stiffness differences, during treadmill running, the surface moves automatically, while an individual propels himself over the surface during overground running49. Several studies suggested that the lack of air resistance is the main reason for the difference in oxygen uptake between overground and treadmill running6,7. Our results showed that there was no significant difference between the energy consumption of overground and treadmill running at the speed of 7 km/h; however, the difference in energy consumption increased as speed increased. The increased differences in energy consumption may be attributed to the effect of air resistance, which becomes more pronounced as running speed increases. Energy-expenditure differences between treadmill and overground running have been attributed to running kinematic and kinetic characteristics, surface type, and environmental conditions. Furthermore, the reasons for the energy consumption of overground running being higher than that of treadmill running need further research.
Different exercise modes may result in different metabolic responses on the relative contributions of fat and carbohydrate oxidation. Studies showed that fat oxidation is significantly lower during cycling than running at the same relative intensity14,50. Our results found that there were significant differences in substrate metabolism and heart rate in the two modes; overground running required the runner to utilize considerably more carbohydrates but less fat than treadmill running did. CHO oxidation of overground running was, on average, 27.9% and 17.3% higher than that of treadmill running, whereas fat oxidation was, on average, 25.9% and 16% lower during overground running when compared with treadmill running at speeds of 8 and 9 km/h. These findings were consistent with a study by Kerdok51. Results demonstrated that overground running at speeds of 8 and 9 km/h had a higher stress response on the body than treadmill running did. Substrate metabolism is regulated by the intensity of physical activity, and we found that, the greater the activity intensity was, whatever the type of running, the larger the contribution of CHO oxidation to total energy expenditure would be, which was entirely consistent with results of previous studies13,52. Therefore, we should pay attention to the CHO and fat-oxidation characteristics of overground running in comparison to treadmill running to prescribe for middle-aged individuals according to the exercise aims.
Treadmill running, which is within a limited and controlled space, offers greater control compared to overground running, and is widely used by clinicians, athletes, and general population, making it close to overground running for the diagnosis and rehabilitation of injuries, training, and improving body fitness. This study suggested that overground running requires greater effort than treadmill running does as speed increases. When middle-aged women select slower running speeds than 7 km/h, they can choose overground or treadmill running according to their familiarity and comfort habits. To achieve greater energy consumption, middle-aged women are recommended to select overground over treadmill running at speeds of 8 and 9 km/h or above. If participants choose treadmill running to achieve the same physiological effects, they could increase the treadmill speed or use a 1% treadmill gradient53.
Limitations and Perspectives
The study has some limitations. First, there were no measurements of sports biomechanics, such as electromyography, step, stride, and joint angular kinematics. Biomechanical data from running can provide more information on muscle activation and kinematics on overground and treadmill running. Second, substrate oxidation rates were affected by energy balance and macronutrient diet composition. Diet conditions were not monitored during the day before the test. Third, to prevent daily changes in physiological response, all exercise tests were performed on the same day, which could potentially lead to more or less fatigue for middle-aged women. Finally, the effect of VO2max on metabolic parameters was not analyzed because the VO2max of subjects was not tested in our study. However, several studies indicated that oxygen uptake and metabolic response during different exercises depend on an individual’s maximal aerobic power54. In future studies, VO2max will be tested and used as a factor to analyze its influence on metabolic variables.
Conclusion
In conclusion, VO2, HR, energy expenditure, and carbohydrate oxidation increased with increasing running speed in all running types. As speed increased, energy expenditure and carbohydrate oxidation were markedly higher during overground running than during treadmill running. Any differences between treadmill and overground running may lead to the incorrect prescription of physical-activity intensities. It is critical that these differences are taken into account when prescribing training intensities and choosing training modes for middle-aged women.
Acknowledgements
The authors thank Ministry of Science and Technology of the People’s Republic of China (Grants No. 2013FY114700) for financial support of this study.
Author contributions
S.L. performed the experiment implementation, statistic analysis. S.L. and J.J.X. wrote the manuscript. J.J.X., Z.H.H., C.S. and P.H. reviewed the manuscript. All the authors read and approved the manuscripts.
Data availability
The original data included in this study during the current study is available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee IM, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamatakis E, et al. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019;73:2062–2072. doi: 10.1016/j.jacc.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Hallal PC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 4.Kohl HW, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380:294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 5.Ekelund U, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 6.Bidder OR, et al. Does the Treadmill Support Valid Energetics Estimates of Field Locomotion. Integr. Comp. Biol. 2017;57:301–319. doi: 10.1093/icb/icx038. [DOI] [PubMed] [Google Scholar]
- 7.Aubry RL, Power GA, Burr JF. An Assessment of Running Power as a Training Metric for Elite and Recreational Runners. J. Strength. Cond. Res. 2018;32:2258–2264. doi: 10.1519/JSC.0000000000002650. [DOI] [PubMed] [Google Scholar]
- 8.McMiken DF, Daniels JT. Aerobic requirements and maximum aerobic power in treadmill and track running. Med. Sci. sports. 1976;8:14–17. [PubMed] [Google Scholar]
- 9.Edwards RB, Tofari PJ, Cormack SJ, Whyte DG. Non-motorized Treadmill Running Is Associated with Higher Cardiometabolic Demands Compared with Overground and Motorized Treadmill Running. Front. Physiol. 2017;8:1–11. doi: 10.3389/fphys.2017.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yngve A, Nilsson A, Sjostrom M, Ekelund U. Effect of monitor placement and of activity setting on the MTI accelerometer output. Med. Sci. Sports Exerc. 2003;35:320–326. doi: 10.1249/01.MSS.0000048829.75758.A0. [DOI] [PubMed] [Google Scholar]
- 11.Mooses M, Tippi B, Mooses K, Durussel J, Mäestu J. Better economy in field running than on the treadmill: evidence from high-level distance runners. Biol. Sport. 2015;32:155–159. doi: 10.5604/20831862.1144418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlander AL, Casazza GA, Horning MA, Buddinger TF, Brooks GA. Effects of exercise intensity and training on lipid metabolism in young women. Am. J. Physiol. 1998;275:E853–863. doi: 10.1152/ajpendo.1998.275.5.E853. [DOI] [PubMed] [Google Scholar]
- 13.Romijn JA, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 14.Capostagno B, Bosch A. Higher fat oxidation in running than cycling at the same exercise intensities. Int. J. Sport. Nutr. Exerc. Metab. 2010;20:44–55. doi: 10.1123/ijsnem.20.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Bassett DR, et al. Aerobic requirements of overground versus treadmill running. Med. Sci. Sports Exerc. 1985;17:477–481. doi: 10.1249/00005768-198508000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Bartolomeu RF, et al. The aging influence on cardiorespiratory, metabolic, and energy expenditure adaptations in head-out aquatic exercises: Differences between young and elderly women. Women Health. 2017;57:377–391. doi: 10.1080/03630242.2016.1164272. [DOI] [PubMed] [Google Scholar]
- 17.Edvardsen E, Hansen BH, Holme IM, Dyrstad SM, Anderssen SA. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest. 2013;144:241–248. doi: 10.1378/chest.12-1458. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JA, D’Acquisto LJ, D’Acquisto DM, Cline MG. Metabolic and cardiovascular response to shallow water exercise in young and older women. Med. Sci. Sports Exerc. 2003;35:675–681. doi: 10.1249/01.MSS.0000058359.87713.99. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001;37:153–156. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 20.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc. Sport. Sci. Rev. 2014;42:45–52. doi: 10.1249/JES.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 21.Nilwik R, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer B, Parvataneni K, Olney SJ. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin. Biomech. 2009;24:729–734. doi: 10.1016/j.clinbiomech.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Berryman N, et al. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur. J. Appl. Physiol. 2012;112:1613–1620. doi: 10.1007/s00421-011-2102-1. [DOI] [PubMed] [Google Scholar]
- 24.Branson RD, Johannigman JA. The measurement of energy expenditure. Nutr. Clin. Pract. 2004;19:622–636. doi: 10.1177/0115426504019006622. [DOI] [PubMed] [Google Scholar]
- 25.Matsas A, Taylor N, McBurney H. Knee joint kinematics from familiarised treadmill walking can be generalised to overground walking in young unimpaired subjects. Gait Posture. 2000;11:46–53. doi: 10.1016/S0966-6362(99)00048-X. [DOI] [PubMed] [Google Scholar]
- 26.Dal U, Erdogan T, Resitoglu B, Beydagi H. Determination of preferred walking speed on treadmill may lead to high oxygen cost on treadmill walking. Gait Posture. 2010;31:366–369. doi: 10.1016/j.gaitpost.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Beck ON, Azua EN, Grabowski AM. Step time asymmetry increases metabolic energy expenditure during running. Eur. J. Appl. Physiol. 2018;118:2147–2154. doi: 10.1007/s00421-018-3939-3. [DOI] [PubMed] [Google Scholar]
- 28.Hobara H, Sato T, Sakaguchi M, Sato T, Nakazawa K. Step frequency and lower extremity loading during running. Int. J. Sports Med. 2012;33:310–313. doi: 10.1055/s-0031-1291232. [DOI] [PubMed] [Google Scholar]
- 29.Bramah C, Preece SJ, Gill N, Herrington L. A 10% Increase in Step Rate Improves Running Kinematics and Clinical Outcomes in Runners With Patellofemoral Pain at 4 Weeks and 3 Months. Am. J. Sports Med. 2019;47:3406–3413. doi: 10.1177/0363546519879693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe DA, Kang M, Sutherland R, Holbrook EA, Barreira TV. Evaluation of inactive adults’ ability to maintain a moderate-intensity walking pace. J. Sci. Med. Sport. 2013;16:217–221. doi: 10.1016/j.jsams.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 31.McClave SA, et al. Achievement of steady state optimizes results when performing indirect calorimetry. JPEN J. Parenter. Enter. Nutr. 2003;27:16–20. doi: 10.1177/014860710302700116. [DOI] [PubMed] [Google Scholar]
- 32.Reeves MM, Davies PS, Bauer J, Battistutta D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J. Appl. Physiol. 2004;97:130–134. doi: 10.1152/japplphysiol.01212.2003. [DOI] [PubMed] [Google Scholar]
- 33.Knechtle B, et al. Fat oxidation in men and women endurance athletes in running and cycling. Int. J. Sports Med. 2004;25:38–44. doi: 10.1055/s-2003-45232. [DOI] [PubMed] [Google Scholar]
- 34.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can. J. sport. Sci. = J. canadien des. Sci. du. sport. 1991;16:23–29. [PubMed] [Google Scholar]
- 35.Dumortier M, Thöni G, Brun JF, Mercier J. Substrate oxidation during exercise: impact of time interval from the last meal in obese women. Int. J. Obes. 2005;29:966–974. doi: 10.1038/sj.ijo.0802991. [DOI] [PubMed] [Google Scholar]
- 36.Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, N. J., England, Lawrence Erlbaum Associates (1988).
- 37.Niemeyer M, Weber TGJ, Beneke R. Higher energy and carbohydrate demand of interval training at a given average velocity on track vs. treadmill. Appl. Physiol. Nutr. Metab. 2018;44:447–449. doi: 10.1139/apnm-2018-0596. [DOI] [PubMed] [Google Scholar]
- 38.Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin. Biomech. 2009;24:95–100. doi: 10.1016/j.clinbiomech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Firminger CR, et al. Joint kinematics and ground reaction forces in overground versus treadmill graded running. Gait Posture. 2018;63:109–113. doi: 10.1016/j.gaitpost.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Stacoff A, et al. Effects of shoe sole construction on skeletal motion during running. Med. Sci. Sports Exerc. 2001;33:311–319. doi: 10.1097/00005768-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Nigg BM, et al. Effect of shoe inserts on kinematics, center of pressure, and leg joint moments during running. Med. Sci. Sports Exerc. 2003;35:314–319. doi: 10.1249/01.MSS.0000048828.02268.79. [DOI] [PubMed] [Google Scholar]
- 42.Saibene F, Minetti AE. Biomechanical and physiological aspects of legged locomotion in humans. Eur. J. Appl. Physiol. 2003;88:297–316. doi: 10.1007/s00421-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 43.Uematsu A, et al. Preferred step frequency minimizes veering during natural human walking. Neurosci. Lett. 2011;505:291–293. doi: 10.1016/j.neulet.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 44.Hunter I, Smith GA. Preferred and optimal stride frequency, stiffness and economy: changes with fatigue during a 1-h high-intensity run. Eur. J. Appl. Physiol. 2007;100:653–661. doi: 10.1007/s00421-007-0456-1. [DOI] [PubMed] [Google Scholar]
- 45.Cavagna GA, Mantovani M, Willems PA, Musch G. The resonant step frequency in human running. Pflug. Arch. 1997;434:678–684. doi: 10.1007/s004240050451. [DOI] [PubMed] [Google Scholar]
- 46.Greenhalgh A, Sinclair J. Influence of footwear choice, velocity and surfaces on tibial accelerations experienced by field hockey participants during running. Footwear Sci. 2012;4:213–219. doi: 10.1080/19424280.2012.696725. [DOI] [Google Scholar]
- 47.Garcíapérez JA, Pérezsoriano P, Llana BS, Lucascuevas AG, Sánchezzuriaga D. Effects of treadmill running and fatigue on impact acceleration in distance running. Sports Biomech. 2014;13:259–266. doi: 10.1080/14763141.2014.909527. [DOI] [PubMed] [Google Scholar]
- 48.JAH S, McKerrow AD, Kohn TA. Metabolic cost of running is greater on a treadmill with a stiffer running platform. J. Sports Sci. 2017;35:1592–1597. doi: 10.1080/02640414.2016.1225974. [DOI] [PubMed] [Google Scholar]
- 49.Fellin RE, Manal K, Davis IS. Comparison of lower extremity kinematic curves during overground and treadmill running. J. Appl. Biomech. 2010;26:407–414. doi: 10.1123/jab.26.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Achten J, Venables MC, Jeukendrup AE. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metab. Clin. Exp. 2003;52:747–752. doi: 10.1016/S0026-0495(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 51.Kerdok AE, Biewener AA, McMahon TA, Weyand PG, Herr HM. Energetics and mechanics of human running on surfaces of different stiffnesses. J. Appl. Physiol. 2002;92:469–478. doi: 10.1152/japplphysiol.01164.2000. [DOI] [PubMed] [Google Scholar]
- 52.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J. Appl. Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- 53.Jones AM, Doust JH. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sports Sci. 1996;14:321–327. doi: 10.1080/02640419608727717. [DOI] [PubMed] [Google Scholar]
- 54.Haramura M, Takai Y, Yoshimoto T, Yamamoto M, Kanehisa H. Cardiorespiratory and metabolic responses to body mass-based squat exercise in young men. J. Physiol. Anthropol. 2017;36:14. doi: 10.1186/s40101-017-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data included in this study during the current study is available from the corresponding author on reasonable request.