Abstract
Liver-resident NK cells are distinct from conventional NK cells and play an important role in the maintenance of liver homeostasis. How liver-resident NK cells participate in autoimmune cholangitis remains unclear. Here, we extensively investigated the impact of NK cells in the pathogenesis of autoimmune cholangitis utilizing the well-established dnTGFβRII cholangitis model, NK cell-deficient (Nfil3−/−) mice, adoptive transfer and in vivo antibody-mediated NK cell depletion. Our data demonstrated that disease progression was associated with a significantly reduced frequency of hepatic NK cells. Depletion of NK cells resulted in exacerbated autoimmune cholangitis in dnTGFβRII mice. We further confirmed that the DX5−CD11chi liver-resident NK cell subset colocalized with CD4+ T cells and inhibited CD4+ T cell proliferation. Gene expression microarray analysis demonstrated that liver-resident NK cells had a distinct gene expression pattern consisting of the increased expression of genes involved in negative regulatory functions in the context of the inflammatory microenvironment.
Key words: Liver-resident NK, Cholangitis, CD4+ T cell, Suppression
Subject terms: Autoimmunity, Innate immune cells
Introduction
Primary biliary cholangitis (PBC) is an autoimmune liver disease characterized by lymphocytic infiltration in portal tracts, the destruction of intrahepatic small bile duct epithelial cells, and the production of anti-mitochondrial antibodies (AMAs).1–4 Clinical studies suggest that the frequency and absolute number of NK cells are increased in both the liver and peripheral blood in PBC patients. The cytotoxicity and perforin expression of PBC NK cells are increased, but inflammatory cytokine secretion by NK cells is decreased.5 These studies indicate a role for NK cells in the pathogenesis of PBC.
Dominant-negative transforming growth factor β receptor II (dnTGFβRII) mice are transgenic for the directed expression of a dominant-negative form of the type II TGFβ receptor under the control of the CD4 promoter lacking the CD8 silencer.6,7 This mouse model mimics several key phenotypic features of human PBC, including the spontaneous production of AMAs, lymphocytic liver infiltration with periportal inflammation and an inflammatory cytokine profile.8 In addition to liver disease, dnTGFβRII mice also develop colitis, and crosstalk between the liver and colon plays an important role in the pathogenesis of autoimmune cholangitis (data not shown). Using this model, we elucidated the function of various immune cells, including CD8+ T cells,9–11 B cells,12 iNKT cells13 and regulatory T cells,14 in the pathogenesis of PBC. In this study, we focused on the role of NK cells, especially liver-resident NK cells, in the pathogenesis of autoimmune cholangitis.
The liver contains an abundance of NK cells, which account for 25–40% of the hepatic lymphocyte population in humans and 10–20% of this population in mice.15–17 NK cells are involved in various liver diseases, including hepatitis C,18–20 hepatitis B,21–23 nonalcoholic fatty liver disease and fibrosis.24,25 Nuclear factor interleukin-3, or Nfil3 (also known as E4-binding protein 4, or E4bp4), is an essential transcription factor for the early development of the NK cell lineage. The ablation of Nfil3 expression results in a dramatic reduction in mature NK cells.26,27 Recently, several groups have reported the existence of a distinct liver-resident NK subset (CD49b− or DX5−), which phenotypically and functionally differs from conventional splenic NK cells.28–30 Hepatic NK cells have enhanced cytotoxic activity against autologous biliary epithelial cells.31,32 In contrast, central nervous system-resident NK cells exhibit a protective role in murine experimental autoimmune encephalomyelitis.31,33 Therefore, the precise role of liver-resident NK cells in PBC is unclear.
In this study, we addressed the involvement and underlying mechanisms of NK cells in the pathogenesis of PBC. First, we found that the progression of the disease in dnTGFβRII mice was negatively correlated with the number of liver-resident NK cells. Next, utilizing NK cell deficient (Nfil3−/−) mice, adoptive transfer and antibody-mediated NK cell depletion, we demonstrated that the loss of NK cells in dnTGFβRII mice resulted in aggravated biliary disease associated with an increase in T cells, especially CD4+ T cells. Furthermore, we found that only DX5−NK cells but not DX5+ NK cells inhibited CD4+ T cell proliferation and co-localized with CD4+ T cells. Finally, we demonstrated that the suppressive function of DX5− NK cells was enhanced in an inflammatory environment. Our data demonstrated the immunosuppressive role of liver-resident NK cells in the pathogenesis of biliary disease. Targeting liver-resident NK cells may be a tissue-specific therapeutic strategy for PBC.
Materials and methods
Mice
dnTGFβRII mice (B6.Cg-Tg(Cd4-TGFBR2)16Flv/J) and Rag1−/− mice (B6.129S7-Rag1tmiMom/J) were initially purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). Foxp3GFP mice were kindly provided by Professor A.Y. Rudensky (Memorial Sloan Kettering Cancer Center).34 dnTGFβRII-Foxp3GFP mice were obtained by backcrossing dnTGFβRII mice with Foxp3GFP mice. NK cell-deficient (Nfil3−/−) mice were kindly donated by Professor Zhigang Tian (University of Science and Technology of China). All mice were on a C57BL/6 background. To generate NK−/− dnTGFβRII mice, dnTGFβRII mice were bred with Nfil3+/− mice to obtain Nfil3+/− dnTGFβRII mice; Nfil3+/− dnTGFβRII mice were then bred with Nfil3+/− mice to obtain Nfil3−/− dnTGFβRII (NK−/− dnTGFβRII) mice. Rag1−/− mice were bred with Nfil3+/− mice to obtain Nfil3+/− Rag1+/− mice, which were then backcrossed to obtain Nfil3−/− Rag1−/− mice and Nfil3+/− Rag1−/− mice. Finally, male Nfil3−/− Rag1−/− mice were bred with female Nfil3+/− Rag1−/− mice to generate Nfil3−/− Rag1−/− (NK−/− Rag1−/−) mice. Nfil3 knockout and transgenic TGFβRII mice were identified by PCR. Thirteen to fifteen-week-old male or female dnTGFβRII and NK−/− dnTGFβRII mice were used in all experiments, unless otherwise noted. All mice were housed in individually ventilated cages under specific pathogen-free conditions in a controlled environment (22 °C, 55% humidity, and a 12-h day/night cycle). All animal experimental protocols conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals by the Laboratory Animal Center, School of Life Sciences, University of Science and Technology of China.
Experimental protocol
Four experimental protocols were followed. (a) First, mice with different stages of disease, including the asymptomatic stage (3–4 weeks), early stage (6-8 weeks) and typical symptom stage (13–15 weeks), were used to analyze the changes in NK cells during the progression of liver inflammation. We performed linear regression analysis of the percentage of hepatic NK cells versus the number of hepatic MNCs to assess the relationship between liver NK cells and inflammation in the typical symptom stage. (b) Second, to demonstrate the role of NK cells in liver disease, we used three mouse models, including NK−/− dnTGFβRII mice, NK−/− Rag1−/− mice with adoptive transfer of splenic T cells and mice with PK136 antibody-mediated depletion of NK cells. Histopathology, flow cytometry and ELISAs were used to assess the severity of liver disease. (c) Third, we performed immunofluorescence staining to analyze the distribution of liver NK cells in dnTGFβRII mice and in vitro suppression assays to test the immunosuppressive function of liver-resident NK cells. We also compared liver-resident NK cell populations between WT mice and dnTGFβRII mice. (d) Finally, we performed gene expression microarray analysis of liver-resident NK cells from WT and dnTGFβRII mice and then applied gene set enrichment analysis (GSEA) to confirm the enhanced immunosuppressive function of liver-resident NK cells in dnTGFβRII mice.
Adoptive transfer
Splenic CD8+ T cells and CD4+ T cells were isolated from 10-14-week old dnTGFβRII mice and transferred as described.9 Briefly, CD8+ T cells or CD4+ T cells were purified from splenic mononuclear cells by positive selection with anti-CD8 or anti-CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). A total of 1 × 106 CD8+ T cells and 1 × 106 CD4+ T cells were cotransferred into Rag1−/− mice or NK−/− Rag1−/− mice by intravenous injection. After 3 to 4 weeks, the recipients were sacrificed. Liver tissue sections were prepared for H&E staining and flow cytometric analysis.
Depletion of NK cells by an anti-PK136 antibody
The PK136 hybridoma was kindly donated by Professor Zhigang Tian (University of Science and Technology of China). To obtain the anti-PK136 antibody, 5 × 105 PK136 hybridoma B lymphocytes were injected intraperitoneally into naïve pretreated Rag1−/− mice.35,36 After 2-3 weeks, peritoneal fluid was collected, and 200 μl of peritoneal fluid or PBS was injected into the peritoneal cavity of dnTGFβRII mice (8 weeks old) every two weeks. Mice were sacrificed at 12 weeks of age for H&E staining and flow cytometric analysis.
Histopathology
Liver tissues were fixed in 10% formaldehyde for 24 h, embedded in paraffin and cut into 4-µm sections, stained with H&E, and evaluated using light microscopy. The severity of tissue damage and inflammation were evaluated by a pathologist in a blinded fashion as previously described.37 First, the degree of portal inflammation was evaluated and scored according to the most severe lesions, as follows: 0, no change; 1, minimal inflammation; 2, mild inflammation; 3, moderate inflammation; and 4, severe inflammation. In addition, the degree of inflammation frequency in a specimen was determined by the percentage of affected tissue within all hepatic lobules per specimen and scored as follows: 0, none; 1, 1–10%; 2, 11–20%; 3, 21–50%; and 4, more than 50%. Finally, a summary score that accounted for both the severity and frequency analyses was generated as the sum of these scores. We note that the lobular inflammation was scored in the same fashion as the portal inflammation. Second, bile duct damage was evaluated first by the degree of severity in the most severe lesions, as follows: 0, no change; 1, epithelial damage (only cytoplasmic changes); 2, epithelial damage with cytoplasmic and nuclear changes; 3, nonsuppurative destructive cholangitis (CNSDC); and 4, bile duct loss. To obtain an integrated evaluation, the severity and frequency scores were summed.
Serum and liver tissue cytokines
Serum and liver tissue were collected from 13-15-week-old dnTGFβRII and NK−/− dnTGFβRII mice. IFN-γ, IL-6 and TNF-α levels in serum and liver tissues were measured using a BDTM Cytometric Bead Array (CBA) Mouse Inflammation Kit (BD Bioscience, San Jose, CA, USA). Data were collected using a FACSVerse flow cytometer with CBA software.
ELISA for anti-mitochondrial antibodies (AMAs)
Serum was collected from 13 to 15-week-old dnTGFβRII and NK−/− dnTGFβRII mice. Serum AMA levels were measured by ELISA using recombinant E2 component of pyruvate dehydrogenase (PDC-E2), 2-oxoglutarate dehydrogenase (OGDC-E2) and branched chain 2-oxo-acid dehydrogenase (BCOADC-E2).7
Flow cytometry
Liver mononuclear cells were prepared as previously described.38 The spleen was disrupted between two glass slides, suspended in PBS and passed through a nylon mesh, and erythrocytes were lysed. After blocking with anti-FcR blocking reagent (BioLegend, San Diego, CA, USA), cells were incubated with the indicated fluorescence-labeled monoclonal Abs in the dark at 4˚C for 20 min and were then washed with PBS. The stained cells were analyzed using the BD FACSVerse™ (BD Biosciences). mAbs included anti-CD44 FITC (IM7), anti-CD62L PerCP/Cy5.5 (MEL-14), anti-CD3 Alexa Fluor 647 (17A2), anti-CD19 APC/Cy7 (6D5), anti-NK1.1 PE/Cy7 (PK136), anti-CD4 Pacific Blue (RM4-5), anti-CD8α V500 (53-6.7), anti-CD69 FITC (H1.2F3), anti-DX5 PE (DX5), anti-TRAIL PE (N2B2), anti-CD11c PerCP/Cy5.5 (N418), anti-PD-L1 APC (10 F.9G2), anti-KLRG1 APC (2F1/KLRG-1). All antibodies were purchased from BioLegend except anti-CD8α V500 (BD Biosciences). Brilliant Violet 421-labeled PBS-57-loaded and unloaded mCD1d tetramers (#22189, #22191) were kindly provided by the NIH Tetramer Core Facility. Acquired data were analyzed with FlowJo software (Tree Star, Inc., Ashland, USA).
Immunofluorescence analysis
Immunofluorescence staining was performed as previously described.36 Briefly, livers from 8-week-old dnTGFβRII mice were fixed with 4% paraformaldehyde for 4 h and dehydrated in a 30% sucrose solution overnight. Liver tissues embedded with OCT were cut into 7-μm sections and blocked with 10% homologous serum at RT for 1 h. Antibodies, including BV421-conjugated anti-CD4 (RM4−5), Alexa Fluor 647-conjugated anti-NKp46 (29A1.4), PE-conjugated anti-DX5 (DX5) and PE-conjugated anti-TRAIL (N2B2), (BioLegend) were incubated with the liver sections overnight in a humidified chamber. Images were acquired and visualized by confocal microscopy (LSM710, Carl Zeiss, Germany).
Cell sorting
To obtain hepatic NK cells, hepatic mononuclear cells were first isolated from 6-8-week-old dnTGFβRII mice. Cells were stained with FITC-conjugated anti-CD3, PE/Cy7-conjugated anti-NK1.1, PE-conjugated anti-DX5 and PerCP/Cy5.5-conjugated anti-CD11c antibodies. Total hepatic NK cells were first enriched for NK1.1+CD3−cells using a FACSAria (BD Biosciences). Two NK subsets were then sorted: DX5+CD11clo NK cells and DX5−CD11chi NK cells. The purity of the sorted cells was greater than 95%. Conventional splenic CD4+ T cells were isolated from 6-8-week-old female dnTGFβRII-Foxp3GFP mice. First, total CD4+ T cells were magnetically sorted with anti-CD4 microbeads (Miltenyi Biotech, Germany). Total CD4+ T cells were labeled with PE-conjugated CD4 antibody, and conventional CD4+ T cells were sorted as CD4+Foxp3− cells. Supporter cells were prepared by labeling the splenic cells from dnTGFβRII-Foxp3GFP mice with PE/Cy7-conjugated anti-NK1.1 and PE-conjugated anti-CD4 and anti-CD8. Then, supporter cells were sorted as NK1.1-CD4-CD8- cells. The cells were treated with 25 μg/mL mitomycin C (Sigma, MO, USA) for 30 min at 37 °C, followed by four washes.
In vitro suppression assay
Conventional splenic CD4+Foxp3- T cells sorted from 6-8-week-old female dnTGFβRII-Foxp3GFP mice were labeled with Cell Trace Violet (Life Technologies, Carlsbad, CA, USA). DX5−CD11chi or DX5+CD11clo NK cells were cultured with Cell Trace Violet-labeled CD4+Foxp3- conventional T cells (5 × 104) at the indicated ratios in the presence of soluble anti-CD3 (1 μg/ml), anti-CD28 (1 μg/ml) (BioLegend) and mitomycin C-treated (Sigma, MO, USA) splenic supporter cells (1 × 105). After 72 h of coculture, cells were stained with a propidium iodide solution (BioLegend) for 15 min to exclude dead cells, and T cell proliferation was assessed by Cell Trace Violet dilution. Acquired data were analyzed with the FlowJo software proliferation module.
Microarray gene expression study
Hepatic DX5-CD11chi NK cells were sorted from dnTGFβRII and WT mice by fluorescence-activated cell sorting (FACS) to a purity of greater than 95%. Total RNA was extracted with a RNAiso Plus Kit (Takara, Dalian, China). Purified RNA was subjected to microarray analysis with an Affymetrix GeneChip® Mouse Transcriptome Assay 1.0, performed by Shanghai Biotechnology Corporation. All microarray data are available in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE99809. Expression fluorescence values were log2 transformed. Heat map analysis was conducted using TreeView and Cluster 3.0 software. Gene set enrichment analysis (GSEA) was performed by the JAVA program (http://www.broadinstitute.org/gsea) using the MSigDB C5:BP GO biological process collection (1320 gene sets available). A total of 1000 random gene set permutations were carried out, and the significance threshold was set at P < 0.05 with a FDR of <0.2.
Statistical analysis
All data are shown as the means ± SEMs. Statistical significance was determined by a two-tailed unpaired Student’s t-test. Correlations between parameters were assessed using linear regression analysis in GraphPad Prism. P-values of <0.05 were considered statistically significant.
Results
Correlation between PBC progression and hepatic NK cells
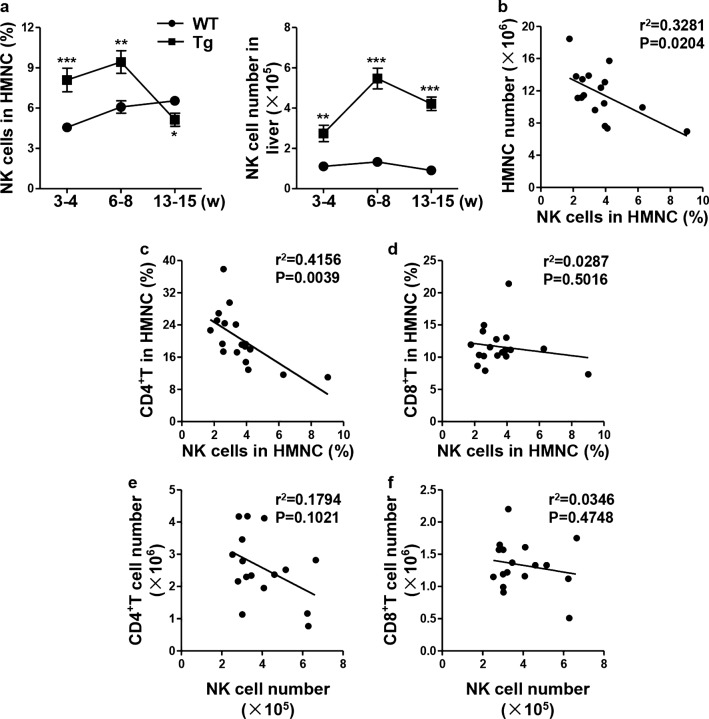
The percentage of dnTGFβRII hepatic NK cells was significantly higher in the asymptomatic stage (3–4 weeks; the number of hepatic MNCs was less than 3 × 106, similar to that in WT mice) (P = 0.0005) and early stage (6–8 weeks, hepatic MNC number 5–10 × 106) (P = 0.0036) but lower in the symptomatic stage of disease (13–15 weeks, hepatic MNC number > 10 × 106) (P = 0.0305) compared to that in age-matched WT littermate controls (Fig. 1a, left panel). However, there were higher numbers of NK cells in the livers of dnTGFβRII mice at all stages than in the livers of the WT littermate controls (P = 0.0011; P < 0.0001; P < 0.0001) (Fig. 1a, right panel). Correlation analysis showed that the number of hepatic mononuclear cells was negatively correlated with the frequency of liver NK cells at the age of 13–15 weeks (r2 = 0.3281, P = 0.0204) (Fig. 1b). The frequency of liver NK cells was negatively correlated with the number of hepatic CD4+ T cells (r2 = 0.4156, P = 0.0039) (Fig. 1c) but not with the number of hepatic CD8+ T cells (r2 = 0.0287, P = 0.5016) (Fig. 1d). The statistical analysis of cell numbers also demonstrated a negative correlation between CD4+ T cells and NK cells but not CD8+ T cells (Figs. 1e, f). These results suggested that NK cells may play an important role in the immunological pathogenesis of autoimmune cholangitis.
Fig. 1.
Evolution of hepatic NK cells in the progression of cholangitis. a The percentage (left) and absolute number (right) of NK1.1+CD3- cells in the liver from dnTGFβRII (Tg) and littermate control wild-type (WT) mice at different stages of disease progression (3–4 weeks: asymptomatic; 6–8 weeks: early stage; 13–15 week: clinical disease) (n = 5–8). b Linear regression analysis of the percentage of hepatic NK1.1+CD3- cells versus the hepatic MNC numbers. c-d Percentage of hepatic CD4+ T cells (c) and CD8+ T cells (d) versus that of hepatic NK1.1+CD3- cells in 13–15-week-old Tg mice (n = 16-18). e-f Number of hepatic CD4+ T cells (e) and CD8+ T cells (f) versus that of NK1.1+CD3- cells in 13–15-week-old Tg mice (n = 16–18). *P < 0.05; **P < 0.01; ***P < 0.001
Deletion of NK cells exacerbates autoimmune cholangitis in dnTGFβRII mice
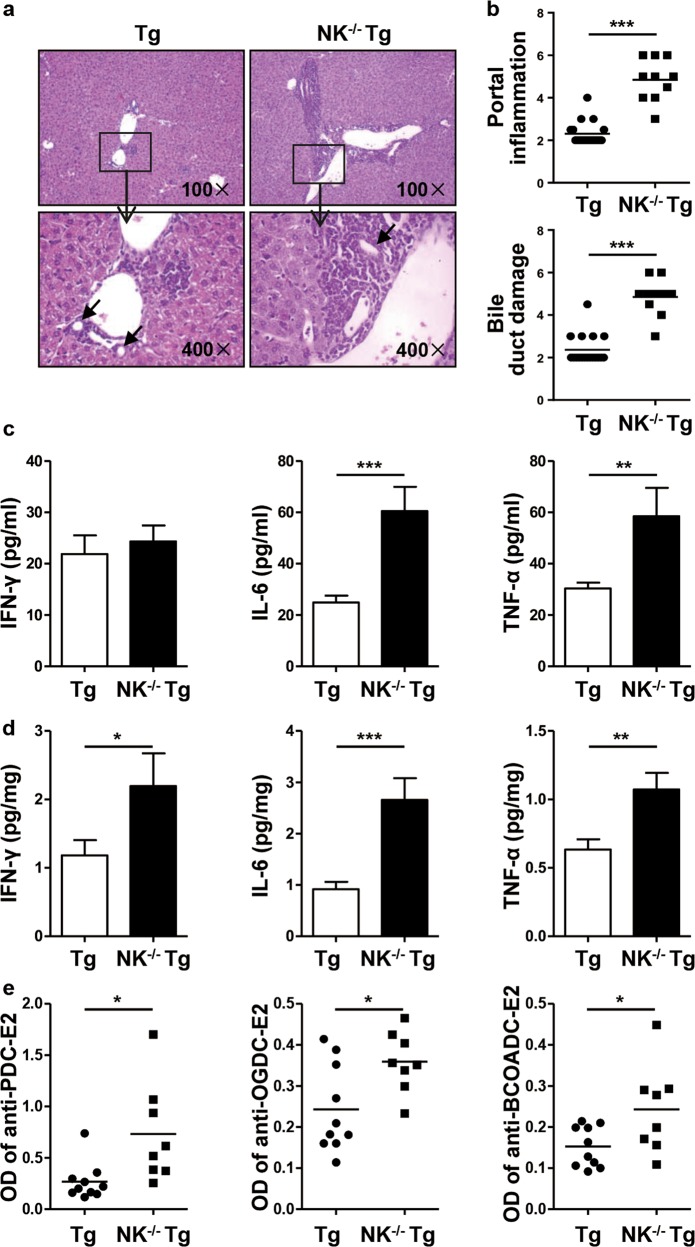
Nfil3 is required for the development and maturation of NK cells.26 We generated Nfil3−/− dnTGFβRII mice as NK−/−dnTGFβRII mice. There were few NK cells in the livers of NK-/-dnTGFβRII mice. Histological examination of liver tissue sections indicated that deletion of NK cells aggravated liver inflammation in dnTGFβRII mice at the age of 13–15 weeks (Fig. 2a). These mice exhibited more lymphocytes infiltrating around the portal tracts (P < 0.0001) and more severe bile duct damage (P < 0.0001) than dnTGFβRII mice (Fig. 2b). The expression levels of inflammatory cytokines, including IL-6 (P = 0.0005, P = 0.0002) and TNF-α (P = 0.0067, P = 0.0042), were significantly higher in both serum and liver tissues, while IFN-γ (P = 0.0458, P = 0.6505) expression was elevated in the liver tissues but not in the serum of NK−/−dnTGFβRII mice compared with that in dnTGFβRII mice (Fig. 2c, d). PBC is characterized by the presence of anti-mitochondrial Abs (AMAs).39 AMAs can react with an epitope on the E2 subunit of the pyruvate dehydrogenase enzyme complex (PDC-E2), the E2 subunit of the functionally related 2-oxo-acid dehydrogenase complex branched chain 2-oxo-acid dehydrogenase (BCOADC-E2), and the E2 subunit of 2-oxoglutarate dehydrogenase (OGDC-E2).40 Indeed, here, we observed higher levels of serum autoantibodies against PDC-E2 (P = 0.0122), OGDC-E2 (P = 0.0188) and BCOADC-E2 (P = 0.0298) in NK−/−dnTGFβRII mice than in dnTGFβRII mice (Fig. 2e).
Fig. 2.
Autoimmune cholangitis was exacerbated in NK-/-dnTGFβRII mice compared to dnTGFβRII mice. a Representative H&E staining of liver sections from dnTGFβRII (Tg) and NK-/-dnTGFβRII mice (NK−/− Tg). The black arrows indicate the damaged bile duct. b Portal inflammation and bile duct damage scores in Tg mice (n = 18) and NK−/− Tg mice (n = 9). c-d The expression levels of IFN-γ, IL-6 and TNF-α in serum (c) and liver tissue homogenate (d) from Tg (n = 10) and NK−/− Tg mice (n = 6). e Serum levels of anti-PDC-E2, anti-OGDC-E2 and anti-BCOADC-E2 in Tg mice (n = 10) and NK−/− Tg mice (n = 8). *P < 0.05; **P < 0.01; ***P < 0.001
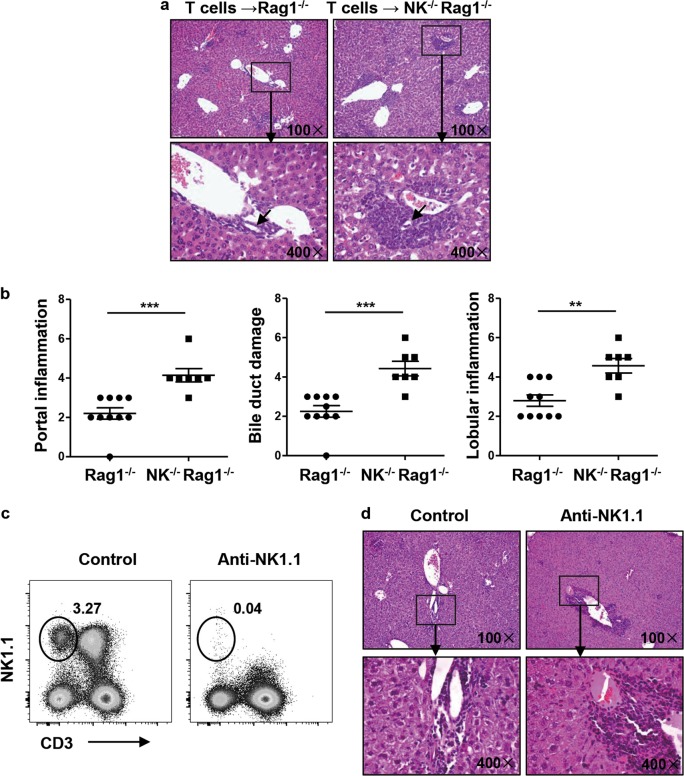
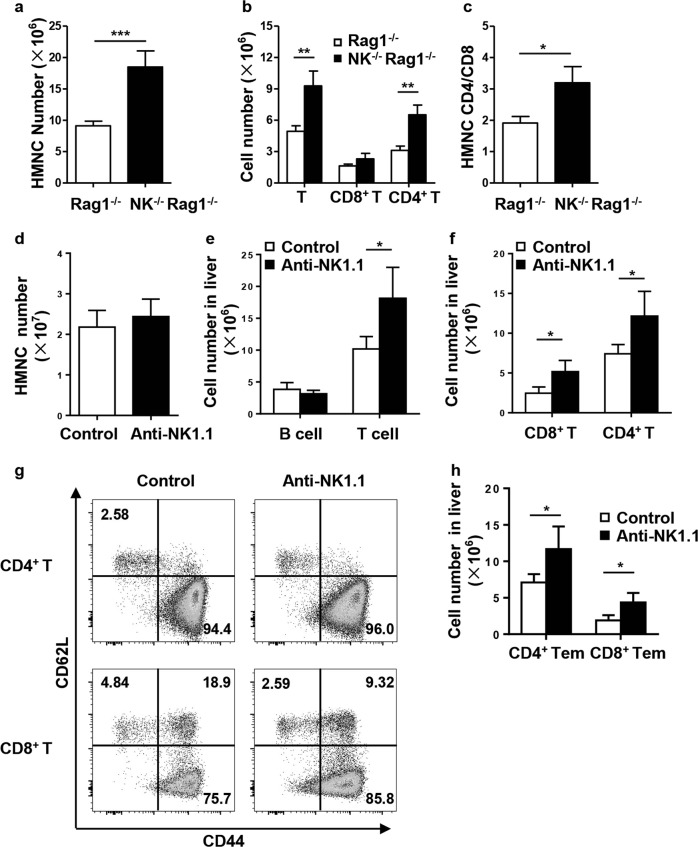
To investigate the regulatory function of NK cells, we cotransferred splenic CD4+ T cells and CD8+ T cells derived from dnTGFβRII mice into Rag1−/− or NK−/−Rag1−/− mice. Histopathological analysis revealed that NK−/−Rag1−/− recipients developed more severe liver inflammation than Rag1−/− recipients (Fig. 3a). NK−/−Rag1−/− recipients also exhibited more lymphocytes infiltrating around the portal tracts (P = 0.0006) and more severe bile duct damage (P = 0.0003) and lobular inflammation (P = 0.0017) than Rag−/− recipients (Figs. 3a, b). In addition, we used an anti-PK136 antibody to deplete NK cells and further confirmed the role of NK cells in the pathogenesis of PBC. The anti-PK136 antibody effectively depleted NK cells in the liver (Fig. 3c). Histopathological analysis of liver sections revealed that NK-depleted mice developed more severe liver inflammation around the portal vein than control mice (Fig. 3d and Supplementary Figure 1a).
Fig. 3.
Autoimmune cholangitis was exacerbated in the adoptive transfer and antibody-mediated NK cell depletion model. a-b Splenic CD4+ T and CD8+ T cells from dnTGFβRII mice were transferred into NK−/−Rag1−/− mice or Rag1−/− mice, and the recipient mice were sacrificed 3–4 weeks later. a Representative H&E staining of liver sections from recipient mice. The black arrows indicate the damaged bile duct. b Scores for liver portal inflammation, bile duct damage and lobular inflammation in NK−/−Rag1−/− mice (n = 7) and Rag1−/− mice (n = 10). c-d An anti-PK136 antibody was intraperitoneally injected into dnTGFβRII mice every two weeks, and the mice were sacrificed 4 weeks later. c Representative depleting effect of the anti-PK136 antibody on liver NK cells. d Representative H&E staining of liver sections from anti-PK136 antibody-treated and control mice
The CD4+ T cell response is enhanced in NK−/−dnTGFβRII mice
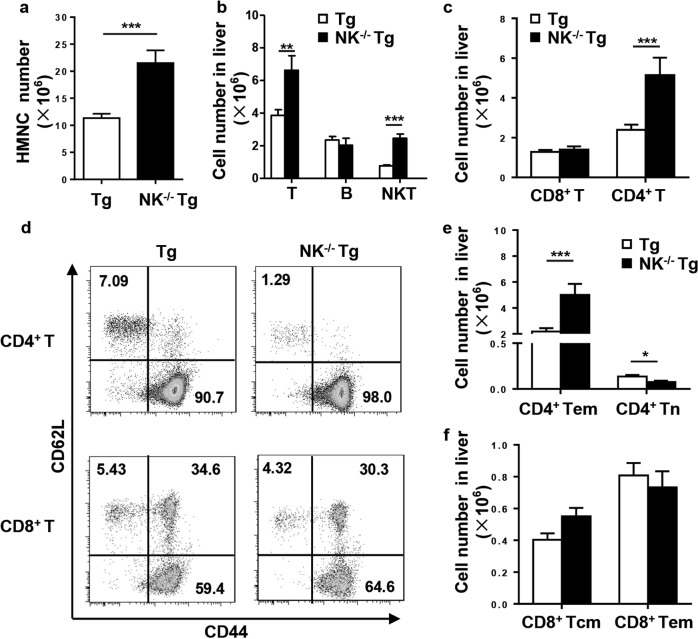
Consistent with the liver histopathology, the number of infiltrating cells in NK−/−dnTGFβRII mice was significantly increased compared with that in dnTGFβRII mice (P < 0.0001) (Fig. 4a). In parallel, the number of T cells (CD3+NK1.1−) (P− = 0.0017) and iNKT cells (CD3+CD1d-tetramer+) (P < 0.0001) was markedly increased after NK deletion in dnTGFβRII mice, while the number of B cells was unchanged (P = 0.4742) (Fig. 4b). The expanded T cells mainly consisted of CD4+ T cells (P = 0.0006) rather than CD8+ T cells (P = 0.5479) in NK−/−dnTGFβRII mice with respect to the population in dnTGFβRII mice (Fig. 4c). We found that the number of effector memory CD4+ T cells (CD44+CD62L−) was significantly increased in NK−/−dnTGFβRII mice (P = 0.0003) (Figs. 4d, e). In contrast, the numbers of cells in the central memory CD8+ T (CD44+CD62L+) and effector memory CD8+ T (CD44+CD62L−) subsets were not altered (Figs. 4d, f). Similar results were obtained in the adoptive transfer model; the numbers of hepatic MNCs (P = 0.0346) and CD3+ T cells (P = 0.0318) were significantly increased in NK−/−Rag1−/− recipients (Fig. 5a). CD4+ T cells (P = 0.0067) but not CD8+ T cells (P = 0.5946) were dominant in CD3+ T cell expansion (Fig. 5b). The ratio of CD4+ T to CD8+ T cells was markedly higher in NK−/−Rag1−/− mice than in Rag1−/− control mice (P = 0.0057) (Fig. 5c). In mice depleted of NK cells with the anti-NK1.1 antibody, we found that the total number of hepatic mononuclear cells (P = 0.4168) was not changed (Fig. 5d), but the number of hepatic T cells (P = 0.0225), including CD4+ T cells (P = 0.0288) and CD8+ T cells (P = 0.0149), was significantly increased (Figs. 5e, f). The number of effector memory CD4+ T cells (P = 0.0320) as well as that of CD8+ T cells (CD44+CD62L−) (P = 0.0156) was significantly increased in NK-depleted mice (Figs. 5g, h).
Fig. 4.
Profile of hepatic lymphocyte subsets from NK−/−dnTGFβRII and dnTGFβRII mice. a)Total number of hepatic MNCs. b Number of T cells (CD3+NK1.1-), B cells (CD19+) and iNKT cells (CD3+CD1d-tetramers+). c Number of CD4+ T and CD8+ T cells. d Expression of CD44 and CD62L on hepatic CD4+ T and CD8+ T cells. e Number of effector memory (CD44+CD62L-) and naive (CD44−CD62L+) CD4+ T cells. f Number of hepatic central memory (CD44+CD62L+) and effector memory (CD44+CD62L−) CD8+ T cells. *P < 0.05; **P < 0.01; ***P < 0.001
Fig. 5.
T cell numbers were increased after NK cell depletion. a Total number of liver mononuclear cells from NK−/−Rag1−/− mice (n = 3) and Rag1−/− mice (n = 5). b Absolute number of total hepatic T cells, CD4+ T cells and CD8+ T cells from NK−/−Rag1−/− mice (n = 3) and Rag1−/− mice (n = 5). c CD4/CD8 ratio in NK−/−Rag1−/− mice (n = 3) and Rag1−/− mice (n = 5). d Total number of liver mononuclear cells from anti-PK136 antibody-treated and control mice. e, f Representative numbers of B cells, T cells, CD4+ T cells, and CD8+ T cells in control (n = 4) and anti-PK136 antibody-treated mice (n = 4). g Expression of CD44 and CD62L on hepatic CD4+ T and CD8+ T cells. h Number of effector memory (CD44+CD62L−) CD4+ T and CD8+ T cells in anti-PK136 antibody-treated and control mice. *P < 0.05; **P < 0.01
DX5−CD11chi liver-resident NK cells inhibit CD4+ T cell proliferation in vitro
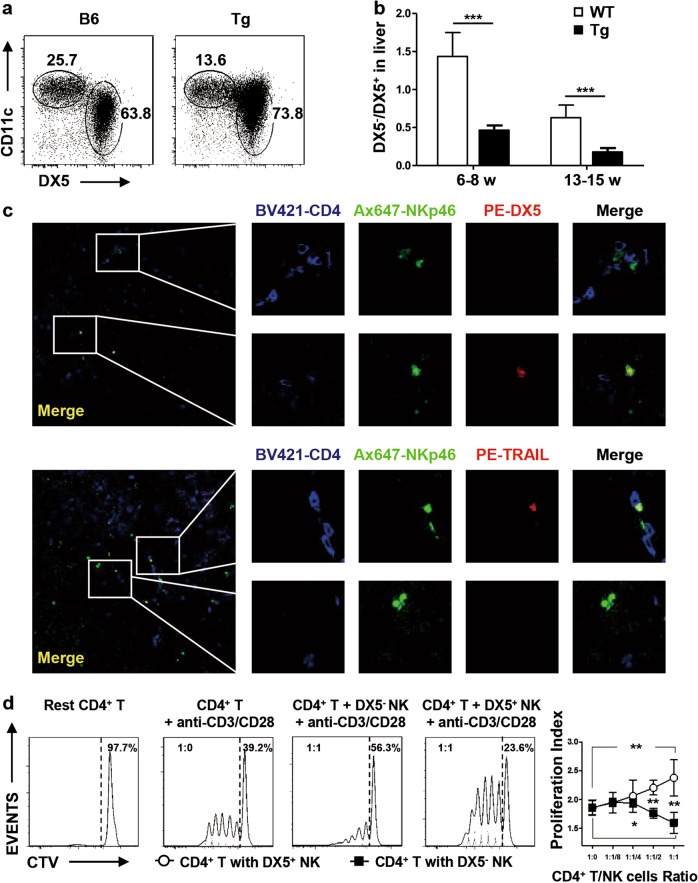
Liver-resident NK cells are distinct from other NK cell populations. DX5 is typically used to distinguish these two groups of cells, and hepatic DX5− NK cells express high levels of CD11c.28 Hence, CD11c and DX5 were used to define DX5−CD11chi cells as liver-resident NK cells and DX5+CD11clo cells as conventional NK cells in WT mice and dnTGFβRII mice (Fig. 6a). The ratio of DX5−CD11chi to DX5+CD11clo NK cells was significantly lower in dnTGFβRII mice than in wild-type mice both in the early stage (6-8 weeks) (P = 0.0009) and typical symptom stage (13–15 weeks) (P = 0.0003) (Fig. 6b). Liver-resident NK cells are also defined as TRAIL+DX5− cells in the mouse liver.41 By immunofluorescence staining of DX5 and TRAIL, we found a divergent distribution of conventional NK cells and resident NK cells in the liver of dnTGFβRII mice. NKp46+DX5− and NKp46+TRAIL+ liver-resident NK cells but not NKp46+DX5+ or NKp46+TRAIL− NK cells, were colocalized with CD4+ T cells (Fig. 6c), suggesting interactions between liver-resident NK cells and CD4+ T cells. Next, hepatic DX5-CD11chi or DX5+CD11clo NK cells from dnTGFβRII mice were sorted and cocultured with conventional CD4+ T cells from dnTGFβRII mice. Liver-resident NK cells but not conventional NK cells effectively suppressed CD4+ T cell proliferation in an in vitro suppression assay (Fig. 6d).
Fig. 6.
Liver-resident NK cells suppressed CD4+ T cell proliferation in vitro. a Hepatic NK cells were classified into DX5−CD11chi and DX5+CD11clo cell subsets by flow cytometry. b Representative ratio of DX5− to DX5+ NK cells in the early stage (n = 8) and typical symptom stage (n = 5–6) referenced in Fig. 6(a). c Representative immunostaining of frozen liver sections from Tg mice labeled with anti-CD4 BV421, anti-NKp46 Ax647, anti-DX5 PE and anti-TRAIL PE; confocal microscopy was then used to assess the location of NK cells and CD4+ T cells (200×). Here, NKp46+DX5− or NKp46+TRAIL+ represents liver-resident NK cells. d Representative FACS histograms of Cell Trace Violet-labeled conventional CD4+ T cells cocultured with hepatic DX5− or DX5+ NK cells from Tg mice under stimulation by 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28. The data are representative of the results of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001
The expression of genes involved in the negative regulation of the immune response is enhanced in dnTGFβRII mice
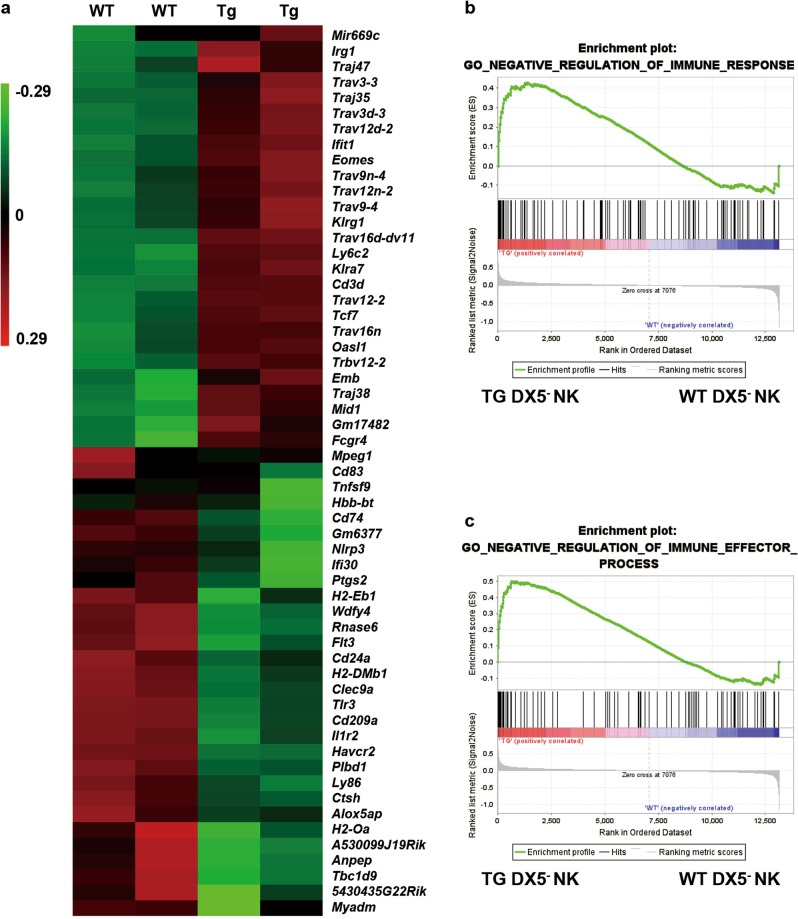
We sorted hepatic DX5−NK cells from WT and dnTGFβRII mice and performed microarray analysis. There were 36,996 transcripts upregulated and 28,954 transcripts downregulated in DX5− NK cells from dnTGFβRII mice relative to their expression levels in DX5− NK cells from WT mice. Transcripts with a fold change of 3 or higher are listed in Fig. 7a. We evaluated the effects of the inflammatory environment on liver-resident NK cells by comparing the gene expression profiles using gene set enrichment analysis (GSEA). Genes involved in the negative regulation of the immune response (P = 0.02, FDR = 0.13, ES = 0.43) and the immune effector process (P = 0.005, FDR = 0.08, ES = 0.5) were enriched in liver-resident NK cells from dnTGFβRII mice (Figs. 7b, c) compared with their expression in liver-resident NK cells from WT mice, suggesting that the suppressive function of liver-resident NK cells is associated with the inflammatory hepatic microenvironment.
Fig. 7.
Gene expression profile of liver-resident NK cells in an inflammatory environment. a Gene expression comparison of DX5−NK cells from wild-type (WT) and dnTGFβRII mice (Tg). The heat map shows the genes with expression differences of greater than 3-fold. The genes in the upper section were upregulated in Tg mice, and the genes in the lower section were downregulated in Tg mice. b, c GSEA analysis of the GO negative regulation of immune response and immune effector process terms in differentially expressed genes in hepatic DX5- NK cells from WT and Tg mice. Microarray analyses were performed with two biological replicates per group
Discussion
We previously reported that mice expressing a dominant negative form of TGFβRII under the control of the CD4 promoter lacking the CD8 silencer spontaneously develop autoimmune cholangitis.7 dnTGFβRII mice exhibit the major serological and histological characteristics of human PBC. Conventional immune cells, including CD4+ T cells, CD8+ T cells and iNKT cells, and inflammatory cytokines, such as IL-6, IL-12p35, IL-12p40 and IFN-γ, play important roles in the pathogenesis of autoimmune cholangitis in dnTGFβRII mice.1 The interaction between immune cell subsets is still not well defined. NK cells are a relatively abundant population among liver-resident immune cells. Clinical studies have demonstrated an increase in the frequency and absolute number of hepatic NK cells in patients with PBC, but the functional significance of this difference is unclear.5
Herein, we used Nfil3-deficient mice backcrossed to dnTGFβRII mice to obtain NK−/−dnTGFβRII mice. Although the number of hepatic NK1.1+ cells was markedly decreased in Nfil3-/-dnTGFβRII mice, there was a small residual population of hepatic NK1.1+ cells. These data are consistent with those of a recent study showing that Nfil3 is crucial for the development, phenotype, fitness and survival of circulating NK cells but is not strictly required for the development of tissue-resident NK cells.29 We used two additional models to further confirm the role of NK cells and obtained similar results showing that NK cell deficiency exacerbated autoimmune cholangitis. Notably, we reported that iNKT cells exacerbate liver injury in dnTGFβRII mice and that a lack of CD1d-restricted iNKT cells resulted in significantly decreased hepatic lymphoid cell infiltration and liver injury in CD1d-/-dnTGFβRII mice.13 In the present study, anti-PK136 effectively depleted NK cells as well as NK1.1+CD3+ NKT cells in the liver. However, portal inflammation was not alleviated when both NK and NKT populations were depleted in anti-PK136-treated dnTGFβRII mice. Instead, an increased number of cellular infiltrates in the liver, including effector memory CD4+ and CD8+ T cells, was observed, suggesting an important regulatory function of NK cells in controlling hepatic T cells. More importantly, we demonstrated that unlike their conventional counterpart, DX5−CD11chi liver-resident NK cells protected against liver inflammation, colocalized with CD4+ T cells, and inhibited CD4+ T proliferation in dnTGFβRII mice. Utilizing our mouse models, we explored the functions of liver-resident NK cells, which cannot be accomplished in a clinical study. However, we cannot exclude the possibility that DX5− NK cells are a heterogeneous population. In addition, the reason that only DX5− NK cells colocalized with CD4+ T cells in an inflammatory environment needs to be further explored. In addition to inhibiting CD4+ T cell responses, NK cells suppress liver damage by other means. For example, it has been shown that the antifibrotic activity of NK cells in experimental liver injury occurs through the killing of activated hepatic stellate cells (HSCs) in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-dependent manners.25,42 Additionally, CXCL10 can promote liver fibrosis by preventing NK cell-mediated HSC inactivation. Additionally, NK cells can participate in crosstalk with other immune cells. NK cells may perform protective roles in either suppressing cholestatic liver injury by stimulating Kupffer cell-dependent IL-6 production43 or inhibiting T cell hepatitis by inducing T and NKT cell apoptosis.16,44
Recent studies have demonstrated that NK cells exhibit distinct phenotypic and functional characteristics in different tissues.45 One unique aspect of liver immunology is the large number of liver-resident NK cells. Previously, many studies of liver NK cells in PBC were based on bulk liver NK cells from rodents or human peripheral blood, and the role of different subsets of liver NK cells was less defined. We explored the functions of conventional NK cells and liver-resident NK cells separately and demonstrated the immunosuppressive role of liver-resident NK cells. We found higher levels of PD-L1 on liver-resident NK cells from dnTGFβRII mice than on those from WT mice (Supplementary Figure 1b). The interaction between NK cells and CD4+ T cells may play an important role in the suppressive function of liver-resident NK cells. DX5− NK cells had a more suppressive effect in an inflammatory environment than in a normal liver environment. GSEA analysis showed enhanced negative regulation of liver-resident NK cells in dnTGFβRII mice. The total numbers of NK cells were increased, but the ratio of DX5−:DX5+ NK cells was significantly decreased, indicating that the balance between these regulatory and effector NK cell subsets was disrupted in dnTGFβRII mice. We acknowledge that two limitations remain in our study. First, we note that there were no adoptive transfer experiments to further address the regulatory function of DX5-CD11chi liver-resident NK cells that we observed in Nfil3−/− dnTGFβRII mice and in the in vitro inhibition assays. It has been indicated that NK cells can adjust their reactivity through the altered engagement and ligation of receptors.46,47 Thus, it is still not clear whether the regulatory function of liver-resident NK cells depends on the inflammatory environment in dnTGFβRII mice. Second, we demonstrated that liver-resident NK cells colocalized with CD4 ± T cells in inflamed liver tissues. However, it is still unknown how this NK population interacts with CD4 ± T cells. Further studies are needed to fully understand NK cell biology and the interaction and crosstalk between different subsets of NK cells and other immune cells.
In summary, we demonstrated the immunosuppressive function of liver-resident NK cells. Targeting liver-resident NK cells by enhancing their regulatory function may be a tissue-specific therapeutic strategy for PBC.
Supplementary information
Acknowledgements
This study was supported by the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S054), the National Natural Science Foundation of China (81601416, 81430034, 91542123), the National Key R&D Program of China (2017YFA0205600) and a National Institutes of Health grant (DK090019).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhi-Bin Zhao, Fang-Ting Lu, Hong-Di Ma.
Contributor Information
Jie Cao, Email: eycaojie@scut.edu.cn.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Zhe-Xiong Lian, Phone: +86-020-39380962, Email: zxlian@scut.edu.cn.
Electronic supplementary material
The online version of this article (10.1038/s41423-019-0199-z) contains supplementary material.
References
- 1.Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu. Rev. Pathol. 2013;8:303–330. doi: 10.1146/annurev-pathol-020712-164014. [DOI] [PubMed] [Google Scholar]
- 2.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 3.Selmi C, et al. Experimental evidence on the immunopathogenesis of primary biliary cirrhosis. Cell. Mol. Immunol. 2010;7:1–10. doi: 10.1038/cmi.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomiyama T, et al. The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cell. Mol. Immunol. 2017;14:276–284. doi: 10.1038/cmi.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang YH, et al. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J. Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/S1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 7.Oertelt S, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J. Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 8.Ma HD, et al. Chemokine receptor CXCR3 deficiency exacerbates murine autoimmune cholangitis by promoting pathogenic CD8(+) T cell activation. J. Autoimmun. 2017;78:19–28. doi: 10.1016/j.jaut.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang GX, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawata K, et al. Clonality, activated antigen-specific CD8( + ) T cells, and development of autoimmune cholangitis in dnTGFbetaRII mice. Hepatology. 2013;58:1094–1104. doi: 10.1002/hep.26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Weici, Zhang Ren, Zhang Jun, Sun Ying, Leung Patrick SC, Yang Guo-Xiang, Shuai Zongwen, Ridgway William M, Gershwin M Eric. Proteomic analysis reveals distinctive protein profiles involved in CD8+ T cell-mediated murine autoimmune cholangitis. Cellular & Molecular Immunology. 2018;15(8):756–767. doi: 10.1038/cmi.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moritoki Y, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang YH, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, et al. Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J. Autoimmun. 2015;59:26–37. doi: 10.1016/j.jaut.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwada M, Pham NL, Pewe LL, Harty JT, Rothman PB. NFIL3/E4BP4 is a key transcription factor for CD8alpha(+) dendritic cell development. Blood. 2011;117:6193–6197. doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B. Basic liver immunology. Cell. Mol. Immunol. 2016;13:265–266. doi: 10.1038/cmi.2016.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier S, et al. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J. Hepatol. 2010;53:805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen C, et al. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell. Mol. Immunol. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn C, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Yinli, Han Qiuju, Hou Zhaohua, Zhang Cai, Tian Zhigang, Zhang Jian. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cellular & Molecular Immunology. 2016;14(5):465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell. Mol. Immunol. 2016;13:328–336. doi: 10.1038/cmi.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laso FJ, et al. Chronic alcohol consumption is associated with an increased cytotoxic profile of circulating lymphocytes that may be related with the development of liver injury. Alcohol. Clin. Exp. Res. 2010;34:876–885. doi: 10.1111/j.1530-0277.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 25.Radaeva S, et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 26.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Male V, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J. Exp. Med. 2014;211:635–642. doi: 10.1084/jem.20132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sojka DK, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda Shinji, Harada Kenichi, Niiro Hiroaki, Shirabe Ken, Taketomi Akinobu, Maehara Yoshihiko, Tsuneyama Koichi, Nakanuma Yasuni, Leung Patrick, Ansari Aftab A., Gershwin M. Eric, Akashi Koichi. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53(4):1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao B, Bertola A. Natural killer cells take two tolls to destruct bile ducts. Hepatology. 2011;53:1076–1079. doi: 10.1002/hep.24275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z, Gershwin ME, Zhang C. Regulatory NK cells in autoimmune disease. J. Autoimmun. 2012;39:206–215. doi: 10.1016/j.jaut.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Li L, et al. Natural killer cells-produced IFN-gamma improves bone marrow-derived hepatocytes regeneration in murine liver failure model. Sci. Rep. 2015;5:13687. doi: 10.1038/srep13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D, et al. Characterization and application of monoclonal antibodies against Mycoplasma hyorhinis pyruvate dehydrogenase E1 complex subunit alpha. Appl. Microbiol. Biotechnol. 2016;100:3587–3597. doi: 10.1007/s00253-015-7263-0. [DOI] [PubMed] [Google Scholar]
- 37.Yang W, et al. Differential modulation by IL−17A of Cholangitis versus Colitis in IL-2Ralpha deleted mice. PLoS One. 2014;9:e105351. doi: 10.1371/journal.pone.0105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Y, et al. Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Ralpha(−/−) mice. J. Autoimmun. 2014;51:99–108. doi: 10.1016/j.jaut.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin. Gastroenterol. Hepatol. 2003;1:297–302. doi: 10.1016/S1542-3565(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 40.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J. Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 41.Zhang LH, Shin JH, Haggadone MD, Sunwoo JB. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 2016;213:2249–2257. doi: 10.1084/jem.20151998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melhem A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J. Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Cheng CW, et al. NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J. Hepatol. 2011;54:746–752. doi: 10.1016/j.jhep.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, et al. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J. Hepatol. 2006;44:446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y, Tian Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell. Mol. Immunol. 2017;14:321–330. doi: 10.1038/cmi.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bern Michael D., Parikh Bijal A., Yang Liping, Beckman Diana L., Poursine-Laurent Jennifer, Yokoyama Wayne M. Inducible down-regulation of MHC class I results in natural killer cell tolerance. The Journal of Experimental Medicine. 2018;216(1):99–116. doi: 10.1084/jem.20181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.