Abstract
OX40L is one of the co-stimulatory molecules that can be expressed by splenic lymphoid tissue inducer (Lti) cells, a subset of group 3 innate lymphoid cells (ILC3s). OX40L expression in subsets of intestinal ILC3s and the molecular regulation of OX40L expression in ILC3s are unknown. Here, we showed intestinal ILC3s marked as an OX40Lhigh population among all the intestinal leukocytes and were the dominant source of OX40L in Rag1–/– mice. All ILC3 subsets expressed OX40L, and NCR–ILC3s were the most abundant source of OX40L. The expression of OX40L in ILC3s could be upregulated during inflammation. In addition to tumor necrosis factor (TNF)-like cytokine 1A (TL1A), which has been known as a trigger for OX40L, we found that Poly (I:C) representing viral stimulus promoted OX40L expression in ILC3s via a cell-autonomous manner. Furthermore, we demonstrated that IL-7-STAT5 signaling sustained OX40L expression by ILC3s. Intestinal regulatory T cells (Tregs), most of which expressed OX40, had defective expansion in chimeric mice, in which ILC3s were specifically deficient for OX40L expression. Consistently, co-localization of Tregs and ILC3s was found in the cryptopatches of the intestine, which suggests the close interaction between ILC3s and Tregs. Our study has unveiled the crosstalk between Tregs and ILC3s in mucosal tissues through OX40–OX40L signaling, which is crucial for the homeostasis of intestinal Tregs.
Keywords: Group 3 innate lymphoid cells, OX40L, Regulatory T cells, Intestinal immunity
Subject terms: Innate lymphoid cells, Mucosal immunology
Introduction
Group 3 innate lymphoid cells (ILC3s), featured by the expression of master regulator RORγt (RAR-related orphan receptor gamma t), are a subset of ILCs that lack antigen-specific T- and B-cell receptors.1,2 ILC3s are widely distributed in mucosal tissues of both humans and mice, particularly in the intestine, and play important roles in inflammation and immune regulation.3–5 NCR+ILC3, NCR–ILC3 and Lti cells (lymphoid tissue inducer cells) are three subsets of ILC3s that are defined according to the characteristic expression of surface molecules.6
Although ILCs belong to the lymphoid lineage, they express a series of molecules that are typically expressed by antigen-presenting cells, which enable their interaction with T cells in the microenvironment.7–9 OX40L (encoded by Tnfsf4), one of the members of the tumor necrosis factor (TNF) superfamily, has been found to be expressed by mouse splenic Lti cells and ILC3s in the meninges.10–12 Moreover, in humans, OX40L+ ILC3s have been found in the tonsil.13,14
In mice, the expression of OX40L on splenic Lti cells can be barely detected in neonates, whereas it increases after birth.15 The mechanism underlying this time-dependent expression is unclear. TL1A, another protein of the TNF superfamily, has been reported to be a strong trigger for OX40L expression in Lti cells.16 However, the expression of TL1A in the spleen is comparable between neonates and adults,16 which suggests TL1A may not be accountable for the differential expression of OX40L in ILC3s from young and adult mice. Environmental factors may affect the expression of OX40L in ILC3s.
OX40, the receptor for OX40L, could be expressed by effector and memory CD4+ T cells.17 Previous reports suggest that OX40L derived from Lti cells is essential for the development and maintenance of CD4 T-cell memory, which suggests there is a crosstalk between Lti cells and T cells through OX40L–OX40 signaling.10,11 With the exception of Lti cells, the signal of OX40L can also be derived from other cell types, particularly from DCs (dendritic cells).18 Moreover, the association of T cells with both Lti cells and DCs could be observed at the B:T cell borders of B-cell follicles.10 However, in mice that lack Lti cells, the CD4 T-cell memory is defective despite the intact OX40L expression in DCs.11 This finding suggests that Lti cells provide an irreplaceable signal for CD4 T-cell memory, potentially through OX40L–OX40 signaling.
Among CD4+ T cells, OX40 is mainly expressed by regulatory T cells (Tregs) in both the periphery and the intestine.19 Tregs are characterized by the expression of transcription factor Foxp3 (forkhead box P3) and play central roles in immune regulation by suppressing inflammatory responses.20,21 OX40 signaling has been reported to be critical for the development and maintenance of Tregs.19,22,23 Forced expression of OX40L in vivo caused an expansion of Tregs in the thymus and spleen.22 Moreover, OX40-deficient mice have a reduced number of Tregs in the spleen of young but not adult mice.22 However, a defective maintenance of intestinal Tregs has been observed in OX40-deficient mice at an adult age.19 These findings indicate that OX40 is particularly important for the homeostasis of intestinal Tregs. The source of OX40L in the intestine remains elusive. Whether ILC3s serve as sources of intestinal OX40L, or their crosstalk with Tregs in the intestine through OX40L, has not been reported so far.
In this study, we investigated the regulation of the expression and function of OX40L in intestinal ILC3s. We found ILC3s were the major source of OX40L among intestinal leukocytes. The expression of OX40L in intestinal ILC3s was gradually increased after birth, and NCR–ILC3s were the most abundant source of OX40L. The expression of OX40L on intestinal ILC3s was triggered by inflammation and suppressed by CD4+ T cells. Moreover, we demonstrated that IL-7-STAT5 signaling sustained the OX40L expression by intestinal ILC3s. Finally, we showed that ILC3-derived OX40L was essential for Treg homeostatic expansion in the intestine, potentially through a direct interaction with OX40 expressed by Tregs. Our study indicates the supportive role of ILC3s on Tregs in the intestine through OX40L–OX40 interaction.
Results
OX40L is expressed by all subsets of intestinal ILC3s in adult mice
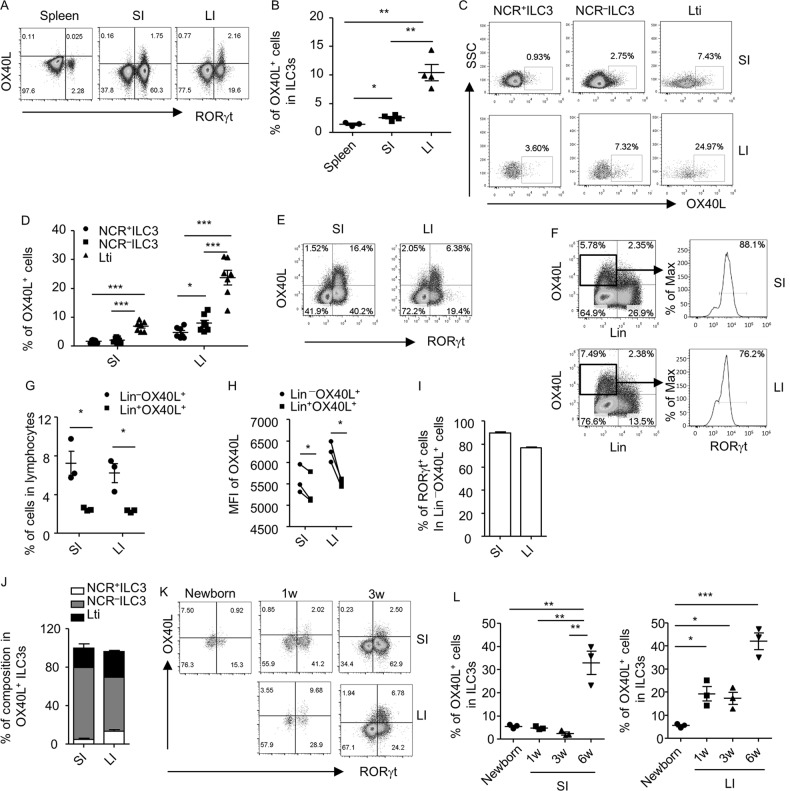
OX40L has been shown to be expressed by splenic Lti cells of both wild-type and Rag1–/– mice.10,15,16 We analyzed the expression of OX40L in all subsets of ILC3s in the intestine of Rag1–/– mice, which have more ILC3s than wild-type mice.24 Consistent with a previous report, the expression of OX40L in splenic ILC3s could hardly be detected when cells were freshly isolated (Fig. 1a).10 Compared to splenic ILC3s, intestinal ILC3s expressed a significantly higher level of OX40L (Fig. 1a, b and Supplementary Fig. S1A). Moreover, the percentage of OX40L+ cells was higher in large than in small intestinal ILC3s (Fig. 1a, b). To gain a full view of OX40L-expressing cells, we analyzed the expression of OX40L in lineage-negative (Lin–, mainly representing innate lymphoid cells) and Lin+ cells (mainly representing myeloid cells and NK cells). Consistently, we observed a higher proportion of Lin–OX40L+ cells than Lin+OX40L+ cells gated on lymphocytes (Supplementary Fig. S1B and S1C). Furthermore, the majority of Lin–OX40L+ cells were ILC3s (Lin–RORγt+) (Supplementary Fig. S1B and S1D). Moreover, the mean fluorescence intensity (MFI) of OX40L+ cells, which reflects the OX40L expression at a per-cell-based level, in Lin–OX40L+ cells was higher than in Lin+OX40L+ cells (Supplementary Fig. S1E). Therefore, we conclude that ILC3s mark as an OX40Lhigh population in the intestine and are the dominant source of OX40L among intestinal leukocytes in Rag1–/– mice.
Fig. 1.
OX40L is highly expressed by intestinal ILC3s of adult mice. Splenocytes and small (SI) and large intestinal (LI) LPLs were isolated from 6-week-old (a–j and l) or young-age (k and l) Rag1–/– mice. Cells were analyzed when freshly isolated (a–d) or after being cultured overnight (e-l). a, e, and k The expression of OX40L and RORγt gated on lineage-negative (Lin–, CD3–B220–CD11b–CD11c–) cells in live lymphocytes was analyzed by flow cytometry. b and l Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. c The expression of OX40L in NCR+ILC3s (Lin–RORγt+NKp46+), NCR–ILC3s (Lin–RORγt+NKp46–CD4–), and Lti cells (Lin–RORγt+NKp46–CD4+) was analyzed by flow cytometry. d Percentages of OX40L+ cells gated on indicated ILC3 subsets are shown. f Expression of Lin and OX40L gated on live lymphocytes was analyzed by flow cytometry. Histogram of RORγt expression gated on Lin–OX40L+ cells is shown. g Percentages of Lin–OX40L+ (upper left quadrant in (f)) and Lin+OX40L+ cells (upper right quadrant in (f)) gated on live lymphocytes are shown. h MFI of OX40L of Lin–OX40L+ and Lin+OX40L+ cells in (f) is shown, respectively. Connected dots were paired data from biological repeats. Statistical analysis was performed with paired t test. i Percentages of RORγt+ cells in Lin–OX40L+ cells in (f) are shown. j Percentages of the composition of different ILC3 subsets within OX40+ILC3s are shown. a–l Data are representative of three independent experiments. LPLs, lamina propria lymphocytes; MFI, mean fluorescence intensity
Notably, OX40L expression could be detected on all subsets of ILC3s, including NCR+ILC3s, NCR–ILC3s, and Lti cells, in Rag1–/– mice (Fig. 1c, Supplementary Fig. S1F and S1G). Although the highest ratio of OX40L expression was detected in Lti cells, NCR–ILC3s contributed most to the OX40L-expressing cells among ILC3 subsets in the small intestine (Fig. 1c, d and Supplementary Fig. S1G). This finding is probably due to the greater abundance of NCR–ILC3s than Lti cells in cell number. Notably, NCR–ILC3s expressed a higher level of OX40L than NCR+ILC3s in the large intestine (Fig. 1c, d).
The expression of OX40L on splenic Lti cells could be detected at a substantially higher level in overnight-cultured cells than in freshly isolated cells, which is considered to be due to the degradation of OX40L by metalloproteinases during isolation.10 To detect OX40L expression in a more sensitive way, we cultured lamina propria lymphocytes (LPLs) overnight before examining the expression of OX40L by flow cytometry. The expression of OX40L was detected at a higher level after culture in Rag1–/– mice (Fig. 1a, e, f and Supplementary Fig. S1A) than in freshly isolated cells (Supplementary Fig. S1A and S1B). Similarly, the percentages and MFI of OX40L expression were significantly higher in Lin– cells than in Lin+ cells in both the small and large intestines (Fig. 1f–h), and the majority of Lin–OX40L+ cells were ILC3s (89.6 ± 0.93% in the small intestine and 76.9 ± 0.35% in the large intestine) (Fig. 1f and i). Among all the ILC3 subsets, NCR–ILC3s were the top constituent of OX40L-expressing cells (Fig. 1j). Therefore, the analysis of OX40L expression by ILC3s could be obtained with a similar trend in both “freshly isolation” and “overnight-culture” systems. NCR–ILC3s could be further categorized as NKp46–CCR6+ILC3s and NKp46–CCR6–ILC3s. NKp46–CCR6+ILC3s (including Lti cells) are important for lymphoid organogenesis, whereas NKp46–CCR6–ILC3s have the capacity to convert to NCR+ILC3s.25,26 We found that NKp46–CCR6+ILC3s expressed a significantly higher level of OX40L than NKp46–CCR6–ILC3s (Supplementary Fig. S2).
We subsequently analyzed the expression of OX40L in ILC3s from mice at different ages. We found that few OX40L could be detected on intestinal ILC3s from newborn mice (Fig. 1k, l). In the small intestine, the expression of OX40L on ILC3s did not clearly increase until the mice were 6-week old (Fig. 1e, k and l). In the large intestine, the OX40L expression in ILC3s started to increase when the mice were 1-week old, with the level maintained at 3 weeks of age and further increased at 6 weeks of age (Fig. 1e, k and l). The differential expression of OX40L on ILC3s in time and space implies regulation involving environmental factors during development.
CD4+ T cells suppress the expression of OX40L by ILC3s
Intriguingly, we found that the expression of OX40L in intestinal ILC3s from wild-type mice was substantially lower than from Rag1–/– mice (Fig. 1e and Supplementary Fig. S3A). In mice of the wild-type background, a higher proportion of OX40L+ cells was found in Lin– cells than in Lin+ cells in both small and large intestinal LPLs (Supplementary Fig. S3B and S3C). The MFI of OX40L was higher in Lin–OX40L+ cells than in Lin+OX40L+ cells in the small intestine but not in the large intestine (Supplementary Fig. S3D). Moreover, more than 75% of the Lin–OX40L+ cells were Lin–RORγt+ ILC3s (Supplementary Fig. S3E). The proportion of Lin–OX40L+ cells was only slightly, although significantly, higher than Lin+OX40L+ cells among the large intestinal LPLs (Supplementary Fig. S3C). These data suggest that ILC3s are a critical, although not a dominant, source of OX40L in the intestinal leukocytes of wild-type mice.
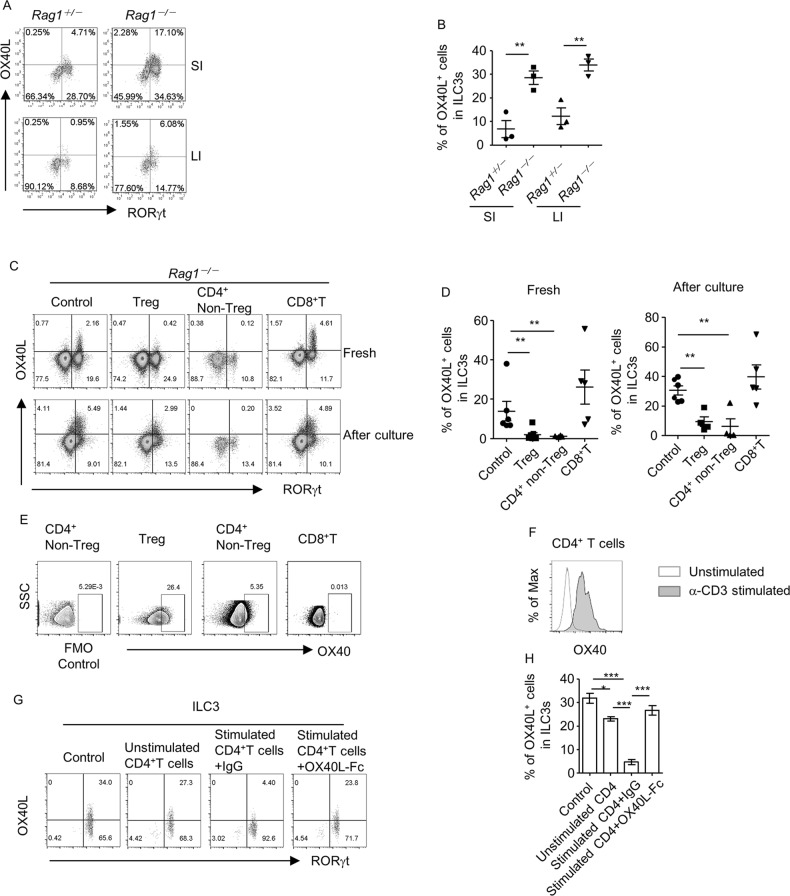
To eliminate the impact of microflora from different breedings, we analyzed the OX40L expression in littermate and co-housed Rag1+/– and Rag1–/– mice. Consistently, in both small and large intestines, the OX40L expression in ILC3s was significantly higher in Rag1–/– mice than in Rag1+/– mice (Fig. 2a, b). We purified the subsets of ILC3s from littermate Rag1–/–Rorcgfp/+ and Rag1+/–Rorcgfp/+mice, in which GFP indicated the expression of RORγt, and analyzed the OX40L mRNA expression via real-time reverse transcription polymerase chain reaction (RT-PCR).27 We found that the OX40L mRNA expression in ILC3s of Rag1–/–Rorcgfp/+ mice was higher than that in Rag1+/–Rorcgfp/+ mice, mainly manifested by NCR–ILC3 and NCR+ILC3 from the small intestine and Lti cells from the large intestine (Supplementary Fig. S4A). Flow cytometry analyses indicated that the percentages of OX40L in Lti cells and NCR–ILC3s were significantly higher in Rag1–/–Rorcgfp/+ than in Rag1+/–Rorcgfp/+ mice (Supplementary Fig. S4B). The differential expression of OX40L in subsets of ILC3s in Rag1–/–Rorcgfp/+ and Rag1+/–Rorcgfp/+ mice was partially consistent, although it did not exactly match the differential level of mRNA expression (Supplementary Fig. S4A and S4B), probably due to the regulation of OX40L expression at the translational or post-translational level. These data indicate that adaptive immune cells may suppress the expression of OX40L by intestinal ILC3s at both the protein and mRNA levels.
Fig. 2.
CD4+ T cells suppress OX40L expression by ILC3s. a and b LPLs were isolated from small and large intestines of littermate Rag1+/– and Rag1–/– mice and cultured overnight prior to analysis. a The expression of OX40L and RORγt gated on Lin– cells in live lymphocytes was analyzed by flow cytometry. b Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. c–e 4 × 105 Tregs (CD3+CD4+Foxp3-YFP+), non-Tregs (CD3+CD4+Foxp3-YFP–), and CD8+ T (CD3+CD8+) cells from the spleen were sorted from Foxp3Cre-YFP mice and transferred to Rag1–/– mice. Large intestinal LPLs were isolated 5 weeks after transfer and analyzed freshly (c-e) or after being cultured overnight (c and d). The expression of OX40L and RORγt gated on Lin– in live lymphocytes was analyzed by flow cytometry. d Percentages of OX40L+ cells gated on ILC3s in (c) are shown. e The expression of OX40 gated on Tregs (CD3+CD4+Foxp3-YFP+), non-Tregs (CD3+CD4+), and CD8+ T cells (CD3+CD8+) was analyzed by flow cytometry. An FMO control for OX40 staining gated on CD3+CD4+ cells was performed using cells from the group transferred with non-Tregs. f CD4+ T cells were purified from the spleen of wild-type mice and stimulated with or without α-CD3 and α-CD28 for 18 h. Expression of OX40 on CD4+ T cells was analyzed by flow cytometry. An overlay of the expression of OX40 gated on CD4+ cells from indicated groups is shown in histogram. g and h ILC3s were cultured alone (control) or co-cultured with unstimulated CD4+ T cells or α-CD3/α-CD28-stimulated CD4+ T cells, which were pre-incubated with IgG or mouse recombinant OX40L (OX40L-Fc), respectively, for 20 h prior to analysis. g Expression of RORγt and OX40L gated on Lin– cells was analyzed by flow cytometry and is shown. h Percentages of OX40L+ cells among ILC3s from indicated groups are shown. a–h Data are representative of three independent experiments. FMO, fluorescence minus one; LPLs, lamina propria lymphocytes
T cells have been suggested to suppress the function of ILC3s in the intestine.24 We therefore transferred different subsets of T cells to Rag1–/– mice to determine whether any type of T cells could inhibit the expression of OX40L by ILC3s. Compared to the control group, Rag1–/– mice transferred with CD4+Foxp3+Tregs or CD4+Foxp3–non-Tregs had a substantially reduced level of OX40L expression in ILC3s under both freshly isolated and cultured conditions (Fig. 2c, d). In contrast, CD8+ T cells had no effect on OX40L expression in ILC3s (Fig. 2c, d). These findings suggest that CD4+, but not CD8+, T cells specifically suppress OX40L expression in ILC3s.
The purity of the CD4+nonTregs and CD8+ T cells was more than 90% at the time of analysis (Supplementary Fig. S4C). The rate of expansion was higher for CD4+nonTregs than Tregs or CD8+ T cells, as indicated by the percentages of indicated T-cell subsets in lymphocytes at the time point of analysis (Supplementary Fig. S4D). However, Tregs, but not CD8+ T cells, could suppress the OX40L expression by ILC3s, which suggests that cell abundance was not a determining factor for this inhibitory function (Fig. 2c, d). A previous study suggests a mutual suppression between OX40L and OX40, which is likely due to the ligand–receptor interaction.22 Compared to the level of OX40 expression on Tregs and CD4+non-Tregs, the expression of OX40 on CD8+ T cells was substantially lower (Fig. 2e). To determine whether OX40 was required for the suppression of OX40L expression on ILC3s by CD4+ T cells, we co-cultured CD4+ T cells with purified ILC3s. The expression of OX40 was upregulated on α-CD3-stimulated CD4+T cells compared with unstimulated CD4+ T cells (Fig. 2f). Compared with unstimulated CD4+ T cells, α-CD3-activated CD4+ T cells had an enhanced ability to suppress OX40L expression by ILC3s (Fig. 2g, h). Moreover, the suppression of OX40L on ILC3s by activated CD4+ T cells could be substantially reversed by the pre-incubation of α-CD3-treated CD4+ T cells with OX40L-Ig but not control IgG (Fig. 2g, h). These data suggest that OX40 is important for the inhibition of OX40L expression on ILC3s by CD4+ T cells, potentially through the ligand–receptor interaction.
Inflammation boosts OX40L expression in ILC3s
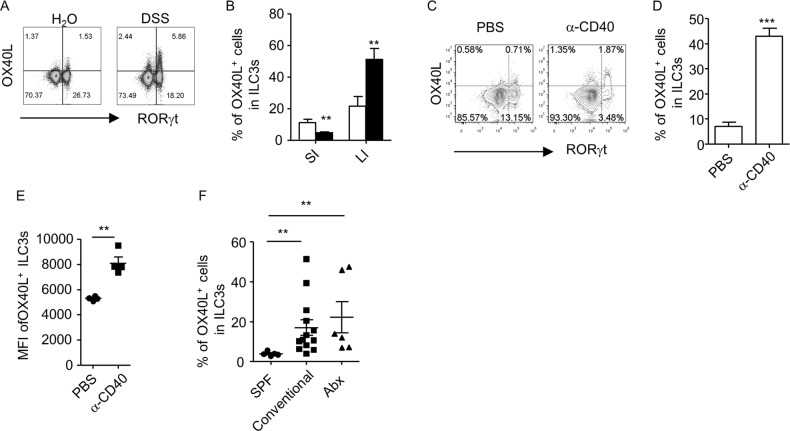
The age-relevant expression of OX40L by ILC3s, as well as the suppression of OX40L by T cells, leads us to think that factors from the microenvironment may regulate the expression of OX40L by intestinal ILC3s. We thus analyzed the expression of OX40L in ILC3s during inflammation. We induced colitis with DSS (dextran sulfate sodium) or α-CD40 agonistic antibody in Rag1–/– mice.28,29 We found that the expression of OX40L in ILC3s from freshly isolated large intestinal LPLs was substantially enhanced in mice induced with both types of colitis compared to the expression under a steady state (Fig. 3a–d). In DSS-induced colitis, OX40L expression on ILC3s was not induced in the small intestine, which typically is not the site of inflammation, suggesting a site-specific induction of OX40L expression on ILC3s by inflammatory cues (Fig. 3b). Consistent with a previous report, the absolute numbers of ILC3s were reduced following α-CD40 treatment (data not shown).30 However, the MFI of OX40L for OX40L+ILC3s was higher in α-CD40-treated mice (Fig. 3e). Together, these data indicate that inflammatory signals can promote OX40L expression in ILC3s. Consistently, a significantly higher level of OX40L expression could be detected in ILC3s from conventional mice than in mice housed in specific pathogen-free conditions (Fig. 3f). However, broad-spectrum antibiotic treatment failed to bring down OX40L expression in ILC3s (Fig. 3f). Inflammatory cues triggered by antibiotic-resistant bacteria or viral factors might sustain OX40L expression in ILC3s.
Fig. 3.
Inflammation induces OX40L expression by ILC3s. Littermate Rag1–/– mice were treated with 3% DSS (a, b) or 200 µg of α-CD40 (c–e). f Rag1–/– mice were housed in SPF or conventional conditions for 4 weeks. A group of mice in conventional conditions were further treated with antibiotics for 2 weeks prior to analysis. a–f Large intestinal LPLs (LI) were freshly isolated for analysis, except for in (b), where small intestinal LPLs (SI) were also freshly isolated for analysis. a, c The expression of OX40L and RORγt gated on Lin– cells in live lymphocytes was analyzed by flow cytometry. b, d, f Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. e MFI of OX40L gated on OX40L+ILC3s (Lin–RORγt+OX40L+) is shown. a–f Data are representative of two independent experiments. DSS, dextran sulfate sodium; LPLs, lamina propria lymphocytes; MFI, mean fluorescence intensity; SPF, specific pathogen-free
Poly (I:C) stimulates OX40L expression in ILC3s in a cell-intrinsic manner
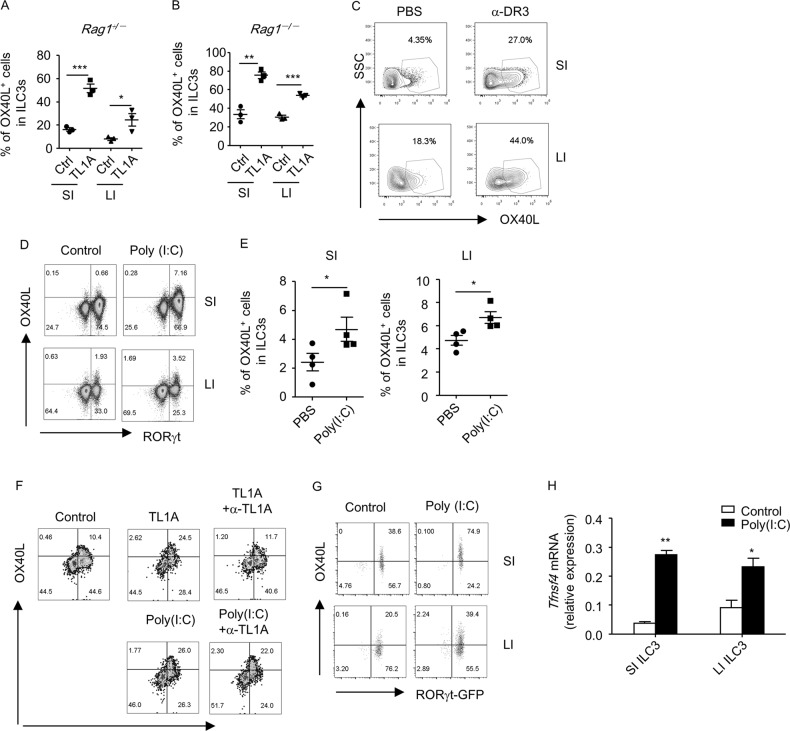
To determine the factors that stimulated OX40L expression in ILC3s during inflammation, we first tested the effect of TL1A, which has been shown to be produced by CX3CR1+ macrophages and to strongly induce OX40L expression by splenic Lti cells.16,31 We found that TL1A substantially boosted the OX40L expression in both small and large intestinal ILC3s of Rag1–/– and Rag1+/– mice in vitro (Fig. 4a, b, Supplementary Fig. S5A and S5B). Furthermore, Rag1–/– mice treated with an agonistic antibody against the receptor of TL1A, DR3 (death receptor 3), had a significantly increased level of OX40L expression in small and large intestinal ILC3s (Fig. 4c, Supplementary Fig. S5C and S5D). Having found to be upregulated in multiple forms of colitis in both humans and mice, TL1A could potentially serve as a crucial factor that drives OX40L expression in ILC3s under inflammatory conditions.32
Fig. 4.
Poly (I:C) induces OX40L expression in ILC3s in a cell-intrinsic manner. a, b Small and large intestinal LPLs were isolated from littermate Rag1+/– and Rag1–/– mice. Cells were then treated with or without TL1A (100 ng/ml) for 24 h prior to analysis. Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. c Rag1–/– mice were treated with 1 µg control IgG or α-DR3 agonistic antibody and sacrificed for analysis after 2 days. Expression of OX40L gated on ILC3s (Lin–RORγt+) in small or large intestinal LPLs was analyzed by flow cytometry and is shown. d, e Littermate Rag1–/– mice were treated with PBS or 200 µg Poly (I:C). d The expression of OX40L and RORγt gated on Lin– cells in live lymphocytes was analyzed by flow cytometry. e Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. f Small intestinal LPLs from wild-type mice were treated with TL1A (100 ng/ml) or Poly (I:C) (100 µg/ml) in the presence or absence of α-TL1A (0.6 µg/ml) for 24 h. The expression of OX40L and RORγt gated on Lin– cells in live lymphocytes was analyzed by flow cytometry. g, h Small and large intestinal ILC3s (Lin–RORγt-GFP+) from Rorcgfp/+ mice were sorted and treated with Poly (I:C) (100 µg/ml) for 24 h. g Expression of OX40L and RORγt-GFP gated on live lymphocytes was analyzed by flow cytometry. h mRNA expression of OX40L was analyzed by real-time RT-PCR. a–h Data are representative of two independent experiments. LPLs, lamina propria lymphocytes; SPF, specific pathogen-free
The failure of antibiotics to downregulate OX40L expression in ILC3s led us to question whether a non-bacterial stimulus could trigger OX40L expression. We subsequently screened a panel of ligands for pattern recognition receptors, which sense different microbes, including bacteria, viruses, and fungi.33 Among the all tested ligands for pattern recognition receptors, Poly (I:C) but not the ligands for TLR1, TLR2, TLR5, TLR7, TLR9, or Dectin-1 significantly elevated the OX40L expression in Lti cells in vitro (Supplementary Fig. S6A-S6C). Consistently, Rag1–/– mice treated with Poly (I:C) in vivo had an increased OX40L expression in both small and large intestinal ILC3s (Fig. 4d, e). As indicated by the similar percentages of lymphocytes, the percentages of live cells in lymphocytes, the percentages of ILC3s gated on lymphocytes, and the absolute numbers of ILC3s in the TL1A- or Poly (I:C)-treated group compared to the control group, the induction of OX40L expression on ILC3s by TL1A or Poly (I:C) in vitro or in vivo was not due to the possible cytotoxic effects on ILC3s (Supplementary Figs.S5D, S6D-S6F). These data suggest that Poly (I:C) representing environmental double-stranded RNA viruses may be a trigger for the expression of OX40L in ILC3s.
The effect of Poly (I:C) on OX40L expression in ILC3s could be direct or indirect through the upregulation of TL1A expression from antigen-presenting cells.34 To elaborate this, we blocked TL1A with a neutralization antibody when LPLs were stimulated with Poly (I:C) in vitro. While α-TL1A antibody sufficiently blocked the promotive effect of TL1A on OX40L expression by ILC3s, it had no effect on Poly (I:C)-induced OX40L expression (Fig. 4f). This finding suggests that Poly (I:C) stimulates OX40L expression in ILC3s independent of TL1A. We then purified ILC3 from the LPLs and treated the cells with Poly (I:C) to determine whether OX40L expression could be induced in an “ILC3-only” system. We found the mRNA and protein levels of OX40L expression were substantially promoted by Poly (I:C), which suggests the cell-intrinsic role of Poly (I:C) on OX40L expression by ILC3s (Fig. 4g, h).
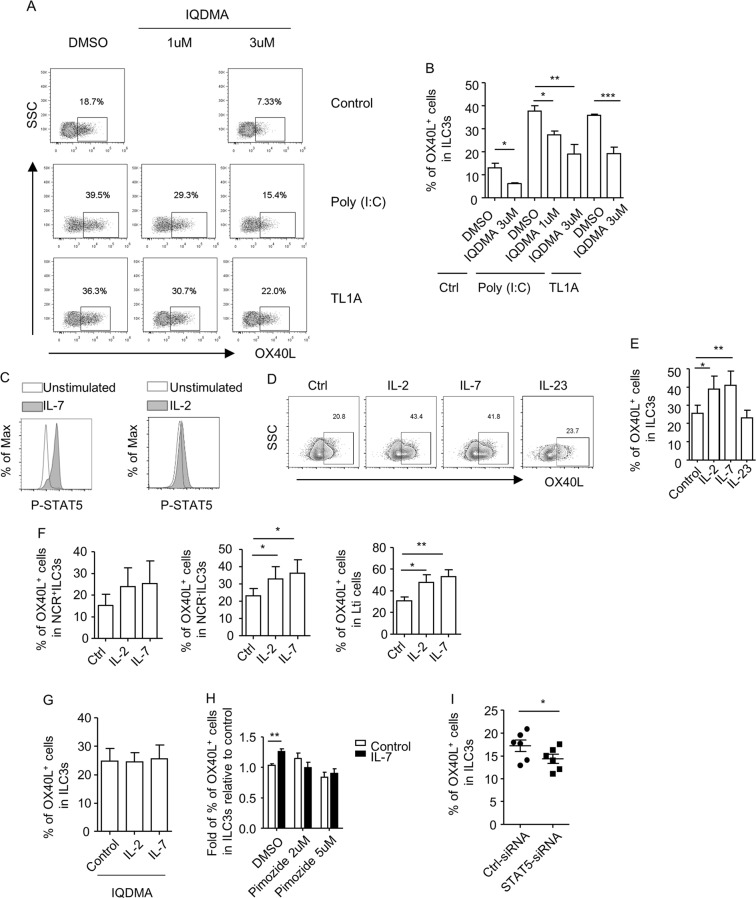
IQDMA suppresses TL1A/Poly (I:C)-induced OX40L expression by ILC3s
Recognized by innate receptors, including TLR3, RIG-I, and MDA5, Poly (I:C) signals through a series of downstream kinases and ultimately activates MAPK (mitogen-activated protein kinase), TBK1 (TANK-binding kinase 1), and NF-κB.35-37 TL1A binds to DR3 and signals through MAPK and NF-κB.38 To determine the molecules that are essential for the induction of OX40L expression in ILC3s, we tested the effect of a panel of inhibitors that targeted key transcription factors and kinases downstream of Poly (I:C) and TL1A. Intriguingly, the inhibitors of TBK1 had no effect on Poly (I:C)-induced OX40L expression by ILC3s (Supplementary Fig. S7A). In addition, the inhibitors of p38, JNK, or NF-κB did not block the induction of OX40L in ILC3s by TL1A or Poly (I:C) (Supplementary Fig. S7B and S7C).
We subsequently evaluated a group of inhibitors that targeted key transcription factors functionally relevant to ILC3s. We found that the inhibitors of RORγt, RORα, NFAT (nuclear factor of activated T cells), or STAT3 did not inhibit the OX40L expression induced by Poly (I:C) (Supplementary Fig. S7D).39–41 Nevertheless, IQDMA, an inhibitor of STAT5, significantly suppressed the expression of OX40L by ILC3s induced by TL1A or Poly (I:C) (Fig. 5a, b and Supplementary Fig. S7E). Furthermore, the basal level of OX40L expression by ILC3s was also suppressed by IQDMA (Fig. 5a, b). As indicated by the similar percentages of lymphocytes, the percentages of live cells in lymphocytes and the percentages of ILC3s gated on lymphocytes in the IQDMA-treated groups and control groups, the suppression of OX40L on ILC3s by IQDMA was not due to the possible cytotoxic effect of IQDMA on ILC3s (Supplementary Fig. S7F). Intriguingly, the induction of OX40L expression on ILC3s by TL1A or Poly (I:C) was not suppressed by pimozide, another inhibitor of STAT5 (Supplementary Fig. S7G). One possibility is that IQDMA and pimozide inhibit STAT5 in different manners. Otherwise, some STAT5-independent mechanisms may play a role in the suppression of OX40L on ILC3s by IQDMA.
Fig. 5.
STAT5 is essential for OX40L expression in ILC3s. a, b Small intestinal LPLs were isolated from wild-type mice. Cells were treated with inhibitor for STAT5 (IQDMA: low, 1 µM; high, 3 µM) for 2 h followed by stimulation with or without Poly (I:C) (100 µg/ml) or TL1A (100 ng/ml) for an additional 18 h. c Small intestinal LPLs from Rag1–/– were treated with or without (unstimulated) IL-2 (20 ng/ml) or IL-7 (20 ng/ml) for 15 min at 37 °C. Expression of lineage markers, RORγt and p-STAT5, was analyzed by flow cytometry. An overlay of p-STAT5 expression gated on ILC3s (Lin–RORγt+) from indicated groups is shown in histogram. d–g Small intestinal LPLs from Rag1–/– mice were treated with indicated cytokines at a concentration of 100 ng/ml for 20 h in the absence (d–f) or presence of 3 µM of IQDMA (g). a, d The expression of OX40L gated on ILC3s (Lin–RORγt+) was analyzed by flow cytometry and is shown. b, e, g Percentages of OX40L+ cells gated on ILC3s (Lin–RORγt+) are shown. f Percentages of OX40L+ cells gated on NCR+ILC3 (Lin–RORγt+NKp46+), NCR–ILC3 (Lin–RORγt+NKp46–CD4–), and Lti cells (Lin–RORγt+NKp46–CD4+) are shown. h Purified ILC3s (Lin–RORγt-GFP+) from Rag1–/–Rorcgfp/+ mice were treated with or without pimozide in the presence or absence (control) of IL-7 (100 ng/ml) for 20 h. i Purified ILC3s (Lin–RORγt-GFP+) from Rag1–/–Rorcgfp/+ mice were transfected with control siRNA or siRNA targeting mouse STAT5 in the presence of IL-7 (20 ng/ml). h The relative fold of percentages of OX40L+ cells gated on ILC3s (Lin–RORγt-GFP+) in the IL-7-treated group relevant to the control group was calculated and shown. i Percentages of OX40L+ cells gated on ILC3s ((Lin–RORγt-GFP+) are shown. Statistical analyses were performed with one-way ANOVA analyses with repeated measures tests for (e) and (f) and paired t test for (h) and (i). a–i Data are representative of at least two independent experiments. LPLs, lamina propria lymphocytes
IL-7-STAT5 signaling promotes OX40L expression by ILC3s
To further investigate the role of STAT5 in regulating OX40L expression by ILC3s, we treated intestinal LPLs with IL-2 or IL-7, the receptors of which are expressed on ILC3s and signal through STAT5.42,43 We found that both IL-2 and IL-7 promoted the phosphorylation of STAT5 (p-STAT5) in ILC3s (Fig. 5c). Moreover, the OX40L expression by ILC3s, specifically by NCR–ILC3 and Lti cells, was enhanced by IL-2 and IL-7 stimulation (Fig. 5d–f). Furthermore, the induction of OX40L by IL-2 and IL-7 could be blocked in the presence of IQDMA (Fig. 5g). As a control, the activation of STAT3 by IL-23 had no effect on the OX40L expression in ILC3s (Fig. 5d, e). Notably, the percentages of lymphocytes, the percentages of live cells in lymphocytes, and the percentages of ILC3s gated on lymphocytes were comparable in the cytokine-treated groups compared to the controls (Supplementary Fig. S8A). This finding suggests that IL-2/IL-7-mediated upregulation of OX40L expression on ILC3s is not due to the expansion of ILC3s or non-specific cytotoxic effects. In purified ILC3s, the induction of OX40L expression by IL-7 was also consistently observed (Fig. 5h). Moreover, the ablation of p-STAT5 by a different compound inhibitor decreased the IL-7-induced OX40L expression on ILC3s (Fig. 5h). The knockdown of STAT5 by small interfering RNA (siRNA) partially reduced the expression of STAT5 (Supplementary Fig. S8B). However, we observed a suppression of IL-7-sustained OX40L expression on STAT5 siRNA-transfected ILC3s (Fig. 5i). Together, the data suggest that STAT5 is important for IL-7-induced OX40L expression by ILC3s.
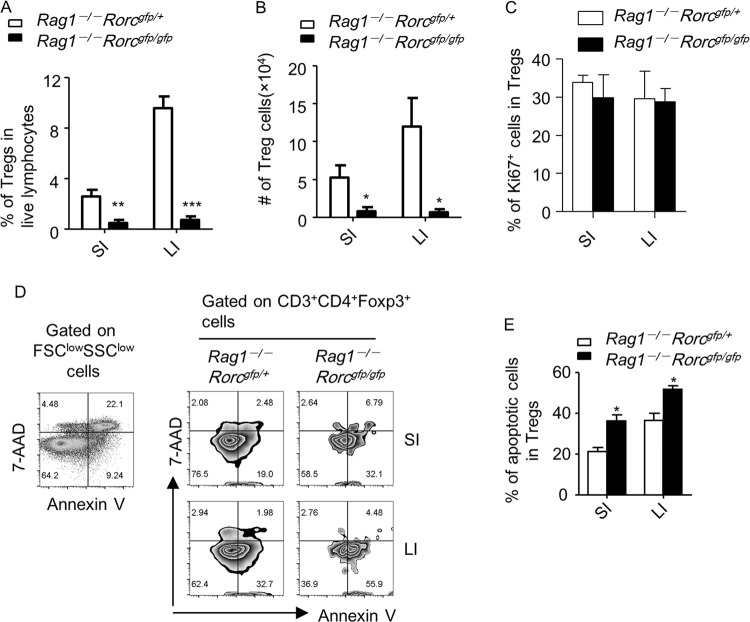
ILC3 is required for homeostatic expansion of Tregs
Considering the essential role of OX40L in supporting Tregs and ILC3s being the main source of OX40L in the intestine, we reasoned that ILC3s could be important for intestinal Treg homeostasis. We bred Rag1–/– mice with Rorcgfp/gfp mice, which lacked ILC3s. We subsequently transferred purified Treg cells to mice with (Rag1–/–Rorcgfp/+) or without (Rag1–/–Rorcgfp/gfp) ILC3s and examined the expansion of Tregs in the host. We found in both the small and large intestines, the percentages of Tregs in lymphocytes and the total numbers of Tregs were significantly decreased in Rag1–/–Rorcgfp/gfp recipients compared to Rag1–/–Rorcgfp/+ mice (Fig. 6a, b and Supplementary Fig. S9A). These data suggest that ILC3s are required for the homeostatic expansion of Tregs in lymphopenic hosts.
Fig. 6.
ILC3s are required for the homeostatic expansion of Tregs in the intestine. In total, 4 × 105 Tregs (CD3+CD4+Foxp3-YFP+) were sorted from the spleen of Foxp3Cre-YFP mice and intravenously injected in littermate Rag1–/–Rorcgfp/+ and Rag1–/–Rorcgfp/gfp mice. Small and large intestinal LPLs from recipients were isolated for analysis after 5 weeks of transplantation. Data are representative of two independent experiments. a, b Expressions of CD3, CD4, and Foxp3 were analyzed by flow cytometry. a Percentages of Tregs (CD3+CD4+Foxp3+ cells) in live lymphocytes were calculated and shown. b The total number of Tregs of indicated groups was calculated and shown. c Expression of CD3, CD4, Foxp3, and Ki67 was analyzed by flow cytometry. Percentages of Ki67+ cells gated on Tregs (CD3+CD4+Foxp3+) are shown. d Cell apoptosis gated on Tregs (CD3+CD4+Foxp3-YFP+) was analyzed by staining with Annexin-V and 7-AAD followed by flow cytometry. Forward scatter (FSC)lowside scatter (SSC)low region enriched for dead cells was used as a control for setting up the gates of Annexin-V and 7-AAD. e Percentages of apoptotic cells (Annexin V+7-AAD–) gated on Tregs (CD3+CD4+Foxp3-YFP+) from indicated groups are shown. a–f Data are representative of two independent experiments. LPLs, lamina propria lymphocytes
To determine the mechanism of defective Treg homeostasis in Rag1–/–Rorcgfp/gfp mice, we analyzed the proliferation and apoptosis of intestinal Tregs after transplantation. No difference in the rate of proliferation among total Tregs was found in Rag1–/–Rorcgfp/gfp compared to Rag1–/–Rorcgfp/+ hosts, as indicated by the percentage of Ki67+ cells among Tregs (Fig. 6c). Strikingly, Tregs in Rag1–/–Rorcgfp/gfp mice underwent a higher rate of apoptosis than the control group, which could contribute to the defective expansion of Tregs (Fig. 6d, e).
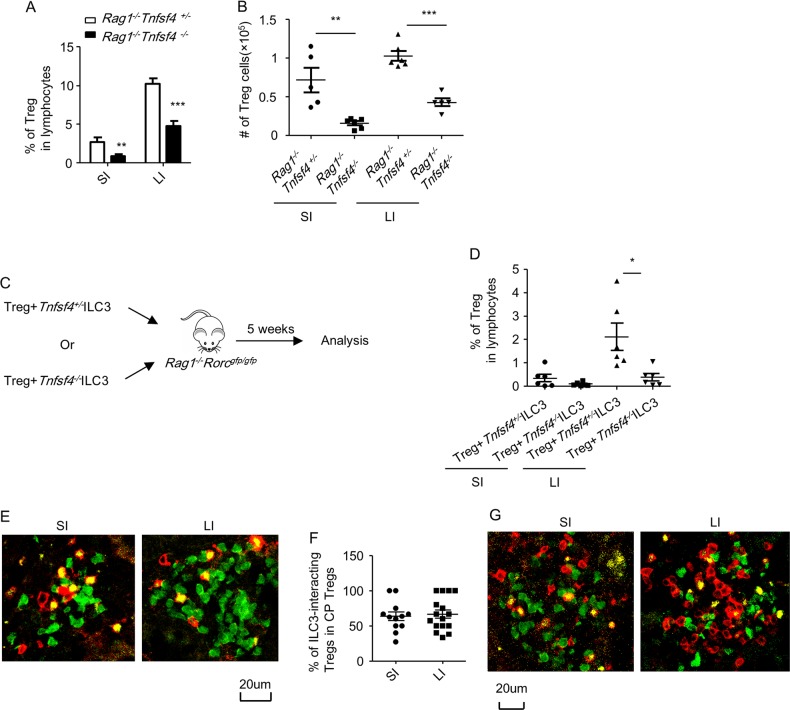
ILC3-derived OX40L is crucial for intestinal Treg homeostasis in immunodeficient mice
A previous study has shown that Treg failed to expand in Rag2–/–Tnfsf4–/– mice.22 Considering ILC3s marked as an OX40Lhigh population in the intestine, the dual requirement of both ILC3s and OX40L for Treg homeostasis led us to reason that OX40L expression in ILC3s was critical.
To determine the role of ILC3-derived OX40L in supporting Tregs, we constructed bone marrow chimeric mice by mixing the bone marrow of Rag1–/–Rorcgfp/gfp mice with Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– bone marrow cells and transferring them to lethally irradiated Rag1–/– mice (Supplementary Fig. S9B). In this system, bone marrow from Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– mice would supply Rag1–/–Rorcgfp/gfp with ILC3s that were sufficient or deficient of OX40L. Furthermore, the deficiency of OX40L in non-ILC3s in Rag1–/–Tnfsf4–/– mice would be supplemented by Rag1–/–Rorcgfp/gfp donors that have an intact OX40L expression in non-ILC3s.11 Therefore, these chimeric mice could be considered to have defective OX40L expression specifically in ILC3s. To confirm this, we analyzed ILC3s and the expression of OX40L after bone marrow reconstitution. We found no difference in the percentages of ILC3s among lymphocytes in the LPLs of the two chimeric mice, which implies the minimal effect of OX40L on the development of ILC3s (Supplementary Fig. S9C). Defective expression of OX40L in ILC3s but not non-ILC3s was observed in mice reconstituted with Rag1–/–Rorcgfp/gfp plus Rag1–/–Tnfsf4–/– bone marrows compared to the control group (Supplementary Fig. S9D and S9E). The residual OX40L+ILC3s from Rag1–/–Tnfsf4–/– bone marrow chimeric mice were from radio-resistant ILC3s from recipients (Supplementary Fig. S9D and S9E).44 After the transfer of Tregs, in mice restored with Rag1–/–Rorcgfp/gfp plus Rag1–/–Tnfsf4–/– bone marrow, the percentages of Tregs in lymphocytes and the total cell number of Tregs were significantly decreased compared to mice restored with Rag1–/–Rorcgfp/gfp plus Rag1–/–Tnfsf4+/– bone marrow (Fig. 7a, b). Therefore, the supplementation of Rag1–/–Rorcgfp/gfp bone marrow cells with OX40L-sufficient ILC3s but not OX40L-deficient ILC3s could better support the homeostatic expansion of Tregs.
Fig. 7.
ILC3-derived OX40L is important for Treg homeostasis in the intestine. a, b Rag1–/– mice were lethally irradiated and reconstituted with bone marrow mixed at 1:1 ratio from Rag1–/–Rorcgfp/gfp mice plus Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– mice. After 6 weeks, 4 × 105 Tregs (CD4+Foxp3-YFP+) were sorted from splenocytes of Foxp3Cre-YFP mice and transferred to bone marrow chimeric mice. c, d Rag1–/–Rorcgfp/gfp mice were transferred with 3 × 105 Tregs (CD4+Foxp3-YFP+) together with 2×105 ILC3s (Lin–Thy1.2highCD45.2intermediate) purified from small intestinal LPLs of Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– hosts. a, b, d Small and large intestinal LPLs from recipient mice were isolated for analysis 5 weeks after the transfer of Tregs. Expressions of CD3, CD4, and Foxp3 were analyzed by flow cytometry. a, d Percentages of Tregs (CD3+CD4+Foxp3+ cells) in live lymphocytes were calculated and shown. b Total number of Tregs of indicated groups was calculated and shown. e, f Approximately 4 × 105 Tregs (CD4+Foxp3-YFP+) were sorted from the spleen of Foxp3Cre-YFP mice and transferred to Rag1–/–Rorcgfp/+ mice. Mice were sacrificed for analysis 6 weeks after the transfer of Tregs. g Eight-week-old Rorcgfp/+ mice were sacrificed for analysis. e, g Expression of CD3 (red), Foxp3 (yellow), and RORγt-GFP (green) in cryptopatches was analyzed by immunofluorescence. f Percentages of Tregs (CD3-Red+, Foxp3-Yellow+) with location associated with ILC3s (CD3-Red–, RORγt-GFP-Green+) among all Tregs observed per CP were calculated and shown. Data were pooled from 12 (SI) and 15 (LI) cryptopatches of four mice. a–g Data are representative of two independent experiments. CP, cryptopatch; LPLs, lamina propria lymphocytes
To more stringently limit the supportive effect on Tregs to ILC3-derived OX40L, we transferred Tregs together with ILC3s purified from Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– mice to Rag1–/–Rorcgfp/gfp recipients (Fig. 7c). We observed a decreased proportion of Tregs among lymphocytes in the large intestine of recipients that received Rag1–/–Tnfsf4–/– ILC3s compared to the control group (Fig. 7d). In the small intestine, the percentages of Tregs in the hosts that received the Rag1–/–Tnfsf4–/– ILC3s showed a trend toward a reduction; however, the difference was not significant. These data solidify that OX40L expression by ILC3s is critical for the supportive role of ILC3s on large intestinal Treg homeostasis. Previous reports indicate that MHC class II (MHC II) on ILC3s mediates a suppressive function on the proliferation of T cells.45 We found that the percentages of MHC II expression on ILC3s from LPLs of Rag1–/–Tnfsf4+/– and Rag1–/–Tnfsf4–/– mice were comparable (Supplementary Fig. S10). These data suggest that the crosstalk between ILC3s and T cells in Rag1–/–Tnfsf4–/– compared to Rag1–/–Tnfsf4+/– mice is not possibly confounded by differential signals from ILC3-derived MHC II expression.
During the homeostatic expansion of Tregs in lymphopenic hosts, we observed the presence of Tregs in both small and large intestinal cryptopatches, where ILC3s were enriched (Fig. 7e). Approximately 63.5 and 66.7% of Tregs co-localized with ILC3s in the analyzed small and large intestinal cryptopatches, respectively (Fig. 7e, f). In addition, an association of Tregs with ILC3s was also detected in small and large intestinal cryptopatches in immune-competent mice under a steady state (Fig. 7g). These data support the direct interaction of Tregs and ILC3s in the cryptopatches, potentially through OX40L–OX40 interaction, which is essential for intestinal Treg homeostasis.
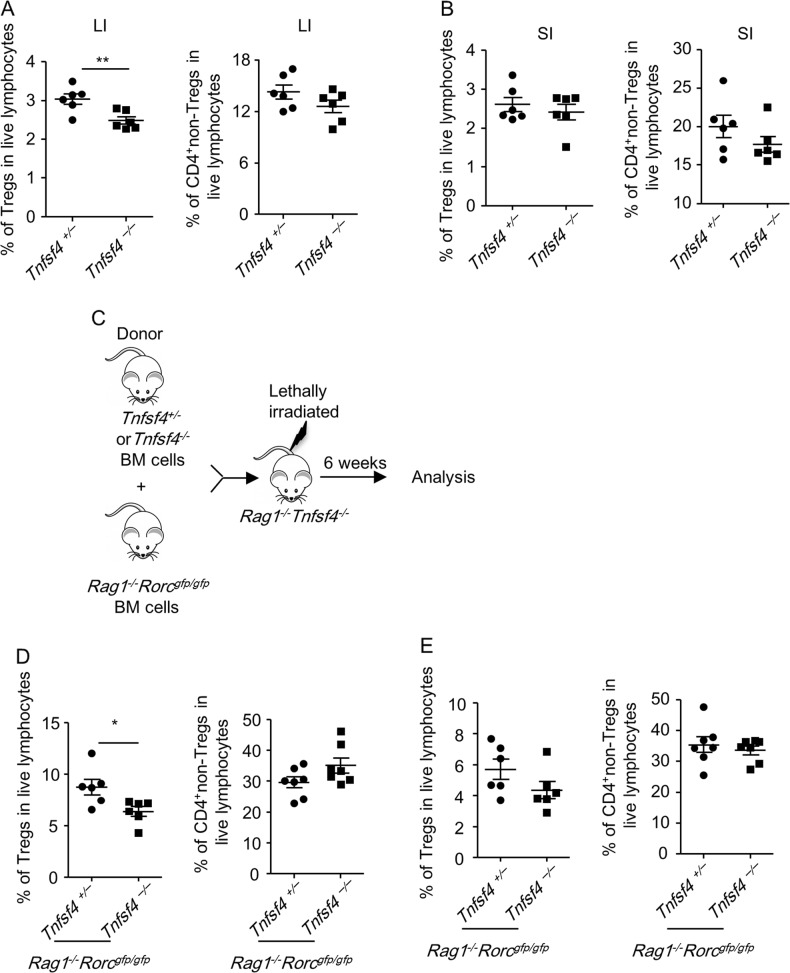
ILC3-derived OX40L is crucial for intestinal Treg homeostasis in immunosufficient mice
A previous study has suggested that colonic Tregs are reduced in OX40-deficient mice.19 We found that in immune-sufficient Tnfsf4–/– mice, the percentages of Tregs but not CD4+non-Tregs among large intestinal LPLs were reduced compared to Tnfsf4+/– littermate controls under a steady state (Fig. 8a). However, the percentages of Treg and CD4+non-Tregs among small intestinal LPLs were comparable between Tnfsf4–/– and Tnfsf4+/– mice (Fig. 8b). This finding suggests that a redundancy exists for the requirement of OX40L signals in supporting Treg homeostasis in the small intestine.
Fig. 8.
ILC3-derived OX40L signaling supports large intestinal homeostasis in immunosufficient mouse. a, b Small and large intestinal LPLs were isolated from littermate Tnfsf4+/– and Tnfsf4–/– mice. Expression of CD3, CD4, and Foxp3 was analyzed by flow cytometry. Percentages of Tregs (CD3+CD4+Foxp3+ cells) and CD4+non-Tregs (CD3+CD4+Foxp3- cells) in live lymphocytes from the large intestine (a) or small intestine (b) are shown. c–e Rag1–/–Tnfsf4–/– mice were lethally irradiated and reconstituted with bone marrow mixed at 1:1 ratio from Rag1–/–Rorcgfp/gfp mice plus Tnfsf4+/– or Tnfsf4–/– mice. Small and large intestinal LPLs were isolated for analysis after 6 weeks. c The protocol for the construction of bone marrow chimeric mice is shown. Percentages of Tregs (CD3+CD4+Foxp3+ cells) and CD4+non-Tregs (CD3+CD4+Foxp3- cells) in live lymphocytes from the large intestine (d) or small intestine (e) are shown. a–e Data are representative of two independent experiments. LPLs, lamina propria lymphocytes
To further investigate the role of ILC3-derived OX40L signaling in intestinal Tregs in an immunosufficient environment, we constructed chimeric mice by transferring bone marrow mixed from Tnfsf4–/– mice (or Tnfsf4+/– mice) and Rag1–/–Rorcgfp/gfp mice to Rag1–/–Tnfsf4–/– hosts (Fig. 8c). In these chimeric mice, ILC3s were deficient for OX40L expression, whereas OX40L on antigen-presenting cells could be compensated from the Rag1–/–Rorcgfp/gfp host. Although T and B cells in the chimeric mice that received Tnfsf4–/– bone marrow cells were also deficient for OX40L, we suggest it had a minimal impact on Tregs as ILC3s were a more important source of OX40L than Lin+ cells in wild-type mice (Supplementary Fig. S3B-S3D). Therefore, the chimeric mouse receiving Tnfsf4–/– bone marrow cells could be considered an immunosufficient mouse with OX40L deficiency specifically on ILC3s. Similarly, we observed a decreased proportion of Tregs, but not CD4+non-Tregs, in the large but not small intestinal LPLs (Fig. 8d, e). Together, the data suggest that the ILC3-derived OX40L signal is important for the homeostasis of large intestinal Tregs.
Discussion
In this study, we investigated the molecular regulation and function of OX40L expression in intestinal ILC3s and unveiled a crosstalk between Tregs and ILC3s through OX40L–OX40, which is critical for Treg homeostasis. In the intestine, we found that ILC3 was marked as an OX40Lhigh population and was the dominant source of OX40L in Rag1–/– mice. Notably, the expression of OX40L in ILC3s gradually increased after birth, which indicates that environmental factors may determine the OX40L expression by ILC3s. Moreover, the OX40L expression in ILC3s could be upregulated by inflammation or when mice were housed in conventional conditions. We have further demonstrated that TL1A and Poly (I:C), representing an inflammatory stimulus, promoted the OX40L expression on ILC3s. Notably, Poly (I:C) could enhance the OX40L expression in ILC3s in a cell-intrinsic manner independent of TL1A. According to a previous report, murine ILC3s lack the expression of PRRs.46 The receptors and signaling cascades downstream of Poly (I:C) in ILC3s remain to be determined. Our finding indicates the possibility that double-stranded RNA viruses could act on ILC3s directly and modulate their function.
We have surprisingly found that IQDMA, as an inhibitor of STAT5, which is typically not expected to be downstream of TL1A or Poly (I:C), effectively suppressed the induction of OX40L expression by ILC3s. Intriguingly, we showed that pimozide, another inhibitor of STAT5, failed to ablate the induction of OX40L expression on ILC3s by TL1A or Poly (I:C). It is likely that the mechanism of action was different for different types of STAT5 inhibitors. Alternatively, in addition to STAT5, some non-STAT5 targets nonspecifically suppressed by IQDMA but not pimozide mediate the suppression of OX40L expression on ILC3s induced by TL1A and Poly (I:C). However, both IQDMA and pimozide dampened the IL-7-induced OX40L expression by ILC3s. Moreover, the ablation of STAT5 by siRNA reduced the IL-7-sustained OX40L expression by ILC3s, which suggests that STAT5 is essential for IL-7-induced OX40L expression. Other STAT5-independent signaling pathways that potentially regulate TL1A- and Poly (I:C)-induced OX40L expression by ILC3s remain to be further investigated.
We found that the OX40L expression on intestinal ILC3s was higher in Rag1–/– mice than in littermate Rag1+/– controls. Specifically, CD4+ but not CD8+ T cells suppressed the OX40L expression by ILC3s in Rag1–/– mice. Macrophages are an important source of TL1A, which is a strong trigger for OX40L expression.31 The functional suppression of myeloid cells by T cells may lead to the downregulation of TL1A.47 However, this is less likely to cause the differential expression of OX40L in ILC3s from Rag1+/– and Rag1–/– mice, considering that the inhibition of myeloid cells could be similarly achieved by both CD4+ and CD8+ T cells.47 By a co-culture of ILC3s together with CD4+ T cells in vitro, we have demonstrated that OX40 is required for the suppression of OX40L expression on ILC3s by CD4+ T cells, which supports a mechanism for the inhibition involving OX40L–OX40 interaction.
Our data have indicated the critical role of ILC3-derived OX40L expression in the homeostasis of intestinal Tregs. Previous studies have shown that ILC3s promote the conversion of non-Tregs to Tregs by secreting GM-CSF, which is important for the accumulation of TGF-β-producing myeloid cells.48,49 Using Rag1–/–Rorcgfp/gfp mice and adoptive transfer of Tregs, we have proven that ILC3s are also important for intestinal Treg homeostatic expansion. Mechanistically, ILC3s supported the survival but not the proliferation of Tregs in the intestine, which matches the function of OX40.19 These findings collectively indicate a supportive role for ILC3s in intestinal Treg homeostasis.
It is noteworthy that several studies suggest the suppressive effect of OX40 signaling on Tregs. The action of OX40 signaling prevents the antigen- and TGF-β-induced conversion of naive CD4+ T cells to Tregs.50,51 Other studies have shown that stimulating OX40 on Tregs inhibited the expression of Foxp3 and abrogated the inhibitory function of Tregs.52,53 Moreover, as OX40L–OX40 signaling has been shown to be important for both CD4+ memory T cells and Tregs, the activation of OX40 signaling could inhibit the generation of Tregs by promoting the expansion of IFN-γ-producing memory T cells.50 Targeting OX40L–OX40 to treat autoimmune diseases could lead to opposite effects based on the balanced role of OX40L signaling on pathogenic T cells and Tregs.54 Signals from agonistic antibodies, ligand-coated magnetic beads or accompanied co-stimulatory signals from interacting cells may also result in differential effects of the activation of OX40 signaling on Tregs. Therefore, it will be important to evaluate the role of OX40L signal on Tregs in a spatial, temporal, and cell type-specific manner.
Using bone marrow chimeric mice, we showed that ILC3 but unlikely other cell-derived OX40L was critical for Treg homeostasis. Consistently, the co-transfer of Tregs with Tnfsf4–/–ILC3s manifested a defective expansion compared to Tregs transferred with Tnfsf4-sufficient ILC3s, which suggests OX40L is critical for the supportive function of ILC3s in Treg expansion in the large intestine. In this system, a reduction in the expansion of Tregs was not observed in the small intestine. This is probably because the reconstitution of ILC3s in the host might be less efficient by the transfer of mature intestinal ILC3s, compared to the reconstitution of ILC3s by the adoptive transfer of bone marrow cells.
Notably, in both immunosufficient Tnfsf4–/– mice and bone marrow chimeric mice that lacked OX40L on ILC3s, we found that the percentage of Tregs in the small intestine under a steady state was comparable to the controls. In contrast, a reduction in the percentage of Tregs was consistently observed in the large intestine in the mice from these experiments that belong to the Tnfsf4–/– group. This finding suggests that there is a redundancy in the requirement of OX40L signals in supporting Treg homeostasis in the small intestine. Numerous environmental factors, including the microbiota and other types of immune cells, will affect the composition of functional types of Tregs in the small and large intestines, such as microbiota-induced Tregs, dietary antigen-induced Tregs, and thymic-derived Tregs.55,56 This may lead to differential requirements of OX40L signaling for the small and large intestinal Treg homeostasis.
Interestingly, a recent study has shown that ILC2-derived OX40L is important for Treg homeostasis in the lung but not in the intestine following IL-33 treatment.57 The authors have shown that, first, OX40L is detected on ILC2s from the lung but not the intestine under a steady state or following IL-33 stimulation. Second, ILC2 but not DC-derived OX40L is important for Treg homeostasis in the lung. Our study, together with this finding, highlights that ILC but not classically recognized DC-derived OX40L is indispensable for Treg homeostasis in mucosal tissues. In addition, the co-localization of Tregs and ILC3s could be detected in intestinal cryptopatches, which indicates a direct interaction between Tregs and ILC3s. Our research broadens our understanding of ILC3s in supporting intestinal Tregs by a direct interaction through OX40L–OX40.
Methods
Mice
Wild-type mice were purchased from Shanghai SLAC Laboratory Animal Co. Rag1−/−, Tnfsf4−/−, Rorcgfp/gfp, and Foxp3Cre-YFP mice were purchased from Jackson laboratory. All the mice used in this study are on a C57BL/6 background and were maintained in specific pathogen-free conditions unless otherwise described. Adult mice used in the experiments were 6–10 weeks old unless otherwise noted. Gender-matched male or female mice were used in the study. For conventional housing, mice were housed in a non-barrier environment with open cages. Animals were then moved to a class II biosafety cabinet (Thermo Scientific; 1388) during the treatment with antibiotics. All animal experiments were performed in compliance with the guide for the care and use of laboratory animals and were approved by the institutional biomedical research ethics committee of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Isolation of intestinal LPLs
The isolation of LPLs was performed as previously described.58 Briefly, small and large intestines were dissected. Fat tissues were removed. Intestines were cut open longitudinally and washed in phosphate-buffered saline (PBS). The intestines were subsequently cut into three pieces, washed and shaken in PBS that contained 1 mM dithiothreitol for 10 min at room temperature (RT). The intestines were incubated with shaking in PBS that contained 30 mM Ethylenediaminetetraacetic acid (EDTA) and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at 37 °C for 10 min for two cycles. The tissues were then digested in RPMI1640 medium (Invitrogen) that contained DNase I (Sigma; 150 µg/ml) and collagenase VIII (Sigma; 150 U/ml) at 37 °C in a 5% CO2 incubator for 1.5 h. The digested tissues were homogenized by vigorous shaking and passed through a 100 µm cell strainer. Mononuclear cells were then harvested from the interphase of an 80 and 40% Percoll gradient after a spin at 2500 rpm for 20 min at RT.
Cell culture medium
For all the cell culture experiments in this study, the medium was IMDM medium (Hyclone), supplemented with 5% fetal bovine serum (Gibco), 2 mM L-glutamine (Gibco), 1 mM sodium pyruvate (Gibco), 50 µM β-ME (Sigma-Aldrich), 100 U/ml penicillin (Gibco), and 100 µg/ml streptomycin (Gibco) at 37 °C with 5% CO2.
Flow cytometry
Anti-mouse CD16/32 antibody was used to block the non-specific binding to Fc receptors prior to all surface staining. Antibodies used for regular flow cytometry were purchased from Thermo Fisher Scientific (eBioscience). For nuclear staining, cells were fixed and permeabilized using a Mouse Regulatory T Cell Staining Kit (Thermo Fisher Scientific). Dead cells were stained with a live and dead violet viability kit (Invitrogen) and were gated out in the analysis. For the detection of p-STAT5, cells were first fixed with a BD Cytofix kit (BD Biosciences) and permeabilized with 100% ice-cold methanol followed by staining with polyclonal α-p-STAT5 (Tyr694; Cell Signaling Technology). Flow cytometry data were collected using the Gallios flow cytometer (Beckman Coulter) and analyzed by FlowJo software (Tree Star Inc.). The lineage markers (Lin) were CD3, B220, CD11b, and CD11c in all the experiments. In some experiments, Treg (CD3+CD4+YFP+), CD4+ non-Treg (CD3+CD4+YFP−), or CD8+ (CD3+CD8+) T cells from the spleen of Foxp3cre-YFP mice were sorted by BD FACSARIAIII. ILC3s (Lin−RORγt-GFP+), NCR+ILC3s (Lin−RORγt-GFP+NKp46+), NCR−ILC3s (Lin−RORγt-GFP+NKp46−CD4−), and Lti cells (Lin−RORγt-GFP+NKp46−CD4+) from Rag1+/–Rorcgfp/+ or Rag1–/–Rorcgfp/+ mice were sorted using MoFlo (Beckman Coulter). ILC3s (Lin−Thy1.2highCD45.2intermediate) from small intestinal LPLs of Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– mice were sorted using MoFlo (Beckman Coulter).59
Adoptive transfer of T cells
Unless otherwise noted, for the adoptive transfer of T cells, 4 × 105 indicated donor cells were transferred to recipients by a retro-orbital injection.
Induction of innate colitis
Littermate Rag1−/− mice were fed with 3% DSS in drinking water for 8 days, and the mice were sacrificed on day 13 of DSS-induced colitis for analysis. In another group, littermate Rag1−/− mice were intraperitoneally injected with 200 µg α-CD40 agonist antibody (FGK4.5; Bioxcel), and the mice were sacrificed for analysis on day 7.
Antibiotic treatment
Mice were gavaged with 200 µl of autoclaved water supplemented with antibiotics (ampicillin 1 g/l, gentamicin 1 g/l, metronidazole 1 g/l, neomycin 1 g/l, and vancomycin 0.5 g/l) every day for 14 days.
Co-culture of CD4+ T cells and ILC3s
CD4+ T cells were purified from the spleen of naive wild-type mice using Dynabeads™ Untouched™ Mouse CD4 Cells Kit (Thermo Fisher Scientific). The purified CD4+ T cells were stimulated in vitro in an α-hamster antibody (MP Biomedicals)-coated plate with soluble α-CD3 (0.25 µg/ml) and α-CD28 (1 µg/ml) for 18 h (96-well plate, 3 × 104 cells/well). The activated CD4+ T cells were subsequently incubated with recombinant mouse OX40L-Ig (Sino Biological; 53582-M04H) or control mouse IgG (Sangon Biotech; BBI, D110503) for 4 h (5 µg/well) prior to being co-cultured with purified ILC3s. Culture supernatants that contained IgG or OX40L-Ig were discarded before CD4+ T cells were pooled together with ILC3s. ILC3s (Lin–GFP+) were sorted from small intestinal LPL of Rag1−/−Rorcgfp/+ mice. Approximately 3 × 104 of the treated CD4+ T cells were cultured with ILC3s at 1:1 ratio in a 96-well round-bottom plate for 20 h prior to analysis.
Transfection of ILC3s with siRNA
ILC3s (Lin−GFP+) were sorted from small intestinal LPLs of Rag1−/−Rorcgfp/+ mice and cultured in a 96-well round-bottom plate (3 × 104 cells/well) in the presence of IL-7 (20 ng/ml). After 12 h, 0.6 µg of STAT5 siRNA that targeted the common region of both STAT5a and STAT5b (sense: 5′-CCCACGACCUGCUCAUCAATT-3′; anti-sense: 5′-UUGAUGAGCAGGUCGUGGGTT-3′), or negative control oligos (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; anti-sense: 5′-ACGUGACACGUUCGGAGAATT-3′), was transfected into cells from each well with Lipofectamine™ 3000 transfection reagent (Thermo Fisher Scientific). Two days after transfection, the cells were harvested for analysis with flow cytometry or real-time RT-PCR.
Reagents
Pam3CSK4 (BioVision), Poly (I:C) (Tocris Bioscience), Flagellin (FLA-ST Ultrapure; InvivoGen), Imiquimod (Enzo Life Sciences), ODN2395 (TLRgrade™synthetic; Enzo Life Sciences), and Curdlan AL (InvivoGen) were used for the in vitro or in vivo experiments. Inhibitors for p38 (SB203580; Selleck Chemicals), JNK (SP600125;, Selleck Chemicals), NF-κB (Bay11-7085; Selleckchem), RORγt (GSK805; provided by Dr. Yonghui Wang from Fudan University),60 RORγt/RORα (SR1001; provided by Dr. Yonghui Wang from Fudan Univeristy), NFAT (Cyclosporin A; Abcam), STAT3 (STA-21; Enzo Life Sciences), and STAT5 (IQDMA; Abcam) were used for the in vitro experiments. TL1A, neutralization antibody for TL1A, and IL-23 were ordered from R&D. IL-2, IL-7, and Flt3-L were obtained from Peprotech.
Detection of mRNA by real-time RT-PCR
RNA was isolated with Trizol reagent (Invitrogen). cDNA was synthesized using a GoScript™ Reverse Transcription kit (Promega). Real-time PCR was performed using SYBR Green (Bio-rad). Reactions were run with the Mx 3000P Q-PCR System (Angilent). The results were displayed as relative expression values normalized to β-actin. The primers for β-actin were β-actin FW 5′-cttctttgcagctccttcgtt-3′ and β-actin RV 5′-aggagtccttctgacccattc-3′. The primers for Tnfsf4 were Tnfsf4 FW 5′-ctgcctgcaactctcttcct-3′ and Tnfsf4 RV 5′-tgacaaccgaattgttctgc-3′. The primers for Stat5 were Stat5 FW 5′-gtcccagttcaaccgggagaa-3′ and Stat5 RV 5′- ctgaagcgcagcaggaaggt-3′.
Construction of bone marrow chimeric mice
In total, 5 × 106 donor bone marrow cells from Rag1–/–Rorcgfp/gfp plus Rag1–/–Tnfsf4+/– or Rag1–/–Tnfsf4–/– mice were mixed at a ratio of 1:1. The cells were then transferred by retro-orbital injection into Rag1–/– recipients that were lethally irradiated at 550 rads twice with a 5 h interval. Approximately 4 × 105 Tregs (CD3+CD4+Foxp3-YFP+) cells from the spleen of Foxp3Cre-YFP mice were transferred to recipient mice 6 weeks after bone marrow reconstitution. The recipient mice were analyzed 5 weeks after Treg transfer.
In other experiments, 5 × 106 donor bone marrow cells from Rag1–/–Rorcgfp/gfp plus Tnfsf4+/– or Tnfsf4–/– mice were mixed at a ratio of 1:1. The cells were then transferred by retro-orbital injection into Rag1–/–Tnfsf4–/– recipients that were lethally irradiated at 550 rads twice with a 5 h interval. The mice were sacrificed for analysis after 6 weeks.
Immunofluorescence staining
Mice were perfused with 20 ml 1 × PBS followed by 20 ml 2% paraformaldehyde (PFA) in PBS. Small and large intestines were further fixed for 1 h in 2% PFA–PBS solution at RT, washed three times with 1 × PBS at 4 °C, cut open, rolled up with inside out, and dehydrated in 30% sucrose overnight at 4 °C. The rolled intestines were embedded in OCT (Tissue-Tek®) at -80 °C for at least 6 h. The frozen tissue blocks were cut into 5 µm slices. The sections were incubated overnight with Hamster-anti-mouse CD3 (145-2C11; Bioxcel), anti-GFP-Alexa Fluo®488 (Thermo Fisher Scientific), and anti-Foxp3-eFluor 660 (eBioscience). The sections were then stained with anti-hamster Cy3 (Jackson ImmunoResearch) for 1 h at RT. Confocal images were acquired using a Zeiss LSM710 laser scanning confocal head with a Zeiss LSM T-PMT microscope (40 × oil-objective) followed by analysis using Zeiss ZEN software. The associations of Tregs with ILC3s in cryptopatches were enumerated and represented as the percentage of ILC3-associated Tregs among the total number of Tregs in each cryptopatch.
Statistical methods
Unless otherwise noted, statistical analysis was performed using the unpaired Student’s t test on individual biological samples using GraphPad Prism 5.0 program. Data from these experiments are presented as mean values ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary information
Acknowledgements
The authors would like to thank Dr. Dechun Feng for experimental support. We thank the entire Q.J. laboratory team for their help and suggestions. This study was supported by grants 2015CB943400 and 2014CB943300 from the Ministry of Science and Technology of China, grant XDB19000000 from the “Strategic priority research program of the Chinese Academy of Sciences”, grants 91542102 and 31570887 from the National Natural Science Foundation of China, and China's Youth 1000 Talent Program to Q.J.
Author contributions
Q.J. and D.T. designed the research. D.T. and S.C. conducted the experiments and analyzed the data. Q.J. and D.T. wrote the manuscript. Y.R., C.J, L.J. and C.T. helped with the experiments and writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41423-019-0200-x) contains supplementary material.
References
- 1.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cording S, Medvedovic J, Cherrier M, Eberl G. Development and regulation of RORgt(+) innate lymphoid cells. FEBS Lett. 2014;588:4176–4181. doi: 10.1016/j.febslet.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Montaldo E, Juelke K, Romagnani C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur. J. Immunol. 2015;45:2171–2182. doi: 10.1002/eji.201545598. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell. Mol. Immunol. 2018;15:697–709. doi: 10.1038/cmi.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Pavert SA, Vivier E. Differentiation and function of group 3 innate lymphoid cells, from embryo to adult. Int. Immunol. 2016;28:35–42. doi: 10.1093/intimm/dxv052. [DOI] [PubMed] [Google Scholar]
- 7.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hepworth MR, et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MY, et al. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/S1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 11.Withers DR, et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J. Immunol. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatfield JK, Brown MA. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell. Immunol. 2015;297:69–79. doi: 10.1016/j.cellimm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Chang JH, et al. The chronicity of tonsillitis is significantly correlated with an increase in an LTi cell portion. Inflammation. 2014;37:132–141. doi: 10.1007/s10753-013-9721-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, et al. CD117+ CD3− CD56− OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur. J. Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- 15.Kim MY, et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J. Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 16.Kim MY, et al. Neonatal and adult CD4+CD3− cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15) J. Immunol. 2006;177:3074–3081. doi: 10.4049/jimmunol.177.5.3074. [DOI] [PubMed] [Google Scholar]
- 17.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu. Rev. Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen AI, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/S1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 19.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudensky AY. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda I, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 23.Kumar Prabhakaran, Marinelarena Alejandra, Raghunathan Divya, Ragothaman Vandhana K, Saini Shikha, Bhattacharya Palash, Fan Jilao, Epstein Alan L, Maker Ajay V, Prabhakar Bellur S. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cellular & Molecular Immunology. 2018;16(2):138–153. doi: 10.1038/cmi.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korn LL, et al. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal Immunol. 2014;7:1045–1057. doi: 10.1038/mi.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORgt+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 26.Sawa S, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 27.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 28.Wirtz S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 29.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Pearson C, et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife. 2016;5:e10066. doi: 10.7554/eLife.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longman RS, et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 34.Shih DQ, et al. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur. J. Immunol. 2009;39:3239–3250. doi: 10.1002/eji.200839087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramnath D, Powell EE, Scholz GM, Sweet MJ. The toll-like receptor 3 pathway in homeostasis, responses to injury and wound repair. Semin. Cell. Dev. Biol. 2017;61:22–30. doi: 10.1016/j.semcdb.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Levitzki A. Targeting the immune system to fight cancer using chemical receptor homing vectors carrying polyinosine/cytosine (PolyIC) Front. Oncol. 2012;2:4. doi: 10.3389/fonc.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosallanejad K, et al. The DEAH-box RNA helicase DHX15 activates NF-kB and MAPK signaling downstream of MAVS during antiviral responses. Sci. Signal. 2014;7:ra40. doi: 10.1126/scisignal.2004841. [DOI] [PubMed] [Google Scholar]
- 38.Pobezinskaya YL, Choksi S, Morgan MJ, Cao X, Liu ZG. The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling. J. Immunol. 2011;186:5212–5216. doi: 10.4049/jimmunol.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao S, et al. Small-molecule RORgt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40:477–489. doi: 10.1016/j.immuni.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulero MC, et al. Inhibiting the calcineurin-NFAT (nuclear factor of activated T cells) signaling pathway with a regulator of calcineurin-derived peptide without affecting general calcineurin phosphatase activity. J. Biol. Chem. 2009;284:9394–9401. doi: 10.1074/jbc.M805889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonard WJ, et al. Signaling via the IL-2 and IL-7 receptors from the membrane to the nucleus. Cold Spring Harb. Symp. Quant. Biol. 1999;64:417–424. doi: 10.1101/sqb.1999.64.417. [DOI] [PubMed] [Google Scholar]
- 43.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goc, J. et al. Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer. Int Immunol. 28, 43–52 (2016). [DOI] [PMC free article] [PubMed]
- 46.Crellin NK, et al. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Kim KD, et al. Adaptive immune cells temper initial innate responses. Nat. Med. 2007;13:1248–1252. doi: 10.1038/nm1207-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SH, Cho BH, Kiyono H, Jang YS. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer's patches. Sci. Rep. 2017;7:3980. doi: 10.1038/s41598-017-02729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao X, et al. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J. Immunol. 2008;181:3193–3201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 51.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, et al. OX40 costimulation inhibits Foxp3 expression and Treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 2018;24:607–618. doi: 10.1016/j.celrep.2018.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruby CE, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J. Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KS, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 56.Ohnmacht C, et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORgt+ T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 57.Halim TYF, et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48:1195–1207 e1196. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo X, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40:25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, et al. Discovery of biaryl amides as potent, orally bioavailable, and CNS penetrant RORgt inhibitors. ACS Med. Chem. Lett. 2015;6:787–792. doi: 10.1021/acsmedchemlett.5b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.