Abstract
Diapause is an endocrine controlled arrested metabolic state to delay development or reproduction under unfavorable conditions. To gain an understanding of importance of diapause for ecological adaptation, it is important to study regulation of diapause in insects. We examined genetics of diapause in Chilo partellus by crossing the hibernating (HD), aestivating (AD), post-hibernating (PHD), post-aestivating (PAD), and nondiapause (ND) strains. Reciprocal crosses were also made to gain full understanding of diapause regulation and the maternal effects, if any. Data were recorded on fecundity, egg hatching, larval survival, diapause induction and termination, adult emergence, and morphometrics of larvae, pupae and adults in the parents (P1, P2), F1 hybrids, and the reciprocal crosses. Genetic analysis showed that AD strain is general combiner, which also improved egg hatching, larval survival, diapause termination, adult emergence and proportion of females in the progenies. Incidence of diapause was highest in HD × AD, whereas termination was greatest in PHD × AD. However, ND strain and its reciprocal crosses with other strains did not exhibit any noticeable developmental response associated with diapause. Specific combining ability analysis suggested that where PHD and AD strains exist together there will be likely reduction in diapause incidence, increased survival with greater fitness and faster multiplication of their progenies resulting in outbreak of C. partellus. Degree of dominance estimates revealed that diapause, developmental and morphometric traits in C. partellus are governed by over dominance gene effects, and mainly depend on parental diapause history.
Subject terms: Ecology, Genetics
Introduction
Diapause is an endocrine controlled physiological state of arrested metabolic activity during a particular stage of insect development to survive under predictable adverse climatic conditions1–4. This happens to control the physiological processes and morphological development during particular stage of life cycle. The insects undergoing diapause pass through a series of physiological events such as suppression of development and reproductive functions, arrested metabolic activity to conserve the reserves, and resumption of normal developmental process on the onset of optimum climatic conditions3,5–7. Differences in genetic basis of various components of diapause also vary in different insect species8.
Diapause being an adaptive but genetically regulated trait provide phenotypic plasticity to insects in response to environmental conditions6. Abiotic factors like cooling and freezing, and rates of temperature change influence developmental and physiological alterations9–11, which also lead to several morphological changes such as body color, length, weight and width of various hard sclerotized structures like head capsule, mandible and body appendages12–15. Moreover, wider geographic distribution leads to behavioral and physiological differences in populations inhabiting different ecological niches16. Existence of ecotypes in different insect species is widely prevalent, and the inheritance of such traits may be due to long-term genetic differentiation or direct physiological response to environmental conditions17,18.
Diapause has been reported to follow a simple Mendelian inheritance (3:1 segregation ratio in the F1 progenies) in some insect species such as linden bug, Pyrrhocoris apterus L19., spider mite, Tetranychus pueraricola Ehara & Gotoh20, and flesh fly, Sarcophaga bullata (Parker)21. The incidence and duration of diapause in many insect species is under polygenic control6,22. Sex-linkage, maternal or paternal effects, and epistasis also play a significant role in diapause regulation in several other insect species16,23–26. Genetic and genetic-environmental interaction has been found to be involved in induction and termination of larval diapause in Ostrinia furnacalis (Guenée)27.
The spotted stem borer, Chilo partellus (Swinhoe) is widely distributed in tropical and temperate areas in Asia and Africa, and is a serious pest of maize and sorghum14,28. It undergoes facultative diapause as mature larvae inside the stems or stubbles of sorghum and maize29,30. Our earlier studies have generated comprehensive information on critical threshold conditions for induction and termination of diapause, duration of diapause, phenology of diapausing larvae, supernumerary moults, effects of diapause on post-diapause development and reproductive physiology, population buildup after diapause, and temperature-based development model of diapausing larvae in C. partellus11,14,15,31,32. Recent studies although revealed strong gene by environment interaction effects on diapause induction and incidence in some lepidopteran insect species, where inheritance patterns and dominance were found dependent on the photoperiod27,33,34. These studies further suggested that the use of varying photoperiod and temperature regimes, and different cross-mating combinations are integral to better understand the inheritance of diapause in insects. In this view our recent studies revealed that both temperature and photoperiod combine are critical for induction and termination of diapause in C. partellus14,15. Dhillon and Hasan32 elaborated that the larvae of C. partellus pass through hibernation under North Indian and aestivation under South Indian environmental conditions. The cross-mating among the adults of diapause and nondiapause strains and their F1 progenies within and across geographical regions is very likely, and there is a possibility of genetic polymorphism within and/or among the C. partellus populations. Thus, to understand such a complex population regulation mechanism, there is an urgent need for intensive genetic research on insects to explore alternative ways to manage insect pests of economic importance. None of the earlier studies attempted such a comprehensive genetic research to understand the genetic regulation of diapause and associated developmental traits in C. partellus under different geographical and population scenarios. Therefore, present studies were planned to investigate: i) the genetic components of diapause regulation and developmental traits, ii) effect of parental genetic background on diapause and related traits, and iii) effect of frequency of type(s) of pre-existing strain(s) on diapause incidence, developmental traits, and likely rate of C. partellus population buildup under given climatic conditions.
Results
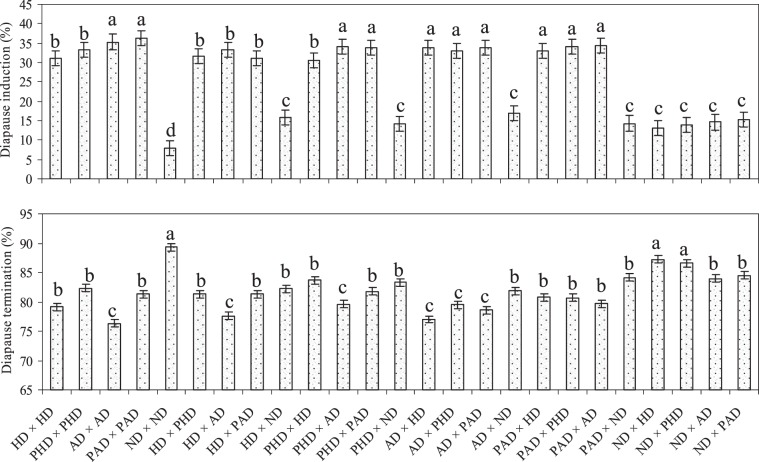
Developmental response and incidence of diapause in the diapause and nondiapause parental progenies and the diallel cross populations of C. partellus
There were significant differences in numbers of eggs laid (F = 12.26; df = 24, 96; P < 0.001), percentage egg hatching (F = 4.77; df = 24, 96; P < 0.001), larval survival (F = 13.28; df = 24, 96; P < 0.001) and adult emergence (F = 13.52; df = 24, 96; P < 0.001) (Table 1) among the parents and the diallel cross progenies of C. partellus. Similarly, incidence of diapause (F = 108.15; df = 24, 96; P < 0.001) and diapause termination (F = 13.42; df = 24, 96; P < 0.001) was also significantly different among the parents and the diallel cross progenies (Fig. 1). Among the parental populations, the numbers of eggs/female, percentage egg hatching, larval survival, diapause termination and adult emergence were significantly greater, while diapause incidence was significantly lower in nondiapause strain as compared to the diapause (HD and AD) and post-diapause (PHD and PAD) strains (Table 1). Egg laying by C. partellus females, egg hatching and larval survival were also significantly higher in the cases where either of the parent from nondiapause strain was crossed with a parent from diapause or post-diapause strains as compared to other cross combinations, although there were a few exceptions (Table 1). Conversely, incidence of diapause was significantly greater in the larvae obtained from the crosses involving diapause and/or post-diapause strains as compared to the nondiapause strain. However, the diapause termination and adult emergence were comparatively more in the progenies where either of the parent from nondiapause was crossed with a parent from diapause or post-diapause strains as compared to other cross combinations (Table 1; Fig. 1). The number of eggs, egg hatching, larval survival, incidence of diapause, diapause termination and adult emergence were similar in the progenies of the crosses involving diapause and/or post-diapause strains, except in a few cases (Table 1; Fig. 1).
Table 1.
Mean values of developmental response and diapause incidence recorded in the progenies of parents and the intermated strains of C. partellus.
| Parents and their diallel crosses | Total pairs observed (No.) | Eggs/ female | Eggs hatching (%) | Larval survival (%) | Adult emergence (%) |
|---|---|---|---|---|---|
| Parents (♀ × ♂) | |||||
| HD × HD | 59 | 126.9o | 53.3 f | 48.3 g | 88.0d |
| PHD × PHD | 41 | 135.8n | 57.7d | 50.9 f | 92.2b |
| AD × AD | 54 | 159.7j | 59.8c | 53.4e | 83.6e |
| PAD × PAD | 45 | 208.8e | 63.0b | 54.4d | 87.0d |
| ND × ND | 75 | 306.2a | 78.0a | 66.9a | 95.0a |
| Diallel crosses (♀ × ♂) including reciprocals | |||||
| HD × PHD | 54 | 132.8n | 53.7 f | 49.6 f | 89.8d |
| HD × AD | 68 | 143.5 l | 52.3 f | 50.2 f | 87.8d |
| HD × PAD | 48 | 150.9k | 56.2e | 49.5 f | 88.0d |
| HD × ND | 62 | 230.3d | 61.4b | 49.1 f | 91.8c |
| PHD × HD | 50 | 129.7o | 56.0e | 51.6 | 91.5c |
| PHD × AD | 50 | 139.8 m | 58.6d | 55.5d | 89.2d |
| PHD × PAD | 42 | 166.5i | 53.6e | 51.8e | 90.6c |
| PHD × ND | 45 | 248.3b | 62.7b | 55.1d | 94.4a |
| AD × HD | 62 | 140.2 l | 55.2e | 51.7e | 87.0d |
| AD × PHD | 70 | 130.5n | 61.2b | 56.1d | 88.5d |
| AD × PAD | 62 | 182.2 g | 60.6c | 53.8e | 87.0d |
| AD × ND | 61 | 227.9d | 64.3b | 62.5a | 92.0b |
| PAD × HD | 43 | 151.6k | 58.4d | 51.0e | 87.0d |
| PAD × PHD | 39 | 173.5 h | 55.9e | 52.7e | 89.2d |
| PAD × AD | 42 | 189.5 f | 63.9b | 49.7 f | 88.6d |
| PAD × ND | 63 | 228.7d | 60.0c | 59.9c | 90.3b |
| ND × HD | 68 | 244.6c | 62.9b | 52.7d | 93.5a |
| ND × PHD | 44 | 249.8b | 61.8c | 57.1c | 94.6a |
| ND × AD | 69 | 231.2d | 62.8b | 60.8b | 92.3b |
| ND × PAD | 48 | 249.8b | 64.2b | 61.9b | 90.6c |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| LSD (P = 0.05) | 40.89 | 6.91 | 3.74 | 2.15 | |
The values in a column with different letters are significantly different (Tukey’s HSD; P > 0.05).
Figure 1.
Diapause cycle recorded in the progenies of parents and the intermated populations of C. partellus. Diapause incidence (%) out of total larvae survived including diapausing and nondiapausing. Term “Diapause termination” means formation of pupa. (HD = Hibernation population; PHD = Post-hibernation population; AD = Aestivation population; PAD = Post-aestivation population; ND = Nondiapause population). Bars with different letters are significantly different (Tukey’s HSD; P > 0.05).
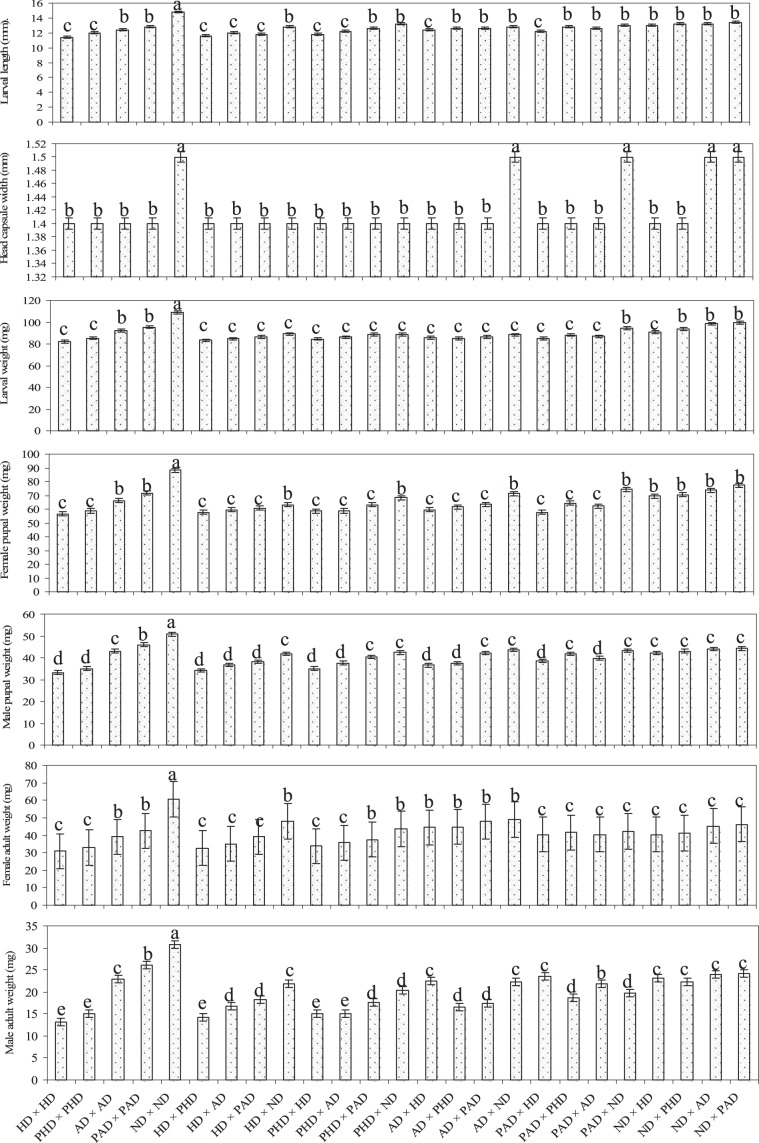
Morphometric differences in various developmental stages of the progenies of diapause and nondiapause parents, and the diallel cross populations of C. partellus
There were significant differences in larval length (F = 3.13; df = 24, 96; P < 0.001), head capsule width (F = 8.56; df = 24, 96; P < 0.001), larval weight (F = 11.53; df = 24, 96; P < 0.001), female pupal weight (F = 57.75; df = 24, 96; P < 0.001), male pupal weight (F = 16.35; df = 24, 96; P < 0.001), female adult weight (F = 28.56; df = 24, 96; P < 0.001), and male adult weight (F = 16.35; df = 24, 96; P < 0.001) across parents, and the diallel cross progenies of C. partellus. The larval length, head capsule width, larval weight, female pupal weight, male pupal weight, female and male adult weights were significantly greater in nondiapause strains as compared to the diapause (HD and AD) and post-diapause [PHD and PAD) parental strains of C. partellus (Fig. 2). Furthermore, larval weight, female and male pupal weights, female and male adult weights were significantly greater in the progenies of aestivation and post-aestivation parental strains as compared to hibernation and post-hibernation strains of C. partellus. The larval length and head capsule width in the progenies of all the diallel crosses were similar with each other, although there were a few exceptions. Larval, female and male pupal, and female and male adult weights were significantly higher in the cases where either of the parent from nondiapause strain was crossed with another parent from diapause or post-diapause strain as compared to other cross combinations, except in a few cases (Fig. 2).
Figure 2.
Mean values of morphometric parameters recorded on different developmental stages of diapausing and non-diapausing parents and intermated populations of C. partellus. (HD = Hibernation population; PHD = Post-hibernation population; AD = Aestivation population; PAD = Post-aestivation population; ND = Nondiapause population). Bars with different letters are significantly different (Tukey’s HSD; P > 0.05).
Genetic parameters of diapause, developmental and morphometric traits of C. partellus
The genetic analysis of incidence and termination of diapause, and adult emergence revealed significantly high variance due to specific combining ability (σ2sca) than general combining ability (σ2gca) effects (Table 2). The diapause incidence and adult emergence showed higher additive variance than the dominance variance, while the diapause termination showed higher dominance variance than the additive variance (Table 2). Egg hatching and larval survival (Table 2), and morphological traits viz., larval weight, and female and male pupal and adult weights (Table 3) also showed high variance due to σ2sca effects and dominance variance than the variance due to σ2gca and additive variance, respectively. The estimates for narrowsense (hns2) and broadsense (hb2) heritability of diapause and associated developmental and morphometric traits ranged from 0.04 to 0.99 (Tables 2, 3). Incidence and termination of diapause and adult emergence exhibited moderate to high ( > 0.30) hns2 (Table 2), while egg hatching, larval survival, larval length, head capsule width, larval weight, and female and male pupal and adult weights, showed very low ( < 0.20) hns2 (Tables 2, 3). However, all the diapause and associated developmental and morphometric traits exhibited very high ( > 0.80) hb2 (Tables 2, 3). The estimates of degree of dominance varied from 1.49 to 30.27 across diapause strains and the associated developmental and morphometric traits (Tables 2, 3).
Table 2.
Estimates of various genetic parameters for different developmental and diapause traits of C. partellus.
| Genetic parameters | Egg hatching (%) | Larval survival (%) | Diapause incidence (%) | Diapause termination (%) | Adult emergence (%) |
|---|---|---|---|---|---|
| GCA variance (σ2gca) | 4.02 | 0.61 | 24.16 | 2.02 | 2.68 |
| SCA variance (σ2sca) | 34.47 | 24.58 | 39.48 | 7.29 | 4.43 |
| σ2gca/ σ2sca | 0.12 | 0.16 | 0.61 | 0.28 | 0.61 |
| Additive variance (σ2a) | 8.04 | 1.22 | 48.32 | 4.04 | 5.37 |
| Dominance variance (σ2d) | 34.47 | 24.58 | 39.48 | 7.30 | 4.43 |
| Degree of dominance | 4.94 | 7.19 | 1.49 | 2.50 | 1.62 |
| Proportion of dominant and recessive alleles in the parents | 0.75 | 1.26 | 0.89 | 0.76 | 0.73 |
| Proportion of alleles with increasing and decreasing effects | 0.24 | 0.24 | 0.22 | 0.24 | 0.23 |
| Broad sense heritability (hb2) | 0.83 | 0.92 | 0.99 | 0.93 | 0.94 |
| Narrow sense heritability (hns2) | 0.16 | 0.04 | 0.54 | 0.33 | 0.52 |
Table 3.
Estimates of various genetic parameters for different morphometric traits of C. partellus.
| Genetic parameters | Larval length (mm) | Head capsule width (mm) | Larval weight (mg) | Female pupal weight (mg) | Male pupal weight (mg) | Female adult weight (mg) | Male adult weight (mg) |
|---|---|---|---|---|---|---|---|
| GCA variance (σ2gca) | 0.07 | 0.00 | 2.10 | 6.13 | 1.76 | 7.20 | 1.76 |
| SCA variance (σ2sca) | 0.56 | 0.00 | 50.96 | 72.54 | 26.24 | 60.09 | 26.24 |
| σ2gca/ σ2sca | 0.13 | 0.07 | 0.04 | 0.08 | 0.07 | 0.12 | 0.07 |
| Additive variance (σ2a) | 0.14 | 0.00 | 4.19 | 12.56 | 3.52 | 14.41 | 3.52 |
| Dominance variance (σ2d) | 0.56 | 0.00 | 50.96 | 72.54 | 26.24 | 60.04 | 26.24 |
| Degree of dominance | 30.27 | 8.95 | 10.31 | 4.75 | 4.79 | 3.97 | 4.79 |
| Proportion of dominant and recessive alleles in the parents | 1.71 | 1.77 | 2.23 | 1.73 | 1.79 | 1.08 | 1.79 |
| Proportion of alleles with increasing and decreasing effects | 0.18 | 0.20 | 0.19 | 0.19 | 0.20 | 0.20 | 0.20 |
| Broad sense heritability (hb2) | 0.84 | 0.93 | 0.93 | 0.99 | 0.96 | 0.98 | 0.96 |
| Narrow sense heritability (hns2) | 0.17 | 0.11 | 0.07 | 0.14 | 0.11 | 0.19 | 0.11 |
General combining ability effects of diapause and nondiapause populations for diapause, developmental and morphometric traits of C. partellus
The GCA effects were significant and positive for egg hatching and larval survival in AD, diapause incidence in HD, PAD and ND, and diapause termination and adult emergence in PHD and AD strains (Table 4). However, the GCA effects were significant and negative for egg hatching and larval survival in PAD, diapause incidence in AD, and diapause termination and adult emergence in HD and PAD strains (Table 4). The GCA effects were significant and positive for larval length, head capsule width, larval weight, female and male pupal weights, female and male adult weights in AD, female pupal weight in PHD and female adult weight in ND strains of C. partellus (Table 5). However, the GCA effects were significant and negative for larval length, head capsule width, larval weight, female and male pupal weights, female and male adult weights in PAD, female pupal and adult weights in HD, female pupal weight in ND, and female adult weight in PHD strains of C. partellus (Table 5).
Table 4.
General combining ability of diapause experienced and nondiapause parental strains for various developmental and diapause traits of C. partellus.
| Parental populations and their crosses (×) | Egg hatching (%) | Larval survival (%) | Diapause incidence (%) | Diapause termination (%) | Adult emergence (%) |
|---|---|---|---|---|---|
| HD × HD | −0.33 | 0.06 | 4.28** | −1.15** | −1.21** |
| PHD × PHD | −1.21 | 0.16 | −0.39 | 0.67* | 0.60* |
| AD × AD | 3.68** | 1.27* | −8.19** | 2.19** | 2.58** |
| PAD × PAD | −2.43* | −1.45** | 3.25** | −1.38** | −1.57** |
| ND × ND | 0.29 | −0.03 | 1.06** | −0.34 | −0.40 |
| Gi-Gj | 7.31** | 0.65** | 2.46*** | 2.33** | 1.91** |
*,** = The GCA values significant at P = 0.05 and 0.01, respectively. HD = Hibernation; PHD = Post-hibernation; AD = Aestivation; PAD = Post-aestivation; ND = Nondiapause.
Table 5.
General Combining ability of diapause experienced and nondiapause parental strains for morphometric parameters of various developmental stages of C. partellus.
| Parental populations and their crosses (×) | Larval length (mm) | Head capsule width (mm) | Larval weight (mg) | Female pupal weight (mg) | Male pupal weight (mg) | Female adult weight (mg) | Male adult weight (mg) |
|---|---|---|---|---|---|---|---|
| HD × HD | −0.22 | −0.01 | −0.46 | −1.49** | −0.42 | −3.30** | −0.42 |
| PHD × PHD | 0.06 | 0.00 | 0.80 | 1.06** | 0.06 | −0.93* | 0.06 |
| AD × AD | 0.46** | 0.02** | 2.40** | 3.77** | 2.18** | 3.95** | 2.18** |
| PAD × PAD | −0.31* | −0.01* | −1.80* | −2.66** | −1.68** | −0.96* | −1.68** |
| ND × ND | 0.01 | 0.00 | −0.94 | −0.69* | −0.14 | 1.24** | −0.14 |
| Gi-Gj | 0.92** | 0.03** | 5.00** | 2.45** | 2.76** | 3.34** | 2.76** |
*,** = The GCA values significant at P = 0.05 and 0.01, respectively. HD = Hibernation; PHD = Post-hibernation; AD = Aestivation; PAD = Post-aestivation; ND = Nondiapause.
Specific combining ability effects of diapause experienced and nondiapause populations for diapause, developmental and morphometric traits of C. partellus
There were significant and positive SCA effects of PHD female × AD male for egg hatching and larval survival; HD female × AD male, PHD female × ND male and AD female × PAD male for diapause incidence; PHD female × AD male and PAD female × ND male for diapause termination; and PHD female × AD male and PAD female × ND male for adult emergence (Table 6). Great number of PHD and AD strains together resulted into reduced diapause incidence, and increased fitness related traits such as egg hatching, larval survival, diapause termination and adult emergence. Significant and negative SCA effects were found in cases of HD female × PHD male, HD female × AD male, AD female × PAD male and AD female × ND male for larval survival; PHD female × AD male, AD female × ND male and PAD female × ND male for diapause incidence; HD female × PAD male and PHD female × ND male for diapause termination; and HD female × PAD male, PHD female × ND male and AD female × PAD male for adult emergence (Table 6). Furthermore, the SCA effects of HD female × PAD male, HD female × ND male and PHD female × AD male were significant and positive; while that of HD female × PHD male, HD female × AD male, PHD female × PAD male, PHD female × ND male, AD female × PAD male, AD female × ND male and PAD female × ND male were significant and negative for all the test morphometric parameters viz., larval length, head capsule width, larval weight, female and pupal weights, and female and male adult weights, except in a few cases where these effects were non-significant (Table 7).
Table 6.
Specific combining ability of diapause experienced and nondiapause crosses for various developmental and diapause traits of C. partellus.
| Crosses | Egg hatching (%) | Larval survival (%) | Diapause incidence (%) | Diapause termination (%) | Adult emergence (%) |
|---|---|---|---|---|---|
| HD × PHD | −4.07 | −4.81** | −1.76 | −1.44 | −1.01 |
| HD × AD | −4.48 | −3.36* | 8.08** | 0.22 | 1.24 |
| HD × PAD | 3.68 | 1.90 | −1.25 | −2.16* | −3.24** |
| HD × ND | 4.18 | 1.45 | 1.78 | 1.73 | −1.03 |
| PHD × AD | 16.97** | 12.59** | −12.66** | 5.37** | 2.22** |
| PHD × PAD | −1.49 | −2.06 | −0.28 | 0.93 | 1.14 |
| PHD × ND | −5.67 | −2.84 | 3.55** | −3.81*** | −1.98* |
| AD × PAD | −3.89 | −3.24* | 7.86** | −0.56 | −2.67** |
| AD × ND | −1.44 | −5.12** | −6.14** | −0.72 | −0.03 |
| PAD × ND | −0.63 | 0.09 | −2.83** | 4.38** | 3.79** |
*,** = The SCA values significant at P = 0.05 and 0.01, respectively. HD = Hibernation; PHD = Post-hibernation; AD = Aestivation; PAD = Post-aestivation; ND = Nondiapause.
Table 7.
Specific combining ability of diapause experienced and nondiapause crosses for various morphometric parameters of various developmental stages C. partellus.
| Crosses | Larval length (mm) | Head capsule width (mm) | Larval weight (mg) | Female pupal weight (mg) | Male pupal weight (mg) | Female adult weight (mg) | Male adult weight (mg) |
|---|---|---|---|---|---|---|---|
| HD × PHD | −0.87* | −0.05** | −6.68** | −6.71** | −5.67*** | −5.02** | −5.67** |
| HD × AD | −0.67 | −0.03** | −5.08* | −7.02** | −5.98** | −7.71** | −5.98** |
| HD × PAD | 0.51 | 0.03* | 6.12** | 6.81** | 5.88** | 3.41* | 5.88** |
| HD × ND | 0.59 | 0.03** | 8.47** | 10.44** | 7.33** | 4.61** | 7.33** |
| PHD × AD | 1.85** | 0.09** | 17.47** | 20.04** | 9.33** | 17.52** | 9.33** |
| PHD × PAD | −0.58 | −0.02* | −4.13* | −4.13** | −3.41** | −5.56** | −3.41** |
| PHD × ND | −0.50 | −0.02 | −3.59 | −4.31** | −2.35* | −5.36** | −2.35* |
| AD × PAD | −0.78* | −0.01 | −2.53 | −3.85** | −1.52 | −3.85** | −1.52 |
| AD × ND | −0.10 | −0.00 | −0.79 | −3.42** | 0.53 | 2.75* | 0.53 |
| PAD × ND | −0.32 | −0.01 | −1.19 | −1.59 | −2.41* | −6.53** | −2.41* |
*,** = The SCA values significant at P = 0.05 and 0.01, respectively. HD = Hibernation; PHD = Post-hibernation; AD = Aestivation; PAD = Post-aestivation; ND = Nondiapause.
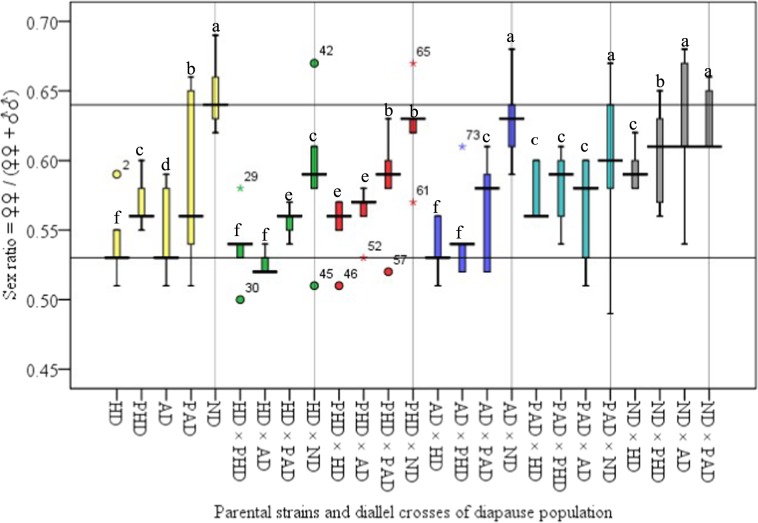
The present studies found a female biased sex-ratio in parental strains and their diallel crosses (Fig. 3). The proportion of females was significantly greater in ND and lower in HD as compared to other strains (F = 3.81; df = 24,96; P < 0.001). The sex-ratio in other parental strains and their diallel crosses varied between ND and HD strains (Fig. 3).
Figure 3.
Sex ratio (♀♀/(♀♀ + ♂♂)) of parental strains and diallel crosses of diapause population of C. partellus. The limits of a box denote the upper and lower quartiles, the horizontal bar is the median, and the 1.5 Interquartile Ratio (IQR) criteria has been used to classify outliers. (F = 3.81, df = 24, 96, P < 0.001; LSD = 0.18). Box plots with different letters are significantly different (Tukey’s HSD; P > 0.05).
Discussion
Diapause has evolved independently in various insect species, encompassing different life styles to adapt their life cycles to the seasonal changes35,36. Greater incidence of diapause in the progenies of diapause and post-diapause strain and their diallel crosses than those from the nondiapause strain in the present studies suggest that the mating between diapause strain greatly increase the propensity of diapause in the progenies. The sex-linked effects for induction of diapause have also been reported in many insect species due to influence of either or both the diapause parents as observed in the present studies16,25,37–40. Further, the greater fecundity, egg hatching, larval survival, termination of diapause and adult emergence in the progenies of nondiapause strain and the crosses involving either of the parent from nondiapause in comparison to diapause and post-diapause strains and their crosses suggest that the parental experience play an important role in inheritance of diapause in the progenies. Earlier reports of 15 to 65% diapause in C. partellus under different temperature and photoperiod conditions in the laboratory shows that apart from parental diapause history, asynchrony in diapause inducing stage and environmental conditions, and genetic segregation could also be influencing the induction of diapause in the progenies14,15. Diapause in C. partellus has also been found to affect certain morphological features like larval weight, length, and head capsule width9,10,14,32. Present studies revealed that the lower larval weight, shorter length and head capsule width in the progenies of diapause and post-diapause parental strains and their crosses lead to lower weight pupae and adults as compared to nondiapause counterparts (Fig. 2). Small size of the insects in the diapause strain could be because of utilization of body reserves to prepare for diapause12,13. Interestingly, it is to be noted that larval weight, female and male pupal weights, female and male adult weights were significantly greater in aestivation experienced strain as compared to hibernation experienced strain of C. partellus. It might be due the fact that larvae of C. partellus exhibited a prolonged duration during hibernation as compared to aestivation, which resulted into considerable reduction of weight in hibernating experienced strain as compared to aestivating experienced strains. Further, reduction of larval weight in diapausing insects also depends on the duration of diapause, the prolonged the duration of diapause the greater reduction of body weight due to utilization of body reserve during diapause12,13.
Significant and positive GCA effects of the C. partellus from HD and PAD strains for incidence of diapause suggested that it is genetically controlled and the parental experience play an important role in diapausing behavior (Table 4). Incidence of diapause in C. partellus was found to be influenced by both the parents, and the F1 progenies exhibited intermediate response as in other insect species38,41,42. Moreover, cross-mating of strains from different geographical areas may also result in genetic polymorphism within insect populations for various diapausing traits. Varying diapause responses have also been reported in the progenies of crosses between different geographic and laboratory strains43,44, and genetic studies related to diapause have shown different modes of inheritance6,21,45,46.
In present studies, the significant and positive SCA effects in certain crosses for various morphometric parameters indicated the involvement of at least one parent from AD and/or HD strain for induction of diapause (Table 7). These findings suggest that if the frequency of PAD and ND type of mixed strains is higher, there are chances of increased fitness in their progenies resulting in built up of the active population at a faster rate. However, the pre-existence of PHD and ND or AD and PAD type of strains under unfavorable climatic conditions could result in drastic reduction in the active population, since such combinations were found to result in increased frequency of diapause incidence in C. partellus (Fig. 1). The results further indicate that under the situations where PHD and AD strains exist together there will be reduced diapause induction, increased survival with greater fitness, and faster multiplication of their progenies resulting in outbreak of C. partellus. Female biased sex ratio suggested the involvement of either of the parent from nondiapause. Sexual differences in diapause propensity might be due to effect of either of the parents, as males and females are quite dissimilar in life history traits47. Generally, the insect females have longer development time than the males48, which profits females growing into larger body size and ultimately influences fecundity49.
Polygenic inheritance of diapause characteristics has been reported in many insect species, wherein hybrids have often been found intermediate between the parents45. Inheritance of diapause induction in European corn borer, O. nubilalis has earlier been reported to be regulated by multigenetic, sex-linked, and lack of dominance24,50,51. In present studies, the large variance due to SCA than GCA for diapause incidence and adult emergence indicated dominance gene action, while greater variance due to additive than dominance gene effects suggested the involvement of genetic and G × E interactions for these traits (Table 2). Greater SCA variance and dominance gene effects for diapause termination, egg hatching, larval survival and weight, and pupal and adult weights of both the sexes suggested that these traits are controlled by dominance gene action. High broadsense heritability of diapause and associated developmental and morphometric traits, and moderate narrowsense heritability of diapause incidence, termination and adult emergence indicate uniform breeding under laboratory conditions, while the estimated heritability under natural conditions could be over-estimated. Heritability in the laboratory reared strains provides a reasonable estimate in terms of magnitude and significance52,53. Heritability estimates for diapause intensity vary from low to very high, depending upon insect species, type of insect population, and experimental conditions54–59. Very high heritability estimates for various life history traits could be because of strong natural selection for the traits relevant to fitness, which under diapausing conditions tend to reduce additive genetic variance60–63. Further, the degree of dominance estimates for diapause, developmental and morphometric traits revealed that these traits are governed by overdominance gene effects in C. partellus, wherein the genetic variability and diapause incidence could be prerequisite for rapid adaptation to prevailing environmental conditions. These studies have implications for exploring appropriate genetic means of C. partellus management under different agro-ecological conditions.
Materials and Methods
Cultures of Chilopartellus
The C. partellus larvae were collected from northern Indian region, New Delhi (28°3823’N, 77°0927’E; AMSL 228.61 m) for hibernation population; southern Indian region, Hyderabad (17.3850°N, 78.4867°E; AMSL: 505 m) for aestivation population; and reared on maize stalks till pupation in the insectary at Division of Entomology, ICAR-Indian Agricultural Research Institute, New Delhi under 27 ± 1 °C, 70 ± 5% RH and 12 L:12D conditions. The adults obtained from these cultures were paired in oviposition cages and provided with water soaked in a cotton swab. The oviposition cages were wrapped with a wax-paper on the outer surface to serve as oviposition substrate. The wax-papers were changed daily. The wax-papers with eggs were kept at 27 ± 1 °C, 70 ± 5% RH and 12 L:12D h for egg hatching. Freshly hatched C. partellus larvae were inoculated in a plastic container (1000 ml capacity) containing artificial diet64, and kept in walk-in insect growth chambers (RINAC Make, 1.83 m L × 1.83 m W × 2.44 m H) at 27 ± 1 °C, 70 ± 5% RH and 12 L:12D). The culture of C. partellus was initially obtained from North and South India were pooled and maintained year-round in the insectory at Division of Entomology, ICAR-Indian Agricultural Research Institute, New Delhi, and used as nondiapause strain.
Formation of different diapause and nondiapause C. partellus populations
The late 4th to early 5th instar larvae of C. partellus from the above described respective cultures were exposed to earlier standardized hibernation (10 ± 1 °C + 10 L:14D) and aestivation (32 ± 1 °C + 13 L:11D) inducing conditions along with dry diet14,15. As a result of diapause treatment, the larvae showing any kind of diapause symptoms described by Dhillon and Hasan32 were considered to be in diapause. In general, the dormancy duration in C. partellus varies from 45 to 50 days in different agro-ecosystems14,15. The diapausing larvae thus obtained were kept undisturbed at the respective treatment conditions up to 45 days, and afterwards exposed to diapause termination conditions (fresh artificial diet; 27 ± 1 °C, 70 ± 5% RH and 12 L:12D conditions). Three batches of C. partellus larvae were exposed to hibernation and aestivation inducing conditions at three-week intervals so that the adults obtained from these diapause strains could be picked for mating in different cross combinations. The afore-mentioned year-round laboratory-maintained C. partellus culture was used as a nondiapause strain. In one set, the adults obtained from the hibernating, aestivating and nondiapausing larvae were designated as hibernation (HD), aestivation (AD) and nondiapause (ND) strains. In another set, the progenies obtained from the hibernation and aestivation strains were reared under laboratory conditions at 27 ± 1 °C, 70 ± 5% RH and 12 L:12D till adult emergence, and the adults thus obtained were delineated as post-hibernation (PHD) and post-aestivation (PAD) strains, respectively. In this way, there were five parental strains, i.e., HD, AD, PHD, PAD and ND.
Mating scheme
The simultaneous existence of different strains viz., hibernation, aestivation, nondiapause and their progenies is very likely, and the cross-mating among the adults of these strains within and across geographical regions could result in genetic polymorphism in C. partellus populations. Thus, present investigations on genetic components of diapause involved hibernation (HD), aestivation (AD), post-hibernation (PHD), post-aestivation (PAD) and nondiapause (ND) strains of C. partellus. The adults of these five parental strains were used in making all possible crosses including reciprocals in a diallel fashion (Fig. 4). The virgin females and males were paired individually in the oviposition cages as per the mating scheme described in Fig. 4. A total of 75 adult pairs were produced for each parental population and their crosses.
Figure 4.
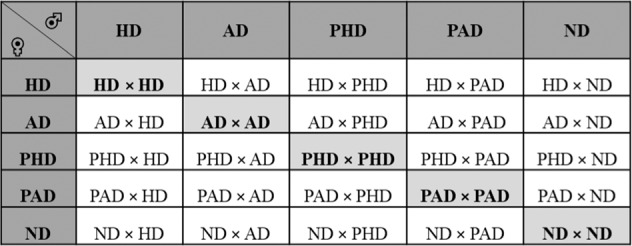
Mating scheme of different diapause and nondiapause C. partellus parental populations in all possible combinations including reciprocals in a diallel fashion. The abbreviations used in the mating scheme are elaborated as: HD = Hibernation, AD = Aestivation, PHD = Post-hibernation, PAD = Post-aestivation, and ND = Nondiapause. The diagonal cells marked in light grey color are parental crosses.
Experimental conditions and observations recorded
The aforesaid 75 male and female pairs of each parental and the diallel crosses were placed such that there was a cohort of 15 pairs per replication, and there were five replications in a completely randomized design (CRD) for each cross combination. The oviposition cages were kept in walk-in insect growth chamber at 27 ± 1 °C, 70 ± 5% RH and 12 L:12D for mating and oviposition. Wax-paper was wrapped around the oviposition cages to serve as oviposition substrate. The wax-papers were changed daily. Eggs laid by each female from each cross combination were recorded daily till the females died. The pairs which did not lay eggs were not included in the experiment. Total numbers of eggs laid by the females in a cohort were divided by the number of egg laying females in each replication, and the data were expressed as number of eggs per female for each mating combination. The wax-papers having egg masses were kept in a plastic bucket having water at the bottom to maintain humidity till the black head stage. At black head stage, the eggs were removed from the bucket and kept in plastic jars for egg hatching. Percentage of egg hatching was recorded for each replication separately. All the neonate larvae from each replication were inoculated on artificial diet64 poured in plastic containers (1000 ml capacity), and kept at 27 ± 1 °C and 12 L:12D. After 30 days, five randomly selected C. partellus larvae displaying diapause symptoms were removed from each replication, and data were recorded on larval weight, length and head capsule width, and data averaged for each replication. Observations on larval length and head capsule width were recorded using Leica StereoZoom Microscope (Leica Microsystems Ltd, Switzerland), and weight on an electronic balance (Precision balance, CB-Series, Contech). Thereafter, the larvae were placed back in respective jars.
After 45 days, the numbers of larvae transformed into pupae, and the larvae showing diapause symptoms or entered diapause were recorded for each replication in each cross. Larval survival was expressed as numbers of larvae (transformed into pupae + the larvae displaying symptoms or entered diapause) survived/numbers of larvae released × 100. Diapause incidence was calculated as numbers of larvae entering diapause/numbers of larvae released × 100. The numbers of diapause larvae molted into pupae were counted and expressed as diapause termination (%): Numbers of pupae from diapause larvae/numbers of diapause larvae × 100. The number of adults emerged from diapause larvae were also counted and expressed as a percentage of adults emerged from diapause larvae/total number of diapause larvae × 100. The weights of five randomly selected female and male pupae, and the female and male moths emerging from the diapause larvae in each cross combination were measured on an electronic balance (Contech, CB-120) after 24 h of pupation, and at adult death, respectively. The data were averaged for each replication, and expressed as mg/pupa or adult.
Statistical analysis
Mean values of all the test parameters of progenies of parents and the diallel crosses were used for statistical analysis. Sex ratio was calculated according to Wilson and Hardy65 formula [Sex ratio = ♀♀ / (♀♀ + ♂♂)] for each diallel cross, and expressed as proportion of offsprings that were females. Data on developmental parameters, morphometrics during different developmental stages, and sex ratio in the progenies of parents and the diallel cross populations were subjected to one-way analysis of variance (ANOVA) followed by Tukey–Kramer honestly significant difference (HSD) test using SPSS v. 15.0 statistical package (SPSS Inc. Chicago, IL, US). Analysis of variance, component analysis for estimation of degree of dominance, proportion of dominant and recessive alleles, proportion of alleles with positive and negative effects, heritability, and combining ability analysis were performed as per the method suggested by Hayman66,67 using Windostat Version 9.2.
Acknowledgements
Laboratory assistance of Mr. Suraj Sharma, Rajender Kumar and S.S. Rawat during experiments is gratefully acknowledged. Funding for research was provided by Department of Science and Technology, New Delhi, India (SERB No: SB/SO/AS-020/2013) and National Agricultural Science Fund, Indian Council of Agricultural Research, New Delhi, India (NASF/ABP-5017/2016-17).
Author contributions
M.K.D. and H.C.S. conceived research. M.K.D., F.H. and N.S. designed experiments. F.H., A.K.T. and J.J. performed experiments. M.K.D., F.H. and N.S. analyzed data and wrote manuscript. All authors approved manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nijhout HF. A threshold size for metamorphosis in the tobacco horn worm, Manduca sexta. Biological Bulletin. 1975;149:214–225. doi: 10.2307/1540491. [DOI] [PubMed] [Google Scholar]
- 2.Denlinger DL. Regulation of diapause. Annual Review of Entomology. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 3.Yocum GD, Rinehart JP, Larson ML. Monitoring diapause development in the Colorado potato beetle, Leptinotarsa decemlineata, under field conditions using molecular biomarkers. Journal of Insect Physiology. 2011;57:645–652. doi: 10.1016/j.jinsphys.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Fischer K, Klockmann M, Reim E. Strong negative effects of simulated heat waves in a tropical butterfly. Journal of Experimental Biology. 2014;217:2892–2898. doi: 10.1242/jeb.106245. [DOI] [PubMed] [Google Scholar]
- 5.Beck, S.D. Insect Photoperiodism, 2nd ed. Academic Press, New York (1980).
- 6.Tauber, M.J., Tauber, C.A. & Masaki, S. Seasonal Adaptations of Insects, Oxford University Press, London (1986).
- 7.Arbab A. Spatial distribution and minimum sample size for overwintering larvae of the rice stem borer, Chilo suppressalis (Walker) in paddy fields. Neotropical Entomology. 2014;43:415–420. doi: 10.1007/s13744-014-0232-y. [DOI] [PubMed] [Google Scholar]
- 8.Hoy Marjorie A. Proceedings in Life Sciences. New York, NY: Springer US; 1978. Variability in Diapause Attributes of Insects and Mites: Some Evolutionary and Practical Implications; pp. 101–126. [Google Scholar]
- 9.Sinclair BJ, Vernon P, Klok CJ, Chown SL. Insects at low temperatures: an ecological perspective. Trends in Ecology and Evolution. 2003;18:257–262. doi: 10.1016/S0169-5347(03)00014-4. [DOI] [Google Scholar]
- 10.Tamiru A, Getu E, Jembere B, Bruce T. Effect of temperature and relative humidity on the development and fecundity of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) Bulletin of Entomological Research. 2012;102:9–15. doi: 10.1017/S0007485311000307. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon MK, Hasan F. Consequences of diapause on post-diapause development, reproductive physiology and population growth of Chilo partellus (Swinhoe) Physiological Entomology. 2018;43:196–206. doi: 10.1111/phen.12243. [DOI] [Google Scholar]
- 12.Kostal V, Sula J, Simek P. Physiology of drought tolerance and cold hardiness of the Mediterranean tiger moth Cymbalophora pudica during summer diapause. Journal of Insect Physiology. 1998;44:165–173. doi: 10.1016/S0022-1910(97)00047-4. [DOI] [PubMed] [Google Scholar]
- 13.Singtripop T, Wanichacheewa S, Tsuzuki S, Sakurai S. Larval growth and diapause in a tropical moth Omphisa fuscidentalis Hampson. Zoological Science. 1999;16:725–733. doi: 10.2108/zsj.16.725. [DOI] [Google Scholar]
- 14.Dhillon MK, Hasan F, Tanwar AK, Bhadauriya APS. Effects of thermo-photoperiod on induction and termination of hibernation in Chilo partellus (Swinhoe) Bulletin of Entomological Research. 2017;107:294–302. doi: 10.1017/S0007485316000870. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon MK, Hasan F, Tanwar AK, Bhadauriya APS. Factors responsible for aestivation in spotted stem borer, Chilo partellus (Swinhoe) Journal of Experimental Zoology A: Ecological and Integrative Physiology. 2019;331(6):326–340. doi: 10.1002/jez.2271. [DOI] [PubMed] [Google Scholar]
- 16.Ikten C, Skoda SR, Hunt TE, Molina-Ochoa J, Foster JE. Genetic variation and inheritance of diapause induction in two distinct voltine ecotypes of Ostrinia nubilalis (Lepidoptera: Crambidae) Annals of the Entomological Society of America. 2011;104:567–575. doi: 10.1603/AN09149. [DOI] [Google Scholar]
- 17.Baldwin JD, Dingle H. Geographic variation in the effects of temperature on life-history traits in the large milkweed bug Oncopeltus fasciatus. Oecologia. 1986;69:64–71. doi: 10.1007/BF00399039. [DOI] [PubMed] [Google Scholar]
- 18.Blanckenhorn WU. Altitudinal life history variation in the dung flies Scathophaga stercoraria and Sepsis cynipsea. Oecologia. 1997;109:342–352. doi: 10.1007/s004420050092. [DOI] [PubMed] [Google Scholar]
- 19.Dolezel D, Vanecková H, Sauman I, Hodkova M. Is period gene causally involved in the photoperiodic regulation of reproductive diapause in the linden bug, Pyrrhocoris apterus? Journal of Insect Physiology. 2005;51:655–659. doi: 10.1016/j.jinsphys.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Suwa A, Gotoh T. Geographic variation in diapause induction and mode of diapause inheritance in Tetranychus pueraricola. Journal of Applied Entomology. 2006;130:329–335. doi: 10.1111/j.1439-0418.2006.01050.x. [DOI] [Google Scholar]
- 21.Han B, Denlinger DL. Mendelian inheritance of pupal diapause in the flesh fly Sarcophaga bullata. Heredity. 2009;100:251–255. doi: 10.1093/jhered/esn082. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Xiao L, He HM, Xu J, Xue FS. A genetic analysis of diapause in crosses of a southern and a northern strain of the cabbage beetle Colaphellus bowringi (Coleoptera: chrysomelidae) Bulletin of Entomological Research. 2014;104:12–18. doi: 10.1017/S0007485313000266. [DOI] [PubMed] [Google Scholar]
- 23.King ABS. Photoperiodic induction and inheritance of diapause in Pionea forficalis (Lepidoptera: Pyralidae) Entomologia Experimentalis et Applicata. 1974;17:397–409. doi: 10.1111/j.1570-7458.1974.tb00358.x. [DOI] [Google Scholar]
- 24.Reed GL, Guthrie WD, Showers WB, Barry BD, Cox DF. Sex-linked inheritance in diapause of the European corn borer and its significance to diapause physiology. Annals of the Entomological Society of America. 1981;74:1–8. doi: 10.1093/aesa/74.1.1. [DOI] [Google Scholar]
- 25.McWatters HG, Saunders DS. The influence of each parent and geographic origin on larval diapause in the blow fly, Calliphora vicina. Journal of Insect Physiology. 1996;42:721–726. doi: 10.1016/0022-1910(96)00030-3. [DOI] [Google Scholar]
- 26.McWatters HG, Saunders DS. Inheritance of the photoperiodic response controlling larval diapause in the blow fly, Calliphora vicina. Journal of Insect Physiology. 1997;43:709–717. doi: 10.1016/S0022-1910(97)00051-6. [DOI] [PubMed] [Google Scholar]
- 27.Fu S, Chen C, Xiao L, He H, Xue H. Inheritance of diapause in crosses between the northernmost and the southernmost strains of the Asian corn borer Ostrinia furnacalis. PLoS ONE. 2015;10:e0118186. doi: 10.1371/journal.pone.0118186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khadioli N, et al. Effect of temperature on the phenology of Chilo partellus (Swinhoe) (Lepidoptera, Crambidae); simulation and visualization of the potential future distribution of C. partellus in Africa under warmer temperatures through the development of life-table parameters. Bulletin of Entomological Research. 2014;104:809–822. doi: 10.1017/S0007485314000601. [DOI] [PubMed] [Google Scholar]
- 29.Ofomata VC, Overholt WA, Egwuatu RI. Diapause termination of Chilo partellus (Swinhoe) and Chilo orichalcociliellus Strand (Lepidoptera: Pyralidae) Insect Science of its Application. 1999;19:187–191. [Google Scholar]
- 30.Kfir R, Overholt WA, Khan ZR, Polaszek A. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annual Review of Entomology. 2002;47:701–731. doi: 10.1146/annurev.ento.47.091201.145254. [DOI] [PubMed] [Google Scholar]
- 31.Dhillon MK, Hasan F. Temperature-dependent development of diapausing larvae of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) Journal of Thermal Biology. 2017;69:213–220. doi: 10.1016/j.jtherbio.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Dhillon MK, Hasan F. Morphological changes in Chilo partellus (Swinhoe) undergoing diapause. Journal of Entomology and Zoology Studies. 2017;5:1658–1661. [Google Scholar]
- 33.Xiao L, et al. Inheritance of photoperiodic control of larval diapause in the Asian corn borer Ostrinia furnacalis (Guenée) Bulletin of Entomological Research. 2015;105:326–334. doi: 10.1017/S0007485315000140. [DOI] [PubMed] [Google Scholar]
- 34.Pruisscher P, et al. Sex‐linked inheritance of diapause induction in the butterfly Pieris napi. Physiological Entomology. 2017;42(3):257–265. doi: 10.1111/phen.12194. [DOI] [Google Scholar]
- 35.Danks HV. Life cycle pathways and the analysis of complex life cycles in insects. Canadian Entomologist. 1991;123:23–40. doi: 10.4039/Ent12323-1. [DOI] [Google Scholar]
- 36.Masaki S. Geographical variation of life cycle in crickets (Ensifera: Grylloidea) European Journal of Entomology. 1996;93:281–302. [Google Scholar]
- 37.Raina AK, Bell RA, Klassen W. Diapause in the pink bollworm: preliminary genetic analysis. International Journal of Tropical Insect Science. 1981;1(3):231–235. doi: 10.1017/S1742758400000461. [DOI] [Google Scholar]
- 38.Sims SR. Inheritance of diapause induction and intensity in Papilio zelicaon. Heredity. 1983;51:495–500. doi: 10.1038/hdy.1983.60. [DOI] [Google Scholar]
- 39.Soderlind L, Nylin S. Genetics of diapause in the comma butterfly Polygonia c-album. Physiological Entomology. 2011;36:8–13. doi: 10.1111/j.1365-3032.2010.00756.x. [DOI] [Google Scholar]
- 40.Xia QW, Chen C, Tu XY, Yang HZ, Xue FS. Inheritance of photoperiodic induction of larval diapause in the Asian corn borer, Ostrinia furnacalis. Physiological Entomology. 2012;37:185–191. doi: 10.1111/j.1365-3032.2011.00810.x. [DOI] [Google Scholar]
- 41.Wipking W, Kurtz J. Genetic variability in the diapause response of the burnet moth Zygaena trifolii (Lepidoptera: Zygaenidae) Journal of Insect Physiology. 2000;46:127–134. doi: 10.1016/S0022-1910(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 42.Tan RH, Zhu DH, Yang YP. The diapause rate of hybrid offspring among three geographic populations in a grasshopper, Oxya chinensis. Chinese Bulletin of Entomology. 2008;45:394–397. [Google Scholar]
- 43.Yang D, Lai XT, Sun L, Xue FS. Parental effects: physiological age, mating pattern, and diapause duration on diapause incidence of progeny in the cabbage beetle Colaphellus bowringi Baly (Coleoptera: Chrysomelidae) Journal of Insect Physiology. 2007;53:900–908. doi: 10.1016/j.jinsphys.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Lai XT, Yang D, Wu SH, Zhu XF, Xue FS. Diapause incidence of progeny in relation to parental geographic origin, host plant and rearing density in the cabbage beetle, Colaphellus bowringi. Entomologia Experimentalis et Applicata. 2008;129:117–123. doi: 10.1111/j.1570-7458.2008.00749.x. [DOI] [Google Scholar]
- 45.Danks, H.V. Insect Dormancy: An Ecological Perspective, vol. 1. Biological Survey of Canada (1987).
- 46.Takeda M. Genetic basis of photoperiodic control of summer and winter diapause in geographic ecotypes of the rice stem maggot, Chlorops oryzae. Entomologia Experimentalis et Applicata. 1998;86:59–70. doi: 10.1046/j.1570-7458.1998.00265.x. [DOI] [Google Scholar]
- 47.Wiklund C, Wickman PO, Nylin S. A sex difference in the propensity to enter direct/diapause development: a result of selection for protandry. Evolution. 1992;46:519–528. doi: 10.1111/j.1558-5646.1992.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 48.Abrams PA, Leimar O, Nylin S, Wiklund C. The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. American Naturalist. 1996;147:381–395. doi: 10.1086/285857. [DOI] [Google Scholar]
- 49.Nylin S, Gotthard K. Plasticity in life history traits. Annual Review of Entomology. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- 50.Showers WB, Brindley TA, Reed GL. Survival and diapause characteristics of hybrids of three geographic races of the European corn borer. Annals of the Entomological Society of America. 1972;65:450–457. doi: 10.1093/aesa/65.2.450. [DOI] [Google Scholar]
- 51.McLeod DGR. Genetics and diapause induction and termination in the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae) in southwestern Ontario. Canadian Entomologist. 1978;110:1351–1353. doi: 10.4039/Ent1101351-12. [DOI] [Google Scholar]
- 52.Falconer DS. Introduction to Quantitative Genetics. 4th edn. London: Longman; 1996. [Google Scholar]
- 53.Weigensberg I, Roff DA. Natural heritabilities: can they be reliably estimated in the laboratory? Evolution. 1996;50:2149–2157. doi: 10.1111/j.1558-5646.1996.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 54.Morris RF, Fulton WC. Heritability of diapause intensity in Hyphantria cunea and correlated fitness responses. Canadian Entomologist. 1970;102:927–938. doi: 10.4039/Ent102927-8. [DOI] [Google Scholar]
- 55.Holtzer TO, Bradley JR, Raab RL. Geographic and genetic variation in time required for emergence of diapausing Heliothis zea. Annals of the Entomological Society of America. 1976;69:261–265. doi: 10.1093/aesa/69.2.261. [DOI] [Google Scholar]
- 56.Dingle H, Brown CK, Hegmann JP. The nature of genetic variance influencing photoperiodic diapause in a migrant insect, Oncopeltus fasciatus. American Naturalist. 1977;111:1047–1059. doi: 10.1086/283237. [DOI] [Google Scholar]
- 57.Palmer JO, Dingle H. Direct and correlated responses to selection among life history traits in milkweed bugs (Oncopeltus fasciatus) Evolution. 1986;40:767–777. doi: 10.1111/j.1558-5646.1986.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 58.Bradshaw, W.E. & Holzapfel, C.M. Evolution of phenology and demography in the pitcher plant mosquito Wyeomyia smithii. 47–67 in Gilbert, F. (Ed.) Genetics, Evolution and Coordination of Insect Life Cycles. Springer-Verlag, London (1990).
- 59.Simons AM, Carriere Y, Roff DA. The quantitative genetics of growth in a field cricket. Journal of Evolution Biology. 1998;11:721–733. doi: 10.1007/s000360050115. [DOI] [Google Scholar]
- 60.Fisher, R.A. The Genetical Theory of Natural Selection. Clarendon Press, Oxford, UK (1930).
- 61.Gustafsson L. Lifetime reproductive success and heritability: empirical support for Fisher’s fundamental theorem. American Naturalist. 1986;128:761–764. doi: 10.1086/284601. [DOI] [Google Scholar]
- 62.Mousseau TS, Roff DA. Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution. 1987;43:1483–1396. doi: 10.1111/j.1558-5646.1989.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 63.Roff DA, Mousseau TA. Quantitative genetics and fitness: lessons from Drosophila. Heredity. 1987;58:103–118. doi: 10.1038/hdy.1987.15. [DOI] [PubMed] [Google Scholar]
- 64.Sharma, H.C., Taneja, S.L., Leuschner, K. & Nwanze, K.F. Techniques to screen sorghum for resistance to insect pests. Information Bulletin No. 32, 48 International Crops Research Institute for the Semi-Arid Tropics, Patancheru 502 324, Andhra Pradesh, India (1992).
- 65.Wilson, K. & Hardy, I.C.W. Statistical analysis of sex ratios: an introduction. 48-92 in Hardy, I.C.W. (Ed.) Sex Ratios: Concepts and Research Methods. Cambridge University Press, New York (2002).
- 66.Hayman BI. The analysis of variance of diallel tables. Biometrics. 1954;10:235–244. doi: 10.2307/3001877. [DOI] [Google Scholar]
- 67.Hayman BI. The theory and analysis of diallel crosses. Genetics. 1954;39:789–809. doi: 10.1093/genetics/39.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]