Abstract
Despite all the research done on the first Zika virus (ZIKV) epidemics, it was only after the Brazilian epidemic that the Congenital Zika Syndrome was described. This was made possible due to the large number of babies born with microcephaly in the Northeast region (NE) in a narrow time. We hypothesize that the fivefold difference in the rate of microcephalic neonates between the NE and other regions is partially an effect of the population prior immunity against Dengue viruses (DENV), that cross-react with ZIKV. In this ecological study, we analysed the interaction between dengue fever epidemics from 2001 to 2014 and the 2015/2016 microcephaly epidemic in 400 microregions in Brazil using random-effects models under a Bayesian approach. The estimated effect of the time lag between the most recent large dengue epidemic (>400/100,000 inhabitants) and the microcephaly epidemic ranged from protection (up to 6 years prior) to an increased risk (from 7 to 12 years). This sustained window of protection, larger than described in previous longitudinal studies, is possibly an effect of herd immunity and of multiple exposures to DENV that could boost immunity.
Subject terms: Viral infection, Epidemiology
Introduction
One of the reasons that made it possible for researchers to associate the microcephaly epidemic in the Northeast (NE) region of Brazil with the Zika virus (ZIKV) was the high rate of dispersion of the virus and the large number of babies born with similar characteristics in the same maternity wards, in the same week, and sometimes even in the same day. This repeatedly happened in different cities of the region in a short time1. Despite all the research done on the first epidemics of ZIKV in Yap Islands and later in French Polynesia2–4, it was only after the Brazilian epidemic that Congenital Zika Syndrome (CZS) was described5. This could be due to the small population in the previously affected areas and to the large number of births concentrated in the same hospitals of the Brazilian Unified Health System (SUS). Even so, a robust analysis using secondary databases estimated a peak of 49.9 microcephaly cases per 10,000 live births in the NE region, whereas for the other regions the peak did not surpass 10 cases per 10,000 live births6. So far, microcephaly ratios as high as in the Brazilian NE region have not been described in any other region in the world that had undergone a Zika epidemic7–9. Several reasons for this discrepancy were suggested – poor data quality10–12, the unreliability of secondary data sources13,14, virus genetic variation15, socioeconomic factors16, dengue immunity profile of the population17, and women postponing pregnancy or having abortions18–20.
The antigenic similarity between ZIKV and dengue virus (DENV), sharing approximately 54% of their amino acid envelope proteins, results in immunological cross-reactivity21. In vitro studies have shown that anti-DENV antibodies can both enhance and neutralize ZIKV infection22–26. In animal models, mice that received plasma with a low level of anti-DENV antibodies had a higher mortality rate after ZIKV infection than mice that received plasma without antibodies. However, all mice that received plasma with a high level of anti-DENV antibodies survived after ZIKV challenge and presented milder symptoms22. Human plasma collected ≤100 days after PCR-confirmed DENV infection binds and cross-neutralizes ZIKV in vitro. On the other hand, late-convalescent-phase plasma does not harbour durable, high levels of cross-neutralizing antibodies against ZIKV27. However, the understanding of cross-neutralizing antibody responses among individuals with prior DENV exposure, particularly how these responses evolve over time and in various transmission contexts in flavivirus-endemic countries is quite unknown28.
In summary, it is known that ZIKV shares structural similarities with other flaviviruses, especially DENV, and co-circulates in dengue-endemic regions. ZIKV and DENV cross-immunity can be either protective or enhancing in experimental models. However, many questions still need to be addressed, particularly by placing in vitro and in vivo findings in an epidemiological context29,30. In this article, we analyse the interaction between dengue fever epidemics from 2001 to 2014 and the 2015/2016 microcephaly epidemic in 400 microregions in Brazil.
Methods
Data
This is an ecological study using data gathered by the Health Informatics Department of the Brazilian Ministry of Health (DATASUS). All data are publicly available at the DATASUS website (http://www.datasus.gov.br/DATASUS/index.php/index.php?area=02).
The neonates’ data are collected by the Brazilian Live Births Information System (SINASC), which includes a field to inform the presence of an observed malformation and five fields to classify the condition. Since, at the time, CZS did not have a specific code, we considered as a microcephaly case a live-born with the microcephaly code “Q02” (10th edition of the ICD) in any of the five fields. We compared the number of microcephaly cases between 2015–2016, considered the epidemic period, to the 2014 data, both in the exploratory analysis and the maps.
Dengue data are collected by the Brazilian Information System for Notifiable Diseases (SINAN). We obtained the number of dengue fever cases by municipality and epidemiological week from 2001 to 2014. For the same period, annual population data by municipality estimated by the Brazilian Institute of Geography and Statistics (IBGE), the census bureau, were also obtained.
We excluded the North region (where the Amazon forest is located) due to poorer data quality and incomplete birth records, and the South region, not endemic for dengue fever or any arboviral disease.
Maps were made using QGIS (version 3.6.3)31 and layers from IBGE (available at https://downloads.ibge.gov.br/downloads_geociencias.htm), and terrain background by Stamen Design (data by OpenStreetMap).
In accordance with the Brazilian Research Ethics, ethical approval is not mandatory for the use of publicly available datasets.
Exploratory analysis
Live-born data was aggregated by 400 socioeconomically homogeneous microregions, as defined by the census bureau. As dengue fever counts are organized by epidemiological week, the first approach was to use this time scale (Fig. 1a,b). Maps (Fig. 2a,b) present the overall microcephaly rate per 10,000 live-born babies and dengue fever incidence per 100,000 inhabitants by microregion in the three selected Brazilian macroregions: NE, Central-West (CW) and Southeast (SE).
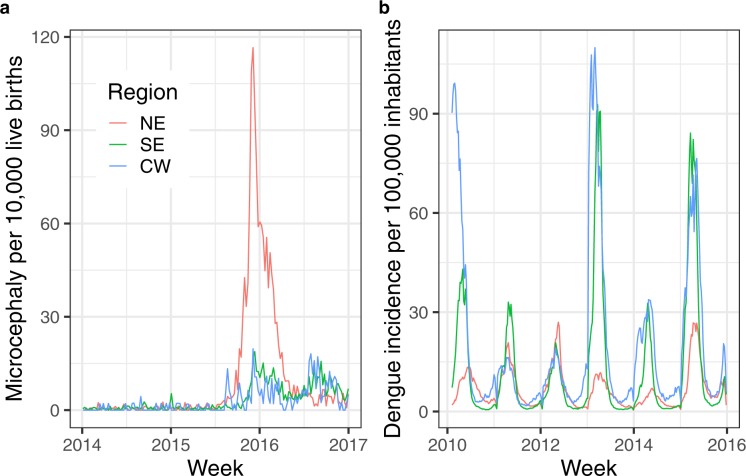
Figure 1.
Microcephaly and dengue fever indicators per week per macroregion, Brazil. Frame (a) presents the rate of microcephaly cases per 10,000 live-births by week in each region analysed, and frame (b) depicts the dengue fever incidence rate per 100,000 inhabitants, in the same time scale.
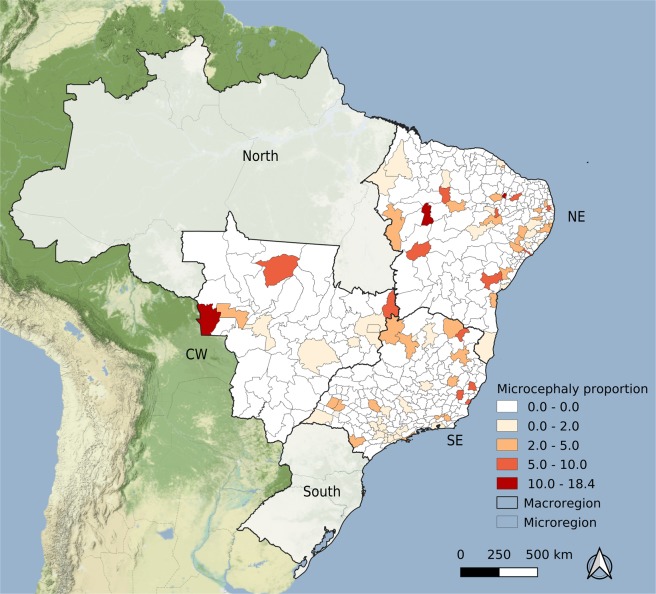
Figure 2.
Rate of microcephaly cases per 10,000 live-born infants per microregion, Brazil, 2014. Map created using QGIS version 3.6.3 (QGIS Development Team 2019. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org). Map tiles by Stamen Design, under CC BY 3.0. (https://creativecommons.org/licenses/by/3.0/) Data by OpenStreetMap, under CC BY SA.
Models
Using the microcephaly counts as the dependent variable and the total number of live births as offset, we fitted four random-effects models varying the likelihood: Poisson, Negative Binomial, zero-inflated Poisson and zero-inflated Negative Binomial. Two random-effects components were included in the model: the first is a time-varying coefficient for the time since the last big dengue epidemic modelled as a second-order random walk to allow a smooth non-linear behaviour. The second random-effects component was an independent and identically distributed Gaussian random effect by microregion. Vague priors were used and the inference was done under the Bayesian approach using the integrated nested Laplace approximation (INLA)32.
To evaluate the time lag between the microcephaly epidemic and a previous dengue fever epidemic, we assessed several rate thresholds (100, 200, 300, 400, 500, and 600 cases per 100,000 inhabitants) and statistical distributions (Supplemental Material). The best fit, according to the Watanabe-Akaike information criterion (WAIC)33, was obtained with the zero-inflated Poisson model and 400 cases per 100,000 inhabitants as the threshold of a dengue epidemic.
In brief, the model evaluates the number of microcephaly cases, considering the total number of live births as offset, and dengue fever epidemics with an incidence above 400/100,000 between one and fourteen years prior as an explanatory variable. The model included two random effects: (1) microregion random effects considering both a spatial structured random effect and an unstructured one, allowing for spatial dependence and extra-variability, and (2) spline-like random effects for the time, in years, since the last dengue epidemic in each region.
We used the R software34 with the tidyverse library35 to organize the data and present the results, and R-INLA36 for the analysis. The R code is available in the Supplemental Material.
Results
The total number of microcephaly cases increased more than 23-fold between 2014 and 2015 in the NE region, and more than tenfold in both the SE and CW regions between 2015 and 2016 (Table 1). The number of live births in 2016 decreased 5% in the NE and CW regions, and 6% in the SE, compared with 2015, following the declaration of public health emergency by the Brazilian Health authorities. In just one week, the number of microcephaly cases in 2015 in the NE region reached 29, more than half of the total number of microcephaly cases from the previous year. The number of microregions with no microcephaly cases decreased first in the NE, the dispersion centre of Zika epidemic, followed by the SE and CW regions.
Table 1.
Number of microcephaly cases in neonates, number of live births, the maximum number of microcephaly cases in one week, and proportion of microregions with zero microcephaly cases, by year and macroregion, Brazil.
| Year | Northeast | Southeast | Central-West | |
|---|---|---|---|---|
| Microcephaly cases | 2014 | 55 | 76 | 12 |
| 2015 | 1294 | 283 | 62 | |
| 2016 | 1071 | 844 | 138 | |
| Live births | 2014 | 838094 | 1189848 | 246450 |
| 2015 | 844590 | 1193720 | 247069 | |
| 2016 | 799760 | 1123013 | 233888 | |
| Maximum number of microcephaly cases in one week | 2014 | 2 | 2 | 1 |
| 2015 | 29 | 15 | 5 | |
| 2016 | 19 | 10 | 3 | |
| Percentage of microregions with zero microcephaly cases | 2014 | 79.3% | 78.8% | 82.7% |
| 2015 | 22.3% | 61.3% | 69.2% | |
| 2016 | 19.7% | 37.5% | 37.7% |
The rate of microcephaly in neonates per 10,000 live births per week by Brazilian macroregions is presented in Fig. 1a. The increase of microcephaly rate beginning at the end of 2015 is observed in all regions. However, in the NE region, by August/2015 the rate had surpassed 30 cases per 10,000 live births, while in other regions it was never higher than 20/10,000 live births. In the SE and CW regions, a second peak occurred at the end of 2016. Comparing the incidence rates of dengue fever (Fig. 1b) in the SE and CW regions, three epidemic years (2010, 2013 and 2015) are present, while in the NE region, in the same period, we observed just a seasonal pattern with no outbreaks.
The map presented in Fig. 2 shows the usual pattern of microcephaly rate among live-born neonates in 2014, before the Zika epidemic. Among the 400 microregions included in the analysis, in 318 (80%) no microcephalic neonate was reported, and the highest rate was due to two babies born in an area with a small population.
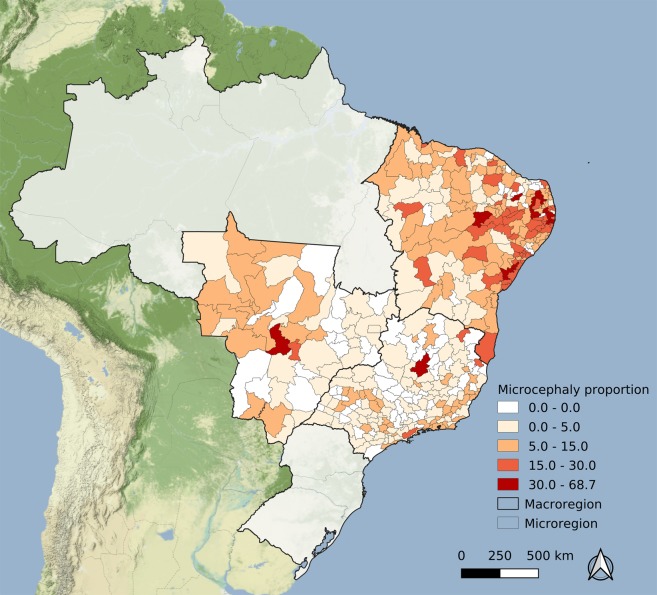
The microcephaly epidemic data map (Fig. 3) depicted a different pattern: more than 60% of the areas in the SE and CW regions did not present any cases, while in the NE this proportion was just 20% (Table 1).
Figure 3.
Rate of microcephaly cases per 10,000 live-born infants per microregion, Brazil, 2015–2016. Map created using QGIS version 3.6.3 (QGIS Development Team, 2019, QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org). Map tiles by Stamen Design, under CC BY 3.0. (https://creativecommons.org/licenses/by/3.0/) Data by OpenStreetMap, under CC BY SA.
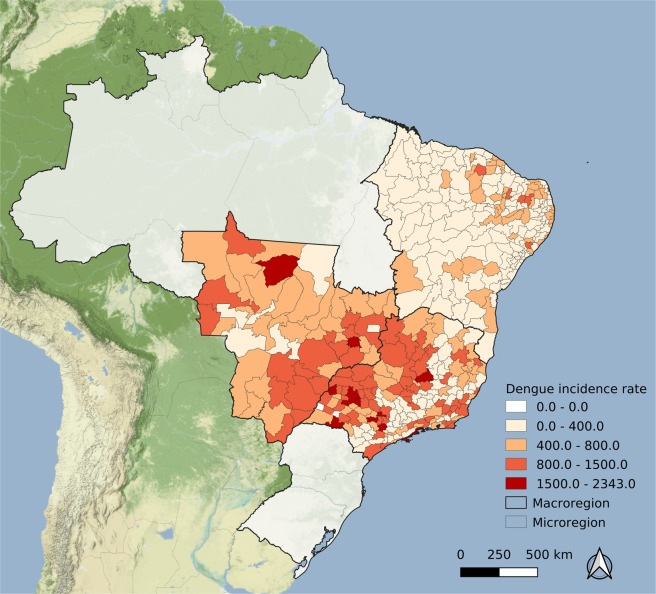
Figure 4 depicts the mean dengue fever incidence rate over five years (2010–2014). The spatial pattern, as compared with Fig. 3, is inverted: in areas with high microcephaly rates, dengue fever incidence rates were smaller.
Figure 4.
Average annual dengue fever incidence rate per 100,000 over 2010–2014 by microregion, Brazil. Map created using QGIS version 3.6.3 (QGIS Development Team, 2019. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org). Map tiles by Stamen Design, under CC BY 3.0. (https://creativecommons.org/licenses/by/3.0/) Data by OpenStreetMap, under CC BY SA.
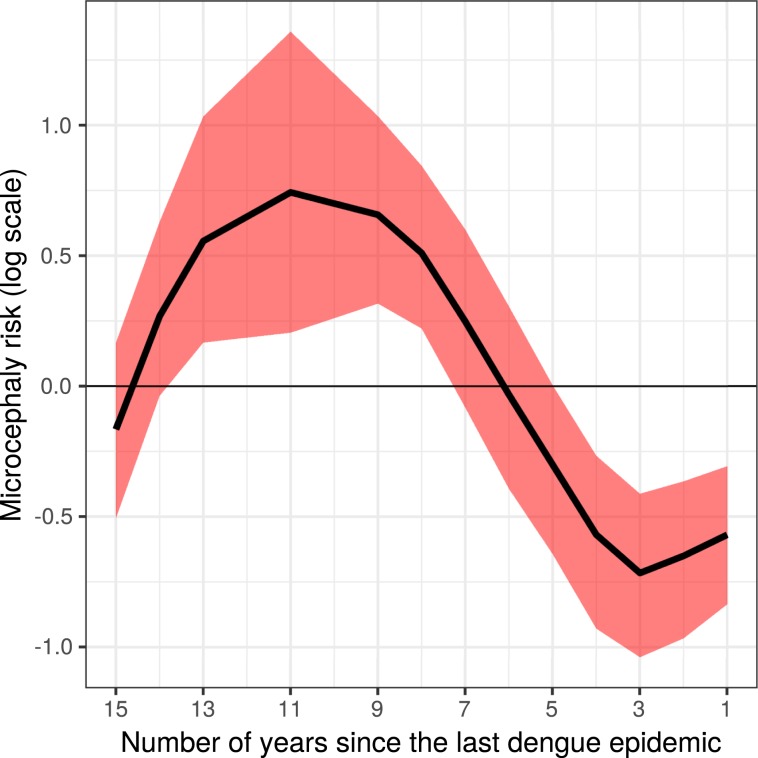
To estimate the association between both indicators – microcephaly rate and previous dengue fever incidence – we estimated the effect of the time lag between the most recent large dengue epidemic and the microcephaly epidemic. Figure 5 presents the main results of this article: the credibility time interval since the last dengue fever epidemic above 400 cases/100,000 inhabitants over the rate of microcephalic neonates. This finding suggests a protective effect up to 6 years prior to an increased risk from 7 to 12 years.
Figure 5.
Estimated microcephaly risk (log) and credibility interval by number of years since the last dengue fever epidemic (incidence >400/100,000).
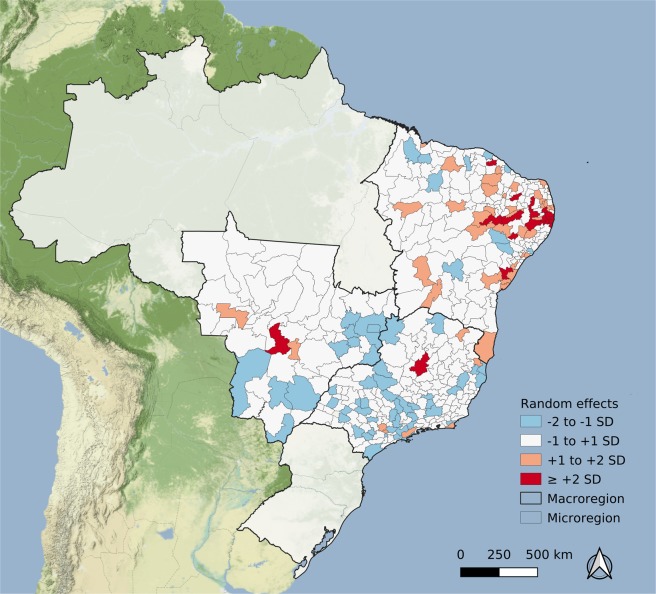
The random effect estimated for each microregion, as presented in Fig. 6, suggests that the dengue fever epidemic effect is not enough to explain the large number of microcephaly cases in some areas in the NE. The Supplemental material presents the results of different models, exploring dengue epidemic definitions from 100 to 600/100,000 inhabitants and other statistical distributions. Blue areas are those with a lower rate of microcephaly cases than predicted by the time lag after the last dengue fever epidemic, while orange/red areas are the opposite. In some areas, the estimated random effect is above two standard deviations, mostly spatially concentrated in the NE region.
Figure 6.
Microregion random effects estimated by the fitted model, presented in standard deviation units. Map created using QGIS version 3.6.3 (QGIS Development Team, 2019. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org). Map tiles by Stamen Design, under CC BY 3.0. (https://creativecommons.org/licenses/by/3.0/) Data by OpenStreetMap, under CC BY SA.
Discussion
This is the first article, to the best of our knowledge, in which an association, at the ecological level, between previous dengue fever epidemics and the Zika-related microcephaly epidemic was described. It should be noted that the notification system for Zika was only implemented in February 2016, when the Zika epidemic was decreasing in the NE region. Therefore, we could not control for Zika incidence in the models. It is possible that the differences in microcephaly rates were a consequence of different ZIKV attack rates across the regions, as suggested in a recent paper37. In this study, after testing for other concurrent factors, ZIKV was assumed to be the only cause of the microcephaly epidemic, with no unmeasured confounders or effect modifiers. However, if Zika incidence alone explains the excess microcephaly rates, then microcephaly would simply be a proxy for Zika, and it remains unclear as to why the NE region would have presented with a Zika incidence more than ten times larger than anywhere else in Brazil37.
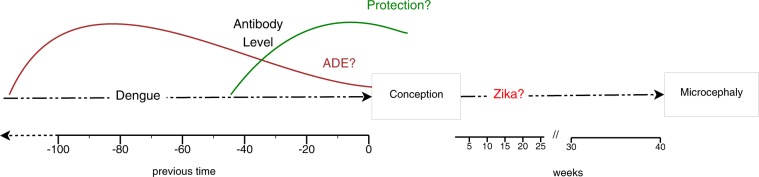
Our hypothesis considers that the antibody decay due to the time interval between infections changes the role of anti-DENV antibodies on ZIKV infection, either to protect or to increase the risk (possibly via antibody-dependent enhancement – ADE), as illustrated in Fig. 7. This hypothesis is based on what is described for sequential heterotypic DENV infections (there are four DENV serotypes). It has been established that a narrow range of pre-existing anti-DENV antibody titres is associated with the risk of severe dengue disease, while high titres protect against clinical illness29,38. As the antibody titres decay over time, there is a window of cross-protection between the DENV serotypes. A previous study found that a shorter interval between first and second infections was associated with protection from clinical illness39. Additionally to antibodies, DENV-specific CD8+ T cells from previous exposure also seems to play a role against ZIKV. Mice primed with DENV and 30 days later challenged with ZIKV during pregnancy presented reduced burden in maternal and fetal tissues, and increased fetal viability compared to non-immune mice. DENV-immune CD8+ T cells were required for this cross-protection. However, mice challenged 80 days after DENV priming were not protected, indicating the transient nature of cross-protection again40.
Figure 7.
Hypothesized interaction between dengue and Zika infections on the development of microcephaly.
Most research on the effect of previous DENV-immunity on the severity of clinical symptoms of ZIKV infection is based on experimental models41,42. Cohort studies are the golden standard to epidemiological designs to estimate disease incidence and would give the most reliable understanding of the role of previous DENV exposure on ZIKV infection. However, the reduction in the incidence of Zika after the last epidemic in Brazil in 2016 limits the ability to use prospective studies43–45. Some cohorts previously designed to study dengue and that have been adapted to also study Zika are of great value. For example, a recent study in the Pau da Lima cohort in Salvador, a large city of the NE region, Brazil, found that the titres of DENV-antibodies measured before the Zika epidemic were inversely associated with the risk of ZIKV infection46. In a paediatric cohort in Nicaragua, prior DENV infection was inversely associated with the risk of symptomatic ZIKV infection47.
In a cohort of ZIKV-infected pregnant women, previous DENV infection was not associated with disease severity and abnormal birth outcomes48. However, the time interval between the infections and the anti-DENV antibody titres were not considered in the analysis. Our findings indicate that a previous dengue fever epidemic may be related to microcephaly incidence and reinforce the idea of a window of cross-protection and a window of increased risk. Our window of protection is wider than detected in a longitudinal analysis of human immune sera, which showed a cross-neutralizing antibody response to ZIKV on DENV-immune subjects 1–3 years after DENV infection28. The larger window at the ecological level is possibly an effect of herd immunity and of multiple exposures to DENV that would boost immunity. This is consistent with a case-control study in which the DENV seroprevalence and the mean number of neutralized serotypes (indicative of multiple exposures) were significantly lower among mothers of neonates with CZS49.
We applied a powerful modelling strategy using the INLA approach32, which allowed us to simultaneously take into account the non-linear behaviour of the time since the last dengue epidemic (Fig. 5) and the excess variation in each area, as discussed above and shown in Fig. 6. The 18 areas highlighted with higher risks than predicted by the model should be further investigated.
In the easternmost area of the NE region there was an excess of two standard deviations over the number of microcephalic neonates predicted by the model (Fig. 6). In this area, the involvement among researchers and clinicians that led to the early discovery of the causal link between Zika and microcephaly may have generated a hyper-awareness of the problem. The more strict head circumference measurements counterbalanced the usual under-reporting11,50. It should be noted that the main surveillance system for neonatal congenital malformation (Latin American Collaborative Study of Congenital Malformations – ECLAMC) did not detect the microcephaly epidemic in the early stages14, possibly due to the geographical constraints of the sentinel hospitals. The use of a secondary database such as SINASC, despite its accuracy limitations, together with the more specialized ECLAMC could reinforce a surveillance system for neonatal malformation clustered in space and time.
Dengue epidemics in the previous 15 years were used as a proxy of the herd immunity level among women of reproductive age. The choice of the threshold that we considered high enough to either protect or increase microcephaly risk (of 400/100,000 inhabitants) was data-driven, based on the best fit. The threshold of 300 cases/100,000 inhabitants as high incidence areas, as defined by the Brazilian Ministry of Health, is a number defined ad hoc, not sustained by any scientific argument in any guideline of the dengue control program published since 2002.
Other possible factors in the pathway between Zika infection and microcephaly have been suggested, the most important of which is the socioeconomic deprivation in the Brazilian NE16,51. This is a typical confounding problem, not easily addressed, mainly due to the larger reservoirs of Aedes aegypti in poor areas and increased awareness of the microcephaly risk in the upper socioeconomic classes, inducing either postponing pregnancy during epidemic periods or voluntary abortion, not legal in Brazil52.
The main limitations of this study, in addition to not controlling for Zika incidence, are well known: accuracy of microcephaly classification and completeness of dengue fever notification. We recognize the potential inaccuracy on the measurement of neonates’ cephalic perimeter. Considering 10% fewer neonates classified as microcephalic in the NE and 10% more in other regions, the difference would be fourfold, instead of five. As for the under notification of dengue fever, we have no reason to believe there was a trend over the years that would affect our results.
The detection of either ADE or cross-protection between DENV and ZIKV is not a trivial task, either in animal models or humans42. The increase in congenital malformations is not easily studied, considering a confluence of different risk factors and the large range of time lags between the potentially harmful event and the outcome. We believe that we brought new evidence that will contribute to building the knowledge regarding the relationship between DENV, ZIKV and congenital Zika syndrome.
Supplementary information
Acknowledgements
MSC was supported by the Conselho Nacional de Pesquisa – CNPq (grant 304101/2017-6) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (grant E-26/203.073/2017). LPF was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil – CAPES (Finance Code 001). PB was supported by Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (grant E-26/202.862/2018), Conselho Nacional de Pesquisa - CNPq (grant 307282/2017-1) and Fondation Christophe et Rodolphe Mérieux.
Author contributions
O.G.C. and L.S.B. were responsible for data organization and statistical modelling. M.S.C., P.B. and L.P.F. carried out the literature review and wrote the main manuscript text. M.S.C. was responsible for the project. All authors reviewed the manuscript.
Data availability
The original datasets are publicly available at http://datasus.saude.gov.br, and the data organised for the present analysis at 10.5281/zenodo.3489428.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58407-7.
References
- 1.Lowe R, et al. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public. Health. 2018;15:96. doi: 10.3390/ijerph15010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy MR, et al. Zika virus outbreak on Yap Island, federated states of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016;94:675–686C. doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouannic J-M, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016;387:1051–1052. doi: 10.1016/S0140-6736(16)00625-5. [DOI] [PubMed] [Google Scholar]
- 5.Miranda-Filho DEB, et al. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public. Health. 2016;106:598–600. doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira WK, et al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet. 2017;390:861–870. doi: 10.1016/S0140-6736(17)31368-5. [DOI] [PubMed] [Google Scholar]
- 7.Coelho A, Crovella S. Microcephaly Prevalence in Infants Born to Zika Virus-Infected Women: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017;18:1714. doi: 10.3390/ijms18081714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel G. One year later, Zika scientists prepare for a long war. Sci. 2016;354:1088–1089. doi: 10.1126/science.354.6316.1088. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque, M. de F. P. M. de et al. Epidemia de microcefalia e vírus Zika: a construção do conhecimento em epidemiologia. Cadernos de Saúde Pública34 (2018). [DOI] [PubMed]
- 11.Silva AA, et al. Prevalence and Risk Factors for Microcephaly at Birth in Brazil in 2010. Pediatrics. 2018;141:e20170589. doi: 10.1542/peds.2017-0589. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues Laura C. Microcephaly and Zika virus infection. The Lancet. 2016;387(10033):2070–2072. doi: 10.1016/S0140-6736(16)00742-X. [DOI] [PubMed] [Google Scholar]
- 13.Butler D. Microcephaly surge in doubt. Nat. 2016;530:13–14. doi: 10.1038/nature.2016.19259. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Camelo, J. S., Orioli, I. M. & Castilla, E. ECLAMC FINAL DOCUMENT (2015).
- 15.Rossi SL, Ebel GD, Shan C, Shi P-Y, Vasilakis N. Did Zika Virus Mutate to Cause Severe Outbreaks? Trends Microbiology. 2018;26:877–885. doi: 10.1016/j.tim.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza, W. V. de et al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. BMC Public Health18 (2018). [DOI] [PMC free article] [PubMed]
- 17.Halstead SB. Biologic Evidence Required for Zika Disease Enhancement by Dengue Antibodies. Emerg. Infect. Dis. 2017;23:569–573. doi: 10.3201/eid2304.161879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiken ARA, et al. Requests for Abortion in Latin America in the Wake of Zika Virus. N. Engl. J. Med. 2016;375:396–398. doi: 10.1056/NEJMc1605389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Quijano FA, Pelissari DM, Chiavegatto Filho ADP. Zika-Associated Microcephaly Epidemic and Birth Rate Reduction in Brazilian Cities. Am. J. Public. Health. 2018;108:514–516. doi: 10.2105/AJPH.2017.304260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro MC, Han QC, Carvalho LR, Victora CG, França GVA. Implications of Zika virus and congenital Zika syndrome for the number of live births in Brazil. Proc. Natl Acad. Sci. 2018;115:6177–6182. doi: 10.1073/pnas.1718476115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priyamvada L, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl Acad. Sci. USA. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardina SV, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Sci. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin AP. Dengue Antibody and Zika: Friend or Foe? Trends Immunology. 2016;37:635–636. doi: 10.1016/j.it.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Miner JJ, Diamond MS. Dengue Antibodies, then Zika: A Fatal Sequence in Mice. Immun. 2017;46:771–773. doi: 10.1016/j.immuni.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Robbiani DF, et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell. 2017;169:597–609.e11. doi: 10.1016/j.cell.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen J, Shresta S. ADE-ing and Abetting Zika. Cell Host Microbe. 2017;21:557–558. doi: 10.1016/j.chom.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins MH, et al. Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg. Infect. Dis. 2017;23:773–781. doi: 10.3201/eid2305.161630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoya Magelda, Collins Matthew, Dejnirattisai Wanwisa, Katzelnick Leah C, Puerta-Guardo Henry, Jadi Ramesh, Schildhauer Samuel, Supasa Piyada, Vasanawathana Sirijitt, Malasit Prida, Mongkolsapaya Juthathip, de Silva Aruna D, Tissera Hasitha, Balmaseda Angel, Screaton Gavin, de Silva Aravinda M, Harris Eva. Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas. The Journal of Infectious Diseases. 2018;218(4):536–545. doi: 10.1093/infdis/jiy164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzelnick LC, et al. Antibody-dependent enhancement of severe dengue disease in humans. Sci. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade Daniela V., Harris Eva. Recent advances in understanding the adaptive immune response to Zika virus and the effect of previous flavivirus exposure. Virus Research. 2018;254:27–33. doi: 10.1016/j.virusres.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.QGIS Development Team. QGIS Geographic Information System. (Open Source Geospatial Foundation Project 2019).
- 32.Rue H, Martino S, Chopin N. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. Journal of the Royal Statistical Society. Ser. B. 2009;71:319–392. [Google Scholar]
- 33.Watanabe S. Asymptotic Equivalence of Bayes Cross Validation and Widely Applicable Information Criterion in Singular Learning Theory. J. Mach. Learn. Res. 2010;11:3571–3594. [Google Scholar]
- 34.The R Foundation for Statistical Computing. R. (The R Foundation 2019).
- 35.Wickham, H. & RStudio. tidyverse: Easily Install and Load the ‘Tidyverse’ (2017).
- 36.The R-INLA project http://www.r-inla.org/home.
- 37.Brady OJ, et al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: An observational analysis of over 4 million births. PLOS Med. 2019;16:e1002755. doi: 10.1371/journal.pmed.1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relat. Dis. severity Antib. response virus recovered. Yale J. Biol. Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson KB, et al. A Shorter Time Interval Between First and Second Dengue Infections Is Associated With Protection From Clinical Illness in a School-based Cohort in Thailand. J. Infect. Dis. 2014;209:360–368. doi: 10.1093/infdis/jit436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regla-Nava, J. A. et al. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nature Communications9 (2018). [DOI] [PMC free article] [PubMed]
- 41.Martín-Acebes, M. A., Saiz, J.-C. & Jiménez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Frontiers in Cellular and Infection Microbiology8 (2018). [DOI] [PMC free article] [PubMed]
- 42.Khandia, R. et al. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front Immunol9 (2018). [DOI] [PMC free article] [PubMed]
- 43.Cohen J. Where has all the Zika gone? Sci. 2017;357:631–632. doi: 10.1126/science.357.6352.631. [DOI] [PubMed] [Google Scholar]
- 44.Siedner MJ, Ryan ET, Bogoch II. Gone or forgotten? The rise and fall of Zika virus. Lancet Public. Health. 2018;3:e109–e110. doi: 10.1016/S2468-2667(18)30029-X. [DOI] [PubMed] [Google Scholar]
- 45.Netto, E. M. et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. mBio8 (2017). [DOI] [PMC free article] [PubMed]
- 46.Rodriguez-Barraquer, I. et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. 5 (2019). [DOI] [PMC free article] [PubMed]
- 47.Gordon A, et al. Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. PLOS Med. 2019;16:e1002726. doi: 10.1371/journal.pmed.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halai U-A, et al. Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin. Infect. Dis. 2017;65:877–883. doi: 10.1093/cid/cix472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedroso Celia, Fischer Carlo, Feldmann Marie, Sarno Manoel, Luz Estela, Moreira-Soto Andrés, Cabral Renata, Netto Eduardo Martins, Brites Carlos, Kümmerer Beate M., Drexler Jan Felix. Cross-Protection of Dengue Virus Infection against Congenital Zika Syndrome, Northeastern Brazil. Emerging Infectious Diseases. 2019;25(8):1485–1493. doi: 10.3201/eid2508.190113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Victora CG, et al. Microcephaly in Brazil: how to interpret reported numbers? Lancet. 2016;387:621–624. doi: 10.1016/S0140-6736(16)00273-7. [DOI] [PubMed] [Google Scholar]
- 51.Campos SS, et al. Zika virus can be venereally transmitted between Aedes aegypti mosquitoes. Parasites vectors. 2017;10:605. doi: 10.1186/s13071-017-2543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum, P. et al. Ensuring a rights-based health sector response to women affected by Zika. Cadernos de Saúde Pública32 (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original datasets are publicly available at http://datasus.saude.gov.br, and the data organised for the present analysis at 10.5281/zenodo.3489428.