Abstract
Dysregulation of miRNA expression has been implicated in cancer. Numerous strategies have been explored to modulate miR but sub-optimal delivery and inability to concurrently target multiple pathways involved in tumor progression have limited their efficacy. In this study, we explored the potential co-modulation of upregulated miR-21 and downregulated miR-7 to enhance therapeutic outcomes in heterogenic tumor types. We first engineered lentiviral (LV) and adeno-associated viral (AAV) vectors that preferentially express anti-sense miR against miR-21(miRzip-21) and show that modulating miR-21 via miRzip extensively targets tumor cell proliferation, migration and invasion in vitro in a broad spectrum of cancer types and has therapeutic efficacy in vivo. Next, we show a significantly increased expression of caspase-mediated apoptosis by simultaneously downregulating miR-21 and upregulating miR-7 in different tumor cells. In vivo co-treatment with AAV-miRzip-21 and AAV-miR-7 in mice bearing malignant brain tumors resulted in significantly decreased tumor burden with a corresponding increase in survival. To our knowledge, this is the first study that demonstrates the therapeutic efficacy of simultaneously upregulating miR-7 and downregulating miR-21 and establishes a roadmap towards clinical translation of modulating miRs for various cancer types.
Subject terms: Cancer therapy, Targeted therapies
Introduction
MicroRNAs (miRs) are a class of small noncoding RNA that are involved in biological processes such as proliferation, differentiation, apoptosis and development1,2. Several studies have shown dysregulation of miRs in human cancers as a consequence of gene amplification, aberrant expression and gain of function mutations of oncogenic miRs (oncomiRs), as well as deletion or inactivation of tumor suppressor miRs (suppressor miRs)3.
miR-based therapeutic strategies are promising for cancer therapy4. However, targeting single miR is not sufficient to elicit significant therapeutic benefits in vivo. miR-21 is a key oncomiR, which is expressed highly in various cancer types5–16 and several studies have shown over-expression miR-21 promotes proliferation, invasion, and migration by repressing expression of a range of tumor suppressor genes5–8. Down regulation of miR-21 induces apoptosis through targeting PI3K/AKT and JAK/STAT3 signaling pathways7 and has a potential to collaborate or synergistically act with other miRs and fine-tune protein output17. microRNA-7 (miR-7) is an intronic microRNA that resides in the first intron of the heterogeneous ribonuclear protein K gene on chromosome 918 and is down regulated in different cancer types19–25. Recently, we have shown that forced expression of miR-7 in GBM cells results in down-regulation of EGFR and p-AKT, leading to activation of the NFkB signaling26. As both miR-21 and miR-7 activate different yet synergistic pathways and could be a contributing factor in tumor heterogeneity, it is essential to explore the potential of co-modulation of miR-7 and miR-21 that can target complementary pathways affecting tumor growth and development.
Two different strategies for miR-targeting therapy have been explored namely, activation or upregulation of tumor suppressor miRs and inhibiting the function of oncomiRs4. The strategies employed to block oncomiRs include: (1) anti-miR, (2) miR sponges, (3) miR masking, and (4) small molecule inhibitors27. Among these strategies anti-miRNA is widely used27,28. However, suboptimal delivery, poor stability, toxicity and off targets have limited their applications in vivo. Therefore, it is important to use effective and safe systems for successful delivery of anti-miR or miR mimics to cancer cells. Alternative delivery methods have been utilized in experimental and preclinical studies, such as liposomes, extracellular vesicles (EVs), polymer-mediated delivery systems, viral vectors, cell-based delivery systems and bacteriophage-based virus-like particles (VLPs)28,29. However, traditional methods have presented numerous caveats such as their transient nature of inhibition and several stoichiometric restrictions that have limited the success of the anti-miR approach. Novel expression systems such as miRzip have been developed to alleviate these issues30. miRzip anti-sense microRNAs are stably expressed RNAi hairpins that produce short, single-stranded anti-microRNAs that competitively bind their endogenous microRNA and inhibit its function30.The competitive nature of miRzip binding to its target results in the de-repression and subsequent changes in the transcripts targeted by the “zipped” microRNA. On the other hand, tumor suppressor miR can serve as therapeutic strategies classified as “miR-replacement” platforms and several studies have reported positive outcomes that are comparable to or greater than gene therapies for cancer31.
In this study, we first assessed the role of modulating oncogenic miR-21 via miRzip in a broad spectrum of cancer types and ultimately explored the therapeutic effects elicited by simultaneous targeting miR-21 and tumor suppressor, miR-7 in various cancer types in vivo.
Results
miRzip-21 effectively downregulates miR-21, reduces invasion of cancer cells and induces apoptosis via suppression of AKT pathway
The Cancer Genome Atlas data shows that miR-21 is up regulated in various human cancers and predominantly overexpressed in colon, prostate cancers and brain tumors (Fig. 1A). RT-PCR analysis of human prostate colon cancer and brain tumor (GBM) cell lines confirmed that miR-21 is widely overexpressed across different cancer types (Fig. 1B,C). To downregulate miR-21 in tumors cells, we packaged a lentiviral (LV) miRzip anti-sense miR-21 to stably express RNAi hairpins and investigated the efficacy of miRzip-21 mediated downregulation in different tumor cell types (Fig. 1D). RT-PCR analysis on LV-miRzip-21 transduced cells showed that miR-21 expression levels were significantly reduced as compared to control groups (Fig. 1E,F) resulting in a significant reduction in cell viability (Fig. 1G) in all cancer types including the primary patient derived GBM stem cells (GSCs). Furthermore, to understand the effect of LV-miRzip-21 following silencing of critical upstream targets, we transfected HCT116 and GBM8 tumor cells with siRNA for EGFR and AKT (Supplementary Fig. 1A,B) prior to LV-miRzip-21 treatment. siEGFR or siAKT transfected HCT116 and GBM8 cancer cells when transduced with LV-miRzip-21 showed a reduced response in cell viability as compared to wild type cells (Supplementary Fig. 1C). Morphological observations by light microscopy showed features of apoptotic cell death and reduced number of cells in miRzip-21 transduced cells as compared to controls (Supplementary Fig. 2).
Figure 1.
miR-21 downregulation via miRzip-21 reduces cancer cell viability in vitro (A) TCGA data showing alteration frequency of miR-21 in various cancer types. (B) RT-PCR analysis showing expression of miR-21 levels in various cancer types. (C) Plot showing relative changes in expression in miR-21 levels in tumor cells as compared to control HEK 293T cells. (D) Schematic representation of the experimental plan for proof-of-principle studies using the LV-miRzip-21. (E-F) RT-PCR showing changes in miR-21 levels post transduction with LVs bearing scramble miR, miRzip-21 or untreated (UT) cells. (E) and quantified in (F). (G) Plot showing viability of different cancer cell lines 72 h post transduction with LVs bearing scramble miR, miRzip-21 or left untreated (UT). Data are presented as mean ± SD. Significant differences between miRzip-21 transduced cells and control groups are indicated by ***(P < 0.001), **(P < 0.01) and *(P < 0.05).
Next, we examined the role of miRzip-21 expression in survival, migration and invasion of tumor cells. Clonogenic assays revealed that miRzip-21 significantly inhibited the colony formation of tumor cells compared to control groups (Fig. 2A and Supplementary Fig. 3). Wound-healing assay and transwell-based cell invasion assay indicated that downregulation of miR-21 robustly inhibits the migration and invasion of cancer cells compared to scramble and untreated control cells (Fig. 2B and Supplementary Fig. 4). Western blot analyses showed that downregulation of miR-21 via miRzip-21 leads to reduced levels of activated AKT (p-AKT), a downstream target in the PTEN/AKT signaling pathway, in most cancer cells screened except Gli36d as compared to control treated cells. Moreover, the expression of PTEN, a known tumor suppressor protein, was significantly increased in tumor cells expressing miRzip-21. This resulted in tumor cells undergoing apoptosis as indicated by higher levels of cleaved-PARP (cl-PARP) and activation of caspases 3/7 and caspase 9 in miRzip-21 expressing cells compared to scramble and untreated control cells (Fig. 2C–G). A diminished caspase 9 activation was observed when miRzip-21 was -expressed in GBM8 and HCT116 tumor cells treated with siEGFR or siAKT in, the as compared to wildtype cells (Supplementary Fig. 1D). These data show that miRzip-21 effectively downregulates miR-21 and results in inducing caspase-mediated apoptosis via suppression of AKT pathway in a broad spectrum of tumor cell lines.
Figure 2.
miRzip-21 results in reduced cell clonogenicity and invasion in cancer cell lines. (A) Plot showing changes in clonogenicity of various cancer cells following treatment with miRzip-21. (B) Plot showing changes in invasion of various cancer cells following treatment with miRzip-21 (C) Western blot analysis showing the effect of miRzip-21 in various cancer lines. (D,E) Plots showing changes in caspase 9 activity following treatment with miRzip-21 in various cancer cell types. (F,G) Plots showing changes in caspase 3/7 activity following treatment with miRzip-21 in various cancer cells. Data are shown as mean ± SD. ***(P < 0.001), **(P < 0.01) and *(P < 0.05) indicate statistical differences between miRzip-21 infected group and their relevant controls.
AAV-miRzip-21 but not MSC-miRzip-21 attenuates cancer growth in vitro and in vivo
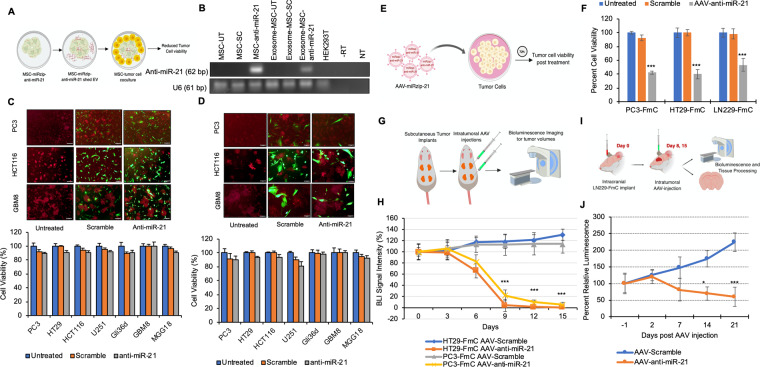
Mesenchymal stem cells (MSCs) have been demonstrated to be a safe and effective delivery vehicle for miRs, due to their ability to specifically target cancer cells and to transfer molecules via exosomal trafficking32,33. We hypothesized that MSC-shed exosomes will serve as carriers for anti-miR-21 and these MSC can be utilized to deliver miRzip-21 to the tumors in vivo (Fig. 3A). To determine the exosome enrichment of anti-miR-21 from transduced MSCs, exosomes were harvested from MSCs transduced with LV-miRzip-21 and control MSC. RT-PCR analysis revealed that MSCs shed exosomes were enriched in miRzip-21 (Fig. 3B). To investigate the therapeutic efficacy of MSC-miRzip-21, different cancer cells were cocultured with MSCs at 1:1 and 3:1 ratio. No change in tumor cell viability was observed in tumor cells post-co-culture with MSC-miRzip-21 in both tested ratios (Fig. 3C,D). To further investigate enrichment of anti-miRzip-21 western blot analysis of the MSC and isolated exosomes was performed. Western blot analysis using CD63 marker to identify exosomes revealed that exosomes are enriched from MSC engineered to express anti-miRzip-21 (Supplementary Fig. 5). These data reveal that although MSC-shed exosomes are enriched in anti-miR-21, they are unable to influence tumor cell viability in vitro.
Figure 3.
AAV-miRzip-21 effectively reduces cancer cell progression in vivo (A) Illustration showing the proposed hypothesis of MSC based delivery of anti-miR-21 to tumor cells. (B) RT-PCR assay showing miR-21 expression in LV-miRzip-21 transduced-MSCs and exosomes extracted from transduced MSC. Negative and RT-minus controls are indicated by NT and -RT, respectively. UT and SC represent untreated and scramble control groups, respectively. (C) Plots and representative fluorescent micrographs of cancer cells cocultured with LV-miRzip-21 expressing MSCs at 3:1 ratio showing cell viability at 120 h. Scale bars: 100 uM (D) Plots and representative fluorescent micrographs of cancer cells cocultured with LV-miRzip-21 infected-MSCs at 1:1 ratio and corresponding cell viability at 120 h. Scale bars: 50 uM (E) Illustration showing the model for AAV transduction of tumor cells (F) Plot showing changes in tumor cell viability at 72 h post transduction with AAV-miRzip-21 and control. (G) Illustration of the in vivo subcutaneous model of colon and prostate cancers. (H) Plot showing changes in bioluminescence signal intensity overtime following AAV-miRzip-21 injection. (I) Illustration of the intracranial LN229-FmC animal model. (J) Plot showing changes in bioluminescence signal intensity overtime following AAV injection. Data presented as mean ± SD. ***(P < 0.01) and *(P < 0.5).
The delivery of transgenes via AAV provides long-term stable in vivo gene expression in both dividing and non-dividing cells with low risk of related genotoxicity, which makes it a useful and highly suitable option for cancer gene therapy34–38. AAV gene transfer technology has also shown promise in clinical trials34,39. To create an efficient delivery vehicle for targeting miR-21 in tumors, we created AAV bearing miRzip-21 (Fig. 3E) and tested its efficacy in vitro. AAV-miRzip-21 transduced cells showed a reduction in cell viability (P < 0.01) as compared to control AAV treated or untreated cells (Fig. 3F). To evaluate the efficacy of AAV-miRzip-21 in vivo, we first created imageable tumors lines of prostate cancer (PC3-FmC), colon cancer (HT29-FmC) and GBM (LN229-FmC) by transducing them with LVs bearing a dual fluorescent and bioluminescent marker (Fluc mCherry; FmC). Next, mice bearing flank tumors of HT29-FmC and PC3-FmC (n = 10/tumor type) were injected intratumorally with three doses of 1 × 106 pfu of either AAV-scramble or AAV-miRzip-21 (n = 5/group) and mice were followed for changes in tumor volumes (Fig. 3G, H). A significant reduction in tumor volumes was seen in mice treated with AAV-miRzip-21 (P < 0.01) as compared to scramble treated groups (Fig. 3H). To evaluate the effect of AAV-miRzip-21 in intracranial GBM models, mice bearing LN229-FmC tumors were stereotactically injected intratumorally with two doses of 1 × 106 pfu of either AAV-scramble or AAV-miRzip-21 (n = 5/group) (Fig. 3I,J). A significant reduction in tumor volumes (P < 0.01) was seen in mice treated with AAV-miRzip-21 as compared to scramble treated groups (Fig. 3J). These data indicate that AAV-miRzip-21 effectively targets colon, prostate and brain cancer growth. However, the progression of colon and prostate tumor growth is halted more efficient than the GBM growth.
Co-modulation of miR-21 and miR-7 prolongs survival of mice bearing GBM xenografts derived from patient GSC
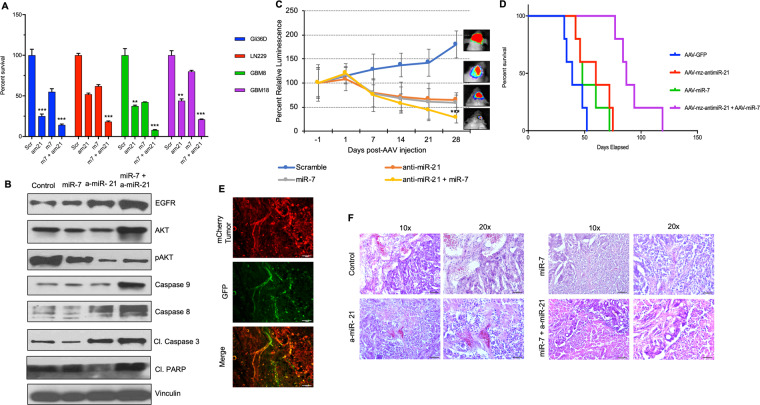
We have previously shown that forced expression of miR-7 in GBM cells results in down-regulation of EGFR and p-AKT and activation of the NFkB signaling26. In an effort to evaluate the effect of miR co-modulation on GBM tumor progression as compared to modulation of miR-21 alone (Fig. 3J), we explored the possibility of combing the downregulation of miR-21 and upregulation of miR-7 in GBMs. We tested a cohort of established and primary patient-derived GBM stem cells (GSC) for their response to a combination of miRzip-21 and miR-7. A significant reduction in GBM cell viability (P < 0.001) was seen in all the tested GBM cell lines post-combined treatment with AAV-miR-7 and AAV-miRzip-21 treatment as compared to monotherapy and control (Fig. 4A). Western blot analysis showed that combination treatment leads to substantial changes in the proteins involved in caspase-mediated apoptosis of tumor cells, in particular, caspase-3, caspase-8 and caspase-9 cleavage and subsequent PARP cleavage (Fig. 4B). Next, we evaluated the therapeutic efficacy in vivo using a primary patient derived GBM model. Specifically, mice bearing patient primary GSC (GBM18) expressing a bimodal imaging marker FmC, GBM18-FmC were challenged with 1 × 106 pfu of either AAV-GFP, AAV-miR-7, AAV-miRzip-21 or a combination of AAV-miR-7 and AAV-miRzip-21. A significant reduction (P < 0.001) in tumor burden was observed in mice treated with a combination of AAV-miR-7 and AAV-miRzip-21 as compared to the monotherapy and control (Fig. 4C). Kaplan Meier survival analysis showed a significant extension in survival of mice treated with the combination of AAV-miR-7 and AAV-miRzip-21 as compared to the other groups (Fig. 4D). Fluorescence imaging of brain sections revealed a robust infection of the GBM tumor following AAV injection (Fig. 4E) H&E analysis revealed a reduction in tumor burden in mice brains following dual modulation of miR-7 and miR-21 (Fig. 4F). These data reveal that modulation of miR-7 and miR-21 presents a therapeutic benefit in mice bearing GBM.
Figure 4.
Combination of AAV delivered anti-miR-21 and miR-7 prolongs survival of mice bearing patient derived GBM xenografts. (A) Plot showing changes in viability of various GBM cells following combination treatment with AAV-miR-7 and AAV-miRzip-21. (B) Western blot analysis showing changes in various cell proliferation and death markers following treatment with AAV-miR-7 and AAV-miRzip-21. (C) Plot showing changes in tumor volumes of GBM-18-FmC overtime following AAV-miR-7 and AAV- miRzip-21 injections as compared to controls. (D) Kaplan-Meier survival curves following AAV-miR-7 and AAV-miRzip-21 injections as compared to controls. (E) Photomicrographs showing AAV-GFP infection of GBM tumors at day 4 post AAV injection. (F) H&E staining showing changes in tumor burden following a combination treatment of AAV delivered miR-7 and anti-miR-21. Data are presented as mean ± SEM. ***(P < 0.001), **(P < 0.01) and *(P < 0.05). Scale bars represent 100 uM.
Discussion
In this study we show that delivery of miRzip-21 effectively induced apoptosis in different cancer types in vitro and mouse xenografts of prostate cancer, colon cancer and brain tumors. We also demonstrate a robust improvement in therapeutic benefit in mice bearing GBM brain tumors by co-modulating miR-21 and miR-7 thereby presenting a unique approach to target heterogenic tumors.
Several studies have shown miR-21 is among the most predominantly dysregulated miR in different cancer types including prostate cancer, colon cancer and highly malignant brain tumors and there is positive correlation between miR-21 expression levels and tumor stages5–8. miR-21 activates the EGFR/AKT signaling pathway via targeting tumor suppressor genes such as PTEN, PDCD4, APAF1, TPM1, TIMP3, RECK, FOXO1 and SPRY240. Different systems have been used to inhibit oncomiR functions such as anti-miRs, miR sponges, miR-masking and small molecule inhibitors27. Despite great promise of available therapeutic anti-miR systems, the effectiveness of current anti-miR strategies is hindered by their transient nature of inhibition. In this study, we used the miRzip anti-sense microRNAs which are stably expressed RNAi hairpins that have anti-microRNA activity to downregulate miR-21. miRzip systems overcome the drawbacks by providing permanent expression of short hairpin anti-sense miRNAs and have been shown to efficiently prevent recurrence of cancers without adverse effects41. Our findings indicate that intracellular constitutive expression of miRzip-21 has the potential to be more effective than other means of downregulating miRs in cancer cells with high level of miR-2142. microRNA-7 (miR-7) is an intronic microRNA that resides in the first intron of the heterogeneous ribonuclear protein K gene on chromosome 918. We previously shown that forced expression of miR-7 via lentiviral vectors suppresses the EGFR/PI3K/AKT signaling pathway in GBM26. miR-7 is also known to induce apoptosis through inhibition of BCL243 and inhibition of miR-21 activates caspase 9 and 37,44.
Cancer cells activate multiple anti-apoptotic pathways to escape programmed cell death through overexpressing many of proteins. Therefore, targeting a single pathway maybe not be adequate for complete tumor control. miR-21 downregulation is known to induce apoptosis through inhibition of PI3K/AKT and JAK/STAT37. Previous studies have also shown that miR-21 activates RAS/MAPK pathway through targeting antagonists of this pathway45 and can simultaneously inhibit PI3K/AKT and RAF/MEK/ERK in GBM46. miR-7 is also known to induce apoptosis through inhibition of BCL243 and inhibition of miR-21 activates caspase 9 and 344,47. In order to improve outcomes, simultaneously targeting both miR-21 and miR-7 pathways offers a promising strategy as they target parallel cell survival pathways and produce a synergistic effects48,49. Although there are few reports on forced co-expression or co-inhibition of miRNAs in cancer study50–53, none of the previous studies have shown simultaneous inhibition and overexpression of different miRs in the same tumor. In one study, Dong et al. showed co-inhibition of miR-21 and miR-10b can significantly induce apoptosis and reduce cell invasion in human GBM50. In an effort to improve therapeutic outcomes by miR modulation, we have explored co-modulation of miR-21 and miR-7 in this study as a strategy to target heterogenic tumors. Our findings indicate that simultaneous suppression of miR-21 and upregulation of miR-7 exert synergistic anti-cancer effects on human GBM through inhibition of EGFR and p-AKT and activation of caspase- mediated pathways, lead to prolonged survival rate of mice bearing xenografts model of patient-derived GBM26.
To gain insights into the molecular mechanisms underlying apoptotic effects of miRzip-21, our data showed the expression levels of cleaved-PARP, PTEN and caspase 3/7 and 9 activity were increased, while the level of p-AKT was decreased after miR-21 suppression. These results suggest that downregulation of miR-21 can increase PTEN which acts as negative regulator of PTEN/p-AKT pathway. Elevated level of PTEN results in activation of caspases 9 and 3/7. Previous studies have shown that inhibition of miR-21 in PTEN mutant cell lines suppresses activated AKT and EGFR7 and leads to decreased levels of STAT3 and p-STAT37. In our studies, we showed miRzip-21 mediated reduced levels of activated AKT and increased level of cleaved-PARP, PTEN and caspase 3/7 and 9 activity in PTEN mutant cell lines. We have also shown that silencing critical upstream receptors and targets diminished effects of LV-miRzip-21. This validates the involvement of the EGFR/PI3K/AKT pathway and proposed mechanism of action is depicted in Supplementary Fig. 6. This is in line with the previous studies showing that downregulation of miR-21 can induce apoptosis through inhibition of EGFR in JAK/STAT3 pathway, independent of the PTEN status29.
Our data indicates that modulation of specific miRs such as miR-21 could be an effective therapeutic strategy for personalized cancer treatment. However, sub-optimal delivery systems have hindered their progress into clinical settings. Exosomes offer a non-toxic and non-immunogenic delivery systems, however, efficiency of their drug-loading and retention is not sufficient to have substantial therapeutic effects in vivo32,33. Previously published studies have shown that intravenously injected exosomes are rapidly cleared from the blood and accumulate in lung, liver and spleen54,55. Industrial scale production of exosomes is a big hurdle to overcome before exosomes are tested in the clinic for treatment of a vast array of diseases32. In addition, there are no published results of trials, which used exosomes as miR carriers yet56. Previous studies have shown that MSCs can effectively deliver miRs to cancer cells via their exosomes57–59. We investigated the therapeutic efficacy of miRzip-21-carrrying MSC as source of exosomes containing miRzip-21. Our results showed no significant decrease in tumor cell numbers when co-cultured with MSC-miRzip-21 probably due to insufficient amount of anti-miR-21 in exosomes derived from MSCs.
The delivery of transgenes via AAV provides long-term stable in vivo gene expression in both dividing and non-dividing cells with low risk of related genotoxicity, which makes it a useful and highly suitable option for cancer gene therapy34–38. AAV gene transfer technology has also shown promise in clinical trials34,39. Currently, AAV-mediated gene delivery is performed in clinical trials of several human diseases such as Parkinson’s, hemophilia A and B, cystic fibrosis, muscular dystrophy, and ocular disease35–38. Additionally, AAVs have been engineered to deliver anti-angiogenic, tumor suppressor, DNA repair, cytotoxic or suicide genes, cytokines, shRNAs and antibodies. These studies showed promising results in preclinical stage4. So far, different serotypes of AAVs including AAV1, AAV-2, AAV1-AAV2 hybrids, AAV-6, AAV-7, AAV-8, AAV-9 and AAVrh10 have been applied in more than 200 gene delivery trial studies. AAV serotype-9 (AAV9) has the ability for effective cross the blood-brain barrier (BBB) and infects CNS cells60. Using AAV delivered miRzip-21 was shown to have efficacy both in vitro and in vivo in a broad spectrum of tumor lines, thus offering a potential delivery system for oncomiR silencing. Among all naturally occurring AAV serotypes, AAV2 is the best characterized and extensively studied and has served as the archetype for AAV biology36. AAV2 serotype additionally has a broad tissue tropism as compared to the other known serotypes thus making them a choice of selection for gene therapy36. In this study, we have used AAV2 serotype based on our previously published studies26 and its efficacy in effectively delivering miR to brain tumors.
In conclusion, our data strongly reveal that AAV mediated stable silencing of miR-21 in combination with forced expression of miR-7 exerts superior anti-tumor outcomes and will serve as templates for a novel therapeutic approach in treating various cancer types.
Materials and Methods
Cell lines
Human Prostate (PC3) and colon (HT29 and HCT116) cancer, glioblastoma (U251 and Gli36d), human embryonic kidney transformed with large T antigen cells (HEK293T) and human mesenchymal stem cells (MSC) were utilized in this study. U251, Gli36d and HEK293T were grown in low glucose Dulbecco’s modified Eagle’s medium (LG-DMEM), HT29 and HCT116 were cultured in RPMI-1640 and McCoy’s 5 A medium (Gibco, Carlsbad, CA), respectively. All these media were supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Glioblastoma (GBM) stem cells (GBM4, GBM8, GBM12, GBM18, GBM23, GBM31, GBM44 and BT74) were grown in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 3mM L-glutamine (Invitrogen, Carlsbad, CA), B27 (Invitrogen, Carlsbad, CA), 2 µg/ml heparin (Stem Cell Technologies, Vancouver, BC), 20 ng/ml human EGF (Invitrogen, Carlsbad, CA), and 20 ng/ml human FGF-2 (Invitrogen, Carlsbad, CA) as described previously61. Ad-MSCs were also cultured in high glucose DMEM supplemented with 10% exosome-depleted FBS (Gibco, Carlsbad, CA) with 3 mM L-glutamine and penicillin/streptomycin (Gibco, Carlsbad, CA). All cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Tumor cells were transduced with LV-Pico2-Fluc-mCherry (FmC) at a multiplicity of infection (MOI) of 2 in medium containing protamine sulfate (2 µg/ml). Cells were visualized by fluorescence microscopy for mCherry and selected by puromycin selection as described earlier26. To generate therapeutic and control MSC, MSC were transduced with LV-miRzip-21 or LV-scramble (Scr) at MOI of 5 in medium containing protamine sulfate (2 µg/ml). Cells were visualized by fluorescence microscopy for GFP expression 36 hours post transduction.
Reverse Transcription-PCR
To determine the miR-21 levels, miRNAs were isolated from cancer cells and Ad-MSCs using miRNeasy Mini Kit (Qiagen). Stem-loop PCR was used for determining the U6, mature miR-21 and anti-miR-21 expressions. RT- PCR was performed. Reverse transcriptase reactions contained RNA samples, 50 nM stem–loop RT primer (IDT Technologies), 2x reverse transcriptase buffer (NEB, USA), 0.25 mM each of dNTPs (NEB Labs) 200 U/µl M-MLV reverse transcriptase (NEB, USA) and 40 U/µl RNase inhibitor (Thermo Fisher Scientific, USA). Total 20 µl reactions were incubated in a 384-well plate for 30 min at 16 °C, 30 min at 42 °C, and 5 min at 85 °C. PCR Reaction contained 5 µl cDNA, 25 µl Taq 2x master mix (NEB, USA), 0.5 µM of each forward and universal reverse primers. The reactions were incubated in at 95 °C for 30 sec., followed by 40 cycles of 95 °C, 55 °C for 30 s, 72 °C for 30 s and 75 °C for 5 min. No template and RT minus controls were included in each experiment.
Cell viability assays
5 × 103 cells per well were plated in 96 well plates. After 24 h, cells were infected with viruses. Cell viabilities were determined using Cell Titer-Glo Luminescent Cell Viability Assay (Promega, USA) and calculated according to the following equation for each treatment. Cell viability (%) = the mean of fluorescence in infected cells/the mean of fluorescence in control (untreated) cells × 100
Colongenic and migration assays
1 × 103 adherent cells were seeded in each well of a six well plates. 24 hours later, cells were transduced with either LV-miRzip-21 or LV-Scr. Cells were incubated for ten days, colonies were fixed and stained with 0.2% crystal violet (Sigma-Aldrich) and counted.
Wound healing assay is a widely used technique to analyze the ability of cell migration in vitro. Adherent cancer cells were plated in 24 well plates and then transduced with LV-miRzip-21 or LV-Scr. After 24 hours, a straight scratch was introduced using a pipette tip. Cell migration was monitored at 4 different time points and quantified using Image J1.48 v.
Invasion assay
1 × 105 LV-miRzip-21 or LV-Scr transduced cells in serum-free media were seeded on Matrigel-coated chambers of the 24 h well trans well plates (pore size 0.8 μm, Corning, USA). 700 µl of medium containing 15% FBS was used in the lower chamber. After 24 hours, cells were fixed with methanol and stained using 0.2% crystal violet. Cells were removed from upper side of the filters and stained cells on the lower side of the membranes were counted under a light microscope.
Silencing EGFR and AKT using siRNA
HCT116 and GBM8 tumor cells were transfected with either siRNA for AKT (Thermo Fisher Scientific, Waltham, MA), siRNA for EGFR (Thermo Fisher Scientific, Waltham, MA) or scramble RNA using Lipofectamine RNAiMax reagent (Life Technologies, Carlsbad, CA) per manufacturer’s recommendations. 72 hours post transfection, cells were assayed for gene knockdown using western blotting as described below.
Western blotting
Total protein was extracted from tumor cells following transduction with either LV-miRzip-21 or LV-Scr and quantified using Bradford protein assay. For siRNA studies, the total protein from GBM8 or HCT116 tumor cells was extracted 48 h post transfection and quantified using Bradford Assay. For exosome analysis, total protein was extracted from MSC-miRzip-21 or MSC-Scr and also from exosomes purified from culture medium by ultracentrifugation.
20 µg of the protein lysates from these samples were loaded onto 10% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad, USA) and conducted at 110 V for 75 mins. Flowing the separation step, proteins were transferred to nitrocellulose membranes at 100 V for 1 h. Membranes were blocked with 5% dry milk or 5% bovine serum albumin (BSA) and 0.1% Tween-20 in TBS for 1 h at room temperature (RT), and incubated with primary antibodies in TBST overnight at 4 °C. After treated with HRP-conjugated secondary antibodies in TBST for 1 h at RT, membranes were developed with Super Signal West Pico system (Thermo Fisher Scientific, Waltham, MA). Then, signals were visualized using autoradiographic films and quantified with Image J. We used Anti- α-Tubulin or Vinculin as a loading and internal control.
Caspase assays
5 × 103 cells/well were seeded in 96 well plates and 48 h after viral infection, caspase activities were assessed by caspase-3/7 and caspase-9 Glo reagents (Promega, USA).
MSC Coculture experiments
Tumor cells expressing FmC and LV-miRzip-21 transduced or LV-Scr transduced -MSC were seeded at ratios of 1:1 and 3:1 in 96 well plates. Cell viabilities of the tumor cells viability were determined 96 and 144 h post coculture using bioluminescence-based Cell Viability Assay as described previously29.
Adeno-associated virus packaging
Recombinant AAV was produced in HEK293T cells by standard triple transient transfection system with some modification. Briefly, three plasmids of pAAV-RC, pHelper and AAV transfer plasmid were co-transfected into HEK293T cells using polyethylenimine (PEI). Cells containing viral particles were collected 72 h after transfection, and the AAV particles were purified by iodixanol gradient (15%, 25%, 40% and 60%) ultracentrifugation, concentrated and formulated in phosphate buffered saline (PBS) containing 0.001% Pluronic F68 (Gibco). Virus titer was determined by measuring DNaseI-resistant genome copies using quantitative PCR.
In vivo assessment of AAV-anti-miR-21 efficacy in colon and prostate cancer
Nude mice (3 week of age, Charles River Laboratories, Wilmington, MA) were implanted subcutaneously with HT29-FmC and PC3-FmC (1 × 105 cells per flank; n = 10/group). Mice were then injected with 1 × 106 pfu AAV-miRzip-21 (n = 5) or AAV-scramble (n = 5) on days 3, 5 and 7 post tumor implantations. tumor cell fate was imaged by Fluc activity as described previously26.
Illustrations
All illustrations have been created by D.B. using biorender.com through an academic subscription (D.B.).
Intracranial GBM cell implantation and in vivo bioluminescence imaging
To understand the effect of AAV-miRzip-21 on GBM, LN229-FmC (2.5 × 105 cells per mouse, n = 10) were stereotactically implanted into the brains (right striatum, 2.5-mm lateral from bregma and 2.2-mm deep) of 8-week-old nude mice. Mice were then stereotactically injected with 1 × 106 pfu AAV-miRzip-21 (n = 5) or AAV-scramble (n = 5) on days 8 and 15-post tumor implantation. GBM burden was followed in in real time by bioluminescence imaging as described previously. To assess therapeutic benefit of the combination of anti-miR-21 and miR-7 modulation, GBM18-FmC cells (5 × 105 cells per mouse, n = 32) were stereo tactically implanted into the brains (right striatum, 2.5-mm lateral from bregma and 2.2-mm deep) of 8-week old nude mice. Mice were then stereotactically injected with 1 × 106 pfu AAV-miRzip-21 (n = 8), 1 × 106 pfu AAV-miR-7 (n = 8), 1 × 106 pfu AAV-miRzip-21 and 1 × 106 pfu AAV-miR-7 (n = 8) or AAV-scramble (n = 8) on days 15 and 22-post tumor implantation and followed for the GBM burden in real time by bioluminescence imaging (BLI) as described previously26 and also followed for survival analysis. All in vivo procedures were approved by the Institutional Animal Care and Use Committee at BWH. Mice demonstrating any visual signs of distress as seen by impaired locomotion or difficulty in accessing food and water were excluded from the study. Treatment groups were randomly assigned from the total number of mice that were included in the study and the investigator was not blinded while administering the treatment.
Tissue processing, H&E staining and immunohistochemistry
GBM were prefused and brains were removed and sectioned for histological analyses. Brain sections were mounted on slides, washed in PBS and mounted and visualized for fluorescence on a confocal microscope (LSM Pascal, Zeiss, Oberkochen, Germany)26. For H&E staining, sections were incubated with Hematoxylin and EosinY (1% alcohol), dehydrated with 95% and 100% ethanol and mounted in xylene-based media26.
Statistical analysis
For in vitro experiments, one-way ANOVA was used to calculate the P values using SPSS 16.0 software. Pair-wise comparisons were conducted using two-sample T-Test with Bonferonni adjusted P-values for the cases of multiple pair-wise tests. Data were expressed as mean ± S.E.M. or S.D. and differences were considered significant at P < 0.05. For survival analysis, Kaplan-Meier estimates and the Log-Rank test for the comparison of survival curves were conducted for all time to event outcomes.
Supplementary information
Acknowledgements
This study was supported by funding from NIH-R01-CA204720 (KS)
Author contributions
D.B.: design, data collection and assembly, data analysis and interpretation, manuscript writing; N.A.: design, data collection and assembly, data analysis and interpretation; manuscript writing; E.R.L.: data collection and assembly; Y.Y.: data collection and assembly; S.A.: Data collection; F.B.: data analysis and interpretation; M.M.: data analysis and interpretation; K.S.: conception and design, data analysis and interpretation, financial support, manuscript writing.
Competing interests
K.S. owns equity in and is a member of the Board of Directors, AMASA Therapeutics, Inc., a company developing cell-based therapies for cancer. K.S.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. D.B. is a consultant at AMASA Therapeutics. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Deepak Bhere and Nahid Arghiani.
Change history
7/3/2024
A Correction to this paper has been published: 10.1038/s41598-024-66236-1
Supplementary information
is available for this paper at 10.1038/s41598-020-58072-w.
References
- 1.Miao X, Wu X, Shi W. MicroRNA-346 regulates neural stem cell proliferation and differentiation by targeting KLF4. Am. J. Transl. Res. 2017;9(12):5400–5410. [PMC free article] [PubMed] [Google Scholar]
- 2.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5(7):522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 3.Leichter AL, et al. MicroRNA expression patterns and signalling pathways in the development and progression of childhood solid tumours. Mol. Cancer. 2017;16(1):15. doi: 10.1186/s12943-017-0584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JK, et al. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol. Oncol. 2014;132(3):739–44. doi: 10.1016/j.ygyno.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Shang YM, Wang QW. MicroRNA-21 promotes the proliferation and invasion of neuroblastoma cells through targeting CHL1. Minerva Med. 2016;107(5):287–93. [PubMed] [Google Scholar]
- 6.Quintavalle C, et al. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2013;32(34):4001–8. doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–36.. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 9.Ribas J, et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69(18):7165–9. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markou A, et al. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin. Chem. 2008;54(10):1696–704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, et al. miR-21: A gene of dual regulation in breast cancer. Int. J. Oncol. 2016;48(1):161–72. doi: 10.3892/ijo.2015.3232. [DOI] [PubMed] [Google Scholar]
- 12.du Rieu MC, et al. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin. Chem. 2010;56(4):603–12.. doi: 10.1373/clinchem.2009.137364. [DOI] [PubMed] [Google Scholar]
- 13.Sondermann A, et al. MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin. Exp. Metastasis. 2015;32(6):521–30. doi: 10.1007/s10585-015-9724-3. [DOI] [PubMed] [Google Scholar]
- 14.Gaudelot K, et al. Targeting miR-21 decreases expression of multi-drug resistant genes and promotes chemosensitivity of renal carcinoma. Tumour Biol. 2017;39(7):1010428317707372. doi: 10.1177/1010428317707372. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Chen X, Gao L. Knockdown of miR-21 as a novel approach for leukemia therapy. J. Formos. Med. Assoc. 2010;109(9):621–3. doi: 10.1016/S0929-6646(10)60101-5. [DOI] [PubMed] [Google Scholar]
- 16.Fu, R., C. Li, and Z. Shao, Aberrant Overexpression and Regulatory Mechanism of Mir-21 in Diffuse Large B Cell Lymphoma. Blood, p. 5171-5171. 2014.
- 17.Chen X, et al. MicroRNAs tend to synergistically control expression of genes encoding extensively-expressed proteins in humans. PeerJ. 2017;5:e3682. doi: 10.7717/peerj.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123(7):1267–77. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Kefas B, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68(10):3566–72.. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 20.Shea A, et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5(8):1917–46. doi: 10.1002/cam4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu DG, et al. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin. Med. J. 2011;124(17):2616–21. [PubMed] [Google Scholar]
- 22.Cui YX, et al. MicroRNA-7 suppresses the homing and migration potential of human endothelial cells to highly metastatic human breast cancer cells. Br. J. Cancer. 2017;117(1):89–101. doi: 10.1038/bjc.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng MW, et al. Prognostic Significance of microRNA-7 and its Roles in the Regulation of Cisplatin Resistance in Lung Adenocarcinoma. Cell Physiol. Biochem. 2017;42(2):660–672. doi: 10.1159/000477884. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, et al. MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int. 2015;15:103. doi: 10.1186/s12935-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan H, et al. Overexpression of Circular RNA ciRS-7 Abrogates the Tumor Suppressive Effect of miR-7 on Gastric Cancer via PTEN/PI3K/AKT Signaling Pathway. J. Cell Biochem. 2018;119(1):440–446. doi: 10.1002/jcb.26201. [DOI] [PubMed] [Google Scholar]
- 26.Bhere D, et al. microRNA-7 upregulates death receptor 5 and primes resistant brain tumors to caspase-mediated apoptosis. Neuro Oncol. 2018;20(2):215–224. doi: 10.1093/neuonc/nox138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6(7):851–64. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estanqueiro, M. et al. Delivering miRNA modulators for cancer treatment. Targeting and Stimuli Sensitive Drug Delivery Systems, 517–565 (2018).
- 29.Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virol. J. 2010;7:248. doi: 10.1186/1743-422X-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao, D. D., Li, L. & Chan, W. Y. MicroRNAs: Key Regulators in the Central Nervous System and Their Implication in Neurological Diseases. Int J Mol Sci, 17(6) (2016). [DOI] [PMC free article] [PubMed]
- 31.Hosseinahli N, et al. Treating cancer with microRNA replacement therapy: A literature review. J. Cell Physiol. 2018;233(8):5574–5588. doi: 10.1002/jcp.26514. [DOI] [PubMed] [Google Scholar]
- 32.Antimisiaris, S. G., Mourtas, S. & Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics, 10(4). 2018. [DOI] [PMC free article] [PubMed]
- 33.Yamashita T, Takahashi Y, Takakura Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol. Pharm. Bull. 2018;41(6):835–842. doi: 10.1248/bpb.b18-00133. [DOI] [PubMed] [Google Scholar]
- 34.Ai J, et al. Adeno-associated virus serotype rh.10 displays strong muscle tropism following intraperitoneal delivery. Sci. Rep. 2017;7:40336. doi: 10.1038/srep40336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J, et al. Adeno-associated virus-mediated cancer gene therapy: current status. Cancer Lett. 2015;356(2 Pt B):347–56. doi: 10.1016/j.canlet.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malecki M, Swoboda P, Pachecka J. Recombinant adeno-associated virus derived vectors (rAAV2) efficiently transduce ovarian and hepatocellular carcinoma cells–implications for cancer gene therapy. Acta Pol. Pharm. 2009;66(1):93–9. [PubMed] [Google Scholar]
- 37.Santiago-Ortiz JL, Schaffer DV. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Rel. 2016;240:287–301. doi: 10.1016/j.jconrel.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun E, Han R, Lu B. Gene therapy of renal cancer using recombinant adeno-associated virus encoding human endostatin. Oncol. Lett. 2018;16(3):2789–2796.. doi: 10.3892/ol.2018.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naso MF, et al. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31(4):317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melnik BC. MiR-21: an environmental driver of malignant melanoma? J. Transl. Med. 2015;13:202. doi: 10.1186/s12967-015-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu D, et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 2011;193(2):409–24. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leone E, et al. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth. Clin. Cancer Res. 2013;19(8):2096–106.. doi: 10.1158/1078-0432.CCR-12-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong S, et al. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int. J. Biol. Sci. 2011;7(6):805–14. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Si ML, et al. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 45.Hatley ME, et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18(3):282–93. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, et al. miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int. J. Oncol. 2014;44(5):1571–80. doi: 10.3892/ijo.2014.2322. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol. Rep. 2010;24(1):195–201. doi: 10.3892/or_00000846. [DOI] [PubMed] [Google Scholar]
- 48.Mohammad RM, et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015;35(Suppl):S78–s103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Reilly MS. Targeting multiple biological pathways as a strategy to improve the treatment of cancer. Clin. Cancer Res. 2002;8(11):3309–10. [PubMed] [Google Scholar]
- 50.Dong CG, et al. Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic inhibition on the proliferation and invasion of human glioma cells. Int. J. Oncol. 2012;41(3):1005–12. doi: 10.3892/ijo.2012.1542. [DOI] [PubMed] [Google Scholar]
- 51.Chen B, et al. Simultaneously expressed miR-424 and miR-381 synergistically suppress the proliferation and survival of renal cancer cells–Cdc2 activity is up-regulated by targeting WEE1. Clin. 2013;68(6):825–33.. doi: 10.6061/clinics/2013(06)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orso F, et al. miR-214 and miR-148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Cancer Res. 2016;76(17):5151–62.. doi: 10.1158/0008-5472.CAN-15-1322. [DOI] [PubMed] [Google Scholar]
- 53.Feng SD, et al. Simultaneous overexpression of miR-126 and miR-34a induces a superior antitumor efficacy in pancreatic adenocarcinoma. Onco Targets Ther. 2017;10:5591–5604. doi: 10.2147/OTT.S149632. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Takahashi Y, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013;165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Morishita M, et al. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 2015;104(2):705–13.. doi: 10.1002/jps.24251. [DOI] [PubMed] [Google Scholar]
- 56.de Castro LL, et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017;8(1):151. doi: 10.1186/s13287-017-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimbo K, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014;445(2):381–7. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Lee HK, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4(2):346–61.. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz JL, et al. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bostick B, et al. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14(22):1605–9. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 61.Sasportas LS, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl Acad. Sci. USA. 2009;106(12):4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.