Abstract
Toxicity studies in the aquatic ecosystem have shown that petrol and its product have adverse effects on aquatic biota. They are able to easily absorb these toxic substances into their bodies from sediment, water or even food items that are contaminated, thus impacting the food chain. In this study, water, sediment and fish (Heterotis niloticus) from the Epe Lagoon were investigated for the presence of BTEX (benzene, toluene, ethylbenzene and the three xylene isomers m, o and p-xylenes), and PAHs using GC-MS. Total concentration of BTEX in sediment and water was respectively 32.37 ± 1.07 μg/g, 49.86 ± 7.30 μg/L; while total concentration of BTEX in fish liver and intestine was 33.75 ± 10.09 and 40.16 ± 9.64 μg/g respectively. Benzene, 1,2-Dichlorobenzene and 1,3-Dichlorobenzene components of BTEX were not detected in both sediment and water. Total concentration of PAH in sediment and water was 7.46 ± 1.73 μg/g and 19.33 ± 1.31 μg/L respectively. Total PAHs concentration in liver and fish intestine was 141.23 ± 5.89 and 173.34 ± 4.677 μg/g respectively. Majority of the PAHs congeners were found to be higher than the acceptable limits. Findings from this study shows the need for continuous monitoring of our natural waters as the present situation portends a potential concern for ecological risk.
Keywords: Environmental science, Environmental hazard, Environmental management, Environmental pollution, Environmental risk assessment, BTEX, PAH, Heterotis niloticus, Sediment, Lagoon
Environmental science; Environmental hazard; Environmental management; Environmental pollution; Environmental risk assessment; BTEX; PAH; Heterotis niloticus; Sediment; Lagoon
1. Introduction
PAHs are a class of environmental pollutants that are formed when organic materials such as fossil fuels, coal and wood do not combust completely. They are also derived from volcanoes, petroleum seeps and forest fires [1, 2, 3, 4, 5]. They are among the priority pollutant list of US Environmental Protection Agency (EPA) and European Union because they exhibit carcinogenic and mutagenic properties [6, 7]. Damage to the liver, kidney disease, jaundice and cataracts are some of the consequences of exposure to PAHs on human health. One of the major PAHs, Naphthalene, is capable of destroying the erythrocytes if taken in large quantities [8].
BTEX are a group of pollutants that find their way into surface and groundwater through improper discharge of contaminated industrial effluents and accidental oil and pipeline spills and leakages respectively [9, 10, 11, 12]. BTEX compounds have also been reported to be persistent in air and are easily transported into the natural water bodies during rains [13, 14]. Some of the health impacts that may arise from exposure to BTEX compounds include cancer, liver cirrhosis, and organ irritation [14, 15].
The petroleum hydrocarbons causing adverse effects in aquatic organisms are considered to be BTEX and PAH [16, 17]. They are capable of triggering multiple disturbances at varying biological organization levels in the aquatic environment [16, 18, 19, 20]. PAHs and BTEX continues to cause a menace to existence of the organisms and the state of the environment. The entry of BTEX and PAHs into the aquatic food chain via the process of bioaccumulation results in morbidities and mortalities.
The contribution of anthropogenic activities to the continuing rise of these pollutants have been documented. According to Zhang and Tao [21], in 2004, about 520 000 tons of the 16 priority PAHs were released in Africa alone corresponding to about 19% of the total emissions globally. Man-made activities such as bad management of water resources has largely impacted the aquatic ecosystem leading to pollution, and consequently reducing the quality of ecosystem health [22].
The aquatic ecosystem which is a source of livelihood and food to a large population living along the coastal region. The Lagos lagoon, especially the Epe fishing community and the neighbouring communities rely on the fish harvested from her waters as a source of food. It is therefore imperative to investigate the extent to which human activities have affected the pollution status of the particular water body. Hence, this study was designed to determine the level of BTEX and PAHs accumulated in water, sediment and fish from the Epe Lagoon and establish whether or not it is within limit maximally acceptable by international regulatory agencies.
2. Materials and method
2.1. Study area
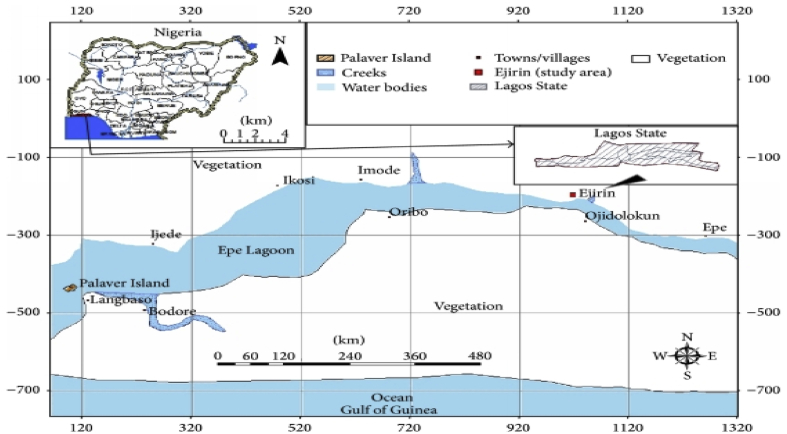
Lagos is located in the low-lying coastal zone of Nigeria (Figure 1); rivers, creeks lagoons and estuaries being dominant in this coastal landscape. Twenty five percent of the land mass of the State is water [23]. Wastes from industries located in the state are disposed of in these water bodies [24]. Epe, Lekki, Lagos and Ologe lagoons are the four major lagoons in the state. Epe Lagoon lies between latitudes 6°29 N and 6°38 N; and longitudes 3°30 E and 4°05 E [25]. A large part of the lagoon has maximum depth of about 6.0 m. The area is a rural settlement, where human population are being concentrated along the lagoon bank. Raphia palms and shrubs characterize the plant community in the lagoon area. There are lots of floating vegetation around the edges of the lagoon; at the same time, there is an abundance of coconut trees that surround the villages nearby.
Figure 1.
Map of the Epe axis of Lagos Lagoon.
2.2. Fish, water and sediment collection
A total of 100 samples of Heterotis niloticus were freshly obtained (randomly for a period of six months) from Epe axis of Lagos lagoon, using appropriate fishing gears with the assistance of fishermen and were immediately transported to the laboratory. Morphometric and meristic features were measured and recorded to the nearest centimetres. Sex of the fish was determined by visual examination of the anal opening and further determined by physical observation of the gonads after dissection. Sediment and water samples were collected in triplicates from the Lagos lagoon at low tide at a distance of >100m from the shoreline under sunny weather conditions using acid-washed glass jar and plastic container and samples were further refrigerated and stored in pre-cleaned bottles at 4 °C, prior to laboratory analysis. The in-situ parameters of pH, temperature, electrical conductivity, salinity, total dissolved solids and dissolved oxygen were read immediately using a multimeter probe. Chemical oxygen demand and biological oxygen demand were measured using closed reflux titration and titrimetric method respectively. A spectrophotometer was used to analyse other parameters.
2.3. Extraction of BTEX and PAHs in samples
For water samples, 100mL of the water sample was measured into 250mL funnel and extracted three times with 20mL of methylene chloride, giving ~60mL of final extracting solvent. The water and DCM mixture was shaken vigorously in a separatory funnel for 1–2 min, periodically venting the funnel to remove excess pressure. The water phase and organic layer were then given time to separate and thereafter decanted into a flask. The extracts further concentrated to about 1–2 mL using a rotary evaporator prior to cleanup. Finally, 1–3 spatula full of activated sodium sulphate was added to the concentrated extract so as to remove the liquid portions, where present.
For sediment samples, about ten grams of sediment was weighed into a Teflon bottle of 250mL. Activated sodium sulphate of about 1–3 spatula full was mixed with the samples in the Teflon bottles to ensure removal of any liquid that may be present. Extraction was done using 20 ml of acetone/hexane in the ration 1:1 three times, totalling 60 ml of the extraction solvent. Teflon bottles that had been covered were vigorously shaken for 30 min at 70 °C in an ultrasonic bath. Sodium sulphate was thereafter used to dry the decanted organic layer.
For biological samples, the method used was the KOH Refluxing/Vortex Extraction EPA Method 3611C where 30g–50g wet weight of tissue sample was weighed, macerated and homogenized. 10g–15g of the homogenized tissue was placed in a 50mL centrifuge tube. 15mL of 6N KOH was added to it and transferred into sealed tubes and incubated for 18h in a 35 °C water bath, while shaking vigorously for 30 s for every ½ hour for the first 4 h. Thereafter, 15mL of methylene was added to the centrifuge tube and centrifuged at 2000rpm for 5 min to facilitate phase separation. The upper aliquot layer was removed using Pasteur pipettes into a 250mL round-bottom flask. Sample extracts concentration to about 5–10 mL is carried out by rotary evaporator prior to fractionation for aliphatic hydrocarbons and PAHs using alumina gel column fractionation and clean up procedure.
2.3.1. Sample purification
To purify samples, EPA Method 3630C (Silica Gel Cleanup) and 3610B (Alumina Gel Cleanup) was employed. To prepare a column, 11g ± 0.01g of activated silica/alumina gel was weighed into a beaker, Hexane/Methylene chloride was added to form a slurry that is poured into the chromatographic column with a based glass wool, rinsing all the silica/alumina gel into the column with the solvent used. 10mL of hexane was used to condition the column, while 20mL of methylene chloride used for elution at a rate of 1 drop/sec. The elution was stopped when the solvent reaches the top of the column after which the eluate was concentrated to 2ml using rotary evaporator, and about 1g Sodium sulphate was added to remove any remaining water in the extract which was stored in a 2 ml GC vial below 4 °C prior to GC analysis.
2.4. Chemical analysis
Agilent 7890B gas chromatograph coupled to flame ionization detector (FID) was used for PAH determination. To separate the compounds, HP-5 capillary column coated with 5% Phenyl Methyl Siloxane was used as the stationary phase. Injection was done using 1μL of the samples, injected at 300 °C, 13.74psi temperature and pressure respectively with a total flow of 21.364 mL min-1. To split vent, purge flow was set for 0.75 min at 15 mL min−1. Oven was programmed at 40 °C for a minute, later at 12 °C min−1 and finally set to 300 °C for 10 min. The temperature of the FID was 300 °C with Hydrogen: Air flow at 30 mL min−1: 300 mL min−1 while the makeup gas, nitrogen was set at a flow rate of 22 mL/min. Thereafter, the samples were analyzed and the concentration of the corresponding PAHs was obtained.
BTEX was analysed using the EPA method 8260B Agilent 7890B gas chromatograph coupled to a mass spectrophotometer (GC-MS) was used. The stationary phase of separation of the compounds used was a DB-5 capillary column coated with 95% dimethyl - 5% Diphenyl polysiloxane (30 m length × 0.32 mm diameter × 1.0 μm film thickness) (Agilent Technologies). 1μL of the samples was injected in split mode with split ratio 100:1 at an injection temperature of 125°, oven was initially programmed at 35 °C (held for 2 min) then ramped at 4 °C/min to 50 °C and then 10 °C/min to 220 °C, helium flow rate was set at 1.5 mL min−1, while the mass range was set at 35–260 amu and scan time at 0.6*2 sec/scan.
2.5. Statistical analysis
Student's t-test was used to compare BTEX and PAH values between water and sediment and also between fish liver and intestine. All analyses were considered significant at 5% (p < 0.05) confidence level of using SPSS, version 20. MS Office Excel was also used where necessary.
3. Result
3.1. Physio chemical parameter of water and sediment in Lekki Lagoon
The values recorded for the water parameters measured are presented in Table 1. Parameters measured in water from the Epe lagoon include pH which was found to be within neutral range, dissolved oxygen was observed to be relatively high at 8.93 mg/lO2, while a low value 32 mg/L of chemical oxygen demand was recorded. A relatively high turbidity value of 35 NTU, salinity of 6.452 ppt which is characteristic of estuaries were recorded. Ammonia was the major form of inorganic compound measured at 8.949 mg/L while Nitrite NO2–N and Nitrate both occurred in trace concentrations of 0.12 and 0.07 mg/L respectively. Acidity and alkalinity of the water complemented the pH values of 7.61 and 78 respectively. Total organic hydrocarbon was also low in concordance with the low COD.
Table 1.
Physiochemical parameter of water sample in Epe lagoon.
| Parameters | Values |
|---|---|
| pH | 7.54 ± 0.51 |
| Electrical Conductivity (μS/cm) | 28305 ± 22.41 |
| Total Dissolved solids (mg/L) | 14125 ± 12.22 |
| Salinity (ppt) | 17.33 ± 1.22 |
| Dissolved Oxygen (mg/L) | 8.93 ± 3.22 |
| Chloride (mg/L) | 9571.50 ± 8.54 |
| Sulphate (mg/L) | 138.60 ± 5.68 |
| Total Suspended solids (mg/l) | 28.50 ± 3.21 |
| Turbidity (NTU) | 35.00 ± 3.33 |
| Ammonia (mg/L) | 8.95 ± 2.65 |
| Nitrate (mg/l) | 0.1202 ± 0.01 |
| Nitrite (mg/L) | 0.073 ± 0.01 |
| Acidity (mg/L) | 7.61 ± 1.64 |
| Alkalinity (mg/L) | 78 ± 4.88 |
| Bicarbonate (mg/L) | 95 ± 5.88 |
| Phosphorus (mg/L) | 0.1426 ± 0.02 |
| Total hydrocarbon (mg/L) | 2.12 ± 0.57 |
| Chemical oxygen demand (mg/L) | 32 ± 3.36 |
The values observed for the sediment physicochemical parameters as seen in Table 2 also showed a neutral pH of 7, electrical conductivity of 7439 μS/cm with a moderate sulphate concentration of 3528.49ppm, total organic carbon of 0.351%, chloride concentration of 2378.50ppm, total nitrogen of 2.06%, total phosphorus of 0.005% and total organic matter of 2.822ppm.
Table 2.
Physiochemical parameter of sediment sample in Epe lagoon.
| Parameter | Values |
|---|---|
| pH | 7.0 ± 1.11 |
| Electrical Conductivity (μS/cm) | 7439 ± 12.05 |
| SO42- (ppm) | 3528.49 ± 10.45 |
| TOC (%) | 0.351 ± 0.05 |
| CL− (ppm) | 2378.50 ± 10.15 |
| Total Nitrogen (%) | 2.058 ± 0.78 |
| Total Phosphorus (%) | 0.005 ± 0.000 |
| Exchangeable acidity (cMol/Kg) | 0.10 ± 0.01 |
| Total hydrocarbon (ppm) | 2.822 ± 0.08 |
3.2. Mean concentration of BTEX in sediment and water samples
The concentrations of the individual components of BTEX and the total concentration is presented in Table 3. Total concentration of BTEX even though higher in water than in sediment, this difference is not significant. 1,2-Dichlorobenzene, 1,3-dichlorobenzene and Benzene were not detected in both sediment and water samples. In addition, Chlorobenzene was also not detected in sediment.
Table 3.
Mean concentration of BTEX in Sediment (μg/g) and Water (μg/L).
| Components | Sediment | Water |
|---|---|---|
| Benzene | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Toluene | 10.07 ± 0.03 | 23.06 ± 2.19* |
| Chlorobenzene | 0.00 ± 0.00 | 2.09 ± 0.95* |
| Ethylbenzene | 7.45 ± 0.52 | 8.19 ± 1.60 |
| m+p-Xylene | 7.72 ± 0.14 | 8.53 ± 1.35 |
| O-Xylene | 6.14 ± 0.27 | 7.02 ± 0.87 |
| 1,3-Dichlorobenzene | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1,4-Dichlorobenzene | 0.98 ± 0.11 | 0.98 ± 0.04 |
| 1,2-Dichlorobenzene | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sum | 32.37 ± 1.07 | 49.86 ± 7.30 |
Asterisked values are significant.
3.3. Mean concentration of BTEX in fish tissues
BTEX concentration in the liver of fish shows that ethylbenzene had the highest concentration, followed by benzene while toluene, chlorobenzene, xylene, and O-xylene were all in a similar range of values. 1,4-dichlorobenzene had the least concentration of BTEX in fish liver. Also, 1,2-Dichlorobenzene and 1,3-dichlorobenzene were also not detected in fish liver.
Concentration of BTEX in the intestine of Heterotis niloticus shows that Ethylbenzene had the highest value, followed by Benzene and then m+p xylene. 1,3-dichlorobenzene had the lowest value while as observed in both intestine and liver, 1,2-Dichlorobenzene was not detected at all (Table 4).
Table 4.
Mean concentration of BTEX (μg/g) in fish wet weight.
| Components | Fish Liver | Fish Intestine |
|---|---|---|
| Benzene | 5.8 ± 1.6 | 7.71 ± 1.89 |
| Toluene | 4.2 ± 1.3 | 5.89 ± 1.10 |
| Chlorobenzene | 4.1 ± 1.58 | 4.17 ± 1.14 |
| Ethylbenzene | 9.51 ± 2.16 | 10.5 ± 1.97 |
| m+p-Xylene | 4.8 ± 1.5 | 6.15 ± 1.42 |
| O-Xylene | 4.48 ± 1.2 | 4.67 ± 1.30 |
| 1,3-Dichlorobenzene | ND | 0.03 ± 0.09 |
| 1,4-Dichlorobenzene | 0.86 ± 0.75 | 1.04 ± 0.73 |
| 1,2-Dichlorobenzene | ND | ND |
| Sum | 33.75 ± 10.09 | 40.16 ± 9.64 |
3.4. Mean concentration of PAH in sediment and water samples
The result of the presence of PAH in sediment and water samples are presented in Table 5. The total concentration of PAH was significantly higher in water than in sediment. Dibenzo(a,h)pyrene and Dibenzo(a,i)pyrene were not detected in both water and sediment. Furthermore, Acenaphthene and Benzo(j)fluoranthene were not detected in sediment and water sample respectively. Pyrene was the lowest in sediment while Benzo(c)phenanthrene was the lowest in water. The PAH values are higher than maximum values acceptable by international regulatory agencies (Table 6).
Table 5.
Mean concentration of PAHs in Sediment (μg/g) and Water (μg/L).
| Components | Sediment | Water |
|---|---|---|
| Naphthalene | 0.10 ± 0.01 | 0.23 ± 0.014 |
| Acenaphthylene | 0.00 ± 0.00 | 0.12 ± 0.02 |
| Acenaphthene | 0.08 ± 0.01 | 0.18 ± 0.03 |
| Fluorene | 0.23 ± 0.05 | 0.52 ± 0.05 |
| Anthracene | 0.10 ± 0.01 | 0.18 ± 0.00 |
| Phenanthrene | 0.21 ± 0.02 | 0.17 ± 0.01 |
| Fluoranthene | 0.10 ± 0.03 | 0.24 ± 0.12 |
| Pyrene | 0.02 ± 0.03 | 0.55 ± 0.08 |
| Benzo(c)phenanthrene | 0.03 ± 0.00 | 0.01 ± 0.01 |
| Chrysene | 0.24 ± 0.07 | 0.46 ± 0.03 |
| Benz(a)anthracene | 0.23 ± 0.03 | 0.49 ± 0.17 |
| Benzo(e)pyrene | 2.55 ± 0.71 | 12.38 ± 0.22 |
| Benzo(b)fluoranthene | 0.22 ± 0.02 | 0.47 ± 0.05 |
| Benzo(j)fluoranthene | 0.22 ± 0.01 | 0.00 ± 0.00 |
| Benzo(a)pyrene | 0.21 ± 0.01 | 0.38 ± 0.02 |
| Benzo(k)fluoranthene | 0.42 ± 0.01 | 0.20 ± 0.03 |
| 7,12-Dimethylbenz(a)anthracene | 0.36 ± 0.04 | 0.69 ± 0.14 |
| 3-Methylcholanthrene | 0.31 ± 0.06 | 0.38 ± 0.04 |
| Indo(1,2,3-c, d)pyrene | 0.13 ± 0.09 | 0.30 ± 0.02 |
| Dibenz(a,h)anthracene | 0.51 ± 0.07 | 0.52 ± 0.10 |
| Benzo(g,h,i)perylene | 0.70 ± 0.38 | 0.49 ± 0.13 |
| Dibenzo(a,l)pyrene | 0.49 ± 0.07 | 0.37 ± 0.03 |
| Dibenzo(a,h)pyrene | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Dibenzo(a,i)pyrene | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sum | 7.46 ± 1.73 | 19.33 ± 1.31* |
Asterisked values are significant at 5% confidence level.
3.5. Mean concentration of PAH in fish tissues
The mean concentration of PAHs in the liver and intestine of fish are as presented in Table 7. Total PAHs was higher in fish intestine than in fish liver; however, this difference was found not to be significant. Indo(1,2,3-cd)pyrene and Pyrene were the highest in fish liver and fish intestine respectively. Furthermore, benzo(j)fluoranthene values were found to be same in both fish liver and intestine.
Table 7.
Mean concentration of PAHs (μg/g) in fish wet weight.
| Components | Fish Liver | Fish Intestine |
|---|---|---|
| Naphthalene | 2.1 ± 0.12 | 3.2 ± 0.06 |
| Acenaphthylene | 2.3 ± 0.06 | 3.1 ± 0.17 |
| Acenaphthene | 3.5 ± 0.27 | 3.8 ± 0.23 |
| Fluorene | 6.1 ± 0.18 | 7.6 ± 0.26 |
| Anthracene | 3.5 ± 0.21 | 7.3 ± 0.06 |
| Phenanthrene | 3.6 ± 0.20 | 5.4 ± 0.15 |
| Fluoranthene | 22.0 ± 0.50* | 10.9 ± 0.18 |
| Pyrene | 8.5 ± 0.16 | 29.2 ± 1.05* |
| Benzo(c)phenanthrene | 4.1 ± 0.16 | 7.8 ± 0.12 |
| Chrysene | 3.9 ± 0.17 | 9.8 ± 0.18* |
| Benz(a)anthracene | 2.7 ± 0.18 | 2.4 ± 0.09 |
| Benzo(e)pyrene | 3.1 ± 0.07 | 3.4 ± 0.11 |
| Benzo(b)fluoranthene | 2.9 ± 0.04 | 3.0 ± 0.019 |
| Benzo(j)fluoranthene | 1.7 ± 0.03 | 2.3 ± 0.03 |
| Benzo(a)pyrene | 3.4 ± 0.09 | 4.3 ± 0.14 |
| Benzo(k)fluoranthene | 2.8 ± .0.07 | 2.8 ± 0.04 |
| 7,12-Dimethylbenz(a)anthracene | 6.9 ± 0.18* | 3.0 ± 0.016 |
| 3-Methylcholanthrene | 10.3 ± 0.38 | 6.0 ± 0.105 |
| Indo(1,2,3-c,d)pyrene | 5.13 ± 1.02 | 18.8 ± 1.09* |
| Dibenz(a,h)anthracene | 13.6 ± 1.02 | 10.9 ± 0.07 |
| Benzo(g,h,i)perylene | 8.0 ± 0.16 | 7.01 ± 0.05 |
| Dibenzo(a,l)pyrene | 5.7 ± 0.05 | 4.47 ± 0.097 |
| Dibenzo(a,h)pyrene | 5.6 ± 0.19 | 7.96 ± 0.22 |
| Dibenzo(a,i)pyrene | 9.8 ± 0.38 | 8.9 ± 0.14 |
| Sum | 141.23 ± 5.89 | 173.34 ± 4.677 |
Asterisked values are significant at 5% confidence level.
Table 6.
USEPA regulatory standards for polycyclic aromatic hydrocarbons (PAHs) in water.
| PAH | LEVEL (mg/L) |
|---|---|
| Benz(a)anthracene | 0.0001 |
| Benzo(a)pyrene, Benzo(b)fluoranthene, Benzo(k)fluoranthene, Chrysene | 0.0002 |
| Dibenz(a,h)anthracene | 0.0003 |
| Indo(1, 2,3-c, d)pyrene | 0.0004 |
4. Discussion
The values recorded for the water parameters measured as seen in Table 1 do not show too much variability from standard range. The relatively high dissolved oxygen content of 8.93 mg/lO2 can be attributed to the shallowness of the water body, lack of thermal stratification and an effective regular mixing due to tidal movement which are features of a well aerated water body and a corresponding low COD value of 32 mg/L. Total suspended solids value of 28.50 mg/l infers a reduced visibility as confirmed by the high turbidity of 35 NTU, total dissolved solids value of 14125 mg/l is probably an indication of high nutrient content. Total organic hydrocarbon content was low, suggesting little presence of organic compound at the time of sample collection in concordance with the low COD.
Comparison of the total BTEX content in water and sediment shows that it was higher in the former (Table 3). One plausible reason for this might be that the BTEX will easily get into water first before reaching sediment. Even when they bind in sediment, they can also easily be released back into water. In addition, effluents from industries release BTEX into water bodies, as they are being used as solvents especially those that are involved in oil and gas. Furthermore, even domestic hazardous wastes have also been implicated as sources of BTEX. Robinson et al. [26] reported the presence of BTEX in human care products, pesticides, pharmaceuticals, some detergents. All these increase the concentration of these compounds in municipal waters, hence may explain the reason for the higher BTEX content in water over sediment. Doherty and Otitoloju [27] also reported higher total BTEX content in water in comparison with sediment from the Lagos lagoon. According to Fayemiwo [28], the occurrence of BTEX compounds in water is an alarming trend, especially in vulnerable parts of the world such as South-East Asia and Africa where these water bodies are directly relied upon for drinking and other potable uses. The presence of these chemicals in sediment is also on the whole, not a good indication. These chemicals may get into underground water via seepage and contaminate it. Underground water serves a lot of domestic purposes. Hence the implication of the presence of these chemicals in either water or sediment, and the consequent health risks posed cannot be overemphasized.
The acceptable limits for Benzene, Toluene, Ethylbenzene and Xylene in water are 10, 700, 300 and 500 μg/L respectively [29]. Although the presence of the BTEX compounds in water and sediment may seem low in comparison with standards, the potential for bioaccumulation cannot be taken for granted. The presence of these chemicals in fish pose some problems for normal functioning of the fish. The branchial tissue regulates water, ions and the balance of acid-base in fish and this organ has direct contact with water [30, 31]. Wendelaar-Bonga and Lock [32], opined that toxic agents in water have the ability to impede hydromineral regulation at the gills. Therefore, a reduction in O2 uptake by fish under these conditions is likely, as branchial function will greatly decrease. Additionally, there may be an increase in mean blood-to-water or decrease in mean water-to-blood diffusion distance while O2 pressure gradient will also be affected [31].
Averagely, the concentration of the chemicals are higher in fish than in water. This may mean that the fish have taken up these chemicals from water. The effect of this bioaccumulation would even be higher in fish-eating birds and other animals, and even in man. However, benzene was not detected in water but was detected in fish. This would mean that there are other solid substances in water that the fish feed upon that harbours these chemicals. Furthermore, some other aquatic animals may have accumulated these chemicals in their bodies from other habitats, and have overtime migrated to the water body sampled in this study. The liver of fish averagely accumulated lower concentrations of BTEX than the intestine. This may be due to the detoxification function of the liver, whether by making the chemical more water soluble or by bio transforming it to less harmful substances. Akinsanya et al. [33] also reported lower average total concentration of BTEX in liver of the fish Chrysichthys nigrodigitatus compared to the intestine.
The presence of PAH in water column and sediment in the Lagoon is established in this study (Table 5). It has been reported, Balcıoğlu [34], that majority of the input of PAHs into the aquatic ecosystem are not unconnected to human activities – wastes and waters from industries, urbanization, vehicular emissions and spills from oil fields. All of these sources can be said to be true of human influence on the Epe lagoon, hence, the likelihood for it to be polluted with these chemicals. Apart from Benzo(k)fluoranthene, which had exactly the same maximum concentration limit (0.0002 mg/L) with USEPA standard, and Indo (1, 2,3-c, d) pyrene, which was lower (at 0.0003 mg/L), all the other PAH congeners have higher concentrations than permissible standards as seen in Table 6. This presents a very worrying scenario as there exist the possibility for further buildup of these chemicals along the food chain. The total PAH in sediment was lower than in water. This may mean that most of these chemicals in water have been dispersed or gone into solution, evaporate or biodegrade where possible. Hence, the sediment is not able to concentrate as much PAHs as is present in water. Two of the high molecular weight PAHs, Dibenzo(a,h)pyrene and Dibenzo(a,i)pyrene were not detected in both water and sediment. They may have been lost as a result of photo-oxidation. Benzo(e)pyrene has the highest concentration of all the PAH components both in water and in sediment. This may be suggestive of some of the physical and chemical features of the high molecular weight PAH viz-a-viz decreasing resistance to oxidation and reduction, decreased aqueous and vapor pressure. However, it has been reported that PAHs of varying molecular weights substantially differ in their distribution and behavioral ability in the environment [34].
The presence of PAHs in the tissues of Heterotis niloticus corroborates other studies that have reported [35] these chemicals in fish from other natural waters. Benson et al. [36] previously reported the presence of PAH in commercially sold fish. The total PAH concentrations reported in this study are higher than those reported by Nwaichi and Ntorgbo [37] in fish caught from coastal waters in Nigeria. It has been reported [38, 39] that the physiological mechanism of fish is very efficient in the quick biotransformation of PAH, which can be influenced by factors varying from length and frequency and route of exposure, fat content in tissues of the fish, environmental factors, exposure to multiple contaminants, sex, age and species differences [40] and health conditions of the animals. It may be that these influencing factors do not favour Heterotis niloticus to successfully transform these chemicals, hence the relatively high PAHs values. Benzo[a]pyrene is usually taken as an indicator of PAH level in samples [6]. The value for Benzo[a]pyrene in this study is 3.4 and 4.3 μg/g in fish liver and intestine respectively. This is higher that the permissible limit of 0.01 μg/g in food samples by the Joint FAO/WHO Expert Committee on Food Additives. The liver harbors lower concentrations of total PAH in comparison with the intestine. This may be seen in the light of the function of the liver in its ability to detoxify and bio transform toxic chemicals.
5. Conclusion
The presence of BTEX and PAHs in water, sediment and fish in the Epe lagoon, Lagos is established in this study, although not all detected in levels above allowed limits. Anthropogenic activities have been thought to contribute largely to the pollution of the water body. Physical characteristics of these toxicants, like hydrophobicity and stability can progressively accumulate these chemicals in the food chain, a situation that may negatively impact the health of man. There is need for a focused, concerted effort towards ensuring that activities that easily discharge these chemicals into the environment are checked.
Declarations
Author contribution statement
Isaac O Ayanda: Analyzed and interpreted the data; Wrote the paper.
Bamidele Akinsaya: Conceived and designed the experiments. Benson Onwuka: Performed the experiments.
Joseph K Saliu: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Neff J.M. Applied Science Publishers; United Kingdom: 1979. Polycyclic Aromatic Hydrocarbons in the Aquatic Environment. Sources, Fates and Biological Effects; p. 262. [Google Scholar]
- 2.Yunker M.B., Macdonald R.W., Vingarzan R., Mitchell R.H., Goyette D., Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002;33:489–515. [Google Scholar]
- 3.Durand C., Ruban V. Characterization of the organic matter of sludge: determination of lipids, hydrocarbons and PAHs from road retention/infiltration ponds in France. Environ. Pollut. 2004;132:375–384. doi: 10.1016/j.envpol.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Nadal M., Schuhmacher M., Domingo J.L. Levels of PAHs in soil and vegetation samples from “Tarragona County” Spain. Environ. Pollut. 2004;132:1–11. doi: 10.1016/j.envpol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Oros D.R., Ross J.R.M. Polycyclic aromatic hydrocarbons in San Francisco estuary sediments. Mar. Chem. 2004;86:169–184. [Google Scholar]
- 6.Anyakora C., Ogbeche A., Palmer P., Coker H. Determination of polynuclear aromatic hydrocarbons in marine samples of Siokolo Fishing Settlement. J. Chromatogr., A. 2005;1073:323–330. doi: 10.1016/j.chroma.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Huang J., Yu G., Hong H. Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui river of Beijing, China. Environ. Pollut. 2004;130:349–361. doi: 10.1016/j.envpol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Scientific Committee on Food . 2002. Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. Brussels, Belgium. [Google Scholar]
- 9.Castill M., Oubiña A., Barceló D. Evaluation of ELISA kits followed by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry for the determination of organic pollutants in industrial effluents. Environ. Technol. 1998;32:2180–2184. [Google Scholar]
- 10.Alberici R.M., Zampronio C.G., Poppi R.J., Eberlin M.N. Water solubilization of ethanol and BTEX from gasoline: on-line monitoring by membrane introduction mass spectrometry. Analyst. 2002;127:230–234. [Google Scholar]
- 11.Mazzeo D.E.C., Levy C.E., De Angelis D.D.F., Marin-Morales M.A. BTEX biodegradation by bacteria from effluents of petroleum refinery. Sci. Total Environ. 2010;408:4334–4340. doi: 10.1016/j.scitotenv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Costa S., Romăo L.P.C., Araujo B.R., Lucas S.C.O., Maciel S.T.A., Wisniewski A., Alexandre M.D.R. Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 2012;105:31–39. doi: 10.1016/j.biortech.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 13.Dutta C., Som D., Chatterjee A., Mukherjee A.K., Jana T.K., Sen S. Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 2009;148:97–107. doi: 10.1007/s10661-007-0142-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Mu Y., Liu J., Mellouki A. Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J. Environ. Sci. 2012;24:124–130. doi: 10.1016/s1001-0742(11)60735-3. [DOI] [PubMed] [Google Scholar]
- 15.Tunsaringkarn T., Siriwong W., Rungsiyothin A., Nopparabundit S. Occupational exposure of gasoline station workers to BTEX compounds in Bangkok, Thailand. Int. J. Occup. Environ. Med. 2012;3:117–125. [PubMed] [Google Scholar]
- 16.Barron M.G., Podrabsky T., Ogle S., Ricker R.W. Are aromatic hydrocarbons the primary determinant of petroleum toxicity to aquatic organisms? Aquat. Toxicol. 1999;46:253–268. [Google Scholar]
- 17.Albers P.H. Petroleum and individual polycyclic aromatic hydrocarbons. In: Hoffman D.J., Rattner B.A., others, editors. Handbook of Ecotoxicology. Lewis Publishers; New York: 2003. p. 1315p. [Google Scholar]
- 18.French D.P. Estimation of exposure and resulting mortality of aquatic biota following spills of toxic substances using a numerical model. In: Mayes M.A., Barron M.G., editors. Aquatic Toxicology and Risk Assessment. 1st. American Society for Testing and Materials; Philadelphia: 1991. pp. 35–47. [Google Scholar]
- 19.Heath G. second ed. Lewis Publishers; 1995. Water Pollution and Fish Physiology. [Google Scholar]
- 20.Van Der Oost R., Beyer J., Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Tao S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons. Atmos. Environ. 2009;43:812–819. [Google Scholar]
- 22.Brand M., Maina J., Mander M., O’Brien G. 2009. Characterisation of the Social and Economic Value of the Use and Associated Conservation of the Yellowfishes in the Vaal River. Water Research Commission Report No KV 226/09. Pretoria. [Google Scholar]
- 23.Ndimele P.E. University of Ibadan; Nigeria: 2003. The prospect of Phytoremediation of Polluted Natural Wetlands by Inhabiting Aquatic Macrophytes (Water Hyacinth) MSc dissertation. [Google Scholar]
- 24.Anetekhai M.A., Akin-Oriola G.A., Aderinola O.J., Akintola S.L. Trace metal concentration in Macrobrachium vollenhovenii from Ologe lagoon, Lagos, Nigeria. J. Afrotropical Zool. 2007;(Special Issue):25–29. [Google Scholar]
- 25.Agboola J.I., Anetekhai M.A. Length-weight relationships of some fresh and brackish water fishes in Badagry Creek, Nigeria. J. Appl. Ichthyol. 2008;24:623–625. [Google Scholar]
- 26.Robinson H.D., Knox K., Bone B.D., Picken A. Leachate quality from landfilled MBT waste. Waste Manag. 2005;25:383–391. doi: 10.1016/j.wasman.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Doherty V.F., Otitoloju A.A. Occurrence and distribution of monocyclic aromatic hydrocarbons (BTEX) and the impact on macrobenthic community structure in Lagos lagoon, Nigeria. Environ. Monit. Assess. 2016;188 doi: 10.1007/s10661-016-5576-9. [DOI] [PubMed] [Google Scholar]
- 28.Fayemiwo O.M., Daramola M.O., Moothi K. BTEX compounds in water – future trends and directions for water treatment. Water S.A. 2017;43:602–614. [Google Scholar]
- 29.World Health Organization . 2008. Guidelines for Drinking-Water Quality Incorporating 1st and 2nd Addenda 1. [Google Scholar]
- 30.Evans D.H. The fish gill: site of action and model for toxic effects of environmental pollutants. Environ. Health Perspect. 1987;71:47–58. doi: 10.1289/ehp.877147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood C.M. Toxic responses of the gill. In: Schlenk D., Benson W.H., editors. Target Organ Toxicity in Marine and Freshwater Teleosts. Taylor & Francis; New York: 2001. pp. 1–101. [Google Scholar]
- 32.Wendelaar-Bonga S.E., Lock R.A.C. The osmoregulatory system. In: Giulio R.T.D., Hinton D.E., editors. The Toxicology of Fishes. CRC Press; Florida: 2008. pp. 401–411. [Google Scholar]
- 33.Akinsanya B., Isibor P.O., Kuton M.P., Saliu J.K., Dada E.O. Aspidogastrea africanus Infections, comparative assessment of BTEX and heavy metals Bioaccumulation, and histopathological alterations as biomarker response in Chrysichthys nigrodigitatus (Lacépède, 1803) of Lekki Lagoon, Nigeria. Sci. Afr. 2019;3 [Google Scholar]
- 34.Balcıoğlu Esra Billur. Potential effects of polycyclic aromatic hydrocarbons (PAHs) in marine foods on human health: a critical review. Toxin. Rev. Early Online. 2016:1–8. [Google Scholar]
- 35.Obiakor M.O., Okonkwo J.C., Ezeonyejiaku C.D., Okonkwo C.N. Polycyclic aromatic hydrocarbons (PAHs) in freshwater media: factorial effects and human dietary exposure risk assessment. Resour. Environ. 2014;4:247–259. [Google Scholar]
- 36.Nsikak U., Anake W.U., Adedapo A.E., Fred-Ahmadu O.H., Eke K.P. Polycyclic aromatic hydrocarbons in imported Sardinops sagax: levels and health risk assessments through dietary exposure in Nigeria. J. Food Compos. Anal. 2016 [Google Scholar]
- 37.Nwaichi E.O., Ntorgbo S.A. Assessment of PAHs levels in some fish and seafood from different coastal waters in the Niger Delta. Toxicol. Rep. 2016;3:167–172. doi: 10.1016/j.toxrep.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porte C., Albaigés J. Bioaccumulation patterns of hydrocarbons and polychlorinated biphenyls in bivalves, crustaceans, and fishes. Arch. Environ. Contam. Toxicol. 1994;26:273–281. doi: 10.1007/BF00203552. [DOI] [PubMed] [Google Scholar]
- 39.Deb S.C., Araki T., Fukushima T. Polycyclic aromatic hydrocarbons in fish organs. Mar. Pollut. Bull. 2000;40:882–885. [Google Scholar]
- 40.Varanasi U., Stein J.E., Nishimoto M. Chemical carcinogenesis in feral fish: uptake, activation, and detoxication of organic xenobiotics. Environ. Health Perspect. 1987;71:155–170. doi: 10.1289/ehp.8771155. [DOI] [PMC free article] [PubMed] [Google Scholar]