Abstract
Constipation is one of the most common gastrointestinal disorders among patients with chronic kidney disease (CKD) partly because of their sedentary lifestyle, low fiber and fluid intake, concomitant medications (e.g., phosphate binders), and multiple comorbidities (e.g., diabetes). Although constipation is usually perceived as a benign, often self-limited condition, recent evidence has challenged this most common perception of constipation. The chronic symptoms of constipation negatively affect patients’ quality of life and impose a considerable social and economic burden. Furthermore, recent epidemiological studies have revealed that constipation is independently associated with adverse clinical outcomes, such as end-stage renal disease (ESRD), cardiovascular (CV) disease, and mortality, potentially mediated by the alteration of gut microbiota and the increased production of fecal metabolites. Given the importance of the gut in the disposal of uremic toxins and in acid-base and mineral homeostasis with declining kidney function, the presence of constipation in CKD may limit or even preclude these ancillary gastrointestinal roles, potentially contributing to excess morbidity and mortality. With the advent of new drug classes for constipation, some of which showing unique renoprotective properties, the adequate management of constipation in CKD may provide additional therapeutic benefits beyond its conventional defecation control. Nevertheless, the problem of constipation in CKD has long been underrecognized and its management strategies have scarcely been documented. This review outlines the current understanding of the diagnosis, prevalence, etiology, outcome, and treatment of constipation in CKD, and aims to discuss its novel clinical and therapeutic implications.
Keywords: cardiovascular disease, chronic kidney disease, constipation, end-stage renal disease, gut microbiota, laxative
Constipation is the prototype of functional gastrointestinal disorders and one of the most prevalent conditions encountered in daily clinical practice. Approximately 30% of individuals in the general population experience problems with constipation during their lifetime, with elderly people and women being mostly affected.1 In patients with CKD, particularly in its advanced stages, the prevalence of constipation has been reported to be higher than in the general population,2, 3, 4 presumably due in part to dietary restrictions (e.g., limited fiber and fluid intake), chronic medication use (e.g., phosphate binders), and high prevalence of comorbidities (e.g., diabetes mellitus).5,6 Increased uremic toxins and altered gut microbiota, both of which are commonly seen in patients with advanced CKD stages,7,8 also have been linked to the high prevalence of constipation in CKD.9,10
Constipation is usually perceived as a benign and often self-limited or treatable condition,11 but its chronic, multiple symptoms negatively affect patients’ quality of life and may impose a considerable social and economic burden.12,13 Furthermore, recent evidence also has revealed that constipation is independently associated with adverse clinical outcomes such as CKD progression, CV events, and mortality,14, 15, 16, 17, 18 which, in turn, suggests that constipation could potentially serve as a new therapeutic target for these outcomes. The advent of new drug classes for constipation, some of which shows unique renoprotective properties with improvement of gut microbiota,19, 20, 21 has further expanded the potential of constipation management as a novel therapeutic strategy for diseases related to altered gut microbiota like CKD.
Notwithstanding the increased recognition of the excess social and clinical burden of constipation in the general population, studies investigating the characteristics and outcomes of constipation in the CKD population remain scarce. Considering the potential clinical relevance of constipation in CKD and the growing awareness of the mechanisms underlying the “gut-kidney axis,” perhaps the time has come to uncover previously unappreciated roles of this frequently overlooked, unpleasant gastrointestinal condition in CKD. In this review, we summarize the current evidence on the epidemiology of constipation among patients with CKD and discuss its clinical and therapeutic implications.
Prevalence of Constipation in CKD
Diagnostic Criteria
Currently, various diagnostic tools and risk assessment questionnaires are available for constipation,1,22, 23, 24, 25, 26, 27, 28 among which the Rome criteria and the Bristol Stool Form Scale are the most widely used to identify patients with functional constipation in the primary care settings.28 The Rome criteria (currently in its fourth version) are mainly composed of 6 constipation-related symptoms, and the diagnosis of constipation is established by the presence of 2 or more symptoms for at least 3 months (Table 1).28 Meanwhile, the Bristol Stool Form Scale is a 7-level scale visual inspection of feces based on its texture and morphology, which correlates with gastrointestinal transit time and is used independently of the Rome criteria (Table 2 and Figure 1).24,25,29,30 Physicians often prefer using objective and physical factors when defining constipation, whereas patient dissatisfaction may not necessarily be related to these factors.31 In this regard, these diagnostic tools, designed based on patients’ subjective symptoms, may overcome the inherent differences in perception of constipation between physicians and patients and thus are recommended to assess constipation for both clinical practice and research purposes.28 In fact, a recent cross-sectional study reported that more than half of patients with CKD (n = 180) who had less frequent bowel movements (defined as bowel frequency of once every 4 to 6 days per week or less) with abnormal stool form and gastrointestinal symptom(s) perceived their bowel health as “normal” or “more normal than abnormal.”32 This finding clearly tells us that sole reliance on self-reporting of constipation in CKD may lead to the underestimation of the clinical problem, strongly suggesting the need for an active and standardized assessment of constipation in CKD by using subjective diagnostic tools such as the Rome criteria and the Bristol Stool Form Scale, which have yet rarely been used in clinical practice in the CKD population.
Table 1.
| 1. Must include 2 or more of the following: |
| a. Straining during more than one-fourth (25%) of defecations |
| b. Lumpy or hard stools (Bristol stool form scale 1 or 2) more than one-fourth (25%) of defecations |
| c. Sensation of incomplete evacuation more than one-fourth (25%) of defecations |
| d. Sensation of anorectal obstruction/blockage more than one-fourth (25%) of defecations |
| e. Manual maneuvers to facilitate more than one-fourth (25%) of defecations (e.g., digital evacuation, support of the pelvic floor) |
| f. Fewer than 3 spontaneous bowel movements per week |
| 2. Loose stools are rarely present without the use of laxatives |
| 3. Insufficient criteria for irritable bowel syndrome |
Criteria fulfilled for the past 3 months with symptom onset at least 6 months before diagnosis.
Table 2.
Bristol Stool Form Scale24
| Type 1. Separate hard lumps, like nuts |
| Type 2. Sausage-shaped but lumpy |
| Type 3. Like a sausage or snake but with cracks on its surface |
| Type 4. Like a sausage or snake, smooth and soft |
| Type 5. Soft blobs with clear-cut edges |
| Type 6. Fluffy pieces with ragged edges, a mushy stool |
| Type 7. Watery, no solid pieces |
Figure 1.
Visual illustration of Bristol Stool Form Scale. Reprinted with permission from Chumpitazi BP, Self MM, Czyzewski DI, et al. Bristol Stool Form Scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol Motil. 2016;28:443–448.30
Prevalence
The reported prevalence of constipation varies substantially across studies. In a systematic review of 68 studies, the prevalence of constipation in the worldwide general population ranged from 0.7% to 79%, with a median of 16% in adults and 34% in the elderly aged 60 years or older.33 This wide variability in prevalence across studies may be due to the differences in diagnostic criteria (e.g., patient- or health care professional–reported diagnosis), study populations (e.g., young or elderly adults), and research settings (e.g., community or hospital settings).33 In addition, given that only a minority of patients with constipation seek medical care,34 its exact prevalence in the general population is difficult to ascertain and hence remains unclear.
In the CKD population, the prevalence of constipation has been most extensively examined among patients with ESRD receiving either peritoneal dialysis (PD) or hemodialysis (HD) treatment.3,6,35, 36, 37, 38, 39, 40 In a recent systematic review of 30 observational studies, Zuvela et al.41 investigated gastrointestinal symptoms in a total of 5161 dialysis (3804 HD and 1507 PD) patients and found that constipation was one of the most common gastrointestinal symptoms, with its prevalence ranging from 1.6% to 71.7% and from 14.2% to 90.3% in HD and PD patients, respectively (Table 33,4,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48). According to a few previous studies comparing the prevalence of constipation between HD and PD patients, constipation has been found to be more prevalent in HD than PD patients.3,4,37,39 In a single-center study of 268 HD and 204 PD patients, Yasuda et al.3 conducted a questionnaire survey related to bowel habits and demonstrated that HD patients had a 3.1 times higher risk of having constipation than those on PD. In another study of 56 HD and 63 PD patients and 25 healthy controls, Wu et al.6 showed that both segmental and total colonic transit times, as measured by radiopaque markers, were significantly longer in HD patients than in PD patients and healthy controls, especially in the right and rectosigmoid segments, with respective mean total colonic transit times of 43.0, 32.7, and 24.3 hours. Despite this evidence on constipation in patients with ESRD, information is scarce on the prevalence of constipation among patients with nondialysis-dependent CKD. In a small cross-sectional study including 21 patients with advanced stages of nondialysis-dependent CKD (estimated glomerular filtration rate [eGFR] <15 ml/min per 1.73 m2), the prevalence of constipation was reported to be 4.8% and 19.0% based on the Rome III criteria and the Bristol Stool Form Scale, respectively (Table 3).48 No larger-scale descriptive studies are available, and thus the real-world prevalence of constipation in nondialysis-dependent CKD remains largely unknown.
Table 3.
Reported prevalence of constipation in patients with CKD/ESRD across studiesa
| Authors | Year | n | Age, mean (SD) | Male sex, % | Symptom assessment tool | Constipation prevalence, % |
|---|---|---|---|---|---|---|
| HD | ||||||
| Chong and Tan38 | 2013 | 123 | 52 (13) | 47 | Questionnaire | 1.6 |
| Salamon et al.42 | 2013 | 172 | 63 (14) | 66 | Interview by dietitian | 23.8 |
| Bossola et al.36 | 2011 | 110 | 65 (15) | 64 | Questionnaire | 27.3 |
| Ramos et al.43 | 2015 | 50 | 51 (12) | 58 | Rome III questionnaire | 32.8 |
| Cano et al.4 | 2007 | 100 | 21–86 (range) | 52 | Locally validated Rome II | 33.0 |
| Dong et al.39 | 2014 | 182 | 59 (14) | 59 | Modified GSRS | 36.3 |
| Wang et al.44 | 2001 | 20 | 64 (11) | 40 | Self-reporting diaries | 38.0 |
| Hammer et al.35 | 1998 | 105 | N/A | N/A | Questionnaire | 40.0 |
| Daniels et al.45 | 2015 | 120 | 60 (15) | 47 | GSRS | 52.5 |
| Yasuda et al.3 | 2002 | 268 | 56 (2) | 62 | Questionnaire | 63.1 |
| Ikee et al.40 | 2016 | 136 | 67 (12) | 68 | Laxative use | 66.2 |
| Zhang et al.37 | 2013 | 478 | 53 (14) | 54 | Rome III questionnaire | 71.7 |
| PD | ||||||
| Zhang et al.37 | 2013 | 127 | 45 (13) | 54 | Rome III questionnaire | 14.2 |
| Dong and Guo46 | 2010 | 112 | 60 (14) | 54 | Modified GSRS | 17.9 |
| Cano et al.4 | 2007 | 48 | 19–87 (range) | 65 | Locally validated Rome II | 27.0 |
| Salamon et al.42 | 2013 | 122 | 61 (14) | 61 | Interview by dietitian | 28.7 |
| Yasuda et al.3 | 2002 | 204 | 50 (14) | 63 | Questionnaire | 28.9 |
| Mitrovic and Majster47 | 2015 | 72 | N/A | N/A | GSRS | 90.3 |
| NDD-CKD | ||||||
| Lee et al.48 | 2016 | 21 | 64 (14) | 48 | Rome III questionnaire | 4.8 |
| Bristol Stool Scale | 19.0 |
CKD, chronic kidney disease; ESRD, end-stage renal disease; GSRS, Gastrointestinal Symptom Rating Scale; HD, hemodialysis; N/A, not available; NDD, non–dialysis-dependent; PD, peritoneal dialysis.
Modified with permission from Zuvela J, Trimingham C, Le Leu R, et al. Gastrointestinal symptoms in patients receiving dialysis: a systematic review. Nephrology (Carlton). 2018;23:718–727.41
Pathophysiology and Etiology of Constipation in CKD
The pathophysiology of constipation is multifactorial and involves complex interactions of various etiological factors.27 Generally, chronic constipation is classified as primary or secondary.49, 50, 51 According to its pathophysiological characteristics, primary constipation can be further classified as normal-transit constipation, slow-transit constipation, outlet obstruction, and a combination of these.1 The normal-transit constipation is associated with symptoms such as straining and abdominal discomfort despite adequate colonic transit on objective evaluation; slow-transit constipation is characterized by prolonged colonic transit time with physiologic impairment of colonic motor activity.52 Structurally, patients with slow-transit constipation have been shown to have reduced numbers of interstitial cells of Cajal53 and myenteric plexus neurons expressing the excitatory neurotransmitter substance P54 and abnormalities in the inhibitory transmitters vasoactive intestinal peptide and nitric oxide.55 Outlet obstruction may result from a failure in synergic movements of pelvic floor muscles.56 Although the precise mechanisms underlying these conditions have yet to be fully elucidated, several extrinsic and intrinsic factors, such as food/diet,57,58 gut microbiota,10 and behavioral (e.g., stool withholding)59 and psychological (e.g., anxiety) factors,60 have been suggested to be involved in the pathogenesis. On the other hand, secondary constipation is an entity in which clinical assessment and workup can identify intestinal or extraintestinal predisposing factors, such as concomitant medications and comorbidities.61
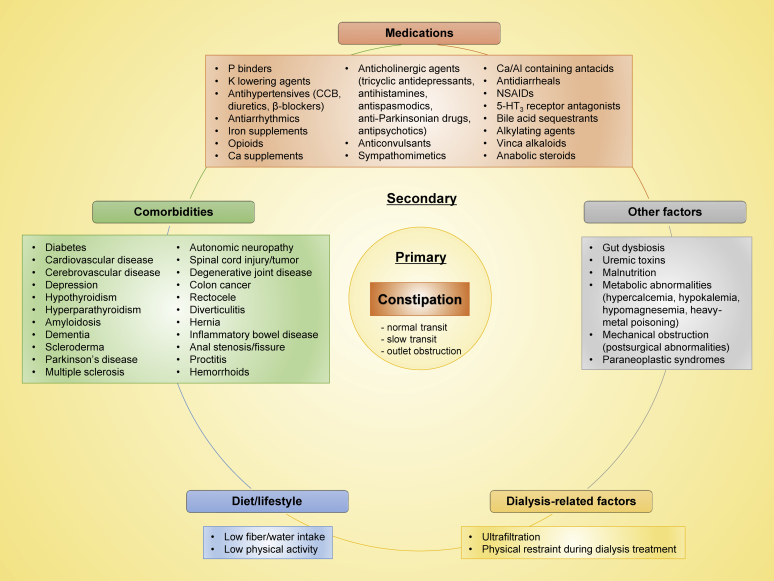
The 2 forms of constipation (i.e., primary or secondary) often coexist and are usually indistinguishable from one another.27 In particular, among patients with CKD who are typically characterized by an immense burden of medications, comorbidities, and metabolic abnormalities,62 the cause of constipation is highly multifactorial, involving many complex pathophysiological mechanisms, as summarized in Figure 2.1,5,63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 Although there is a lack of consensus in the published literature, most of these predisposing factors (listed in Figure 2) appear to be shared by the general population. With a few notable exceptions, the strict dietary restrictions (e.g., low-fiber diets [to avoid hyperkalemia] and limited fluid intake [to avoid volume overload]), frequent use of constipation-inducing medications (e.g., phosphate binders, potassium-lowering agents, calcium channel blockers, opioids, iron supplements, and antidepressants), uremic toxins, and altered gut microbiota, all of which are typically present in patients with CKD, may further contribute to increase the prevalence of constipation in this particular population.6,77, 78, 79 The greater reported prevalence of constipation in patients on HD (vs. PD) may be attributable to their stricter dietary restriction, longer physical restraint during dialysis treatment (and potential resultant stool withholding), and/or more rapid and greater volume removal (ultrafiltration).6
Figure 2.
Medical conditions associated with constipation in chronic kidney disease. CCB, calcium channel blocker; NSAID, nonsteroidal anti-inflammatory drug.
Constipation and Gut Microbiota in CKD
Over the past few decades, the characteristics and functions of gut microbiota have been extensively studied, and emerging evidence has revealed the biological links of altered gut microbiota (i.e., gut dysbiosis) with both constipation and CKD.80,81 The alterations of fecal microbiota in patients with chronic constipation have been characterized by a relative decrease in obligate anaerobic bacteria (e.g., Lactobacillus and Bifidobacterium genera) and a parallel increase in potentially pathogenic microorganisms (e.g., Enterobacteriaceae family).80,82,83 A recent study also reported that the overall composition of the colonic mucosal (but not fecal) microbiota was associated with constipation, and that constipated patients (vs. healthy controls) had a significantly higher abundance of phylum Bacteroidetes in the colonic mucosal microbiota.84 These alterations of gut microbiota have been suggested to influence intestinal motility potentially through the following mechanisms: (i) release of bacterial substances or end-products of bacterial fermentation, (ii) intestinal neuroendocrine factors, and (iii) mediators released by the gut immune response.85 For example, the decrease in relative abundance of anaerobic bacteria could result in reduced production of short-chain fatty acids such as butyrate, acetate, and propionate, which are bacterial fermentation products of plant-derived carbohydrates and stimulate ileal and colonic smooth muscle contractility, and could therefore contribute to constipation.80
Meanwhile, patients with CKD also have been recognized as having a substantial alteration of the gut microbiota (e.g., increased relative abundance of phylum Proteobacteria), along with impaired intestinal barrier function, partly due to the uremic milieu.81,86 Bacterial urease in the gut, for example, hydrolyzes urea and produces large quantities of ammonia and ammonium hydroxide, which increases luminal pH and results in the alteration of gut microbial compositions and the disruption of intestinal epithelial tight junctions, characterized by the depletion of occludin, claudin-1, and zona occludens proteins.87, 88, 89 Importantly, these alterations in turn allow translocation of gut-derived toxins, bacterial fragments, and intact bacteria through the bowel wall into the systemic circulation, which has been considered as a key contributor to the activation of host inflammatory responses, potentially leading to excess morbidity and mortality in CKD.90 Although the differences in gut microbial profiles and barrier function between constipated and nonconstipated patients with CKD remain unknown, given the close relationship between constipation and altered gut microbiota, it is possible that the presence of constipation could further exacerbate conditions associated with the gut dysbiosis and barrier dysfunction in patients with CKD.
Constipation and Hyperkalemia in CKD
Given the high prevalence of hyperkalemia and its significant association with increased mortality in patients with CKD, it is important to recognize the potential impact of constipation on hyperkalemia management in this population.91 Dietary potassium is absorbed mostly in the duodenum and jejunum and the net intestinal potassium absorption is approximately 90%. Under physiologic circumstances, intestinal potassium excretion is quite constant at approximately 10 mmol/d, with a maximum level of 15 to 20 mmol/d91; however, when the kidneys are unable to excrete the dietary potassium load (i.e., oliguria/anuria), the gut becomes especially important for maintaining potassium balance.92 A series of potassium balance studies have demonstrated that potassium excretion in stool was 3 times higher in HD patients compared with healthy controls, reaching approximately 80% of dietary potassium (up to 3000 mg/d) for some HD patients.93 The increase in intestinal potassium excretion in CKD was later shown not to be the result of reduced dietary potassium absorption in the small intestine but primarily because of increased potassium secretion into the gut, an adaptation that may be attributable to greater high-conductance potassium channels on the apical surface of colonic epithelial cells.94,95 It is therefore conceivable that slow intestinal transit time and impaction of feces with high potassium content can enhance intestinal potassium absorption, whereas conditions with faster intestinal transit time, such as diarrhea, can reduce potassium absorption, sometimes leading to profound hypokalemia. Among patients with CKD, it is also possible that a low-fiber diet (to avoid hyperkalemia) leads to constipation, which leads to hyperkalemia, which then leads to prescription of potassium-lowering agents that will lead to further worsening of constipation. These mechanisms may, in turn, explain why an increase in serum potassium or overt hyperkalemia in patients with CKD is a rare finding even in those on potassium-rich vegetarian diets that typically contain high fiber,96, 97, 98, 99 and also could support the active constipation management as a potential preventive measure against hyperkalemia in CKD.
Clinical Impact of Constipation in CKD
Impact on Economy and Quality of Life
In the United States, constipation accounts for 2.5 million physician visits annually,100 significantly contributing to health care financial burden. The cost is estimated at approximately $3000 for diagnostic workup per patient101 and at approximately $82 million for over-the-counter laxatives every year102; albeit without any relevant data in the CKD population. In addition, the unpleasant clinical symptoms and psychological preoccupations related to constipation can exert a profound negative impact on quality of life, affecting both physical and emotional well-being.11 In fact, in a study of 605 dialysis patients assessing health-related quality of life by the 12-item short-form, patients with constipation had significantly lower physical and mental health scores than those without constipation.37
Impact on Clinical Outcomes
Constipation has been increasingly recognized as a potentially serious condition, particularly in patients with ESRD receiving PD, affecting the mechanical properties of dialysis techniques and predisposing to bacterial intestinal translocation and eventual enteric peritonitis.76 Although little attention has been paid to the clinical impact of constipation beyond its gastrointestinal complications (e.g., diverticulitis, perforation, and peritonitis),6,103,104 recent studies have disclosed its independent associations with the risk of several clinical outcomes, such as Parkinson’s disease,105 ESRD,14 CV disease,15, 16, 17, 18 and mortality.18
Constipation and CV Disease and Mortality
In an initial pioneering study on the association between constipation and CV outcomes, Salmoirago-Blotcher et al.15 assessed self-reported symptoms of constipation in 93,676 postmenopausal women enrolled in the Women’s Health Initiative observational study and demonstrated that, compared with women without constipation, those with severe constipation had a 23% higher risk of CV events independent of known CV risk factors. Subsequently, using a cohort of 45,112 Japanese men and women aged 40 to 79 years, Honkura et al.17 reported that a lower defecation frequency was significantly associated with higher CV mortality (21% and 39% higher mortality for defecation frequency of 1 time every 2–3 days and ≤1 time every 4 days [vs. ≥1 time per day], respectively). In a recent large observational study of 3,359,653 US veterans with an eGFR ≥60 ml/min per 1.73 m2, Sumida et al.18 reported that patients with (vs. without) constipation had 11% and 19% higher incidence of coronary heart disease and ischemic stroke, respectively, and also experienced a 12% higher all-cause mortality.
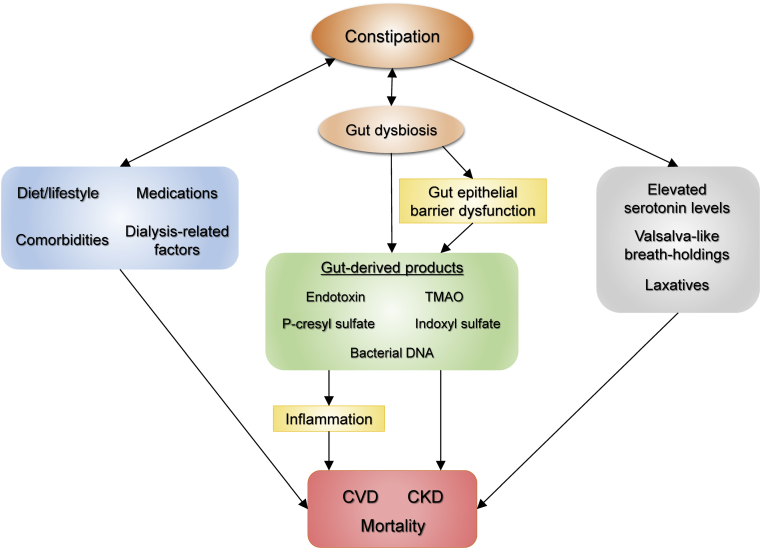
Although the precise mechanisms for these associations remain elusive, several potential explanations have been proposed, one of which is through the process mediated by altered gut microbiota. Because gastrointestinal motility and gut microbiota are closely interrelated and exert reciprocal effects on each other,9,10,106 constipation, one of the clinical forms of altered gut microbiota,73,77,107,108 is thought to be involved in the pathogenesis of atherosclerosis partly through chronic inflammation induced by bacterial endotoxins109 and/or gut metabolites (e.g., trimethylamine-N-oxide),110 consequently contributing to adverse CV outcomes. Although the median follow-up period ranging from 6.7 to 13.3 years in the aforementioned 3 studies appears to be relatively short to evaluate a long-term effect of constipation on the outcomes,15,17,18 given the chronic nature of this particular disease condition, it is possible that the patients identified as constipated had been exposed for a much longer time to these potential causative factors up to the time when the study follow-up was started. Some other factors, such as autonomic dysfunction,111 increased blood serotonin levels,112,113 and repeated Valsalva-like breath-holdings (a well-recognized cause of “defecation syncope”),114 associated with constipation may also serve as potential explanations for the observed associations (Figure 3). Nonetheless, it is important to acknowledge that there is one observational study that reported no significant association between constipation and CV outcomes.115 In addition, all of these previous studies included only individuals with normal kidney function, and hence it is unclear whether constipation can add such prognostic information in the CKD population.
Figure 3.
Schematic representation of potential mechanisms underlying the association between constipation and adverse outcomes in chronic kidney disease (CKD). CVD, cardiovascular disease; TMAO, trimethylamine-N-oxide.
Constipation and CKD Progression
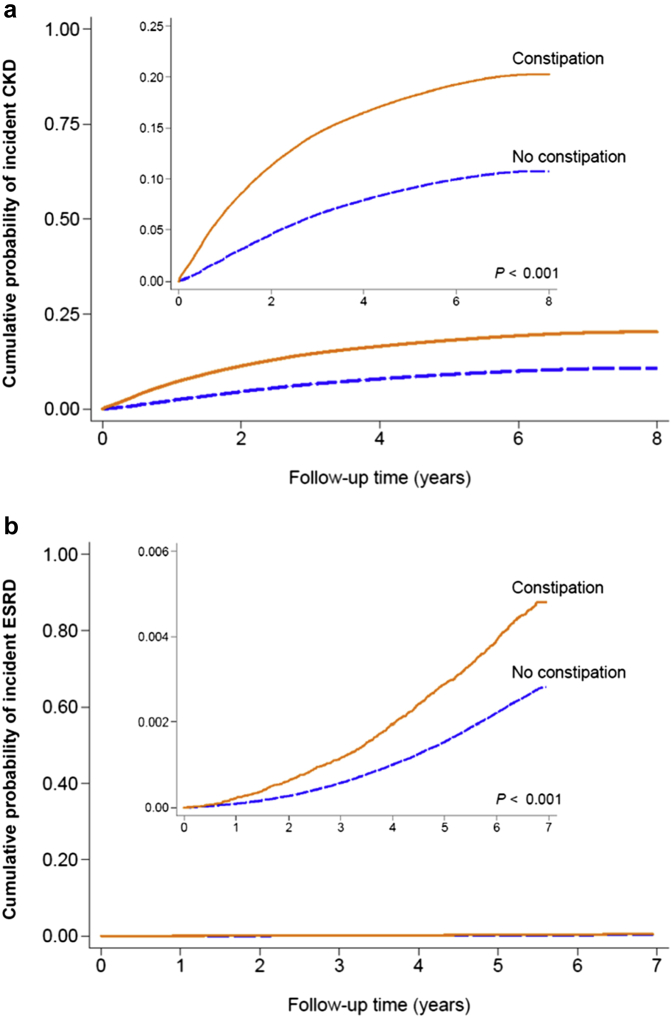
Despite the wide recognition of higher prevalence of constipation in patients with CKD than in the general population, it was not until very recently that studies investigated whether the presence of constipation per se worsens kidney function and increases the risk of developing de novo kidney disease. In a recent study using a nationwide cohort of 3,504,732 US veterans with an eGFR ≥60 ml/min per 1.73 m2, Sumida et al.14 examined the association of constipation with incident CKD, incident ESRD, and change in eGFR over time during a median follow-up of 7 years, and demonstrated that patients with (vs. without) constipation had a significantly higher risk of incident CKD and ESRD and were also at a greater risk of experiencing more progressive eGFR decline (Figure 4). In addition to the aforementioned potential mechanisms for the association with CV outcomes, the increased production of uremic toxins, including p-cresyl sulfate and indoxyl sulfate, may also contribute to the increased risk of renal events (Figure 3).116 As is well known, these uremic toxins are primarily excreted by the kidney and hence accumulate in CKD as kidney function declines.117 Of interest, they may accumulate more as patients get constipated. In a recent cross-sectional study including 43 nondiabetic nondialysis-dependent patients with CKD with a mean eGFR of 21.3 ml/min per 1.73 m2, Ramos et al.118 investigated the association of bowel habits with gut-derived uremic toxins and demonstrated that constipation was significantly associated with higher levels of urinary p-cresyl sulfate, a surrogate of intestinal production of the toxin.
Figure 4.
Cumulative probability of (a) incident chronic kidney disease (CKD) and (b) incident end-stage renal disease (ESRD) according to constipation status. Reprinted with permission of the American Society of Nephrology from Constipation and incident CKD, Sumida K, Molnar MZ, Potukuchi PK, et al. J Am Soc Nephrol., volume 28, issue 4, Copyright © 2017; permission conveyed through Copyright Clearance Center, Inc.14
Taken together, these findings suggest that, as a clinical form of heterogeneous health conditions, constipation may not only be a harbinger of ominous prognosis, but perhaps more importantly, it may serve as a novel therapeutic target for adverse outcomes like CKD and CV disease beyond its conventional defecation management.
Management of Constipation in CKD
To date, published literature on the management of constipation in patients with CKD is scarce. It is therefore assumed that constipation in CKD has been managed by physicians based primarily on their clinical experience and/or on the general therapeutic recommendations for constipation (vide infra),1 or was simply left untreated as a common, ignorable condition. Some patients with CKD might perceive constipation as self-manageable and thus may not even seek special medical attention.33 However, given the potential clinical impact of constipation, its adequate management may be more important than previously considered. The fundamental key to adequate management is identifying the etiologic, pathophysiologic, and/or symptomatic factors causing constipation in each individual case and modifying such causative factors, if any.76 Nonpharmacological and pharmacological interventions would then need to be considered for the management of constipation in CKD.
Nonpharmacological Treatment
Nonpharmacological treatment is traditionally considered the first step of a comprehensive management of constipation,50 which mainly consists of dietary and lifestyle modifications, such as fiber supplements and increased physical activity.1,119, 120, 121 Patients with CKD, however, are typically advised to restrict the intake of fiber-rich foods to prevent hyperkalemia and are also often limited in physical capacity due to multiple comorbidities,41 and hence, these dietary and lifestyle modifications may not always be practical in this population. That being said, given the fact that a recent meta-analysis showed a lower mortality risk associated with a more plant-based, fiber-rich diet (with less red meat, sodium, and refined sugar intake) in adults with CKD (including those on dialysis),122 the potential health and gastrointestinal benefits of dietary fiber, along with its low cost, may justify consideration of dietary fiber supplementation as a first step in the management of constipation even in patients with CKD. It is noteworthy that both prebiotics (i.e., nondigestible substances such as oligosaccharides) and probiotics (i.e., live microorganisms such as Lactobacilli and Bifidobacteria), which have become familiar to the public as the components of dietary supplements and bioyogurt,75 also can be treatment options for constipation in CKD. Although the evidence of these supplements in constipation management in CKD remains limited, given their beneficial effects on reducing inflammation and uremic toxins among patients with CKD,123,124 the effectiveness of pre- and probiotics for constipation in CKD may deserve future in-depth clinical trials.
Pharmacological Treatment
Pharmacological treatment is generally recommended when simple changes to diet and lifestyle fail to ameliorate symptoms of constipation.1 In contrast to primary constipation, secondary (e.g., drug-induced) constipation, which is predominant among patients with CKD, is unlikely to respond well to nonpharmacological treatment alone.58 Pharmacological interventions may therefore often be required for constipation management in patients with CKD.
A wide range of pharmacological treatments is currently available, including commonly used laxative compounds (e.g., bulk-forming and osmotic laxatives, stimulants, stool softeners, and lubricants) and relatively new agents (e.g., chloride channel activators, guanylate cyclase C receptor agonists, selective serotonin 5-HT4 receptor agonists, and ileal bile acid transporter inhibitors).125,126 This heterogeneous group of drugs differs substantially in their pharmacological characteristics and mechanisms of action (Table 4). Recent recommendations from the American College of Gastroenterology Evidence-Based Monograph and a practice guideline from the American Gastroenterological Association suggest the use of bulk-forming and osmotic agents (e.g., psyllium and polyethylene glycol) first, supplemented by stimulant laxatives as needed (as “rescue” agents), before considering the use of newer agents with more physiological mechanisms of action.1,126
Table 4.
Pharmacological treatment options for constipation
| Types | Agents | Mechanisms of action and effects | Common side effects |
|---|---|---|---|
| Commonly used laxatives | |||
| Bulk-forming laxatives | Psyllium, methylcellulose, polycarbophil | Increase water-absorbing properties of stool and decrease stool consistency | Bloating, flatulence |
| Osmotic laxatives | Sodium phosphate, polyethylene glycol, sorbitol, lactulose, magnesium hydroxide, magnesium citrate, magnesium sulfate | Osmotically increase intraluminal fluids by nonabsorbable ions and molecules and decrease stool consistency | Bloating, flatulence, abdominal cramps, electrolyte disturbance |
| Stimulants | Diphenylmethane derivatives (bisacodyl, sodium picosulfate), anthraquinones (sennoside, aloe, cascara) | Stimulate mucosa or myenteric plexus to trigger peristaltic contractions and inhibit absorption of water and electrolytes | Abdominal discomfort, pain, and cramps, nausea, incontinence |
| Stool softeners | Docusate sodium, docusate calcium | Enhance interaction of stool and water | Abdominal cramps, diarrhea |
| Lubricants | Mineral oil | Lubricate stool and ease passage | Lipid pneumonia, malabsorption of fat-soluble vitamins, incontinence |
| Newer agents | |||
| Chloride channel activators | Lubiprostone | Selectively activate enterocyte type 2 chloride channels (CCl2), resulting in chloride secretion into intestinal lumen followed by passive diffusion of sodium and water | Diarrhea, nausea |
| Guanylate cyclase C receptor agonists | Linaclotide, plecanatide | Stimulate intestinal epithelial cell guanylate cyclase C receptors, resulting in secretion of chloride, bicarbonate, and water into intestinal lumen and acceleration of stool transit | Diarrhea, nausea |
| Selective serotonin 5-HT4 receptor agonists | Prucalopride, cisapride, tegaserod | Stimulate intestinal fluid secretion and motility through activation of 5-HT4 receptors of myenteric plexus | Diarrhea, nausea, headache |
| Ileal bile acid transporters inhibitors | Elobixibat | Reduce ileal reabsorption of bile acids and enhance colonic secretion and motility | Abdominal cramps, diarrhea |
These pharmacological approaches also may be applicable to individuals with CKD, and in fact, previous studies have reported the beneficial effects of some of these agents on constipation in dialysis patients.74,127 Nevertheless, because of the lack of clear management guidelines for constipation in CKD and the availability of many types of agents as over-the-counter therapeutics, it can still be difficult for physicians and patients alike to choose one specific agent over another to treat constipation in CKD. In addition, the potential safety concerns about the use of these agents (e.g., drug-induced nephrotoxicity and electrolyte disturbances)5,128 may lead to possible undertreatment of constipation in patients with CKD, which could potentially contribute to their excess morbidity and mortality. In this context, the unique pharmacological properties of a few constipation agents, such as lactulose, a chloride channel activator (lubiprostone), and a guanylate cyclase C agonist (linaclotide),19–21,129 may deserve special attention. In a study using an adenine-induced renal failure mouse model, Mishima et al.19 demonstrated that lubiprostone ameliorated the progression of CKD and the accumulation of uremic toxins by improving the gut microbiota and intestinal environment, suggesting its therapeutic potential for CKD. Recently, similar renoprotective properties have also been demonstrated for both lactulose and linaclotide in animal studies,20,21 with a notable reduction in plasma trimethylamine-N-oxide levels additionally observed in linaclotide-treated (vs. untreated) mice.21 Furthermore, recent findings on the efficacy of fecal microbiota transplantation for the treatment of slow-transit constipation have underscored the importance of fecal microbial composition on the gut motility, implying the need for the development of novel gut microbiota–targeted strategies for the treatment of constipation.130,131 From the standpoint of practical application, lactulose typically has fewer adverse effects than other commonly used laxatives (e.g., stimulants) and may be easily available at an affordable cost.132 Although the issues of high drug costs and long-term safety profiles of both lubiprostone and linaclotide remain to be addressed,126 the more physiological mechanisms of action of these emerging agents (vs. commonly used laxatives) have made them attractive therapeutic options for constipation in CKD. But perhaps most importantly, given the substantial alterations of gut microbiota in CKD, the gut microbiota–targeted approach using these drugs seems particularly relevant to the constipation management in patients with CKD.
Future Research Implications
Although constipation is one of the most prevalent gastrointestinal conditions, there remains a substantial knowledge gap in its epidemiology in CKD. Future research efforts are therefore needed to improve our understanding of the characteristics of constipation in CKD, with a particular focus on its biological interactions with gut environment (e.g., microbiota composition, barrier function, and metabolites). In addition, it should be addressed whether the presence of constipation in CKD provides clinically meaningful prognostic information, as seen in individuals without CKD, and if so, to what extent and through what mechanisms. Furthermore, because there are currently no clinical trials that examined the effectiveness of aggressive management of constipation on hard clinical outcomes like mortality, future studies should evaluate the effectiveness and safety profiles of various therapeutic agents for constipation in CKD, as well as their potential benefits on subsequent outcomes of CKD. In particular, given the increasing roles of the gut in the acid-base and mineral homeostasis and the disposal of uremic toxins with declining kidney function,117 the detailed examination of therapeutic effectiveness across the spectrum of CKD may provide novel clinical implications of constipation management in CKD.
Conclusions
Constipation has long been recognized as a benign and often self-manageable condition, which has thus been overlooked and understudied as a complication of CKD. Recent evidence has, however, challenged this most common perception of constipation, reinforcing its importance as a major public health issue that is highly relevant not only to primary care providers and gastroenterologists, but also to nephrologists. In addition, the advent of new constipation agents that may possess unique therapeutic properties has paved the way for a new understanding of constipation management in CKD, making it a potentially appealing and impactful therapeutic strategy for kidney and CV outcomes. In an ongoing quest to improve outcomes in CKD, the time has come to advance our understanding of this overlooked, unpleasant, and hazardous gastrointestinal condition of CKD and to explore its therapeutic potential beyond conventional constipation management.
Disclosure
All the authors declared no competing interests.
Acknowledgments
CPK is an employee of the US Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
References
- 1.Bharucha A.E., Pemberton J.H., Locke G.R., 3rd American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strid H., Simren M., Johansson A.C. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17:1434–1439. doi: 10.1093/ndt/17.8.1434. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda G., Shibata K., Takizawa T. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis. 2002;39:1292–1299. doi: 10.1053/ajkd.2002.33407. [DOI] [PubMed] [Google Scholar]
- 4.Cano A.E., Neil A.K., Kang J.Y. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102:1990–1997. doi: 10.1111/j.1572-0241.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 5.Shirazian S., Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol. 2010;6:480–492. doi: 10.1038/nrneph.2010.84. [DOI] [PubMed] [Google Scholar]
- 6.Wu M.J., Chang C.S., Cheng C.H. Colonic transit time in long-term dialysis patients. Am J Kidney Dis. 2004;44:322–327. doi: 10.1053/j.ajkd.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22:636–643. doi: 10.1111/j.1525-139X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramezani A., Massy Z.A., Meijers B. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attaluri A., Jackson M., Valestin J., Rao S.S. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407–1411. doi: 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quigley E.M. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol. 2011;25:119–126. doi: 10.1016/j.bpg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Dennison C., Prasad M., Lloyd A. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sun S.X., Dibonaventura M., Purayidathil F.W. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56:2688–2695. doi: 10.1007/s10620-011-1639-5. [DOI] [PubMed] [Google Scholar]
- 13.Guerin A., Carson R.T., Lewis B. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J Med Econ. 2014;17:577–586. doi: 10.3111/13696998.2014.919926. [DOI] [PubMed] [Google Scholar]
- 14.Sumida K., Molnar M.Z., Potukuchi P.K. Constipation and incident CKD. J Am Soc Nephrol. 2017;28:1248–1258. doi: 10.1681/ASN.2016060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmoirago-Blotcher E., Crawford S., Jackson E. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. 2011;124:714–723. doi: 10.1016/j.amjmed.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubota Y., Iso H., Tamakoshi A. Bowel movement frequency, laxative use, and mortality from coronary heart disease and stroke among japanese men and women: The Japan Collaborative Cohort (JACC) Study. J Epidemiol. 2016;26:242–248. doi: 10.2188/jea.JE20150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honkura K., Tomata Y., Sugiyama K. Defecation frequency and cardiovascular disease mortality in Japan: the Ohsaki cohort study. Atherosclerosis. 2016;246:251–256. doi: 10.1016/j.atherosclerosis.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Sumida K., Molnar M.Z., Potukuchi P.K. Constipation and risk of death and cardiovascular events. Atherosclerosis. 2019;281:114–120. doi: 10.1016/j.atherosclerosis.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishima E., Fukuda S., Shima H. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol. 2015;26:1787–1794. doi: 10.1681/ASN.2014060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sueyoshi M., Fukunaga M., Mei M. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol. 2019;23:908–919. doi: 10.1007/s10157-019-01727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanto-Hara F, Kanemitsu Y, Fukuda S, et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfz126. Accessed December 4, 2019. [DOI] [PubMed]
- 22.Svedlund J., Sjodin I., Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 23.Agachan F., Chen T., Pfeifer J. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- 24.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 25.Riegler G., Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech Coloproctol. 2001;5:163–164. doi: 10.1007/s101510100019. [DOI] [PubMed] [Google Scholar]
- 26.Krogh K., Christensen P., Sabroe S., Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- 27.Basilisco G., Coletta M. Chronic constipation: a critical review. Dig Liver Dis. 2013;45:886–893. doi: 10.1016/j.dld.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Mearin F., Lacy B.E., Chang L. Bowel disorders. Gastroenterology. 2016;150:1393–1470. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Saad R.J., Rao S.S., Koch K.L. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010;105:403–411. doi: 10.1038/ajg.2009.612. [DOI] [PubMed] [Google Scholar]
- 30.Chumpitazi B.P., Self M.M., Czyzewski D.I. Bristol Stool Form Scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol Motil. 2016;28:443–448. doi: 10.1111/nmo.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley E.M.M., Horn J., Kissous-Hunt M. Better Understanding and Recognition of the Disconnects, Experiences, and Needs of Patients with Irritable Bowel Syndrome with Constipation (BURDEN IBS-C) study: results of an online questionnaire. Adv Ther. 2018;35:967–980. doi: 10.1007/s12325-018-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trimingham C., McDonald S., Dansie K. Bowel health in chronic kidney disease: patient perceptions differ from clinical definitions. J Ren Care. 2018;44:65–72. doi: 10.1111/jorc.12230. [DOI] [PubMed] [Google Scholar]
- 33.Mugie S.M., Benninga M.A., Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3–18. doi: 10.1016/j.bpg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Galvez C., Garrigues V., Ortiz V. Healthcare seeking for constipation: a population-based survey in the Mediterranean area of Spain. Aliment Pharmacol Ther. 2006;24:421–428. doi: 10.1111/j.1365-2036.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- 35.Hammer J., Oesterreicher C., Hammer K. Chronic gastrointestinal symptoms in hemodialysis patients. Wien Klin Wochenschr. 1998;110:287–291. [PubMed] [Google Scholar]
- 36.Bossola M., Luciani G., Rosa F., Tazza L. Appetite and gastrointestinal symptoms in chronic hemodialysis patients. J Ren Nutr. 2011;21:448–454. doi: 10.1053/j.jrn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Huang C., Li Y. Health-related quality of life in dialysis patients with constipation: a cross-sectional study. Patient Prefer Adherence. 2013;7:589–594. doi: 10.2147/PPA.S45471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong V.H., Tan J. Prevalence of gastrointestinal and psychosomatic symptoms among Asian patients undergoing regular hemodialysis. Nephrology (Carlton) 2013;18:97–103. doi: 10.1111/nep.12000. [DOI] [PubMed] [Google Scholar]
- 39.Dong R., Guo Z.Y., Ding J.R. Gastrointestinal symptoms: a comparison between patients undergoing peritoneal dialysis and hemodialysis. World J Gastroenterol. 2014;20:11370–11375. doi: 10.3748/wjg.v20.i32.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikee R., Toyoyama T., Endo T. Clinical factors associated with constipation in hemodialysis patients. Int Urol Nephrol. 2016;48:1741–1742. doi: 10.1007/s11255-016-1363-3. [DOI] [PubMed] [Google Scholar]
- 41.Zuvela J., Trimingham C., Le Leu R. Gastrointestinal symptoms in patients receiving dialysis: a systematic review. Nephrology (Carlton) 2018;23(8):718–727. doi: 10.1111/nep.13243. [DOI] [PubMed] [Google Scholar]
- 42.Salamon K., Woods J., Paul E., Huggins C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J Ren Nutr. 2013;23:114–118. doi: 10.1053/j.jrn.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Ramos C.I., Andrade de Lima A.F., Grilli D.G., Cuppari L. The short-term effects of olive oil and flaxseed oil for the treatment of constipation in hemodialysis patients. J Ren Nutr. 2015;25:50–56. doi: 10.1053/j.jrn.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang H.F., Lim P.S., Kao M.D. Use of isomalto-oligosaccharide in the treatment of lipid profiles and constipation in hemodialysis patients. J Ren Nutr. 2001;11:73–79. doi: 10.1016/s1051-2276(01)92591-9. [DOI] [PubMed] [Google Scholar]
- 45.Daniels G., Robinson J.R., Walker C. Gastrointestinal symptoms among African Americans undergoing hemodialysis. Nephrol Nurs J. 2015;42:539–548. quiz 549. [PubMed] [Google Scholar]
- 46.Dong R., Guo Z.Y. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: multivariate analysis of correlated factors. World J Gastroenterol. 2010;16:2812–2817. doi: 10.3748/wjg.v16.i22.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitrovic M., Majster Z. The prevalence, severity and diversity of gastrointestinal symptoms in hemodialysis and peritoneal dialysis patients. Nephrol Dial Transplant. 2015;30:SP706. [Google Scholar]
- 48.Lee A., Lambert K., Byrne P., Lonergan M. Prevalence of constipation in patients with advanced kidney disease. J Ren Care. 2016;42:144–149. doi: 10.1111/jorc.12157. [DOI] [PubMed] [Google Scholar]
- 49.American Gastroenterological Association. Bharucha A.E., Dorn S.D. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Tack J., Muller-Lissner S., Stanghellini V. Diagnosis and treatment of chronic constipation--a European perspective. Neurogastroenterol Motil. 2011;23:697–710. doi: 10.1111/j.1365-2982.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 52.Longstreth G.F., Thompson W.G., Chey W.D. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 53.He C.L., Burgart L., Wang L. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 54.Tzavella K., Riepl R.L., Klauser A.G. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol. 1996;8:1207–1211. doi: 10.1097/00042737-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Cortesini C., Cianchi F., Infantino A., Lise M. Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci. 1995;40:2450–2455. doi: 10.1007/BF02063253. [DOI] [PubMed] [Google Scholar]
- 56.Rao S.S., Welcher K.D., Leistikow J.S. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 57.Muller-Lissner S.A. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988;296:615–617. doi: 10.1136/bmj.296.6622.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voderholzer W.A., Schatke W., Muhldorfer B.E. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol. 1997;92:95–98. [PubMed] [Google Scholar]
- 59.Mugie S.M., Di Lorenzo C., Benninga M.A. Constipation in childhood. Nat Rev Gastroenterol Hepatol. 2011;8:502–511. doi: 10.1038/nrgastro.2011.130. [DOI] [PubMed] [Google Scholar]
- 60.Haug T.T., Mykletun A., Dahl A.A. Are anxiety and depression related to gastrointestinal symptoms in the general population? Scand J Gastroenterol. 2002;37:294–298. doi: 10.1080/003655202317284192. [DOI] [PubMed] [Google Scholar]
- 61.Lindberg G., Hamid S.S., Malfertheiner P. World Gastroenterology Organisation global guideline: constipation--a global perspective. J Clin Gastroenterol. 2011;45:483–487. doi: 10.1097/MCG.0b013e31820fb914. [DOI] [PubMed] [Google Scholar]
- 62.Zoccali C., Tripepi G., Mallamaci F. Predictors of cardiovascular death in ESRD. Semin Nephrol. 2005;25:358–362. doi: 10.1016/j.semnephrol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Evenepoel P., Meijers B.K., Bammens B.R., Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009:S12–S19. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 64.Campbell A.J., Busby W.J., Horwath C.C. Factors associated with constipation in a community based sample of people aged 70 years and over. J Epidemiol Community Health. 1993;47:23–26. doi: 10.1136/jech.47.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouchoucha M., Devroede G., Bon C. Difficult defecation in constipated patients and its relationship to colonic disorders. Int J Colorectal Dis. 2016;31:685–691. doi: 10.1007/s00384-016-2528-3. [DOI] [PubMed] [Google Scholar]
- 66.Chang J.Y., Locke G.R., Schleck C.D. Risk factors for chronic constipation and a possible role of analgesics. Neurogastroenterol Motil. 2007;19:905–911. doi: 10.1111/j.1365-2982.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 67.Fosnes G.S., Lydersen S., Farup P.G. Drugs and constipation in elderly in nursing homes: what is the relation? Gastroenterol Res Pract. 2012;2012:290231. doi: 10.1155/2012/290231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wein S. Opioid-induced constipation. J Pain Palliat Care Pharmacother. 2012;26:382–384. doi: 10.3109/15360288.2012.734907. [DOI] [PubMed] [Google Scholar]
- 69.Bennett W.E., Jr., Heuckeroth R.O. Hypothyroidism is a rare cause of isolated constipation. J Pediatr Gastroenterol Nutr. 2012;54:285–287. doi: 10.1097/MPG.0b013e318239714f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ragno A., Pepe J., Badiali D. Chronic constipation in hypercalcemic patients with primary hyperparathyroidism. Eur Rev Med Pharmacol Sci. 2012;16:884–889. [PubMed] [Google Scholar]
- 71.Boobes K., Rosa R.M., Batlle D. Hypokalemia associated with acute colonic pseudo-obstruction in an ESRD patient. Clin Nephrol. 2017;87:152–156. doi: 10.5414/CN109002. [DOI] [PubMed] [Google Scholar]
- 72.Weng Y.M., Chen S.Y., Chen H.C. Hypermagnesemia in a constipated female. J Emerg Med. 2013;44:e57–e60. doi: 10.1016/j.jemermed.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Arnaud M.J. Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003;57(Suppl 2):S88–S95. doi: 10.1038/sj.ejcn.1601907. [DOI] [PubMed] [Google Scholar]
- 74.Sutton D., Ovington S., Engel B. A multi-centre, randomised trial to assess whether increased dietary fibre intake (using a fibre supplement or high-fibre foods) produces healthy bowel performance and reduces laxative requirement in free living patients on peritoneal dialysis. J Ren Care. 2014;40:157–163. doi: 10.1111/jorc.12056. [DOI] [PubMed] [Google Scholar]
- 75.De Giorgio R., Ruggeri E., Stanghellini V. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosmadakis G., Albaret J., Da Costa Correia E. Constipation in peritoneal dialysis patients. Perit Dial Int. 2019;39:399–404. doi: 10.3747/pdi.2018.00169. [DOI] [PubMed] [Google Scholar]
- 77.Palmer S.C., Gardner S., Tonelli M. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis. 2016;68:691–702. doi: 10.1053/j.ajkd.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 78.Hoibian E., Florens N., Koppe L. Distal colon motor dysfunction in mice with chronic kidney disease: putative role of uremic toxins. Toxins (Basel) 2018;10(5) doi: 10.3390/toxins10050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y., Yu Y.B. Intestinal microbiota and chronic constipation. Springerplus. 2016;5:1130. doi: 10.1186/s40064-016-2821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabatino A., Regolisti G., Brusasco I. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30:924–933. doi: 10.1093/ndt/gfu287. [DOI] [PubMed] [Google Scholar]
- 82.Khalif I.L., Quigley E.M., Konovitch E.A., Maximova I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37:838–849. doi: 10.1016/j.dld.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Simren M., Barbara G., Flint H.J. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parthasarathy G., Chen J., Chen X. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 2016;150:367–379.e1. doi: 10.1053/j.gastro.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbara G., Stanghellini V., Brandi G. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y.Y., Chen D.Q., Chen L. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. 2019;17:5. doi: 10.1186/s12967-018-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaziri N.D. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens. 2012;21:587–592. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaziri N.D., Wong J., Pahl M. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 89.Wong J., Piceno Y.M., DeSantis T.Z. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–237. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sumida K., Kovesdy C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol Int. 2019;106:195–206. doi: 10.1556/2060.106.2019.19. [DOI] [PubMed] [Google Scholar]
- 91.Cupisti A., Kovesdy C.P., D'Alessandro C., Kalantar-Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018;10(3) doi: 10.3390/nu10030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26:282–287. doi: 10.1053/j.jrn.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayes C.P., Jr., McLeod M.E., Robinson R.R. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- 94.Martin R.S., Panese S., Virginillo M. Increased secretion of potassium in the rectum of humans with chronic renal failure. Am J Kidney Dis. 1986;8:105–110. doi: 10.1016/s0272-6386(86)80120-2. [DOI] [PubMed] [Google Scholar]
- 95.Mathialahan T., Maclennan K.A., Sandle L.N. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 96.Barsotti G., Morelli E., Cupisti A. A low-nitrogen low-phosphorus vegan diet for patients with chronic renal failure. Nephron. 1996;74:390–394. doi: 10.1159/000189341. [DOI] [PubMed] [Google Scholar]
- 97.Cupisti A., Morelli E., Meola M. Vegetarian diet alternated with conventional low-protein diet for patients with chronic renal failure. J Ren Nutr. 2002;12:32–37. doi: 10.1053/jren.2002.29595. [DOI] [PubMed] [Google Scholar]
- 98.Moorthi R.N., Armstrong C.L., Janda K. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am J Nephrol. 2014;40:582–591. doi: 10.1159/000371498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Iorio B.R., Di Micco L., Marzocco S. Very low-protein diet (VLPD) reduces metabolic acidosis in subjects with chronic kidney disease: the “nutritional light signal” of the renal acid load. Nutrients. 2017;9(1) doi: 10.3390/nu9010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sonnenberg A., Koch T.R. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989;34:606–611. doi: 10.1007/BF01536339. [DOI] [PubMed] [Google Scholar]
- 101.Rantis P.C., Jr., Vernava A.M., 3rd, Daniel G.L., Longo W.E. Chronic constipation--is the work-up worth the cost? Dis Colon Rectum. 1997;40:280–286. doi: 10.1007/BF02050416. [DOI] [PubMed] [Google Scholar]
- 102.Menees S.B., Guentner A., Chey S.W. How do us gastroenterologists use over-the-counter and prescription medications in patients with gastroesophageal reflux and chronic constipation? Am J Gastroenterol. 2015;110:1516–1525. doi: 10.1038/ajg.2015.156. [DOI] [PubMed] [Google Scholar]
- 103.Su C.Y., Pei J., Lu X.H. Gastrointestinal symptoms predict peritonitis rates in CAPD patients. Clin Nephrol. 2012;77:267–274. doi: 10.5414/cn107249. [DOI] [PubMed] [Google Scholar]
- 104.Chang S.S., Huang N., Hu H.Y. Patients with end-stage renal disease were at an increased risk of hospitalization for acute diverticulitis. Medicine (Baltimore) 2016;95:e4881. doi: 10.1097/MD.0000000000004881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adams-Carr K.L., Bestwick J.P., Shribman S. Constipation preceding Parkinson's disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:710–716. doi: 10.1136/jnnp-2015-311680. [DOI] [PubMed] [Google Scholar]
- 106.Ponnusamy K., Choi J.N., Kim J. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60(Pt 6):817–827. doi: 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim S.E., Choi S.C., Park K.S. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J Neurogastroenterol Motil. 2015;21:111–120. doi: 10.5056/jnm14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tap J., Derrien M., Tornblom H. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152:111–123.e8. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 109.Bowman J.D., Surani S., Horseman M.A. Endotoxin, Toll-like receptor-4, and atherosclerotic heart disease. Curr Cardiol Rev. 2017;13:86–93. doi: 10.2174/1573403X12666160901145313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang W.H., Wang Z., Levison B.S. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kop W.J., Stein P.K., Tracy R.P. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72:626–635. doi: 10.1097/PSY.0b013e3181eadd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vikenes K., Farstad M., Nordrehaug J.E. Serotonin is associated with coronary artery disease and cardiac events. Circulation. 1999;100:483–489. doi: 10.1161/01.cir.100.5.483. [DOI] [PubMed] [Google Scholar]
- 113.Costedio M.M., Coates M.D., Brooks E.M. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol. 2010;105:1173–1180. doi: 10.1038/ajg.2009.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pstras L., Thomaseth K., Waniewski J. The Valsalva manoeuvre: physiology and clinical examples. Acta Physiol (Oxf) 2016;217:103–119. doi: 10.1111/apha.12639. [DOI] [PubMed] [Google Scholar]
- 115.Ma W., Li Y., Heianza Y. Associations of bowel movement frequency with risk of cardiovascular disease and mortality among US women. Sci Rep. 2016;6:33005. doi: 10.1038/srep33005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lekawanvijit S., Kompa A.R., Wang B.H. Cardiorenal syndrome: the emerging role of protein-bound uremic toxins. Circ Res. 2012;111:1470–1483. doi: 10.1161/CIRCRESAHA.112.278457. [DOI] [PubMed] [Google Scholar]
- 117.Poesen R., Meijers B., Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial. 2013;26:323–332. doi: 10.1111/sdi.12082. [DOI] [PubMed] [Google Scholar]
- 118.Ramos C.I., Armani R.G., Canziani M.E. Bowel habits and the association with uremic toxins in non-dialysis-dependent chronic kidney disease patients. J Ren Nutr. 2020;30:31–35. doi: 10.1053/j.jrn.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 119.De Schryver A.M., Samsom M., Smout A.I. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig Dis Sci. 2003;48:1206–1212. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 120.Muller-Lissner S.A., Kamm M.A., Scarpignato C., Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–242. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 121.Suares N.C., Ford A.C. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33:895–901. doi: 10.1111/j.1365-2036.2011.04602.x. [DOI] [PubMed] [Google Scholar]
- 122.Kelly J.T., Palmer S.C., Wai S.N. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2017;12:272–279. doi: 10.2215/CJN.06190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koppe L., Mafra D., Fouque D. Probiotics and chronic kidney disease. Kidney Int. 2015;88:958–966. doi: 10.1038/ki.2015.255. [DOI] [PubMed] [Google Scholar]
- 124.Koppe L., Fouque D. Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med. 2017;59:173–187. doi: 10.23736/S0031-0808.16.03282-1. [DOI] [PubMed] [Google Scholar]
- 125.Andresen V., Layer P. Medical therapy of constipation: current standards and beyond. Visc Med. 2018;34:123–127. doi: 10.1159/000488695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ford A.C., Moayyedi P., Lacy B.E. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–S26. doi: 10.1038/ajg.2014.187. quiz S27. [DOI] [PubMed] [Google Scholar]
- 127.Mimidis K., Mourvati E., Kaliontzidou M. Efficacy of polyethylene glycol in constipated CAPD patients. Perit Dial Int. 2005;25:601–603. [PubMed] [Google Scholar]
- 128.Xing J.H., Soffer E.E. Adverse effects of laxatives. Dis Colon Rectum. 2001;44:1201–1209. doi: 10.1007/BF02234645. [DOI] [PubMed] [Google Scholar]
- 129.Tayebi-Khosroshahi H., Habibzadeh A., Niknafs B. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev. 2016;5:162–167. doi: 10.15171/jrip.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian H., Ding C., Gong J. Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J Clin Gastroenterol. 2016;50:865–870. doi: 10.1097/MCG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 131.Tian H., Ge X., Nie Y. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ford A.C., Suares N.C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut. 2011;60:209–218. doi: 10.1136/gut.2010.227132. [DOI] [PubMed] [Google Scholar]