Abstract
The present study was designed to investigate the therapeutic efficacy of metal chelator and anticancer drug in the treatment of colorectal cancer (CRC). Pellets containing Phytic acid, 5- Fluorouracil (5-FU), Microcrystalline cellulose (MCC) PH 101, Hydroxypropyl Methylcellulose (HPMC) and Barium sulfate were prepared by using extrusion spheronization technique. Prepared pellets were coated with Eudragit S100 to achieve colon-specific drug delivery. Pellets were characterized for various pharmaceutical and micromeritic attributes. The in vivo therapeutic efficacy comprising of both pharmacokinetic and pharmacodynamic parameters was determined in Ehrlich ascites carcinoma (EAC) induced cancer animal model. Phytic acid and 5-FU combinations seem to exert higher cytotoxic activity via increased reactive oxygen species (ROS) level by chelating manganese. Further pharmacokinetic studies reveled approximately 50% lower Cmax in the finished formulation, indicates lower systemic exposure to the drug. X-ray radiography ensures the localized delivery of the encapsulated drug. Histopathological studies indicated no significant local toxicity compared to the uncoated formulation. Results inferred that the proposed combination has superior anticancer activity with minimum systemic and local toxicity and it opens a new avenue in the treatment of colorectal cancer.
Keywords: Materials science, Pharmaceutical science, Controlled drug delivery, 5-flurouracil, Phytic acid, Colon cancer, Pellets
Materials science; Pharmaceutical science; Controlled drug delivery; 5-flurouracil; Phytic acid; Colon cancer; Pellets
1. Introduction
The incidences of colorectal cancer (CRC) have increased rapidly in young patients. Further, drinking alcohol increases the risk of developing CRC (Vuik et al., 2019). The second leading cause of death responsible all over the world is cancer, accounting for 8.9 million deaths globally per year (Fitzmaurice et al., 2018). 5-fluorouracil (5-FU) has been widely used as oral chemotherapy for early and advanced CRC. Although 5-FU presents to be a promising drug for CRC however, short elimination half-life requires multiple administration, limits the therapeutic potential of the drug (Alibolandi et al., 2017). Multiple administration of 5-FU increases the risk of chemotherapy toxicity and resistance. Colon-specific sustained drug delivery system improves the efficacy and safety of the drug. Further sustained-release minimizes the risk of drug plasma fluctuation and reduced systemic toxicity (Sreelatha and Brahma, 2013). Therefore, specific Eudragit® acrylic polymers have been developed for peroral dosage forms with the step-wise release of active ingredients in the digestive tract (Amidon et al., 2015). Eudragit® coatings get dissolved as a function of environmental pH and release of active ingredients occurs near the colon in a pH range 6.5–7.5. Eudragit S 100 has been extensively used as a pH-sensitive polymer for colon drug targeting (Wairkar and Gaud, 2016). In the current study, we have formulated Eudragit S100 coated pellets to achieve colon-specific sustained drug delivery system. Superoxide dismutase (SOD) is a critical enzyme responsible for the elimination of superoxide radicals and is considered to be a key anti-oxidant in aerobic cells (Hwang et al., 2007). Reactive oxygen species (ROS) production including the superoxide radicals (o2.-) and hydrogen peroxide (H2O2) occurs when oxygen is taken up by cells for oxidative phosphorylation during ATP (adenosine triphosphate) generation in mitochondria. Accumulation of ROS results in cellular oxidative stress and if not corrected, can lead to damage of important biomolecules such as membrane lipids, proteins, and DNA (deoxyribonucleic acid). Prolonged accumulation of high levels of free radicals in cells may cause irreversible cellular injury and ultimately results in cell death. Since SOD is the key enzyme involved in the management of free radicals (Hileman et al., 2001), silencing of SOD causes increased accumulation of O2. -subsequently lead to cell death. Thus, silencing of SOD might offer a promising alternative to kill cancer cells (Birben et al., 2012). SODs are the most important antioxidant defense systems against O2. -, that incorporates three isoforms of SOD in mammals (Perry et al., 2010): the cytoplasmic Cu/ZnSOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular Cu/ZnSOD (SOD3). All isoforms need catalytic metal (Cu or Mn) for their activation. Cancer cells usually express high level of antioxidant enzymes to protect cells from ROS. It has been reported that colon cancer has elevated levels of MnSOD. Chelating agents are capable of binding to free metal ions, thereby makes them unavailable for enzyme activity (Borrelli et al., 2014). Inactivation of SOD increases the level of ROS, which leads to cell lysis. Cui et al., reported that copper chelates penicillamine enhances the level of intrinsic oxidative stress in variety of cancers, leading to cell lysis (Cui et al., 2005).
Phytic acid (myoinositol-hexaphosphate, IP6) is a constituent of dietary fiber in cereals. It has a high capacity to form insoluble complexes (phytates) with divalent metal ions present in food (Persson et al., 1998). The relative order of metal chelation with phytic acid at pH 7.4 was found to be Cu2+>Zn2+>Co2+>Mn2+>Fe2+>Ca2+ (Reddy et al., 1982). Phytic acid supplementation is known to exhibit anti-cancer, anti-inflammatory and antioxidant activity, therefore, metal chelates are of considerable interest to improve the anticancer activity of chemotherapeutic drugs in combination or alone (Narayanaswamy and Mohd, 2018). Accordingly, the present study focused on investigating the therapeutic potential of combination strategies (phytic acid with 5-FU) to treat cancer in xenograft animal model.
In addition to the above, the proposed formulation consists of a contrast agent, barium sulfate to track the drug carrier in the gastrointestinal tract. In the present study, we have formulated Eudragit coated pellets containing phytic acid, 5-FU and barium sulfate using extrusion spheronization technique. Critical formulation parameters like weight ratio of coating polymer, 5-FU, Hydroxypropyl methylcellulose and process parameters including spheronizer speed and time were optimized. Selected critical attributes should perform an effect on the morphology of pellets. X-ray radiography of the treated animals was performed to establish the targeting potential of the formulation. Plasma drug distribution and histopathology studies were performed to access the toxicological outcomes of the optimized formulation. Tumor regression study was performed to measure the therapeutic efficacy of the prepared system in Ehrlich ascites carcinoma (EAC) induces cancer model in Wistar rats.
2. Materials and methods
2.1. Materials
The following materials were used to prepare the formulations: The drug 5-FU was obtained from the United Biotech Pvt. Ltd., Baddi (India); Eudragit S 100 polymer was obtained as gift sample from Evonik India Pvt. Ltd., Mumbai; HPMC E 5 LV premium and Barium Sulfate were procured from Loba Chem. Pvt. Ltd., Mumbai; MCC PH 101 was purchased from DFE Pharma India LLP, EAC cell line was purchased from NCCS Pune. All other chemicals used in this research were of analytical grade.
2.2. Method of preparation of uncoated pellets
Sustained released 5-FU loaded pellets were prepared by using instrument extruder and spheronizer using barium sulfate as high-density X-ray imaging contrast material, phytic acid as a chelating agent, HPMC as a binder, MCC as a bulking agent and Eudragit S 100 as enteric coating polymer. Pellets were produced with 6.62 g of powder blend containing 1.81% of anticancer drug (5-FU), 3% of phytic acid, 4.5% of barium sulfate, 7.5% of HPMC and 83.19% of MCC. Pellets were prepared by mixing the components with distilled water and made into suitable dough mass for palletization process. Nine different formulations F1, F2, F3,……,F9 were prepared with varying concentrations of binder and bulking agent up to a certain limit as described in Table 1 to achieve the required size of pellets. F9 formulation was the optimized formulation among the nine formulations prepared. This ninth formulation was then loaded with 5-fluorouracil and labeled as F10 formulation. The F10 formulation was then coated with Eudragit S 100 enteric-coated polymer and labeled as F11. The optimization of speed of rotation and time of rotation was as given in Table 2. The yield was computed as a percentage of the weight of dried pellets collected divided by the initial weight of solid mass prior to extrusion and spheronization (Wairkar and Gaud, 2016) using the following equation:
Table 1.
Composition of different pellet formulations.
| Formula Code | Phytic acid (g) | Barium sulfate (g) | HPMC (g) | MCC (g) | Water (ml) | Drug (g) |
|---|---|---|---|---|---|---|
| F1 | 0.2 | 0.3 | 2 | 4 | 2 | - |
| F2 | 0.2 | 0.3 | 1 | 5 | 3 | - |
| F3 | 0.2 | 0.3 | 0.5 | 5 | 4 | - |
| F4 | 0.2 | 0.3 | 0.5 | 5.5 | 4.5 | - |
| F5 | 0.2 | 0.3 | 0.5 | 5.5 | 5 | - |
| F6 | 0.2 | 0.3 | 0.5 | 6 | 5 | - |
| F7 | 0.2 | 0.3 | 0.5 | 5.5 | 5 | - |
| F8 | 0.2 | 0.3 | 0.5 | 5.5 | 5 | - |
| F9 | 0.2 | 0.3 | 0.5 | 5.5 | 5 | - |
| F10 | 0.2 | 0.3 | 0.5 | 5.5 | 5 | 0.12 |
Table 2.
Optimization of process variables involved in spheronization process.
| F1, F2, F3, F4, F5, F6, F9 & F10 |
F7 & F8 |
F7 |
F8 |
|
|---|---|---|---|---|
| Rotation speed (RPM) | Rotation time (min) | Rotation speed (RPM) | Rotation time (min) | Rotation time (min) |
| 400 | 2 | 500 | 5 | 10 |
| 700 | 4 | 200 | 10 | - |
| 700 | 4 | 300 | 10 | 15 |
| 700 | 4 | 700 | 10 | 15 |
| 700 | 4 | 700 | 10 | 15 |
| 700 | 4 | - | - | - |
| 700 | 4 | - | - | - |
2.3. Preparation of coated pellets
Eudragit S100 was used for the coating of 5-FU loaded pellets. Accurately weighed enteric coating polymer (Eudragit S 100) was taken to make three different concentrations i.e. 5%, 10% and 15% to get optimized coating concentration as given in Table 3. Eudragit S 100 was dissolved in isopropyl alcohol. The solution was homogenized until it became clear. Then, the pipe of the peristaltic pump was rinsed with an organic solvent. Solution Pipe was filled with a coating solution and ensured that there were no air bubbles in the pipe and pipe was attached with spray gun of Accela Cota. The door was closed of coating pan and all the process parameters like; flow rate, temperature, drum speed and air pressure were then adjusted. 5.9 g of 5-FU loaded pellets of F10 formulation were firstly heated in the coating pan and then these preheated pellets were subjected to coating. A total of 1 ml coating solution was atomized by spray coating for 1 min, in time intervals of 1 min to allow sufficient time for solvent evaporation. Likewise, this was repeated 3 times and the resulted formulation was labelled as F11 (Pandey et al., 2018).
Table 3.
Optimization of formulation variables to obtain coated pellets.
| S. no. | Concentration (%) | Flow rate (ml/min) | Drum speed (rpm) | Temperature (°C) | Air pressure (kg/cm2) | Inference |
|---|---|---|---|---|---|---|
| 1. | 5 | 1 | 20 | 50 | 5 | No significant difference in in vitro drug release profile |
| 2. | 10 | 1 | 20 | 50 | 10 | Required in vitro drug release |
| 3. | 15 | 1 | 20 | 50 | 10 | Droplet formation on the tip of atomizer |
2.4. In-vitro characterization of 5-FU loaded pellets
The optimization of the amount of HPMC, barium sulfate and MCC and selection of the best formulation was done on the basis of the shape & size of pellets, percentage yield, friability and angle of repose.
2.4.1. Pellets morphology
Pellet size and morphological defects of the prepared pellets were examined under the Motic microscope. Pellet size was determined by using a 10X objective lens. A clean glass slide was taken and few drops of glycerin were spread over the slide. A pellet was placed over the slide and the size of 20 pellets was determined likewise (Pundlikrao and Rajput, 2017; Wairkar and Gaud, 2016).
2.4.2. Sphericity studies
The shape and the area of pellets were investigated by optical microscopic image analysis. Pellet size and shape were measured using an optical DMW2-223 digital microscope (Motic Instruments) equipped with a 1/3″ CCD camera imaging accessory and computer-controlled image analysis software (Motic Images, 2000, 1.3 version). 20 pellets from each batch were selected and analyzed by microscopic image analysis technique and a variety of parameters like aspect ratio, roundness and circularity have been used to assess the shape of the pellets. The pellet size data was further processed to get aspect ratio, circularity factor and roundness using following equations:
Where, Dmax. –maximum diameter of the pellet, Dmin. –the minimum diameter of the pellet
Where, P – perimeter of the pellet, A – area of the pellet.
2.4.3. Micrometric properties
Pellets were characterized for flow properties such as the angle of repose, bulk density, tapped density, Hausner's ratio, Carr's index compressibility index and flow behavior was studied. The results of the above parameters were expressed as mean values of three observations.
2.4.3.1. Angle of repose
The flow property could be decided from the angle of repose as given in Table 4. Briefly, the sample was allowed to fall gently through a funnel on to a hard surface from the height of 2.5 cm. The height and diameter of the pile were noted. The angle of repose was determined by using the following formula.
| Angle of repose (θ) = tanˉ1 (h/r) |
Where h is the height of the pile and r is the radius of the pile.
Table 4.
Empirical relation between the flow properties and angle of repose (Al-Hashemi and Al-Amoudi, 2018).
| Flow properties | Angle of repose (◦) |
|---|---|
| Excellent | <25 |
| Good | 25–30 |
| Passable | 30–40 |
| Very poor | >40 |
2.4.3.2. Bulk density
Pellets were accurately weighed to 10 g and were gently poured through a glass funnel into a calibrated 100 ml measuring cylinder. The surface was made smooth carefully with the application of pressure. The volume occupied by the sample was recorded and bulk density (g/ml) was calculated and recorded in the table by using the following formula.
2.4.3.3. Tapped density
Similar to the bulk density, tapped density was observed by tapping the cylinder 100 times from 3-inch height by using Electrolab tapped density apparatus after pouring the pellets into the measuring cylinder and the tapped volume was recorded. Finally, the tapped density was recorded by using the formula.
2.4.3.4. Hausner's ratio
The Hausner's ratio is a number that is correlated to the flowability of pellets. It was calculated by the following formula:
Where ρt is tapped density and ρb is bulk density.
2.4.3.5. Carr's index
The Carr's index is an indication of the compressibility of pellets. It was calculated by the following formula:
Where ρt is the tapped density of the powder and ρb is the freely settled bulk density of the powder.
2.4.4. Friability
Accurately weighed 5.9 g of pellets were placed on a sieve having 0.85 mm aperture with 20 pellets of 3 mm diameter and then placed in Roche's friabilator (Veego Scientific, India). Then friabilator was subjected for 100 revolutions at 25 rpm speed. The pellets were collected from the friabilator and again placed on the sieve. The pellets having a smaller diameter than the aperture of sieve will pass through the sieve and then the pellets have reweighed. The friability was determined as the percentage loss of mass of pellets after the test was recorded.
2.4.5. Karl Fischer titration
The moisture content of prepared formulations was chemically quantified by Karl Fischer (KF) coulometric titration. The sample (0.1 g) was placed in titration cell. The iodine is generated from oxidation of iodide contained in the KF reagent. Then reaction was started. Once the reaction consumes all the water present, the presence of excess iodine was detected by the indicator electrode. Then percentage of water present in the sample was calculated automatically and displayed on the screen of the KF titration apparatus.
2.4.6. Determination of entrapment efficiency
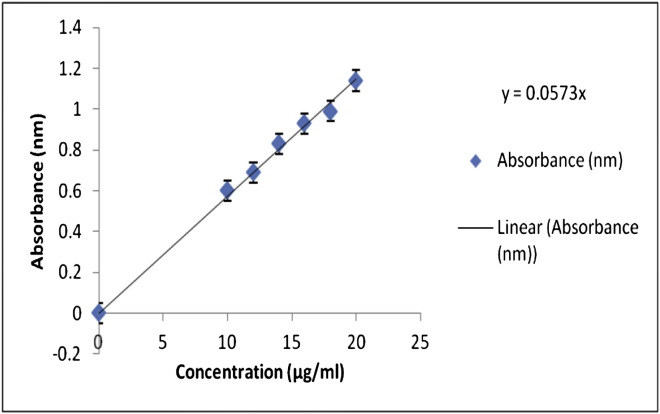
Sustained release pellets equivalent to 5 mg of the drug were dissolved in a suitable medium i.e. PBS 7.4. The resulting solution was briefly vortexed and treated in a bath sonicator for 10 min and then centrifuged for 10 min at 5000 rpm. The amount of drug in the supernatant was determined using the formula shown by the standard curve shown in Figure 1 and Table 5 at λmax. 266 nm by using UV-Spectrophotometer (Shanmugam et al., 2013). Using the following formula:
Figure 1.
Standard curve of 5-FU in PBS 7.4.
Table 5.
Calibration curve of 5-FU in PBS 7.4.
| Concentration (μg/ml) | Absorbance (nm) |
|---|---|
| 0 | 0.00 ± 0.00 |
| 10 | 0.60 ± 0.05 |
| 12 | 0.69 ± 0.05 |
| 14 | 0.83 ± 0.06 |
| 16 | 0.93 ± 0.05 |
| 18 | 0.99 ± 0.04 |
| 20 | 1.14 ± 0.06 |
2.4.7. Uniformity of drug content
The drug content for the 5-FU loaded pellets was determined by soaking pellets in water for 30 min, the pellets were broken with the spatula, vortexed for 5 min, centrifuged for 10 min at 2000 rpm, diluted to 50 ml with PBS 7.4 pH and then the drug was estimated by Shimadzu UV/visible spectrophotometer at 266 nm.
2.4.8. In vitro drug release study
In vitro release study of 5-FU loaded pellets was carried out using the dialysis bag method (Zhao et al., 2018). In this method firstly the dialysis bag was activated by washing it in running water for 3–4 h for removal of glycerin. Further, it was treated with 0.3 % (w/v) sodium sulfide solution at 70 °C for 1 min to remove sulfur compounds and then it was again washed with hot water (60 °C) for 2 min followed by acidification with 0.2% sulfuric acid and rinsed with hot water to remove the acid. Accurately weighed 0.24 g pellets were placed into a previously activated dialysis bag in a 5 ml solution of the donor compartment. The receptor compartment was filled with 100 ml of simulated gastric fluid (SGF) pH 1.2 in a conical flask. This flask was kept in incubator shaker and speed of shaker was maintained at 60 rpm at 37 ± 1 °C. At specific time intervals for 2 h, samples (5ml) were withdrawn and filtered. The same volume (5ml) was replaced after each sampling with SGF. Then, after 2 h the dialysis bag was placed into PBS pH 7.4. The same sampling procedure was followed for specific time intervals for 10 h and sink condition was maintained. The drug content in the sample was determined by the UV method being developed at 266 nm (Kamal et al., 2017, Shanmugam, S et al., 2013).
2.4.9. Compatibility studies and physical state of formulation (XRD and DSC)
The crystallinity of 5-FU loaded pellets was evaluated using an X-ray Diffractometer. The X-ray diffraction (XRD) patterns were determined for the 5-FU loaded pellets. Samples were exposed to monochromatic nickel-filtered copper radiation (45 kV, 40 mA) in a wide-angle X-ray Diffractometer (D8 Advance, BRUKER, Germany) with 2θ angle. A thermo-analytical technique that is DSC, which is used to examine the degree of crystallinity and polymorphic transitions or thermal transitions involving energy changes throughout the method of formulation. The phase transition temperature of 5-FU in drug-loaded pellets was analyzed by Differential Scanning Calorimetry (SIIO 6300, SIIO, Japan) in perforated aluminum sealed pans at a heating rate of 5 °C/min from 30 to 400 °C using nitrogen as blanket gas (50 ml/s) (Tan et al., 2018).
2.4.10. In vitro cytotoxicity assay and cancer cell growth inhibition
In the present studies, EAC cells were used to determine the cytotoxicity of the experimental formulation. Briefly, EAC cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 50 μg/ml Gentamicin. The cells were cultured in a 5% CO2 atmosphere at 37 °C. Culture flasks were transferred to a sterile working area and the medium was discarded. Trypsin (0.1 ml/cm2) was added to the side of the flasks. Flasks were then turned over and laid down. Ensuring the monolayer was completely covered, the flasks were left stationary for 10–30s. Flasks were then rinsed to remove the trypsin from the monolayer. Then it was incubated in the flasks lying flat until the cells rounded up when the flask was tilted. RPMI 1640 medium was added (0.1–0.2 ml/cm2) and cells were dispersed by repeated pipetting over the surface bearing the monolayer. Finally, the cell suspension up and down was pipetted out for a few times, with the help of the pipette resting on the bottom corner of the bottle, taking care not to create foam. Cells were then counted using hemocytometer.
2.4.11. MTT assay
% cell viability of prepared formulations was determined against EAC cell lines purchased from NCCS, Pune. EAC cell lines were seeded in 96-well microculture plates at 1 × 10 7 EAC cells in RPMI 1640 media supplemented with 10% heat-inactivated FBS and incubated for 24 h at 37 °C and CO2 was maintained at 5%. Plain drug, drug-loaded coated and uncoated Pellets were dissolved in PBS pH 7.4 to prepare a solution. This solution was added in culture medium and EAC cells were incubated for 24 h. Then the percent cell growth inhibition was measured using MTT assay standard protocol.
2.5. In vivo studies
2.5.1. Induction of colon cancer and regimen of treatment
Colon cancer was induced in Wistar rats weighing 200–250 g and the dose of 1*107 EAC cells was injected intrarectal, procured from NCCS Pune. Wistar rat was considered to have colon cancer after 14 days of disease induction which was confirmed by X-ray. Formulations were then administered according to animal dose as described in Table 6. All animals were procured from the Animal House of ISF College of Pharmacy, Moga for behavioral parameters assessment as well as for biochemical estimation before the administration of the drug as well as after the administration of the drug. Rats were subjected to standard laboratory conditions (i.e. room temperature, 25 ± 2 °C; relative humidity, 55 ± 5%; 12/12 h light/dark cycles) with free access to a commercial rodent diet and water. For the pharmacokinetic study, animals were fasted overnight and for 8 h after the dosing. The protocol for studies was approved by the Institutional Animal Ethical Committee (IAEC) at ISF College of Pharmacy, Moga, India. The experiments were conducted as per CPCSEA (Committee for Prevention, Control and Supervision of Experimental Animals ISFCP/IAEC/CPCSEA/Meeting No.22/2018/Protocol No. 367) guidelines. Various pharmacokinetic parameters were obtained after oral administration of tested formulation (plain drug, phytic acid, 5-FU-Eudragit S100 formulation, phytic acid-Eudragit S 100 formulation and final formulations) on day 14. Blood samples were withdrawn at 0hr, 2hr, 4hr, 8hr, 12 h, 24 h and 48 h following single oral dosing of above experimental formulations. Total 35 animals will be divided into five groups. Route of administration dose and sample used for the animal study are described in Table 6.
Table 6.
Experimental layout of animal study represents groups of animal, dose and route of administration.
| Group No. | Description | Name of animal | No. of animals | Dose (mg/kg) | Route of administration |
|---|---|---|---|---|---|
| 1 | 5-FU | Male Wistar rat | 7 | 5 | Oral |
| 2 | Phytic acid | 7 | 330 | ||
| 3 | Phytic acid + Eudragit based pellets | 7 | |||
| 4 | 5-FU + Eudragit based pellets | 7 | 5 | ||
| 5 | Final formulation | 7 |
2.5.2. Bioanalytical method development by HPLC for pharmacokinetic studies
High performance liquid chromatographic (HPLC) technique for the quantification of 5-FU in rat plasma has been developed. Chromatographic analysis was performed in the isocratic mode. The sample was analyzed by reverse-phase C18 Grace Vydac (250 × 4.6 mm, 5 μ) as a stationary phase. The mobile phase consisted of a mixture of methanol and water (92:8, v/v), which was pumped at a flow rate of 1.0 ml/min. The sample injection volume was 20μl and the UV detection at wavelength 266 nm. The method run time was 4 min, and all experiments were performed at 25 °C. The method was validated as per ICH guidelines. Linearity was determined by calculating a regression line from the plot of peak area vs. concentration for the six standard solutions in water (i.e. 2.0, 4.0, 6.0, 8.0 and 10.0 μg/ml) using the linear least squares methodology. The specificity was evaluated by comparing the representative chromatograms of samples containing possible interfering substances and samples containing 5-FU. Additionally, specificity was demonstrated by performing stress studies (i.e., light stability, pH variation, temperature and oxidation). Precision was assessed at two levels: repeatability and intermediate precision. The repeatability was assessed by testing three different standard solutions on the same day and using a standard solution with a concentration of 10.0 μg/ml analyzed 10 times in succession. The intermediate precision was evaluated by analyzing three different standard samples on two different days. These results were reported as the standard deviation (SD). Robustness was evaluated by deliberately varying the temperature of the analytical column (35 °C), the flow rate (0.9 ml/min and 1.1 ml/min) and using a similar C18 column (5 μm particle size, 4.6 mm internal diameter, and 250 mm length; Vertical Chromatography).
2.5.3. X-ray transmission radiography to determine the retention potential of the formulation
The behavior of optimized pellet formulation in the rat was determined using a radiographic imaging technique. Prior to this, X-ray study animals were fasted overnight, with free access to water. It involved the use of radio-opaque marker barium sulfate, incorporated in the formulation to determine the position of the pellets. The amount of the barium sulfate was optimized for no effect on the physical characteristics of the optimized pellets formulation. X-ray images of the colon of the treated rats were taken at various time points namely just after administration 2 h and 4 h to trace the in vivo movement and behavior of the pellets in the GIT. X-ray images of the rats in prone positions were captured using L&T Vision 100 (C-arm) X-ray machine, at 64 mAs and 63 kV techniques.
2.5.4. Tumor regression analysis
Tumor reduction analysis was performed on the 21st day after the initiation of treatment. The tumor regression was monitored by the tumor volume calculation using Vernier Caliper on the 21st day of study. The two largest perpendiculars in X and Y plane which was removed by a skilled surgeon was measured by independent 4 observers using Vernier Caliper. The depth was assumed to be equivalent to the shortest perpendicular axis defined by Y. The volume was calculated using the following equation (Sharma et al., 2018).
2.5.5. Histopathology study
It was performed on the 21st day of treatment. For histopathology examination, local tissue including the tumor was successfully excised from Wistar rat and was fixed in 10% formalin immediately after its removal and then dehydrated and embedded in paraffin. Cross-sections were made with a size of about 4–5 mm and stained with hematoxylin and eosin. The excised section of colon was viewed under the microscope to examine the change occur due to action of the prepared formulations.
2.6. Statistical analysis
Statistical analysis of observation was performed using ANOVA followed by Tukey's multiple composition tests. The results were expressed as mean ± SD and showing the number of repeats. P-value less than 0.05 was considered a statistically significant value.
3. Results and discussions
3.1. Preparation and optimization of coated 5-FU loaded pellets
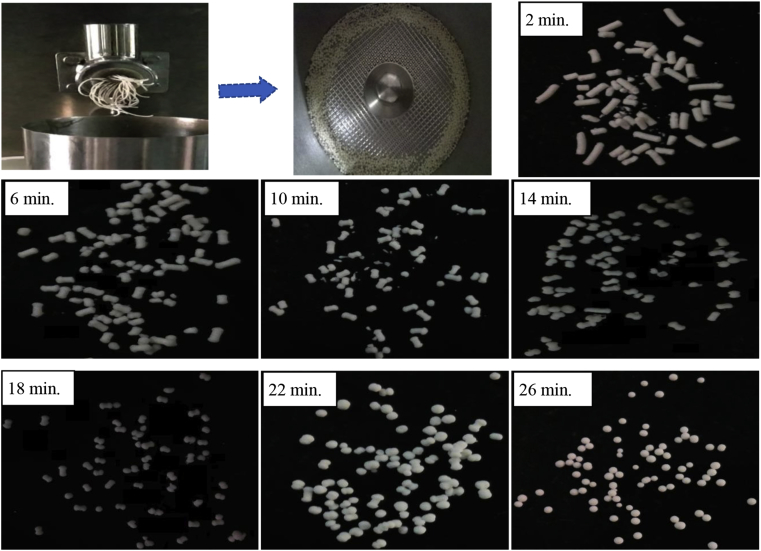
Process variable i.e. weight ratio of 5-FU, phytic acid, barium sulfate, HPMC and MCC were optimized on the basis of their effect on % yield, moisture and pellet shape as shown in Figure 2. In this study, the collective level of formulation variables, rotation speed & rotation time of spheronization and extrusion conditions was required to produce 5-FU loaded pellets with desirable pharmaceutical properties (% highest yield, optimum moisture content and lowest pellet size). At the initial stage, the concentration of binder, water and bulking agent were optimized under a define extrusion spheronization parameters (rotation speed and time). Results show that as the concentration of HPMC decreases from 10.5% to 5% w/w against a fixed concentration of MCC, it affects the pellet morphology and percentage yield. However, percent yield was found to decrease with an increase in MCC concentration. Further, the higher water content increases inter particular attractive force which is enough to cause the spheronization of pellets with decreasing particle size. From the results, 5% w/w concentration of binder was taken forward for further optimization. Keeping the binder concentration constant then MCC concentration was optimized. MCC concentration at 47.8% w/w has shown the highest product yield with optimum moisture content and lowest pellet size. As the MCC concentration was increased further, there was a decrease in % yield with an increase in particle size. 47.8% w/w percentage of MCC considered optimized and taken forward for further optimization. Further, the formulation was screened for the water level on the basis of pellets size and shape keeping the concentration of binder and bulking agent constant. Results suggested that the pellet size was significantly reduced above a total water level of 43.4% w/v. Further, the formulation was screened for rotation speed and time for spheronization. The changes in rotation speed and time caused the reduction in % yield. Therefore optimized speed and time rotation were considered to formulate drug loaded pellets as described in Table 2. Particle size, moisture content and percentage yield were the criteria considered for the selection of final formulation. It has been observed that the shape and size of the pellets were reduced with increasing concentration of water. The results of the optimized process suggested that the formulation F9 (containing 3% w/w phytic acid, 4.5% w/w barium sulfate, 7.5% w/w HPMC and 83.19% w/w MCC) demonstrated all the required characteristics and was therefore selected for drug-loaded formulation development.
Figure 2.
Steps involved in pellets manufacturing and shape heterogeneity with time. First step in the process of palletization is to form extrude then with the help of minute and sharp blades in the disc of spheroniser, the extrude is cut into small pieces. As we continue spheronization process with the time rod shaped extrudes results into spherical shaped pellets.
3.2. Optimization of the pellet coating process
Eudragit S100 was used to coat 5-FU loaded targeted and sustained release pellets. Eudragit S100 was used at three different concentrations. From three different conc. 10%w/v Eudragit S100 coating was found to be the best coating for targeted and sustained release of 5-FU from pellets. The concentration of coating material above 10% leads to aggregation and that could be related to slow drying under the specified experimental conditions. Further, a significant change in sphericity and morphology was observed with increased polymer concentration that could be attributed to improving atomization as a function of polymer viscosity. 10% w/v polymer conc. produced and pellets were coated (Tan et al., 2018).
3.3. In-vitro characterization of 5-FU loaded pellets
In- vitro characterization of 5-FU loaded pellets included parameters like particle morphology, in-vitro drug release, entrapment efficiency, X-ray diffraction studies, differential scanning calorimetry and morphology.
3.3.1. Pellets morphology
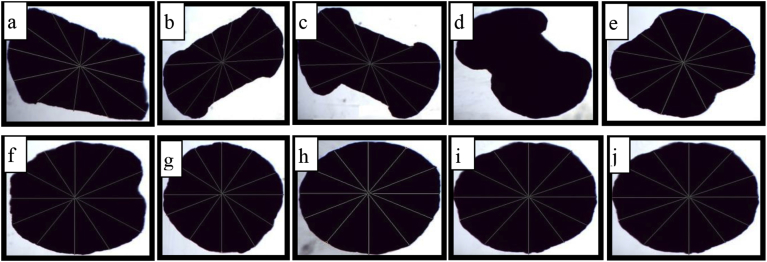
The particle morphology is shown in Figure 3 and the mean particle size of pellets is shown below in Table 7. The size of optimized pellets of F9, F10 and F11 was found to be 500 μm, 480 μm and 530 μm respectively.
Figure 3.
Microscopical analysis of different pellet formulations. a) F2, b) F3, c) F4, d) F5, e) F6, f) F7, g) F8, h) F9, i) Drug loaded uncoated pellets and j) drug loaded coated pellets. This figure indicates the improvement in sphericity of pellets due to variations in the binder and vehicle (water).
Table 7.
Shape and size analysis of different pellet formulations.
| Formula Code | Shape | Aspect ratio | Roundness Factor | Circularity factor | Average diameter (um) |
|---|---|---|---|---|---|
| F1 | Rod shaped | 1.359 ± 0.15 | 1.059 ± 0.11 | 0.964 ± 0.39 | - |
| F2 | Rod shaped | 1.325 ± 0.21 | 1.050 ± 0.25 | 0.969 ± 0.22 | 660 ± 20.6 |
| F3 | Rod shaped | 1.305 ± 0.11 | 1.047 ± 0.29 | 0.971 ± 0.27 | 680 ± 22.3 |
| F4 | Dumbbell | 1.286 ± 0.23 | 1.045 ± 0.06 | 0.975 ± 0.18 | 730 ± 23.7 |
| F5 | Dumbbell | 1.272 ± 0.21 | 1.039 ± 0.17 | 0.976 ± 0.21 | 680 ± 24.3 |
| F6 | Elongated spheroids | 1.245 ± 0.16 | 1.024 ± 0.10 | 0.977 ± 0.15 | 740 ± 24.2 |
| F7 | Elongated spheroids | 1.230 ± 0.24 | 1.021 ± 0.23 | 0.979 ± 0.26 | 610 ± 24.5 |
| F8 | Elongated spheroids | 1.225 ± 0.21 | 1.019 ± 0.19 | 0.981 ± 0.10 | 560 ± 25.4 |
| F9 | Spheroids | 1.120 ± 0.19 | 1.0015 ± 0.13 | 0.988 ± 0.27 | 500 ± 24.1 |
| F10 | Spheroids | 1.109 ± 0.24 | 1.0009 ± 0.11 | 0.988 ± 0.16 | 480 ± 23.5 |
| F11 | Spheroids | 1.102 ± 0.09 | 1.0005 ± 0.09 | 0.990 ± 0.09 | 530 ± 24.2 |
3.3.2. Sphericity studies
Pellets with the sphericity value of 1 were considered to be exactly spherical and pellets with sphericity values near to 1 were considered nearly spherical. The aspect ratio, roundness factor and circularity factor for the optimized formulation F9 were found to be 1.120 ± 0.19, 1.0015 ± 0.13 and 0.988 ± 0.27 respectively as shown in Table 7.
3.3.3. Micrometric properties
The results of flow properties like Angle of repose, Bulk density, Tapped density, Hausner ratio and Carr's index of various batches of pellet formulations are shown in Table 8. The angle of repose (θ) for the pellets was observed in the range of 18.51°±1.1–25.13°±1.26 which indicated excellent flow properties of 5-FU loaded sustained pellet formulations. Similarly, values of Carr's index and Hausner ratio were found in between 7.320 ± 0.63% to 20.31 ± 0.02% and 1.00 ± 0.04 to 1.38 ± 0.22 respectively. Carr's index and Hausner ratio are commonly used parameters to predict flow characteristics and can be correlated with size, shape, surface area and cohesiveness of the substance. Pellets containing a low quantity of HPMC, an appropriate amount of water and MCC have shown lower values of Carr's index as well as Hausner ratio and can be attributed to their regular and spherical shape. Therefore, it is evident that water and MCC can improve the shape and size of sustained release pellets which will further show better micrometric properties (Kamalaldin et al., 2017).
Table 8.
Micrometric properties for different pellet formulations.
| Formula code | Angle of repose (θ) | Bulk density (g/cm3) | Tapped density (g/cm3) | Hausner's ratio | Carr's index (%) | % Friability |
|---|---|---|---|---|---|---|
| F1 | 36.300 ± 0.360 | 0.711 ± 0.003 | 0.823 ± 0.003 | 1.267 ± 0.005 | 19.780 ± 0.208 | 0.82 ± 0.15 |
| F2 | 33.600 ± 0.360 | 0.704 ± 0.003 | 0.818 ± 0.001 | 1.253 ± 0.004 | 19.003 ± 0.325 | 0.77 ± 0.19 |
| F3 | 32.033 ± 0.208 | 0.716 ± 0.003 | 0.845 ± 0.002 | 1.256 ± 0.004 | 17.263 ± 0.482 | 0.68 ± 0.11 |
| F4 | 31.566 ± 0.251 | 0.735 ± 0.002 | 0.844 ± 0.035 | 1.197 ± 0.003 | 15.976 ± 0.123 | 0.56 ± 0.18 |
| F5 | 31.333 ± 0.416 | 0.727 ± 0.003 | 0.837 ± 0.002 | 1.188 ± 0.003 | 14.170 ± 0.056 | 4.51 ± 0.14 |
| F6 | 30.666 ± 0.416 | 0.744 ± 0.002 | 0.850 ± 0.002 | 1.142 ± 0.006 | 12.453 ± 0.331 | 0.46 ± 0.10 |
| F7 | 29.433 ± 0.472 | 0.755 ± 0.003 | 0.861 ± 0.003 | 1.140 ± 0.004 | 12.296 ± 0.388 | 0.32 ± 0.12 |
| F8 | 26.633 ± 0.472 | 0.774 ± 0.003 | 0.823 ± 0.002 | 1.126 ± 0.004 | 11.903 ± 0.651 | 0.36 ± 0.10 |
| F9 | 25.066 ± 0.404 | 0.767 ± 0.002 | 0.843 ± 0.002 | 1.101 ± 0.008 | 8.573 ± 0.285 | 0.19 ± 0.07 |
| F10 | 25.005 ± 0.351 | 0.764 ± 0.002 | 0.832 ± 0.002 | 1.097 ± 0.005 | 8.023 ± 0.332 | 0.18 ± 0.06 |
| F11 | 24.332 ± 0.312 | 0.775 ± 0.003 | 0.837 ± 0.003 | 1.077 ± 0.002 | 7.320 ± 0.633 | 0.18 ± 0.05 |
3.3.4. Friability
The friability test was carried out on all optimized formulation(s) to check their mechanical strength. The F10 and F11 formulations possessed excellent mechanical strength and were observed in the range of 0.18 ± 0.05% w/w to 0.82 ± 0.15% w/w. Highest friability was seen in the F1 formulation in which hard, rod-shaped pellets were formed as shown in Table 8.
3.3.5. Karl Fischer titration
The percentage moisture content of each formulation was evaluated by the Karl Fischer (KF) coulometric titration method. The results of percentage moisture content and percentage yield are tabulated in Table 9.
Table 9.
Percentage moisture content and percentage yield of all the formulation prepared while optimization of formula.
| Formulation code | % Yield | % Moisture content |
|---|---|---|
| F2 | 89.3 ± 0.15 | 3.31 ± 0.01 |
| F3 | 81.62 ± 0.21 | 3.44 ± 0.02 |
| F4 | 81.36 ± 0.21 | 3.52 ± 0.02 |
| F5 | 81.48 ± 0.17 | 4.21 ± 0.04 |
| F6 | 71 ± 0.28 | 4.23 ± 0.06 |
| F7 | 86 ± 0.08 | 4.27 ± 0.03 |
| F8 | 83.5 ± 0.09 | 4.31 ± 0.04 |
| F9 | 89.5 ± 0.06 | 4.32 ± 0.03 |
| F10 | 89.5 ± 0.05 | 4.32 ± 0.04 |
3.3.6. Entrapment efficiency
The entrapment efficiency of drug loaded pellets was determined and repeated thrice. The high water solubility of 5-FU and the physicochemical proportion of excipients play a key role in achieving high entrapment efficiency in the experimental formulation. Percentage Entrapment efficiency was calculated according to the formula given in section 2.4.6 and was found to be 87.5 ± 0.05% for uncoated pellets and 82 ± 0.05% for coated pellets. A little loss in entrapment efficiency in coated pellets attributed to drug loss associated with coating polymer would adhere to the coating surface during the coating process.
3.3.7. Uniformity of drug content
Drug content was found to homogenous in the formulation and the prepared pellets showed uniformity in drug content and was within the permissible range 99.89 ± 0.74.
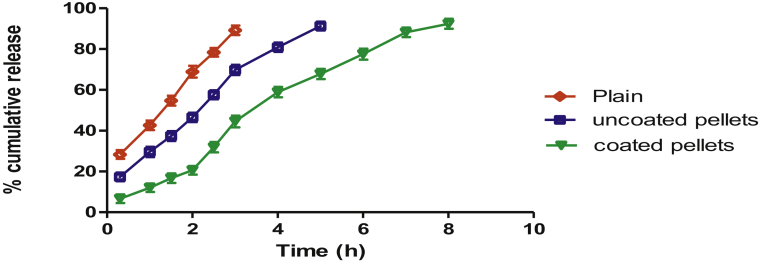
3.3.8. In-vitro drug release
The drug release data obtained for plain drug, uncoated and coated formulations are shown in Figure 4. The cumulative percent drug release versus time plot showing the comparison of uncoated and coated pellets, the release of anticancer drug from enteric coated formulation was prolonged over a period of 8 h. Eudragit is of polyanionic nature so it inhibits the burst release of 5-FU in gastric region. This was followed by the approximated zero-order release of the formulated system because that shows a steady drug release due to pH-dependent drug release behavior of Eudragit S 100. Due to the pH-sensitive nature of polymer used to coat the formulation the rate of drug release depends on the swelling of Eudragit S100 in an alkaline pH 7.4 (Pundlikrao P. et al., 2017). As the Eudragit S 100 swells in alkaline pH that will lead to the deterioration of the pores of coating layer. Then ultimately pore size and pore density was influenced the release rate. There was a significant difference in drug release pattern of the coated and uncoated 5-FU loaded anti-cancer pellets.
Figure 4.
In vitro drug release profile of the plain drug, 5-FU uncoated pellets and 5-FU enteric coated pellets in SGF and SIF. Plain drug shows release upto 3 h and 60% of drug gets released into SGF. In case of uncoated pellets the release gets sustained upto 5 h due to incorporation of drug into carrier system while coated pellets exhibit sustained release of 5-FU upto 8 h due to enteric polymer coating.
3.3.9. Compatibility studies and physical state of formulation (XRD and DSC)
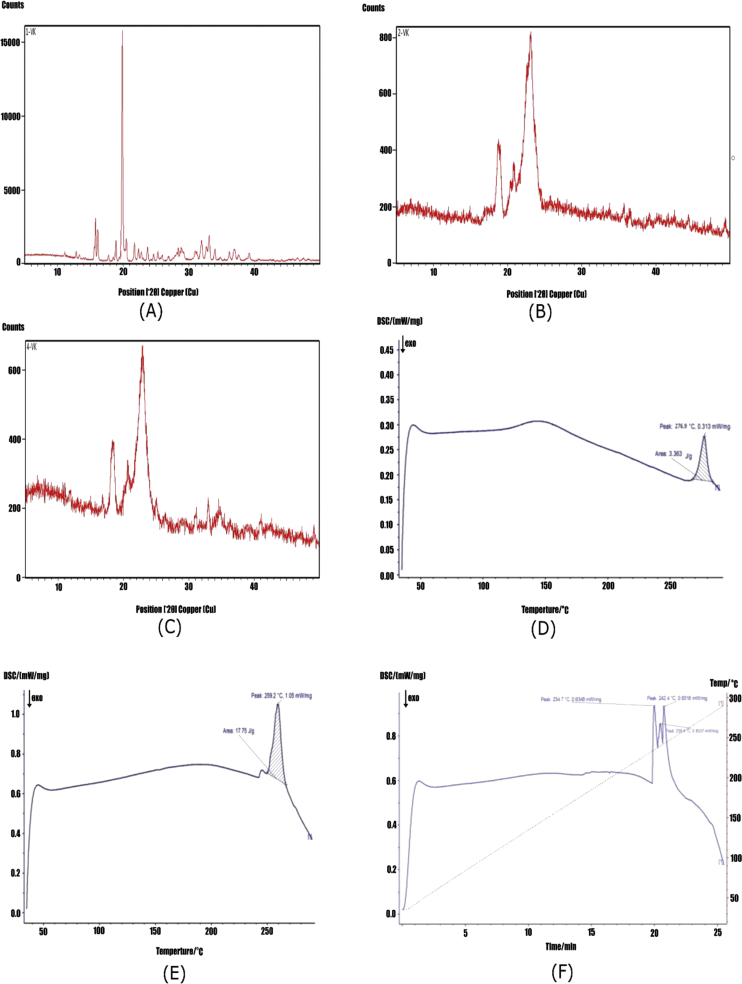
Diffractograms of the plain drug, uncoated 5-FU loaded formulation and coated 5-FU formulation had shown peaks at 23° which can be seen in Figure 5 (A), (B) and (C) respectively. Due to the amorphous nature of polymer used for coating, there is a little decrease in the peak intensity was observed in the X-ray diffractogram of enteric-coated polymeric pellets. . There was slight disappearance of the 5-FU peak because the encapsulated drug was dispersed at molecular level in the carrier system which indicates the uniform coating of the formulation with the enteric polymer having amorphous nature and also indicates the crystalline state of the encapsulated 5-FU (Lu et al., 2016). Eudragit coated pellets DSC thermogram had shown peaks at 234.7 °C and 239.4 °C and 242.4 °C the area where 5-FU was present as seen in Figure 5 (c). These two compatibility studies further strengthen the evidence that there is chance of encapsulation of drug into the carrier system and the compatibility of polymer and drug.
Figure 5.
Comparative assessment of physical and thermal properties between plain drug, 5-FU uncoated and 5-FU coated pellets. XRD diffractogram of (A) plain 5-FU, (B) uncoated 5-FU loaded formulation, (C) 5-FU-Eudragit S-100 loaded formulation, plain drug the peak shows very sharp at 23° due to crystalline nature of drug but due to amorphous nature of MCC and eudragit S 100 Figure 5 (B) and (C) shows decreased intensity of peaks at 23°. DSC thermogram of (D) plain 5-FU, (E) uncoated formulation and (F) coated formulation. Thermograms indicating the partial conversion of the crystalline form of the drug during the pelletization process.
3.3.10. Cytotoxic study (MTT assay)
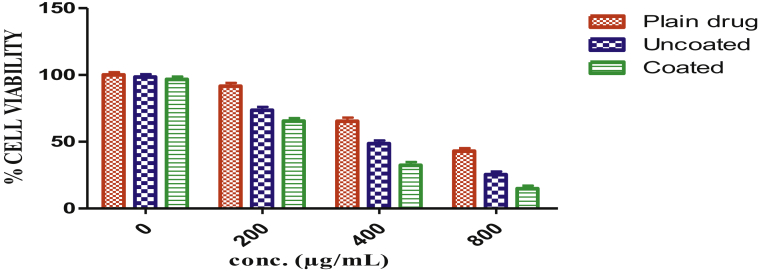
The growth medium RPMI 1640 was routinely used for human epithelial colorectal adenocarcinomas cell line EAC cells and the growth medium was supplemented with 10% FBS (fetal bovine serum), 1% Antibiotic antimycotic solution, and 1% L-glutamine. For maintenance of the cell lines, they were kept in humidified 5% CO2 incubator and temperature was maintained at 37 °C till a good confluency of cells observed. After the incubation for 24 h of EAC cells the cytotoxic activity of the plain drug, drug-loaded uncoated formulation and the drug-loaded coated formulation was determined using MTT assay. Cytotoxicity of drug-loaded uncoated pellets and drug-loaded coated pellets were investigated in comparison with different concentrations of plain anti-cancer drug to explicate the anticancer activity of developed formulation with respect to dose and time (Elyagoby et al., 2013). A positive correlation was observed. As the concentration of anticancer drug and time of contact with formulation increases the cytotoxic effect also increases. But as in the comparison of the entire formulations, drug loaded coated formulation showed a drastic increase in cytotoxicity at the same conditions. The plain anticancer drug at the highest tested dose that is 800 μg/ml, showed 42.9% cell viability after 24 h. The final formulation showed higher cytotoxicity at corresponding concentration of 5–FU having a percentage cell viability of 14.8% after incubation for 24 h (Figure 6) (Kim. et al., 2010).
Figure 6.
Percent cell viability of EAC cells treated with various formulations after 24 h indicating the increased potency of anticancer oral therapy in the presence of chelating agents due to synergism.
3.4. In vivo studies
3.4.1. Analytical method development for estimation of 5-FU
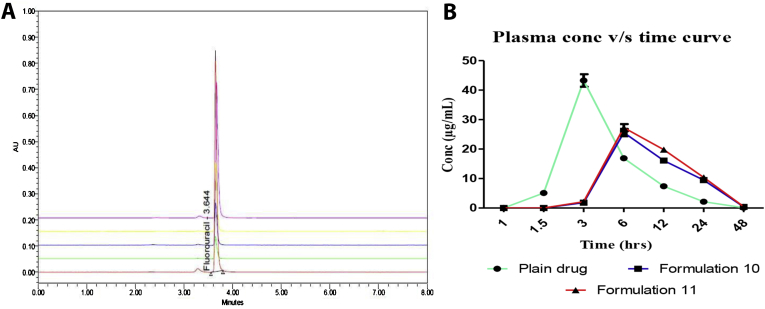
A sensitive analytical method for the determination of 5-FU has been developed and validated as per the method described earlier; Figure 7a depicts the overlay spectra of absorbance peaks of drug concentration in plasma. HPLC method was developed in animal plasma for the estimation of 5-FU. A linear response was obtained in the range of 0.1–10 μg/ml with correlation coefficient r2 = 0.999. The reproducibility of the analytical method was measured in terms of linearity, Lower Limit of Quantitation (LLOQ), specificity and precision. The minimum limit of reliable quantification within the range 0.1–10 μg/ml was assessed, and the minimum limit found to be 0.1 μg/ml with a correlation coefficient r2 = 0.999. The precision of developed analytical method for intra and inter-day was analyzed as the percent variation over the concentration range of LLOQ and (medium quality control) MQC. The accuracy and precision at LLOQ were seemed to be 98.38% and 2.55% respectively. The accuracy under the experimental stress condition for 5-FU was found to be 103.2% as low quality control (LQC) and 102.02% for MQC. The above observations indicated that the developed analytical method was suitable to determine the plasma concentration at different time intervals following administration of 5-FU.
Figure 7.
(A): Overlay spectra of 5-FU used for estimation of plasma drug concentration and (B) Represents plasma drug concentration against different time point.
From the given results, it was observed that the developed optimized formulation showed extended plasma concentration with T1/2 7.05 over the plain drug T1/2 5.60 (Table 10). Further, the plasma concentration took a longer time to reach its maximum value in the case of 5-FU-Eudragit S100 formulation followed by plain drug showing sustained release in behavior in the experimental formulation. Observed mean Cmax 27.25 μg/ml and longer half-life of Eudragit S 100 coated formulation indicated lower systemic drug exposure, which may reduce the risk of drug dependent systemic toxicity (Figure 7b), however further in-depth toxicological study is required to support the statement. Pharmacokinetic finding established the controlled drug release behavior of experimental formulation which offered a more effective modality of chemotherapy by providing higher local concentration of the drug at the target sites and minimizing systemic drug absorption.
Table 10.
Plasma Pharmacokinetic parameters of different formulations.
| Parameters | (F11) | (F10) | Plain drug (5-FU) |
|---|---|---|---|
| Cmax (μg/ml) | 27.25 ± 0.35 | 25.60 ± 0.28 | 43.26 ± 0.87 |
| Tmax (hour) | 6.00 ± 0.14 | 6.00 ± 0.11 | 3.00 ± 0.21 |
| T1/2 (hour) | 7.05 ± 0.19 | 7.03 ± 0.21 | 5.60 ± 0.38 |
| AUCtotal (μg/μl/h) | 444.12 ± 11.12 | 389.84 ± 7.89 | 258.16 ± 2.23 |
| MRT | 16.31 ± 0.17 | 16.31 ± 0.21 | 9.43 ± 0.54 |
Abbreviations: AUC= Area under the curve, Cmax= maximum concentration of the drug, Tmax= Peak time when drug achieves Cmax, MRT= Mean residence time.
3.4.2. X-ray study to determine the targeting potential
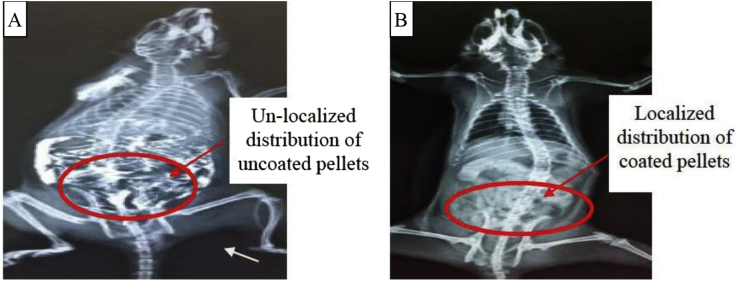
X-ray studies have shown that the Eudragit S 100 coated formulation successfully targeted to the colon. Polyanionic nature of Eudragit retarded the release of encapsulated material in upper GIT. pH-dependent drug release behavior of Eudragit ensured colon-specific drug delivery as shown in Figure 8B. It can be concluded from the X-ray images that the enteric coated pellets release the drug in the colon. Hence, it proves that the formulation is ideal for colon targeting.
Figure 8.
(A): X- Ray image of rat without coating system and (B) X- Ray image of rat with coating system indicating the distribution of prepared formulation after oral administration.
3.4.3. Tumor regression studies
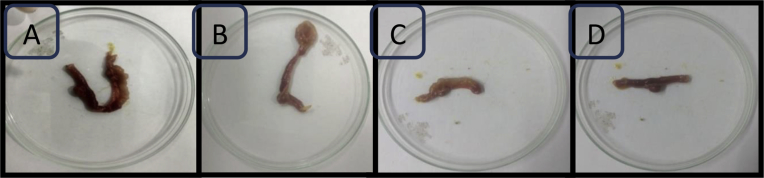
Tumor regression studies were performed on the 21st day with the help of Vernier Caliper. A tumor excised on the 21st day from various groups is shown in Figure 9 (A), (B), (C) and (D). Results obtained from the tumor regression studies indicated that there was a notable difference in tumor volume.
Figure 9.
Tumor regression analysis (A) Disease control group, (B) 5-FU treated group, (C) Uncoated pellets treated group and (D) Coated pellets treated group. Result of tumor volume indicating the increased therapeutic efficacy of coated pellets compared to disease control group.
The volume of induced tumors in different groups is shown in Table 11. The decrease in tumor volume in all experimental groups indicated the presence of 5-FU in the active state in the final formulation (He et al., 2008). The group treated with uncoated pellets resulted in not much reduction in tumor volume which might be due to the release of drug in upper GIT. The group treated with coated formulation showed increased therapeutic efficacy against colon cancer as compared to plain drug and uncoated formulation. The coated pellets decreased the tumor size for 5-FU to 3.42 ± 0.32 mm3 relative to uncoated pellets having the tumor volume of 3.97 ± 0.35 mm3 (P < 0.01), whereas the tumor size in plain drug decreased to 3.75 ± 0.28 mm3after 21 days (P < 0.05).This might be due to the oral delivery of the formulation and synergistic action of the drug and the chelating agent. The phytic acid might show their action by chelation of manganese and then inhibiting the SOD enzyme. Also, the cell viability studies have shown that the coated formulation had lower cell viability than the formulation with the plain drug.
Table 11.
Tumor volume comparing the effectiveness of the different investigated treatment (Data are presented as mean ± SD. **represents highly significant difference in tumor volume in coated pellets compare to uncoated pellets (p < 0.01). * represents statistical difference in tumor volume between plain drug and uncoated pellets (p < 0.05).
| S.No. | Group | Tumor volume (mm3) |
|---|---|---|
| 1. | Disease control | 4.5 ± 0.25 |
| 2. | Plain 5-FU | 3.75 ± 0.28* |
| 3. | Uncoated pellets | 3.97 ± 0.35 |
| 4. | Coated pellets | 3.42 ± 0.32** |
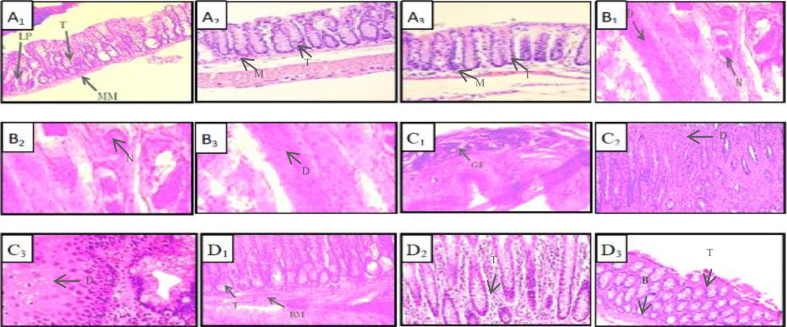
3.4.4. Histopathological study
The tumor was frozen at -20 °C and then examined under a bright-field microscope at a magnification level of 100X to check the synergistic effect of the prepared formulation in comparison with plain anticancer drug. The test was repeated 3 times for test samples to get more precise results. This study indicates that there is drastic damage to the lamina propria as shown in diseased control specimen (Figure 10 A and B). The targeted release of the anticancer drug with chelating agent shows a better restoration of basement membrane and lamina propria than the plain anticancer drug (Figure 10 C and D). The sample treated with the final formulation shows a lamina propria that regained its original finger-like structure, indicating a synergistic effect of final formulation. Similar observations were reported by Foroushani and co-workers, wherein grapheme oxide conjugated polydopamine shows improved localization of pay-load without any sign of tissue abnormality (Foroushani et al., 2019).
Figure 10.
Histopathological analysis, A1), A2),A3) Normal colon, B1), B2), B3) Disease control, C1), C2), C3) Plain 5-FU and D1), D2), D3) drug loaded coated formulation. The results showing the responses of prepared formulations to the colon cancer characterized by finger-like tubular structures in lamina propria.; Abbreviations: LP: lamina propria, T: finger-like tubular structure, MM: muscularis mucosa, D: intraepithelial dysplasia, N: hyperchromatic nuclei, GE: glandular epithelium, BM: basement membrane.
4. Conclusion
Metal chelators are found to be an attractive channel to improve the cytotoxic activity of the chemotherapeutic drug. The study further highlights inhibiting redox enzymes, which could be an effective strategy to break the defense mechanism of cancer cells, leading to improve therapeutic activity. Delivery of anticancer drugs using pH-sensitive polymer, ensure high local drug concentration to achieve selective cytotoxic activity and also minimizes systemic exposure of drug, could help to reduce the risk of drug dependent systemic toxicity. The present studies open a new avenue for effective treatment in colon cancer. However, organ-specific toxicity will need to be investigated for further clinical prospects.
Declarations
Author contribution statement
Veerpal Kaur, Amit Goyal, Goutam Ghosh, Sudamsi Chandra, Goutam Rath: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al-Hashemi H.M.B., Al-Amoudi O.S.B. A review on the angle of repose of granular materials. Powder Technol. 2018;330:397–417. [Google Scholar]
- Alibolandi M., Rezvani R., Farzad S.A., Taghdisi S.M., Abnous K., Ramezani M. Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int. J. Pharm. 2017;532(1):581–594. doi: 10.1016/j.ijpharm.2017.09.039. [DOI] [PubMed] [Google Scholar]
- Amidon S., Brown J.E., Dave V.S. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16(4):731–741. doi: 10.1208/s12249-015-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli A., Schiattarella A., Bonelli P., Tuccillo F.M., Buonaguro F.M., Mancini A. BioMed research international; 2014. The functional role of MnSOD as a biomarker of human diseases and therapeutic potential of a new isoform of a human recombinant MnSOD; p. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Lockman P.R., Atwood C.S., Hsu C.H., Gupte A., Allen D.D., Mumper R.J. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer's and other CNS diseases. Eur. J. Pharm. Biopharm. 2005;59(2):263–272. doi: 10.1016/j.ejpb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Elyagoby A., Layas N., Wong T.W. Colon-specific delivery of 5-fluorouracil from zinc pectinate pellets through in Situ intracapsularethylcellulose–pectin plugs formation. J. Pharm. Sci. 2013;102(2):604–616. doi: 10.1002/jps.23388. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., Alsharif U., Alvis-Guzman N., Amini E., Anderson B.O., Aremu O. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA oncology. 2018;4(11):1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroushani M.S., Shervedani R.K., Kefayat A., Torabi M., Ghahremani F., Yaghoobi F. Folate-graphene chelate manganese nanoparticles as a theranostic system for colon cancer MR imaging and drug delivery: in-vivo examinations. J. Drug Deliv. Sci. Technol. 2019;54:101223. [Google Scholar]
- He W., Du Q., Cao D.Y., Xiang B., Fan L.F. Study on colon-specific pectin/ethyl cellulose film-coated 5-fluorouracil pellets in rats. Int. J. Pharm. 2008;348(1-2):35–45. doi: 10.1016/j.ijpharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hileman E.A., Achanta G., Huang P. Superoxide dismutase: an emerging target for cancer therapeutics. Expert Opin. Ther. Targets. 2001;5(6):697–710. doi: 10.1517/14728222.5.6.697. [DOI] [PubMed] [Google Scholar]
- Hwang I.T., Chung Y.M., Kim J.J., Chung J.S., Kim B.S., Kim H.J., Kim J.S., Do Yoo Y. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem. Biophys. Res. Commun. 2007;359(2):304–310. doi: 10.1016/j.bbrc.2007.05.088. [DOI] [PubMed] [Google Scholar]
- Kamal T., Sarfraz M., Arafat M., Mikov M. Cross-linked guar gum and sodium borate based microspheres as colon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak. J. Pharm. Sci. 2017;30 [PubMed] [Google Scholar]
- Kamalaldin N.A., Jaafar M., Zubairi S.I., Yahaya B.H. 2017. Physico-Mechanical Properties of HA/TCP Pellets and Their Three-Dimensional Biological Evaluation in Vitro. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee J.H., Kim S.J., Oh G.S., Moon H.D., Kwon K.B., Park C., Park B.H., Lee H.K., Chung S.Y., Park R. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci. 2010;30(11):3933–3946. doi: 10.1523/JNEUROSCI.6054-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Kan S., Zhao Y., Zhang W., Liu J. Novel naproxen/esomeprazole magnesium compound pellets based on acid-independent mechanism: in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2016;42(9):1495–1503. doi: 10.3109/03639045.2016.1151029. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy R., Mohd E.S.A.N. Phytic acid (MYO-INOSITOL hexaphosphate)- a promising pharmaceutical agent: a review. Asian J. Pharmaceut. Clin. Res. 2018;11(11):42–46. 2018. [Google Scholar]
- Pandey S., Swamy S.V., Gupta A., Koli A., Patel S., Maulvi F., Vyas B. Multiple response optimization of processing and formulation parameters of pH sensitive sustained release pellets of capecitabine for targeting colon. J. Microencapsul. 2018:1–13. doi: 10.1080/02652048.2018.1465138. [DOI] [PubMed] [Google Scholar]
- Perry J.J.P., Shin D.S., Getzoff E.D., Tainer J.A. The structural biochemistry of the superoxide dismutases. BiochimicaetBiophysicaActa (BBA)-Proteins and Proteomics. 2010;1804(2):245–262. doi: 10.1016/j.bbapap.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Türk M., Nyman M., Sandberg A.S. Binding of Cu2+, Zn2+, and Cd2+ to inositol tri-, tetra-, penta-, and hexaphosphates. J. Agric. Food Chem. 1998;46(8):3194–3200. [Google Scholar]
- Pundlikrao P., Rajput P.M. Stability study of microemulsion and their use in formulation of pellets with enhanced solubility and dissolution efficiency of nevirapine. Indian J. Nov. Drug Deliv. 2017;9(4):223–235. [Google Scholar]
- Reddy N.R., Sathe S.K., Salunkhe D.K. Vol. 28. Academic Press; 1982. Phytates in legumes and cereals; pp. 1–92. (Advances in Food Research). [DOI] [PubMed] [Google Scholar]
- Shanmugam S., Reddy J.S., Vetrichelvan T. 2013. Formulation and in Vitro Evaluation of 5-Fluorouracil Microcapsules by Using Different Methods of Micro Encapsulation. [Google Scholar]
- Sharma A., Goyal A.K., Rath G. Development and characterization of gastroretentive high density pellets lodged with zero valent iron nanoparticles. J. Pharm. Sci. 2018 doi: 10.1016/j.xphs.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Sreelatha D., Brahma C.K. Colon targeted drug delivery–a review on primary and novel approaches. J. Glob. Trends Pharm. Sci. 2013;4(3):1174–1183. [Google Scholar]
- Tan Y.L., Huang C.H., Guo Z.X., Yu J. Morphology and mechanical properties of polyamide 6/polystyrene blends prepared by diffusion and subsequent polymerization of styrene in polyamide 6 pellets. Materials. 2018;11(5):776. doi: 10.3390/ma11050776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuik F.E., Nieuwenburg S.A., Bardou M., Lansdorp-Vogelaar I., Dinis-Ribeiro M., Bento M.J., Zadnik V., Pellisé M., Esteban L., Kaminski M.F., Suchanek S. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019 doi: 10.1136/gutjnl-2018-317592. gutjnl-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wairkar S.M., Gaud R.S. Formulation and IN-vitro characterisation OF sustained release matrix pellets OF nateglinide. Int. J. Pharm. Sci. Res. 2016;7(7):2925. [Google Scholar]
- Zhao H., Sun D., Tang Y., Yao J., Yuan X., Zhang M. Thermo/pH dual-responsive core–shell particles for apatinib/doxorubicin controlled release: preparation, characterization and biodistribution. J. Mater. Chem. B. 2018;6(46):7621–7633. doi: 10.1039/c8tb02334d. [DOI] [PubMed] [Google Scholar]