Abstract
The present work investigated the ability of algal biomass Chlorella vulgaris to remove mercury from aqueous solutions. The mercury biosorption process was studied through batch experiments 35 °C temperature with regard to the influence of contact time, initial mercury concentration, pH and desorption. The maximum adsorption capacity was registered at pH 6. The adsorption conduct of Hg(II) was defined by pseudo second order well rather pseudo first order as the experimental data (qe) come to an agreement with the calculated value. The kinetics of adsorption was fast and a high capacity of adsorption occurred within only 90 min. The adsorption data were signified by many models but Langmuir (qmax = 42. mg g−1) & Freundlich fitted well having regression coefficients near to unity. The thermodynamic parameters were also suited well as negative value of free energy cope up to spontaneity, positive value of the randomness described by ΔS attributed to affinity of Hg+2 towards algal bioadsorbant and high positive value of heat of enthalpy designates that the adsorption process is expected due to robust interactions between the Hg(II) ions and various functional groups on surface of algal bioadsorbant. Field emission scanning electron microscopy integrated with energy dispersive X-ray spectroscopy analysis before and after adsorption of Hg(II) reveals the adsorption of metallic ions over the surface. FTIR study supported the existence of various functional groups (carboxylix, amines, hydroxyls, amides etc.) helped in adsorption. Continuous adsorption desorption experiments proved that algal cells was excellent biosorbents with potential for further development.
Keywords: Environmental science, Engineering, Toxicology, Environmental engineering, Environmental toxicology, Sustainable development, Waste, Wastewater management, Water pollution, Water quality, Thermodynamics, Mercury ion, Adsorption isotherms, Biosorption, Chlorella vulgaris
Environmental science; Engineering; Toxicology; Environmental engineering; Environmental toxicology; Sustainable development; Waste; Wastewater management; Water pollution; Water quality; Thermodynamics; Mercury ion, Adsorption isotherms; Biosorption; Chlorella vulgaris.
1. Introduction
Heavy metals are natural elements of the earth but negatively affect aquatic life as well as human beings if found more than a permissible limits (Sathe et al., 2020; Souri et al., 2019; Goswami et al., 2019a, b; Areco et al., 2012). They enter in plant, animals and human through inhalation and aqueous media. Industrial operations like mining, metallurgy, electroplating etc. are responsible to discharge these toxic metals extensively in aqueous medium and industrial effluents (Kushwaha et al., 2018, 2017; Bind et al., 2018; Liu et al., 2009). Heavy metals like cobalt (Co), chromium (Cr), cadmium (Cd), mercury (Hg) and lead (Pb) are known to be toxic on the ecosystem by accumulating in food chain at various tropic levels (Kushwaha et al., 2019, 2015; Hatamian et al., 2019; Souri et al., 2018). These elements are non biodegradable and once enter in our body they neither degrade nor destroyed, cause serious threat to our life (Goswami et al., 2018a, Goswami et al., 2018b; Kumar et al., 2016). EPA (Environmental Protection Agency) find mercury as highly threating metal due to its perpetual and bioaccumulating nature in the ecosystem. It is third most toxic metal decalered by International agency of 21st century. The elemental form of mercury (Hg) affect living system by its transport routes whereas the most toxic form is oxidized Hg (II) which binds to the cysteinein proteins causing mercury poisoning affecting neural system, cardiovascular system, bones and nephrons of the body (USEPA, 1997). LED's, CFL, thermometer, measuring, wiring and control devices, paper and pulp industry, oil refining and battery manufacturing industries are important sources of mercury pollution. Bureau of Indian standards set mercury permissible limit in drinking water as 1 μg L−1 (BIS, 2005).
The accumulation of the heavy metals in food chain demands attention to develope the sustainable techniques for its effective remove from the industrial effluents. Several techniques viz. adsorption, coagulation, advanced oxidation and membrane separation have been used for the removal of heavy metals from the aqueous system (Alyüz and Veli, 2009). Among these methods biosorption is the best and economical method as biosorbents are low cost materials, easily available and highly efficient (Barakat, 2011).
Extensive research are going on treating pollutents from liquid medium by various adsorbant viz., microorganisms (bacteria, fungi, algae), industrial wastes, lignocellulosic materials, biopolymers and nanoadsorbants etc. (Goswami et al., 2017a, b; Fu and Wang, 2011). Few of the well-known utilized adsorbents particularly for heavy metals contaminated wastewater are chitosans, nanoadsorbents, barks, wastes from olive oil industry, coconut based materials, agricultural peels, zeolites, clay soils and betonites and vermiculites (Wuana and Okieimen, 2011). Researchers have been recently published their work with primary goal of removal of toxic heavy metals from industrial effluents using adsorbent materials.
Algae have been widely studied for heavy metal removal because of their global occurrence. A number of microalgal strains (Chlorella vulgaris, Chlorella fuscas, Spirogyra species, Spirulina sp., which are potentially suitable for heavy metals removal in aqueous solution, were used in several studies and showed varying removal efficiencies (Bailey et al., 1999). Chlorella vulgaris has recently gained greater attention for the treatment of heavy metal contamination in aqueous solutions. Chlorella vulgaris is a chlorophyll containing organism, autotrophic in nature size vary from microscopic to giant macroscopic play a significant role in food chain and in maintaining the oxygen supply on our planet, and can easily grow anywhere (Yadav et al., 2013). Many researchers have reported that algal cells and may have a very high capacity for binding with metals due to the presence of polysaccharides, proteins, or lipids on the surface of cell walls, possessing the functional groups viz. aminos, hydroxyls, carboxyls and sulfhydryl, which can act as binding sites for metals (Kumar et al., 2019; Souri and Hatamian, 2019).
The aim of this work is to study the ability of Chlorella vulgaris to remove mercury from aqueous solutions. It identifies the functional groups involved in the interaction between algae and metal (Hg (II)). The purpose of this study is to develop a low cost adsorbent for treatment of toxic heavy metal (Hg) from industrial effluents. Algal biomass (Chlorella vulgaris) as a potential bioadsorbant was characterized by FTIR (Fourier transform infrared spectroscopy) for various functional groups, SEM (Scanning electron microscope) equipped with EDX (Energy dispersive spectroscopy) for morphological characters and elemental analysis along with physical, chemical and thermal parameters. The effects of several operating parameters such as pH, initial Hg (II) ion concentration, contact time, and adsorbent dosage were investigated in batch system. Adsorption isotherms and different kinetic models were examined to correlate the experimental data. The results of this work would contribute to a better understanding of the metal uptake by Chlorella vulgaris and can be beneficial in the development of potential biosorbents with high capacity for heavy-metal (mercury) uptake from aqueous environment. Investigation study of regeneration also have performed.
2. Materials and methods
2.1. Materials
All the chemicals and reagents used were procured from Merck (Mumbai, India). A stock solution (1000 ppm) of Hg (II) was prepared by dissolving approximately 1.35 g of mercury (II) chloride in 1.0 L of Milli-Q deionized water. Aqueous solutions of various concentrations were prepared from HgCl2 and used as a source for Hg (II). The solutions were then diluted to the desired concentrations and analyzed.
2.2. Microalgae strain
The microalga Chlorella vulgaris UTEX 2714 was used for this study. The strain was obtained through the Culture Collection of Algae of IARI New Delhi and was revived in 10 ml Fog's Medium suitable for Chlorella vulgaris.
2.3. Preperation of adsorbent biomass
The algal biomass was deactivated by heating in an autoclave at 121 °C for 10 min (Gupta et al., 2015). Biomass was then harvested by filtering the cultured medium through cellulose nitrate Whatman membrane filters with pore size of 45 μm, and washed with deionized water. The biomass was then sun dried for 3 days, followed by oven drying at 55 °C for 24 h, and ground in a stone mortar pestle to get uniform particle size. The sieved material (195-148, 148-132, and 132-98 μm) was stored in air tight container for further use.
2.4. Adsorption experiments
Adsorption experiments were conducted at various solutions pH (1-8), dose (0.5–2.0 g), contact time (10 - 120 min) and initial concentration (10-200 mg/L) under batch mode. The solution pH was maintained using 0.1 M NaoH and 0.1 M H2SO4 before adsorption experiment using Labman digital pH meter (LBPH-10, India). Adsorption experiment were performed in 250 ml volumetric flask using 100 ml Hg (II) solution at room temperature i.e., 35 °C. The equilibrium time was maintained at 90 min in a rotary incubator at an agitation speed of 120 rpm. After completing the experiment the bioadsorbent was filtered and analyzed by spectrophotometric method using 2-Acetylpyridine thiosemicarbazone (APT) (Admasu et al., 2016). Double beam spectrophotometer (2375, Electronics India, India) was used for absorbance measurement. Experiments were carried out in tripilicate and for data analysis, mean values were used.
The mercury (Hg+2) adsorbed by the adsorbent was calculate using the equations (Supplementary file).
2.5. Elemental analysis
The elemental compositions of the algal biosorbant were examined by ELTRA AD 2400 elemental analyzer, Germany. The percentage (%) of carbon (C), Hydrogen (H), Nitrogen (N) and oxygen (O) in the sample was determined accordingly.
2.6. Total ash content, density and surface area analysis of algal bioadsorbant
The ash content was determined by putting the algal bioadsorbant in the muffle furnance at 500 °C for 10 h. The Bulk density (weight per unit volume of algal biosorbant) was calculated by filling known weight of algal adsorbant in the calibrated glass cylinder. To determine surface area measurements, Brunauer-Emmet-Teller (BET) analysis was performed using BET surface analyzer (Horiba scientific SA-9600 series) (Goswami et al., 2019c).
2.7. FTIR and SEM analyses
To examine the functional groups of virgin algal bioadsorbant and Hg(II) loaded algal bioadsorbant were further recorded by using Fourier transform infrared (FTIR) spectrophotometer (Bruker Tensor 27, Germany) in the range of 400–4000 cm−1. The morphological examination of algal bioadsorbant was performed by scanning electron (SEM) microscope (Zeiss, Sigma VP, Germany).
2.8. Desorption studies of algal bioadsorbant
Recycling of algal bioadsorbant is necessary to make the bioadsorbant industrially feasible. Repeated sorption-desorption experiments were carried out using the same bioadsorbant to examine the extent of sorption. It was studied in batch system in which algae (1 g) loaded with merucury (100 ppm) were employed in desorption medium of 100 ml in 150 ml conical flask for one hour at 35 °C and was stirred at 120 rpm. All glasswares were dunked in 0.1 N HNO3 and cleaned with water to remove metal salt residue if any for highly sensitive experiments with metals. Desorption ratio was calculated as:
| Desorption ratio = (Amount of metal ions desorbed/ Amount of metal ions adsorbed) × | 100 |
3. Results and discussion
3.1. Characterization of the algal bioadsorbant
3.1.1. BET surface area and elemental analyses
The accessibility of surface of organic materials is a key factor in heavy metal adsorption. The mean surface area of algal bioadsorbant was analysed 12.03 m2 g−1 which is far better than many bioadsorbant recorded in the studies till now. It has good porosity of surface, pore structure, pore volume (0.197 cc g−1), pore size (107-186 Å). These factors provide significant insight for adsorption of Hg (II) over algal bioadsorbant. The ionic radii of Hg (II) in the present study was 0.3 Å, very much suits the adsorption process with mean pore size (147 Å) of algal adsorbant.
The major elements (Carbon (C): Hydrogen (H): Nitrogen (N): Sulphur (S): Oxygen (O)) in the powered algal bioadsorbant by percentage basis were presented in Table 1. The percentage of important contributed elements showed the various type of functional groups present on the algal bioadsorbant surface viz: carboxyl, amines, alcohol, phenol etc. responsible for Hg (II) adsorption.
Table 1.
Percentage of Major Elements in powered form of algal bioadsorbant.
| Element | Carbon | Hydrogen | Nitrogen | Sulphur | Oxygen |
|---|---|---|---|---|---|
| Percentage | 48.94 | 7.89 | 5.76 | .93 | 36.93 |
3.1.2. Scanning electron microscopy
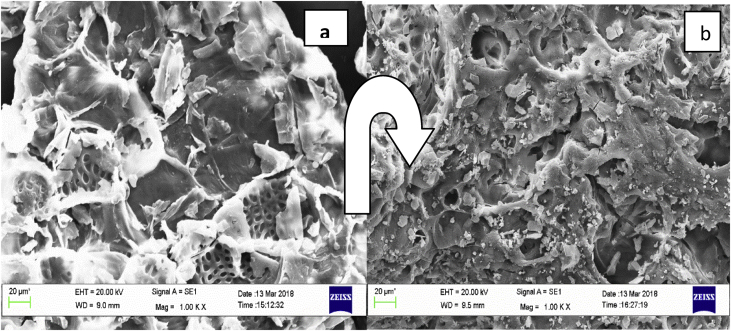
The surface morphology of plain algal and mercury loaded algal biomass and image with EDX graph was showing in (Figure 1 (a) & (b)) and (Figure 2), respectively. Presence of hetrogenous pores structure, its previous structures with rough cavities which are essential for prospective adsorbent were seen in unloaded SEM (Figure 1(a)) micrograph (Manikandan et al., 2016; Yadav et al., 2015). Loaded particles (Figure 1 (b)) are visually assume throughout the image with close, compact and smoother structure due to hostage of Hg (II). Emergence of new peak of Hg (II) along with peaks of virgin biomass in the EDX graph (Figure 2) confirm the presence of metal ions Hg (II). EDS layered and compositional images showed the presence of Hg (II) along with other elements validate the adsorption of metal ions strongly.
Figure 1.
SEM micrographs of (a) Raw algal biomass and (b) Mercury loaded biomass.
Figure 2.
EDX graph and mapping of Hg(II) adsorbed algal biomass; (i) EDX spectra of the Hg-loaded biomass, (ii) elemental mapping of the biomass and (iii) Hg-mapping on to the adsorbed biomass.
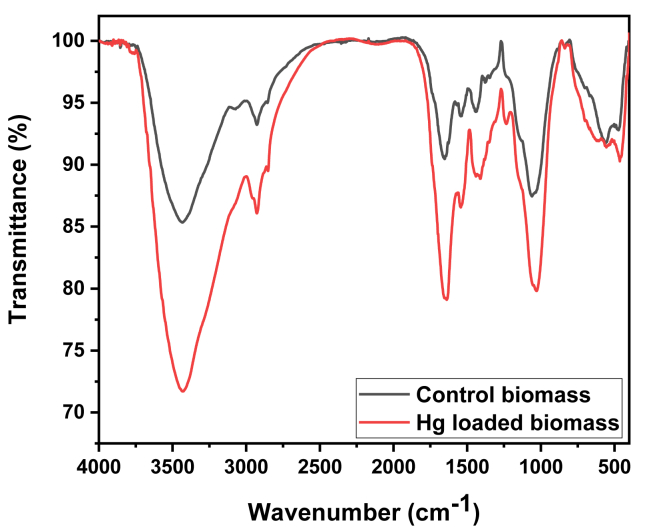
3.1.3. FTIR analysis
Different types of vibrational frequencies because of different functional groups were shown in FTIR spectra of the algal bioadsorbant and is presented in Figure 3. The strong, extended (2934 cm−1) and broad adsorption peak belong to N-H stretching and bending vibrations (Goswami et al., 2017c). The alcohol group (-OH) also come in this range. The stretched peak seen to be shifted in loaded biomass compared to unloaded algal biomass (Gupta et al., 2014). The peak at 1614.8 cm−1 attributed to (C=O) carbonyl group shifted to 1770 cm−1. Larger shifting clearifiy the strong bonding of metal ions (Hg(II)) to the algal surface. The shifting of band from 1498 cm−1 to 1400 cm−1 corresponds to –CH stretching vibrations (Bhasney et al., 2019). The peak at 1210.10 cm−1 attributed to –SO3 with stretching of aliphatic amines (C-N) (Plazinski et al., 2009).
Figure 3.
FTIR spectra of Chlorella biomass & Hg(II) loaded biomass.
3.2. Effect of various process parameters on adsorption of Hg (II) on algal bioadsorbant
3.2.1. Role of pH on adsorption of Hg (II) on algal bioadsorbant
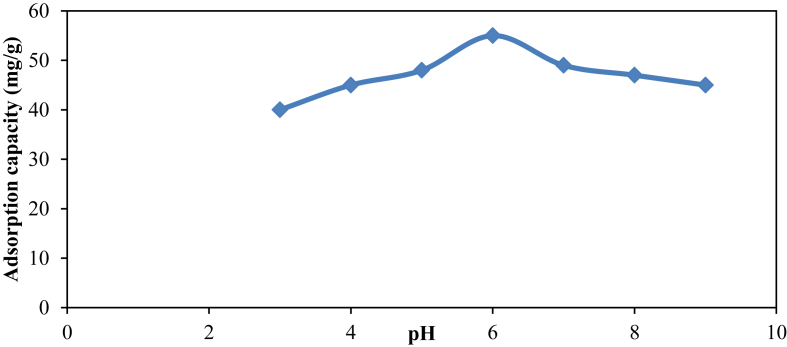
The effect of pH on Hg (II) removal was examined by batch sorption studies. The study was performed using algal biomass at the initial concentration was 100 μg/L, biomass dose of 1 g and contact time of 120 min. Hydrogen ion concentration plays important task in affecting the adsorption of heavy metals over adsorbant because it itself compete to the metal ions while adsorption. Degree of ionization of H+ and surface charge ratio of adsorbant affect the binding of metal ions (Mason et al., 1996). It is evidenced from Figure 4 that Hg (II) adsorption was maximum at pH 6, study was conducted between pH 3 to 8. The inference of this result suggested that at lower pH (<6) hydrogen ion concentrations were busy with the negative ligands of the adsorbant causing low adsorption as less number of vacant sites were available for heavy metal ions (Gupta et al., 2011). pH around 6 fascilitate deprotonation of negative ligands which created vacant sites to promote adsorption. Thus, in further experiments the initial pH was maintained at pH 6. This study is similar to the work reported earlier by Gupta et al. (2011). Efforts were not taken to maintain the pH during the course of the experiment.
Figure 4.
Effect of pH on adsorption study of Hg(II) ions on algal bioadsorbant: pH = 6, Ci = 100 mg/L, dose = 1 g/L, contact time = 2 h at 35 °C.
3.2.2. Role of adsorbant dosage on adsorption of Hg(II) on algal bioadsorbant
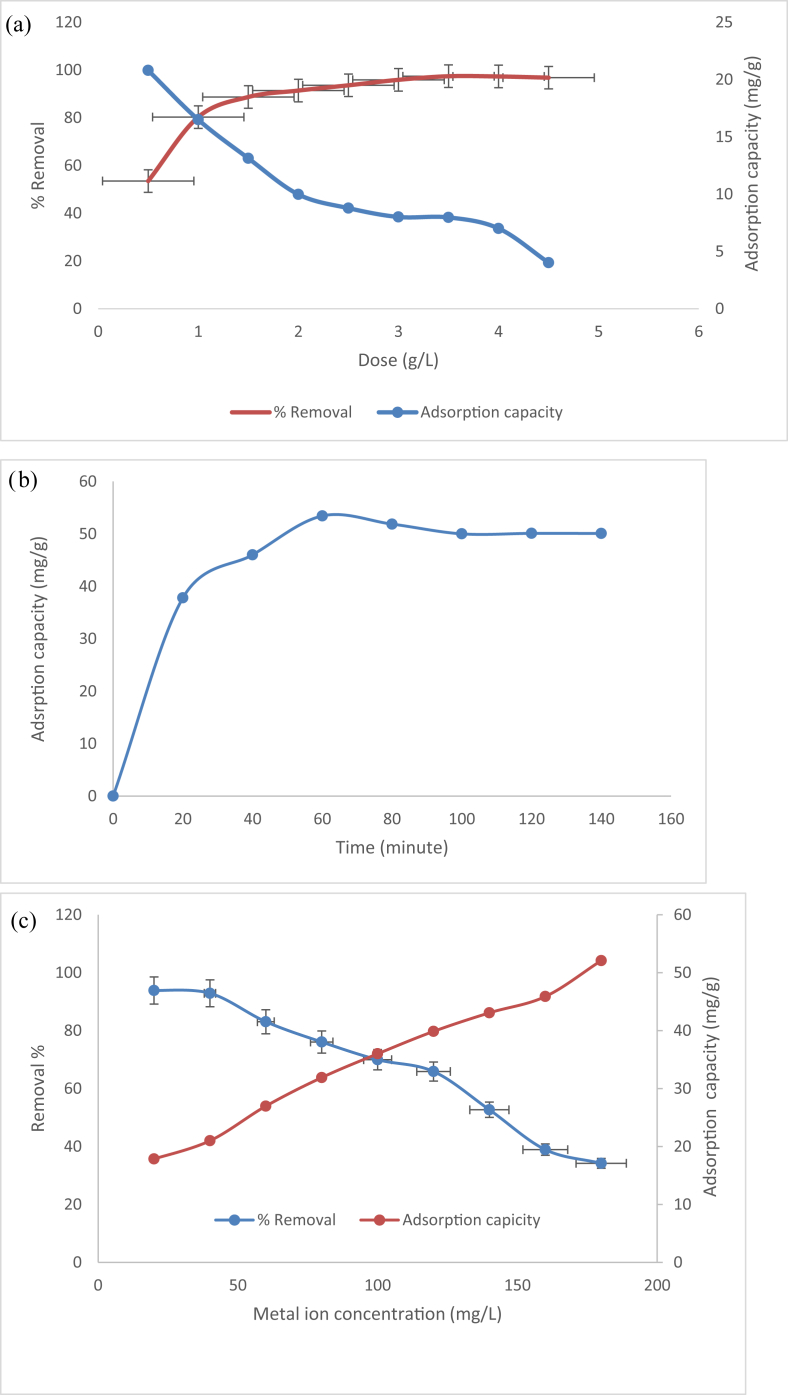
Increase of percentage removal of Hg (II) was seen with increasing adsorbant dose. Sharp increase in removal of Hg (II) was observed when we increased the algal adsorbant dose from 0.5 g to 1.5 g and beyond 2 g of adsorbant dose, no significant percentage removal was observed in Figure 5 (a) that probably because of increase in adsorbant dose, provide large number of available active sites at the surface of algal bioadsorbant which recognized as various functional groups leads to adsorption of Hg (II) ions. However further increase in adsorbant dose does not affect the sorption due to constant initial concentration. It was noticed that the adsorption capacity (qe) which attributed amount of metal adsorbed per unit of mass of the adsorbent (mg/g) decreases with increase in adsorbant dose because the fixed initial metal ion concentration do not meets need to extend over all the available sites on the adsorbant (Wilde and Benemann, 1993).
Figure 5.
(a) Role of adsorbant dosage on the adsorption of Hg(II) on algal adsorbant: pH = 6,Ci = 100 mg/L, contact time = 2 hoursat 35 °C (b) Role of contact time on the adsorption of Hg(II) on to algal bioadsorbant: pH = 6, Ci = 100 mg/L, dose = 1 g/L at 35 °C (c) Role of initial metal concentration on the adsorption on to algal bioadsorbant: pH = 6, dose = 1 g/L, contact time = 2 h at 35 °C.
3.2.3. Role of contact time on adsorption of Hg(II) on algal bioadsorbant
The effect of contact time for the adsorption of Hg (II), onto algal biomass was investigated. Initially, the amount of heavy metal ions adsorbed rapidly within one hour due to presence of extended surface area. After this adsorption followed with constant rate, reached equilibrium at 90 min for Hg (II) on to algal biomass (Figure 5b). Fast adsorption rate followed by slower rate and then equilibrium is characterstic results obtained by most of studies (Veglio and Beolchini, 1997). The rate of adsorption of heavy metal occurred from outer side of the adsorbant to inner side of the adsorbant and in that event became insignificant afterwards (Kapoor and Viraraghavan, 1998). The curve having plateau portion relates to pore diffusion and the linear portion of the curve reveals surface layer diffusion (Saifuddin and Kumaran, 2005) Equilibrium stage attributated saturation of vacant sites. Thus, all the experiments were carried out at 90 min equilibration time.
3.2.4. Role of initial metal concentration on adsorption of Hg(II) on algal bioadsorbant
Role of initial metal concentration over range of 20–180 mg/L fixing pH 6 and agitation time 90 min for removal of Hg (II) was studied (Figure 5c). The adsorption capacity showed increasing trend from 17.87 to 52.09 mg/g and removal percentage decreased from 93% to 34.21% with increase of initial metal concentration. At specific fixed dose with the low initial concentration adequate sites are available for adsorption of Hg (II) however at higher initial concentration, there is increase in number of mole of metal ions compared to available adsorption sites resulting in the decrease in removal percentage (Nassar, 2010).
3.2.5. Kinetics of biosorption of Hg(II)on algal bioadsorbant
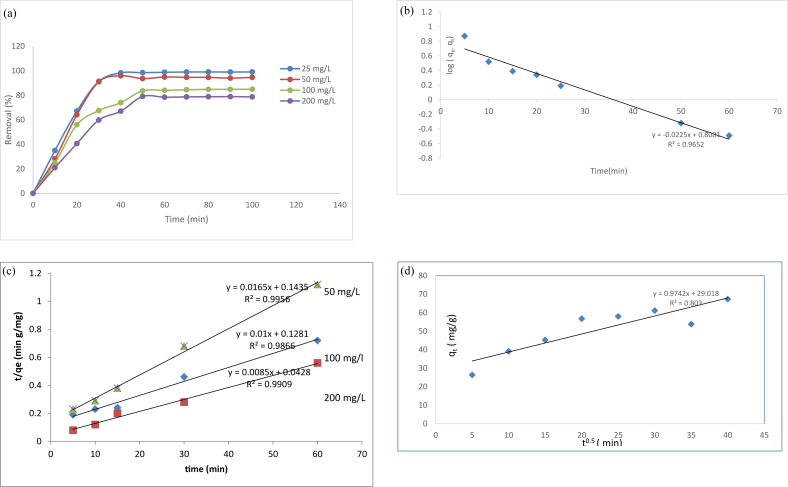
Adsorption equilibria studies are vital to regulate the effectiveness of adsorption. In spite of this, it is also necessary to identify the adsorption mechanism type in a given system. The study of adsorption have two components viz., equilibrium and kinetic study. The rate of uptake in the adsorption process is realted to adsorption equilibrium and is explained by various equilibrium isotherms whereas kinetic study provide insight to rate limiting mechanism & deduce operating coditions (Deng et al., 2007). These two factors facilitate to recognize operationg conditions and marginalize resistance of mass transfer to forcast efficacy of adsorbant. For determining the biosorption mechanism and its potential rate-controlling steps that includes: mass transport and chemical reaction processes, kinetic models have been exploited to test the experimental data (Simons and van Beem, 1990). Kinetics studies were conducted at an initial concentration (25-200 mg/L) of Hg (II) in batch operation to investigate intrinsic kinetics. High agitation speed to reduce film thickness and small particle size for reduction of pore diffusion resistance were applied to ease mass transfer effect to ascertain the time required to attain the equilibrium between the adsorbent and the adsorbate. Figure 6 (a) illustrated Hg (II) percentage removal vs time sketche by Chlorella vulgaris at various initial Hg (II) concentrations. Initially the rapid removal percentage reveals that the high initial concentration overcome the mass transfer resistance between the aqueous and solid phases (i.e., availibility of large surface sites) are initially speed up the process and with course of time, remiaing sites are tough to be employed due to repulsion between solute molecules of solid and bulk phases. Higher initial metal concentration showed less removal percentage due to higher number of surface sites accessible per mole of metal ions but increased number of moles can not access the required surface sites and hence the removal percentage decreses (Wilde and Benemann, 1993). Straight line plot for Hg(II) and correlation coefficient values (R² = 0.9652 for 100 mg/L initial concentration) showed in Figure 6 (b) suggest that the removal of metal ions by adsorption follows Pseudo first order kinetics in certain extent. The values of the adsorption parameters qe and k2 were determined from the slope and intercept of the plot shown in Figure 6 (c), respectively. The regression coefficients (R2) at different initial metal concentrations indicate very close proximity to unity, illustrated better fit to experimental data (Table 2). The value of ki was calculated from slope of plot qt vs t 0.5 (Figure 6 (d)) and are presented in Table 2. Larger the intercept, greater the involvement of surface sorption in controlling the rate of adsorption.
Figure 6.
(a) Kinetic plot of the adsorption of Hg(II) on to algal bioadsorbant: pH = 6, Ci=(25,50,100,200) mg/L, dose = 1 g/L at 35 °C (b) Pseudo first order plot for the adsorption of Hg(II): pH = 6, 100 mg/L, dose = 1 g/L at 35 °C (c) Pseudo second order plot for the adsorption of Hg(II) on to algal bioadsorbant: pH = 6, Ci=(50,100,200) mg/L, dose = 1 g/L at 35 °C (d) Intra particle diffusion model for the adsorption of Hg(II) on to algal bioadsorbant: pH = 6, Ci = 100 mg/L, dose = 1 g/L at 35 °C.
Table 2.
Pseudo first order, pseudo second order & intra-particle diffusion rate for adsorption of Hg(II) on algal Bioadsorbant.
| Constants | Initial concentration (mg/L) |
||
|---|---|---|---|
| 50 | 10 | 200 | |
| qe exp. (mg/g) | 38.02 | 76.39 | 132.98 |
| Pseudo first order | |||
| qe cal. (mg/g) | 21.71 | 46.75 | 81.49 |
| k1 (min−1) | 0.024 | 0.034 | 0.028 |
| R2 | 0.9876 | 0.9652 | 0.9882 |
| Pseudo second order | |||
| qe cal. (mg/g) | 36.69 | 75.41 | 141.46 |
| k2 (g mg −1min−1) | 0.094 | 0.083 | 0.068 |
| R2 | 0.9956 | 0.9866 | 0.9909 |
| Intraparticle diffusion | |||
| Kid (mg g min−1) | 0.024 | 0.034 | 0.028 |
| R2 | 0.876 | 0.803 | 0.890 |
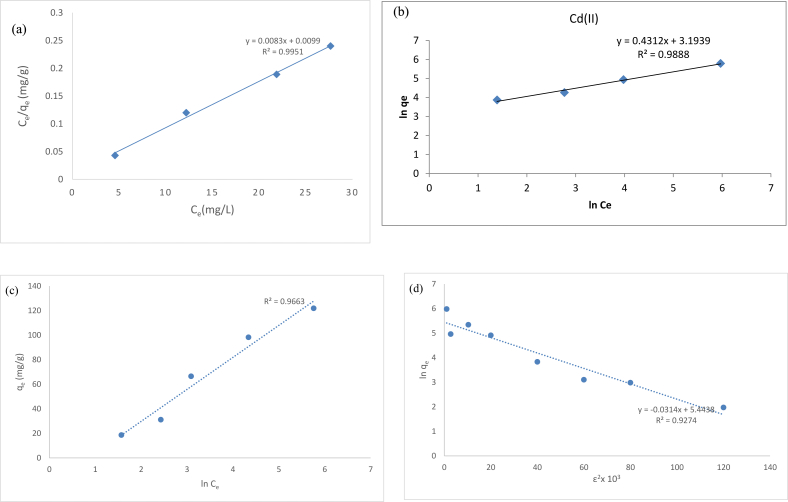
To determine which model to use to describe the adsorption for a particular adsorbent/adsorbate isotherms experiments are usually run. Data from these isotherm experiments are then analyzed based on linearization of the models (Figure 7). A comprision of adsorption capicity by various algal species were shown in Table 4. The data obtained in determing the kinteics and equlibrium studies of the Hg(II) adsorption are tabulated in Tables 3 & 4. To investigate the kinetics of heavy metal biosorption, many models have been developed. Inspite of many alternative models, Pseudo first order, Pseudo second order and intraparticle diffusion model stay the most prevalent models for batch process to evaluate controlling mechanism in bioadsorption system (Supplementary file).
Figure 7.
(a) Langmuir isotherm plot for the adsorption of Hg(II) on to algal biomass:pH = 6, Ci = 25–200 mg/L,dose = 1 g/L, contatct time = 90 min at 35 °C (b) Freundlich isotherm plot for the adsorption of Hg(II) on to algal biomass: pH = 6, Ci = 25–200 mg/L,dose = 1 g/L, contatct time = 90 min at 35 °C (c) Temkin Isotherm plot for adsorption of Hg(II) on to algal biomass: pH = 6, Ci = 25–200 mg/L,dose = 1 g/L, contatct time = 90 min at 35 °C (d) D-R isotherm plot for adsorption of Hg(II) on to algal biomass: pH = 6, Ci = 25–200 mg/L,dose = 1 g/L, contatct time = 90 min at 35 °C.
Table 4.
Comparison of adsorption capacities of adsorbents towards heavy metal removal.
|
Algae |
Metals | Capacity (mg/g) | References |
|---|---|---|---|
| Spirulina plantensis | Cu2+ | 1.64–1.94 | 16 |
| Chlorella vulgaris | Cu2+ | 1.14–1.47 | 16 |
| Arthrospira plantensis | Cu2+ | 17.3 | 17 |
| Spirulina plantensis | Cr6+ | 12.3–16.8 | 18 |
| Chlorella vulgaris | Cr6+ | 10.2–13.7 | 18 |
| Chlorella vulgaris | Cr6+ | 0.5–15 | 18 |
| Chlorella miniata | Cr3+ | 14.7–41.2 | 19 |
| Spirulina plantensis | Zn2+ | 2.1 | 20 |
| Chlorella vulgaris | Zn2+ | 2.2 | 20 |
| Chlorella vulgaris | Hg2+ | 42.1 | Present work |
Table 3.
Isotherm models and their calculated constants for sorption of Hg(II) on algal bioadsorbant.
| Model | Parameters | |
|---|---|---|
| Langmuir | q max (mg g-1) | 42.1 |
| b (L mg−1) | 0.01852 | |
| R2 | 0.9951 | |
| RL | 0.231–0.973 | |
| Freundlich | kf (mg/g) | 3.792 |
| R2 | 0.9888 | |
| n | 2.43 | |
| Temkin | B | 32.76 |
| b (J/mol) | 24.09 | |
| A (L/g) | 2.12 | |
| R2 | 0.9663 | |
| Dubinin-Radushkevich (D-R) | q max (mg g-1) | 38.76 |
| β (mol2 J−2) | 4.21 × 10−6 | |
| E (KJ/mol) | 8.87 | |
| R2 | 0.9274 | |
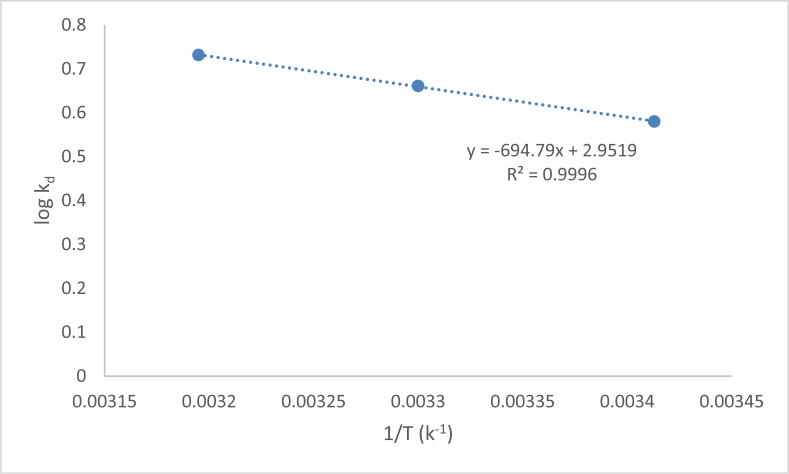
3.2.6. Role of temperature and thermodynamics of adsorption of Hg(II) on algal bioadsorbant
The data obtained from Figure 8 were presented in Table 5. The normal range of free energy for the physical adsorption ranges from -20 KJ/mol to 0 KJ/mol and for chemical adsorption range varies from -80 KJ/mol to -400 KJ/mol [102]. The negative free energies calculated in this study were -0.0268 KJ/mol, -0.0214 KJ/mol, -0.01644 KJ/mol respectively at temperature 293, 303, 313 K indicate the feasibility and spontaneous nature of the process. We can observed here as the temperature increases, the adsorptivity increases (consider the calculated values of Ce & Kd from Table 5). This may be probably credited to the endothermic nature i.e, positive value of ΔH (+27.58 KJ/mol) of the sorption process moreover the endothermic pore diffusion has much influence in the sorption process on both adsorbate and adsorbent over diverse range of temperature. The high positive value of heat of enthalpy designates that the adsorption process is expected due to robust interactions between the Hg (II) ions and various functional groups on surface of algal bioadsorbant (Mohan et al., 2002). The randomness described by ΔS attributed to affinity of heavy metal ions (Hg+2) towards algal bioadsorbant was calculated +56.48 J/mol K deliver suitibility to increased randomness at solid - liquid boundary.
Figure 8.
Thermodynamic plot (log Kd vs. 1/T) of adsorption of Hg(II) ions on to algal biomass: pH = 6, Ci = 100 mg/L,dose = 1 g/L, contatct time = 120 min.
Table 5.
Thermodynamic parameters for the adsorption of Hg(II) on algal bioadsorbant.
| Temp(K) | 1/T | Ce (g/l) | Kd | log kd | ΔH (KJ/mol) | ΔS (J/KMol) | ΔG (KJ/mol) |
|---|---|---|---|---|---|---|---|
| 293 | 0.003413 | 0.045 | 3.88 | 0.5797 | +27.58 | +56.48 | -0.0268 |
| 303 | 0.003300 | 0.048 | 4.583 | 0.6608 | -0.0214 | ||
| 313 | 0.003195 | 0.052 | 5.384 | 0.7311 | -0.02644 |
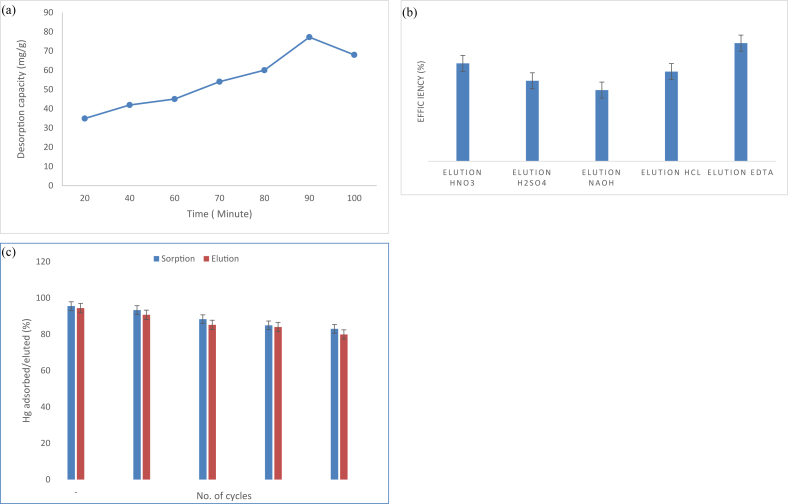
3.2.7. Recyclability & desorption studies
It is expected to reuse and regenerate the algal biomass as it is an important parameter for assessing the viability of adsorption process. In Figure 9 (b) five eluents (0.1 N of each HCl, NaOH, HNO3, H2SO4, EDTA) in one adsorption/desorption cycle were established. Most of the striping solutions showed good eluting capacity (HCl: 72.2%, HNO3: 78.8%, H2SO4: 64.8%) due to at higher concentration of protons, established a robust competition for the active surface sites on the algal bioadsorbant. On the other hand, NaOH did not display good eluting capability. However, EDTA set to attain 95.1% recovery because in basic medium EDTA is deprotonated and with pronounced chealating properties, EDTA characterized exceptional streaping solution for heavy metals bounded to the porous matrix.
Figure 9.
(a) Effect of time on desorption capacity of algal biomass: dose = 0.1 g/L, EDTA concentration = 0.1 mol/L) (b) Percentage efficiency of five eluents 0.1N of each (HCl, NaOH, HNO3, H2SO4, and EDTA) (c) Five Sorption/Desorption cycles: Ci = 100 mg/L, dose = 0.1 g/L, 0.1 N EDTA.
The study also revealed that 90 min of equilibration time was sufficient for the quantitative stripping (Figure 9(a)). Subsequently, the adsorption/desorption cycles were repeated for five cycles using 1 g of the adsorbent. The results obtained are furnished in Figure 9 (c). The sorption ability deceased from 95.5% to 79.87% upto five cycle by 8.36% which could be assume negligible and thus, the adsorbent can be reused.
4. Conclusions
Mercury metal ion biosorption on Chlorella vulgaris is strongly affected by parameters such as contact time, initial metal ion concentration, pH and temperature. The maximum biosorption was found 42.1 at pH 6. Both Langmuir and Freundlich equilibrium isotherm model is proved to be good fit for the experimental data of Hg2 biosorption on Chlorella vulgaris biomass (Kumar et al., 2018). The kinetics of the biosorption of Hg2 on the biomass could be described by a second-order kinetic model (Gupt et al., 2018). Free energy change (ΔG) with negative sign reflects the feasibility and spontaneous nature of the process. The positive enthalpy values indicate endothermic nature and negative entropy value point towards increase in randomness at solid liquid interface. The biomass of Chlorella vulgaris is mostly amorphous in nature. The marine microalgae is promising biosorbent forremoval of metal ions from waste water streams.
Declarations
Author contribution statement
Mahendra Kumar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alak Kumar Singh: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Mohammad Sikandar: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Avinash Gupta, IIT Kanpur, Dr. Lalit Goswami,IIT Guwahati, Dr Sunil Kumar Yadav, Inverstis Univrsity, Mr. Manish Singh Rajput, Dr. AITH Kanpur for their keen interests and IIT Kanpur for providing necessary facilities (FTIR and SEM) for the work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Admasu D., Reddy D.N., Mekonnen K.N. Trace determination of zinc in soil and vegetable samples by spectrophotometry using pyridoxal thiosemicarbazone and 2-acetyl pyridine thiosemicarbazone. Cogent Chemistry. 2016;2(1):1249602. [Google Scholar]
- Alyüz B., Veli S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard Mater. 2009 Aug 15;167(1-3):482–488. doi: 10.1016/j.jhazmat.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Areco M.M., Hanela S., Duran J., Afonso M-d. S. Biosorption of Cu(II), Zn(II) Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard Mater. 2012;213:123–132. doi: 10.1016/j.jhazmat.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Arul Manikandan N., Alemu A.K., Goswami L., Pakshirajan K., Pugazhenthi G. Waste litchi peels for cr (vi) removal from synthetic wastewater in batch and continuous systems: sorbent characterization, regeneration and reuse study. J. Environ. Eng. 2016 Feb 23;142(9):C4016001. [Google Scholar]
- Bailey S.E., Olin T.J., Bricka R.M., Adrian D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999 Aug 1;33(11):2469–2479. [Google Scholar]
- Barakat M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011 Oct 1;4(4):361–377. [Google Scholar]
- Bhasney S.M., Bhagabati P., Kumar A., Katiyar V. Morphology and crystalline characteristics of polylactic acid [PLA]/linear low density polyethylene [LLDPE]/microcrystalline cellulose [MCC] fiber composite. Compos. Sci. Technol. 2019;171:54–61. [Google Scholar]
- Bind A., Goswami L., Prakash V. Comparative analysis of floating and submerged macrophytes for heavy metal (copper, chromium, arsenic and lead) removal: sorbent preparation, characterization, regeneration and cost estimation. Geol. Ecol. Landscapes. 2018;2(2):61–72. [Google Scholar]
- BIS – Bureau of Indian Standards . 2005. IS 10500,http://bis.org.in/sf/fad/fad25(2047)c.pdf FAD25(2047)C. [Google Scholar]
- Deng L., Su Y., Su H., Wang X., Zhu X. Sorption and desorption of lead(II) from wastewater by green algae Cladophora fascicularis. J. Hazard Mater. 2007;143:220–225. doi: 10.1016/j.jhazmat.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011 Mar 1;92(3):407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Goswami L., Namboodiri M.T., Kumar R.V., Pakshirajan K., Pugazhenthi G. Biodiesel production potential of oleaginous Rhodococcus opacus grown on biomass gasification wastewater. Renew. Energy. 2017;105:400–406. [Google Scholar]
- Goswami L., Kumar R.V., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Simultaneous polycyclic aromatic hydrocarbon degradation and lipid accumulation by Rhodococcus opacus for potential biodiesel production. J. Water Process Eng. 2017;17:1–10. [Google Scholar]
- Goswami L., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech. 2017;7(1):37. doi: 10.1007/s13205-016-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami L., Kumar R.V., Borah S.N., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: a review. J. Water Process Eng. 2018;26:314–328. [Google Scholar]
- Goswami L., Manikandan N.A., Dolman B., Pakshirajan K., Pugazhenthi G. Biological treatment of wastewater containing a mixture of polycyclic aromatic hydrocarbons using the oleaginous bacterium Rhodococcus opacus. J. Clean. Prod. 2018;196:1282–1291. [Google Scholar]
- Goswami L., Kumar R.V., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Anthracene biodegradation by oleaginous Rhodococcus opacus for biodiesel production and its characterization. Polycycl. Aromat. Comp. 2019:1–13. [Google Scholar]
- Goswami L., Kumar R.V., Pakshirajan K., Pugazhenthi G. A novel integrated biodegradation-microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard Mater. 2019;365:707–715. doi: 10.1016/j.jhazmat.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Goswami L., Manikandan N.A., Taube J.C.R., Pakshirajan K., Pugazhenthi G. Novel waste-derived biochar from biomass gasification effluent: preparation, characterization, cost estimation, and application in polycyclic aromatic hydrocarbon biodegradation and lipid accumulation by Rhodococcus opacus. Environ. Sci. Pollut. Control Ser. 2019;26(24):25154–25166. doi: 10.1007/s11356-019-05677-y. [DOI] [PubMed] [Google Scholar]
- Gupt C.B., Yamsani S.K., Prakash A., Medhi C.R., Sreedeep S. Appropriate liquid-to-solid ratio for sorption studies of Bentonite. J. Environ. Eng. 2018;145(2) [Google Scholar]
- Gupta V.K., Jain R., Saleh T.A., Nayak A., Malathi S., Agarwal S. Equilibrium and thermodynamic studies on the removal and recovery of safranine-T dye from industrial effluents. Separ. Sci. Technol. 2011 Mar 22;46(5):839–846. [Google Scholar]
- Gupta A., Vidyarthi S.R., Sankararamakrishnan N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard Mater. 2014 Jun 15;274:132–144. doi: 10.1016/j.jhazmat.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Gupta A., Vidyarthi S.R., Sankararamakrishnan N. Studies on glutaraldehyde crosslinked xanthated chitosan towards the removal of mercury (ii) from contaminated water streams. Environ. Eng. Manag. J. (EEMJ) 2015 May 1;14(5) [Google Scholar]
- Hatamian M., Rezaie Nejad A., Kafi M., Souri M.K., Shahbazi K. Growth characteristics of ornamental Judas tree (cercis siliquastrum L.) seedling under different concentrations of lead and cadmium in irrigation water. Acta Scientiarum polonorum-Hortorum Cultus. 2019;18(2):87–96. [Google Scholar]
- Kapoor A., Viraraghavan T. Removal of heavy metals from aqueous solutions using immobilized fungal biomass in continuous mode. Water Res. 1998 Jun 1;32(6):1968–1977. [Google Scholar]
- Kumar R.V., Goswami L., Pakshirajan K., Pugazhenthi G. Dairy wastewater treatment using a novel low cost tubular ceramic membrane and membrane fouling mechanism using pore blocking models. J. Water Process Eng. 2016;13:168–175. [Google Scholar]
- Kumar M., Singh A.K., Sikandar M. Study of sorption and desorption of Cd (II) from aqueous solution using isolated green algae Chlorella vulgaris. Appl. Water Sci. 2018 Dec 1;8(8):225. [Google Scholar]
- Kumar M., Goswami L., Singh A.K., Sikandar M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon. 2019;5(10) doi: 10.1016/j.heliyon.2019.e02562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha A., Rani R., Kumar S., Gautam A. Heavy metal detoxification and tolerance mechanisms in plants: implications for phytoremediation. Environ. Rev. 2015;24(1):39–51. [Google Scholar]
- Kushwaha A., Rani R., Kumar S., Thomas T., David A.A., Ahmed M. A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ. Sci. Pollut. Control Ser. 2017;24(11):10652–10661. doi: 10.1007/s11356-017-8752-8. [DOI] [PubMed] [Google Scholar]
- Kushwaha A., Hans N., Kumar S., Rani R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018;147:1035–1045. doi: 10.1016/j.ecoenv.2017.09.049. [DOI] [PubMed] [Google Scholar]
- Kushwaha A., Rani R., Patra J.K. Adsorption kinetics and molecular interactions of lead [Pb (II)] with natural clay and humic acid. Int. J. Environ. Sci. Technol. 2019:1–12. [Google Scholar]
- Liu Y., Cao Q., Luo F., Chen J. Biosorption of Cd2+, Cu2+ Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J. Hazard Mater. 2009;163:931–938. doi: 10.1016/j.jhazmat.2008.07.046. [DOI] [PubMed] [Google Scholar]
- Mason R.P., Reinfelder J.R., Morel F.M. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 1996 May 23;30(6):1835–1845. [Google Scholar]
- Mohan S.V., Rao N.C., Prasad K.K., Karthikeyan J. Treatment of simulated Reactive Yellow 22 (Azo) dye effluents using Spirogyra species. Waste Manag. 2002;22:575–582. doi: 10.1016/s0956-053x(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Nassar N.N. Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. J. Hazard Mater. 2010 Dec 15;184(1-3):538–546. doi: 10.1016/j.jhazmat.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Plazinski W., Rudzinski W., Plazinska A. Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv. Colloid Interface Sci. 2009 Nov 30;152(1-2):2–13. doi: 10.1016/j.cis.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Saifuddin M., Kumaran P. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005 Apr;8(1):43–53. [Google Scholar]
- Sathe S.S., Goswami L., Mahanta C., Devi L.M. Integrated factors controlling arsenic mobilization in an alluvial floodplain. Environmental Technol. Innovat. 2020;17:100525. [Google Scholar]
- Simons J., van Beem A.P. Spirogyra species and accompanying algae from pools and ditches in The Netherlands. Aquat. Bot. 1990;37:247–269. [Google Scholar]
- Souri M.K., Hatamian M. Aminochelates in plant nutrition; a review. J. Plant Nutr. 2019;42(1):67–78. [Google Scholar]
- Souri M.K., Alipanahi N., Hatamian M., Ahmadi M., Tesfamariam T. Elemental profile of heavy metals in garden cress, coriander, lettuce and Spinach, commonly Cultivated in Kahrizak, South of Tehran-Iran. Open Agric. 2018;3(1):32–37. [Google Scholar]
- Souri M.K., Hatamian M., Tesfamariam T. Plant growth stage influences heavy metal accumulation in leafy vegetables of garden cress and sweet basil. Chem. Biol. Technol. Agric. 2019;6(1):25. [Google Scholar]
- USEPA . United States Environmental Protection Agency; 1997. Mercury Study Report to Congress. Volume III: Fate and Transport of Mercury in the Environment. EPA-452/R-97-005. [Google Scholar]
- Veglio F., Beolchini F. Removal of metals by biosorption: a review. Hydrometallurgy. 1997 Mar 1;44(3):301–316. [Google Scholar]
- Wilde E.W., Benemann J.R. Bioremoval of heavy metals by the use of microalgae. Biotechnol. Adv. 1993 Jan 1;11(4):781–812. doi: 10.1016/0734-9750(93)90003-6. [DOI] [PubMed] [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. 2011 Oct 24:2011. [Google Scholar]
- Yadav S.K., Singh D.K., Sinha S. Adsorption study of lead (II) onto xanthated date palm trunk: kinetics, isotherm and mechanism. Desalination Water Treat. 2013 Oct 1;51(34-36):6798–6807. [Google Scholar]
- Yadav S.K., Sinha S., Singh D.K. Chromium (VI) removal from aqueous solution and industrial wastewater by modified date palm trunk. Environ. Prog. Sustain. Energy. 2015 Mar 4;34(2):452–460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.