Abstract
The prescription of hemodialysis (HD) in patients with incident end-stage kidney disease (ESKD) is fundamentally empirical. The abrupt transition from nondialysis chronic kidney disease (CKD) to thrice-weekly in-center HD of much the same dialysis intensity as in those with prevalent ESKD underappreciates the progressive nature of kidney disease whereby the decline in renal function has been gradual and ongoing—including at the time of HD initiation. Adjuvant pharmacologic treatment (i.e., diuretics, acid buffers, potassium binders), coupled with residual kidney function (RKF), can complement an initial HD regimen of lower intensity. Barriers to less intensive HD in incident ESKD include risk of inadequate clearance of uremic toxins due to variable and unexpected loss of RKF, lack of patient adherence to assessments of RKF or adjustment of HD intensity, increased burden for all stakeholders in the dialysis units, and negative financial repercussions. A stepped dialysis regimen with scheduled transition from time-delineated twice-weekly HD to thrice-weekly HD could represent an effective and safe strategy to standardize incremental HD in patients with CKD transitioning to early-stage ESKD. Patients’ adherence and survival as well as other clinical outcomes should be rigorously evaluated in clinical trials before large-scale implementation of different incremental schedules of HD. This review discusses potential benefits of and barriers to alternative dialysis regimens in patients with incident ESKD, with emphasis on twice-weekly HD with pharmacologic therapy, and summarizes in-progress clinical trials of incremental HD schedules.
Keywords: clinical trials, hemodialysis, incremental dialysis, transition, twice-weekly
More than half a century from the construct and introduction of hemodialysis (HD) therapy as treatment for end-stage kidney disease (ESKD), the optimal prescription (i.e., frequency, duration, and dose) of HD treatment is unsettled. In most developed countries, the vast majority of patients with ESKD receiving HD are treated with a conventional regimen of thrice-weekly in-center HD of full intensity (i.e., to achieve a minimum dialysis single pool Kt/Vurea [spKt/Vurea] ≥1.2 and urea reduction ratio [URR] ≥65%), whether the patient is new to dialysis treatment (incident) or has been undergoing dialysis therapy for a while (prevalent). Unlike the science used behind HD prescription for prevalent patients with ESKD, who often have little or no remaining kidney function, rigorous trials of standardized HD prescription in patients with incident ESKD have not been conducted, especially for those who still make considerable amounts of urine.1,2 Furthermore, health care resources have a bearing on practice patterns. In economically disenfranchised countries (e.g., Asia, Africa, and Latin America), incremental in-center HD (e.g., twice- or even once-weekly HD) is prescribed to 25% to 50% of the HD population, being as common as near 100% of patients with ESKD in some regions of India.3, 4, 5 Therefore, less intensive schedules of HD can be afforded in ESKD, owing to persistent low levels of kidney function; this kidney function, not uncommonly associated with 1 to 2 L of urine output per day, has been dubbed the residual kidney function (RKF).
Incremental HD denotes gradual adjustments in the dialysis prescription (i.e., frequency, treatment time, blood flow, dialysate flow, etc.), guided by changes in RKF. Under this umbrella concept, several regimens of HD have been probed: twice-weekly HD with later conversion to thrice-weekly HD dictated by changes in RKF or changes in clinical status in the absence of assessments of RKF; once-weekly HD with low-protein diet followed by later changes to more frequent HD schedules; or thrice-weekly HD with short treatment sessions (<4 hours per session) followed by increase in treatment length (≥4 hours per session).6 Although protein-restricted diets with nitrogen-free ketoacid analogues have been reported as effective and safe means to reduce the rate of kidney function decline and delay dialysis initiation,7,8 the application of these dietary modifications in patients receiving HD remains to be investigated. Nutrition-related indexes are recognized as strong prognostic factors for all-cause and cardiovascular morbidity and mortality in patients on chronic HD.9 The rise in prevalence rates of protein-energy wasting according to HD duration ought to be acknowledged,10 yet the optimal level and composition of protein intake in patients with incident ESKD and its effects on RKF, cardiovascular outcomes, and survival have not been studied. Thus, until research brings more clarity, a dietary approach for incremental HD should be regarded with caution.
Irrespective of the incremental HD schedule used at dialysis initiation, regular assessment of endogenous renal function is an integral part in its prescription. Although a regimen of incremental HD consisting of adjustment in treatment length rather than treatment frequency may have least have an impact on the current infrastructure of the dialysis units, incremental dialysis requires a very high level of commitment from the nephrologist, the dialysis unit staff, and foremost the patient. Shorter HD treatments will yield subtarget dialysis clearance metrics, which, in the absence of concomitant renal clearance measurements, will be challenged as inadequate dialysis in the United States, within the mandatory federal reporting of urea clearance metrics for patients undergoing thrice-weekly HD. To this date, the safety and efficacy of 1 regimen of incremental HD over another is not known, as these regimens have not been directly compared. In this article, we focus our debate on the prescription of twice-weekly versus conventional thrice-weekly in-center HD and propose a regimen of stepped HD prescription consisting of time-delineated twice-weekly in-center HD with adjuvant pharmacologic therapy in patients with incident ESKD.
The Pervasive Unknowns of HD Dose
A one-size-fits-all HD therapy approach is in discord with one of the tenets of medical practice: treatment of disease based on illness severity. The target HD treatment dose applied in conventional HD prescription was derived from clinical trials that involved solely prevalent HD patients with dialysis vintage >2 years and very low to no RKF1,2; this was then extrapolated as an optimal dialysis dose to all dialysis patients, including those with incident ESKD and appreciable RKF. Barring the endeavor of recent Frequent Hemodialysis Network trials that compared more-frequent HD with conventional HD, no randomized controlled trial has examined whether less frequent HD treatments (or other schedules of incremental HD) would be practicable, beneficial, or harmful in patients with incident ESKD.11,12
As such, in the current state of clinical practice, HD prescription for patients with ESKD is stereotypical and oblivious of RKF, most commonly consisting of thrice-weekly HD to specific targets of dialysis spKt/Vurea and URR.
The schedule of HD therapy has, however, transformed over years. At its inception, nearly 7 decades ago, HD was performed as 24-hour weekly treatments; the practice gradually changed to 12-hour twice-weekly sessions, 8- to 12-hour thrice-weekly sessions, and then finally to short 3- to 4-hour thrice-weekly schedules, changes largely made possible by technological advances in dialysis engineering and shaped by logistical operations.13,14 In early 1970s, Babb and Milutinovic et al. called attention to residual glomerular filtration rate, affirming that “it is [now] apparent that it is necessary to consider the effect of RKF in prescribing a treatment protocol for a given patient”; their recommendation did not permeate clinical practice.15,16 Alongside, the prescription of HD progressed from bedside assessment of uremic symptoms (e.g., reversal of uremic coma) to serum-based HD dose quantification (i.e., time-averaged concentration of blood urea nitrogen [BUN], Kt/Vurea, URR). The generalized acquiescence to a conventional dialysis schedule and target and minimum urea clearance metrics in patients treated with HD is a composite result of two clinical trials (the National Cooperative Dialysis Study [NCDS] and the Effect of Dialysis Dose and Membrane Flux in Maintenance Hemodialysis [HEMO] study),1,2 bolstered by clinical practice guidelines,17,18 intertwined with government regulations.19 In the NCDS, patients with prevalent ESKD and residual creatinine clearance ≤3 ml/min on thrice-weekly HD were randomized to test the effect of achieving higher and lower BUN concentrations. The trial, based on 151 patients, showed that the groups with time-averaged BUN concentrations of approximately 50 mg/dl fared better (i.e., longer time to first hospitalization) than those with time-averaged BUN concentrations of approximately 80 mg/dl.1 Gotch and Sargent converted time-averaged BUN as a function of spKt/Vurea, and their re-analysis of the NCDS data showed that patients with spKt/Vurea <0.8 had a high relative probability of failure (composite endpoint of death, hospitalization, and de novo uremic symptoms) irrespective of BUN or normalized protein catabolic rate.20 These results set the first benchmark for a minimum dialysis dose delivery at spKt/Vurea ≥1.0; this target was set 25% higher than the so-called spKt/Vurea break point identified in the NCDS trial. The HEMO study used the construct Kt/Vurea as the principal measure of the dose of dialysis, and showed that an increase in spKt/Vurea from approximately 1.4 to 1.7 on a thrice-weekly HD regimen, in patients with prevalent ESKD and residual urea clearance <1.5 ml/min per 35 L of urea, afforded no clinical outcome benefit.2
In the aftermath of the aforementioned landmark trials, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommended a target spKt/Vurea of 1.4 per HD session for patients treated thrice weekly, with a minimum delivered spKt/Vurea of 1.2.17 The upward drift in the target spKt/Vurea from 1.0 to 1.4, which occurred without the support of a clinical trial, is reinforced in the United States by the regulatory bodies of the Centers for Medicare and Medicaid Services (CMS). Intricacies of the implicit social contract between the population of the United States and its government regarding treatment of ESKD put aside, many positive aspects are to be highlighted, such as government-financed entitlement to HD care and cost containment.19 Although it may seem that part of the reason that countries other than the Unites States are successful at performing and studying less frequent HD is lax federal oversight on dialysis metrics, the reporting of dialysis dose adequacy in the United States is not required by the CMS for patients undergoing HD at schedules other than thrice weekly. In fact, should adequate research prove the effectiveness of incremental HD, a change in CMS rules could force adaptation of timely urine collections and measurement of RKF in clinical practice. Meanwhile, the financial drawbacks to dialysis units’ stakeholders brought about by less frequent HD schedules are curbing the acceptance of performing and studying less frequent HD.
Unlike treatment of ESKD with peritoneal dialysis (PD), the ease of achieving adequate solute clearance with HD allows the treating physicians to disregard RKF quantification. Because of the inherent nature of less efficient solute clearance with PD, patients started on renal replacement therapy with this modality tend to have higher levels of RKF at dialysis initiation than those started on HD. The reliance on RKF to achieve adequate solute clearance in patients initiated on PD compels providers to obtain periodic measurements of intrinsic kidney function. The prescription of PD is subsequently titrated based on changes in RKF; for this reason, RKF has been referred to as the heart of PD.21 In contrast, the inclusion of RKF in HD prescription has been left up to the discretion of the nephrologist, with scant guidance on how to perform incremental HD (discussed below). Although we acknowledge the efficiency, quality, and current acceptance of a streamlined, thrice-weekly HD approach—independent of RKF—for medical professionals, recasting ESKD under a progression model, with its own stages, may compel more recognition to a regressive kidney function in patients who transition from predialysis chronic kidney disease (CKD) to early-stage ESKD.
Early-Stage ESKD
Residual Kidney Function
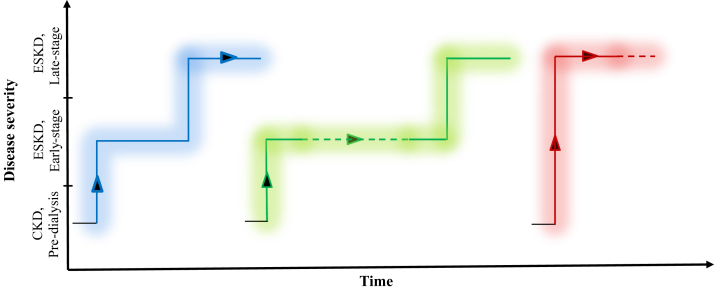
It has been estimated that nearly half of the patients with CKD who progress to ESKD and require initiation of renal replacement therapy have an appreciable amount of RKF present at the time of dialysis.22,23 Although the ESKD designation lends the inference of comparable kidney function deficit in those afflicted, we argue that the final stage of kidney disease can be broadly divided, for clinical purposes, into 2 substages: early-stage ESKD in the initial period of HD therapy (present in up to 50% of incident patients), and late-stage ESKD (prevalent patients) demarcated by a shortfall in RKF. Just as the progression of kidney disease tracks heterogeneous patterns in adult patients with CKD,24 kidney function regression from predialysis CKD to ESKD may follow several paths, and perhaps not all patients reach late-stage ESKD (Figure 1). Our speculation of transition patterns from CKD to different stages of ESKD needs to be verified with clinical data obtained in prospective studies.
Figure 1.
Hypothetical plots of different patterns of progression from chronic kidney disease (CKD) to end-stage kidney disease (ESKD): gradual transition from CKD to late-stage ESKD, passing through early-stage ESKD; possible candidate for incremental dialysis (blue-line trajectory); intermediate transition from CKD to early-stage ESKD with prolonged dwell in early-stage ESKD; ideal candidate for incremental dialysis (green-line trajectory); and abrupt transition from CKD to late-stage ESKD; likely not a candidate for incremental dialysis (red-line trajectory).
In the year 2015, national statistics from the United States Renal Data System (USRDS) reported a mean estimated glomerular filtration rate (eGFR) at dialysis initiation of 9.8 ml/min per 1.73 m2; 39% of all patients with incident ESKD had an eGFR at ≥10 ml/min per 1.73 m2 at dialysis initiation.25,26 Based on a USRDS registry cohort of 971,481 patients who initiated dialysis between 1995 and 2012, Li et al. showed that the mean eGFR at dialysis initiation rose monotonically from 7.7 in 1995 to 11.1 in 2009 and 10.9 ml/min per 1.73 m2 in 2012; >90% of all incident dialysis patients had an eGFR of ≥5 ml/min per 1.73 m2 at dialysis initiation.22 As a result, a large proportion of patients with incident ESKD have substantial RKF that may confer, at least provisionally, partial solute clearance and volume control. Initiation of HD with a less frequent regimen is conceivably a suitable dialysis schedule in patients with early-stage ESKD and clinically evident RKF.27 Chin et al. estimated the feasibility of twice-weekly HD for patients in the United States with incident ESKD. The authors examined the proportion of patients that could have started maintenance HD with a twice-weekly schedule based on 4 criteria: weekly urea clearance (i.e., standard Kt/Vurea [stdKt/Vurea]); interdialytic weight gain; intradialytic blood pressure; and intradialytic symptoms such as nausea and vomiting. On the basis of total stdKt/Vurea (delivered by dialysis and RKF), ultrafiltration rate, and blood pressure stability, the authors calculated that approximately one-third of incident hemodialysis patients (i.e., 219 of 646) would qualify for an initial schedule of twice-weekly HD.23
Adjuvant Pharmacologic Therapy
The key to providing less frequent HD safely in patients with incident, early-stage ESKD is patient selection, with the core criterion revolving around the level of RKF. Residual renal solute clearance is calculated as the amount of solute in interdialytic urine collection divided by the average of plasma solute concentration at the beginning and end of the collection period. A detailed discussion about RKF is beyond the scope of this article, and readers are referred to recent review articles for this topic.28,29 When present, RKF provides fluid and salt removal, phosphorus excretion, endogenous vitamin D and erythropoietin production, and greater clearance of protein-bound solutes and middle molecules.30, 31, 32, 33 In addition, RKF provides continuous solute clearance either by filtration or by tubular secretion, which is not provided by HD.34 In a recent study, plasma concentrations of secreted solutes (hippurate, phenylacetylglutamine, indoxyl sulfate, and p-cresol sulfate) were as well controlled in patients with mean (standard deviation) residual renal urea clearance (Kru) 2.8 (1.5) ml/min on twice-weekly HD as in anuric patients on thrice-weekly HD.35 Remarkably, a previously considered clinically negligible level of RKF (i.e., urinary urea clearance ≤1.5 ml/min) may in fact make a significant clinical difference. Toth-Manikowski et al. analyzed serum levels of uremic solutes and clinical outcomes in 1280 participants from the HEMO study, 34% of whom had average residual kidney urea clearance of 0.7 ± 0.4 ml/min per 35 L total body water, and 66% had no RKF.36 Among those with RKF, 7 of 8 measured non-urea solutes were in lower concentrations compared with levels in patients without RKF, irrespective of the HD prescription; the difference ranged from 24% to 3.7% lower (P < 0.05). Having RKF at baseline was associated with a trend toward a 14% lower risk of cardiovascular events; and a maintained RKF at 1 year of follow-up was associated with a 19% lower risk of death, 25% lower risk of cardiac death, and 16% lower risk of first cardiovascular event.36 Therefore, even at very low levels, RKF is consequential.
A tendency in clinical practice is to decrease the dose of or to discontinue the medications that were heavily relied upon in advanced CKD stages: diuretics, potassium-binding agents, and acid buffers. Should a regimen of twice-weekly HD be used at dialysis initiation, these medications can be used to augment and supplement RKF, and to bridge transition from less frequent to thrice-weekly or more frequent HD. When the issue of diuretic prescription was analyzed by Chin et al., the findings suggested that an additional 25% of patients with incident ESKD could have been managed by twice-weekly HD if they had received diuretic therapy.37 In contrast, diuretics are much more commonly used in patients who are treated with PD at dialysis initiation, for whom an escalating regimen of PD frequency and intensity is commonly prescribed.38,39 In 1 trial, 61 patients new to dialysis were randomly assigned to either loop diuretic medication furosemide 250 mg every day or no furosemide at the time of PD training.40 The results were expressed on an intention-to-treat basis, and showed that long-term furosemide produced a significant increase in urine volume over 12 months of follow-up (+176 ml/24 h in the diuretic group vs. −200 ml/24 h in the control group at 6 months and +48.8 vs. −305 ml/24 h, respectively, at 1 year; P < 0.05) and resulted in clinically significant improvement in fluid balance.40 It can be advanced that in patients with progressive CKD and incident ESKD receiving HD, diuretics can ameliorate weight gains between dialysis sessions and lessen unsafe ultrafiltration rates while patients are receiving a time-limited regimen of lower-frequency HD.
Safer medications can now be used to maintain control of serum potassium in patients treated with less frequent HD. Two newer potassium binders, patiromer calcium and sodium zirconium cyclosilicate, have been safely used for potassium imbalance treatment in patients with CKD and heart failure while on therapy with renin−angiotensin−aldosterone system (RAAS) inhibitors. Patiromer calcium has been shown to effectively reduce serum potassium levels,41 to maintain long-term normokalemia,42,43 and to prevent hyperkalemia.44, 45, 46 In 1 trial using zirconium, a dose-dependent increase in the incidence of edema was observed.47 Based on these data, it can be anticipated that the use of patiromer can overcome the risk of hyperkalemia and enhance the tolerability of RAAS inhibitor in patients with incident ESKD treated with less frequent HD regimens.
Timed Urine Collection
A set of clinical criteria to aid in the identification of patients with ESKD suitable for less frequent HD has been proposed, with the core requirement being the presence of urine output >600 ml/d or Kru >3.0 ml/min, and suggest that an incremental transition from twice- to thrice-weekly would be necessary if urine output drops to <500 ml/d or Kru drops to <2.0 ml/min.48,49 Other clinical factors have been considered, such as absence of severe hyperkalemia and hyperphosphatemia, absence of severe cardiovascular and pulmonary disease, lack of profound anemia, and fluid retention <2.5 kg between 2 consecutive HD treatments done 3 to 4 days apart.50,51
In the opinion of the KDOQI Work Group, less frequent HD could be practiced as long as Kru remains >2.0 ml/min, provided that Kru is measured periodically. Because RKF declines over time and large variations in change in RKF exist, the Group set a practical cut-off Kru of 2.0 ml/min per 1.73 m2, below which Kru should be ignored.17 As schedules of less frequent HD rely on objective quantification of RKF, baseline and follow-up interdialytic urine collections (when the collection period begins and ends within an interdialysis interval) at intervals of 1 to 3 months stand as prerequisites in patient identification and modeling of HD prescription. This criterion is a daunting task, given uncertainties in obtaining appropriate urine collections. Regular urine collection between 2 dialysis sessions and its coordination are cumbersome, both for the patient and for the staff. In addition, under- or overcollection of urine is common in practice, and no methods can reliably assess the accuracy of timed urine collection, especially among patients in whom native clearance data may be confounded by the added clearance from the HD therapy.52
Studies that looked at the frequency of timed urine collection to assess Kru showed a low rate in attaining these tests, even at dialysis units that more commonly perform less frequent HD. In a study by Mathew et al., the median prevalence of urine collection during the first 91 days of dialysis was 33%, 42%, and 56% among dialysis facilities that had a prevalence of incremental HD of 0%, >0% to 3%, and >3%, respectively; the trend for higher prevalence of urine collection with rising facility prevalence of incremental HD was statistically significant (P trend <0.001).53 The regularity of timed urine collection was estimated in a cohort of 23 patients with incident ESKD, initiated on a regimen of twice-weekly HD and later switched to thrice-weekly HD; timed urine collection at dialysis initiation was identified only in 12 patients, with recorded mean urine output of 1128 (233) ml/d; at follow-up, only 2 patients had completed a 24-hour urine collection during the course of treatment with twice-weekly HD.54
Methods to assess RKF in patients on dialysis without reliance on urine collections have been explored. Several investigators developed mathematical formulae for cross-sectional estimation of RKF in patients on chronic HD.55, 56, 57 The equations, based on serum biomarkers (urea, creatinine, β-trace protein, β2-microglobulin, cystatin C), were compared against measured urinary clearance of urea and creatinine collected as part of clinical care. In those studies, the precision in estimating RKF was significantly better for β-trace protein- and β2-microglobulin−based equations, whereas the accuracy was significantly greater for β-trace protein−, β2-microglobulin−, and cystatin C−based equations. Overall, RKF estimation equations had low sensitivity but high specificity to classify patients above or below Kru of 2 ml/min. However, bias between measured and estimated RKF was approximately ∼0.5 ml/min with relatively wide limits of agreement, suggesting that β-trace protein− and β2-microglobulin−based estimating equations may not be accurate enough to replace timed urine collections. Furthermore, the equations underestimated the change in kidney function over time. Other limitations of the estimating equations include lack of validation against inulin clearance, and lack of laboratory standards for β-trace protein and β2-microglobulin measurement.29,58 To date, the goal of using equations that estimate RKF from serum markers without urine collection in patients on dialysis remains unmet.
Prescribing Less Frequent HD
The metric of stdKt/Vurea was advanced to normalize the HD prescription by the frequency of dialysis treatments, constructed around the target of dialysis spKt/Vurea in patients with prevalent ESKD on thrice-weekly HD.59,60 The 2015 KDOQI opinion-based guidelines for HD adequacy recommend a target stdKt/Vurea of 2.3 across all schedules of HD, with a minimum delivered dose of 2.1; for less frequent HD schedules, a method of total stdKt/Vurea calculation that includes the combined contributions of ultrafiltration and RKF was proposed.17
In the United States, when less frequent HD is prescribed, patients are generally started on twice-weekly HD, but some clinicians in other countries initiate dialysis with a regimen of once-weekly HD, including in combination with a low-protein diet on nondialysis days.61 A Kru of 2 ml/min confers a weekly renal urea clearance of 0.50 for a volume of distribution of urea of 40 L, 0.57 for a volume of distribution of urea of 35 L, and 0.67 for a volume of distribution of urea of 30 L. Using the urea kinetic modeling according to different dialysis modalities developed by Gotch, residual renal clearance at dialysis initiation can supplement an initial HD prescription of lower frequency but higher dose (i.e., twice-weekly HD with dialysis spKt/Vurea ≥1.60). In this scenario, twice-weekly HD prescription would involve longer-than-usual treatment times (i.e., 240−270 minutes vs. 200−230 minutes).62 Less frequent HD is maintained until Kru is <2.0 ml/min; stdKt/Vurea cannot be maintained ≥2.1 when combining dialysis Kt/Vurea and Kru; and/or clinical manifestations and/or laboratory findings suggest suboptimal dialysis. Practiced in this manner, the success of incremental HD depends on the accomplishment of accurate, frequent and timely measurements of RKF with interdialytic urine collection. Keeping pace with regular, timed urine collection tests may not be a tenable task in real life or busy clinical practice, and especially not until large dialysis organizations operationalize the process.
It is important to mention here that there have been no clinical trials conducted to assess the association of achieved stdKt/Vurea with clinical outcomes in patients undergoing HD, regardless of ESKD stage (incident or prevalent) or dialysis frequency. In 1 retrospective study, Rivara et al. evaluated the predictive value of stdKt/Vurea for clinical outcomes.63 In their study, using nationally representative data from a large dialysis provider in the United States, dialysis stdKt/Vurea ≥2.3 was associated with a modestly lower risk for all-cause mortality compared with stdKt/Vurea 2.1 to <2.3 (adjusted hazard ratio = 0.97, 95% confidence interval [CI] = 0.94–0.99). In individuals with available data on RKF and models adjusting for kidney stdKt/Vurea, dialysis, stdKt/Vurea ≥2.3 was not associated with decreased risk of mortality or hospitalization compared with the referent category.63
Among the main challenges related to the prescription of incremental HD are an arbitrary use of infrequent regimens and the lack of clear (and undemanding) standards for incorporating RKF in the assessment of HD dose. There is a large variability in the practice of incremental HD between countries, across practices in the same country, and between providers at the same practice. In a nationally representative sample of 434 incremental and 50,162 conventional HD patients from 1737 dialysis facilities across the United States, 74% (1293) of the dialysis facilities did not have any patients treated with incremental HD between January 2007 and December 2011; 17% (288) of the facilities had between 0% and 3% of the incident HD patients treated with incremental HD, and 9% (156) of the facilities had >3% of their incident population treated with incremental HD.53 Not surprisingly, patients prescribed twice-weekly HD had better baseline Kru and higher urine volume than those prescribed conventional thrice-weekly HD; no significant difference was noted in age and comorbidities between patients treated with incremental HD and those treated with a conventional HD regimen.64
The complexity of prescribing less frequent HD is augmented by the fact that the rate of RKF decline is unpredictable and exhibits large variations across patients, and clinical events can affect its trajectory (Figure 1). Patients may have slow deterioration of kidney function, moving from predialysis CKD to phases of early-stage ESKD when requiring HD initiation; these patients are good candidates for prescription of incremental HD (Figure 1, green-line trajectory). However, some patients have direct or rapid change in kidney function from predialysis CKD to late-stage CKD, and these patients would not be considered good candidates for prescription of incremental HD (Figure 1, red- and blue-line trajectories). Factors associated with faster decline in RKF are female sex, nonwhite race, diabetes, congestive heart failure, uncontrolled hypertension, proteinuria, bioincompatible membrane, intradialytic hypotension, and dialysis frequency65, 66, 67, 68, 69, 70; RKF is inversely correlated with dialysis vintage.68
Apart from stdKT/Vurea, other methods have been proposed and are used for evaluation of the equivalency of different schedules of HD, such as weekly urea reduction ratio, weekly solute removal index, weekly fractional solute removal, and equivalent renal clearance.62,71 The European guidelines recommend the use of stdKt/Vurea, EKR, and solute removal index.72 Because the solute removal index has conflicting definitions, EKRd, which represents the dialysis equivalent urea clearance per dialysis session, is used as suggested by Casino and Lopez.73 Opinion-based clinical practice guidelines advise to achieve a total EKR (EKRd + RKF) at least equal to the adequacy value corresponding to an equilibrated Kt/Vurea of 1.2 in anuric patients on a thrice-weekly HD regimen, that is, 12 ml/min per 35 L.17,74 As with other measurements of dialysis dose, the shortcoming of total EKR is lack of parameter validation in prospective clinical trials and regimens of incremental HD.75
Several other models have been proposed for prescription of incremental HD. The latest, called the Variable Target Model (VTM), takes into account the dynamic changes of Kru and the ability for fine tuning Kd, thus giving more clinical weight to the contribution of RKF to the total solute clearance.76 Casino and Basile showed that once-weekly HD would virtually not be accomplished under a conventional fixed target model of urea clearance, but would be accomplished with VTM for Kru ∼ 5 ml/min per 1.73 m2 (corrected for end-dialysis urea distribution). Using the VTM, once-weekly HD was achievable until the corrected Kru fell to <4 ml/min per 1.73 m2, at which point the schedule needed to change to twice-weekly HD, which, in turn, was maintained until the corrected Kru fell to <2 ml/min per 1.73 m2.76 Although the VTM may allow for less frequent HD treatments at lower levels of RKF, limitations of this model are to be acknowledged, such as the need for high-intensity monitoring and computation, and lack of prospective studies to test VTM feasibility at larger scale.
Together, these studies highlight the complexity of prescribing incremental HD, the large variability in the practice of less frequent HD, and the irregularity in obtaining periodic assessments of RKF. For practical reasons, it can be argued that patients with CKD who progress to ESKD and have urine output ≥500 ml/d (i.e., early-stage ESKD), without a recent abrupt decline in kidney function (i.e., eGFR <30 ml/min per 1.73 m2 in the 90 days preceding dialysis initiation) will inherently harbor sufficient RKF to permit prescription of twice-weekly HD, at least for a limited period. The concept of time-limited stepped HD will be tested in a proof-of-concept clinical trial and is discussed below. The study of a practical HD prescription that considers the progressive nature of CKD and overcomes practice-based limitations (i.e., assessments of RKF with interdialytic urine collection) is urgently needed.
Potential Advantages of Less Intensive HD
Introduction of HD befalls a vulnerable state and heralds high rates of adverse events. Mortality is highest in the first 6 months of maintenance HD therapy, with many factors converging to yield 80% higher death risk in the first 2 months of renal replacement therapy.77,78 Confounding by indication as a result of clinical deterioration that may prompt the physician to initiate HD can explain, to a certain extent, the exceptionally high death rate in the first months of conventional dialysis. It has also been argued that abrupt transition from non−dialysis-dependent CKD to thrice-weekly HD could contribute to the high mortality rate.
Indeed, loss of RKF after HD initiation can be a factor in the high mortality rate.79, 80, 81 Hemodialysis can cause episodic ischemic damage to the kidneys, leading to repetitive bouts of ischemic events that may accelerate the decline in RKF. It has also been theorized that, with progressive loss of kidney function, the remaining functioning nephrons are activated to undergo compensatory adaptations; initiation of dialysis may lead to the deactivation of functioning nephrons and deterioration of RKF.82 Volume expansion is thought to be a stimulus for the functioning nephrons, and its loss by dialysis may play a role in RKF decline after dialysis initiation.83 It has been further speculated that the diseased kidney’s adaptation might remain stimulated when dialysis is gently and incrementally introduced, a concept called “the intact nephron hypothesis in reverse.”82 Consistent with the hypothesis that HD-dependent factors contribute to changes in RKF are the observations that RKF declines more rapidly in patients on 6-times-per-week nocturnal HD than 3-times-per-week nocturnal HD, and declines in patients on in-center thrice-weekly HD more rapidly than those on PD therapy.68,81 Retrospective and observational studies showed that a regimen of twice-weekly HD at dialysis initiation may confer better RKF preservation than thrice-weekly HD treatment.65,69 The potential salutary effects of less frequent HD on intrinsic kidney function can also be gleaned from the studies that involved patients with dialysis-dependent acute kidney injury. A post hoc analysis of the Acute Renal Failure Trial Network study compared the rate of renal recovery at day 28 between participants randomized to intermittent HD 3 times per week (n = 138) and 6 times per week (n = 108).84 This study indicated that patients treated with more frequent HD experienced lower rates of renal function recovery (creatinine clearance >20 ml/min) by day 28 (odds ratio = 0.49, 95% CI = 0.28–0.87).84
The long-standing observations that patients treated with PD, relative to those treated with HD, have slower decline of RKF bring additional support to the effect of dialysis intensity on RKF. Compared to HD, PD is a less intensive form of renal replacement therapy. In 1 prospective observational study of 50 patients with incident ESKD divided into 2 groups and matched for baseline creatinine clearance (4.3 ± 2.4 ml/min) prior to dialysis initiation, patients in group 1 (n = 25) treated with continuous ambulatory PD had slower decline of creatinine clearance over a period of 18 months compared to those in group 2 treated with intermittent HD (4.4 ± 2.2 vs. 2.7 ml/min ± 1.8 at 6 months, 3.8 ± 1.8 vs. 2.1 ± 1.5 at 12 months, and 4.0 ± 2.3 vs. 1.3 ± 1.2 at 18 months of dialysis).85 Moreover, a large body of literature has shown that the level of RKF in patients treated with PD has a strong correlation with patient survival, and mathematically equivalent rates of solute removal with RKF and PD do not render equivalent patient survival. In 1995, Maiorca et al. reported a 50% reduction in mortality in patients on PD patients who had some ongoing kidney function (i.e., glomerular filtration rate 10 L/wk per 1.73 m2).86 Later, larger prospective studies reported a strong correlation between residual renal creatinine clearance and patient survival, and lack of a similar correlation between dialysis peritoneal clearance and survival. Diaz-Buxo et al. observed a dose−response association between RKF and outcomes; each increase in renal creatinine clearance of 5 L/wk per 1.73 m2 was associated with a 10% decrease in mortality, and no association was seen between peritoneal creatinine clearance and mortality.87 Subsequent reanalyses of landmark multicenter clinical trials confirmed that the endogenous clearance (i.e., RKF) is an essential marker of patient survival, whereas the exogenous clearance (i.e., dialysis dose delivered via PD) has much less of an effect on clinical outcomes.88, 89, 90, 91
Research on changes in RKF in patients with incident ESKD treated with HD and the associated effects on patient outcomes is scant. Patients on HD with higher levels of RKF have generally reported better quality of life,81 and longer survival than those with lower levels of RKF.92 Confounding the results is selection bias, whereby patients perceived as healthier, more compliant with treatment recommendations, and with better kidney function at dialysis initiation are offered less frequent HD. These very plausible clinical scenarios select patients with inherent biological advantage for better clinical outcomes, whereas statistical methods used in observational studies are unable to adjust for covert biological factors.
A few retrospective studies have examined survival in patients with incident ESKD initiated on maintenance dialysis with twice-weekly HD and have reported mixed results. A study done in Lithuania compared survival between patients initiated on once-per-week HD (n = 109) and twice-per-week HD (n = 747) with patients who began dialysis with 3 sessions of HD per week (n = 1207), and concluded that HD frequency of less than 3 times per week was an independent risk factor for death after adjustment for age, sex, and primary kidney disease (relative risk = 1.92, 95% CI = 1.643–2.24, P < .001).93 A larger study analyzed incident ESKD population from the USRDS, initiated on HD between 2007 and 2011, including 434 patients treated with less frequent (twice-weekly or less) HD and 50,162 patients treated with conventional (thrice-weekly) HD, matched by demographic characteristics (age, sex, race, and central venous catheter as vascular access) and comorbidity burden. After adjustment for RKF, all-cause mortality was not significantly different in the less frequent compared with conventional HD group (hazard ratio = 0.88, 95% CI = 0.72–1.08).53 In another retrospective study of patients initiated on renal replacement with twice-weekly HD, those who survived longer than 3 months had higher mortality if the they had baseline Kru ≤3 ml/min per 1.73 m2, urine volume ≤600 ml/d, or interdialytic weight gains ≥6% of the dry weight; importantly, follow-up Kru levels were not evaluated in this study.64 Together, these reports suggest the possibility that twice-weekly HD, when maintained for an unchecked duration after dialysis initiation, could impose negative effects on patient outcomes, likely due to occult erosion of RKF.
Apart from better preservation of intrinsic kidney function, other potential advantages of an initial regimen of less intensive HD (e.g., less frequent or shorter dialysis) include better patient satisfaction with health-related quality of life and end-of-life concerns, while containing costs and resources.94 Some studies, however, did not find a significant difference in the overall patient-reported quality of life and depression screening in patients treated with incremental HD versus those receiving conventional thrice-weekly HD.95,96 Two sessions of HD per week upon initiating dialysis therapy means less frequent cannulations of a new arteriovenous access, which may prolong fistula or graft longevity. Vascular access−related events (first event of repair, loss, or access-related hospitalization) were analyzed in a trial that randomly assigned 245 prevalent dialysis patients to receive in-center 6-times-per-week HD or HD 3-times-per-week HD and 87 patients to receive home nocturnal 6-times-per-week HD or 3-times-per-week HD for 12 months.97 Across all participants, frequent HD significantly increased the risk of vascular access complications (hazard ratio = 1.76, 95% CI = 1.11–2.79); in the subgroup of 198 patients using an arteriovenous fistula or graft at randomization, there was a similarly increased risk of vascular access−related events with frequent HD (hazard ratio = 1.90, 95% CI = 1.11–3.25).97 The converse—namely, that less frequent access cannulation would lead to better vascular access outcomes—cannot be inferred, and at present there is no evidence that twice-weekly versus thrice-weekly access cannulation has an impact on vascular access longevity. It can also be conjectured that buttonhole access cannulation might be safely applied in patients who undergo once- or twice-weekly HD; however, access outcomes using this cannulation technique with less frequent HD are still to be studied.
Potential Disadvantages and Barriers in Prescribing Less Intensive HD
To achieve widespread scientific recognition and clinical implementation of incremental HD, many obstacles ought to be overcome. Table 198 summarizes the main barriers to incremental HD as well as potential solutions, some of which have been discussed above. It is of utmost importance that patient adherence and survival are rigorously evaluated in clinical trials before large-scale implementation of different schedules of HD. Undoubtedly, some apprehension in prescribing twice-weekly HD may stem from the reports of increased mortality after longer interdialytic intervals, possibly caused by higher interdialytic weight gains and/or hyperkalemia.99,100 These data, nevertheless, come from the analysis of the prevalent dialysis population, most of whom have little or no RKF. It can be contended that, in incident patients with early-stage ESKD and RKF, proper use of pharmacologic measures and timely conversion from twice-weekly to thrice-weekly HD can prevent large interdialytic weight gains and severe hyperkalemia. Indeed, the variability in RKF loss, coupled with the low success rate of obtaining serial measurements in RKF, create room for error and uncertainty in deciding on the timing of transition from a less frequent to a thrice-weekly HD schedule. In a cohort of 434 patients with incident ESKD treated with less frequent incremental HD, 155 patient (36%) transitioned to conventional HD treatment frequency after a median of 9 months (interquartile range, 3−15) of a less frequent HD treatment schedule (i.e.,<2.5 HD treatment sessions per week).53 In another small cohort of 23 patients with incident ESKD initiated on twice-weekly HD, time to transition to thrice-weekly HD was highly variable, after a range of 30 to 1255 days on twice-weekly HD.54 Virtually all other studies on incremental in-center HD did not record the point of transition from less frequent to thrice-weekly HD.64,69,93,94,101,102
Table 1.
Perceived barriers to incremental hemodialysis
| Obstacle | Reason | Potential solution |
|---|---|---|
| Concern for inadequate clearance of uremic solutes (including solutes other than urea) due to insidious and unpredictable loss of RKF | No study done to determine the required minimum amount of solute clearance in patients with incident ESKD, with or without appreciable RKF, starting HD. At risk for untimely transition from less frequent to conventional thrice-weekly HD treatments |
Time-delineated incremental HD |
| Concern for insidious onset of volume overload and adverse clinical outcomes | In patients on conventional thrice-weekly HD, patient mortality is higher after the longer interdialytic interval. | Aggressive combined diuretic therapy Timely adjustment in target weight and dialysis duration/frequency |
| Undefined effects on patient survival and other important clinical outcomes (e.g., changes in RKF, rate of cardiovascular events, hospitalization, nutrition, vascular access complications, quality of life, control of uremic symptoms) | Retrospective, observational data on incremental HD produced heterogeneous results. | Clinical trials powered to determine the effects of different schedules of incremental HD on patient survival |
| Uncertain patient adherence to serial urine collections | Reliance on potentially inaccurate urine collections. Retrospective studies showed that RKF assessment is not routinely done, even at dialysis units with structural organization for incremental HD. |
An incentive for patients to collect urine is incremental dialysis (less frequent and/or shorter HD sessions) |
| Uncertain patient adherence to recommended changes in HD treatment frequency or length | Many nephrologists experience patient refusal when increasing the dialysis frequency/time; this risk has not been systematically quantified. | Set expectations from the outset: when the time comes to increase HD dose, the discussion is about how to do it, not whether it will be done. Leadership and firmness must accompany the empathy for the added dialysis burden.98 |
| Faulty identification of patients who can undergo incremental HD | Assessment of RKF may be inaccurate. Confusion among nephrologists about the suitable tests to assess RKF A large panel of criteria to identify patients suitable for incremental HD perceived as not pragmatic |
Apply incremental HD to cases of certain suitability Simplify suitability criteria to consist mainly of urine output volume and patient volume status |
| Added workload for the dialysis staff and nephrologist | Requirement of additional medical team members to monitor serial assessment of RKF and to implement changes in HD prescription in a timely manner | Operationalize the process of serial urine collections Develop an automatic electronic system to calculate RKF, Kru (based on interdialytic urine collection), and the required dialysis spKt/Vurea (based on HD prescription) to achieve desired stdKt/Vurea |
| Shortfall in financial reimbursement for all dialysis stakeholders | Per current reimbursement model, payment is based on the number of dialysis sessions delivered per patient. | Use shorter, thrice-weekly HD as the form of incremental HD; this approach bears no financial shortfalls, unless spKt/Vurea reports <1.2. |
ESKD, end-stage kidney disease; HD, hemodialysis; RKF, residual kidney function.
There is also some confusion among nephrologists about the suitable tests to assess RKF: urea clearance, average urea and creatinine clearance, 24-hour urine output, or interdialytic urine output. The latest literature about incremental HD uses urea clearance and urine output. Concern for lack of patient adherence to changes in HD frequency and/or length hinders the practicability of incremental in-center HD. An open HD regimen of less frequent schedule may generate unrealistic expectations to many dialysis patients that less frequent HD can continue for lengthy periods of time. This practical limitation may be averted by an initial implementation of a stepped HD regimen, in which the boundary of twice-weekly HD is delineated upfront.
A Step Forward
An important feature of the incremental transition to dialysis is its patient-centeredness that is consistent with precision medicine in ESKD.103 To this end, there has been an exponential interest in incremental transition to dialysis among patient groups who believe that this is a more gentle and patient-friendly approach as opposed to the traditional outright 3-times-per-week HD schedule under the current standard of care. Incremental dialysis may become the treatment of choice under the End-stage renal disease Seamless Care Organizations (ESCO) as well as under the US government’s 12 July 2019 Executive Order on “Advancing American Kidney Health Initiative” (AAKHI), given that more home HD therapy means higher utility of incremental dialysis protocols.104
Notwithstanding these developments, the lack of clinical trial−based evidence in the prescription of HD initiation means that the clinical safety, clinical effectiveness, and cost-effectiveness of 1 regimen versus another have not been established; this lack of evidence highlights the need for robust primary research. We and other researchers have launched pilot trials as a first step to moving a new process of HD initiation into the research arena. Table 2105, 106, 107, 108, 109, 110 summarizes the designs of several recently initiated and/or ongoing pilot trials that study safety and effectiveness of less frequent HD in patients with incident ESKD, most of which consist of twice-weekly HD regimens at dialysis initiation. Different research designs will shed different amounts of light on how treatments work under controlled conditions. To reduce the inherent limitations associated with dependence on frequent measurements of RKF, we are studying the feasibility of a regimen of stepped HD as a pragmatic alternative of incremental HD in the TWOPLUS pilot trial (NCT03740048). With this regimen, patients with incident ESKD and clinical evidence of satisfactory RKF (i.e., urine output ≥500 ml/d) are randomized to either a dialysis regimen of time-limited twice-weekly in-center HD schedule and adjuvant pharmacologic therapy (i.e., effective aggressive diuretics, patiromer, and sodium bicarbonate) for 6 weeks, continued with thrice-weekly HD, or a standard regimen of thrice-weekly in-center HD. We believe that a time-delineated prescription of twice-weekly HD, whether of 6 weeks or longer, will prevent extension of less frequent HD schedules into late-stage ESKD when there is a high risk of unexpected, sudden loss of RKF. Although the timeframe of 6 weeks for twice-weekly in-center HD used in the TWOPLUS pilot trial may seem arbitrary, a conservative approach was elected because of untested clinical and financial repercussions. To our knowledge, there has been no study to prospectively record, in patients with incident ESKD, longitudinal changes in RKF based on different schedules of HD frequency. Based on other reports and our experience, enough RKF to decrease the dialysis burden at HD initiation may last from 4 to 6 weeks at a minimum to as much as 4 to 6 years, with an average timeframe of 4 to 12 months.98 Given the intention to apply less frequent in-center HD on a larger scale in the TWOPLUS trial, agnostic of eventual fiscal ramifications and patient compliance with timed urine collections and changes in HD frequency, we cautiously structured time-delineated twice-weekly HD by choosing the shorter duration of time when meaningful RKF would be present to allow safe prescription of less frequent HD.
Table 2.
Summary of randomized pilot trials in the study of less frequent hemodialysis
| Principal investigator(s), study, N = planned participant enrollment (per arm) |
Intervention arm | Comparator arm | Key enrollment criteria | Primary Outcome | Country and date of application | Clinical Trial Registry number |
|---|---|---|---|---|---|---|
| Diera et al.105 Incremental Hemodialysis in Incident Patients (IHDIP) N = 75 |
Once-weekly HD. The number of HD sessions per week is increased to 2 and later to 3 per criteria for progression. | Thrice-weekly HD |
|
Patient survival | Spain, August 2017 |
NCT03239808 |
| Fernándex and Teruel106 Incremental Hemodialysis as a Starting Way of Renal Replacement Therapy N = 42 |
Twice-weekly HD | Thrice-weekly HD |
|
Change in RKF | Spain, October 2017 |
NCT03302546 |
| Vilar107 Incremental HD N = 25 |
Twice-weekly HD. The dialysis dose is adjusted according to measurement of RKF. | Thrice-weekly HD |
|
Feasibility | United Kingdom, February 2018 |
NCT03418181 |
| Murea108 The TWOPLUS-HD Pilot Trial N = 101 |
Twice-weekly HD for 6 wk plus adjuvant pharmacologic therapy (diuretics, potassium binder, sodium bicarbonate) followed by thrice-weekly HD. | Thrice-weekly HD |
|
Feasibility | North Carolina, USA, November 2018 |
NCT03740048 |
| White109 Dialysis-Less Frequently In The Elderly (D-LITE) N = 20 |
Twice-weekly HD | Thrice-weekly HD |
|
Feasibility | Canada, December 2018 |
NCT03787719 |
| Sirich110 Efficacy of Twice Weekly Hemodialysis in Patients With Residual Kidney Function N = 25 |
Twice-weekly HD for 4 wk, cross-over design | Thrice-weekly HD for 4 wk; cross-over design |
|
Kidney disease−related QoL | California, USA, March 2019 |
NCT03874117 |
CKD, chronic kidney disease; ESKD, end-stage kidney disease; HD, hemodialysis; Kru, residual renal urea clearance measured by timed urine collection; QoL, quality of life; RKF, residual kidney function.
Conclusions
Despite growing evidence of potential benefits of less frequent HD in early-stage ESKD, changes in HD practice will require results from randomized trials. To do less for today’s patients than for the patients of the past may make clinicians feel exposed. Less frequent HD might restrict revenue streams. Until more data and knowledge are accrued, most clinicians will treat CKD progression to ESKD with long-standing practice conventions. Pending clinical trials, the ideal incremental HD, which implies that the dose and frequency of dialysis treatments can be lower at dialysis inception, in the presence of a substantial RKF, but should be gradually and timely increased to compensate for subsequent reductions in RKF—determined by interdialytic urine collections—may not be tenable on an immediate and large scale. A stepped regimen of time-delineated twice-weekly in-center HD with adjuvant pharmacologic regimen may set the stage for streamlined practice of less frequent HD and obviate the need for repetitive (and unreliable) urine collections.
Disclosure
The TWOPLUS trial is sponsored by Relypsa Inc/Vifor Pharma (MM). All the other authors declared no competing interests.
References
- 1.Lowrie E.G., Laird N.M., Parker T.F., Sargent J.A. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305:1176–1181. doi: 10.1056/NEJM198111123052003. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G., Beck G.J., Cheung A.K. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 3.Bieber B., Qian J., Anand S. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics and quality of life in the China Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant. 2014;29:1770–1777. doi: 10.1093/ndt/gft472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee C.M., Unruh M., Chen J. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26:720–727. doi: 10.1111/sdi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan R., Mendonca S. Adequacy of twice weekly hemodialysis in end stage renal disease patients at a tertiary care dialysis centre. Indian J Nephrol. 2015;25:329–333. doi: 10.4103/0971-4065.151762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caria S., Cupisti A., Sau G., Bolasco P. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014;15:172. doi: 10.1186/1471-2369-15-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garneata L., Stancu A., Dragomir D. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol. 2016;27:2164–2176. doi: 10.1681/ASN.2015040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garneata L., Mocanu C.A., Mocanu A.E. Vegetarian severe hypoproteic diet supplemented with keto-analogues for predialysis chronic kidney disease patients: the influence on long term prognosis. Nephrol Dial Transplant. 2019;34(suppl 1):i5. [Google Scholar]
- 9.Herselman M., Esau N., Kruger J.M. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition. 2010;26:10–32. doi: 10.1016/j.nut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Lim H.S., Kim H.S., Kim J.K., Park M., Choi S.J. Nutritional status and dietary management according to hemodialysis duration. Clin Nutr Res. 2019;8:28–35. doi: 10.7762/cnr.2019.8.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow G.M., Levin N.W., Beck G.J. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocco M.V., Lockridge R.S., Jr., Beck G.J. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis J.R., Eastwood J.B., Smith E.K. Maintenance haemodialysis. Q J Med. 1969;38:49–89. [PubMed] [Google Scholar]
- 14.Blagg C.R. Hemodialysis 1991. Blood Purif. 1992;10:22–29. doi: 10.1159/000170070. [DOI] [PubMed] [Google Scholar]
- 15.Babb A.L., Farrell P.C., Strand M.J. Residual renal function and chronic hemodialysis therapy. Proc Clin Dial Transplant Forum. 1972;2:142–148. p. 147. [PubMed] [Google Scholar]
- 16.Milutinovic J., Cutler R.E., Hoover P. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975;8:185–190. doi: 10.1038/ki.1975.98. [DOI] [PubMed] [Google Scholar]
- 17.KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 18.European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association Section II. Haemodialysis adequacy. Nephrol Dial Transplant. 2002;17(suppl 7):16–31. [PubMed] [Google Scholar]
- 19.Wish J.B. Quality and accountability in the ESRD program. Adv Ren Replace Ther. 2001;8:89–94. doi: 10.1053/jarr.2001.23990. [DOI] [PubMed] [Google Scholar]
- 20.Gotch F.A., Sargent J.A. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney Int. 1985;28:526–534. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 21.Wang A.Y. The "heart" of peritoneal dialysis. Perit Dial Int. 2007;27(suppl 2):S228–S232. [PubMed] [Google Scholar]
- 22.Li Y., Jin Y., Kapke A. Explaining trends and variation in timing of dialysis initiation in the United States. Medicine. 2017;96:e6911. doi: 10.1097/MD.0000000000006911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin A.I., Appasamy S., Carey R.J., Madan N. Feasibility of incremental 2-times weekly hemodialysis in incident patients with residual kidney function. Kidney Int Rep. 2017;2:933–942. doi: 10.1016/j.ekir.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caravaca-Fontan F., Azevedo L., Luna E., Caravaca F. Patterns of progression of chronic kidney disease at later stages. Clin Kidney J. 2018;11:246–253. doi: 10.1093/ckj/sfx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Hare A.M., Wong S.P., Yu M.K. Trends in the timing and clinical context of maintenance dialysis initiation. J Am Soc Nephrol. 2015;26:1975–1981. doi: 10.1681/ASN.2013050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Renal Data System Chapter 1: Incidence, prevalence, patient characteristics, and treatment modalities annual data report. Atlas of chronic kidney disease and end-stage renal disease in the United States; 2017. https://www.usrds.org/2017/view/v2 01.aspx Available at:
- 27.Wong J., Vilar E., Davenport A., Farrington K. Incremental haemodialysis. Nephrol Dial Transplant. 2015;30:1639–1648. doi: 10.1093/ndt/gfv231. [DOI] [PubMed] [Google Scholar]
- 28.Shafi T., Mullangi S., Toth-Manikowski S.M. Residual kidney function: implications in the era of personalized medicine. Semin Dial. 2017;30:241–245. doi: 10.1111/sdi.12587. [DOI] [PubMed] [Google Scholar]
- 29.Shafi T., Levey A.S. Measurement and estimation of residual kidney function in patients on dialysis. Adv Chronic Kidney Dis. 2018;25:93–104. doi: 10.1053/j.ackd.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabanda A., Jadoul M., Pochet J.M. Determinants of the serum concentrations of low molecular weight proteins in patients on maintenance hemodialysis. Kidney Int. 1994;45:1689–1696. doi: 10.1038/ki.1994.221. [DOI] [PubMed] [Google Scholar]
- 31.Stompor T., Sulowicz W., Anyszek T. Dialysis adequacy, residual renal function and serum concentrations of selected low molecular weight proteins in patients undergoing continuous ambulatory peritoneal dialysis. Med Sci Monit. 2003;9:Cr500–Cr504. [PubMed] [Google Scholar]
- 32.Delaney M.P., Stevens P.E., Al Hasani M. Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2008;51:278–284. doi: 10.1053/j.ajkd.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Marquez I.O., Tambra S., Luo F.Y. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6:290–296. doi: 10.2215/CJN.06100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K., Kestenbaum B. Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol. 2018;13:1291–1296. doi: 10.2215/CJN.12001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong S.C., Sao J.N., Taussig A. Residual function effectively controls plasma concentrations of secreted solutes in patients on twice weekly hemodialysis. J Am Soc Nephrol. 2018;29:1992–1999. doi: 10.1681/ASN.2018010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toth-Manikowski SM, Sirich TL, Meyer TW, et al. Contribution of 'clinically negligible' residual kidney function to clearance of uremic solutes [e-pub ahead of print]. Nephrol Dial Transplant. 10.1093/ndt/gfz042. Accessed January 2, 2020. [DOI] [PMC free article] [PubMed]
- 37.Obi Y., Kalantar-Zadeh K. Incremental and once- to twice-weekly hemodialysis: from experience to evidence. Kidney Int Rep. 2017;2:781–784. doi: 10.1016/j.ekir.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandrini M., Vizzardi V., Valerio F. Incremental peritoneal dialysis: a 10 year single-centre experience. J Nephrol. 2016;29:871–879. doi: 10.1007/s40620-016-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auguste B.L., Bargman J.M. Incremental peritoneal dialysis: new ideas about an old approach. Semin Dial. 2018;31:445–448. doi: 10.1111/sdi.12712. [DOI] [PubMed] [Google Scholar]
- 40.Medcalf J.F., Harris K.P., Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59:1128–1133. doi: 10.1046/j.1523-1755.2001.0590031128.x. [DOI] [PubMed] [Google Scholar]
- 41.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 42.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 43.Jones R.L., Rubal B., Jones S. Prevalence of early repolarization in a large cohort of young adults. J Am Coll Cardiol. 2015;65:A371. [Google Scholar]
- 44.Pitt B., Anker S.D., Bushinsky D.A. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitt B., Bushinsky D.A., Kitzman D.W. Evaluation of an individualized dose titration regimen of patiromer to prevent hyperkalaemia in patients with heart failure and chronic kidney disease. ESC Heart Failure. 2018;5:257–266. doi: 10.1002/ehf2.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal R., Rossignol P., Garza D., Mayo M.R., Warren S., Arthur S., Romero A., White W.B., Williams B. Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: rationale and design of the AMBER study. Am J Nephrol. 2018;48:172–180. doi: 10.1159/000492622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 48.Hur I., Lee Y.K., Kalantar-Zadeh K., Obi Y. Individualized hemodialysis treatment: a perspective on residual kidney function and precision medicine in nephrology. Cardiorenal Med. 2019;9:69–82. doi: 10.1159/000494808. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y.J., Rhee C.M., Kalantar-Zadeh K. Residual kidney function in twice-weekly hemodialysis: irreplaceable contribution to dialysis adequacy. Ann Translat Med. 2018;6:317. doi: 10.21037/atm.2018.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K., Unruh M., Zager P.G. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 52.John K.A., Cogswell M.E., Campbell N.R. Accuracy and usefulness of select methods for assessing complete collection of 24-hour urine: a systematic review. J Clin Hypertens. 2016;18:456–467. doi: 10.1111/jch.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathew A., Obi Y., Rhee C.M. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90:1071–1079. doi: 10.1016/j.kint.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 54.Bowline I.G., Russell G.B., Bagwell B. Temporal trends in fluid management with incremental hemodialysis. Clin Nephrol. 2019;92:165–173. doi: 10.5414/CN109660. [DOI] [PubMed] [Google Scholar]
- 55.Vilar E., Boltiador C., Wong J. Plasma levels of middle molecules to estimate residual kidney function in haemodialysis without urine collection. PLoS One. 2015;10:e0143813. doi: 10.1371/journal.pone.0143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong J., Sridharan S., Berdeprado J. Predicting residual kidney function in hemodialysis patients using serum beta-trace protein and beta2-microglobulin. Kidney Int. 2016;89:1090–1098. doi: 10.1016/j.kint.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 57.Shafi T., Michels W.M., Levey A.S. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89:1099–1110. doi: 10.1016/j.kint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson M.A., Waikar S.S. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–689. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leypoldt J.K., Jaber B.L., Zimmerman D.L. Predicting treatment dose for novel therapies using urea standard Kt/V. Semin Dial. 2004;17:142–145. doi: 10.1111/j.0894-0959.2004.17212.x. [DOI] [PubMed] [Google Scholar]
- 60.Leypoldt J.K. Urea standard Kt/V(urea) for assessing dialysis treatment adequacy. Hemodialysis international. Int Symp Home Hemodial. 2004;8:193–197. doi: 10.1111/j.1492-7535.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 61.Bolasco P., Cupisti A., Locatelli F. Dietary management of incremental transition to dialysis therapy: once-weekly hemodialysis combined with low-protein diet. J Ren Nutr. 2016;26:352–359. doi: 10.1053/j.jrn.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Gotch F.A. The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transplant. 1998;13(suppl 6):10–14. doi: 10.1093/ndt/13.suppl_6.10. [DOI] [PubMed] [Google Scholar]
- 63.Rivara M.B., Ravel V., Streja E. Weekly standard Kt/V urea and clinical outcomes in home and in-center hemodialysis. Clin J Am Soc Nephrol. 2018;13:445–455. doi: 10.2215/CJN.05680517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obi Y., Streja E., Rhee C.M. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68:256–265. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin Y.F., Huang J.W., Wu M.S. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology. 2009;14:59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 66.Levey A.S., Coresh J., Balk E. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 67.Mathew A.T., Fishbane S., Obi Y., Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int. 2016;90:262–271. doi: 10.1016/j.kint.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daugirdas J.T., Greene T., Rocco M.V. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Wang M., Li H. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40:140–150. doi: 10.1159/000365819. [DOI] [PubMed] [Google Scholar]
- 70.Obi Y., Rhee C.M., Mathew A.T. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27:3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maduell F., Gutierrez E., Navarro V. [Evaluation of methods to calculate dialysis dose in daily hemodialysis] Nefrologia. 2003;23:344–349. [PubMed] [Google Scholar]
- 72.Tattersall J., Martin-Malo A., Pedrini L. EBPG guideline on dialysis strategies. Nephrol Dial Transplant. 2007;22(suppl 2):ii5–ii21. doi: 10.1093/ndt/gfm022. [DOI] [PubMed] [Google Scholar]
- 73.Casino F.G., Lopez T. The equivalent renal urea clearance: a new parameter to assess dialysis dose. Nephrol Dial Transplant. 1996;11:1574–1581. [PubMed] [Google Scholar]
- 74.European Best Practice Guidelines for Renal Transplantation, Part 2. Nephrol Dial Transplant. 2002;17(suppl 4):48–49. doi: 10.1093/ndt/17.suppl_4.48. [DOI] [PubMed] [Google Scholar]
- 75.Vartia A. Equivalent continuous clearances EKR and stdK in incremental haemodialysis. Nephrol Dial Transplant. 2012;27:777–784. doi: 10.1093/ndt/gfr383. [DOI] [PubMed] [Google Scholar]
- 76.Casino F.G., Basile C. The variable target model: a paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant. 2017;32:182–190. doi: 10.1093/ndt/gfw339. [DOI] [PubMed] [Google Scholar]
- 77.Bradbury B.D., Fissell R.B., Albert J.M. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 78.Lukowsky L.R., Kheifets L., Arah O.A. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35:548–558. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perl J., Bargman J.M. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Vilar E., Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 81.Shafi T., Jaar B.G., Plantinga L.C. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golper T.A., Mehrotra R. The intact nephron hypothesis in reverse: an argument to support incremental dialysis. Nephrol Dial Transplant. 2015;30:1602–1604. doi: 10.1093/ndt/gfv271. p. 1603. [DOI] [PubMed] [Google Scholar]
- 83.Davenport A. Will incremental hemodialysis preserve residual function and improve patient survival? Semin Dial. 2015;28:16–19. doi: 10.1111/sdi.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vijayan A., Delos Santos R.B., Li T. Effect of frequent dialysis on renal recovery: results from the Acute Renal Failure Trial Network Study. Kidney Int Rep. 2018;3:456–463. doi: 10.1016/j.ekir.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rottembourg J., Issad B., Gallego J.L. Evolution of residual renal functions in patients undergoing maintenance hemodialysis or continuous ambulatory peritoneal dialysis. Proc EDTA. 1983;19:397. [PubMed] [Google Scholar]
- 86.Maiorca R., Brunori G., Zubani R. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10:2295–2305. doi: 10.1093/ndt/10.12.2295. [DOI] [PubMed] [Google Scholar]
- 87.Diaz-Buxo J.A., Lowrie E.G., Lew N.L. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33:523–534. doi: 10.1016/s0272-6386(99)70190-3. [DOI] [PubMed] [Google Scholar]
- 88.Szeto C.C., Wong T.Y., Leung C.B. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000;58:400–407. doi: 10.1046/j.1523-1755.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- 89.Bargman J.M., Thorpe K.E., Churchill D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 90.Paniagua R., Amato D., Vonesh E. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 91.Termorshuizen F., Korevaar J.C., Dekker F.W. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003;41:1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 92.Termorshuizen F., Dekker F.W., van Manen J.G. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 93.Stankuviene A, Ziginskiene E, Kuzminskis V, Bumblyte IA. Impact of hemodialysis dose and frequency on survival of patients on chronic hemodialysis in Lithuania during 1998-2005. Medicina (Kaunas, Lithuania). 2010;46:516–521. [PubMed]
- 94.Hanson J.A., Hulbert-Shearon T.E., Ojo A.O. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19:625–633. doi: 10.1159/000013533. [DOI] [PubMed] [Google Scholar]
- 95.Park J.I., Park J.T., Kim Y.L. Comparison of outcomes between the incremental and thrice-weekly initiation of hemodialysis: a propensity-matched study of a prospective cohort in Korea. Nephrol Dial Transplant. 2017;32:355–363. doi: 10.1093/ndt/gfw332. [DOI] [PubMed] [Google Scholar]
- 96.Golper T.A. Incremental dialysis: review of recent literature. Curr Opin Nephrol Hypertens. 2017;26:543–547. doi: 10.1097/MNH.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 97.Suri R.S., Larive B., Sherer S. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol. 2013;24:498–505. doi: 10.1681/ASN.2012060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golper T.A. Incremental hemodialysis: how I do it. Semin Dial. 2016;29:476–480. doi: 10.1111/sdi.12530. [DOI] [PubMed] [Google Scholar]
- 99.Foley R.N., Gilbertson D.T., Murray T., Collins A.J. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 100.Bleyer A.J., Russell G.B., Satko S.G. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez-Lucas M., Teruel-Briones J.L., Gomis-Couto A. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32:767–776. doi: 10.3265/Nefrologia.pre2012.Jul.11517. [DOI] [PubMed] [Google Scholar]
- 102.Teruel-Briones J.L., Fernandez-Lucas M., Rivera-Gorrin M. Progression of residual renal function with an increase in dialysis: haemodialysis versus peritoneal dialysis. Nefrologia. 2013;33:640–649. doi: 10.3265/Nefrologia.pre2013.May.12038. [DOI] [PubMed] [Google Scholar]
- 103.Gedney N., Kalantar-Zadeh K. Dialysis patient-centeredness and precision medicine: focus on incremental home hemodialysis and preserving residual kidney function. Semin Nephrol. 2018;38:426–432. doi: 10.1016/j.semnephrol.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 104.Moore L.W., Kalantar-Zadeh K. Implementing the "Advancing American Kidney Health Initiative" by leveraging nutritional and dietary management of kidney patients. J Ren Nutr. 2019;29:357–360. doi: 10.1053/j.jrn.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Diera J., Suárez M.A., López F. IHDIP: a controlled randomized trial to assess the security and effectiveness of the incremental hemodialysis in incident patients. BMC Nephrol. 2019;20:8. doi: 10.1186/s12882-018-1189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernándex L.M., Teruel J.L. Hemodiálisis incremental como forma de inicio del tratamiento sustitutivo renal [Incremental hemodialysis schedule at the start of renal replacement therapy] Nefrologia. 2017;37:1–4. doi: 10.1016/j.nefro.2016.08.002. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 107.Vilar E. Does Incremental Initiation of Haemodialysis Preserve Native Kidney Function? Available at: https://clinicaltrials.gov/ct2/show/NCT03418181. Accessed January 2, 2020.
- 108.Murea M. A Pilot Trial of Twice-weekly Versus Thrice-weekly Hemodialysis in Patients With Incident End-stage Kidney Disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03740048. Accessed January 2, 2020.
- 109.White C. Dialysis-Less Frequently In The Elderly. Available at: https://clinicaltrials.gov/ct2/show/NCT03787719. Accessed January 2, 2020.
- 110.Sirich T. Efficacy of Twice Weekly Hemodialysis in Patients With Residual Kidney Function. Available at: https://clinicaltrials.gov/ct2/show/NCT03874117. Accessed January 2, 2020.