Abstract
Introduction
Autoantibodies against the M-type phospholipase A2 receptor (PLA2R) are important markers in the diagnosis and monitoring of primary membranous nephropathy (pMN). For the detection of anti-PLA2R autoantibodies, a standardized recombinant cell-based indirect immunofluorescence assay (RC-IFA) and enzyme-linked immunosorbent assay (ELISA) are widely used, the former providing higher sensitivity but lacking a finely graduated quantification of antibody titers. In this study, we evaluated the diagnostic performance characteristics of a novel standardized chemiluminescence immunoassay (ChLIA) by comparison with the established anti-PLA2R test systems.
Methods
Sera from 155 patients with biopsy-proven pMN and 154 disease controls were analyzed for autoantibodies against PLA2R by the novel ChLIA as well as by ELISA and RC-IFA.
Results
The clinical sensitivity of the ChLIA (83.9%) was higher compared with ELISA (73.5%) and equaled that of RC-IFA (83.2%), at similar specificities (≥99.4%). Among ELISA-negative pMN samples, ChLIA and RC-IFA yielded positive results in 39.0% and 36.6%, respectively. The qualitative agreement amounted to 94.5% (ChLIA vs. ELISA) and 99.4% (ChLIA vs. RC-IFA).
Conclusion
The novel anti-PLA2R ChLIA outperforms the ELISA in detecting patients with pMN and demonstrates almost perfect agreement with RC-IFA. It thus presents a promising alternative tool for accurate anti-PLA2R testing, with the advantage of rapid turnaround times and fully automated random-access processing.
Keywords: anti-PLA2R, autoantibody, chemiluminescence immunoassay, membranous nephropathy, phospholipase A2 receptor
Graphical abstract
Autoantibodies against M-type PLA2R have become a valuable biomarker to differentiate pMN from secondary disease forms (membranous nephropathy due to another underlying disease).1 Moreover, sequential anti-PLA2R titers are used for monitoring disease activity, assessment of response to therapy, and prognosis.2, 3, 4 In recent years, standardized assays for the detection of autoantibodies against PLA2R have been developed.2,3 The RC-IFA is a highly sensitive method with particular capability in detecting very low anti-PLA2R titers, but semiquantitative results make it less adequate for monitoring disease progression and therapeutic response.5,6 Moreover, the suitability of RC-IFA for routine testing is limited because of error-prone subjective interpretation, a long turnaround time, and the requirement of specialized equipment. A quantitative anti-PLA2R ELISA is widely used for diagnosis and follow-up of pMN. Although it has been shown to provide high correlation with RC-IFA at very high specificity, the ELISA lags slightly behind in sensitivity, particularly in cases of therapeutic or spontaneous remission.6,7
Fast access to test results is crucial in clinical practice because early diagnosis and immediate immunotherapy may be life-saving in autoimmune pathologies. Therefore, diagnostic laboratories are increasingly shifting to fully automated random-access systems with focus on bead-based chemiluminescence technology.8
The objective of this study was to evaluate the performance characteristics of a novel standardized anti-PLA2R ChLIA, and to determine if this assay can close the sensitivity gap between ELISA and RC-IFA.
Methods
Patients and Samples
The study included 155 serum samples from patients with pMN who were referred to the INSERM Unit UMR_S1155 at Tenon Hospital (Paris, France). The clinical diagnosis of pMN was supported by histopathology of kidney biopsy4,9 in the absence of associations suggestive of secondary MN. All sera were sampled at around the time of biopsy in nephrotic patients with active disease. Moreover, 154 disease control sera were collected from patients with other biopsy-proven glomerular diseases and systemic autoimmune disorders (Table 1). Control samples were obtained from INSERM Unit UMR_S1155 (Paris, France) and the Department of Rheumatology, Karolinska University Hospital (Stockholm, Sweden). Individual and ethical approval was not mandatory, as patient data and samples were used anonymously.
Table 1.
Patient characteristics
| Panel | N | Female/male | Mean age (range) |
|---|---|---|---|
| Primary membranous nephropathy | 155 | 39/114a | 55 (18–85)b |
| Disease controls | 154 | 110/44 | 42 (18–88) |
| Secondary membranous nephropathy | 6 | 3/3 | 55 (41–61) |
| IgA nephropathy | 10 | 3/7 | 47 (20–85) |
| Focal segmental glomerular sclerosis | 10 | 2/8 | 54 (26–84) |
| Membranoprolifierative glomerulonephritis | 10 | 4/6 | 56 (23–88) |
| Minimal change disease | 17 | 10/7 | 42 (18–87) |
| Lupus nephritis class I-IV | 33 | 29/4 | 36 (18–79) |
| Lupus nephritis class Vc | 34 | 28/6 | 37 (18–62) |
| Systemic lupus erythematosus | 34 | 31/3 | 42 (20–79) |
Information on sex was not available for 2 patients with primary membranous nephropathy.
Information on age (at the time of blood sampling) was not available for 8 patients with primary membranous nephropathy.
Among the 34 patients classified as lupus nephritis (LN) class V, 28 had pure membranous LN, whereas 6 showed membranous and additional proliferative features. LN class V represents a subtype of secondary membranous nephropathy.
Immunoassays
The anti-PLA2R ChLIA (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany) is based on magnetic beads coated with recombinant human PLA2R1 that was expressed in human embryonic kidney cells and purified as described previously.6 The assay was performed fully automatically on a random-access analyzer (EUROIMMUN). All assay reagents were contained in a reagent cartridge, including PLA2R-coated beads, acridinium ester-conjugated anti-human IgG secondary antibodies (tracer), sample buffer, and diluent. Within the device, sample buffer and beads were transferred into a cuvette and patient sample was added at a dilution of 1:40. After 10 minutes at 37 °C, unbound antibodies were removed by repeated magnetic force–mediated sedimentation and washing of the beads. Acridinium ester-conjugated anti-human IgG was then added and allowed to bind to the immobilized antibodies for 10 minutes at 37 °C. The beads were sedimented and washed to remove unbound conjugate, followed by the addition of alkaline hydrogen peroxide to trigger the emission of light. The luminescence output from this reaction, which is directly proportional to the amount of anti-PLA2R bound to the antigen-coated beads, was measured luminometrically in relative light units over 10 seconds. Using a predefined lot-specific master curve and integrating the results of 2 calibrators, the system generated a standard curve adapted to the device in use. Based on this standard curve, the results were automatically converted from relative light units into chemiluminescent units per milliliter (CU/ml). In accordance with the manufacturer’s recommendations, results ≥10 CU/ml were considered as positive.
The anti-PLA2R ELISA and RC-IFA (both EUROIMMUN) were performed and evaluated as described before using the manufacturer’s cutoff values.6
Statistics
Data were evaluated statistically using GraphPad Prism (GraphPad Software Inc., La Jolla, CA). Confidence intervals (95% CI) were calculated according to the modified Wald method. To examine the discriminatory ability of the assays, receiver operating characteristics curve analysis was carried out. Cohen’s kappa test was performed to analyze the agreement between portions, with kappa (κ) values corresponding to almost perfect (0.81–1.00), substantial (0.61–0.80), moderate (0.41–0.60), fair (0.21–0.40), slight (0.01–0.20), and no (≤0) agreement. Spearman’s rank correlation test was used to determine the degree of correlation between assays. P values <0.05 were considered significant.
Results
Diagnostic Performance Characteristics of ChLIA, ELISA, and RC-IFA
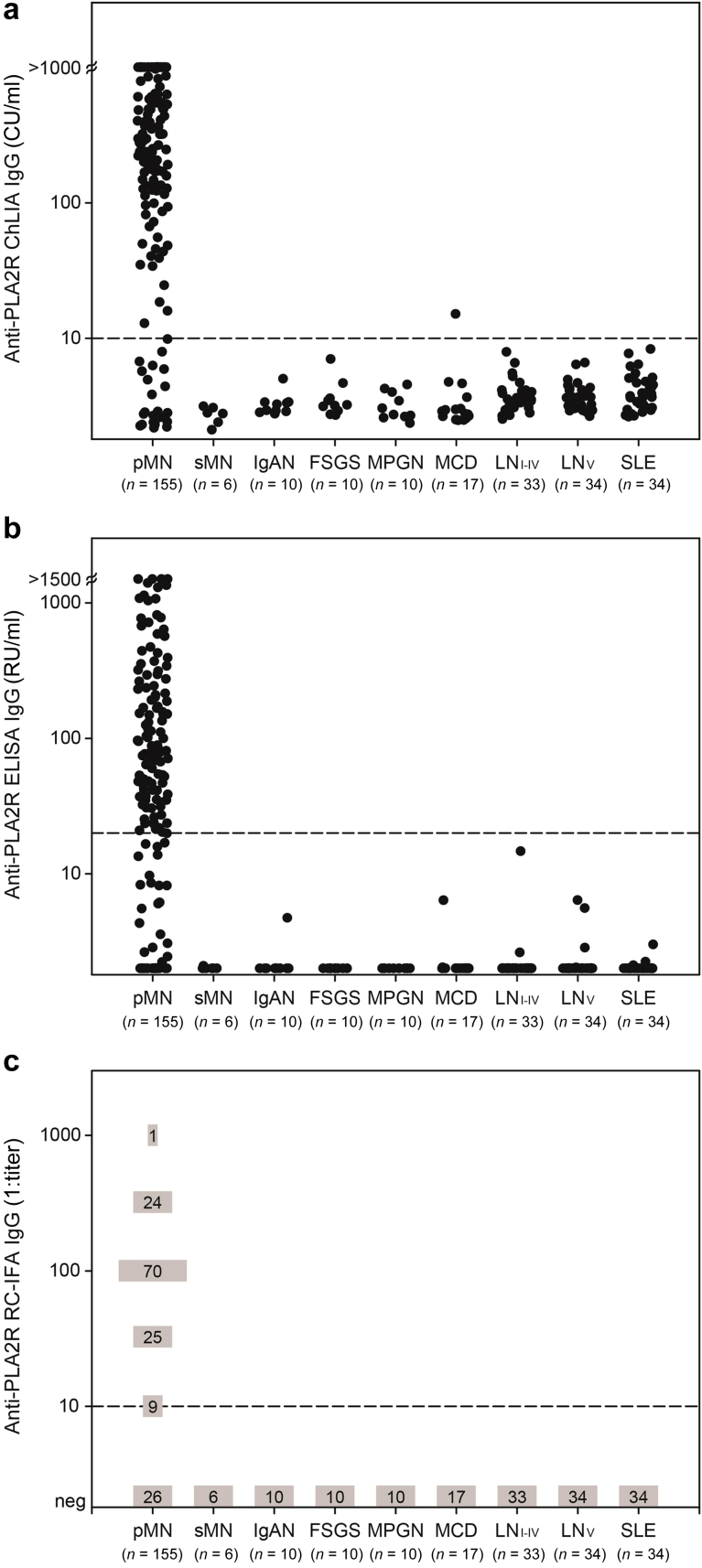
Clinical sensitivity and specificity were assessed in 155 patients with biopsy-proven pMN and 154 disease controls, respectively. The ChLIA was capable of detecting anti-PLA2R autoantibodies in 16 additional patients compared with ELISA and 1 additional patient compared with RC-IFA, who was positive by Western blot and biopsy staining (Supplementary Table S1). Thus, the ChLIA demonstrated a higher sensitivity (83.9%) for diagnosing pMN than ELISA (73.5%) and equalled RC-IFA (83.2%). Specificity was similarly high, ranging between 99.4% (ChLIA) and 100% (ELISA, RC-IFA). Only 1 control sample (minimal change disease) yielded discrepant qualitative results, showing anti-PLA2R reactivity exclusively by ChLIA with antibody levels only marginally above the cutoff; this patient was negative for PLA2R staining in the biopsy (Table 2, Figure 1). Among the 41 pMN samples that tested negative by ELISA, anti-PLA2R reactivity was detectable by ChLIA and RC-IFA in 16 (39.0%) and 15 (36.6%) cases, respectively, with most yielding results in the low to moderate positive range by ChLIA and RC-IFA (Supplementary Table S1).
Table 2.
Clinical sensitivity and specificity of the Anti-PLA2R ChLIA, ELISA, and RC-IFA
| Panel | n | Anti-PLA2R–positive |
||
|---|---|---|---|---|
| Anti-PLA2R ChLIA (cutoff 10 CU/ml)a | Anti-PLA2R ELISA (cutoff 20 RU/ml)a,b | Anti-PLA2R RC-IFA (cutoff titer 1:10)a | ||
| pMN | 155 | 130 | 114 | 129 |
| Sensitivity (95% CI) | 155 | 83.9% (77.2%–88.9%) | 73.5% (66.1%–79.9%) | 83.2% (76.5%–88.3%) |
| sMN | 6 | 0 | 0 | 0 |
| IgAN | 10 | 0 | 0 | 0 |
| FSGS | 10 | 0 | 0 | 0 |
| MPGN | 10 | 0 | 0 | 0 |
| MCD | 17 | 1 | 0 | 0 |
| LN I-V | 33 | 0 | 0 | 0 |
| LN V | 34 | 0 | 0 | 0 |
| SLE | 34 | 0 | 0 | 0 |
| Specificity (95% CI) | 154 | 99.4% (96.1%–100%) | 100% (97.1%–100%) | 100% (97.1%–100%) |
Anti-PLRA2, anti–phospholipase A2 receptor; ChLIA, chemiluminescence immunoassay; CI, confidence interval; CU/ml, chemiluminescent units per milliliter; ELISA, enzyme-linked immunosorbent assay; FSGS, focal segmental glomerular sclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MCD, minimal change disease; MPGN, membranoprolifierative glomerulonephritis; pMN, primary membranous nephropathy; RC-IFA, recombinant cell-based indirect immunofluorescence assay; RU, relative units; SLE, systemic lupus erythematosus; sMN, secondary membranous nephropathy.
Cutoff recommended by the manufacturer.
Borderline results (≥14 to <20 RU/ml) were considered as negative.
Figure 1.
Anti–phospholipase A2 receptor (anti-PLA2R) reactivity as determined in 155 patients with primary membranous nephropathy (pMN) and in 154 disease controls using (a) chemiluminescence immunoassay (ChLIA), (b) enzyme-linked immunosorbent assay (ELISA), and (c) recombinant cell-based indirect immunofluorescence assay (RC-IFA). To avoid excessive overlap of data points at the distinct titer classes (negative, 1:10, 1:32, 1:100, 1:320, 1:1000), the results of RC-IFA are indicated as absolute frequencies. Dashed lines represent the cutoff values for positivity. CU/ml, chemiluminescent units per milliliter; FSGS, focal segmental glomerular sclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MCD, minimal change disease; MPGN, membranoprolifierative glomerulonephritis; RU, relative units; SLE, systemic lupus erythematosus; sMN, secondary membranous nephropathy.
Receiver operating characteristics curve analysis revealed high areas under the curve for ChLIA (0.899), ELISA (0.927), and RC-IFA (0.916), indicating similar discrimination between patients with pMN and disease controls. ChLIA and RC-IFA outperformed the ELISA in terms of the maximum sum of sensitivity and specificity and with regard to sensitivity at predefined specificities. The manufacturer’s cutoff (10 CU/ml) of the novel ChLIA lies slightly above the optimal cutoff (9.1 CU/ml), ensuring a specificity >99% (Table 3, Supplementary Figure S1).
Table 3.
Overall test characteristics
| Test variable | Anti-PLA2R ChLIAa | Anti-PLA2R ELISAa | Anti-PLA2R RC-IFAa |
|---|---|---|---|
| Area under the curve (95% CI) | 0.899 (0.854–0.944) | 0.927 (0.894–0.961) | 0.916 (0.880–0.952) |
| Maximum sum of sensitivity and specificity, % (cutoff) | 183.9 (9.1) | 181.3 (3.0) | 183.2 (1:5) |
| Sensitivity at 98% specificity, % (cutoff) | 85.2 (7.8) | 81.9 (5.8) | NA |
| Sensitivity at 99% specificity, % (cutoff) | 84.5 (9.1) | 80.7 (7.3) | NA |
| Sensitivity at 100% specificity, % (cutoff) | 83.2 (15.5) | 76.1 (15.2) | 83.2 (1:5) |
Anti-PLRA2, anti–phospholipase A2 receptor; ChLIA, chemiluminescence immunoassay; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; NA, not available; RC-IFA, recombinant cell-based indirect immunofluorescence assay; RU, relative units.
Cutoff values are indicated in chemiluminescent units per milliliter (ChLIA), RU/ml (ELISA), and titer (RC-IFA).
Correlation Between ChLIA and ELISA
High overall concordance was found between qualitative anti-PLA2R results obtained by ChLIA and ELISA, as reflected by an agreement of 94.5% (95% CI: 91.3%–96.6%) and a κ-value of 0.885 (95% CI: 0.833–0.938). A total of 114 samples (all pMN) were positive and 178 (25 pMN, 153 controls) negative by both methods (Figure 2). Seventeen samples (16 pMN, 1 minimal change disease) yielded discrepant results, that is, all of them reacted positively by ChLIA, whereas ELISA reactivity was in the borderline range in 4 cases and negative in 13 cases. Spearman’s rank correlation analysis revealed a significant correlation between both assays (r = 0.978, 95% CI: 0.969–0.984, P < 0.001; Figure 3a).
Figure 2.
Venn diagram showing the correlation among chemiluminescence immunoassay (ChLIA), enzyme-linked immunosorbent assay (ELISA), and recombinant cell-based indirect immunofluorescence assay (RC-IFA) for the detection of anti–phospholipase A2 receptor (anti-PLA2R) autoantibodies in a total of 309 sera (155 primary membranous nephropathy, 154 disease controls). Percent values indicate the overall qualitative agreement between 2 adjacent assays.
Figure 3.
Correlation between anti–phospholipase A2 receptor (anti-PLA2R) levels in 155 patients with primary membranous nephropathy measured by (a) chemiluminescence immunoassay (ChLIA) versus enzyme-linked immunosorbent assay (ELISA) and (b) ChLIA versus RC-IFA. Axes are displayed in logarithmic scale. Dashed lines represent cutoff values for positivity. Correlation coefficients and P values were calculated using the Spearman’s rank correlation test. CU/ml, chemiluminescent units per milliliter; RU, relative units.
The analytical imprecision expressed as coefficients of variation for positive, and near cutoff samples were calculated as within-run coefficients of variation (2.1%–6.5%) and total coefficients of variation (5.0%–10.3%) (Supplementary Table S2). These results were similar to repeatability and reproducibility of the ELISA.6
Correlation Between ChLIA and RC-IFA
The anti-PLA2R ChLIA and RC-IFA yielded concordant results in 99.4% (95% CI: 97.5%–100%) of cases, with a κ-value of 0.987 (95% CI: 0.968–1.000), indicating almost perfect agreement. A total of 129 samples (all pMN) were positive and 178 (25 pMN, 153 controls) negative by both methods (Figure 2). There were only 2 samples (1 pMN, 1 minimal change disease) with divergent qualitative results, both showing low positive ChLIA reactivity, whereas RC-IFA was negative. The Spearman’s rank coefficient indicated strong correlation between ChLIA results and RC-IFA titers (r = 0.894, 95% CI: 0.856–0.923, P < 0.001; Figure 3b).
Discussion
The present study investigated the diagnostic performance of a novel anti-PLA2R ChLIA in comparison with the established ELISA and RC-IFA. The clinical sensitivity of the ChLIA exceeded that of ELISA and RC-IFA by 10.4% and 0.7%, respectively, at similar specificities (>99%). The anti-PLA2R–positive rates detected by ChLIA (83.9%), ELISA (73.5%), and RC-IFA (83.2%) were equal to or higher than the prevalence data determined among non-preselected patients with pMN by different methods, such as Western blot (53.0%–81.7%),10,11 RC-IFA (48.0–82.3%),12,13 ELISA (50.0–71.8%),14,15 addressable laser bead immunoassay (51.5–66.9%),16,17 luciferase immunoprecipitation systems assay (53.3%),18 and time-resolved fluoroimmunoassay (71.0–89.7%).19,20 These variations may be due to differences in assay techniques (e.g., epitope exposure, cutoff values, detected Ig subclass) and cohort characteristics (e.g., ethnicity, immunosuppressive treatment). Recently, Burbelo et al.18 reported a quantitative PLA2R-NanoLuc luciferase immunoprecipitation system assay that provides high diagnostic performance (receiver operating characteristics area under the curve = 1.0) and is, just like the novel ChLIA, more sensitive in detecting anti-PLA2R seropositivity than the ELISA. In the respective pMN panels, luciferase immunoprecipitation system found 1 and ChLIA found 16 additional anti-PLA2R–positive samples compared with ELISA.
Most published studies using the EUROIMMUN anti-PLA2R ELISA adopted the manufacturer-recommended cutoff (20 relative units [RU]/ml), resulting in specificities ranging between 89.7% and 100%.7,14,16,21, 22, 23, 24 However, some studies used customized thresholds to increase sensitivity, sometimes leading to adverse effects on specificity.16,21,22,24 In the present study, receiver operating characteristics analysis revealed an optimum cutoff value of 3.0 RU/ml (maximum sum sensitivity and specificity). Applying this cutoff, 17 additional pMN samples would have been positive by ELISA (sensitivity 84.5%), whereas specificity would fall from 100% to 96.8%, which we consider unacceptable. Bobart et al.25 recommended for centers preferentially performing ELISA, that samples giving ELISA values in the range between ≥2 and ≤20 RU/ml should be confirmed by RC-IFA owing to higher assay sensitivity.
In conclusion, the novel ChLIA is superior to ELISA in detecting autoantibodies against PLA2R, indicating its capability to improve diagnosis and follow-up testing of pMN. Its performance characteristics hold the potential for overcoming the sensitivity gap that exists between the established ELISA and RC-IFA, without compromising specificity. Fully automated random-access processing allows for rapid turnaround times and the option to incorporate the ChLIA into testing lines, giving laboratories with different requirements a higher degree of flexibility in their routines.
Disclosure
CD and SS are employees and WS is a board member of EUROIMMUN. EUROIMMUN is exclusive licensee of patents pertaining to the detection of autoantibodies to PLA2R. All the other authors declared no competing interests.
Acknowledgments
The authors gratefully acknowledge Janine Stockdreher, Martina Lehmann, Sarah Konitzer, and Christine Maaßen for excellent technical assistance, and Alexander Kühnl for support in scientific communication. PR is a recipient of European Research Council ERC-2012-ADG_20120314 grant 322947 and 7th Framework Programme of the European Community contract 2012–305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases), and the National Research Agency grant MNaims (ANR17-CE17-0012-01). We are greatly indebted to the clinicians who took care of the patients in the Department of Nephrology and Dialysis at Tenon Hospital, Paris, over the past 20 years. We thank Eva Compérat and all staff members of the Tenon Hospital Biological Resource Center (BRC CANCER HUEP-Paris) for their help in centralizing and managing biological data collection.
Footnotes
Figure S1. Assay comparison using receiver operating characteristics (ROC) curve analysis for the discrimination between patients with pMN (n = 155) and disease controls (n = 154). The diagonal line indicates no discrimination (area under the curve: 0.5).
Table S1. Reactivity in anti-PLA2R ChLIA and RC-IFA among 41 pMN samples that tested negative by anti-PLA2R ELISA. Positive results are highlighted.
Table S2. Coefficients of variation (CV) for the anti-PLA2R ChLIA. Five representative samples with target values covering the positive and near cutoff measuring range were used to determine anti-PLA2R levels using the newly developed ChLIA. CV = SD/mean expressed as a percentage.
Supplementary Material
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vriese A.S., Glassock R.J., Nath K.A. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlumberger W., Hornig N., Lange S. Differential diagnosis of membranous nephropathy with autoantibodies to phospholipase A2 receptor 1. Autoimmun Rev. 2014;13:108–113. doi: 10.1016/j.autrev.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Ronco P., Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 5.Hoxha E., Harendza S., Zahner G. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 6.Dähnrich C., Komorowski L., Probst C. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Wei D., Zhou Z. Anti-PLA2R antibodies in Chinese patients with membranous nephropathy. Med Sci Monit. 2016;22:1630–1636. doi: 10.12659/MSM.896090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinquanta L., Fontana D.E., Bizzaro N. Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Auto Immun Highlights. 2017;8:9. doi: 10.1007/s13317-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debiec H., Ronco P. Immunopathogenesis of membranous nephropathy: an update. Semin Immunopathol. 2014;36:381–397. doi: 10.1007/s00281-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 10.Qin W., Beck L.H., Jr., Zeng C. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama S., Akiyama M., Imai E. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19:653–660. doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei S.Y., Wang Y.X., Li J.S. Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol. 2016;43:129–140. doi: 10.1159/000445361. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y., Miura N., Debiec H. Circulating antibodies to alpha-enolase and phospholipase A2 receptor and composition of glomerular deposits in Japanese patients with primary or secondary membranous nephropathy. Clin Exp Nephrol. 2017;21:117–126. doi: 10.1007/s10157-016-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hihara K., Iyoda M., Tachibana S. Anti-phospholipase A2 receptor (PLA2R) antibody and glomerular PLA2R expression in Japanese patients with membranous nephropathy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofstra J.M., Debiec H., Short C.D. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behnert A., Schiffer M., Muller-Deile J. Antiphospholipase A(2) receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res. 2014;2014:143274. doi: 10.1155/2014/143274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behnert A., Fritzler M.J., Teng B. An anti-phospholipase A2 receptor quantitative immunoassay and epitope analysis in membranous nephropathy reveals different antigenic domains of the receptor. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burbelo P.D., Beck L.H., Jr., Waldman M. Detection and monitoring PLA2R autoantibodies by LIPS in membranous nephropathy. J Immunol Methods. 2017;444:17–23. doi: 10.1016/j.jim.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q., Huang B., Xiaobin L. Ultrasensitive quantitation of anti-phospholipase A2 receptor antibody as a diagnostic and prognostic indicator of idiopathic membranous nephropathy. Sci Rep. 2017;7:12049. doi: 10.1038/s41598-017-12014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang B., Wang L., Zhang Y. A novel time-resolved fluoroimmunoassay for the quantitative detection of antibodies against the phospholipase A2 receptor. Sci Rep. 2017;7:46096. doi: 10.1038/srep46096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmermans S.A., Damoiseaux J.G., Heerings-Rewinkel P.T. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol. 2014;142:29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 22.Dou Y., Zhang L., Liu D. The accuracy of the anti-phospholipase A2 receptor antibody in the diagnosis of idiopathic membranous nephropathy: a comparison of different cutoff values as measured by the ELISA method. Int Urol Nephrol. 2016;48:845–849. doi: 10.1007/s11255-016-1263-6. [DOI] [PubMed] [Google Scholar]
- 23.Pang L., Zhang A.M., Li H.X. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy: a cross-sectional study. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Li X., Ma C. Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin Chim Acta. 2018;476:9–14. doi: 10.1016/j.cca.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Bobart S.A., De Vriese A.S., Pawar A.S. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019;95:429–438. doi: 10.1016/j.kint.2018.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.