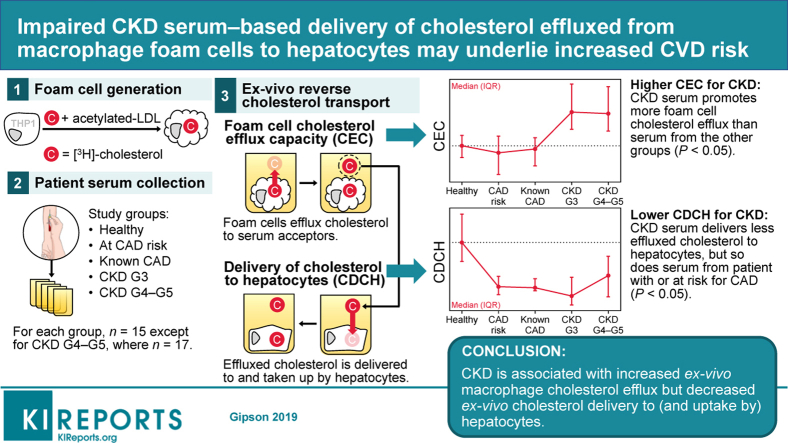
Abstract
Introduction
Although chronic kidney disease (CKD) is associated with increased risk for coronary artery disease (CAD), the underlying mechanisms are not completely defined. In the present study, we tested the hypothesis that flux of cholesterol from macrophage foam cells to liver is impaired in subjects with CKD.
Methods
Consecutive healthy patients, patients with at least 1 CAD risk factor, patients with established CAD, and patients with CKD stages G3 to G5 (n ≥ 15/group) were recruited prospectively. The ability of total patient serum without any modifications to (i) facilitate efflux of cholesterol from human THP1-macrophage foam cells under physiological conditions (cholesterol efflux capacity [CEC]) and (ii) to deliver this effluxed cholesterol to primary hepatocytes with physiological expression of high-density lipoprotein (HDL) receptor SR-BI (capacity to deliver cholesterol to hepatocytes [CDCH]) was evaluated.
Results
Although healthy patients, patients with at least 1 CAD risk factor, and patients with established CAD all showed similar CEC, patients with CKD showed significantly higher CEC. CDCH was significantly lower in all groups compared with the healthy patients; however, when corrected for higher CEC, CDCH in patients with CKD was significantly lower than in patients with CAD. CDCH correlated with age, body mass index, metabolic parameters, inflammatory markers, and kidney function markers (estimated glomerular filtration rate [eGFR], serum creatinine, and serum cystatin C).
Conclusions
These results suggest that aberrations in delivery of cholesterol effluxed from macrophage foam cells to liver for final elimination or the last step of reverse cholesterol transport, may underlie the increased risk of CAD in patients with CKD.
Keywords: cardiovascular disease risk, chronic kidney disease, delivery of cholesterol to the liver, reverse cholesterol transport, serum cholesterol efflux capacity
Graphical abstract
CKD is associated with high risk for CAD, myocardial infarction, and stroke, and collectively cardiovascular disease is the primary cause of morbidity and mortality in patients with chronic, including end-stage, kidney disease.1,2 Several cross-sectional studies have also identified higher prevalence of CAD risk factors in patients with CKD compared with the population with normal kidney function.3,4 Specifically, CAD risk is 1.5- to 3.0-fold greater for patients with CKD compared with patients without CKD even before dialysis.5 Clinical as well as experimental studies have demonstrated that renal injury potentiates hyperlipidemia and subsequent atherosclerosis.6,7 Dyslipidemia in advanced CKD, the most important risk factor for CAD, is associated with increased plasma concentration of very low density lipoprotein and chylomicrons secondary to reduced lipoprotein lipase8 and hepatic lipase9 and underexpression of very low density lipoprotein receptor.10 In addition, reduced hepatic apoA1 secretion and impaired HDL maturation11 diminish the role of the anti-atherogenic HDL. Furthermore, increased oxidation of HDL is also observed in patients with end-stage kidney disease.12 However, the mechanisms underlying the observed dyslipidemia and increased risk for CAD in patients with CKD are not completely defined.
Cellular mechanisms underlying the development of atherosclerotic plaques include unregulated uptake of modified low-density lipoprotein (LDL) by macrophages, formation of macrophage foam cells, and accumulation of these lipid-laden foam cells within the arterial intima. Reduction in plasma LDL-cholesterol, or correction of dyslipidemia, is the most widely used therapeutic strategy to reduce atherosclerotic plaque formation; however, significant residual CAD risk remains despite reaching the target goals of LDL-cholesterol levels.13,14 A paradigm shift has occurred from the focus on “absolute” plasma lipoprotein cholesterol levels to “flux” of cholesterol between relevant tissues.15 In this new paradigm, the ability of plasma lipoproteins to facilitate the flux of cholesterol between tissues becomes more important than the cholesterol content at any given time. Although efflux of unesterified or free cholesterol (FC) to FC acceptors in the plasma, namely, apoA1, HDL, or albumin, is the first step in removal of cholesterol from peripheral tissues, delivery of cholesterol to the liver is the final step of this process of reverse cholesterol transport (RCT). Final removal of cholesterol in feces is the only mechanism for eliminating cholesterol from the body. Therefore, for a more complete assessment of RCT, it is critical to not only assess the ability of plasma components to facilitate efflux of FC from macrophages but also the ability to deliver this effluxed cholesterol to the liver for final elimination.
Several studies have assessed the CEC using various methodologies, some of which compromise physiological relevance and with varying results in patients with CAD.16, 17, 18, 19, 20 However, limited information is currently available on the last step of RCT, namely, delivery of effluxed cholesterol to the liver for final elimination. Although hepatic scavenger receptor BI (SR-BI) facilitates selective uptake of HDL cholesterol (HDL-C), the pathways involved in the delivery of albumin- or phospholipid-associated FC to hepatocytes are not completely defined. Combined hepatocyte uptake of cholesterol effluxed to the total plasma acceptors represents a quantifiable parameter of the last step in RCT.
The present study aimed to evaluate the ability of human serum to not only facilitate macrophage foam cell cholesterol efflux (CEC) but also to deliver the cholesterol so-effluxed to hepatocytes (CDCH). We describe a method using a human THP1 macrophage foam cell system in which intracellular cholesterol is esterified and total (unmodified) serum is the extracellular cholesterol acceptor, all with the goal of recapitulating human in vivo physiological conditions. Our data show that despite significantly higher CEC, CDCH was significantly reduced in patients with CKD. Similarly, lower CDCH also was noted in patients with CAD without any change in CEC, indicating defective delivery of cholesterol to liver by serum from patients with CAD. Taken together, these data suggest that increased CAD-associated morbidity and mortality in patients with CKD may be related to reduced CDCH, identifying aberrant hepatic delivery of cholesterol as a novel, nontraditional CAD risk factor.
Methods
Cholesterol Efflux Assays
PMA-differentiated human THP1 macrophages, a physiological relevant human cell line in which cholesterol efflux is mediated by all known pathways relevant to macrophages, were used to determine CEC as described before.21 In brief, THP1 macrophages were loaded with cholesterol using acetylated human LDL (25 μg/ml) and labeled with [3H]-cholesterol (1 μCi/ml) for 48 hours. To maintain relevance to the physiological conditions (i.e., storage of cholesterol in macrophage foam cells as cholesteryl esters or CE and not FC), no acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor was included. After a 24-hour equilibration in serum-free medium, FC efflux was initiated by the addition of 1% serum or cholesterol acceptors at physiological concentrations present in 1% serum (albumin 0.5 mg/ml or HDL 150 μg/ml). Conditioned medium was collected after 4 hours and percent FC efflux was calculated as described.21 When assessing CEC of serum samples from human subjects, a control pooled serum sample was included while performing these cholesterol efflux assays and CEC of serum samples from human subjects was normalized to the percent efflux obtained using this control serum sample and expressed as a percentage.
Uptake of FC Effluxed From Macrophages by Hepatocytes
To choose the most appropriate cell model for assessing uptake of FC effluxed from macrophages, 4 different cell types were initially compared; 2 of these namely Fu5AH rat hepatoma cells and ldl-SRBI cells express high levels of HDL receptor SR-BI; the other two (human hepatocyte cell line HepG2 and mouse primary hepatocytes) express the physiological levels of SR-BI. Primary mouse hepatocytes were isolated as described before.22 Confluent cell monolayers in 12-well tissue culture dishes were incubated with respective growth media containing 20% of efflux medium obtained after FC efflux assays. After 4 hours, cells were washed twice with phosphate-buffered saline, dried, and total lipids extracted in isopropanol. Lipid extracts were dried under nitrogen and radioactivity associated with cellular lipids determined. The delipidated cells were lysed in 1N NaOH and protein concentration determined by BioRad dye binding assay. Total uptake of effluxed FC by hepatocytes was expressed as disintegrations per minute (dpm)/mg cellular protein. Primary mouse hepatocytes were chosen to be the cell model system to evaluate CDCH of serum from human subjects. Total uptake of effluxed FC by primary hepatocytes (dpm/mg protein) was determined and normalized to uptake observed with 20% efflux medium where control pooled serum was used as the acceptor.
Patient Enrollment
The study was approved by the VCU and Hunter Holmes McGuire VAMC institutional review boards. Seventy-seven consecutive patients were enrolled in the following groups: 1, healthy controls; 2, at CAD risk based on traditional CAD risk factors no but no established CAD and no CKD; 3, angiographically confirmed CAD but “normal” kidney function; 4, mild CKD (stage G3); and 5, advanced CKD (stages G4–5). Precise definitions used for patient classification are found in Table 1.23 Although subjects in groups 1, 2, 4, and 5 were enrolled at VCU, patients in group 3 were enrolled at the Hunter Holmes McGuire VA Medical Center. Body weight and height were measured at baseline to calculate body mass index (BMI) at the time of enrollment. Serum/plasma samples were collected after a minimum of 8 hours of fasting and then analyzed by external reference laboratories (True Health Diagnostics, Frisco, TX).
Table 1.
Definitions of healthy control, coronary artery disease risk factors, coronary artery disease, “normal” kidney function, and chronic kidney disease
| Definition of healthy control |
| Any patient without major systemic illness, including CAD, CKD, or diabetes mellitus; and no risk factors for CAD |
| Definition of coronary artery disease risk factors |
|
| Definition of coronary artery disease |
| Presence of luminal stenosis >50% in 1 or more major coronary arteries. |
| Definition of “normal” kidney functiona |
|
| Definition of chronic kidney diseasea |
|
CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein–associated cholesterol; LDL, low-density lipoprotein cholesterol.
See Kidney Disease: Improving Global Outcomes CKD Work Group.23
Statistical Analysis
CEC and CDCH for each cohort were summarized with medians and interquartile ranges. Overall test for difference between group distributions was achieved with the Kruskal-Wallis H test (nominal significance: α = 0.05). Post hoc pairwise comparisons were done with the joint-rank Dunn method, which incorporates a Bonferroni correction (overall significance: α = 0.05). Exploration of correlations between CEC, CDCH, and a select set of covariates was accomplished using Spearman rank correlation coefficient–based analysis. Ordinary least-squares simple linear regression prediction models also were constructed. All analyses were done with JMP Pro 14.0.0 (SAS Institute, Cary, NC) and all figures were produced with GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Results
Clinical Characteristics
Table 2 summarizes the baseline characteristics of the enrolled subjects, including demographics and biochemical parameters.
Table 2.
Summary of patient characteristics by disease classification
| Characteristic | Healthy (control) | At CAD risk | CAD | CKD G3 | CKD G4–G5 | P |
|---|---|---|---|---|---|---|
| n | 15 | 15 | 15 | 15 | 17 | |
| Sex | 0.7240 | |||||
| Male | 9 (60) | 7 (47) | 10 (67) | 7 (47) | 8 (47) | |
| Female | 6 (40) | 8 (53) | 5 (33) | 8 (53) | 9 (53) | |
| Race | <0.0001 | |||||
| White | 10 (76) | 9 (60) | 10 (67) | 1 (7) | 3 (18) | |
| Black | 1 (8) | 6 (40) | 5 (33) | 14 (93) | 14 (82) | |
| Asian | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Indian | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Age, y | 29 (6) | 51 (32) | 64 (13) | 56 (20) | 63 (16) | <0.0001 |
| BMI, kg/m2 | 24.1 (4.7) | 29.0 (15.3) | 33.7 (8.1) | 32.9 (8.6) | 34.2 (9.9) | 0.0056 |
| HbA1c, % | 5.2 (0.3) | 5.9 (0.7) | 6.5 (2.2) | 6.4 (2.5) | 5.7 (1.7) | <0.0001 |
| Total cholesterol, mg/dl | 179 (43) | 184 (58) | 146 (62) | 202 (38) | 154 (34) | 0.0006 |
| LDL-C, mg/dl | 104 (39) | 114 (55) | 85 (33) | 108 (59) | 72 (31) | 0.0033 |
| HDL-C, mg/dl | 63 (19) | 49 (25) | 38 (19) | 62 (32) | 47 (30) | 0.0006 |
| TAG, mg/dl | 86 (36) | 136 (102) | 108 (90) | 112 (73) | 99 (48) | 0.0331 |
| Non–HDL-C, mg/dl | 112 (45) | 131 (56) | 109 (48) | 140 (50) | 95 (40) | 0.0047 |
| Apo B, mg/dl | 81 (27) | 86 (42) | 90 (28) | 100 (25) | 64 (35) | 0.0138 |
| LDL-P, nmol/l | 1240 (494) | 1486 (908) | 1524 (526) | 1654 (819) | 1219 (732) | 0.0183 |
| Small LDL-P, nmol/l | 487 (543) | 795 (831) | 809 (433) | 767 (654) | 672 (705) | 0.0777 |
| Apo A-I, mg/dl | 151 (39) | 138 (30) | 125 (45) | 156 (42) | 140 (49) | 0.0020 |
| HDL-P, μmol/l | 36.6 (4.7) | 36.4 (7.5) | 33 (12.3) | 36.9 (9.8) | 30.8 (10.6) | 0.0216 |
| Apo B:apo A-I Ratio | 0.54 (0.29) | 0.64 (0.36) | 0.74 (0.28) | 0.62 (0.38) | 0.50 (0.36) | 0.0101 |
| Lp(a)-P, nmol/l | 50 (28) | 50 (122) | 82 (202) | 221 (211) | 102 (270) | 0.0192 |
| Total protein, g/dl | 7.3 (0.4) | 7.3 (0.6) | 6.6 (1.0) | 7.3 (0.8) | 7.0 (0.9) | 0.0011 |
| Albumin, g/dl | 4.7 (0.4) | 4.3 (0.8) | 4.0 (0.8) | 4.1 (0.6) | 4.1 (0.7) | 0.0001 |
| WBC, ×1000/μl | 5.9 (2.1) | 6.1 (3.2) | 7.2 (3.4) | 7.4 (3.8) | 7.3 (1.9) | 0.1011 |
| hs-CRP, mg/l | 0.6 (0.4) | 1.4 (5.2) | 4.8 (9.7) | 5.0 (8.2) | 2.4 (5.7) | 0.0012 |
| eGFR, ml/min per 1.73 m2 | 150 (14) | 125 (48) | 85 (51) | 38 (16) | 19 (16) | <0.0001 |
| SCr, mg/dl | 0.9 (0.2) | 0.9 (0.2) | 1.0 (0.2) | 1.8 (0.3) | 3.4 (2.3) | <0.0001 |
| Cystatin C, mg/l | 0.70 (0.14) | 0.79 (0.21) | 0.99 (0.29) | 1.60 (0.41) | 2.45 (1.36) | <0.0001 |
apo A-I, apolipoprotein A-I; apo B, apolipoprotein B; apo B:apo A-I, apoB-to–apo A-I ratio; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate (computed by 4-variable Modification of Diet in Renal Disease [MDRD] equation); HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein–associated cholesterol; HDL-P, high-density lipoprotein particle; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein–associated cholesterol; LDL-P, low-density lipoprotein particle; Lp(a)-P, lipoprotein(a) particle; SCr, serum creatinine; TAG, triacylglycerol (triglyceride); WBC, white blood cell count.
All characteristics are described using median (interquartile range) except for sex and race, which are described using frequency (percentage). For continuous data, Kruskal-Wallis H test (overall significance level α = 0.05) was used, followed by the Dunn test with joint ranking (overall significance level α = 0.05 with Bonferroni adjustment for multiple comparisons). For categorical data, Fisher-Freeman-Halton exact test was used (overall significance level α = 0.05), followed by pairwise Bonferroni-adjusted 2-sample χ2 tests (pairwise significance level α = 0.0042).
Characterization of a Human THP1 Macrophage System for Determination of FC Efflux Capacity
Under physiological conditions, macrophage ACAT is not inhibited and foam cells store CE and not FC. Therefore, in the present study, no ACAT inhibitor was included to mimic physiological macrophage foam cells. Furthermore, for direct human relevance, human THP1 macrophages with complete repertoire of FC efflux pathways were used to assess FC efflux to physiological concentrations of albumin and HDL in 1% serum. Consistent with the efflux capacity, significantly higher FC efflux was observed when HDL was used as FC acceptor compared with albumin (Figure 1). In addition, significantly higher FC efflux was obtained with 1% serum as the cholesterol acceptor and this was quantitatively additive of FC efflux to albumin and HDL. These data are consistent with the reported association of effluxed FC with albumin and HDL.19
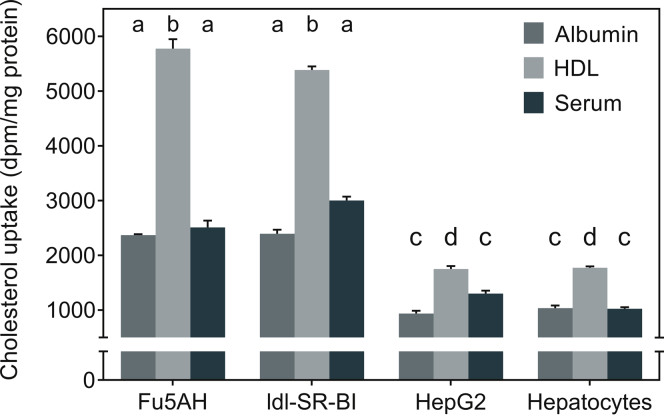
Figure 1.
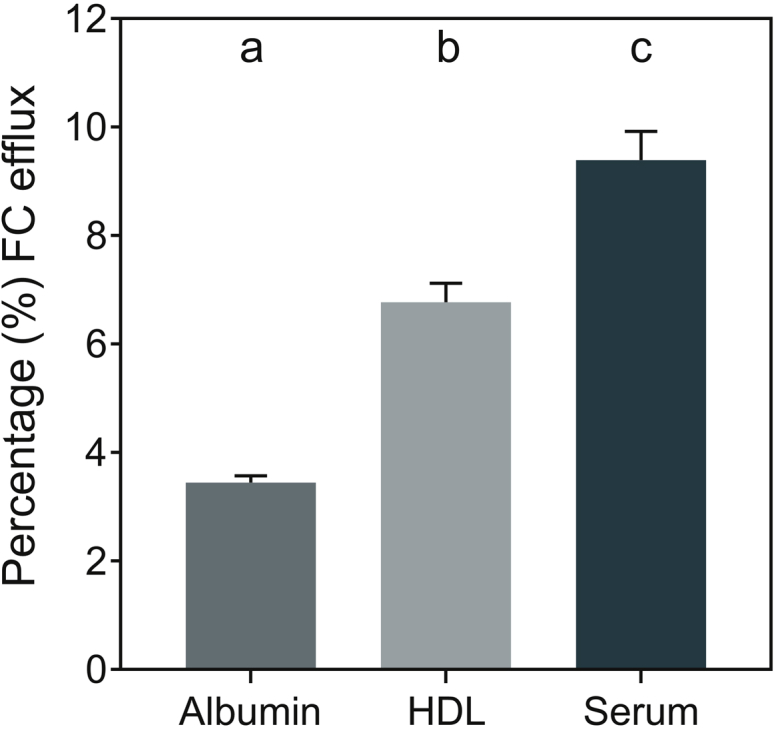
Efflux of cholesterol from lipid-loaded human THP1 macrophages to extracellular acceptors. Free cholesterol (FC) efflux to indicted extracellular acceptors was determined as described in the Methods section and data (% FC efflux) are presented as mean ± SEM, n = 12. Dissimilar letters indicate P < 0.05. HDL, high-density lipoprotein.
Assessment of CDCH
Hepatic uptake of cholesterol returning from the periphery, including from arterial atherosclerotic plaque-associated macrophage foam cells, represents the final step in RCT. Aberration in the final delivery to the hepatocytes despite normal efflux from the macrophages will lead to retention of cholesterol within the body. To establish a suitable cell system to monitor delivery of effluxed FC, we assessed the ability of FC effluxed to albumin, HDL, and serum to deliver to hepatocytes (human hepatocellular carcinoma cell line HepG2 or primary mouse hepatocytes) or cells expressing high levels of HDL receptor SR-BI (namely, rat hepatoma cell line Fu5AH and ldl-SR-BI cells).
Compared with HepG2 and mouse primary hepatocytes, physiologically relevant cells with normal expression of HDL receptor SR-BI, significantly higher uptake was seen in Fu5AH rat hepatoma cells as well as ldl-SRBI cells from FC effluxed to HDL (Figure 2). More importantly, increased uptake of FC effluxed to albumin or serum was also observed, indicating that use of these cell lines apparently overestimates cellular uptake of FC from macrophage efflux medium. Individual P values obtained are given in Table 3. The differences in uptake were significant between cell types or cholesterol acceptors used, and the interactions between cell type and FC acceptor also were significant (P < 0.0001 for all). Comparing the uptake from the 3 different efflux media by a given cell type, significantly higher uptake was seen in all cell types used from FC effluxed to HDL (Table 4). These data not only confirm the role of HDL in delivery of cholesterol from peripheral tissues to the liver, but also establish a novel mechanism for the assessment of HDL functionality in accomplishing this final step in the process of RCT and final cholesterol elimination from the body.
Figure 2.
Uptake of cholesterol effluxed from THP1 macrophages by different cell types. Confluent monolayers of indicated cell types were exposed to effluxed medium (final concentration 20%) and cellular uptake was determined as described in the Methods section. Data (mean ± SEM, n = 6) are expressed as disintegrations per minute (dpm)/mg cellular protein. Significance of observed differences were evaluated by analysis of variance and individual P values of multiple comparisons are shown in Table 1 and 2. HDL, high-density lipoprotein.
Table 3.
Comparison of the uptake of cholesterol effluxed from macrophages by different cell types
| Comparisons | Cholesterol acceptor used |
||
|---|---|---|---|
| Albumin | HDL | Serum | |
| Fu5AH vs. ldl-SR-BI | 0.9944 | 0.0079 | 0.0009 |
| Fu5AH vs. HepG2 | <0.0001 | <0.0001 | <0.0001 |
| Fu5AH vs. Hepatocytes | <0.0001 | <0.0001 | <0.0001 |
| ldl-SR-BI vs. HepG2 | <0.0001 | <0.0001 | <0.0001 |
| ldl-SR-BI vs. hepatocytes | <0.0001 | <0.0001 | <0.0001 |
| HepG2 vs. hepatocytes | 0.8039 | 0.9972 | 0.0826 |
HDL, high-density lipoprotein.
Significance of observed differences was evaluated by analysis of variance and individual P values of multiple comparisons are shown.
Table 4.
Comparison of the delivery of cholesterol effluxed to indicated extracellular acceptors from macrophages to different cell types
| Comparisons | Cell type used |
|||
|---|---|---|---|---|
| Fu5AH | ldl-SR-BI | HepG2 | Hepatocytes | |
| Albumin vs. HDL | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Albumin vs. serum | 0.4033 | <0.0001 | 0.0078 | 0.9944 |
| HDL vs. serum | <0.0001 | <0.0001 | 0.0011 | <0.0001 |
HDL, high-density lipoprotein.
Significance of observed differences was evaluated by analysis of variance and individual P values of multiple comparisons are shown. Nonsignificant differences are indicated in bold.
Based on the observed lack of differences in FC uptake by human HepG2 cells and primary mouse hepatocytes and the reported defects in bile acid synthesis and conjugation associated with HepG2 cells,24 primary mouse hepatocytes were used for all subsequent studies.
CAD Risk Factors Alone or Established CAD Do Not Affect CEC, but Higher CEC Is Associated With Serum From Subjects With CKD
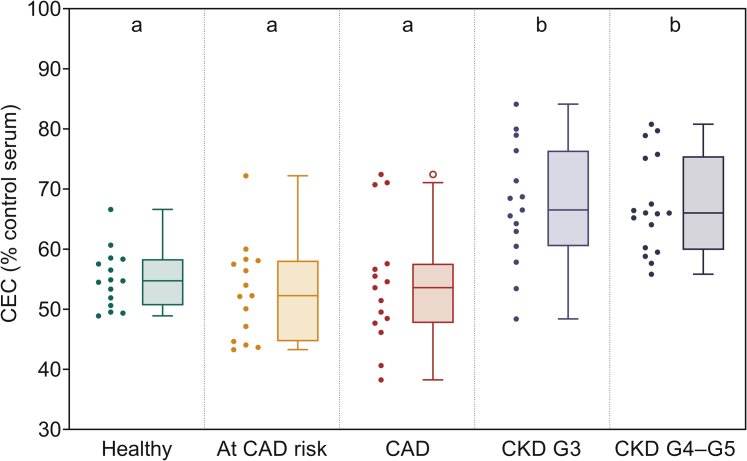
Human THP1 macrophage foam cell system as optimized above was used to evaluate the CEC of serum obtained from human subjects. Final concentration of serum used as the extracellular cholesterol acceptor was 1% and it should be noted that no additional manipulations were performed (e.g., polyethylene glycol precipitation of ApoB containing lipoproteins) to retain physiological relevance. No significant difference in CEC was noted between serum from healthy subjects or patients with CAD risk factors or established CAD (Figure 3). In contrast, significantly higher CEC was associated with serum from all subjects with CKD, suggesting that the increased CAD risk associated with CKD may not be related to the CEC. This is consistent with increased CEC noted in 12 adults with CKD stages G3 to G5 observed by Meier et al.25
Figure 3.
Serum from patients with chronic kidney disease (CKD) has significantly higher cholesterol efflux capacity (CEC) compared with healthy controls and patients with coronary artery disease (CAD). THP1 macrophages were loaded with acetylated human low-density lipoprotein (AcLDL) and radiolabeled with [3H]-cholesterol as described in the Methods section. Serum, at a final concentration of 1%, from subjects in indicated cohorts was used as the extracellular cholesterol acceptor and observed CEC was normalized to a pooled control serum sample and expressed as percentage. Data are presented as Tukey box-and-whisker plots (box divider: median; box ends: interquartile range [IQR]; whiskers: ±1.5 × IQR). Statistical analysis for overall difference between groups was performed using the Kruskal-Wallis H test (overall significance level α = 0.05), followed by the Dunn test with joint ranking (overall significance level α = 0.05 with Bonferroni adjustment for multiple comparisons). Dissimilar letters indicate statistically significant differences.
Reduced CDCH by Serum From Subjects With Presence of CAD Risk Factors, Established CAD, or CKD
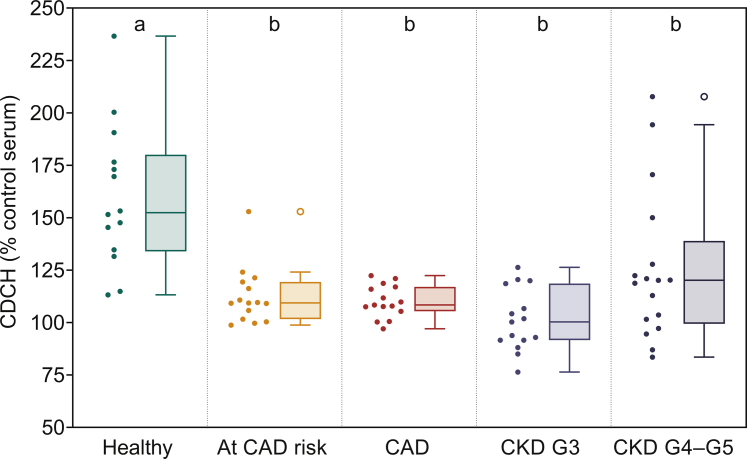
Using mouse primary hepatocytes with physiological levels of SR-BI expression and complete repertoire of pathways involved in cholesterol metabolism, CDCH was assessed as described under Methods. A significant reduction in CDCH was noted in subjects with CAD risk factors, or established CAD (Figure 4). These data suggest that despite similar CEC, serum from subjects with 1 risk factor or CAD was not as effective in delivering the effluxed FC to hepatocytes for the final step of RCT. Similar to CAD subjects, delivery of effluxed FC to hepatocytes was also reduced in subjects with CKD (Figure 4).
Figure 4.
Serum from patients with chronic kidney disease (CKD) as well as patients with coronary artery disease (CAD) has significantly lower capacity to deliver cholesterol to hepatocytes (CDCH). CDCH was evaluated by incubating primary hepatocytes with conditioned medium (20%) containing the [3H]-cholesterol effluxed from THP1 macrophages to 1% serum from subjects in indicted cohorts. After 4-hour incubation to facilitate uptake by hepatocytes, the medium was removed, cells washed with phosphate-buffered saline and total radioactivity associated with cells determined. The observed CDCH was normalized to CDCH obtained when hepatocytes were incubated with macrophage conditioned medium when pooled control serum sample was used as an acceptor. Data are represented here as Tukey box-and-whisker plots (box divider: median; box ends: interquartile range [IQR]; whiskers: ±1.5 × IQR). Statistical analysis for overall difference between groups was done using the Kruskal-Wallis H test (overall significance level α = 0.05), followed by the Dunn test with joint ranking (overall significance level α = 0.05 with Bonferroni adjustment for multiple comparisons). Dissimilar letters indicate statistically significant differences.
Because higher CEC was observed with serum from subjects with CKD compared with subjects with established CAD (Figure 3), and during evaluation of CDCH a fixed volume (20%) of effluxed medium was used, hepatocytes were consequently exposed to higher levels of radioactivity. Therefore, to evaluate differences, if any, between CDCH obtained for CAD and subjects with CKD, CDCH was normalized to observed CEC for each group and the results are shown in Figure 5. These data indicate that CDCH of serum from subjects with CKD is significantly lower than that seen with subjects with CAD. Taken together with the observed CEC, these data indicate that CDCH merits further exploration as one possible factor contributing to development of CAD in subjects with CKD.
Figure 5.
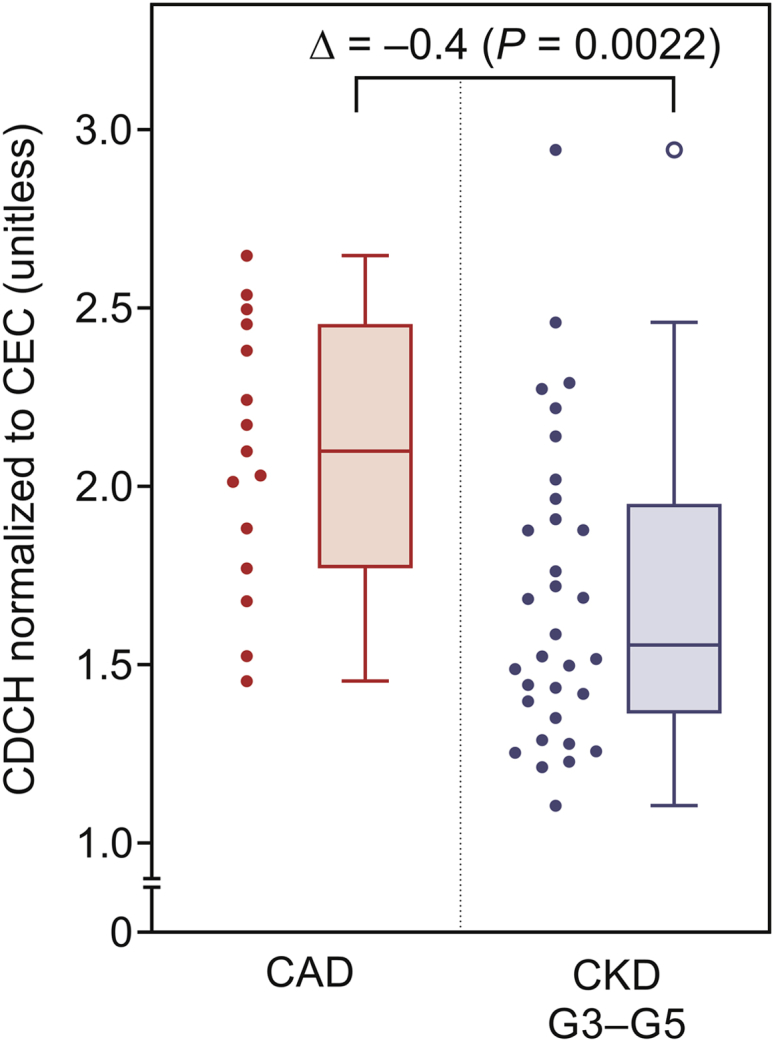
Median cholesterol efflux capacity (CEC)–normalized capacity to deliver cholesterol to hepatocytes (CDCH) is significantly lower for the chronic kidney disease (CKD) group than for the coronary artery disease (CAD) group. Because higher CEC is seen with patients with CKD compared with patients with CAD, observed CDCH was normalized to CEC. Data for all patients with CKD was combined and compared with patients with CAD. Data are represented here as Tukey box-and-whisker plots (box divider: median; box ends: interquartile range [IQR]; whiskers: ±1.5 × IQR). Statistical analysis for difference between groups was done using the Wilcoxon rank-sum test with nominal significance level α = 0.05.
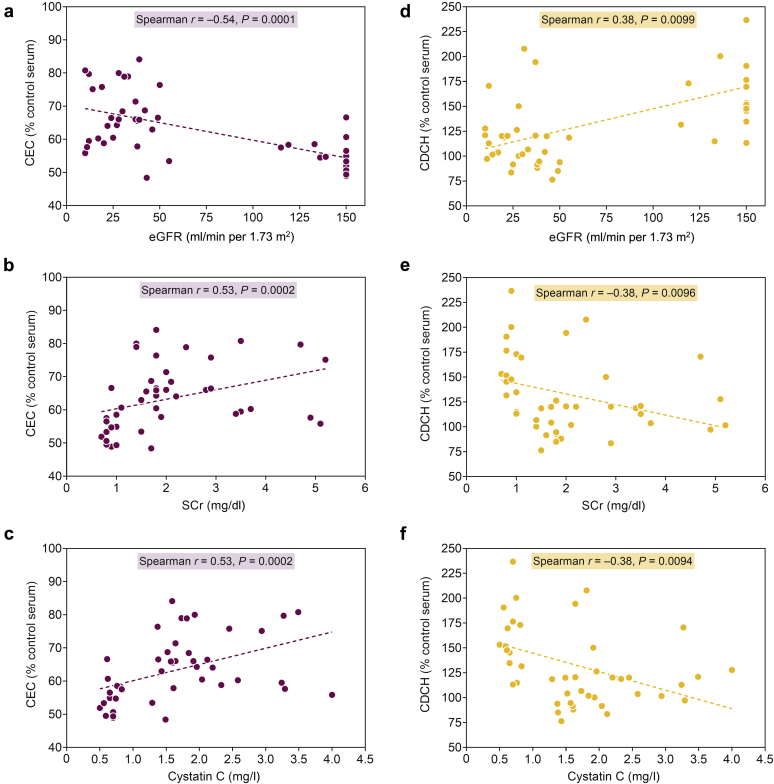
Correlation of CEC and CDCH With Markers of Kidney Function
Declining kidney function is associated with increasing CAD risk and therefore we evaluated the relationship between CEC or CDCH and markers of kidney function in subjects with CKD. The observed CEC and CDCH in healthy individuals as well as subjects with CKD (stage G3 and stages G4–G5) were correlated to eGFR, serum creatinine, and serum cystatin C. Consistent with higher CEC observed in subjects with CKD (Figure 3), CEC was negatively correlated to eGFR (Figure 6a) and positively correlated with serum creatinine (Figure 6b) as well as serum cystatin C (Figure 6c). In contrast, CDCH was positively correlated with eGFR (Figure 6d) and negatively correlated with serum creatinine (Figure 6e) and serum cystatin C (Figure 6f). These analyses indicate that declining kidney function correspondingly modulates CDCH and further supports the concept that impaired CDCH is likely to attenuate RCT in subjects with CKD.
Figure 6.
Correlation of cholesterol efflux capacity (CEC) and capacity to deliver cholesterol to hepatocytes (CDCH) with markers of kidney function. Serum creatinine (SCr) levels were determined and estimated glomerular filtration rate (eGFR) was computed using the 4-variable modification of diet in renal disease equation. Nonparametric correlation analyses between eGFR or SCr or cystatin C and CEC (a–c) or CDCH (d–f) were performed using Spearman rank-based correlation coefficients r (significance level α = 0.05). Dashed line is from ordinary least-squares simple linear regression with acceptable diagnostics. Pearson and Spearman correlation coefficients were comparable.
Correlation Between CEC and CDCH With Age, BMI, and Components of Metabolic Profile
Age and increased BMI are major risk factors for CAD, but no significant correlation was noted between CEC and age or BMI in healthy individuals, those at CAD risk, or patients with CAD (Table 5). In contrast, CDCH was negatively correlated to both age (r = –0.47, P = 0.001) and BMI (r = –0.41, P = 0.0139). In patients with CKD, although CEC was positively correlated with age (r = 0.41, P = 0.004) and BMI (r = 0.35, P = 0.0266), CDCH was negatively correlated with age (r = –0.51, P = 0.0002) and BMI (r = –0.47, P = 0.002). CEC was not significantly correlated with total cholesterol, LDL-cholesterol, or HDL-C; plasma triacylglycerol (triglyceride) levels were positively correlated to CEC albeit in patients with CKD only. In contrast, CDCH was positively correlated to HDL-C in patients with CAD (r = 0.61, P < 0.0001), but this relationship was not statistically significant in patients with CKD. These data indicate that underlying factors that determine HDL-C and CDCH are likely different in patients with CAD and patients with CKD. It is noteworthy that plasma triacylglycerol (triglyceride) levels were negatively correlated to CDCH in patients with CAD (r = –0.35, P = 0.0190), suggesting that the detrimental effects of high plasma triglyceride in increasing CAD risk may be related to its effects on reducing the return of cholesterol to the liver for final elimination.
Table 5.
Correlation analyses for CEC and CDCH with age, BMI, and “metabolism profile” components
| Characteristics | Healthy + at CAD risk + CAD (n = 45) |
Healthy + CKD G3–G5 (n = 47) |
||||||
|---|---|---|---|---|---|---|---|---|
| CEC |
CDCH |
CEC |
CDCH |
|||||
| r | P | r | P | r | P | r | P | |
| Age, y | −0.22 | 0.1558 | −0.47 | 0.0010 | 0.41 | 0.004 | −0.51 | 0.0002 |
| BMI, kg/m2 | −0.18 | 0.3121 | −0.41 | 0.0139 | 0.35 | 0.0266 | −0.47 | 0.0020 |
| Metabolism | ||||||||
| Total cholesterol, mg/dl | 0.25 | 0.1028 | −0.01 | 0.9558 | 0.09 | 0.5442 | −0.16 | 0.2814 |
| LDL-C, mg/dl | 0.06 | 0.7092 | −0.18 | 0.2244 | −0.13 | 0.4030 | −0.20 | 0.1963 |
| HDL-C, mg/dl | 0.10 | 0.5122 | 0.61 | <0.0001 | 0.02 | 0.9056 | 0.26 | 0.0854 |
| Non–HDL-C, mg/dl | 0.20 | 0.1870 | −0.31 | 0.0409 | 0.08 | 0.5850 | −0.29 | 0.0506 |
| TAG, mg/dl | 0.15 | 0.3299 | −0.35 | 0.0190 | 0.38 | 0.0101 | −0.26 | 0.0826 |
BMI, body mass index; CAD, coronary artery disease; CDCH, capacity to deliver cholesterol to hepatocytes; CED, cholesterol efflux capacity; CKD, chronic kidney disease; HDL-C, high-density lipoprotein–associated cholesterol; LDL-C, low-density lipoprotein–associated cholesterol; TAG, triacylglycerol (triglyceride).
Correlation analysis is presented in terms of Spearman rank-based correlation coefficients r (significance level α = 0.05).
Correlation Between CEC and CDCH With Inflammation Profile
In addition to dyslipidemia, systemic inflammation and abnormal glycemic control are risk factors for CAD and, therefore, we examined the relationship between CEC or CDCH with relevant plasma markers (namely, high-sensitivity C-reactive protein and HbA1c). Although CEC and CDCH were negatively correlated with HbA1c in patients with CAD as well as patients with CKD (Table 6), only CDCH in subjects with CAD was negatively correlated with high-sensitivity C-reactive protein (r = –0.25, P = 0.0065) and this correlation did not reach statistical significance in patients with CKD (r = 0.23, P = 0.12). Although plasma HDL as well as lipid-free apoA1 are well recognized circulating cholesterol acceptors, Li et al.19 showed that cholesterol effluxed from J774 murine macrophages to patient serum was also associated with plasma albumin that is a low affinity but high capacity cholesterol acceptor in serum. Interestingly, although plasma albumin levels were not correlated to CEC (r = 0.24, P = 0.10) in patients with CAD, significant negative correlation was noted in patients with CKD (r = –0.49, P = 0.0006). Conversely, albumin levels were positively associated with hepatocyte uptake in CAD (r = 0.40, P = 0.007) as well as in patients with CKD (r = 0.50, P = 0.0005). These data suggest that higher albumin levels may facilitate delivery of effluxed cholesterol to hepatocytes.
Table 6.
Correlation analyses for CEC and CDCH with “inflammation profile” components
| Characteristics | Healthy + at CAD risk + CAD (n = 45) |
Healthy + CKD G3–G5 (n = 47) |
||||||
|---|---|---|---|---|---|---|---|---|
| CEC |
CDCH |
CEC |
CDCH |
|||||
| r | P | r | P | r | P | r | P | |
| Inflammation | ||||||||
| hs-CRP, mg/l | −0.25 | 0.0913 | −0.40 | 0.0065 | 0.20 | 0.1793 | −0.23 | 0.1252 |
| Albumin, mg/dl | 0.24 | 0.1050 | 0.40 | 0.0070 | −0.49 | 0.0006 | 0.50 | 0.0005 |
| HbA1c, % | −0.34 | 0.0221 | −0.56 | <0.0001 | 0.42 | 0.0041 | −0.63 | <0.0001 |
CAD, coronary artery disease; CEC, cholesterol efflux capacity; CDCH, capacity to deliver cholesterol to hepatocytes; CKD, chronic kidney disease; hs-CRP, high-sensitivity C-reactive protein; HbA1c, hemoglobin A1c.
Correlation analysis is presented in terms of Spearman rank-based correlation coefficients r (significance level α = 0.05).
Discussion
The validity of merely increasing plasma HDL-C has been questioned in recent years, and the emphasis has accordingly shifted to the understanding of HDL functions that likely determine its athero-protective effects. Although earlier studies have solely focused on correlating disease status with CEC of HDL or apoB-depleted patient serum (by polyethylene glycol precipitation), the data presented here, for the first time, also examined the ability of whole serum to deliver cholesterol effluxed from macrophages to hepatocytes for final elimination from the body. Using this integrated and comprehensive approach in which the first as well as the final step in the process of RCT is evaluated, our data show a significant decrease in the delivery of cholesterol effluxed from macrophages to hepatocyte by serum (CDCH) from subjects with CKD as well as subjects with CAD risk factors or established CAD. Taken together with no significant difference in the ability of serum to facilitate efflux of cholesterol from human THP1 macrophage foam cells (CEC) in patients with CAD and an increase in CEC in patients with CKD, these data clearly indicate that the potential aberration in the process of RCT is cholesterol delivery to hepatocyte or the final step.
Initial studies to measure CEC used rat hepatoma cell line Fu5AH26 and using this in vitro system, Pajunen et al.27 reported an inverse association between CEC and severity of CAD. Using mouse macrophage cell line J774 loaded with FC (and not CEs that are present in vivo) and apolipoprotein B–depleted plasma, Hafiane et al.16 reported reduced CEC in patients with acute coronary syndrome independent of plasma HDL-C. Khera et al.17 also used FC-labeled (inclusion of ACAT1 inhibitor during the study) and cAMP-treated (to activate expression of ABCA1) mouse J774 macrophage cell line as well as polyethylene glycol–precipitated ApoB-depleted serum to measure CEC as a metric of HDL function and reported a strong inverse association with both carotid intima-media thickness and the likelihood of angiographic CAD. Similarly, Rohatgi et al.18 found that CEC was inversely associated with CAD events in a population-based cohort. In contrast, Li et al.19 found that increased CEC was associated with increased prospective risk for myocardial infarction, stroke, and death. Detailed characterization of efflux medium from FC-loaded J774 mouse macrophages used in this study showed association of FC with albumin in the apolipoprotein-B–depleted serum (and not complete serum) used as the extracellular cholesterol acceptor in this study. In the present study, we used a human THP1 macrophage cell system instead of the murine cell line J774, omitted the use of ACAT1 inhibitor to mimic physiological storage of CE in macrophage foam cells, and monitored efflux of FC to total serum instead of polyethylene glycol–precipitated ApoB-depleted serum. Using this model system, data presented here also show either no change in CEC with serum from subjects with CAD or an increase in this parameter in subjects with CKD. These data are consistent with reported high CEC in patients with impaired renal function paradoxically associated with increased CAD risk,25 suggesting that CEC may not be a reliable predictor of CAD risk in CKD. By evaluating CEC of HDL from patients with CKD, Bauer et al.28 similarly concluded that CEC is not a prognostic CAD risk marker in patients with CKD.
Delivery of FC effluxed from macrophages to liver for final elimination is the final step in RCT, and CDCH of serum has not been evaluated in earlier studies. Hepatic SR-BI facilitates specific uptake of HDL-C as well as positively regulates macrophage to feces RCT in mouse models29 and consistently liver-specific transgenic overexpression of SR-BI reduces atherosclerosis.30 Zanoni et al.31 recently described a rare loss-of-function variant of SR-BI (P376L) in humans and heterozygous carriers of this variant have significantly increased plasma HDL-C as well as CAD. The fact that CEC between control and P376L heterozygotes was similar underscores the importance of evaluating the appropriate delivery of cholesterol to the liver in the development of CAD. In the present study, a standard mouse primary hepatocyte system was used to evaluate the uptake of FC effluxed from macrophages and, therefore, the observed differences in hepatocyte uptake cannot be due to changes in SR-BI expression or other hepatocyte characteristics. It is noteworthy that other cell lines tested, namely, Fu5AH and ldl-SR-BI, showed significantly high uptake of FC effluxed to both albumin as well as HDL, demonstrating the unsuitability of these cell lines with high SR-BI expression for measurement of CDCH. Based on the observed differences in the hepatic delivery of cholesterol associated with albumin or HDL to primary mouse hepatocytes, it is likely that hepatic uptake will be affected by the type of cholesterol acceptor to which FC is effluxed by macrophages. Despite the lack of evidence for the presence of a specific albumin receptor, high-affinity binding domains for albumin exist on hepatocyte membrane that can facilitate rapid transfer of albumin-bound hydrophobic ligands such as cholesterol.32,33 It remains to be seen whether cholesterol delivered via albumin or HDL is equally available for further hepatic metabolism (e.g., conversion to bile acids for fecal elimination).
The presence of CKD profoundly affects HDL composition and functionality,34 but whether abnormal HDL independently contributes to cardiovascular events in patients with CKD remains undefined. Untersteller et al.35 monitored HDL-C, HDL-associated serum amyloid A, paraoxonase, and Lp-PLA2 in patients with CKD and were unable to predict CAD outcomes based on these parameters. Although clear disturbances have been observed for the “functionality” of HDL particles in patients with CKD, this did not necessarily translate into clear-cut associations with outcomes.36 Reduction in LDL/very low density lipoprotein cholesterol with accompanying increase in HDL-C by normalization of chronic renal insufficiency–induced hepatic ACAT37,38 indicates a likely modulation of hepatic lipoprotein metabolism in kidney disease. It needs to be emphasized that although hepatic ACAT or ACAT2 regulates CE incorporation in secreted very low-density lipoprotein, macrophage ACAT or ACAT1 is responsible for CE accumulation in foam cells39 with potential to affect CEC as determined herein.
Uremia associated with CKD leads to modulation of multiple solutes: while indoxyl sulfate, carboxymethyl lysine, N-methyl-4-pyridone-3-carboxamide are significantly increased, bilirubin, reduced glutathione, α1-antitrypsin, arginine, and homoarginine are significantly decreased. How these changes affect cholesterol flux through the body is currently not known. Binder et al.40 observed significant increase in 3-chlorotyrosine, carbamyl lysine, and carboxymethyl lysine content of albumin from hemodialysis patients that acts as an antagonist for HDL uptake receptor, SR-BI. It is therefore likely that serum from patients with CKD decreases SR-BI–mediated HDL uptake by liver (the final step of RCT), resulting in significantly reduced CDCH reported here (Figure 2). Factors other than RCT that likely contribute to CAD risk were not evaluated in the present study, and because bilirubin, reduced glutathione, α1-antitrypsin, arginine, and homoarginine are associated with antioxidant, anti-inflammatory, and vasodilating properties,41 their reduced concentrations in CKD could be involved with adverse effects of uremia on other processes relevant to atherogenesis.
In addition to uremia, the other routine measures of kidney functions, namely, eGFR, serum creatinine, and cystatin C, were also found to be strongly correlated to CDCH and not CEC (Figure 3, Figure 4, Figure 5). It is noteworthy that in a recent meta-analysis from the CKD Prognosis Consortium, eGFR and albuminuria improve cardiovascular disease risk prediction beyond traditional cardiovascular disease risk factors.42 Following up the patients with CKD in the Chronic Renal Insufficiency Cohort (CRIC) study, Bundy et al.43 reported negative correlation between coronary artery calcification and eGFR as well as cystatin C, and Bahrmann et al.44 demonstrated that assessing renal function by cystatin C is useful for predicting cardiovascular disease mortality in patients with CKD admitted to the emergency department with acute coronary syndrome. Lower eGFR and high albumin-to-creatinine ratios were also independently associated with measures of arterial stiffness, an important pathophysiologic phenotype of vascular disease in CKD.45 Taken together, the published evidence for eGFR, creatinine, and cystatin C as valuable predictors of CAD in CKD and the strong correlation between these parameters with CDCH underscore the importance of hepatic delivery of returning cholesterol to liver in patients with CKD. Although currently very limited knowledge is available on biliary and fecal excretion of bile acids and cholesterol (the only routes for cholesterol elimination from the body) in CKD, it is intriguing that uremic solutes modulate hepatic bile acid transporters46 and serum levels of circulating bile acids are high and related to coronary artery calcification in patients with CKD.47
In conclusion, analyses of the plasma compartment or “liquid biopsy” is currently the only available option for assessing whole-body cholesterol homeostasis. The present pilot study extends the scope of these investigations by assessing the ability of serum components to not only facilitate FC efflux from human THP1 macrophages under physiological conditions, but also to quantify the ability to deliver the effluxed cholesterol to hepatocytes for final elimination. Although technically challenging and limited by the small sample size, this study documents the predictive value of measuring CDCH and recognizes impaired delivery and/or handing of cholesterol by the liver as a step likely to be affected in CKD and also CAD. It needs to be emphasized that this a small pilot study and future studies involving a larger cohort of patients with CKD and healthy controls with matched race and age will establish the relative role of CDCH in mediating the development of CAD in patients with CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by Virginia Commonwealth University Department of Internal Medicine’s Academic Enhancement Funds awarded to DEC.
Translational Statement
Mechanisms underlying increased CAD risk associated with CKD remain undefined. The present study identifies aberrations in the final delivery of cholesterol to the liver in CAD and patients with CKD. Measurement of CDCH, therefore, represents a quantifiable parameter to assess CAD risk in patients with CKD.
References
- 1.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Vanholder R., Massy Z., Argiles A. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 3.Sarnak M.J., Coronado B.E., Greene T. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57:327–335. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P., Hamm L.L., Kusek J.W. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P., He J., Astor B.C. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 6.Bro S., Bentzon J.F., Falk E. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S., Zuo Y., Ma J. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2011;26:2491–2497. doi: 10.1093/ndt/gfq759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaziri N.D., Liang K. Down-regulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int. 1996;50:1928–1935. doi: 10.1038/ki.1996.515. [DOI] [PubMed] [Google Scholar]
- 9.Sato T., Liang K., Vaziri N.D. Protein restriction and AST-120 improve lipoprotein lipase and VLDL receptor in focal glomerulosclerosis. Kidney Int. 2003;64:1780–1786. doi: 10.1046/j.1523-1755.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri N.D., Liang K. Down-regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 1997;51:913–919. doi: 10.1038/ki.1997.129. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri N.D. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–F272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 12.Moradi H., Pahl M.V., Elahimehr R., Vaziri N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res. 2009;153:77–85. doi: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Takata K., Nicholls S.J. Tackling residual atherosclerotic risk in statin-treated adults: focus on emerging drugs. Am J Cardiovasc Drugs. 2019;19:113–131. doi: 10.1007/s40256-018-0312-1. [DOI] [PubMed] [Google Scholar]
- 14.Bruzzone G., Corbelli G., Belci P. Cholesterol lowering therapy: treat to target or reduce the global risk? The unresolved problem of residual risk. Curr Pharm Des. 2016;22:5676–5686. doi: 10.2174/1381612822666160822143753. [DOI] [PubMed] [Google Scholar]
- 15.Tuteja S., Rader D.J. High-density lipoproteins in the prevention of cardiovascular disease: changing the paradigm. Clin Pharmacol Ther. 2014;96:48–56. doi: 10.1038/clpt.2014.79. [DOI] [PubMed] [Google Scholar]
- 16.Hafiane A., Jabor B., Ruel I. High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am J Cardiol. 2014;113:249–255. doi: 10.1016/j.amjcard.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Khera A.V., Cuchel M., de la Llera-Moya M. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohatgi A., Khera A., Berry J.D. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X.M., Tang W.H., Mosior M.K. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwala A.P., Rodrigues A., Risman M. High-density lipoprotein (HDL) phospholipid content and cholesterol efflux capacity are reduced in patients with very high HDL cholesterol and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35:1515–1519. doi: 10.1161/ATVBAHA.115.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B., Song J., St Clair R.W., Ghosh S. Stable overexpression of human macrophage cholesteryl ester hydrolase results in enhanced free cholesterol efflux from human THP1 macrophages. Am J Physiol Cell Physiol. 2007;292:C405–C412. doi: 10.1152/ajpcell.00306.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hylemon P.B., Gurley E.C., Kubaska W.M. Suitability of primary monolayer cultures of adult rat hepatocytes for studies of cholesterol and bile acid metabolism. J Biol Chem. 1985;260:1015–1019. [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 24.Everson G.T., Polokoff M.A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986;261:2197–2201. [PubMed] [Google Scholar]
- 25.Meier S.M., Wultsch A., Hollaus M. Effect of chronic kidney disease on macrophage cholesterol efflux. Life Sci. 2015;136:1–6. doi: 10.1016/j.lfs.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Syvänne M., Castro G., Dengremont C. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin-dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127:245–253. doi: 10.1016/s0021-9150(96)05962-x. [DOI] [PubMed] [Google Scholar]
- 27.Pajunen P., Syvänne M., Castro G. Cholesterol efflux capacity in vitro predicts the severity and extent of coronary artery disease in patients with and without type 2 diabetes. Scand Cardiovasc J. 2001;35:96–100. doi: 10.1080/140174301750164736. [DOI] [PubMed] [Google Scholar]
- 28.Bauer L., Kern S., Rogacev K.S. HDL cholesterol efflux capacity and cardiovascular events in patients with chronic kidney disease. J Am Coll Cardiol. 2017;69:246–247. doi: 10.1016/j.jacc.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Da Silva J.R., Reilly M. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arai T., Wang N., Bezouevski M. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 31.Zanoni P., Khetarpal S.A., Larach D.B. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ockner R.K., Weisiger R.A., Gollan J.L. Hepatic uptake of albumin-bound substances: albumin receptor concept. Am J Physiol. 1983;245:G13–G18. doi: 10.1152/ajpgi.1983.245.1.G13. [DOI] [PubMed] [Google Scholar]
- 33.Reed R.G., Burrington C.M. The albumin receptor effect may be due to a surface-induced conformational change in albumin. J Biol Chem. 1989;264:9867–9872. [PubMed] [Google Scholar]
- 34.Kaseda R., Jabs K., Hunley T.E. Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism. 2015;64:263–273. doi: 10.1016/j.metabol.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Untersteller K., Meissl S., Trieb M. HDL functionality and cardiovascular outcome among nondialysis chronic kidney disease patients. J Lipid Res. 2018;59:1256–1265. doi: 10.1194/jlr.P085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronenberg F. HDL in CKD—the devil is in the detail. J Am Soc Nephrol. 2018;29:1356–1371. doi: 10.1681/ASN.2017070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang K., Vaziri N.D. Upregulation of Acyl-CoA: cholesterol acyltransferase (ACAT) in chronic renal failure. Am J Physiol Endocrine Metab. 2002;283:E676–E681. doi: 10.1152/ajpendo.00364.2001. [DOI] [PubMed] [Google Scholar]
- 38.Vaziri N.D., Liang K. ACAT inhibition reverses LCAT deficiency and improves plasma HDL in chronic renal failure. Am J Physiol Renal Physiol. 2004;287:F1038–F1043. doi: 10.1152/ajprenal.00150.2004. [DOI] [PubMed] [Google Scholar]
- 39.Rudel L.L., Lee R.G., Cockman T.L. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Binder V., Ljubojevic S., Haybaeck J. The myeloperoxidase product hypochlorous acid generates irreversible high-density lipoprotein receptor inhibitors. Arterioscler Thromb Vasc Biol. 2013;33:1020–1027. doi: 10.1161/ATVBAHA.113.301235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duranton F., Cohen G., De Smet R. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita K., Ballew S.H., Coresh J. Cardiovascular risk prediction in people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25:518–523. doi: 10.1097/MNH.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bundy J.D., Chen J., Yang W. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: the CRIC study. Atherosclerosis. 2018;271:53–60. doi: 10.1016/j.atherosclerosis.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahrmann P., Bertsch T., Giannitsis E. Quantification of renal function and cardiovascular mortality in patients admitted to the emergency department with suspected acute coronary syndromes. Clin Lab. 2017;63:1457–1466. doi: 10.7754/Clin.Lab.2017.170326. [DOI] [PubMed] [Google Scholar]
- 45.Kim E.D., Tanaka H., Ballew S.H. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2018;72:682–690. doi: 10.1053/j.ajkd.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Weigand K.M., Schirris T.J.J., Houweling M. Uremic solutes modulate hepatic bile acid handling and induce mitochondrial toxicity. Toxicol In Vitro. 2019;56:52–61. doi: 10.1016/j.tiv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovich A., Isakova T., Block G. Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD. Am J Kidney Dis. 2018;71:27–34. doi: 10.1053/j.ajkd.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]