Abstract
Introduction
Kidney transplant (Ktx) recipients are excluded from clinical trials of immune checkpoint inhibitors. The aim of this systematic review was to assess the safety of immune checkpoint inhibitors among Ktx patients.
Methods
A literature search was conducted using MEDLINE, EMBASE, and Cochrane Database from inception through April 2019. We included studies that reported outcomes of Ktx recipients who received immune checkpoint inhibitors for cancer treatment. Outcomes of interest were allograft rejection and/or allograft failure.
Results
Twenty-seven articles with a total of 44 Ktx patients treated with immune checkpoint inhibitor were identified. Of 44 Ktx patients, 18 were reported to have acute rejection. Median time from immune checkpoint inhibitors to acute rejection diagnosis was 24 (interquartile range, 10–60) days. Reported types of acute allograft rejection were cellular rejection (33%), mixed cellular and antibody-mediated rejection (17%), and unspecified type (50%). Fifteen (83%) had allograft failure and 8 (44%) died. Three patients had a partial remission (17%), 1 patient achieved cancer response (6%), and 5 patients had stable disease (28%).
Conclusion
The findings of our study raise awareness of the increased risk for acute allograft rejection/failure following immune checkpoint inhibitors for cancer treatment among Ktx patients, in particular with programmed cell death 1 (PD-1) inhibitors. Future large-scale clinical studies are required to appraise the pathogenesis and plan optimal balanced therapy that helps sustain graft tolerance.
Keywords: allograft rejection, immune checkpoint inhibitors, immunosuppresants, kidney transplant, PD-1 inhibitor, systematic review
Kidney transplantation is a life-saving therapy for patients with end-stage kidney disease with overall improved survival and quality of life.1,2 Despite this, kidney transplant recipients remain at high risk of fatal cardiovascular disease events as compared with the general population and this accounted for approximately 40% to 55% of all deaths.3 However, in the past 30 years, there has been a decline in deaths from cardiovascular disease and a rise in malignancy-related deaths,4 which now accounts for approximately 30% of all deaths. Solid organ transplant patients are at a 2-fold higher risk of malignancy compared with the general population.5 The overall incidence of malignancies is markedly higher in patients who receive a Ktx compared with those who stay on dialysis.6 Immunosuppression, oncogenic viruses, and age are considered a few of the risk factors. The most common cancers are the carcinomas of the skin and lip, anogenital cancers, and non-Hodgkin’s lymphoma.7 Melanoma represents approximately 5% of posttransplant skin cancers compared with 2.7% in the general population.8
The advent of immunotherapy, especially immune checkpoint inhibitors (ICIs) changed the cancer treatment landscape, initially for metastatic melanoma and since then for many other cancer types, and its use has quickly moved to the frontline therapies. However, cancer trials have excluded transplant patients. The core principle of checkpoint inhibitors is to stimulate the immune system to destroy the cancer cells, which contradicts a transplant patient’s need to suppress the immune system to prevent allograft rejection. There are currently no guidelines for the transplant community for the use of ICIs, and in the end, when the question becomes of a life versus graft, mutual discussion between the patient and provider is crucial, and the graft is in many circumstances the one to be sacrificed. Experiences reported in the literature have been mixed, and the objective of our study was to systematically review the literature for safety of ICIs in Ktx patients.
Methods
Search Strategy
The protocol for this study is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42019126777). A systematic search of the published literature was conducted in MEDLINE (1946 to April 2019), EMBASE (1988 to April 2019), and the Cochrane Database of Systematic Reviews (2005 to April 2019) from inception of databases through April 2019. We also obtained pertinent references via manual review of these retrieved references. Review of the abstracts and full text was conducted independently by the investigators SM and CT using the search approach that incorporated the terms of “pembrolizumab” OR “nivolumab” OR “ipilimumab” OR “cemiplimab” OR “atezolizumab” OR “avelumab” OR “durvalumab” AND “kidney transplantation” OR “kidney graft” OR “kidney graft rejection” OR “acute graft rejection” OR “organ transplantation” which is provided in Supplementary Item S1. Differing decisions were solved by mutual consensus. We conducted this systematic review in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.9 The PRISMA checklist is shown in Supplementary Item S2.
Inclusion Criteria
Our intended patient populations were all Ktx patients who received checkpoint inhibitor for cancer therapy. The included studies fulfilled the following criteria: (i) Ktx recipient with an active functioning graft, (ii) received a checkpoint inhibitor therapy like cytotoxic T-lymphocyte-associated-antigen 4 inhibitor (ipilimumab) or PD-1 inhibitor (pembrolizumab or nivolumab) or programmed cell death ligand 1 (PDL-1) inhibitor (avelumab, atezolizumab or durvalumab). We included conference abstracts and observational studies. We excluded any reports that did not fit our inclusion criteria.
Outcomes of Interest
Clinical outcomes of interest were renal allograft rejection and/or failure, cancer outcome, and mortality after treatment with checkpoint inhibitors.
Data Extraction
We used a standardized data collection template to extract the following information: last name of the first author, article title, study type, year of publication, sample size, name of checkpoint inhibitors, indication of treatment (tumor type), age, sex, baseline graft function, year after transplant, lag time from checkpoint inhibitor initiation and development of allograft rejection, renal biopsy features, follow-up time after treatment, cancer, and patient outcome as reported in the study.
Outcome Measures
The outcome terminology “allograft rejection” was used to define any renal adverse event reported by authors as a rejection either by clinical criteria alone or after histological confirmation of rejection based on criteria followed at their individual institutions. The outcome terminology allograft failure was used to define a complete loss of graft requiring the initiation of dialysis.
Statistical Analysis
Descriptive statistics were performed. Continuous variables were reported as mean ± SD. Categorical variables were reports as count and percentage. The differences in patient and cancer characteristic, management, and outcomes between patients who developed and did not develop rejection after ICIs were tested using Wilcoxon rank-sum test for continuous variables, and Fischer’s exact test for categorical variables. All analyses were performed using JMP statistical software (version 10; SAS Institute, Cary, NC).
Results
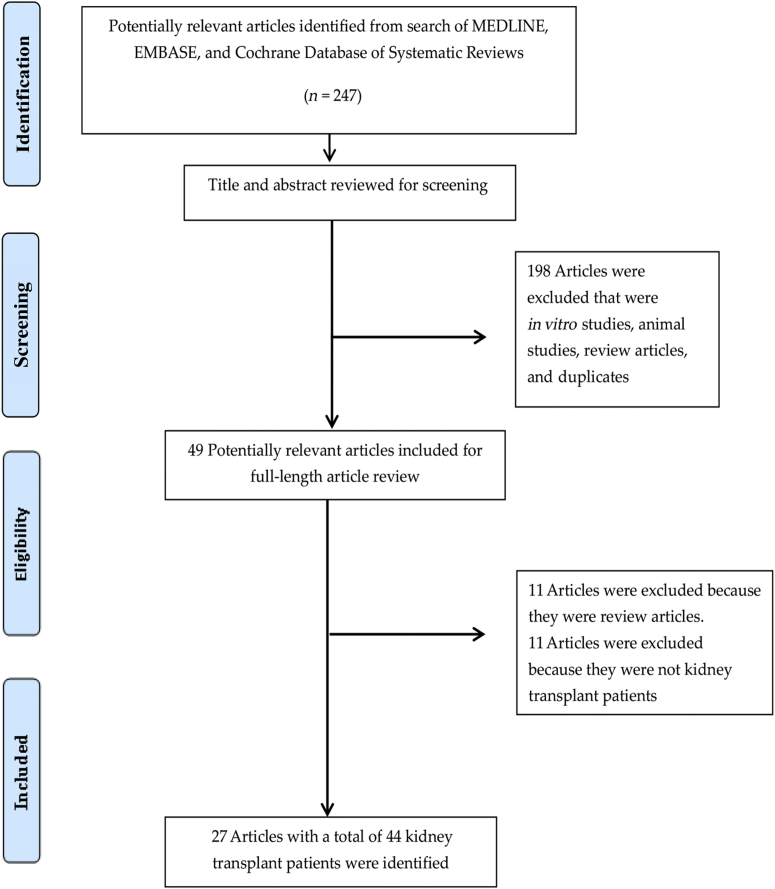
Our search strategy yielded 247 articles. Of these, 198 articles were excluded based on relevance and eligibility criteria after the review of the title and abstract. The remaining 49 articles underwent full-length review. Subsequently, 11 articles were excluded because they were review articles and another 11 articles were excluded because they were not Ktx patients (liver,10, 11, 12, 13, 14, 15, 16 heart,17,18 and hematopoietic stem cell transplantation19,20). Finally, 27 articles with a total of 44 Ktx patients were enrolled in our systematic review.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Figure 1 outlines the search methodology used.
Figure 1.
Outline of our search methodology.
Among these 44 Ktx patients, the average age at the time they received the ICIs was 64 years (SD ±10). Thirty-five of the 44 patients (80%) were male. The predominant underlying cancer being treated was dermatological, with 30 cases (68%) being melanoma and 5 cases (11%) presenting with metastatic squamous cell carcinoma of the skin. Lung cancer was the next most common cancer in 11% (5 of 44) of cases. The other cancers noted were Merkel cell carcinoma (5%), urothelial carcinoma (2%), and duodenal cancer (2%). Patients had developed cancer an average of 107 months (98) after kidney transplantation.
Thirty-six (81%) patients received a single ICI with nivolumab in 15 (34%) cases, pembrolizumab in 11 (25%), ipilimumab in 9 (20%), and avelumab in 1 (2%) patients. Among the different classes, the PD1 inhibitor was the most commonly chosen drug, with use in 26 (59%) patients. Combination checkpoint blockade was conducted in a sequential manner in 8 (18%) patients in the following patterns: ipilimumab followed by pembrolizumab in 2 (5%) or nivolumab in 4 (9%) cases, or pembrolizumab followed by nivolumab in 1 (2%) or ipilimumab in 1 (2%) case. Table 1 summarizes the demographic characteristics and outcomes of Ktx patients with ICIs for cancer treatment.
Table 1.
Demographic characteristics and outcomes of kidney transplant patients with immune checkpoint inhibitors for cancer treatment
| Variable | Total (N = 44) |
|---|---|
| Age, yr | 64±10 |
| Male sex | 35 (80) |
| Immune checkpoint inhibitor | |
| - Nivolumab | 15 (34) |
| - Pembrolizumab | 11 (25) |
| - Ipilimumab | 9 (20) |
| - Avelumab | 1 (2) |
| - Ipilimumab followed by pembrolizumab | 2 (5) |
| - Ipilimumab followed by nivolumab | 4 (9) |
| - Pembrolizumab followed by nivolumab | 1 (2) |
| - Pembrolizumab followed by ipilimumab | 1 (2) |
| Cancer | |
| - Melanoma | 30 (68) |
| - Lung cancer | 5 (11) |
| - Metastatic squamous cell carcinoma of skin | 5 (11) |
| - Merkel cell carcinoma | 2 (5) |
| - Urothelial carcinoma | 1 (2) |
| - Duodenal cancer | 1 (2) |
| Rejection | 18 (41) |
| Graft failure | 16/43a (37) |
| Cancer outcomes | |
| - Complete response | 5 (11) |
| - Partial response | 8 (18) |
| - Stable disease | 8 (18) |
| - Progressive disease | 21 (48) |
| - Not available | 2 (5) |
| Death | 18 (41) |
| Follow-up time | |
| - Less than 1 yr | 15 |
| - 1–3 yr | 5 |
One patient was excluded because he had graft failure due to transplant nephrectomy.
Acute Rejection Following ICIs
Of 44 Ktx patients, 18 (41%) were reported to have acute rejection of renal allograft. Median time from ICI to acute rejection diagnosis was 24 (interquartile range, 10–60) days. Cancer types treated were melanoma (66%), lung cancer (17%), and metastatic squamous cell carcinoma of skin (12%), respectively. There were 83% men with a mean age of 62 ± 13 years (Table 2). Eight (44%) patients received nivolumab, 3 (17%) received pembrolizumab, 2 (11%) received ipilimumab, 2 (11%) received ipilimumab followed by pembrolizumab, 2 (11%) received ipilimumab followed by nivolumab, and 1 (6%) received pembrolizumab followed by nivolumab.
Table 2.
Demographic characteristics and outcomes of kidney transplant patients who developed acute rejection following immune checkpoint inhibitors
| Variable | Total (N = 18) |
|---|---|
| Age, yr | 62 ± 13 |
| Male sex | 15 (83) |
| Immune checkpoint inhibitor, n (%) | |
| - Nivolumab | 8 (44) |
| - Pembrolizumab | 3 (17) |
| - Ipilimumab | 2 (11) |
| - Ipilimumab followed by pembrolizumab | 2 (11) |
| - Ipilimumab followed by nivolumab | 2 (11) |
| - Pembrolizumab followed by nivolumab | 1 (6) |
| Cancer | |
| - Melanoma | 12 (67) |
| - Lung cancer | 3 (17) |
| - Metastatic squamous cell carcinoma of skin | 3 (17) |
| Time from immune checkpoint treatment to rejection, d | 24 (10–60) |
| Type of rejection | |
| - Acute cellular rejection | 6 (33) |
| - Acute mixed rejection | 3 (17) |
| - Unspecified | 9 (50) |
| Graft failure | 15 (83) |
| Cancer outcomes | |
| - Complete response | 1 (6) |
| - Partial response | 3 (17) |
| - Stable disease | 5 (28) |
| - Progressive disease | 7 (39) |
| - Not available | 2 (11) |
| Death | 8 (44) |
Reported types of acute allograft rejection were cellular rejection (33%), mixed cellular and antibody-mediated rejection (17%), and unspecified type (50%). Eventually, 15 (83%) of the 18 patients had allograft failure with subsequent mortality in 8 (44%) patients. From a cancer standpoint, progressive disease was seen in 7 (40%) of these patients. Complete response, partial response, and stable disease was reported in 1 (6%), 3 (17%), and 5 (28%) of patients respectively.
Graft Tolerance Following ICIs
Out of the 44 Ktx patients, 25 (59%) patients did not have a rejection. One of the patients had a transplant nephrectomy (Boyle et al.44) before the use of ICI, as he had donor-derived melanoma. All patients were followed for an average of 9.6 months after receiving ICI. Most of these patients had received a PD-1 inhibitor (57%) with pembrolizumab or nivolumab in 8 (3.07%) and 7 (26.9%) cases, respectively. The predominant cancer indication was noted to be melanoma (69% of patients). From a cancer standpoint, 14 (53%) of them had progressive disease, whereas a favorable response, including complete response, partial response, and stable disease, was noted in 4 (15.3%), 5 (19.2%), and 3 (11.5%) of the patients, respectively. From a renal standpoint, only 2 (7.6%) patients had a graft failure (Table 3).
Table 3.
Characteristics of kidney transplant patients that developed an acute rejection with those that did not develop rejection after immune checkpoint inhibitor therapy
| Variable | Rejection (N = 18) | No rejection (N = 25)a | P value |
|---|---|---|---|
| Age | 61.6 (12.6) | 66 (6.28) | 0.13 |
| Sex, male | 15 (83.3) | 20 (76) | 0.6 |
| Drug class | |||
| CTLA-4 | 2 (11.1) | 7 (26.9) | 0.27b |
| PD-1 | 11 (61) | 15 (57) | 0.8 |
| PDL-1 | — | 1 (3.8) | — |
| Combination CTLA-4 and PD-1 | 4 (22.2) | 3 (11.5) | 0.41b |
| Sequential PD-1 | 1 (5.5) | — | — |
| Drug name | |||
| Ipilimumab | 2 (11.1) | 7 (26.9) | 0.2b |
| Pembrolizumab | 3 (16.6) | 8 (30.7) | 0.2b |
| Nivolumab | 8 (44.4) | 7 (26.9) | 0.22 |
| Avelumab | — | 1 (3.8) | — |
| Ipilimumab followed by pembrolizumab | 2 (11.1) | 1 (3.8) | c |
| Ipilimumab followed by nivolumab | 2 (11.1) | 2 (7.6) | c |
| Pembrolizumab followed by nivolumab | 1 (5.5) | — | — |
| Cancer type | |||
| Melanoma | 12 (66.6) | 18 (69) | 0.85 |
| Metastatic Squamous cell carcinoma of skin | 3 (16.6) | 2 (7.6) | c |
| Lung cancer | 3 (27) | 2 (7.6) | c |
| Duodenal cancer | — | 1 (3.8) | — |
| Urothelial cancer | — | 1 (3.8) | — |
| Merkel cell carcinoma | — | 2 (7.6) | — |
| Follow-up time after immune checkpoint inhibitor therapy, mo | 11.3 (10.3) | 9.6 (8.1) | — |
| Graft failure | 15 (83.3) | 2 (7.6) | <0.0001 |
| Cancer outcome | |||
| Favorable response | 0.52 | ||
| Complete response | 1 (5.5) | 4 (15.3) | — |
| Partial response | 3 (16.6) | 5 (19.2) | — |
| Stable disease | 5 (27.7) | 3 (11.5) | — |
| Progressive disease | 7 (38.8) | 14 (53.8) | 0.32 |
| Not available | 2 | — | — |
| Patient mortality | |||
| Dead | 8 (44.4) | 10 (38.4) | 0.88 |
| Not available | 2 | 5 | — |
CTLA-4, cytotoxic T-lymphocyte-associated-antigen 4; PD-1, programmed cell death 1; PDL-1, programmed cell death ligand 1.
P value < 0.05 was considered statistically significant and is in bold.
One patient excluded as he had a nephrectomy before immune checkpoint blockade.
Nonparametric test was used for statistical analysis.
Too few patients for any clinically meaningful statistical analysis.
Immunosuppression Management
Immunosuppression data were missing in multiple studies. At the time of cancer diagnosis, baseline immunosuppression regimen data were available in 31 of the 44 patients. Of these patients, 15 (48%) were on a triple regimen with a calcineurin inhibitor (CNI), such as tacrolimus or cyclosporine, mycophenolate mofetil (MMF), and a low-dose steroid. A CNI-based regimen was used in 30 of 31 patients, which included the following: CNI alone in 1 (3.2%), CNI/steroid in 6 (19.3%), CNI/MMF in 5 (16.1%), CNI/MMF/steroid in 15 (48.3%), CNI/azathioprine/steroid in 3 (9.6%) of the patients. One patient was on a mammalian target of rapamycin inhibitor with steroids.
The data were again limited on the changes in immunosuppression for allograft after the cancer diagnosis, which was available only in 21 of the 44 patients. Of these, interestingly, 42% (9 patients) had no change done to their immunosuppressive therapy. Nine patients of 21 had their CNI discontinued. Of these, 7 of them were replaced with a mammalian target of rapamycin inhibitor like sirolimus or everolimus, and 3 of them, in addition, also had discontinuation of their MMF. Overall, 4 of 21 patients had their MMF discontinued.
We also reviewed the changes in immunosuppression done at the time of ICI therapy initiation. Data were incomplete for interpretation in 16 patients. Of the remaining 28 patients, interestingly, 5 patients had no change in their immunosuppression both at the time of cancer diagnosis and checkpoint inhibitor therapy initiation. Three of those 5 patients had melanoma and they ended up with progressive disease, whereas the other 2 of 5 patients had other underlying cancers (1 squamous cell carcinoma and 1 urothelial cancer) and they had complete response. On the other hand, 11 (39%) of the 28 patients were tapered down to steroids alone for graft preservation at the time of checkpoint inhibitor initiation and 8 of them subsequently ended up with a rejection. For the 9 patients whose CNI had already been stopped at the time of cancer diagnosis, 6 patients underwent further reduction in therapy, which ranged from overall reduction in dose of other immunosuppressant (n = 2), discontinuation of MMF (n = 1), regimen of steroid alone (n = 3). In the 9 patients described earlier who had undergone no change in immunosuppression at the time of cancer diagnosis, 4 of them were rapidly tapered to steroid-alone regimen at the time of checkpoint inhibitor therapy initiation. Three of the 4 were on triple immunosuppression with CNI/MMF/steroid and 2 of these had a rejection with graft failure (Table 4).
Table 4.
Immunosuppression management in kidney transplant patients around the time of immune checkpoint inhibitor therapy
| Variable | Rejection (N = 18) | No rejection (N = 25a) |
|---|---|---|
| Time from kidney transplantation to cancer development, mo | 135 (103) | 91 (92) |
| Baseline creatinine at the time of immune checkpoint inhibitor therapy, mg/dl | 1.3 (0.56) | 1.4 (0.4) |
| Baseline eGFR at the time of immune checkpoint inhibitor therapy, ml/min | 63.8 (15.4) | 53.2 (16.7) |
| Maintenance immunosuppression at the time of cancer diagnosis | ||
| CNI | 1 (7.6) | — |
| CNI/MMF | 3 (23) | 2 (11.7) |
| CNI/steroid | 2 (15.3) | 4 (23.5) |
| CNI/MMF/steroid | 4 (30.7) | 10 (23.5) |
| CNI/AZA/steroid | 3 (23.0) | — |
| mTOR/steroid | — | 1 (5.8) |
| Not available | 5 | 8 |
| Change in immunosuppression at the time of cancer diagnosis | ||
| No change | 3 (33.3) | 6 (50) |
| Reduction in dose alone | 2 (22.2) | — |
| Stop CNI alone | 1 (11.1) | 1 (8.3) |
| CNI to mTOR alone | 1 (11.1) | 3 (25) |
| CNI to mTOR + stop MMF | 2 (22.2) | 1 (8.3) |
| Not available | 9 | 13 |
| Planned change in immunosuppression at the time of immune checkpoint inhibitor therapy | ||
| No change | — | 8 (34.7) |
| Reduction in dose alone | 2 (11.7) | 1 (4.3) |
| Stop MMF | — | 1 (4.3) |
| CNI to mTOR | 2 (11.7) | 1 (4.3) |
| CNI to mTOR + stop MMF | 1 (5.8) | 1 (4.3) |
| Steroid alone | 8 (47) | 3 (13.0) |
| Baseline immunosuppression data not available for comparison | — | 8 (34.7) |
| Missing data | 1 | 2 |
AZA, azathioprine; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin inhibitor.
One patient excluded due to nephrectomy before immune checkpoint blockade.
Cancer Outcomes
Overall, details regarding cancer outcome was reported in 42 of the 44 patients. Complete response, partial response, and disease stability was noted in 5 (11%), 8 (18%), and 8 (18%) of the patients, respectively. Progressive disease was reported in 21 (48%) of the patients. Three patients had a partial remission (17%), 1 patient achieved cancer response (6%), and 5 patients had stable disease (28%).
Patient Outcomes
Overall, of the 44 Ktx recipients, survival data were reported in 37 patients. Nineteen of 37 (43%) were reported to be alive at the end of follow-up. Among the 18 patients who had a rejection, 8 (44%) were reported alive and the remaining died of various etiologies. The median age of those who were reported alive was lower than those who died (60, interquartile range, 50.5–72.5; vs. 68, interquartile range, 47.5–73.0) but not statistically significant. Both groups had predominantly melanoma patients, but information regarding the stage of cancer and associated comorbidities was not available for review.
Among those who did not have a rejection, data were missing in 6 patients, but interestingly, 9 (36%) of the patients were reported alive at last follow-up. Eighteen patients were reported deceased at the time of last follow-up, and of these, progressive disease was noted in 14 (77.8%) of them, but interestingly only 6 (28%) of them had graft failure. Of the 18 patients who were reported alive at the time of last follow-up, 15 of them had a favorable cancer outcome with complete response in 4, partial response in 4, and stable disease in 7, and this was despite the fact that half of them had allograft failure (1 of whom was the patient with donor-derived melanoma who underwent a nephrectomy).
Discussion
In our systematic review of 27 articles, we found 44 Ktx patients who had received ICI therapy, with 18 of the 44 (41%) culminating in a renal allograft rejection. Of these, 15 (83%) patients eventually had complete graft failure. Evidence of rejection was noted early with an average of 24 days (10–60 days). Management of immunosuppression around the time of ICIs is crucial in balancing the delicate yin-yang of cancer therapy and allograft protection with the multitude of regimen changes reported in literature is a testament to the need for expert consensus guidance to the transplant community.
In immunology, the concept of co-stimulatory signals was a major milestone.48 The immunoglobulin superfamily of co-stimulatory receptors is crucial in the adaptive immune system's ability to create a destructive response to an antigenic target. The role of the “stimulatory” members of this family is the generation of the signal 2, typically CD28 on T cell with CD80 or CD86 of the antigen-presenting dendritic cell, which along with T-cell receptor and peptide major histocompatibility complex trigger (signal 1) this immune cascade. Moreover, to regulate this system, it is critical to have negative regulatory members. This is to be able to restrain an activated immune system and hence induce a tolerance. The inhibitory members of the immunoglobulin superfamily can be either “T-cell intrinsic,” as are cytotoxic T-lymphocyte-associated-antigen 4 and PD-1. CD4 regulatory T cells, T follicular helper cells, memory cells, and exhausted CD8 cells typically have high levels of PD-1 expression.49 The inhibitors that are T-cell extrinsic are present on the tissue (e.g., macrophages), like the ligand of PD-1, PDL-1.50
In organ transplantation, the PD-1:PDL-1 pathway plays a critical role in regulation of peripheral tolerance as well as protective immunity.49 PDL-1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine production. Major histocompatibility complex class II–expressing renal tubular epithelial cells can function as antigen-presenting cells for T cells.51 Renal tubular epithelial cells can regulate T-cell activation and suppress alloreactivity and immunopathology in the kidney, but with the blockade of this PD1: PDL-1 pathway, the kidney allograft is at a higher risk of rejection.52 The immunosuppressive drugs that could be used to alter this balance by generating an environment of graft tolerance, unfortunately, dampen the immune system’s ability to detect and destroy cancer cells. Furthermore, immunogenic cancers, such as melanoma, also induce immunosuppression around it to be able to grow and metastasize. It does this by various mechanisms: downregulation of surface antigens, secretion of immunosuppressive cytokines, and lack of co-stimulatory signals, which in the end induce tolerance and evade immune system detection.53 ICIs take away these tolerance “brakes” and are very effective in cancer therapy but can be detrimental to the allograft.
The use of ICI in organ transplant patients is uncommon, and evidence is limited. De Bruyn et al.,54 in their systematic review, found 48 cases of liver and Ktx recipients who had received ICI. Of the 29 Ktx patients, 45% (13 of 29) had rejection after receiving ICI. Six of them had received dual ICI with a cytotoxic T-lymphocyte-associated-antigen 4 and PD-1 inhibitor, but only 3 had experienced a rejection within 2 to 4 weeks of receiving the second agent. Most of their immunosuppression, around the time of immunotherapy, had been reduced to steroid monotherapy.54 From a cancer outcome standpoint, 7 of 13 (53%) patients who had a rejection had a favorable outcome.54
Abdel-Wahab et al.55 noted in their systematic review, 39 solid organ transplant patients (including 9 from their institution) who had received ICI therapy. This included cardiac (n = 5), liver (n = 11), and kidney (n = 23) recipients. Rejection was highest in the Ktx group reported in 48% of the patients compared with liver (36%) and cardiac (20%). Of those who had histology obtained, approximately 50% of them were T-cell–mediated rejection, and the rest was a combination of cellular and antibody-mediated rejection. They noted that 20 patients had preemptive baseline immunosuppression modifications before ICI use. Patients who were on prednisone <10 mg/d had the highest rate of graft rejection but were also the ones with the best tumor response, with 63% having disease either in remission or stabilization. Those on calcineurin inhibitors had the least rejection rates, at 11%, but also had the lowest tumor response rate at 25%.55 In our study, we noted similar results: 61% of the patients who had a rejection were on a steroid-alone regimen at the time of ICI use and those who had no significant change to their immunosuppression regimen had allograft preservation. Among those who had a favorable cancer outcome, 9 (52%) patients were on a steroid-alone regimen. More recently, Venkatachalam et al.56 reported another case series of 6 Ktx patients receiving ICIs (2 patients with squamous cell cancers, 2 with melanoma, 1 with adenocarcinoma of the lung, and 1 with renal cell cancer). In this case series with a longer follow-up, 3 of 6 patients developed acute kidney injury. Two had biopsy-proven acute allograft rejections and subsequently had allograft loss. The third case had probable acute rejection, which fortunately recovered following the discontinuation of the checkpoint inhibitor. In this case series, 5 of 6 patients died within a year of being on ICI.56
Regardless of the cancer type Abdel-Wahab et al.55 noted that the median overall survival was lower in patients who had a rejection (5 months; 95% confidence interval, 1–9 months) compared with those who did not have a rejection (12 months; 95% confidence interval, 8–16 months). It is important to note that in this study, the authors included liver, kidney, and cardiac transplant patients, which differs from our study that was restricted to Ktx recipients alone. Therefore, we believe the transplant patient overall survival was more closely linked with the cancer outcome rather than the allograft failure because these patients could be transitioned to dialysis in the case of renal allograft failure unlike other solid organ transplant patients. We found 83% of those who were alive at the last follow-up showed evidence of favorable cancer outcome. This highlights the importance of understanding the underlying cancer biology and its aggressiveness and the need for close communication among the patients, their families, oncologists, and transplant nephrologists.
The level of reduction in immunosuppression after a cancer diagnosis alone depends on many factors, for instance, the patient’s age, HLA match, prior history of rejection, allograft source, time after transplantation, prior kidney transplants received, and the medication types and levels. In melanoma, expert consensus guidelines by International Transplant Skin Cancer Collaborative and the Skin Care in Organ Transplant Patients Europe give recommendations of the level of reduction in immunosuppression to consider based on the allograft type and stage of melanoma.57 For Ktx recipients, a mild reduction in immunosuppression is recommended for stage 1A–1B melanoma, moderate reduction for stage 2A–2B, and a severe reduction for those with stage ≥2C melanoma.57 Although not ideal, a more aggressive approach may be needed in some patients with discontinuation of all immunosuppressive agents with a return to dialysis, as a life-saving measure, a life support option that is only possible in Ktx patients as opposed to other solid organ transplant recipients. This would need to be a careful informed mutual decision among the patients, oncologists, hematologists, and transplant nephrologists.
So what immunosuppressant drugs should we consider stopping? In our review, we found that CNIs were the most common drug to be discontinued at the time of cancer diagnosis (42.8%) and at the time of ICI initiation (17.8%). When the CNI was switched, it was typically to the mammalian target of rapamycin agent. MMF was stopped in 19% of patients at the time of cancer diagnosis and again in 10% of patients at the time of ICI initiation.
CNIs changed the allograft survival paradigm in organ transplantation; however, these are also the drugs that have the strongest evidence of heightened cancer risk. Hojo et al.58 demonstrated in their mice model study using noninvasive adenocarcinoma cell lines that exposure to cyclosporine made these cells acquire an invasive nature with the development of metastasis. They also showed that this is related to cyclosporine-induced transforming growth factor-beta production by tumor cells,58 and that when transforming growth factor-beta was blocked, metastatic lesions were prevented. Similar effects have been reported with tacrolimus,59 and some even report that the risk of posttransplant lymphoproliferative disease is higher with tacrolimus than cyclosporine.60 Interestingly, in a recent randomized trial of Ktx recipients, Dantal et al.61 showed that the incidence of cancer in cyclosporine patients might be dose-dependent. The patients who were randomized to receive maintenance immunosuppression after 1 year with cyclosporine at a lower trough (75–125 ng/ml) had a lower incidence of any type of cancer (20% vs. 32%) compared with their standard trough (150–250 ng/ml) group.61 One of the older immunosuppressive drugs, azathioprine, is also known for its cytotoxic and mutagenic potential with an increased risk of posttransplant lymphoproliferative disease.60,62
On the other hand, mammalian target of rapamycin inhibitors, such as rapamycin and sirolimus, have been shown in animal model studies to prevent tumor growth and progression.63 They are also noted to reduce vascular endothelial growth factor levels.64 Subsequent studies by Kauffman et al.65 have shown this to be clinically appreciable as well. In their study, they reported that in 33,249 Ktx recipients, the incidence rate of any de novo malignancy was lowest in sirolimus/everolimus groups at 0.6% compared with 1.8% with cyclosporine/tacrolimus.65 Also, one must not forget that immunosuppression begins with the initial induction regimen and that the risk of malignancies like posttransplant lymphoproliferative disease rose with the use of OKT3 and anti-thymocyte globulin.60 It has also been shown that treatment of rejection episodes with these drugs only has an additive effect on their risk.60 Unfortunately, data regarding this in our review were incomplete and hence unable to make any sufficient conclusions.
The major limitation of our systematic review was that the included articles were case reports and case series, which may have precluded the real evaluation of the acute allograft rejection following the ICI therapy. Certain cases of successful treatment of ICI without allograft rejection/failure among Ktx recipients might not have been published, leading to potential publication bias. We were also limited by the short-term follow-up available to be able to determine the impact of the allograft rejection on overall patient and cancer outcome. Thus, our statistical analysis was limited to descriptive, because of potential significant bias in the reporting source. Further, large cohort studies are required to evaluate the incidence of ICI-associated allograft rejection/failure among Ktx recipients, and the impacts of different immunosuppression regimens around the time of ICI use on patient/outcomes.
In summary, we present a systematic review of all reported Ktx patients who received immune checkpoint inhibitor therapy. Allograft rejection following immune checkpoint inhibitor therapy has been increasingly reported. The time to allograft rejection is rapid and necessitates close follow-up by the nephrologists. The management of immunosuppression around the time of ICI use is difficult and a close collaboration with the treating hematologist/oncologist as well as the goals of the patient are critical while caring for these complex cases.
Disclosure
SMH is supported by National Institutes of Health K08 DK118120 from the National Institute of Diabetes and Digestive and Kidney Diseases and by a Mary Kathryn and Michael B. Panitch Career Development Award. All the other authors declared no competing interests.
Acknowledgments
An abstract of this paper was presented as an oral presentation at the American Society of Nephrology conference 2019. All authors had access to the data and had a role in writing the manuscript.
Footnotes
Item S1. Search terms and strategy for systematic review.
Item S2. PRISMA Checklist.
Supplementary Material
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A., Keown P., Pus N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 3.Briggs J.D. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16:1545–1549. doi: 10.1093/ndt/16.8.1545. [DOI] [PubMed] [Google Scholar]
- 4.Pilmore H., Dent H., Chang S. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89:851–857. doi: 10.1097/TP.0b013e3181caeead. [DOI] [PubMed] [Google Scholar]
- 5.Engels E.A., Pfeiffer R.M., Fraumeni J.F., Jr. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajdic C.M., McDonald S.P., McCredie M.R. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske B.L., Vazquez M.A., Harmon W.E. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1–S86. [PubMed] [Google Scholar]
- 8.Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61:274–278. doi: 10.1097/00007890-199601270-00019. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Toni E.N., Gerbes A.L. Tapering of immunosuppression and sustained treatment with nivolumab in a liver transplant recipient. Gastroenterology. 2017;152:1631–1633. doi: 10.1053/j.gastro.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 11.Ranganath H.A., Panella T.J. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38:211. doi: 10.1097/CJI.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 12.Friend B.D., Venick R.S., McDiarmid S.V. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64:e26682. doi: 10.1002/pbc.26682. [DOI] [PubMed] [Google Scholar]
- 13.Morales R.E., Shoushtari A.N., Walsh M.M. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. doi: 10.1186/s40425-015-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schvartsman G., Perez K., Sood G. Immune checkpoint inhibitor therapy in a liver transplant recipient with melanoma. Ann Intern Med. 2017;167:361–362. doi: 10.7326/L17-0187. [DOI] [PubMed] [Google Scholar]
- 15.Biondani P., De Martin E., Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29:286–287. doi: 10.1093/annonc/mdx548. [DOI] [PubMed] [Google Scholar]
- 16.Kuo J.C., Lilly L.B., Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28:61–64. doi: 10.1097/CMR.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 17.Owonikoko T.K., Kumar M., Yang S. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 2017;66:45–50. doi: 10.1007/s00262-016-1918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gastman B., Ernstoff M. Tolerability of immune checkpoint inhibition cancer therapy in a cardiac transplant patient. Ann Oncol. 2016;27:2304–2305. doi: 10.1093/annonc/mdw293. [DOI] [PubMed] [Google Scholar]
- 19.Covut F., Pinto R., Cooper B. Nivolumab before and after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52:1054. doi: 10.1038/bmt.2017.44. [DOI] [PubMed] [Google Scholar]
- 20.Angenendt L., Schliemann C., Lutz M. Nivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:443. doi: 10.1038/bmt.2015.266. [DOI] [PubMed] [Google Scholar]
- 21.Zehou O., Leibler C., Arnault J.P. Ipilimumab for the treatment of advanced melanoma in six kidney transplant patients. Am J Transplant. 2018;18:3065–3071. doi: 10.1111/ajt.15071. [DOI] [PubMed] [Google Scholar]
- 22.Tamain M., Garrouste C., Aguilera D. Mixed acute kidney allograft rejection after an antiprogrammed cell death protein 1 antibody treatment for lung epidermoid carcinoma. Transplant Int. 2016;29:1247–1248. doi: 10.1111/tri.12834. [DOI] [PubMed] [Google Scholar]
- 23.Jose A., Yiannoullou P., Bhutani S. Renal allograft failure after ipilimumab therapy for metastatic melanoma: a case report and review of the literature. Transplant Proc. 2016;48:3137–3141. doi: 10.1016/j.transproceed.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Gueguen J., Bailly E., Machet L. CMV disease and colitis in a kidney transplanted patient under pembrolizumab. Eur J Cancer. 2019;109:172–174. doi: 10.1016/j.ejca.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Tio M., Rai R., Ezeoke O.M. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137–144. doi: 10.1016/j.ejca.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herz S., Höfer T., Papapanagiotou M. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer. 2016;67:66–72. doi: 10.1016/j.ejca.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Ong M., Ibrahim A.M., Bourassa-Blanchette S. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer. 2016;4:64. doi: 10.1186/s40425-016-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akturk H.K., Alkanani A., Zhao Z. PD-1 inhibitor immune-related adverse events in patients with preexisting endocrine autoimmunity. J Clin Endocrinol Metab. 2018;103:3589–3592. doi: 10.1210/jc.2018-01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipson E.J., Bodell M.A., Kraus E.S., Sharfman W.H. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32:e69–e71. doi: 10.1200/JCO.2013.49.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadaat M., Jang S. Complete tumor response to pembrolizumab and allograft preservation in renal allograft recipient on immunosuppressive therapy. J Oncol Pract. 2018;14:198–199. doi: 10.1200/JOP.2017.027326. [DOI] [PubMed] [Google Scholar]
- 31.Winkler J.K., Gutzmer R., Bender C. Safe administration of an anti-PD-1 antibody to kidney-transplant patients: 2 clinical cases and review of the literature. J Immunother. 2017;40:341–344. doi: 10.1097/CJI.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 32.Kwatra V., Karanth N.V., Priyadarshana K., Charakidis M. Pembrolizumab for metastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: a case report. J Med Case Rep. 2017;11:73. doi: 10.1186/s13256-017-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesouhaitier M., Dudreuilh C., Tamain M. Checkpoint blockade after kidney transplantation. Eur J Cancer. 2018;96:111–114. doi: 10.1016/j.ejca.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Spain L., Higgins R., Gopalakrishnan K. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol. 2016;27:1135–1137. doi: 10.1093/annonc/mdw130. [DOI] [PubMed] [Google Scholar]
- 35.Singh P., Visger Von J., Prosek J. Preserved renal allograft function and successful treatment of metastatic Merkel cell cancer post nivolumab therapy. Transplantation. 2019;103:E52–E53. doi: 10.1097/TP.0000000000002502. [DOI] [PubMed] [Google Scholar]
- 36.Goldman J.W., Abdalla B., Mendenhall M.A. PD 1 checkpoint inhibition in solid organ transplants: 2 sides of a coin - Case report. BMC Nephrol. 2018;19:210. doi: 10.1186/s12882-018-1003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boils C.L., Aljadir D.N., Cantafio A.W. Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant. 2016;16:2496–2497. doi: 10.1111/ajt.13786. [DOI] [PubMed] [Google Scholar]
- 38.Alhamad T., Venkatachalam K., Linette G.P., Brennan D.C. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. 2016;16:1332–1333. doi: 10.1111/ajt.13711. [DOI] [PubMed] [Google Scholar]
- 39.Lipson E.J., Bagnasco S.M., Moore J. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 2016;374:896–898. doi: 10.1056/NEJMc1509268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu C.K., Juang G.D., Lai H.C. Tumor regression and preservation of graft function after combination with anti-PD-1 immunotherapy without immunosuppressant titration. Ann Oncol. 2017;28:2895–2896. doi: 10.1093/annonc/mdx409. [DOI] [PubMed] [Google Scholar]
- 41.Barnett R., Barta V.S., Jhaveri K.D. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab: To the editor. N Engl J Med. 2017;376:191–192. doi: 10.1056/NEJMc1614298. [DOI] [PubMed] [Google Scholar]
- 42.Deltombe C., Garandeau C., Quereux G. Severe allograft rejection and autoimmune hemolytic anemia after anti-PD1 therapy in a kidney transplanted patient. Transplant Int. 2017;30:405. doi: 10.1097/TP.0000000000001861. [DOI] [PubMed] [Google Scholar]
- 43.Coche S., Pieters T., Devresse A., Kanaan N. Immune checkpoint inhibitors in kidney transplant recipients. Transplant Int. 2018;31:13. [Google Scholar]
- 44.Boyle S.M., Ali N., Olszanski A.J. Donor-derived metastatic melanoma and checkpoint inhibition. Transplant Proc. 2017;49:1551–1554. doi: 10.1016/j.transproceed.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Kittai A.S., Oldham H., Cetnar J., Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40:277–281. doi: 10.1097/CJI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 46.Soellradl I., Kehrer H., Cejka D. The use of checkpoint-inhibitors in metastatic melanoma in a combined heart and kidney transplant recipient. A case report. Transplant Int. 2018;31:18–19. doi: 10.1016/j.transproceed.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Miller D.M., Faulkner-Jones B.E., Stone J.R., Drews R.E. Complete pathologic response of metastatic cutaneous squamous cell carcinoma and allograft rejection after treatment with combination immune checkpoint blockade. JAAD Case Rep. 2017;3:412–415. doi: 10.1016/j.jdcr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller D.L., Jenkins M.K., Schwartz R.H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 49.Riella L.V., Paterson A.M., Sharpe A.H., Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peggs K.S., Allison J.P. Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. Br J Haematol. 2005;130:809–824. doi: 10.1111/j.1365-2141.2005.05627.x. [DOI] [PubMed] [Google Scholar]
- 51.Ding H., Wu X., Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–191. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Starke A., Lindenmeyer M.T., Segerer S. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 2010;78:38–47. doi: 10.1038/ki.2010.97. [DOI] [PubMed] [Google Scholar]
- 53.Kubica A.W., Brewer J.D. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87:991–1003. doi: 10.1016/j.mayocp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Bruyn P., Van Gestel D., Ost P. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol. 2019;31:54–64. doi: 10.1097/CCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Wahab N., Safa H., Abudayyeh A. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkatachalam K, Malone AF, Heady B, et al. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients [e-pub ahead of print]. Transplantation. https://doi.org/10.1097/TP.0000000000002914. Accessed November 2, 2019. [DOI] [PubMed]
- 57.Otley C.C., Berg D., Ulrich C. Reduction of immunosuppression for transplant-associated skin cancer: expert consensus survey. Br J Dermatol. 2006;154:395–400. doi: 10.1111/j.1365-2133.2005.07087.x. [DOI] [PubMed] [Google Scholar]
- 58.Hojo M., Morimoto T., Maluccio M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 59.Maluccio M., Sharma V., Lagman M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 60.Opelz G., Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 61.Dantal J., Hourmant M., Cantarovich D. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 62.Yuan B., Wang Y. Mutagenic and cytotoxic properties of 6-thioguanine, S6-methylthioguanine, and guanine-S6-sulfonic acid. J Biol Chem. 2008;283:23665–23670. doi: 10.1074/jbc.M804047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luan F.L., Hojo M., Maluccio M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002;73:1565–1572. doi: 10.1097/00007890-200205270-00008. [DOI] [PubMed] [Google Scholar]
- 64.Luan F.L., Ding R., Sharma V.K. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917–926. doi: 10.1046/j.1523-1755.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 65.Kauffman H.M., Cherikh W.S., Cheng Y. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.