Abstract
Ionizing radiation causes damage to a variety of tissues, especially radiation-sensitive tissues, such as the small intestine. Radiation-induced damage is caused primarily by increased oxidative stress in the body. Studies have shown that trace metal elements play an irreplaceable role in oxidative stress in humans, which may be associated with radiation-induced tissue damage. However, the alteration and functional significance of trace metal elements in radiation-induced injury is not clear. In this study, we explored the association between radiation-induced damage and 7 trace metal elements in mouse models. We found that the concentration of zinc and copper in mice serum was decreased significantly after irradiation, whereas that of nickel, manganese, vanadium, cobalt, and stannum was not changed by inductively coupled plasma mass spectrometry. The role of copper in radiation-induced intestines was characterized in detail. The concentration of copper was increased in irradiated intestine but reduced in irradiated heart. Immunohistochemistry staining showed that copper transporter protein copper transport 1 expression was upregulated in irradiated mouse intestine, suggesting its potential involvement in radiation-induced copper accumulation. At the cellular level, the addition of CuCl2 potentiated radiation-induced reactive oxygen species in intestine-derived human intestinal epithelial cell and IEC-6 cells. Moreover, the level of copper in damaged cells may be related to the severity of radiation-induced damage as evidenced by a cell viability assay. These results indicate that copper may be involved in the progression of radiation-induced tissue damage and may be a potential therapeutic target.
Keywords: radiation, trace metal element, copper, radiation-induced intestinal injury
Introduction
Exposure to radiation from nuclear accidents, terrorist attack, or radiotherapy leads to multiple tissue injuries, which poses serious threats to human public health. 1 Radiation-induced injury is a complex pathophysiological process. The sensitivity of human tissues and organs to radiation is different, and therefore, tissue injuries induced by irradiation differ. The organs with vigorous cell division have high radiation sensitivity, while adult tissues with slow cell division have low sensitivity to radiation. 2 For example, the gastrointestinal tract, especially the small intestine, is particularly sensitive to radiation. 3
Metals play important roles in a wide variety of biological processes of living systems. 4 As essential elements of the human body, metal elements form metal complexes such as metalloproteins and metalloenzymes with various bioligands (proteins, nucleic acids, vitamins, hormones, metabolites, etc.) and play an irreplaceable role in the oxidative stress response of the body. It has been well-documented that several trace metal elements, such as zinc, copper, nickel, manganese, vanadium, cobalt, and stannum, play important roles in maintaining the physiology of human body. For example, zinc is the second most abundant trace element (after iron) necessary for all organisms and is involved in the regulation of many genes and enzyme activities in the body, including cell division, growth, and differentiation, and plays a role in many aspects of cellular metabolism, involving the metabolism of proteins, lipids, and carbohydrates. 4 -6 The essential trace element copper is a cofactor of many enzymes involved in redox reactions, including cytochrome c oxidase, ascorbate oxidase, and superoxide dismutase. In addition to its enzymatic role, copper is used in biological systems for electron transport. 7 Cobalt is an essential component of vitamin B12 and is associated with DNA damage and repair. 6 Nickel and stannum are essential for human growth and energy metabolism. 8,9 Manganese and vanadium are cofactors for proteins and enzymes, both of which play important roles in antioxidant and immune regulation. 10 -13 Radiation-induced damage is largely caused by an increase in oxidative stress in the body. Previous studies have shown that metal elements play an important role in oxidative stress in humans 4 and thus may be related to radiation-induced damage.
However, the alteration and functional significance of trace metal elements in radiation-induced injury is not clear. In this study, we explored the alteration of trace metal elements during radiation-induced damage in mice using total body irradiation (TBI) models.
Materials and Methods
Reagents
Nitric acid (purity >99.8%), hydrochloric acid (purity >99.8%), and hydrogen peroxide (purity >99.8%) were purchased from Sinopharm Group Chemical Reagent Co, Ltd (Shanghai, China). Phosphate-buffered saline (PBS) was purchased from Beyotime (Nantong, China). The standard metal stock solution and scandium standard stock solution were both purchased from China National Nonferrous Metals and Electronic Materials Analysis and Testing Center (Beijing, China). CuCl2 was purchased from Meryer Chemical Technology Co, Ltd (Shanghai, China). A Cu2+-specific fluorescent probe [1-(3′,6′-bis(ethylamino)-2′,7′-dimethyl-3-oxospiro(isoindoline-1,9′-xanthene)-2-yl)thiourea(C27H29N5O2 S)] was purchased from Heliosense Biotech (Xiamen, China).
Animal Treatment
Male C57BL/6N mice (6-7 weeks of age) were purchased from the Shanghai SLAC Laboratory Animal Co, Ltd (Shanghai, China). These animals were housed in a pathogen-free environment at the facilities of the Medical School of Soochow University. Total body irradiation was delivered to mice at a dose rate of 600 mGy/min using 250 kV X-rays (Small Animal Irradiator X-RAD 320; Precision X-ray, North Branford, Connecticut). Mice were irradiated with 0, 2, 4, or 8 Gy X-rays. The animal experiment protocols were approved by the Animal Experimentation Ethics Committee of Soochow University (Suzhou, China).
Metal Element Standard Solution Preparation
Multistandard manganese, vanadium, cobalt, copper, zinc, tin, and nickel standard stock solution (100 μg/mL) and a scandium internal reference standard stock solution (1000 μg/mL) were purchased from China National Nonferrous Metals and Electronic Materials Analysis and Testing Center. Five percent HNO3 was used to obtain multistandard working solutions of 1, 5, 20, 50, 100, 200, and 500 ng/mL and in making solutions of standards, blanks, and samples containing 20 ng/mL scandium internal standard.
Determination of the Metal Element Concentration in Mice Sera and Tissues
Mice were subjected to retro-orbital blood sampling. Collected blood was cooled at 4°C for 2 hours and then centrifuged (13 000g) to obtain the upper layers of serum. The heart and small intestine tissues were rinsed with PBS to remove any residual blood and intestinal contents and then placed on filter paper to absorb any remaining moisture. One hundred microliters of serum or 0.25 g tissue (heart or small intestine) was placed in 25-mL beakers and digested in 10 mL of aqua regia. After 24 hours, the beakers were placed on a temperature-controlled electric heating board, which was heated to 230°C for digestion. Then the samples were neutralized twice with hydrogen peroxide solution (1 mL each time). The digested solution was quantitatively transferred with 5% HNO3 to a 5-mL volumetric flask that had the Sc internal standard solution added, and the volume was adjusted and shaken to obtain the sample solution to be tested. Another clean volumetric flask was used for reagent blanks and standard samples, all of which were made using the above methods to synchronize operations. The metal elements in the serum and tissue were measured by inductively coupled plasma mass spectrometry (ICP-MS; ELEMENT 2; Thermo-Fisher, Waltham, Massachusetts).
Cell Culture and Irradiation
Normal human intestinal epithelial cells (HIEC) and a rat jejunal crypt cell line (IEC-6) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cell lines were confirmed by short tandem repeat DNA profiling. The cells were maintained in Dulbecco’s modified Eagle’s medium. Cells were exposed to different dosages (4 or 10 Gy) of ionizing radiation using an X-ray linear accelerator (RadSource, Suwanee, Georgia) at a fixed dose rate of 1.15 Gy/min.
Intracellular Copper Staining Using a Copper-Specific Probe
Frozen sections were prepared from mouse intestinal tissues 28 days after 0, 2, or 4 Gy radiation. Sections were incubated with 20 μM of the copper-specific probe (C27H29N5O2S) for 20 minutes at 37°C. For visualization of intracellular Cu2+, cells were treated with different concentrations of copper and irradiated at different doses. The cells were then incubated with 20 μM C27H29N5O2S at 37°C for 20 minutes. Images were photographed with a scanning laser confocal microscope (Olympus, Tokyo, Japan), with excitation and emission wavelengths of 488 and 530 nm, respectively.
Immunohistochemistry Staining
Tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Three-micrometer-thick paraffin sections were deparaffinized and heat-treated with citrate buffer (pH 6.0) for 7 minutes as an epitope retrieval protocol. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 minutes at room temperature and tissue nonspecific-binding sites were blocked with 4% skim milk for 30 minutes. Sections were then incubated with the copper transporter protein copper transport 1 (Ctr1) antibody (Abcam, Cambridge, Massachusetts; #129067) and 2% skim milk for 1 hour to reduce the nonspecific staining before being incubated with the biotinylated secondary antibody for 30 minutes. Immunohistochemistry (IHC) staining was visualized with a substrate solution containing diaminobenzidine and hydrogen peroxide. Hematoxylin was used for counterstaining. All steps were performed at room temperature.
Reactive Oxygen Species Assay
Cellular reactive oxygen species (ROS) levels were determined using the ROS-sensitive dye 2,7-dichlorofluoresceindiacetate (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The level of DCF fluorescence, reflecting the concentration of ROS, was measured by a fluorescence microscope.
Cell Viability Assay
The Cell Counting Kit-8 (CCK-8) cell viability assay (Dojindo Laboratories, Kumamoto, Japan) was carried out in 96-well plates. Cells (4 × 103 cells/well) were treated with various concentrations of copper chloride solution. After 24 and 48 hours, respectively, CCK-8 was added, and the plates were placed in a CO2 incubator for an additional 1 hour. The optical density was determined at 490 nm.
Statistical Analysis
Data are expressed as mean ± standard error of the mean. The data were evaluated using either unpaired 2-sided Student t tests or 1-way analysis of variance to determine statistical significance. For all in vitro experiments, 3 biological replicates were analyzed. For all in vivo experiments, 5 biological replicates were analyzed for each condition. Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, Inc, La Jolla, California). Data are considered significant if P < .05.
Results
Changes of Serum Metal Elements at Different Time Points After Irradiation
The time-dependent groups were obtained at 0, 1, 2, 5, and 28 days after irradiation with 4 Gy. Seven metal element concentrations in the serum were measured by ICP-MS. As shown in Figure 1, the concentration of zinc in serum decreased from the initial 34.31 ± 5.29 μg/L to 7.86 ± 1.79 μg/L within 24 hours, then increased to about half of the initial amount gradually over the following 4 days. Although serum zinc level returned to 23.72 ± 3.93 μg/L on the 28th day after radiation, it was still lower than the initial level (Figure 1A). Similar to zinc, copper in serum decreased from the initial 19.71 ± 3.10 μg/L to 6.97 ± 1.41 μg/L on the first day, which returned to about half of the initial concentration on the second day and remained at this level until the 28th day (Figure 1B). Serum levels of nickel, manganese, tin, vanadium, and cobalt did not show significant differences following radiation, with changes less than 1 μM (Figure 1C-G). These results indicated that only the levels of zinc and copper in the serum changed after irradiation, which suggested their involvement in radiation-induced injury.
Figure 1.
Changes of metal elements in serum at different time points after 4 Gy total body irradiation (TBI) in mice. C57BL/6N mice were randomized into 5 groups (n = 4) and irradiated with 4 Gy TBI using 250 kV X-rays. Serum samples were obtained 0, 1, 2, 5, or 28 days after irradiation. Inductively coupled plasma mass spectrometry was used to detect the metal concentration in serum. A, Serum zinc concentration. B, Serum copper concentration. C, Serum nickel concentration. D, Serum manganese concentration. E, Serum vanadium concentration. F, Serum cobalt concentration. G, Serum stannum concentration. *P < .05, **P < .01.
Changes of Serum Metal Elements After Different Doses of Irradiation
To investigate whether changes in metal content changed with radiation doses, mice were irradiated with TBI at doses of 0, 2, 4, and 8 Gy and sampled after 24 hours. The serum metal element level was measured by ICP-MS. As shown in Figure 2A, the serum zinc ion concentration in the nonirradiated group was 34.95 ± 3.02 μg/L at 24 hours and decreased to 15.17 ± 1.64 μg/L after 2 Gy irradiation. The serum zinc concentration decreased to less than 25% of the original concentration after 4 or 8 Gy irradiation (Figure 2A). Similarly, after exposure to different doses, the concentration of copper in serum decreased to less than one-third of the background concentration (Figure 2B). Nickel levels also showed a slight decrease (Figure 2C). Other elements do not change significantly (Figure 2D-G). These results indicate that the level of zinc, copper, and nickel in the serum decreased as the irradiation dose increased.
Figure 2.
Changes of metal elements in serum after different radiation doses in mice. C57BL/6N mice were randomized into 4 groups (n = 4) and irradiated with a single dose of 0, 2, 4, and 8 Gy (total body irradiation) using 250 kV X-rays. Serum samples were obtained 24 hours after irradiation. Inductively coupled plasma mass spectrometry was used to detect the metal content in serum. A, Serum zinc concentration. B, Serum copper concentration. C, Serum nickel concentration. D, Serum manganese concentration. E, Serum vanadium concentration. F, Serum cobalt concentration. G, Serum stannum concentration. *P < .05, **P < .01.
Copper Levels in Small Intestine Tissue Was Increased After Irradiation With an Increased Expression of the Copper Transporter Ctr1
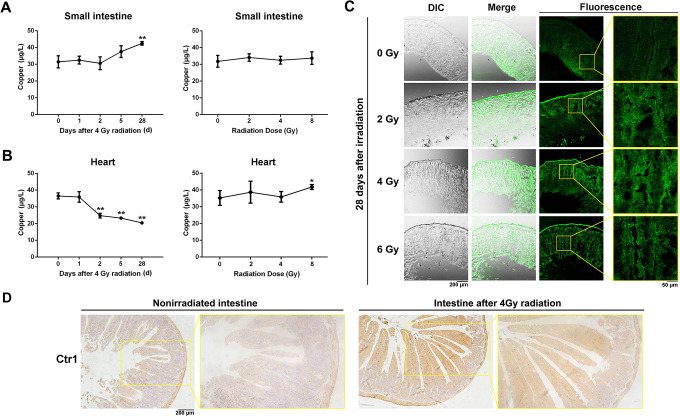
Among the metal elements with significant changes in concentration after irradiation, we focused on copper, an important essential macro metal element in the human body. We wondered if the irradiation modulated copper level in tissues. Radiation-sensitive small intestine and insensitive heart tissue were obtained from irradiated mice. The concentration of copper in tissues was detected by ICP-MS. As shown in Figure 3A, in the small intestine, copper levels increased by ∼2 μg in 24 hours as the irradiation dose increased. However, copper level increased from 31.48 ± 3.63 μg to 42.54 ± 1.09 μg in 28 days after 4 Gy irradiation. In the heart tissue, copper levels were dose dependent and decreased after irradiation gradually (Figure 3B). These results indicated that the change in copper level was tissue-specific.
Figure 3.
Changes of copper levels in heart and small intestine tissues. A, The concentration of copper in small intestine tissue. Mice were either irradiated with 4 Gy X-rays and tissue samples were collected at different time points or irradiated with different doses and collected 24 hours after radiation. B, Changes in copper levels in mouse heart tissue. Mice were either irradiated with 4 Gy X-rays and tissue samples were collected at different time points or irradiated with different doses and collected 24 hours after radiation. C, Frozen sections were prepared from small intestine tissue 28 days after irradiation with different doses. Cu2+ in the sections was determined with a copper-specific probe (C27H29N5O2S). Sections were observed with a confocal microscope. *P < .05, **P < .01. D, Immunohistochemistry staining of Ctr1 in nonirradiated and 4 Gy irradiated mouse intestinal tissues.
To visualize the change of copper level in the small intestine after irradiation, we stained a section of the small intestine with a fluorescent copper-specific probe (C27H29N5O2S). Compared with the nonirradiated control group, small intestine tissues irradiated with 2, 4, or 6 Gy showed stronger fluorescence (Figure 3C), which confirmed that copper homeostasis was disrupted by radiation in mouse small intestine.
To investigate whether the increased copper accumulation in mouse small intestine was attributed to increased copper uptake, we performed an IHC staining of Ctr1. As shown in Figure 3D, Ctr1 expression was upregulated in irradiated mouse intestine, suggesting the involvement of Ctr1 in radiation-induced copper accumulation.
Increased Copper Deposited in Irradiated Intestinal Cells
Since the levels of copper in mouse serum were attenuated and those in mouse small intestine tissue were increased after irradiation, we wondered if radiation could promote the uptake of copper to intestines that were sensitive to radiation. To verify this hypothesis, intestine-derived IEC-6 and HIEC cells were irradiated with 10 or 12 Gy radiation and then 40 μM CuCl2 was added to the culture medium. Intracellular Cu2+ was visualized by a fluorescent copper-specific probe (C27H29N5O2S). The fluorescence intensity of intracellular Cu2+ in the CuCl2-treated group was almost the same as that of the control group, which may be attributed to the regulation of copper homeostasis. In irradiated cells, the intracellular Cu2+ increased slightly (Figure 4A and B). Combined radiation (4 or 6 Gy) and CuCl2 treatment showed stronger intracellular fluorescence intensity, indicating that the concentration of Cu2+ entering the cells increased during irradiation (Figure 4A and B). Taken together, these results indicate that radiation induced the accumulation of copper in intestinal cells.
Figure 4.
Radiation induces Cu2+ accumulation in intestinal cells. Both (A) HIEC and (B) IEC-6 cells were treated with CuCl2 (40 μM) and irradiated with different doses. After 24 hours, the Cu2+ in the cells were stained with C27H29N5O2 S and observed by confocal microscopy. C, Quantitative analysis of Cu2+ accumulation in intestinal cells ImageJ software. *P < .05, **P < .01. HIEC indicates human intestinal epithelial cell; ns, nonsignificant.
Deposition of Copper in Small Intestinal Cells Is Related to the Extent of Cell Damage After Irradiation
Since disruption of metal element homeostasis may lead to oxidative stress, 4 we treated HIEC and IEC-6 cells with radiation plus CuCl2 and measured intracellular ROS. Compared with the control group, the ROS level was not changed after CuCl2 treatment. However, when CuCl2 was added after irradiation, levels of intracellular ROS were markedly increased in both IEC-6 and HIEC cells (Figure 5A). To investigate whether copper accumulation affected radiation-induced cell death, both HIEC and IEC-6 cells were treated with radiation, followed by different concentrations of CuCl2. In nonirradiated HIEC and IEC-6 cells, an increase in copper level reduced cell viability. At 24 hours after radiation, there was no effect of radiation on CuCl2-induced cell death (Figure 5B and C). However, at 48 hours after irradiation, the cell viability of the irradiated plus CuCl2-treated cells was lower than that of the CuCl2-treated group (Figure 5B and C), indicating the synergistic effect of CuCl2 treatment and radiation on cell viability. Taken together, these results indicated that copper treatment aggravated radiation-induced damage in intestinal cells.
Figure 5.
Effect of Cu2+ on the radiation-induced ROS and cell viability after irradiation. A, Both HIEC and IEC-6 cells were treated with CuCl2 (40 μM) and irradiated with different doses, and cell ROS were observed with a fluorescence microscope at 24 and 48 hours. Both HIEC and IEC-6 cells were treated with different concentrations of CuCl2 and irradiated with different doses. Cell proliferation was measured with a CCK-8 cell viability assay (B) 24 and (C) 48 hours after radiation. CCK-8 indicates Cell Counting Kit-8; HIEC, human intestinal epithelial cell; ROS, reactive oxygen species.
Discussion
In this study, we found that the concentration of zinc and copper in mice serum was decreased significantly after irradiation, whereas that of nickel, manganese, vanadium, cobalt, and stannum was not changed by ICP-MS. Zinc and copper are important trace metal elements in the body. 14 -18 Numerous reports have shown the importance of zinc in the growth, development, maintenance, and priming of the immune system, as well as tissue repair. 14 -16 Zinc deficiency may lead to human growth retardation and hypogonadism. 17 Copper is an integral part of many important enzymes involved in a number of vital biological processes, such as catalytic and transport functions in living cells and their organelles. 18 Free copper is able to inactivate cellular proteins by directly binding to cysteine residues 19 and promotes oxidative stress and ROS, which affects multiple cellular functions. 20 Abnormalities in the concentration of copper in cells may cause diseases such as hepatolenticular degeneration, Menkes syndrome, and familial amyotrophic lateral sclerosis. It is reported that the levels of some trace elements could be affected by external factors such as a chronic light/dark cycle. 21,22 In this study, we found that zinc and copper levels in the serum were reduced after TBI in mice. Our results showed that both in the time-dependent or dose-dependent group, the changes in serum zinc and copper levels were consistent with the radiation dose and time of injury after acute exposure, suggesting that serum zinc and copper levels may be potential indicators of biological radiation. Electromagnetic radiation, which is a potentially harmful factor in the human body, has a different effect on the levels of trace elements in serum compared with ionizing radiation. There were no significant changes in the levels of zinc and copper in rat serum after electromagnetic radiation exposure. 23 There was no time–effect or dose–effect relationship between electromagnetic radiation exposure and serum element levels, indicating that serum trace elements are not suitable end points of biological dosimetry of electromagnetic radiation.
Copper is a cofactor for many enzymes involved in redox reactions and is used in biological systems for electron transport, 7 and more importantly, it induces oxidative stress. It directly catalyzes the formation of ROS via a Fenton-like reaction 24,25 and is capable of causing DNA strand breaks and oxidation of bases via ROS. 26 In addition, exposure to elevated levels of copper significantly decreases the levels of glutathione, a substrate for several ROS-removing enzymes, and is present in cells at millimolar concentrations, which suppresses copper toxicity by directly chelating the metal. 27,28 Excessive copper levels lead to an increase in the level of intracellular oxidative stress, which triggers damage that impedes cell proliferation and metabolic activity. 29 Copper is easily absorbed from the diet in the small intestine (∼2 mg/d), bound to either serum albumin or histidine, and is trafficked through the bloodstream for delivery to tissues or stored in the liver. 30 Because the intestine proliferates quickly, it is more vulnerable to radiation-induced injury. 31 Higher doses of radiation (>10 Gy) cause disastrous damage to gastrointestinal tissues. Malabsorption, diarrhea, dehydration, and intestinal bleeding occurred within 15 days after radiation and eventually led to death. 32 Importantly, radiotherapy has been proven to be a mainstay for the treatment of abdominal malignancies. However, radiation-induced gastrointestinal syndrome is a significant limiting factor, which restricts maximum doses of radiation and the efficiency of the therapy. 33 To date, the crucial molecular mechanisms regulating epithelial cell death underlying radiation-induced gastrointestinal injury have not been completely illustrated. Herein, we found that copper potentiated radiation-induced ROS and reduced cell viability of intestinal cells. Therefore, it is likely that the deposition of copper in the small intestine during ionizing radiation may be associated with the progression of radiation-induced intestinal injury. Moreover, copper accumulated in intestinal cells and irradiated mouse intestines with increased expression of copper transporter Ctr1. The Ctr1 is a 23-kDa protein that has 3 transmembrane segments and oligomerizes to form a functional trimer, which has a unique pore for the transport of copper. 34 Our results indicate the involvement of Ctr1 in radiation-induced intestinal damage. However, this link merits further investigation. Thus, dysregulated copper homeostasis by Ctr1 may become a novel therapeutic target for radiation-induced intestinal injury.
Footnotes
Authors’ Note: Li Zhong and Aijing Dong contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China (81803166, 81673100, 31770911, and 81872552), Young Talent Program of China National Nuclear Corporation, Fundation of Jiangsu Province (BE2017634, BE2017652, and BK20161220), and Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. KYCX19_1969).
ORCID iD: Shuyu Zhang  https://orcid.org/0000-0003-1419-3635
https://orcid.org/0000-0003-1419-3635
References
- 1. Imanaka T, Hayashi G, Endo S. Comparison of the accident process, radioactivity release and ground contamination between Chernobyl and Fukushima-1. J Radiat Res. 2015;56(suppl):i56–i61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnet NG, Nyman J, Turesson I, Wurm R, Yarnold JR, Peacock JH. Prediction of normal-tissue tolerance to radiotherapy from in-vitro cellular radiation sensitivity. Lancet. 1992;339(8809):1570–1571. [DOI] [PubMed] [Google Scholar]
- 3. Zhao Y, Zhang J, Han X, Fan S. Total body irradiation induced mouse small intestine senescence as a late effect. J Radiat Res. 2019;60(4):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jomova K, Valko M, Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2-3):65–87. [DOI] [PubMed] [Google Scholar]
- 5. Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95(3):749–784. [DOI] [PubMed] [Google Scholar]
- 6. Berg JM. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990;265(12):6513–6516. [PubMed] [Google Scholar]
- 7. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. [DOI] [PubMed] [Google Scholar]
- 8. Zambelli B, Ciurli S. Nickel and human health. Met Ions Life Sci. 2013;13:321–357. [DOI] [PubMed] [Google Scholar]
- 9. Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol. 2002;42(1):35–56. [DOI] [PubMed] [Google Scholar]
- 10. Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci. 2018;23:1655–1679. [DOI] [PubMed] [Google Scholar]
- 11. Aizenman E, Mastroberardino PG. Metals and neurodegeneration. Neurobiol Dis. 2015;81:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan C, Sudipta C, Somshuvra M, et al. Manganese homeostasis in the nervous system. J Neurochem. 2015;134(4):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tripathi D, Mani V, Pal RP. Vanadium in biosphere and its role in biological processes. Biol Trace Elem Res. 2018;186(1):52–67. [DOI] [PubMed] [Google Scholar]
- 14. Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 2001;79(2):170–177. [DOI] [PubMed] [Google Scholar]
- 15. Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8(3):281–291. [DOI] [PubMed] [Google Scholar]
- 16. Rostan EF, Debuys HV, Madey DL, Pinnell SR. Evidence supporting zinc as an important antioxidant for skin. Int J Dermatol. 2002;41(9):606–611. [DOI] [PubMed] [Google Scholar]
- 17. Prasad AS, Halsted JA, Nadimi M. Nutrition classics. The American Journal of Medicine, volume 31, 1961. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Nutr Rev. 1983;41(7):220–223. [DOI] [PubMed] [Google Scholar]
- 18. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1-2):147–163. [DOI] [PubMed] [Google Scholar]
- 19. Letelier ME, Faúndez M, Jara-Sandoval J, et al. Mechanisms underlying the inhibition of the cytochrome P450 system by copper ions. J Appl Toxicol. 2009;29(8):695–702. [DOI] [PubMed] [Google Scholar]
- 20. Rotilio G, Carrì MT, Rossi L, Ciriolo MR. Copper-dependent oxidative stress and neurodegeneration. IUBMB Life. 2000;50(4-5):309–314. [DOI] [PubMed] [Google Scholar]
- 21. Kayan M, Naziroglu M, Barak C. Effects of vitamins C and E combination on element levels in blood of smoker and nonsmoker radiology X-ray technicians. Biol Trace Elem Res. 2010;136(2):140–148. [DOI] [PubMed] [Google Scholar]
- 22. Karakoc Y, Buruk MS, Aktan B, et al. Effects of chronic light/dark cycle on iron zinc and copper levels in different brain regions of rats. Biol Trace Elem Res. 2011;144(1-3):1003–1007. [DOI] [PubMed] [Google Scholar]
- 23. Li K, Ma S, Ren D, et al. Effects of electromagnetic pulse on serum element levels in rat. Biol Trace Elem Res. 2014;158(1):81–86. [DOI] [PubMed] [Google Scholar]
- 24. Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007;79(12):2325–2338. [Google Scholar]
- 25. Liochev SI, Fridovich I. The Haber-Weiss cycle—70 years later: an alternative view. Redox Rep. 2002;7(1):55–57. [DOI] [PubMed] [Google Scholar]
- 26. Moriwaki H, Osborne MR, Phillips DH. Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicol In Vitro. 2008;22(1):36–44. [DOI] [PubMed] [Google Scholar]
- 27. Speisky H, Gómez M, Burgos-Bravo F, et al. Generation of superoxide radicals by copper–glutathione complexes: redox-consequences associated with their interaction with reduced glutathione. Bioorg Med Chem. 2009;17(5):1803–1810. [DOI] [PubMed] [Google Scholar]
- 28. Mattie MD, Freedman JH. Copper-inducible transcription: regulation by metal- and oxidative stress responsive pathways. Am J Physiol Cell Physiol. 2004;286(2):C293–C301. [DOI] [PubMed] [Google Scholar]
- 29. Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek KJ. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90(1):1–37. [DOI] [PubMed] [Google Scholar]
- 30. Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63(5):797S–811S. [DOI] [PubMed] [Google Scholar]
- 31. Mason KA, Withers HR, Davis CA. Dose dependent latency of fatal gastrointestinal and bone marrow syndromes. Int J Radiat Biol. 1989;55(1):1–5. [DOI] [PubMed] [Google Scholar]
- 32. Leibowitz BJ, Wei L, Zhang L, et al. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kantara C, Moya SM, Houchen CW, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest. 2015;95(11):1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296(3):F505–F511. [DOI] [PMC free article] [PubMed] [Google Scholar]