Abstract
Multidrug resistance has increased globally in the communities. Bacterial infections associated with health care have weakened the existing antimicrobial therapy and demand the search for alternative therapies. In the present investigation, the medicinal plant Pulicaria gnaphalodes from Quetta, Pakistan, has been screened for antimicrobial potential. In vitro antimicrobial efficacy of P gnaphalodes extracts (methanol and ethanol) was quantitatively evaluated on the basis of zone of inhibition against different bacteria and minimum inhibitory concentration (MIC). In vivo, antihypercholesterolemic activity is determined in different rat groups. The results of the study indicated that the ethanol extract of P gnaphalodes showed maximum zone of inhibition for Bacillus subtilis of 12.1 ± 1.1 mm from all others. The methanol extract showed maximum zone of inhibition for Staphylococcus aureus of 11.9 ± 1.0 mm and rifampicin showed maximum zone of inhibition of 23.1 ± 0.9 mm. The results of ethanol and methanol extract of P gnaphalodes against different bacteria revealed that this plant has greater antimicrobial activity. However, the plant extract shows nonsignificant antihypercholesterolemic activity. The extract of this plant can be utilized as medicine to inhibit several infections caused by some bacterial pathogens found in human body.
Keywords: Pulicaria gnaphalodes, antimicrobial, antihypercholesterolemic, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pasteurella multocida
Introduction
The Pulicaria genus has its place in the family Compositae (Asteraceae), which comprises more than 100 species that are commonly dispersed throughout Africa, Asia, and Europe.1,2 The genus contains a number of chemical constituents, including diterpenes and terpenes, such as monoterpenes, xanthanes, germacranes, guaianes, pseudoguaianes, eudesmanes, bisabolenes, caryophyllanes, diterpenoids, triterpenoids, steroids, flavonoids, and essential oils.3,4 Several biological activities of this genus have been described such as antimicrobial, antibacterial, leishmanicidal, and antifungal.3,5-7 Pulicaria plants are usually consumed as flavoring agent, herbal tea, and medicinal plant.
Pulicaria gnaphalodes is an obstinate plant about 30 ± 8 cm high and have gold-yellow flowers. The plant mostly grows on sandy, stony, and abandoned areas in the Saudi Arabia, Afghanistan, Pakistan, Iran, India, Iraq, and Turkey.8 The plant is also known as Kakkosh-Biabani.9 This plant contains numerous chemical components, including flavonoids such as giperoside and pulicarin, phenol acid such as salicylic acid, clerodane diterpenoids such as salvicin and salvifolin, alpha-pinene, and 1-8-cineole.10,11 This plant or its components are utilized globally in folk medicine because of its antibacterial, antidiarrheal, anti-inflammatory, antioxidant, and leishmanicidal properties.6,10,12 It cures many infectious diseases such as diarrhea and cutaneous abscesses. It is also utilized conventionally as a flavoring agent in food. Many studies have reported the antibacterial, antimicrobial, and leishmanicidal activity of essential oils of P gnaphalodes. The current study has been conducted to assess the antimicrobial property of ethanol and methanol extract of P gnaphalodes against different bacteria in vitro and also described its effect on lipid profile in vivo. However, there is no reported study in the past about the effect of P gnaphalodes on the cholesterol level.
Materials and Methods
Microbial Strains
Four bacterial species were cultured for these tests comprising gram-positive Bacillus subtilis, Bacillus thuringiensis, Bacillus thuringiensis, and gram-negative Escherichia coli. At diagnostic laboratory of GC University, Faisalabad, the antibacterial activity tests were accomplished. In nutrient agar, bacteria were cultured at 37°C.
Plant Collection
Stems of the P gnaphalodes were assembled from Gilgit-baltistan. The plant was identified on macroscopic characteristics relating to authenticated sample from the horticulture at Faisalabad, Pakistan.
Preparation of Extract
The stems of the sample plant were washed with distilled water. Then fine powder was prepared and sieved with 150-mesh sieve after air drying at 35°C in a shaded place. Two solvents, methanol and ethanol, were used for extraction of the plant. Thirty grams of this powder was taken for each solvent in 300 mL, which is placed inside the Soxhlet extractor. After extraction, the filtration of solvent having plant material was carried out using Whatman No. 1 filter. After filtration, the solvents were loaded into Petri dishes for evaporation of solvents to get plant extract, or rotary apparatus was used for rapid evaporation.
In Vitro Determination of Antibacterial Activity
Disk diffusion method
For antimicrobial susceptibility testing, disk diffusion method was accomplished as defined by Anand et al13 to determine the existence of antimicrobial activity of the plant extracts. The pure cultures of Staphylococcus aureus, Pasteurella multocida, Bacillus subtilis, and E coli were subcultured in nutrient agar. Positive control was prepared using rifampicin. Sterile paper disks was 6 mm in diameter and made from filter paper and impregnated with drug, comprising solution put on the inoculated agar. These Petri dishes were incubated for 24 hours and then antibacterial activity was deliberated by the diameter measurement of the growth inhibition zone.
Minimum inhibitory concentration assay
Agar well dilution method was utilized for the determination of minimum inhibitory concentration (MIC). In 10% dimethyl sulfoxide, the ethanol and methanol extracts were distinctly dissolved and then diluted to a minimum concentration (500 µg/mL). Nutrient broth (95 µL) was mixed with inoculum (5 µL) to 96-well plate preparation. Plant extract (100 µL) was added in the first well. Then this 100 µL was relocated into 6 consecutive wells. All these plates were incubated for 24 hours and then each plate absorbance was taken at 650 nm by the microplate reader.
In Vivo Determination of Antihypercholesterolemic Activity
Procurement of animals
Thirty male Albino rats of 150 to 225g were purchased from the Animal House of Department Microbiology of Agriculture University, Faisalabad. These rats were kept under the standardized conditions such as stable room temperature of 25°C with alternating 12-hour periods of light and darkness, and the humidity was kept in 50% to 60%. Ethical committee of the Government College University, Faisalabad, approved the in vivo study. World Health Organization guideline has been followed to conduct the animal study.
Hypercholesterolemia induction and treatment protocol
Hypercholesterolemia was introduced into the rats for 30 days by oral administration of cholesterol of 1%. At the end of adaptation period, the rats were separated into 5 groups each having 6 rats.=Group 1 was the control rats group that had no hypercholesterolemia and did not receive any treatment at the chow maintenance diet (48g/kg bw). Group 2 comprises hypercholesterolemic rats administered peanut oil in order to induce cholesterolemia. Group 3 had the hypercholesterolemic rats administered allopathic medicine atorvastatin (10 mg/kg bw/d) along with peanut oil. Groups 4 and 5 had hypercholesterolemic rats administrated P gnaphalodes ethanol extract at a dose of 100 and 250 mg/kg bw/d, respectively, along with administration of peanut oil orally.
Blood sample
After 30 days, all the rats were decapitated, and the blood samples were isolated from all experimental rats. Subsequently, the serum was isolated from all rats for the analysis of serum lipid profile parameters and serum liver profile parameters.
Lipid profile
The total lipid profile levels including total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides were found in the blood serum by utilizing the commercially accessible reagent kits (Randox Laboratories, Kearneysville, West Virginia). Low-density lipoprotein level was found by subtraction of the HDL cholesterol concentration from the concentration of the TC.
Liver profile and bilirubin level
Alanine amino transferase (ALT), alkaline phosphate (ALP), bilirubin direct, bilirubin indirect, and bilirubin total were determined by commercially available kits.
Statistical Analysis
The P gnaphalodes influence was explicated by the 1-way analysis of variance utilization. For this study, the SPSS 23 software was used. P value ≤.05 was considered significant.
Results
Antibacterial Activity of P Gnaphalodes
In this study, the zone of inhibition of bacterial pathogens that are found in human body (E coli, P multocida, B subtilis, and S aureus) was determined using ethanol and methanol extracts of P gnaphalodes and rifampicin. The ethanol and methanol extract of P gnaphalodes and rifampicin showed the zone of inhibition against E coli and was 10.1 ± 0.9 mm and 10.7 ± 0.8 mm and 19.7 ± 0.8 mm, respectively (Table 1). In vitro zone of inhibition of ethanol and methanol extract of this plant and rifampicin against P multocida was 10.8 ± 0.6 mm and 9.7 ± 1.1 mm and 21.7 ± 1.1 mm, respectively. The ethanol and methanol extract of plant and rifampicin against B subtilis showed zone of inhibition of 12.1 ± 1.1 and 11.2 ± 0.9 and 23.1 ± 0.9 mm, respectively. For Staphylococcus aureus, the inhibition zone of ethanol, methanol, and rifampicin plant extract are 11.4 ± 0.8, 11.9 ± 1.0, and 22.3 ± 1.2 mm, respectively (Table 1).
Table 1.
Antibacterial Activity of Pulicaria Gnaphalodes Extracts and Diameter of Inhibition Zone (mm) Including Well Diameter of 6 mm.a
| Bacterial Strains | Ethanol Extract Zone of Inhibition, mm |
Methanol Extract Zone of Inhibition, mm |
Rifampicin Zone of Inhibition, mm |
|---|---|---|---|
| Escherichia coli | 10.1 ± 0.9 | 10.7 ± 0.8 | 19.7 ± 0.8 |
| Pasteurella multocida | 10.8 ± 0.6 | 9.7 ± 1.1 | 21.7 ± 1.1 |
| Bacillus subtilis | 12.1 ± 1.1 | 11.2 ± 0.9 | 23.1 ± 0.9 |
| Staphylococcus aureus | 11.4 ± 0.8 | 11.9 ± 1.0 | 22.3 ± 1.2 |
a Values are the means ± standard deviation of triplicate.
Determination of MIC
a. Escherichia coli
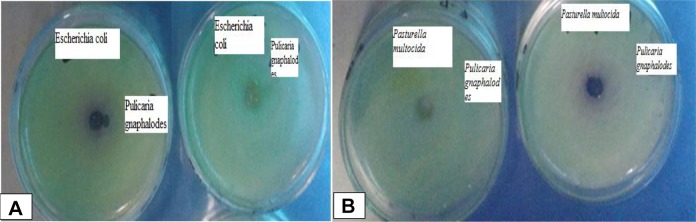
The ethanol and methanol extract of P gnaphalodes and rifampicin kill the E coil with zone of inhibition of 4.1 ± 0.2 mg/mL and 3.9 ± 0.2 mg/mL and 0.5 ± 0.0 mg/mL, respectively (Figure 1).
Figure 1.
Escherichia coli and Pasteurella multocida killed by extract of Pulicaria gnaphalodes. The ethanol and methanol extracts of P gnaphalodes show the zone of inhibition in Escherichia coli (A) and Pasteurella multocida (B) bacterial culture plate.
b. Pasteurella multocida
The ethanol and methanol extract of P gnaphalodes and rifampicin kill the P multocida with MIC value of 3.4 ± 0.1 mg/mL 3.4 ± 0.1 mg/mL, and 0.4 ± 0.0 mg/mL, respectively (Figure 1). The extracts of P gnaphalodes give better results in killing P multocida than rifampicin, which gives minor results (Table 2).
Table 2.
Minimum Inhibitory Activity of Pulicaria gnaphalodes.a
| Bacterial Strains | Ethanol Extract | Methanol Extract | Rifampicin |
|---|---|---|---|
| Escherichia coli | 4.1 ± 0.2 | 3.9 ± 0.2 | 0.5 ± 0.0 |
| Pasteurella multocida | 3.4 ± 0.1 | 3.4 ± 0.1 | 0.4 ± 0.0 |
| Bacillus subtilis | 3.1 ± 0.2 | 2.9 ± 0.3 | 0.2 ± 0.0 |
| Staphylococcus aureus | 2.8 ± 0.3 | 3.1 ± 0.2 | 0.1 ± 0.0 |
Abbreviations: MIC, minimum inhibitory concentration.
a Values are the means ± standard deviation of triplicate.
c. Bacillus subtilis
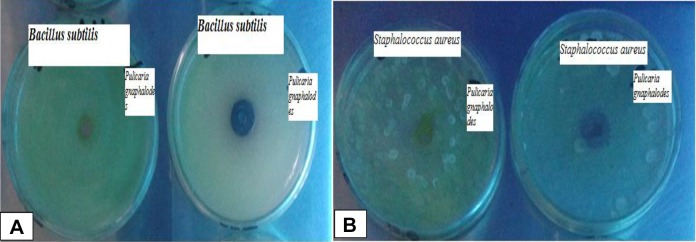
The ethanol and methanol extract of P gnaphalodes and rifampicin kill B subtilis with the zone of inhibition of 3.1 ± 0.2 mg/mL and 2.9 ± 0.3 mg/mL and 0.2 ± 0.0 mg/mL, respectively (Figure 2). The results show that the ethanol extract of P gnaphalodes gives better results to kill B subtilis (Table 2).
Figure 2.
Bacillus subtilis and Staphylococcus aureus killed by extract of Pulicaria gnaphalodes. The ethanol and methanol extracts of P gnaphalodes show the zone of inhibition in Bacillus subtilis (A) and Staphylococcus aureus (B) bacterial culture plate.
d. Staphylococcus aureus
The ethanol and methanol extract of P gnaphalodes and rifampicin kill the S aureus with zone of inhibition of 2.8 ± 0.3 mg/mL, 3.1 ± 0.2 mg/mL, and 0.1 ± 0.0 mg/mL, respectively (Figure 2). The results exposed that the methanol extract of P gnaphalodes gives better results to kill S aureus than the ethanol extract and the rifampicin showed negligible inhibition (Table 2).
Lipid and Liver Profile
The ethanol extract of P gnaphalodes did not show any significant antihypercholesterolic activity in the hypercholesterolemic rats (Table 3). The results of ALT and ALP are also nonsignificant in the experimental groups (Table 4). Bilirubin level in the blood was not improved by the induction of extract in hypercholesterolemic rats (Table 5).
Table 3.
Lipid Profile (mg/dL) in Different Groups of Experimental Rats at Day 0 and 30.
| TC | HDL | LDL | Triglyceride | |
|---|---|---|---|---|
| Day 0 | ||||
| Group 1 | 196 ± 1 | 59 ± 1.1 | 137 ± 2 | 151 ± 1 |
| Group 2 | 220 ± 2 | 45 ± 1 | 175 ± 1.9 | 156 ± 2 |
| Group 3 | 224 ± 1.1 | 43 ± 2 | 181 ± 1 | 158 ± 1.1 |
| Group 4 | 218 ± 1.9 | 50 ± 1 | 168 ± 1.5 | 160 ± 1.9 |
| Group 5 | 214 ± 1.6 | 49 ± 1.9 | 165 ± 1.1 | 154 ± 1 |
| Day 30 | ||||
| Group 1 | 200 ± 0.7 | 61 ± 0.2 | 139 ± 0.0 | 153 ± 0.0 |
| Group 2 | 239 ± 0.2 | 40 ± 0.7 | 199 ± 0.8 | 159 ± 0.2 |
| Group 3 | 211 ± 0.7 | 55 ± 0.0 | 156 ± 0.7 | 150 ± 0.8 |
| Group 4 | 229 ± 0.0 | 45 ± 0.8 | 184 ± 0.8 | 166 ± 0.0 |
| Group 5 | 223 ± 0.0 | 44 ± 0.2 | 179 ± 0.2 | 160 ± 0.7 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol.
Table 4.
Liver Profile (U/L) in Different Groups of Experimental Rats at Day 0 and 30.
| Day 0 | Day 30 | Day 0 | Day 30 | |
|---|---|---|---|---|
| ALT | ALT | ALP | ALP | |
| Group 1 | 39 ± 1.0 | 50.6 ± 0.8 | 246 ± 3.01 | 246.1 ± 3.01 |
| Group 2 | 41 ± 2.0 | 58.9 ± 3.0 | 246 ± 3.01 | 302.9 ± 5.02 |
| Group 3 | 38 ± 1.5 | 45.3 ± 2.9 | 245 ± 3.00 | 347.3 ± 5.55 |
| Group 4 | 42 ± 2.5 | 61.8 ± 3.36 | 245 ± 3.00 | 373.6 ± 6.63 |
| Group 5 | 43 ± 2.6 | 55.0 ± 4.28 | 250 ± 3.05 | 393.1 ± 6.94 |
Abbreviations: ALT, Alanine amino transferase; ALP, alkaline phosphate.
Table 5.
Total Bilirubin, Direct Bilirubin, and Indirect Bilirubin (mg/dL) in Different Groups of Experimental Rats.
| Day 0 | Day30 | Day 0 | Day 30 | Day 0 | Day 30 | |
|---|---|---|---|---|---|---|
| Total | Total | Direct | Direct | Indirect | Indirect | |
| Group 1 | 0.5 ± 0.0 | 0.53 ± 0.1 | 0.23 ± 0.0 | 1.4 ± 0.1 | 0.75 ± 0.0 | 1.8 ± 0.3 |
| Group 2 | 0.4 ± 0.0 | 0.42 ± 0.1 | 0.22 ± 0.0 | 1.2 ± 0.1 | 0.73 ± 0.0 | 1.7 ± 0.2 |
| Group 3 | 0.5 ± 0.0 | 0.53 ± 0.2 | 0.25 ± 0.0 | 1.8 ± 0.3 | 0.68 ± 0.0 | 1.4 ± 0.1 |
| Group 4 | 0.4 ± 0.0 | 0.50 ± 0.0 | 0.22 ± 0.0 | 1.4 ± 0.1 | 0.70 ± 0.0 | 1.6 ± 0.1 |
| Group 5 | 0.5 ± 0.0 | 0.55 ± 0.2 | 0.25 ± 0.0 | 1.8 ± 0.3 | 0.69 ± 0.0 | 1.5 ± 0.1 |
Discussion
Numerous types of herbs and plants are stated to be a worthwhile basis of medicines. Diverse medicinal actions of P gnaphalodes extracts in solvents (methanol and ethanol) was examined. In the present study, the plant was gathered from Quetta, Pakistan, and these stems of the plants were ground to fine powder by grinding the sample. Plant extracts were prepared from the finely ground dry sample of P gnaphalodes in 2 solvents, methanol and ethanol. The present study is designed to evaluate the antimicrobial activity of ethanol and methanol extracts of P gnaphalodes. The antimicrobial effect was determined by measuring the zone of inhibition. Four bacteria including E coli, P multocida, B subtilis, and S aureus strains were selected. Ethanol extract of P gnaphalodes showed the zone of inhibition against E coli is 10.1 ± 0.9, methanol showed zone of inhibition of 10.7 ± 0.8 mm, and rifampicin showed 19.7 ± 0.8 mm. The in vitro zone of inhibition of ethanol and methanol extract of this plant against P multocida is 10.8 ± 0.6 mm and 9.7 ± 1.1 mm, respectively and that of rifampicin is 21.7 ± 1.1 mm. The ethanol and methanol extract of plant and rifampicin against B subtilis showed zone of inhibition of 12.1 ± 1.1 and 11.2 ± 0.9 and 23.1 ± 0.9 mm, respectively, and for S aureus, the inhibition zone of ethanol, methanol, and rifampicin plant extract is 11.4 ± 0.8, 11.9 ± 1.0 and 22.3 ± 1.2 mm, respectively. The results of study revealed that the ethanol extract of P gnaphalodes showed maximum zone of inhibition of 12.1 ± 1.1 mm for B subtilis compared to all others. The methanol extract showed maximum zone of inhibition for S aureus of 11.9 ± 1.0 mm and rifampicin showed maximum zone of inhibition of 23.1 ± 0.9 mm. The results of antibacterial activity of P gnaphalodes revealed that ethanol and methanol extract of this plant have greater antimicrobial activity. The MIC results indicated that ethanol extract of the plant is more affective against Staphylococcus aureus, and methanolic extract of the plant was more effective against the B subtilis. Gandomi et al10 evaluated the antibacterial and antifungal properties of the P gnaphalodes. They demonstrated that ethanolic and methanolic extracts exhibited more inhibition zone as compared to the aqueous extract. With MIC of 0.025%, 0.8%, 0.1%, and 0.1% for essential oil, aqueous, ethanolic, and methanolic extracts, respectively, Bacillus cereus was the utmost sensitive strain. The essential oils have better ability to inhibit the fungal growth as compared to the extracts. The MIC results of the present study showed that ethanolic extract of the plant was more potent against the S aureus with MIC of 2.8 ± 0.3. The methanolic extract of the plant was more effective against the B subtilis with MIC of 2.9 ± 0.3. Hozoorbakhsh et al14 reported the antibacterial activity of P gnaphalodes against the Mycobacterium tuberculosis. They demonstrated that P gnaphalodes essential oil extracts possess strong inhibitory effects on M tuberculosis. The mean of inhibition percentage for P gnaphalodes in 640 μg/mL was 58.1%. In the present investigation, the in vivo study was also conducted on 30 albino rats. The hypercholesterolemia was induced by giving the cholesterol 1% for 30 days. Then the rats were separated into 5 groups each with 6 rats. Group 1 and group 2 were the negative control and positive control, respectively. Groups 3, 4, and 5 were treated group with allopathic medicine atorvastatin (10 mg/kg bw/d) and P gnaphalodes ethanol extract at the dose of 100 and 250 mg/kg bw/d, respectively. After 30 days, blood samples were collected for lipid and liver profile. The extracts of P gnaphalodes did not show any significant effect on lipid and liver profiles. However, minor increase in the bilirubin level shows that plant may reduce the cholesterol level in a dose-dependent manner. In the past, no study was reported which describes the effect of P gnaphalodes on lipid and liver profiles, and more research is required.
Conclusion
Pulicaria gnaphalodes has antibacterial potential against E coli, P multocida, B subtilis, and S aureus. The ethanolic and methanolic extracts of the P gnaphalodes were more effective against the S aureus with MIC of 2.8 ± 0.3 and B subtilis with MIC of 2.9 ± 0.3. However, P gnaphalodes does not have not any significant effect on lipid profile. The antimicrobial activity of the plant suggests possibility of utilizing it in the health care to prevent the growth of bacteria that cause infection and improve the safety of human life.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Syed Ali Raza Naqvi  https://orcid.org/0000-0002-2172-9066
https://orcid.org/0000-0002-2172-9066
Muhammad Saeed  https://orcid.org/0000-0002-8759-6948
https://orcid.org/0000-0002-8759-6948
Muhammad Akram  https://orcid.org/0000-0002-7457-8572
https://orcid.org/0000-0002-7457-8572
References
- 1. Khan M, Khan M, Adil SF, et al. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine. 2013;8:1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderberg AA. Taxonomy and phylogeny of the tribelnuleae (Asteraceae). Plant Syst Evol. 1991;176(1-2):75–123. [Google Scholar]
- 3. Liu L, Yang J, Shi Y. Phytochemicals and biological activities of Pulicaria species. Chem Biodivers. 2010;7(2):327–349. [DOI] [PubMed] [Google Scholar]
- 4. Dendougui H, Benayache S, Benayache F, Connoly JD. Sesquiterpene lactones from Pulicaria crispa . Fitoterapia. 2000;71(4):373–378. [DOI] [PubMed] [Google Scholar]
- 5. Al-Hajj NQ, Wang HX, Ma C, Lou Z, Bashari M, Thabit R. Antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants (Pulicaria inuloides-Asteraceae and Ocimum forskolei-Lamiaceae). Trop J Pharm Res. 2014;13(8):1287–1293. [Google Scholar]
- 6. Asghari G, Zahabi F, Eskandarian A, Yousefi H, Asghari M. Chemical composition and leishmanicidal activity of Pulicaria gnaphalodes essential oil. J Pharmacogn Phytochem. 2014;1(4):27–33. [Google Scholar]
- 7. Znini M, Cristofari G, Majidi L, Paolini J, Desjobert JM, Costa J. Essential oil composition and antifungal activity of Pulicaria mauritanica Coss., against postharvest phytopathogenic fungi in apples. Lwt-Food Sci Technol. 2013;54(2):564–569. [Google Scholar]
- 8. Kamkar A, Ardekani MR, Shariatifar N, Misagi A, Nejad AS, Jamshidi AH. Antioxidative effect of Iranian Pulicaria gnaphalodes L. extracts in soybean oil. S Afr J Bot. 2013;85:39–43. [Google Scholar]
- 9. Chitsazi MR, Korbekandi H, Asghari G, et al. Synthesis of silver nanoparticles using methanol and dichloromethane extracts of Pulicaria gnaphalodes (Vent.) Boiss. aerial parts. Artif Cells Nanomed Biotechnol. 2016;44(1):328–333. [DOI] [PubMed] [Google Scholar]
- 10. Gandomi H, Abbaszadeh S, Rahimikia E, Shariatifar N. Volatile organic compound from Pulicaria gnaphalodes and the antibacterial and antifungal properties of its essential oil and aqueous, ethanolic and methanolic extracts. J Food Process Pres. 2015;39(6):2129–2134. [Google Scholar]
- 11. Eshbakova KA. Chemical constituents of Pulicaria gnaphalodes Boiss. Med Plan Int J Phytomed Relat Indust. 2011;3(2):161–163. [Google Scholar]
- 12. Shariatifar N, Kamkar A, Shamse T, et al. Composition and antioxidant activities of Iranian Pulicaria gnaphalodes essential oil in soybean oil. Pak J Pharm Sci. 2014;27(4):807–812. [PubMed] [Google Scholar]
- 13. Anand SP, Deborah S, Velmurugan G. Antimicrobial activity, nutritional profile and phytochemical screening of wild edible fruit of catunaregam spinosa (Thunb.) triveng. Pharma Innovation. 2017;6(10):106–109. [Google Scholar]
- 14. Hozoorbakhsh F, Esfahani BN, Moghim S, Asghari G. Evaluation of the effect of Pulicaria gnaphalodes and Perovskia abrotanoides essential oil extracts against mycobacterium tuberculosis strains. Adv Biomed Res. 2016;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]