Abstract
Pulmonary hypertension is a fatal disease of which pulmonary vasculopathy is the main pathological feature resulting in the mean pulmonary arterial pressure higher than 25 mmHg. Moreover, pulmonary hypertension remains a tough problem with unclear molecular mechanisms. There have been dozens of studies about endoplasmic reticulum stress during the onset of pulmonary hypertension in patients, suggesting that endoplasmic reticulum stress may have a critical effect on the pathogenesis of pulmonary hypertension. The review aims to summarize the rationale to elucidate the role of endoplasmic reticulum stress in pulmonary hypertension. Started by reviewing the mechanisms responsible for the unfolded protein response following endoplasmic reticulum stress, the potential link between endoplasmic reticulum stress and pulmonary hypertension were introduced, and the contributions of endoplasmic reticulum stress to different vascular cells, mitochondria, and inflammation were described, and finally the potential therapies of attenuating endoplasmic reticulum stress for pulmonary hypertension were discussed.
Keywords: pulmonary hypertension, endoplasmic reticulum stress, unfolded protein response, vascular remodeling
Introduction
Pulmonary hypertension (PH) is a highly hazardous disease, and gradually increasing pulmonary artery (PA) pressure is one of the main characteristics. The symptoms accompanied with high PA pressure include the remodeling of pulmonary vessels, enhanced vasoconstriction, and the compensatory hypertrophy of right ventricle (RV). In the final phase of this disease, heart failure and even death will occur. According to the current clinical classification system from World Health Organization, PH is classified into five categories based on presumed molecular etiologies, histopathology, and clinical associations.1 Group 1 includes a severe form of PH called pulmonary artery hypertension (PAH). Other groups affect a much larger global population and reflect a wide variety of conditions, such as congenital or acquired left heart disease, lung diseases and/or hypoxic, chronic thromboembolism, and unclear multifactorial mechanisms. In the past, landmark studies have shown similar changes of PASMCs (pulmonary artery smooth muscle cells)/ECs (endothelial cells) in the pathology of cancer and PH, which, to a certain extent, explains the pivotal mechanism of PH.2–4 That is to say, some cancer-related studies could provide some references for PH research.5
At present, endoplasmic reticulum (ER) stress is a hot topic in the researches of cancer pathogenesis.6 In recent years, several studies have shown that the glucose-regulated protein 78 kDa (GRP78), a molecular chaperone in ER stress, is involved in proliferation and survival of cancer cells and angiogenesis in tumor tissues.6,7 Angiogenic transformation with tumor angiogenesis was discovered as a downstream target of the unfolded protein response (UPR) pathway, emphasizing the importance of ER stress in tumor angiogenesis.8,9 Therefore, some studies focused on the role of ER stress in PH and the results suggested that UPR functioned in the development of PH.10–12 Nowadays, the inhibition of ER stress was considered as a new potential intervention in clinical treatment of PH. Some studies have already demonstrated that the treatment of using the chemical chaperone, such as 4-phenylbutyrate (PBA), to decrease ER stress could reverse or treat animal models of PH.11,12 However, this therapy has not been used in clinical or pre-clinical studies yet. In a word, the relevant research results of ER stress presented a glimmer of hope for exploring the new targets for the future treatment of PH.
The endoplasmic reticulum stress
Endoplasmic reticulum
ER is the central organelle for intracellular secretion. It is responsible for post-translational modification, folding and maturation, and secretion of transmembrane and secreted proteins. The proteins are then further transported to the Golgi and eventually secreted as the vesicles or displayed on the surface of the plasma.13 Moreover, ER is also crucial for other cellular functions like biosynthesis of lipids (including triglycerides, phospholipids, and cholesterol), Ca2+ buffering, and carbohydrate synthesis. However, the speed of proteins transportation and folding is affected by intracellular and extracellular factors, and changes among different cell types. Thus, by enlarging the entire size of ER preferentially and increasing the production of chaperone proteins, cells will adjust to the need for the entering of numerous nascent proteins into the lumen of ER for folding.14
ER stress
Stress conditions of ER refer to situations such as the status of high glucose or lack of energy, hypoxia, Ca2+ overload, oxidative stress, and exposure to chemicals that will cause imbalances in the homeostasis.15 These stimulations activate the related signals to promote new proteins synthesis for dealing with stress, while these signals will reduce the general protein synthesis.16,17 When the amount of translating proteins exceeds that which ER folding can handle, it will cause misfolded proteins to accumulate in the ER. Due to heaping up of these misfolded proteins in ER, an evolutionarily conserved response of stress which is called unfolded protein response (UPR) will be activated. The adaptive response that takes place in the initial phase of UPR aims to rehabilitate protein folding homeostasis.18–23 When cells cannot recover from ER stress, UPR will terminate this adaptive response and trigger cell apoptosis.19,24–28 The function of UPR prevented the damaged and non-functional proteins heaping up in the ER by re-establishing body homeostasis or triggering cell death.29
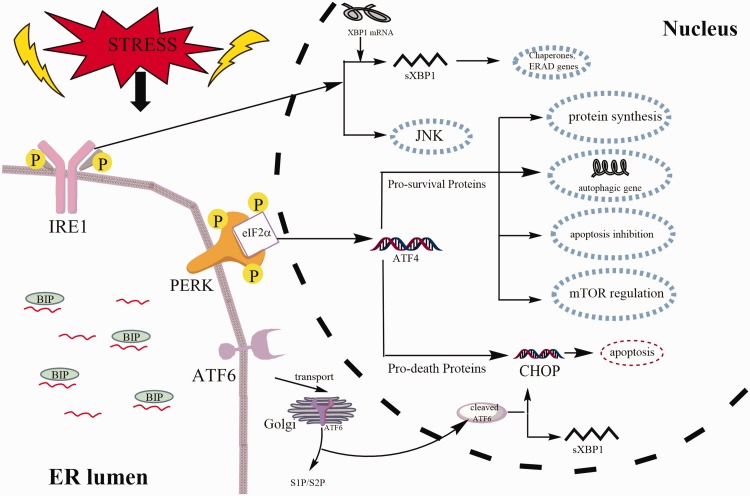
There are three types of UPR signal transducers in mammalian cells, all of which are ER-resident transmembrane proteins. The three proteins are inositol-requiring enzyme 1 α/β (IRE1α/β), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Fig. 1). IRE1 is essential for UPR in plants and animals.30–33 As a multidomain protein of the ER transmembrane, IRE1 has the properties of both a kinase and an endoribonuclease. Under ER stress, IRE1 RNase is activated through autophosphorylation, conformational changes, and higher order oligomerization.34–36 The second sensor of UPR is PERK which primarily attenuates the translation of protein and modulates oxidative stress. This protein can phosphorylate and activate a transcription factor- nuclear factor erythroid-2-related factor 2 (NRF2) and eukaryotic initiation factor 2a (eIF2a) to alleviate unfolded proteins or misfolded proteins.29,37–41 ATF6, the last sensor of the three branches of UPR, is a transmembrane protein and it also functions as a transcription factor when it is cleaved.42–44 The direct target of the cleaved ATF6 is the UPR proteins, such as chaperones.45 At present, there is a more comprehensive understanding of the signaling pathways in ER stress, but the connections between ER stress and many diseases are still waiting for more researchers to explore.
Fig. 1.
The unfolded protein response (UPR) pathway. The three types of UPR signal transducers are PERK, IRE1, and ATF6. IRE1 has both kinase and endoribonuclease identity. When IRE1 is activated, a small intron of XBP1 is removed to form sXBP1, which involves the transcription of genes that restores ER folding ability. PERK phosphorylates eIF2a attenuating mRNA translation, but it specifically induces the transcription factors ATF4 and CHOP, which will induce the occurrence of related reactions such as protein synthesis and apoptosis. ATF6 is translocated into the Golgi where it is cleaved to expose the transcriptionally active cytoplasmic domain of ATF6. The direct target of the cleaved ATF6 is the UPR proteins, such as chaperones. Also, ATF6 can also induce CHOP and XBP1 genes. PERK: protein kinase RNA-like ER kinase; IRE1: inositolrequiring enzyme 1; ATF6: activating transcription factor 6; BIP: Immunoglobulin binding protein; eIF2a: eukaryotic initiation factor 2a; XBP1: X-box binding protein; S1P: protease site 1 protease; S2P: protease site 2 protease; CHOP: transcription factor C/EBP homologous protein; mTOR: mammalian target of rapamycin; JNK: c-Jun N-terminal kinase.
The relationship between ER stress and PH
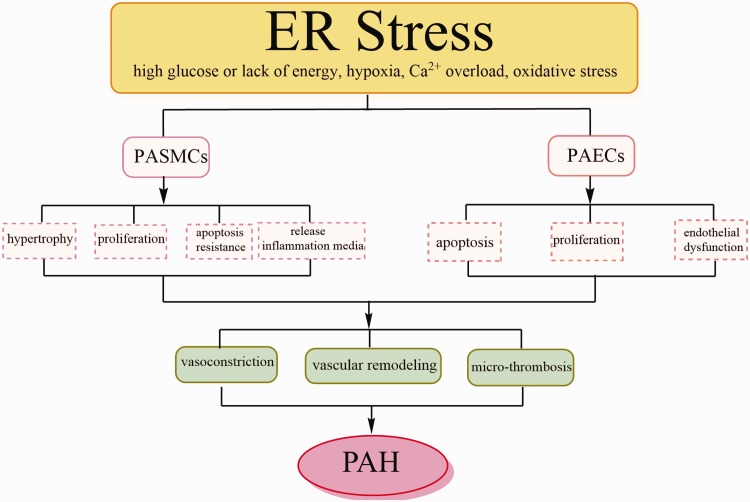
It is widely accepted that the vascular remodeling is a major feature in the pathogenesis of PH and may be present in a wide range of diseased tissues. The corresponding pathological process causing vascular remodeling in PH is very complicated and involves a variety of environmental factors and genes.46–48 This feature was associated with hyperplasia of PASMCs, excessive proliferation of PAECs, microthrombus formation, and persistent pulmonary vasoconstriction. And the uncontrolled over-proliferation of PASMCs and PAECs in complicated vascular lesions will eventually lead to occlusion of the PA, which further cause the adverse rise in pulmonary blood pressure.49–51 In addition, these individual and collective changes were related to excessive cell proliferation, apoptosis resistance, circulating inflammatory cells recruitment, and phenotypic switching.52,53
Studies have shown that vascular remodeling is closely related to ER stress, especially the proliferation of PASMCs.12 Other aspects of PH, such as proliferation of PAECs and the subcellular connections, are also closely related to ER stress.
Pulmonary artery smooth muscle cells
As indicated by the above description, vascular remodeling is one of the significant features in PH, but the mechanisms are still under investigating. In several studies, it has been pointed out that the imbalance of proliferation and apoptosis in PASMCs contributes to medial thickening.50,51 Pathological studies have also shown that some cells in the intraluminal occlusion express SMC markers.54 Therefore, some features such as over-proliferation and anti-apoptosis of PASMCs made the concept of carcinoid appearing. Meanwhile, there was increasing evidence that the hypertrophy, proliferation, and apoptosis resistance of PASMCs were critical components of abnormal vascular remodeling in PH5 (Fig. 2).
Fig. 2.
The main pathological changes of pulmonary artery (PA) during endoplasmic reticulum (ER) stress reaction. ER stress can be induced by multiple adverse physiologic conditions. When ER stress occurs, ECs and SMCs in the PA play an extremely important role. In addition to hypertrophy, hyperplasia and apoptosis resistance, PASMCs also release inflammatory mediators. PAECs are in a dysfunctional state: in the early stage of PH, apoptosis of ECs increased, and in the later period, excessive proliferation occurred. These pathological processes will lead to the generation of microthrombi, persistent pulmonary vasoconstriction, and pulmonary vascular remodeling, eventually leading to PH and even death. PASMCs: pulmonary artery smooth muscle cells; PAECs: pulmonary artery endothelial cells.
Recent studies on the role of ER stress in PH have focused on PASMC. It has been suggested that PASMCs proliferation and resistance to apoptosis are essential for vascular remodeling in PH.55–57 ER stress is a basic cellular response that promotes the proliferation and enhances inflammatory response of PASMCs.58 The abnormal proliferation of PASMCs is the most important cause of pulmonary vascular remodeling in PAH.59,60 Recently, some studies have shown that autophagy is involved in the development of PAH induced by MCT.61,62 And eIF2α can activate ER autophagy after ER stress.37–39 At the same time, eIF2α plays a key role in regulating cell proliferation and hypertrophy, and participated in the regulation of SMC proliferation and migration.63,64 According to Wang et al., they observed that after transfected with eIF2α siRNA in hypoxia-promoted PASMCs proliferation, it can significantly inhibit the proliferation of PASMCs.65,66 Moreover, Cao et al. found that an inhibitor of the IRE1α/XBP1 pathway, 4u8c, promoted apoptosis and repressed cell proliferation and migration of PASMCs.67 All of the above indicate that ER stress is involved in the abnormal proliferation of PASMC in PH.
There was evidence indicating that recruiting the inflammatory cells and continuous development of inflammation in PH are two crucial components of pathological vascular remodeling.68 Recently, it has been reported that ER stress may trigger smooth muscle cells to produce HA.69,70 In the rat PH model induced by MCT, the activity of hyaluronidase-1 (HYAL1) increased significantly at the beginning of the disease, while its degradation and synthesis increased in the late stage of PH.71 However, apart from the increase in the production of HA, there is no systematic description of the mechanism of HA clearance in the (diseased) lungs, and no complete description of the mechanism of the accumulation of HA in a balanced state. It is well known that the clearance of HA is critical to organ homeostasis.72 Previous studies have shown that PH was related to the reducing degradation and increasing synthesis of HA, especially isomers of high molecular weight.71,73 Yeager et al.74 recently pointed out that the activation of UPR and the generation of pro-inflammatory biomolecule were attributed to ET-1 signaling in rat PASMCs. The occurrence of ER stress promotes the proliferation and inflammatory state of PASMCs, which will promote the onset of PH. These experimental results further demonstrate the importance of PASMC in the inflammatory process of PH development, especially in recruiting and preserving the immune cells. The persistent inflammation produced by PASMCs is related to vascular remodeling and inhibition of this process is a potential method of treating PH.
Dromparis et al. found that the ATF6 pathway could be activated in the vasculature of both hypoxic models in vivo and cultured vascular SMCs.11 Surprisingly, they did not mention if the CHOP pathway was also activated. Meanwhile, the ATF6 signaling pathway has been shown to up-regulate Nogo to disrupt mitochondrial-ER units in PASMC, leading to the occurrence of PH.44,75 Although the function of ER stress in PASMCs have been extensively studied, the mechanism is still unknown. In summary, inhibiting ER stress that stimulates the above PASMCs responses is also an extremely appealing treatment option.
Pulmonary arterial endothelial cells
The endothelium secretes many mediators which are necessary for normal vascular functioning, including the mediators of regulating vascular tone and coagulation, modulating immune responses, and controlling vascular cell growth. Moreover, vascular ECs compose a barrier between the vessel lumen and the wall, through which liquid, gas, and macromolecular substances could be selectively transported. At the same time, it also maintains the homeostasis of blood vessels and mediates the passage of nutrients and white blood cells through the vessel wall. Vascular intima can maintain the balance between vasodilation and vasoconstriction in physiological conditions. ECs play an important role in the early stage of PH due to their apoptosis.76 Furthermore, in the studies of late-stage PH, it was confirmed that the over-proliferation endothelial cells, vasoconstriction, the formation of microthrombus, and eventual vascular remodeling are crucial for pathological changes of PH76 (Fig. 2).
Changes of endothelial function in the pulmonary vasculature contribute to the neointimal formation, thickening of the intima, and occlusion of the distal pulmonary artery, which is of great significance for PH.51 One feature in some patients with severe PH is a complex vascular lesion called a plexiform lesion.77 These lesions can be seen in the myofibroblastic stroma in which the monoclonal EC of the lesion is proliferating.78–80 The plexiform or complex vascular lesions are characterized by anti-apoptotic and phenotypic altered EC.81–85
ECs produce the vasodilator/anti-mitotic agents and vasoconstrictors/mitosis, such as prostacyclin, NO, thromboxane A2 and ET-1, to regulate SMCs activity.86 Endothelial dysfunction is thought to be an imbalance that favors the production of vasoconstrictors and proliferative factors, including pro-inflammatory and thrombogenic effects, which has been clearly shown in PH.87 Under the normal conditions, the main players are prostacyclin, nitric oxide (NO), and thrombomodulin. These participants inhibit SMC proliferation, platelet aggregation, and the expression of leukocyte adhesion molecule.88
Some dysfunctions are related to the reducing activity of endothelial NO synthase (eNOS), increasing endothelium-produced ROS, decreasing anticoagulant properties, adhesion molecules up-expression, and release of chemokines and cytokines. It has been confirmed in previous studies that the release of the vasoconstrictor thromboxane A2 increased in these patients, in contrast, the release of prostacyclin is depressed.89
At the same time, more and more evidence showed that the imbalance of signal transduction of ROS and NO was related with PH.90 Moreover, the decreasing expression of prostacyclin synthase in the pulmonary arteries could be detected in some patients with primary PH,91 and the expression of NO synthase decreased in lung ECs in some PH patients.92 Recently, the studies with independent PH model and muscles of CHOP−/− mice have suggested that in the ECs the expression of eNOS induced by ER stress has negative impacts on ECs function.93,94
There are also some patients with elevating plasma ET-1 levels.95 In addition, elevating plasma ET-1 levels have also been reported in experimental models of PH.96 Interestingly, ER stress also induces changes in ECs secretion.97 In a study to confirm whether the UPR pathway was activated in ECs, Lenna et al.98 examined the expression of pulmonary vascular UPR markers in the systemic sclerosis-associated PH (SSC-PH) patients. In this study, they observed that the expressions of BIP and CHOP as markers of ER stress in SSC-PH pulmonary vasculature and macrophages were significantly higher than those in healthy subjects. Interestingly, the elevating CHOP levels existed mainly in the ECs of SSC-PH lung.88 In SSC-PH model of the mouse, the elevating ER stress markers could also be found in Gata6-KO mice.99 The experimental result of Lenna et al.98 suggested that macrophages and ECs might be the reason for increasing ER stress/UPR in hypoxic mice. According to the study of Lenna et al., thapsigargin, the ER stress inducer, could up-regulate the expression of ET-1 by increasing the formation of the ATF4/c-Jun transcription complex in ECs.100 These findings have important implications for the study of the mechanism of ER stress in the endothelium of PH. Perhaps it is a good idea to focus on the ER stress in the treatment of PH.
Subcellular networks
Increasing evidence indicated that the ER stress/UPR pathway had a relationship with other subcellular networks.88 These networks included the ER-mitochondrial units, inflammatory response network and other subcellular factors.
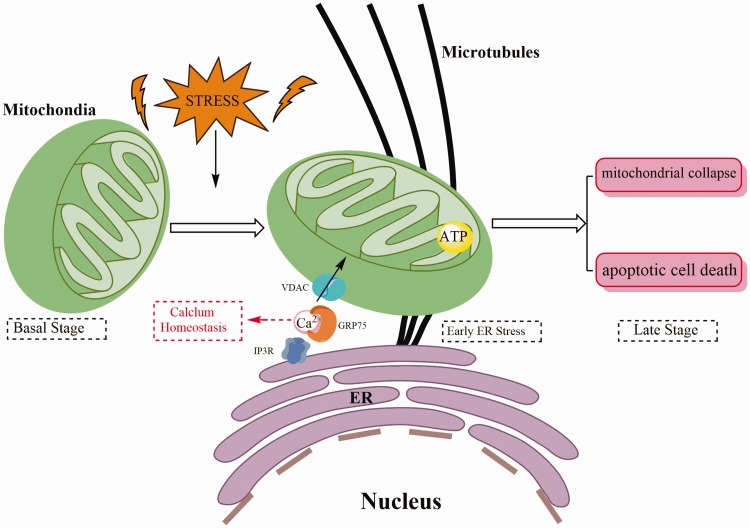
Among all other organelles associated with ER, mitochondria are the most prominent organelle that regulates metabolism and cell survival. Previous studies have shown that mitochondrial dysfunction may lead to vascular remodeling, thus affecting the occurrence of PH.101–103 Mitochondria-associated membrane (MAM) is a special contact formed by the interaction of ER and mitochondrion in physical structure.104 Membrane and luminal components can mix and exchange in the MAM, and the composition of MAM is adapted in response to a variety of internal and external stimuli.104–106 The structure of MAM is complicated and involves a large number of proteins with widely various functions.107 IP3R (inositol 1,4,5-triphate receptor) and VDAC (voltage-dependent anion channel) are the major ER-mitochondrial calcium (Ca2+) transfer channels (Fig. 3). They are located on both the ER side and mitochondria side of the MAM and physically connect the two organelles by forming a complex with the chaperone GRP75, respectively.108 Ca2+ has been identified as an important regulator of cell proliferation and apoptosis, and it also plays an important role in PASMC proliferation and vasoconstriction of PH.109–113 And the ER is the main intracellular reservoir of Ca2+, while many ER chaperone proteins are dependent on Ca2+, thus the regulation of Ca2+ homeostasis in ER is very important.114,115 Alterations of calcium manipulate by the ER/mitochondrial couple providing a pathway to activate apoptosis.116 A key process linking apoptosis to ER-mitochondrial interactions is the change of the Ca2+ homeostasis, which results in massive and/or prolonged mitochondrial Ca2+ overload.107 Moreover, Mitochondria are accepted as indispensable oxygen sensor for hypoxic pulmonary vasoconstriction in cells.117 And mitochondrial signals will promote apoptosis-resistance and proliferative diathesis of vascular remodeling in PH.118
Fig. 3.
Correlation between endoplasmic reticulum (ER) and mitochondria. Under physiological conditions, Ca2 + can be transferred from the ER into the mitochondria. Early stage of ER stress triggers an increase in mitochondrial metabolism which depends critically upon mitochondria-associated membrane (MAMS) and Ca2 + transfer. If stress persists, this response leads to mitochondrial collapse and triggers apoptotic cell death. IP3R: inositol 1,4,5-triphate receptor; VDAC: voltage-dependent anion channel; ATP: adenosine triphosphate.
Recently, several studies have shown that ER stress have involved in the development of inflammation during the PH, but all these are preliminary studies.12,74,98 However, the roles of inflammation both in ER stress and PH are highly correlated. Proinflammatory cytokines and chemokines have been shown to be involved in the animal model of monocrotaline-induced PH.119–122 Most patients with primary PH have evidence of autoimmune and/or active inflammation. In these patients, circulating antinuclear antibodies and increasing serum levels of interleukin (IL)-1 and IL-6 can be detected.123,124 And several studies have demonstrated that in ER stress IRE1 and PERK signaling pathways also participate in the inflammatory response.125,126 The UPR pathway also can trigger the activation of NF-kB, another major inflammatory mediator.127 The specific relationship among ER stress, inflammation and PH could contribute to reveals the pathogenesis of PH, which requires further research.
Among the other various factors affecting PH, the PDGF-β receptor is one of the most concerned focuses in PH researches and it is responsible for proliferation of PASMCs and neointimal hyperplasia of the PA.128–130 Other experiments of cultured PASMCs have indicated that using platelet-derived growth factor (PDGF)-BB to treat cells will activate the UPR pathway.12 These results suggest that PDGF-BB may be one of the contributors to activate the UPR pathway in PH. There has been increasing evidence showing that the ER stress and UPR pathways have a critical effect on regulating the expression of proangiogenic factors. They also serve as novel mediators of angiogenesis. Up-regulation of angiogenic factors such as VEGF-A, FGF, IL-8, and ET-1 has been reported to occur during the UPR.131 Moreover, according to reports, as a regulatory ER structural protein,132,133 Nogo-B is involved in vascular remodeling and plays a role in PAH.75,134 At the same time, it has also been demonstrated that PH-related loss of function mutations of BMPRII and the resultant protein transporting dysfunction can also induce ER stress.135 Furthermore, recent researches on metabolic abnormalities of the pulmonary vascular system and the right ventricle have also attracted more and more attention.136,137
Potential therapies
Recently, several studies have suggested that the chemical chaperones of ER stress, like 4-PBA or tauroursodeoxycholic acid (TUDCA), could reduce the mPAP significantly in PH animal model.10–12 Chemical chaperones are often defined as low molecular weight compounds.138,139 So far, the exact mechanism of chemical chaperones in ER stress has not been elucidated. Most likely, these two molecules exert their effects by stabilizing the structure of incorrectly folded proteins, stimulating molecular chaperones to obtain more efficient protein trafficking, reducing protein aggregation, and preventing the interactions of non-specific proteins and other proteins.140–142 4-PBA, One of the chemical chaperones which most commonly mentioned, is a low molecular weight fatty acid and a non-toxic pharmacological compound.143–146 It has three main pharmacological effects as an ammonia scavenger,143,147 a weak histone deacetylase inhibitor,148,149 and an ER stress inhibitor.150,151 Due to its ammonia scavenger properties, it has been approved by the FDA for clinical use in pathological diseases of the urea cycle147,152,153 At present, it has been widely studied as a small chemical chaperone to modulate restoration of ER homeostasis and multiple concerning pathological conditions.12,154 Furthermore, using 4-PBA can down-regulate several key proteins in ER stress, thereby improving PA and RV remodeling.3,10,143,155 Another chemical chaperone, TUDCA, a safe hydrophilic bile acid. Although the exact mechanism of TUDCA as a chemical chaperone is still unclear, it has been shown to prevent UPR dysfunction and reduce ER stress in various cell types.156–160 Recently, there has been shown that TUDCA binded with the hydrophobic regions of proteins to prevent subsequent protein aggregation and unfolded protein accumulation, thus attenuating ER stress.161,162 And it has been reported that TUDCA also exerts these effects partially by assisting in the transfer of mutant proteins and partially by improving protein folding capacity through the activation of ATF6.163
These above results showed that attenuating ER stress could be an effective treatment strategy to protect the PA from damage. If the association between ER stress and PH was figured out, the use of these drugs which have non-toxic and does not burden other organs would be a nice choice for clinical treatment of PH.
Summary
A review of all the literature mentioned in the text makes it evident that ER stress was involved in the PH, and may play an important role. These above content in this article show that attenuating ER stress may be an effective treatment to protect the PA. It is also clear that the exact molecular mechanisms of ER stress in the pathology of PH remains unclear. More studies are needed in future.
Authors’ contribution
YH and WY contributed in original draft preparation, LX, BL and HL in review and editing, YH and TL in literature correction, and BL and HL in supervision.
Guarantor
Yanan Hu, Bin Liu, Hanmin Liu.
Ethical approval
No ethical statement will be required for this study because there is no direct involvement of human.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was supported by Sichuan Science and Technology Program “Key Research and Development Projects of Sichuan Science and Technology Department” (19ZDYF1169). This research was also supported by Science and technology project of Chengdu Science and Technology Bureau (2016-HM01-00102-SF).
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. [DOI] [PubMed] [Google Scholar]
- 2.Rai PR, Cool CD, King JA, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab 2014; 19: 558–573. [DOI] [PubMed] [Google Scholar]
- 4.Ruffenach G, Bonnet S, Rousseaux S, et al. Identity crisis in pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045893217746054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucherat O, Vitry G, Trinh I, et al. The cancer theory of pulmonary arterial hypertension. Pulm Circ 2017; 7: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong D, Ni M, Li J, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res 2008; 68: 498–505. [DOI] [PubMed] [Google Scholar]
- 7.Cullen SJ, Fatemie S, Ladiges W. Breast tumor cells primed by endoplasmic reticulum stress remodel macrophage phenotype. Am J Cancer Res 2013; 3: 196–210. [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh R, Lipson KL, Sargent KE, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 2010; 5: e9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YG, Alam GN, Ning Y, et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Research 2012; 72: 5396–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Adi D, Long M, et al. 4-Phenylbutyric acid induces protection against pulmonary arterial hypertension in rats. PLoS One 2016; 11: e0157538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dromparis P, Paulin R, Stenson TH, et al. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 2013; 127: 115. [DOI] [PubMed] [Google Scholar]
- 12.Koyama M, Furuhashi M, Ishimura S, et al. Reduction of endoplasmic reticulum stress by 4-phenylbutyric acid prevents the development of hypoxia-induced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2014; 306: H1314–1323. [DOI] [PubMed] [Google Scholar]
- 13.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833: 3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuck S, Prinz WA, Thorn KS, et al. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol 2009; 187: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao SZ, Fan XF, Xue F, et al. Intermedin modulates hypoxic pulmonary vascular remodeling by inhibiting pulmonary artery smooth muscle cell proliferation. Pulm Pharmacol Ther 2014; 27: 1–9. [DOI] [PubMed] [Google Scholar]
- 16.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 17.Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat Cell Biol 2015; 17: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman M, van der Goot FG. Novel ubiquitin-dependent quality control in the endoplasmic reticulum. Trends Cell Biol 2009; 19: 357–363. [DOI] [PubMed] [Google Scholar]
- 19.Merksamer PI, Papa FR. The UPR and cell fate at a glance. J Cell Sci 2010; 123: 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoseki J, Ushioda R, Nagata K. Mechanism and components of endoplasmic reticulum-associated degradation. J Biochem 2010; 147: 19–25. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab 2011; 22: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 23.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 2011; 334: 1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woehlbier U, Hetz C. Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci 2011; 36: 329–337. [DOI] [PubMed] [Google Scholar]
- 25.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 2011; 23: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Bio 2012; 13: 89–102. [DOI] [PubMed] [Google Scholar]
- 27.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 2012; 22: 274–282. [DOI] [PubMed] [Google Scholar]
- 28.Jager R, Bertrand MJM, Gorman AM, et al. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell 2012; 104: 259–270. [DOI] [PubMed] [Google Scholar]
- 29.McQuiston A, Diehl JA. Recent insights into PERK-dependent signaling from the stressed endoplasmic reticulum. F1000Res 2017; 6: 1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1 alpha interactome. Molecular Cell 2009; 35: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetz C, Martinon F, Rodriguez D, et al. The unfolded protein response: integrating stress signals through the stress sensor IRE1 alpha. Physiol Rev 2011; 91: 1219–1243. [DOI] [PubMed] [Google Scholar]
- 32.Nagashima Y, Mishiba K, Suzuki E, et al. Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci Rep 2011; 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YN, Brandizzi F. AtIRE1A/AtIRE1B and AGB1 independently control two essential unfolded protein response pathways in Arabidopsis. Plant J 2012; 69: 266–277. [DOI] [PubMed] [Google Scholar]
- 34.Ali MMU, Bagratuni T, Davenport EL, et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. Embo J 2011; 30: 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aragon T, van Anken E, Pincus D, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 2009; 457: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korennykh AV, Egea PF, Korostelev AA, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature 2009; 457: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11: 619–633. [DOI] [PubMed] [Google Scholar]
- 38.Tsai YC, Weissman AM. The unfolded protein response, degradation from endoplasmic reticulum and cancer. Genes Cancer 2010; 1: 764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013; 15: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.B'chir W, Maurin AC, Carraro V, et al. The eIF2 alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 2013; 41: 7683–7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harding HP, Novoa I, Zhang YH, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000; 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Okada T, Haze K, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 2000; 20: 6755–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta A, Hossain MM, Read DE, et al. PERK regulated miR-424(322)-503 cluster fine-tunes activation of IRE1 and ATF6 during unfolded protein response. Sci Rep 2015; 5: 18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida H, Matsui T, Yamamoto A, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001; 107: 881–891. [DOI] [PubMed] [Google Scholar]
- 45.Shen J, Snapp EL, Lippincott-Schwartz J, et al. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol 2005; 25: 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parpaleix A, Amsellem V, Houssaini A, et al. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur Respir J 2016; 48: 470–483. [DOI] [PubMed] [Google Scholar]
- 47.Pugh ME, Hemnes AR. Metabolic and hormonal derangements in pulmonary hypertension: from mouse to man. Int J Clin Pract 2010; 64: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep 2015, pp. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: 13S–24S. [DOI] [PubMed] [Google Scholar]
- 50.Ranchoux B, Harvey LD, Ayon RJ, et al. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series). Pulm Circ 2018; 8: 2045893217752912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humbert M, Montani D, Perros F, et al. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vasc Pharmacol 2008; 49: 113–118. [DOI] [PubMed] [Google Scholar]
- 52.Schermuly RT, Ghofrani HA, Wilkins MR, et al. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2012; 122: 4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension – a morphometric and immunohistochemical study. Am J Resp Crit Care 2000; 162: 1577–1586. [DOI] [PubMed] [Google Scholar]
- 55.Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol 2008; 153(Suppl 1): S99–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perros F, Montani D, Dorfmuller P, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 81–88. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Liu X, Meng L, et al. Up-regulated lipocalin-2 in pulmonary hypertension involving in pulmonary artery SMC resistance to apoptosis. Int J Biol Sci 2014; 10: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R, Zhong W, Shao C, et al. Docosahexaenoic acid inhibits monocrotaline-induced pulmonary hypertension via attenuating endoplasmic reticulum stress and inflammation. Am J Physiol Lung Cell Mol Physiol 2018; 314: L243–L255. [DOI] [PubMed] [Google Scholar]
- 59.Santos-Ribeiro D, Mendes-Ferreira P, Maia-Rocha C, et al. Pulmonary arterial hypertension: Basic knowledge for clinicians. Arch Cardiovasc Dis 2016; 109: 550–561. [DOI] [PubMed] [Google Scholar]
- 60.Yu L, Tu Y, Jia X, et al. Resveratrol protects against pulmonary arterial hypertension in rats via activation of silent information regulator 1. Cell Physiol Biochem 2017; 42: 55–67. [DOI] [PubMed] [Google Scholar]
- 61.Long L, Yang X, Southwood M, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 2013; 112: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 62.Kim D, George MP. Pulmonary hypertension. Med Clin North Am 2019; 103: 413–423. [DOI] [PubMed] [Google Scholar]
- 63.Jiang L, Zang D, Yi S, et al. A microRNA-mediated decrease in eukaryotic initiation factor 2α promotes cell survival during PS-341 treatment. Sci Rep 2016; 6: 21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Bennett RL, Cheng X, et al. PKR regulates proliferation, differentiation, and survival of murine hematopoietic stem/progenitor cells. Blood 2013; 121: 3364–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang AP, Li XH, Yang YM, et al. A critical role of the mTOR/eIF2α pathway in hypoxia-induced pulmonary hypertension. PLoS One 2015; 10: e0130806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo L, Li Y, Tian Y, et al. eIF2alpha promotes vascular remodeling via autophagy in monocrotaline-induced pulmonary arterial hypertension rats. Drug Des Devel Ther 2019; 13: 2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.X C, Y H, X L, et al. The IRE1α-XBP1 pathway function in hypoxia-induced pulmonary vascular remodeling, is upregulated by quercetin, inhibits apoptosis and partially reverses the effect of quercetin in PASMCs. Am J Transl Res 2019; 11: 641–654. [PMC free article] [PubMed] [Google Scholar]
- 68.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009; 54: S10–19. [DOI] [PubMed] [Google Scholar]
- 69.Majors AK, Austin RC, de la Motte CA, et al. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 2003; 278: 47223–47231. [DOI] [PubMed] [Google Scholar]
- 70.ME L, D M, C F, et al. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem 2009; 284: 5299–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ormiston ML, Slaughter GR, Deng Y, et al. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2010; 298: L148–157. [DOI] [PubMed] [Google Scholar]
- 72.Fischer JW, Schror K. Regulation of hyaluronan synthesis by vasodilatory prostaglandins. Implications for atherosclerosis. Thromb Haemost 2007; 98: 287–295. [PubMed] [Google Scholar]
- 73.Aytekin M, Comhair SA, de la Motte C, et al. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008; 295: L789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeager ME, Belchenko DD, Nguyen CM, et al. Endothelin-1, the unfolded protein response, and persistent inflammation: role of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 2012; 46: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutendra G, Dromparis P, Wright P, et al. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 2011; 3: 88ra55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin 2004; 22: 343–351, v. [DOI] [PubMed] [Google Scholar]
- 77.Voelkel NF Tuder RM and Weir EK. Pathophysiology of primary pulmonary hypertension: from physiology to molecular mechanisms. In: Rubin LJ and Rich S (ed) Primary pulmonary hypertension. New York: Marcel Dekker, Inc, 1997, pp. 83–129.
- 78.Voelkel NF, Tuder RM. Severe pulmonary hypertensive diseases: a perspective. Eur Respir J 1999; 14: 1246–1250. [DOI] [PubMed] [Google Scholar]
- 79.Cool CD, Stewart JS, Werahera P, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Am J Pathol 1999; 155: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2010; 43: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 2007; 49: 803–810. [DOI] [PubMed] [Google Scholar]
- 82.Yuan JXJ, Rubin LJ. Pathogenesis of pulmonary arterial hypertension – the need for multiple hits. Circulation 2005; 111: 534–538. [DOI] [PubMed] [Google Scholar]
- 83.Sakao ST-SL, Cool CD, Tada Y, et al. VEGF-R blockade causes endothelial cell apoptosis, expansion of surviving CD341 precursor cells and transdifferentiation to smooth muscle-like and neuronal-like cells. FASEB J 2007; 21: 3640–3652. [DOI] [PubMed] [Google Scholar]
- 84.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001; 15: 427–438. [DOI] [PubMed] [Google Scholar]
- 85.Sakao S, Taraseviciene-Stewart L, Lee JD, et al. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. Faseb J 2005; 19: 1178. [DOI] [PubMed] [Google Scholar]
- 86.Galie N. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 2004; 61: 227–237. [DOI] [PubMed] [Google Scholar]
- 87.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 2000; 102: 2781–2791. [DOI] [PubMed] [Google Scholar]
- 88.Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 2014; 66: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992; 327: 70–75. [DOI] [PubMed] [Google Scholar]
- 90.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 2012; 52: 1970–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995; 333: 214–221. [DOI] [PubMed] [Google Scholar]
- 92.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Resp Crit Care 1999; 159: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 93.Lenna S, Townsend DM, Tan FK, et al. HLA-B35 upregulates endothelin-1 and downregulates endothelial nitric oxide synthase via endoplasmic reticulum stress response in endothelial cells. J Immunol 2010; 184: 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Loinard C, Zouggari Y, Rueda P, et al. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation 2012; 125: 1014–1026. [DOI] [PubMed] [Google Scholar]
- 95.Stewart DJ, Levy RD, Cernacek P, et al. Increased plasma endothelin-1 in pulmonary-hypertension – marker or mediator of disease. Ann Intern Med 1991; 114: 464–469. [DOI] [PubMed] [Google Scholar]
- 96.Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary-hypertension. Circ Res 1993; 73: 887–897. [DOI] [PubMed] [Google Scholar]
- 97.Galan M, Kassan M, Kadowitz PJ, et al. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta 2014; 1843: 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lenna S, Farina AG, Martyanov V, et al. Increased expression of endoplasmic reticulum stress and unfolded protein response genes in peripheral blood mononuclear cells from patients with limited cutaneous systemic sclerosis and pulmonary arterial hypertension. Arthritis Rheum 2013; 65: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghatnekar A, Chrobak I, Reese C, et al. Endothelial GATA-6 deficiency promotes pulmonary arterial hypertension. Am J Pathol 2013; 182: 2391–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lenna S, Chrobak I, Farina GA, et al. HLA-B35 and dsRNA induce endothelin-1 via activation of ATF4 in human microvascular endothelial cells. PLoS One 2013; 8: e56123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Ten VS, Ratner V. Mitochondrial bioenergetics and pulmonary dysfunction: current progress and future directions. Paediatr Respir Rev 2019, pp. pii: S1526-0542(19)30036–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest 2018; 128: 3704–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marshall JD, Bazan I, Zhang Y, et al. Mitochondrial dysfunction and pulmonary hypertension: cause, effect, or both. Am J Physiol Lung Cell Mol Physiol 2018; 314: L782–L796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 1990; 265: 7248–7256. [PubMed] [Google Scholar]
- 105.Myhill N, Lynes EM, Nanji JA, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 2008; 19: 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bui M, Gilady SY, Fitzsimmons REB, et al. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem 2010; 285: 31590–31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giorgi C, De Stefani D, Bononi A, et al. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell B 2009; 41: 1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szabadkai G, Bianchi K, Varnai P, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 2006; 175: 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hadri L, Kratlian RG, Benard L, et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 2013; 128: 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aguero J, Ishikawa K, Hadri L, et al. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol 2014; 307: H1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aguero J, Ishikawa K, Hadri L, et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J Am Coll Cardiol 2016; 67: 2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonnet S, Rochefort G, Sutendra G, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 2007; 104: 11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao L, Oliver E, Maratou K, et al. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature 2015; 524: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bravo R, Gutierrez T, Paredes F, et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 2012; 44: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci 2008; 65: 862–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pinton P, Giorgi C, Siviero R, et al. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008; 27: 6407–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weir EK, Lopez-Barneo J, Buckler KJ, et al. Acute oxygen-sensing mechanisms. N Engl J Med 2005; 353: 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dromparis P, Paulin R, Sutendra G, et al. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ Res 2013; 113: 126–136. [DOI] [PubMed] [Google Scholar]
- 119.Voelkel NF, Tuder RM, Bridges J, et al. Interleukin-1 receptor antagonist treatment reduces pulmonary-hypertension generated in rats by monocrotaline. Am J Resp Cell Mol 1994; 11: 664–675. [DOI] [PubMed] [Google Scholar]
- 120.Bhargava A, Kumar A, Yuan N, et al. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1999; 1: 126–132. [PubMed] [Google Scholar]
- 121.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 122.Kimura H, Kasahara Y, Kurosu K, et al. Alleviation of monocrotaline-induced pulmonary hypertension by antibodies to monocyte chemotactic and activating factor monocyte chemoattractant protein-1. Lab Invest 1998; 78: 571–581. [PubMed] [Google Scholar]
- 123.Isern RA, Yaneva M, Weiner E, et al. Autoantibodies in patients with primary pulmonary-hypertension – association with anti-Ku. Am J Med 1992; 93: 307–312. [DOI] [PubMed] [Google Scholar]
- 124.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary-hypertension. Am J Resp Crit Care 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 125.Urano F, Wang XZ, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000; 287: 664–666. [DOI] [PubMed] [Google Scholar]
- 126.Zhang KZ, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008; 454: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garg AD, Kaczmarek A, Krysko O, et al. ER stress-induced inflammation: does it aid or impede disease progression?. Trends Mol Med 2012; 18: 589–598. [DOI] [PubMed] [Google Scholar]
- 128.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008; 118: 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians new concepts and experimental therapies. Circulation 2010; 121: 2045–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L, Ma J, Shen TT, et al. Platelet-derived growth factor (PDGF) induces pulmonary vascular remodeling through 15-LO/15-HETE pathway under hypoxic condition. Cell Signal 2012; 24: 1931–1939. [DOI] [PubMed] [Google Scholar]
- 131.Paridaens A, Laukens D, Vandewynckel YP, et al. Endoplasmic reticulum stress and angiogenesis: is there an interaction between them?. Liver Int 2014; 34: e10–18. [DOI] [PubMed] [Google Scholar]
- 132.Hu J, Shibata Y, Voss C, et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 2008; 319: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 133.Zurek N, Sparks L, Voeltz G. Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic 2011; 12: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muñoz JP, Zorzano A. Endoplasmic reticulum stress enters a Nogo zone. Sci Transl Med 2011; 3: 88ps26. [DOI] [PubMed] [Google Scholar]
- 135.Sobolewski A, Rudarakanchana N, Upton PD, et al. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet 2008; 17: 3180–3190. [DOI] [PubMed] [Google Scholar]
- 136.Whyte C, Thies F, Peyrol L, et al. N-3 long-chain polyunsaturated fatty acids inhibit smooth muscle cell migration by modulating urokinase plasminogen activator receptor through MEK/ERK-dependent and -independent mechanisms. J Nutr Biochem 2012; 23: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 137.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metabolism 2014; 19: 558–573. [DOI] [PubMed] [Google Scholar]
- 138.Cortez L, Sim The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014; 8: pii: 28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uppala JK, Gani AR, Ramaiah KVA. Chemical chaperone, TUDCA unlike PBA, mitigates protein aggregation efficiently and resists ER and non-ER stress induced HepG2 cell death. Sci Rep 2017; 7: 3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kusaczuk M. Tauroursodeoxycholate – bile acid with chaperoning activity: molecular and cellular effects and therapeutic perspectives. Cells 2019; 8: 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res 2002; 52: 832–836. [DOI] [PubMed] [Google Scholar]
- 142.Dandage R, Bandyopadhyay A, Jayaraj GG, et al. Classification of chemical chaperones based on their effect on protein folding landscapes. ACS Chem Biol 2015; 10: 813–820. [DOI] [PubMed] [Google Scholar]
- 143.Kolb PS, Ayaub EA, Zhou W, et al. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol 2015; 61: 45–52. [DOI] [PubMed] [Google Scholar]
- 144.Wang WJ, Mulugeta S, Russo SJ, et al. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. J Cell Sci 2003; 116: 683–692. [DOI] [PubMed] [Google Scholar]
- 145.de Almeida SF, Picarote G, Fleming JV, et al. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem 2007; 282: 27905–27912. [DOI] [PubMed] [Google Scholar]
- 146.Chamcheu JC, Navsaria H, Pihl-Lundin I, et al. Chemical chaperones protect epidermolysis bullosa simplex keratinocytes from heat stress-induced keratin aggregation: involvement of heat shock proteins and MAP kinases. J Invest Dermatol 2011; 131: 1684–1691. [DOI] [PubMed] [Google Scholar]
- 147.Lichter-Konecki U, Diaz G, Merritt JL, et al. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol Genet Metab 2011; 103: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miller AC, Cohen S, Stewart M, et al. Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys 2011; 50: 585–596. [DOI] [PubMed] [Google Scholar]
- 149.Shi X, Zheng C, Li C, et al. 4-Phenybutyric acid promotes gastric cancer cell migration via histone deacetylase inhibition-mediated HER3/HER4 up-regulation. Cell Biol Int 2018; 42: 53–62. [DOI] [PubMed] [Google Scholar]
- 150.Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 2011; 60: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hong YP, Guo WY, Wang WX, et al. 4-Phenylbutyric acid attenuates pancreatic beta-cell injury in rats with experimental severe acute pancreatitis. Int J Endocrinol 2016; 2016: 4592346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monteleone JP, Mokhtarani M, Diaz GA, et al. Population pharmacokinetic modeling and dosing simulations of nitrogen-scavenging compounds: disposition of glycerol phenylbutyrate and sodium phenylbutyrate in adult and pediatric patients with urea cycle disorders. J Clin Pharmacol 2013; 53: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee B, Rhead W, Diaz GA, et al. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Mol Genet Metab 2010; 100: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mimori S, Ohtaka H, Koshikawa Y, et al. 4-Phenylbutyric acid protects against neuronal cell death by primarily acting as a chemical chaperone rather than histone deacetylase inhibitor. Bioorg Med Chem Lett 2013; 23: 6015–6018. [DOI] [PubMed] [Google Scholar]
- 155.Park CS, Cha H, Kwon EJ, et al. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun 2012; 421: 578–584. [DOI] [PubMed] [Google Scholar]
- 156.Falasca L, Tisone G, Palmieri G, et al. Protective role of tauroursodeoxycholate during harvesting and cold storage of human liver: a pilot study in transplant recipients. Transplantation 2001; 71: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 157.Xie Q, Khaoustov VI, Chung CC, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 2002; 36: 592–601. [DOI] [PubMed] [Google Scholar]
- 158.Schoemaker MH, Conde de la Rosa L, Buist-Homan M, et al. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 2004; 39: 1563–1573. [DOI] [PubMed] [Google Scholar]
- 159.Lee YY, Hong SH, Lee YJ, et al. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem Bioph Res Co 2010; 397: 735–739. [DOI] [PubMed] [Google Scholar]
- 160.da-Silva WS, Ribich S, Arrojo e Drigo R, et al. The chemical chaperones tauroursodeoxycholic and 4-phenylbutyric acid accelerate thyroid hormone activation and energy expenditure. FEBS Lett 2011; 585: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gani AR, Uppala JK, Ramaiah KV. Tauroursodeoxycholic acid prevents stress induced aggregation of proteins in vitro and promotes PERK activation in HepG2 cells. Arch Biochem Biophys 2015; 568: 8–15. [DOI] [PubMed] [Google Scholar]
- 162.Paridaens A, Raevens S, Colle I, et al. Combination of tauroursodeoxycholic acid and N-acetylcysteine exceeds standard treatment for acetaminophen intoxication. Liver Int 2017; 37: 748–756. [DOI] [PubMed] [Google Scholar]
- 163.Omura T, Asari M, Yamamoto J, et al. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem Bioph Res Co 2013; 432: 689–694. [DOI] [PubMed] [Google Scholar]