Abstract
We aimed to characterize the plasma metabolome of chronic thromboembolic pulmonary hypertension patients using a high-throughput unbiased omics approach. We collected fasting plasma from a peripheral vein in 33 operable chronic thromboembolic pulmonary hypertension patients, 31 healthy controls, and 21 idiopathic pulmonary arterial hypertension patients matched for age, gender, and body mass index. Metabolomic analysis was performed using an untargeted approach (Metabolon Inc. Durham, NC). Of the total of 862 metabolites identified, 362 were different in chronic thromboembolic pulmonary hypertension compared to controls: 178 were higher and 184 were lower. Compared to idiopathic pulmonary arterial hypertension, 147 metabolites were different in chronic thromboembolic pulmonary hypertension: 45 were higher and 102 were lower. The plasma metabolome allowed us to distinguish subjects with chronic thromboembolic pulmonary hypertension and healthy controls with a predictive accuracy of 89%, and chronic thromboembolic pulmonary hypertension versus idiopathic pulmonary arterial hypertension with 80% accuracy. Compared to idiopathic pulmonary arterial hypertension and healthy controls, chronic thromboembolic pulmonary hypertension patients had higher fatty acids and glycerol; while acyl cholines and lysophospholipids were lower. Compared to healthy controls, both idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients had increased acyl carnitines, beta-hydroxybutyrate, amino sugars and modified amino acids and nucleosides. The plasma global metabolomic profile of chronic thromboembolic pulmonary hypertension suggests aberrant lipid metabolism characterized by increased lipolysis, fatty acid oxidation, and ketogenesis, concomitant with reduced acyl choline and phospholipid moieties. Future research should investigate the pathogenetic and therapeutic potential of modulating lipid metabolism in chronic thromboembolic pulmonary hypertension.
Keywords: metabolism, lipolysis, ketogenesis, chronic thromboembolic pulmonary hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by occlusion of large pulmonary arteries by organized thromboembolic material and concomitant microscopic vasculopathy. The pathobiology of CTEPH is complex and incompletely understood. CTEPH and idiopathic pulmonary arterial hypertension (IPAH) share several features, most notable the increased pulmonary vascular resistance and right ventricular failure which lead to premature death. The pulmonary microvascular disease of CTEPH is similar in many respects to the changes observed in PAH.1–3 However, a key difference is that CTEPH starts with one or more thromboembolic events, and that it can be cured by the removal of the fibrotic thromboembolic material via pulmonary endarterectomy (PEA) surgery.
In recent years, metabolic dysregulation has emerged as an important pathway in IPAH.4,5 Altered glucose homeostasis and dyslipidemia have been documented in PAH and associated with worse outcomes.6–10 Metabolism has not been studied in CTEPH. Baseline glycosylated hemoglobin A1c has been found to correlate with right atrial pressure and cardiac index in operable CTEPH. 11 Our group recently reported decreased high-density lipoprotein cholesterol levels in CTEPH patients, which correlated with higher postoperative pulmonary vascular resistance. 12
With the advent of new metabolomic platforms, we now have the ability to measure small molecules in biologic samples using a high-throughput unbiased approach. Metabolomics provides a snapshot of the physiological state of a tissue/organ or the whole body, and can uncover the relative activity in different metabolic pathways.13,14 Plasma metabolomic studies in IPAH have demonstrated alterations in glycolysis, lipid metabolism, and altered bioenergetics.15,16 The objectives of the present study were to describe the plasma metabolome of CTEPH patients and compare it to healthy controls, and to compare the plasma metabolome of CTEPH with that of IPAH patients in order to ascertain whether the observed changes are specific to pathophysiologic process/es related to CTEPH or to pulmonary hypertension and right heart failure.
Methods
This study was approved by the Institutional Review Board (IRB 06-245), and all subjects signed a written informed consent. A fasting blood sample was obtained from a peripheral vein from 33 CTEPH patients, 31 healthy controls, and 21 IPAH patients. The samples were collected in EDTA tubes, processed to isolate plasma, and frozen at −80℃ until analysis. Subjects in the three groups were matched for age, gender, and body mass index (BMI) using data available in our institutional pulmonary hypertension registry (IRB 8097) and biobank (IRB 06-245). All CTEPH patients were evaluated by our multidisciplinary CTEPH team according to current guidelines and deemed technically operable.17,18 New York Heart functional (NYHA) class, 6-min walk distance, N-terminal pro B-type natriuretic peptide (NT-proBNP) levels, and right heart catheterization data were extracted from our registry. Median time from blood sample collection and clinical data was four weeks, interquartile range 1–20 weeks.
Metabolomic analysis was performed by Metabolon Inc. (Durham, NC) as described (see online supplement for details). 19 An aliquot of plasma was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. Another aliquot was also analyzed using acidic positive ion conditions; however, it was chromatographically optimized for more hydrophobic compounds. Another aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The fourth aliquot was analyzed via negative ionization following elution from a hydrophilic interaction liquid chromatography (HILIC) column. Raw data were extracted, and peaks were identified and processed using standardized quality control procedures. Compounds were identified by automated comparison using Metabolon’s reference library entries. We report the annotated metabolites from untargeted metabolomics analysis. Instrument variability was determined by calculating the median relative standard deviation (RSD) for the internal standards that were added to each sample prior to injection into the mass spectrometers, and was 6%. Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e. non-instrument standards) present in technical replicates of a pool created by taking a small aliquot from each sample. Instrument variability was 6%, and total process variability was 9%.
To confirm some of the key metabolomics findings, we used high-performance liquid chromatography (HPLC) Online Tandem Mass Spectrometry (LC/MS/MS) to quantitate plasma fatty acids. For details on the methods used, please see the online supplement.
Statistical analysis
We present data as fold changes between groups. The fold changes are derived from the median scaled intensities without log transformation. Missing values were imputed with the minimum observed value for each compound. Following log transformation of the data, one-way ANOVA was used to identify biochemicals that differed significantly between experimental groups. To account for multiple comparisons, an estimate of the false discovery rate (q-value) was calculated. 20 Statistical significance was declared for p values <0.05 and q values <0.1. We also considered a fold change >2 or <0.5 to be significant, but when most biochemicals in a pathway were statistically different, then we consider that pathway of biologic relevance. The scaled intensity values have undergone a median scaling procedure. 21 We used the scale intensity of selected biochemical (those with the largest differences observed in CTEPH patients) to calculate Pearson correlation coefficients with NYHA functional class, 6-min walk distance, arterial oxygen saturation, right atrial pressure, mean pulmonary artery pressure, cardiac index, total pulmonary vascular resistance (TPR), and NT-proBNP. For the HPLC quantitative analysis of fatty acids, we used one-way ANOVA to compare fatty acid levels across the three experimental groups, and the Tukey test to compare levels between groups while adjusting for multiple comparisons.
We also used principal component analysis to obtain a high-level view of the structure of the data, and random forest analysis to assess if the metabolomics profile could separate the experimental groups, as previously reported. 22 We also performed pathway enrichment analysis to ascertain which metabolic pathways were up- or down-regulated in the experimental groups. Pathway enrichment displays the number of experimentally regulated compounds relative to all detected compounds in a pathway, compared to the total number of experimentally regulated compounds relative to all detected compounds in the study. A pathway enrichment value >1 indicates that the pathway contains more experimentally regulated compounds relative to the study overall.
Results
Study population
The study included 33 CTEPH patients, 31 healthy controls and 21 IPAH patients. Table 1 shows the characteristics of the study population. Age, gender, and BMI were matched in the three groups. Twenty-one (80.8%) of the CTEPH patients had a history of previous pulmonary embolism. Compared with IPAH, CTEPH patients had a lower 6-min walk distance and a lower mean pulmonary artery pressure. There were no other notable differences in right heart hemodynamics. The distribution of metabolic, cardiovascular, and pulmonary comorbidities was similar between CTEPH and IPAH. Liver and kidney function tests were similar. The time from diagnosis until blood sampling for the study was longer in IPAH compared to CTEPH, median (25th, 75th percentile) weeks: 168.4 (82.3, 287.1) versus 7.6 (0.4, 31.7), p < 0.001. A larger proportion of IPAH patients were on PAH-targeted therapies at the time of plasma sampling. As expected, more CTEPH patients were on anticoagulation, particularly the novel oral anticoagulants, compared to IPAH. Use of other medications such as statins, hormones, and diuretics was similar.
Table 1.
Baseline characteristics of the study population.
| Control (n = 31) | CTEPH (n = 33) | IPAH (n = 21) | p | |
|---|---|---|---|---|
| Age (years) | 52.32 ± 7.68 | 52.48 ± 13.91 | 54.71 ± 10.44 | 0.711 |
| Male gender | 20 (64.5) | 20 (60.6) | 12 (57.1) | 0.863 |
| BMI (kg/m2) | 30.23 ± 5.65 | 33.87 ± 10.24 | 32.06 ± 9.69 | 0.287 |
| White race | 26 (83.9) | 20 (60.6) | 20 (95.2) | 0.007 |
| NYHA class | 0.09 | |||
| I–II | 7 (21.2) | 10 (47.6) | ||
| III | 20 (60.6) | 6 (28.6) | ||
| IV | 6 (18.2) | 5 (23.8) | ||
| Resting SpO2 (%) | 93.64 ± 4.29 | 93.63 ± 4.00 | 0.993 | |
| 6MWD (meters) | 335.19 ± 93.96 | 410.89 ± 133.63 | 0.029 | |
| Heart rate (beats/min) | 79.44 ± 13.60 | 83.53 ± 11.55 | 0.316 | |
| Right atrial pressure (mmHg) | 11.06 ± 6.69 | 10.05 ± 5.34 | 0.571 | |
| mPAP (mmHg) | 45.84 ± 12.16 | 53.10 ± 12.79 | 0.045 | |
| PAWP (mmHg) | 11.76 ± 5.14 | 11.05 ± 8.27 | 0.726 | |
| Cardiac index (L/min/m2) | 2.42 ± 0.72 | 2.73 ± 0.75 | 0.176 | |
| PVR (Wood units) | 7.15 ± 4.20 | 7.75 ± 2.70 | 0.608 | |
| NT-proBNP (pg/mL) | 2246 ± 4623 | 640 ± 773 | 0.263 | |
| Diabetes | 6 (18.2) | 5 (23.8) | 0.878 | |
| Hypertension | 19 (57.6) | 11 (52.4) | 0.925 | |
| Dyslipidemia | 12 (36.4) | 8 (38.1) | 1 | |
| Coronary artery disease | 6 (18.2) | 2 (9.5) | 0.631 | |
| COPD | 9 (27.3) | 4 (19.0) | 0.717 | |
| Obstructive sleep apnea | 10 (30.3) | 9 (42.9) | 0.516 | |
| Hemoglobin (g/dL) | 13.92 ± 2.14 | 14.01 ± 2.22 | 0.89 | |
| Albumin (g/dL) | 3.90 ± 0.96 | 3.99 ± 0.49 | 0.691 | |
| Bilirubin (mg/dL) | 0.83 ± 0.77 | 0.81 ± 0.47 | 0.929 | |
| Alanine aminotransferase (U/L) | 27.88 ± 18.90 | 24.14 ± 11.19 | 0.417 | |
| Glucose (mg/dL) | 103.3 ± 62.08 | 98.85 ± 18.16 | 0.558 | |
| Creatinine (mg/dL) | 1.09 ± 0.38 | 1.06 ± 0.25 | 0.781 | |
| PAH-targeted therapy | 9 (27.3) | 17 (81) | <0.001 | |
| Oxygen therapy | 9 (27.3) | 5 (23.8) | 1 | |
| Warfarin | 16 (48.5) | 6 (28.6) | 0.243 | |
| Heparin | 6 (18.2) | 0 (0.0) | 0.261 | |
| Novel oral anticoagulants | 12 (36.4) | 0 (0.0) | 0.005 | |
| Statin | 8 (24.2) | 2 (9.5) | 0.318 | |
| Thyroid hormone | 4 (12.1) | 3 (14.3) | 1 | |
| Beta blocker | 6 (18.2) | 7 (33.3) | 0.346 | |
| Steroids | 4 (12.1) | 4 (19.0) | 0.76 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CTEPH: chronic thromboembolic pulmonary hypertension; IPAH: idiopathic pulmonary arterial hypertension; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; NT-proBNP: N terminal pro B-type natriuretic peptide; NYHA: New York Heart Association; SpO2: oxygen saturation by pulse oximetry; 6MWD: 6-min walk distance.
Data are presented as mean ± standard deviation.
Plasma metabolome
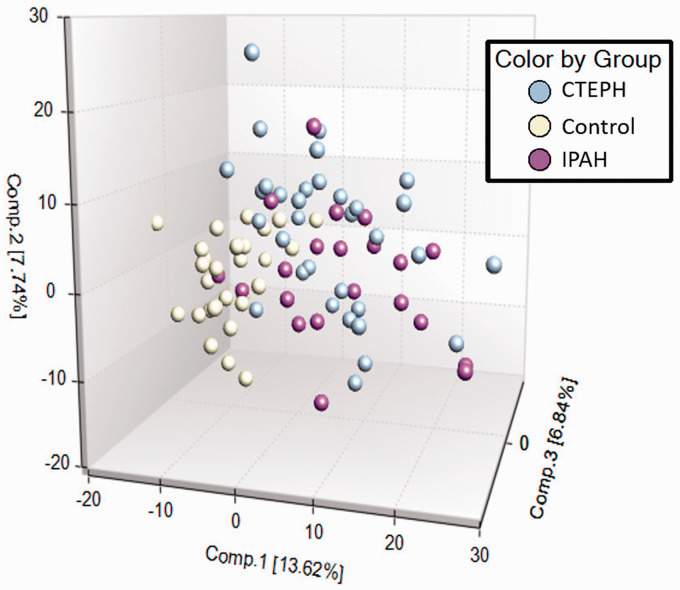
From a total of 862 metabolites identified in plasma, 362 were different in CTEPH compared with healthy controls: 178 were higher and 184 were lower. Compared with IPAH, 147 metabolites were different in CTEPH: 45 were higher and 102 were lower (e-Table 1). Principal component analysis showed separation between healthy controls and patients with CTEPH or IPAH, with overlap between the two groups of patients (Fig. 1).
Fig. 1.
Principle component analysis.
CTEPH: chronic thromboembolic pulmonary hypertension; IPAH: idiopathic pulmonary arterial hypertension.
CTEPH versus healthy controls
Random forest analysis distinguished subjects with CTEPH and healthy controls with a predictive accuracy of 89% (e-Fig. 1). Nucleotides, lipids, and amino acids emerged as metabolites that provided important separation between the groups. Pathway enrichment analysis identified the following pathways as enriched in the CTEPH patients compared to controls: amino sugar metabolism, acyl choline, long-chain fatty acid (LCFA), acylcarnitine, lysophospholipid, and phosphatidylcholine (e-Fig. 2). In the following, we describe the major findings in the super pathways lipids, amino acids, carbohydrates, and nucleosides. For full details, please refer to the complete research data file posted online.
Lipids
Plasma fatty acids were increased in CTEPH compared to controls (Table 2).The LCFAs palmitoleate and eicosenoate, and the polyunsaturated fatty acids hexadecadienoate, linolenate, and dihomo-linoleate were more than two-fold higher in CTEPH. Glycerol, an essential component of triglycerides and an indicator of whole body lipolysis, was 2.4 times higher in the CTEPH patients (e-Fig. 1).
Table 2.
Fatty acids and glycerol.
| Biochemical | CTEPH/ control | p Values | q Values |
|---|---|---|---|
| Myristate (14:0) | 1.93 | 4.28E-06 | 4.11E-05 |
| Myristoleate (14:1n5) | 1.99 | 1.47E-05 | 0.0001 |
| Pentadecanoate (15:0) | 1.43 | 0.0002 | 0.0007 |
| Palmitate (16:0) | 1.64 | 3.95E-07 | 8.25E-06 |
| Palmitoleate (16:1n7) | 2.82 | 7.78E-07 | 1.12E-05 |
| Margarate (17:0) | 1.86 | 1.31E-05 | 0.0001 |
| 10-heptadecenoate (17:1n7) | 2.29 | 1.86E-06 | 2.04E-05 |
| Stearate (18:0) | 1.36 | 8.88E-05 | 0.0004 |
| Oleate/vaccenate (18:1) | 1.89 | 3.05E-07 | 6.67E-06 |
| Nonadecanoate (19:0) | 1.32 | 0.0034 | 0.0081 |
| 10-Nonadecenoate (19:1n9) | 2.19 | 4.68E-07 | 8.93E-06 |
| Arachidate (20:0) | 1.14 | 0.1505 | 0.1516 |
| Eicosenoate (20:1) | 2.42 | 1.42E-07 | 4.01E-06 |
| Erucate (22:1n9) | 1.84 | 0.0022 | 0.006 |
| Heneicosapentaenoate (21:5n3) | 1.56 | 0.4784 | 0.3504 |
| Hexadecadienoate (16:2n6) | 2.07 | 3.41E-05 | 0.0002 |
| Stearidonate (18:4n3) | 1.86 | 0.0049 | 0.0107 |
| Eicosapentaenoate (EPA; 20:5n3) | 1.26 | 0.2509 | 0.2253 |
| Docosapentaenoate (n3 DPA; 22:5n3) | 1.91 | 0.0002 | 0.0009 |
| Docosahexaenoate (DHA; 22:6n3) | 1.07 | 0.7152 | 0.4619 |
| Linoleate (18:2n6) | 1.89 | 1.96E-06 | 2.09E-05 |
| Linolenate (alpha or gamma; (18:3n3 or 6)) | 2.27 | 2.09E-05 | 0.0001 |
| Dihomo-linolenate (20:3n3 or n6) | 1.24 | 0.029 | 0.0431 |
| Arachidonate (20:4n6) | 1.1 | 0.3469 | 0.2796 |
| Adrenate (22:4n6) | 1.88 | 3.83E-05 | 0.0002 |
| Docosapentaenoate (n6 DPA; 22:5n6) | 1.16 | 0.204 | 0.1915 |
| Docosadienoate (22:2n6) | 2.1 | 2.36E-08 | 1.76E-06 |
| Dihomo-linoleate (20:2n6) | 2.42 | 2.56E-08 | 1.76E-06 |
| Glycerol | 2.37 | 2.18E-08 | 1.76E-06 |
Data presented as fold change between CTEPH and control group.
CTEPH: chronic thromboembolic pulmonary hypertension.
Plasma acylcarnitines were increased in CTEPH (Table 3). Hydroxybutyrylcarnitine, suberoylcarnitine, and adipoylcarnitine were more than two-fold higher in CTEPH than controls. CTEPH patients also had an almost six-fold increase in 3-hydroxybutyrate compared to controls, and elevated dicarboxyl fatty acids such as adipate and sebacate (Table 4). All acyl cholines measured, such as palmitoylcholine and linoleoylcholine, were significantly lower in CTEPH compared to controls (e-Table 2).
Table 3.
Acylcarnitines.
| Biochemicals | CTEPH/ control | p Values | q Values |
|---|---|---|---|
| Acetylcarnitine (C2) | 1.73 | 8.85E-08 | 3.55E-06 |
| 3-Hydroxybutyrylcarnitine (1) | 2.95 | 3.34E-08 | 1.78E-06 |
| 3-Hydroxybutyrylcarnitine (2) | 2.11 | 4.76E-07 | 8.93E-06 |
| Hexanoylcarnitine (C6) | 1.33 | 0.0096 | 0.0178 |
| Octanoylcarnitine (C8) | 0.76 | 0.6054 | 0.4109 |
| Decanoylcarnitine (C10) | 0.74 | 0.9095 | 0.5382 |
| Cis-4-decenoylcarnitine (C10:1) | 1.05 | 0.5838 | 0.4015 |
| Laurylcarnitine (C12) | 1.01 | 0.262 | 0.231 |
| Myristoylcarnitine (C14) | 1.25 | 0.0265 | 0.0404 |
| Palmitoylcarnitine (C16) | 1.34 | 0.0023 | 0.0061 |
| Palmitoleoylcarnitine (C16:1) | 1.49 | 0.001 | 0.0031 |
| Stearoylcarnitine (C18) | 1.17 | 0.2795 | 0.2381 |
| Linoleoylcarnitine (C18:2) | 1.42 | 0.0012 | 0.0035 |
| Linolenoylcarnitine (C18:3) | 1.66 | 0.0025 | 0.0064 |
| Oleoylcarnitine (C18:1) | 1.55 | 6.13E-05 | 0.0003 |
| Myristoleoylcarnitine (C14:1) | 1.54 | 0.0055 | 0.0117 |
| Suberoylcarnitine (C8-DC) | 2.91 | 9.18E-07 | 1.26E-05 |
| Adipoylcarnitine (C6-DC) | 2.42 | 0.0006 | 0.0021 |
| 3,4-Methyleneheptanoylcarnitine | 1.9 | 0.0224 | 0.0355 |
| Arachidoylcarnitine (C20) | 0.99 | 0.7449 | 0.4747 |
| Arachidonoylcarnitine (C20:4) | 1.3 | 0.0564 | 0.0737 |
| Behenoylcarnitine (C22) | 0.71 | 0.0095 | 0.0177 |
| Dihomo-linolenoylcarnitine (C20:3n3 or 6) | 1.38 | 0.0024 | 0.0063 |
| Dihomo-linoleoylcarnitine (C20:2) | 1.51 | 0.0014 | 0.0039 |
| Eicosenoylcarnitine (C20:1) | 1.39 | 0.0032 | 0.0076 |
| Lignoceroylcarnitine (C24) | 0.8 | 0.0246 | 0.0384 |
| Margaroylcarnitine (C17) | 1.24 | 0.2508 | 0.2253 |
| Nervonoylcarnitine (C24:1) | 1.24 | 0.0177 | 0.0292 |
| Cerotoylcarnitine (C26) | 1.1 | 0.3129 | 0.2566 |
| Ximenoylcarnitine (C26:1) | 1.2 | 0.048 | 0.0645 |
Data presented as fold change between CTEPH and control group.
CTEPH: chronic thromboembolic pulmonary hypertension.
Table 4.
Dicarboxyl fatty acids and 3-hydroxybutyrate.
| Biochemicals | CTEPH/ control | p Values | q Values |
|---|---|---|---|
| Glutarate (C5-DC) | 1.33 | 0.1313 | 0.1374 |
| 2-Hydroxyglutarate | 1.34 | 0.0054 | 0.0116 |
| 4-Hydroxy-2-oxoglutaric acid | 1.28 | 0.1006 | 0.113 |
| Adipate (C6-DC) | 3.15 | 0.0068 | 0.0137 |
| 2-Hydroxyadipate | 1.12 | 0.5252 | 0.3722 |
| 3-Methyladipate | 1.54 | 0.0163 | 0.0274 |
| Maleate | 0.73 | 0.4599 | 0.3405 |
| Pimelate (C7-DC) | 1.52 | 0.0094 | 0.0176 |
| Suberate (C8-DC) | 1.76 | 0.007 | 0.014 |
| Azelate (C9-DC) | 3.54 | 0.2132 | 0.1974 |
| Sebacate (C10-DC) | 2.05 | 0.0164 | 0.0274 |
| Dodecanedioate (C12-DC) | 1.28 | 0.1741 | 0.17 |
| Tetradecanedioate (C14-DC) | 1.29 | 0.0273 | 0.0414 |
| Hexadecanedioate (C16-DC) | 1.7 | 0.0006 | 0.0022 |
| Octadecanedioate (C18-DC) | 1.44 | 0.01 | 0.0183 |
| Eicosanodioate (C20-DC) | 0.88 | 0.1394 | 0.1444 |
| Docosadioate (C22-DC) | 0.9 | 0.064 | 0.0817 |
| 3-Carboxy-4-methyl-5-propyl-2- Furanpropanoate (CMPF) | 1.32 | 0.0251 | 0.0388 |
| 3-Hydroxybutyrate (BHBA) | 5.81 | 7.80E-07 | 1.12E-05 |
Data presented as fold change between CTEPH and control group.
CTEPH: chronic thromboembolic pulmonary hypertension.
Lysophospholipids were decreased in CTEPH (Table 5). 1-palmitoylglycerol 3-phosphate and 2-palmitoylglycerophosphocholine levels in CTEPH were almost half of those in controls.
Table 5.
Lysophospholipids.
| Biochemicals | CTEPH/ control | p Values | q Values |
|---|---|---|---|
| 1-Palmitoyl-GPA (16:0) | 0.52 | 0.0012 | 0.0035 |
| 1-Linoleoyl-GPA (18:2) | 0.61 | 0.0005 | 0.0018 |
| 1-Arachidonoyl-GPA (20:4) | 0.78 | 0.0762 | 0.0915 |
| 1-Palmitoyl-GPC (16:0) | 0.71 | 1.31E-07 | 3.93E-06 |
| 2-Palmitoyl-GPC (16:0) | 0.67 | 1.50E-07 | 4.01E-06 |
| 1-Palmitoleoyl-GPC (16:1) | 0.73 | 0.0004 | 0.0016 |
| 2-Palmitoleoyl-GPC (16:1) | 0.57 | 0.0016 | 0.0045 |
| 1-Stearoyl-GPC (18:0) | 0.67 | 1.87E-07 | 4.73E-06 |
| 1-Oleoyl-GPC (18:1) | 0.74 | 7.10E-06 | 6.09E-05 |
| 1-Linoleoyl-GPC (18:2) | 0.7 | 1.47E-05 | 0.0001 |
| 1-Linolenoyl-GPC (18:3) | 0.61 | 1.76E-05 | 0.0001 |
| 1-Arachidonoyl-GPC (20:4n6) | 0.8 | 0.0044 | 0.01 |
| 1-Lignoceroyl-GPC (24:0) | 0.65 | 2.97E-05 | 0.0002 |
| 1-Palmitoyl-GPE (16:0) | 0.72 | 0.0003 | 0.0013 |
| 1-Stearoyl-GPE (18:0) | 0.69 | 1.70E-05 | 0.0001 |
| 2-Stearoyl-GPE (18:0) | 0.66 | 1.49E-06 | 1.80E-05 |
| 1-Oleoyl-GPE (18:1) | 0.72 | 0.0007 | 0.0024 |
| 1-Linoleoyl-GPE (18:2) | 0.78 | 0.0065 | 0.0133 |
| 1-Arachidonoyl-GPE (20:4n6) | 1.05 | 0.9128 | 0.5395 |
| 1-Stearoyl-GPS (18:0) | 1.49 | 0.5163 | 0.3676 |
| 1-Palmitoyl-GPG (16:0) | 1 | 0.8944 | 0.5345 |
| 1-Stearoyl-GPG (18:0) | 0.66 | 0.0726 | 0.0889 |
| 1-Oleoyl-GPG (18:1) | 0.88 | 0.1906 | 0.1826 |
| 1-Linoleoyl-GPG (18:2) | 1.02 | 0.983 | 0.5574 |
| 1-Palmitoyl-GPI (16:0) | 0.68 | 0.0117 | 0.0209 |
| 1-Stearoyl-GPI (18:0) | 0.65 | 4.88E-05 | 0.0003 |
| 1-Oleoyl-GPI (18:1) | 0.6 | 0.0003 | 0.0014 |
| 1-Linoleoyl-GPI (18:2) | 0.85 | 0.1267 | 0.1341 |
| 1-Arachidonoyl-GPI (20:4) | 0.83 | 0.0511 | 0.0674 |
Data presented as fold change between CTEPH and control group.
CTEPH: chronic thromboembolic pulmonary hypertension; GPA: glycero-3-phosphate; GPC: glycero-3-phosphocholine; GPE: glycero-3-phosphoethanolamine; GPI: glycero-3-phospho-D-myo-inositol.
Amino acids
Several modified amino acids were elevated in CTEPH, including 3-methylhistidine (2.84-fold, p = 0.0083, q = 0.016), N-acetyl-3-methylhistidine (4.72-fold, p < 0.0001, q = 0.0002), and N2,N5-diacetylornithine (2.7-fold, p = 0.0002, q = 0.0009), as well as the acetylated peptides phenylacetylglutamate (2.34-fold, p = 0.048, q = 0.06) and 4-hydroxyphenylacetylglutamine (2.36-fold, p = 0.0151, q = 0.0258). Related to methionine metabolism, CTEPH patients showed a 2.3-fold higher S-adenosylhomocysteine (SAH) level compared to controls (p = 0.008, q = 0.02). Several amino acids in the arginine pathway were abnormal in CTEPH, with N2,N5-diacetylornithine being 2.7-fold higher in CTEPH versus controls (p = 0.0002, q = 0.0009). Arginine (0.77-fold) and homoarginine (0.66-fold) were low, and dimethylarginine was 1.2-fold higher in CTEPH (all p < 0.05 and q < 0.05)
Carbohydrates
There were no consistent differences in metabolites related to glycolysis, gluconeogenesis, pyruvate, pentose, or glycogen metabolism. The only notable difference in carbohydrates was that amino sugars were elevated in CTEPH compared to controls, including N-acetylglucosaminylasparagine, which was 2.11-fold higher (p = 0.01, q = 0.02).
Nucleosides
Several purine and pyrimidine nucleosides were elevated in CTEPH patients, including xanthosine (2.24-fold, p = 0.004, q = 0.01), N6-succinyladenosine (2-fold, p < 0.0001, q = 0.0004), orotidine (4.6-fold, p < 0.0001, q = 0.0003), and N4-acetylcytidine (2.86-fold, p < 0.0001, q < 0.0001).
CTEPH versus IPAH
From a total of 862 metabolites detected in plasma, 147 were significantly different between CTEPH and IPAH subjects: 102 metabolites were lower in CTEPH and 45 were higher (e-Table 1). Random forest analysis distinguished subjects with CTEPH from IPAH with a predictive accuracy of 80% and suggested key differences in lipids and amino acids (e-Fig. 3). Pathway enrichment analysis identified differences in polyunsaturated fatty acids, acyl cholines, and lysophospholipids (e-Fig. 4). Several polyunsaturated fatty acids were higher in CTEPH compared to IPAH, with stearidonate being 2.17-fold higher (p = 0.006, q = 0.08). Glycerol was 1.6-fold higher in CTEPH (p = 0.002, q = 0.06). Conversely, most acyl cholines and lysophospholipids were lower in CTEPH, most notably oleoylcholine (0.64-fold, p = 0.006, q = 0.08) and 1-palmitoylglycerol 3-phosphate (0.33-fold, p = 0.0001, q = 0.03) and 1-linoleoyl glycerol 3-phosphate (0.42-fold, p < 0.0001, q = 0.009). Regarding amino acids, CTEPH patients had lower levels of ornithine (0.81-fold, p = 0.0003, q = 0.05) and dimethylarginine (0.83-fold, p = 0.004, q = 0.076).
There were several similarities in the metabolomic profile of CTEPH and IPAH patients. Specifically, both groups showed increased fatty acids, acyl carnitines and beta-hydroxybutyrate (β-HB), increased modified amino acids, peptides and nucleosides, increased aminosugars, and increased polyamines.
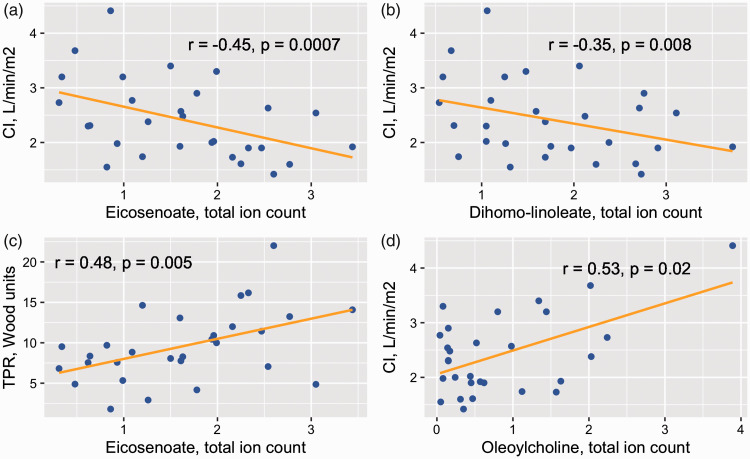
CTEPH clinical correlations
Fig. 2 and e-Table 3 show associations between selected biochemicals and cardiac index and TPR. The LCFAs eicosenoate and dihomo-linoleate correlated negatively with cardiac index and positively with TPR. The acyl cholines palmitoylcholine and oleoylcholine had a positive correlation with cardiac index. These correlations were not observed in the IPAH patients.
Fig. 2.
In CTEPH, the long-chain fatty acids, eicosenoate and dihomo-linoleate, are associated with cardiac index (a and b). Eicosenoate also correlates with total pulmonary resistance (c). The acyl choline oleoylcholine is associated with cardiac index (d). r are the Pearson correlation coefficients. p Values are from a multivariable linear regression model adjusting for age, gender, body mass index, statin use, thyroid replacement therapy, steroids, and diabetes drug therapy.
CTEPH: chronic thromboembolic pulmonary hypertension; CI: cardiac index; TPR: total pulmonary resistance.
Twenty-six of the 33 CTEPH patients (78.8%) underwent PEA. Two patients decided not to pursue surgery, two had comorbidities that increased surgical risk (pulmonary aspergillosis and obstructive lung disease), and three patients did not come back for follow up. We measured the plasma metabolome in five CTEPH patients a median of 6 months (range 3–6 months) after PEA. We found that glycerol and the fatty acids laurate and pimelate decreased significantly after surgery (e-Fig. 5).
Quantitation of fatty acids via HPLC LC/MS/MS
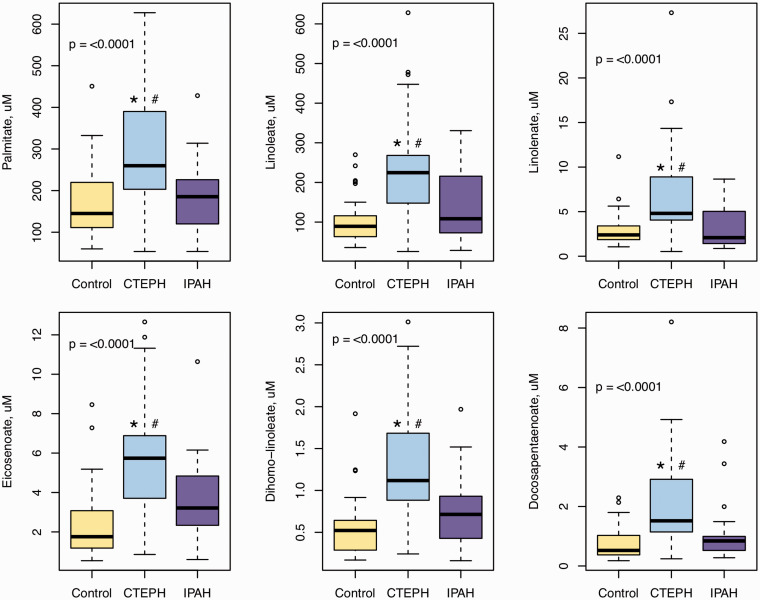
To confirm some of the key findings of the metabolomics analysis, we quantitated long-chain free fatty acids via HPLC (Fig. 3 and e-Table 4). Eighteen saturated and unsaturated fatty acids ranging from 16 to 22 carbons were measured. All were significantly higher in CTEPH compared to healthy controls, except for stearidonate (18:4), eicosanoate (20:0), eicosapentaenoate (20:5), and docosanoate (22:0) (e-Table 3). Palmitate (16:0), linoleate (18:2), gamma-Linolenate (18:3), eicosenoate (20:1), dihomo-linoleate (20:2), and docosapentaenoate (22:5) were also significantly elevated in CTEPH compared to IPAH after adjusting for multiple comparisons (Fig. 3). We also confirmed the correlations between eicosenoate (20:1) and cardiac index (r = –0.44, p = 0.004) and TPR (r = 0.43, p = 0.009), as well as between dihomo-linoleate (20:2) and cardiac index (r = –0.37, p = 0.006), after adjusting for several confounders.
Fig. 3.
Plasma fatty acids measured by high-performance liquid chromatography (HPLC) Online Tandem Mass Spectrometry (LC/MS/MS). Data are box plots with median, 25th and 75th quartile (bottom and top of the box), and minimal and maximal distribution (whiskers). p Value from ANOVA.
*p < .0001 for the CTEPH vs control comparison; #p < 0.01 for the CTEPH vs IPAH comparison; both from Tukey test for multiple comparisons adjustment.
CTEPH: chronic thromboembolic pulmonary hypertension; IPAH: idiopathic pulmonary arterial hypertension.
Discussion
There is reason to believe that metabolic dysregulation is part of the underlying pathophysiology in patients with CTEPH. Herein, we used a global untargeted metabolomics approach to characterize the metabolic phenotype of these patients. We found elevated fatty acids, acyl carnitines, glycerol, and β-HB; while acyl cholines and lysophospholipids were decreased. These changes were observed when compared to both healthy controls and, in the case of fatty acids, glycerol, acyl cholines, and lysophospholipids, also compared to IPAH patients. CTEPH participants also had increased amino sugars and modified nucleosides, as well as increased modified amino acids, changes that were similar to IPAH patients.
Lipids
The observed metabolomic profile in CTEPH suggests altered lipid metabolism in multiple tissues and organs. The elevated plasma levels of fatty acids, glycerol, and acyl carnitines are evidence of increased lipolysis and fatty acid oxidation in CTEPH. The markedly elevated plasma β-HB suggests that the liver is a major site of the increased fatty acid oxidation. β-HB is a ketone produced by the liver during states of elevated fatty acid oxidation or lipid overload. Ketones produced by the liver are released into the circulation and are subsequently oxidized by peripheral tissues, like the heart and skeletal muscle. Elevated ketones in the context of CTEPH is intriguing, as right heart failure ensues with disease progression and ketones represent a more energetically favorable substrate for the heart. It is plausible that elevating ketones is a short-term compensation for declining heart function 23 ; however, this response may create long-term unfavorable adaptations. 24 Other organs, such as the pulmonary vasculature, skeletal muscle, and the right ventricle, may contribute to fatty acid oxidation.
Recent research by our group and others demonstrates dysregulated glucose and insulin metabolism in IPAH6,7,25,26 and CTEPH.11,12 The metabolomic data generated in this report corroborate these findings, evidencing both elevated lipids (a well-known contributor to peripheral insulin resistance) and elevated dicarboxyl fatty acids. Dicarboxyl fatty acids can be generated by a secondary mechanism (ω-oxidation, suggesting lipid availability beyond the rate of tissue-level beta-oxidation) or through lipid peroxidation. The latter mechanism is consistent with the low-grade chronic inflammation and elevated lipid peroxidation that is observed in the insulin-resistant state previously noted in pulmonary vascular disease.27,28 Skeletal muscle, as the primary tissue responsible for peripheral insulin resistance, is likely to be involved in the observed dysregulated nutrient metabolism.
A similar metabolomic signature was observed in lung tissue from PAH patients. 15 Increased fatty acid oxidation in the right heart has been reported in a rat model of right ventricular hypertrophy induced by pulmonary artery banding, 29 but heritable PAH murine models are suggestive of impaired fatty acid oxidation.30,31 Human data point toward decreased fatty acid oxidation in the right ventricle. 30 Plasma evidence of increased lipolysis and fatty acid oxidation has been previously documented in IPAH,16,30,32 and interestingly, in acute pulmonary embolism. 33 Our study is the first to report metabolomic data in CTEPH.
Acyl cholines were significantly lower in CTEPH compared to healthy controls. The metabolic role of acyl cholines is poorly understood. Zeleznik et al. also found lower acyl cholines in patients with intermediate risk acute pulmonary embolism compared to high-risk patients. 33 Further investigation into these metabolic abnormalities is warranted.
Lysophospholipids were decreased in CTEPH. Phospholipids are major components of lung surfactant. Phospholipids decrease in bronchoalveolar lavage fluid of patients 10 days after pulmonary embolism. 34 Low plasma phospholipids have also been documented in a pulmonary embolism model in pigs. 35 Moreover, antiphospholipid antibodies are known risk factors for CTEPH. 36 Recent evidence suggests that these antibodies need to be taken up by cells to effect the pro-thrombotic response, 37 perhaps decreasing the release of phospholipids into the circulation. In our cohort, four patients (12%) had a known diagnosis of the antiphospholipid syndrome, but other antibodies, not currently measured clinically, are being recognized as risk factors for thrombosis. 38 Phospholipids are also the metabolic source for eicosanoids such as prostacyclin, 39 an important molecule for pulmonary vascular health. Reduced lysophospholipid levels have been associated with enhanced hypoxia-induced pulmonary vascular remodeling and endothelin-1 upregulation in a mouse model. 40 Taken together, these data suggest a prominent role for dysregulated phospholipid metabolism in CTEPH.
Other metabolites
CTEPH patients demonstrated dysregulated methionine metabolism with increased SAH. The latter is associated with cardiovascular disease, 41 venous thrombosis, 42 and decreased phosphatidylcholine synthesis.43,44 Arginine levels were low and dimethylarginine levels were high in CTEPH compared to controls, suggesting decrease nitric oxide (NO) activity and supportive of the role of enhancing the NO pathway in CTEPH. IPAH patients had the same abnormalities compared to controls, but higher dimethylarginine levels compared to CTEPH. This suggests that the NO pathway may more impaired in IPAH. This may be related to the fact that this is an operable CTEPH cohort dominated by large vessel thrombofibrotic obstruction and not microscopic vasculopathy. We speculate that the NO abnormalities in inoperable CTEPH patients might resemble more closely IPAH.
The increased amino sugars are a novel observation in CTEPH. Our group has previously reported increased O-linked β-N-acetylglucosamine modification in IPAH that drives pulmonary artery smooth muscle cell proliferation. 45 In the present study, amino sugars were similarly elevated in CTEPH and IPAH.
We found elevated levels of methylated and acetylated amino acids and peptides in CTEPH, as well as increased modified nucleosides. Epigenetic modulation, specifically DNA methylation and histone acetylation and methylation, plays an important role in the initiation and progression of pulmonary hypertension. 46 Modified nucleosides can be released from transfer RNAs (tRNAs), 47 and detected in the circulation given how abundant tRNAs are in human cells. 48 Increased circulating levels of modified nucleosides probably reflect increased cell turnover or stress. 48 This would be consistent with hyperproliferation of pulmonary vascular cells, and has been observed previously in IPAH. 16 Post-translational modification can also occur at the protein level. Dysregulation of protein acetylation has been associated with cancer, neurodegenerative disease, and cardiovascular disorders. 49
Clinical correlations and CTEPH versus IPAH comparison
CTEPH and IPAH share many similarities, particularly the elevation in pulmonary vascular resistance and right ventricular afterload that is responsible for right heart failure and premature death. However, a striking difference is that CTEPH is initiated by one or more episodes of pulmonary embolism, and that PEA surgery can be curative to many patients. 50 In this context, our study found many similarities in the plasma metabolomics signature of IPAH and CTEPH, including increased fatty acids, acyl carnitines, and β-HB, increased modified amino acids, peptides and nucleosides, increased aminosugars, and increased polyamines. These findings are consistent with dysregulated insulin metabolism and cell proliferation, likely common processes in CTEPH and IPAH, perhaps related to right ventricular dysfunction. However, abnormalities in lipid metabolism were more prominent in CTEPH than IPAH. Increased lipolysis and fatty acid oxidation were associated with the degree of hemodynamic compromise in CTEPH, but not in IPAH. The extent of lipolysis was greater in CTEPH than IPAH, and it decreased after PEA surgery. Acyl cholines and lysophospholipids were lower in CTEPH compared to IPAH, and low oleoylcholine levels were associated with lower cardiac index in CTEPH. As lipid signaling plays an important role in thrombosis,51,52 our data suggest that dysregulated fatty acid and phospholipid metabolism are important in the pathobiology of CTEPH.
Limitations
This is a cross sectional study of a relatively small sample size. As such, we cannot rule out the possibility that some differences in the metabolomic profiles are due to shorter duration of disease in CTEPH. For example, it is possible that an earlier identified cohort of IPAH patients would have had correlations between fatty acids and carnitines and right heart hemodynamics. Our study does not allow to conclude firmly the tissue sources of abnormal metabolic pathways, as the plasma metabolomic profile provides a global representation of body metabolism. Finally, we cannot rule out the effect of medications, including PH-targeted therapies.
In conclusion, the plasma global metabolomic profile of CTEPH is characterized by increased lipolysis, fatty acid oxidation, and ketogenesis; decreased acyl cholines and lysophospholipids; elevated amino sugars and modified nucleosides; and altered amino acid metabolism. These data are consistent with alterations in metabolic pathways at many tissue levels, including lungs, heart, liver, adipose tissue, and skeletal muscle. While some changes are common to IPAH and likely related to pulmonary hypertension and right heart failure, several lipid abnormalities are unique to CTEPH. These findings open novel research avenues to investigate the pathogenic and therapeutic implications of modulating lipid metabolism in pulmonary thromboembolic disease.
Supplemental Material
Supplemental material, PUL890553 Supplemental material for Plasma metabolomic profile in chronic thromboembolic pulmonary hypertension by Gustavo A. Heresi, Jacob T. Mey, John R. Bartholomew, Ihab S. Haddadin, Adriano R. Tonelli, Raed A. Dweik, John P. Kirwan and Satish C. Kalhan in Pulmonary Circulation
Author contributions
GAH, ART, and RAD designed the study and collected data. GAH and SCK drafted the initial version of the manuscript. GAH, JTM, JRB, ISH, ART, RAD, and SCK contributed substantially to data analysis and interpretation, and the writing and review of the manuscript.
Conflict of interest
GAH has received fees from Bayer Healthcare for Advisory Boards and Speaker’s Bureau. JTM, JRB, ISH, ART, and RAD: None. SCK is a member of the Biochemistry advisory board of Metabolon Inc. In this capacity, he attends the meetings of the Board and receives financial and stock compensation.
Funding
This work is supported by grants NHLBI K23HL125697 (for GAH) and R01HL130209 (for RAD).
Guarantor statement
GAH and SCK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993; 103: 685–692. [DOI] [PubMed] [Google Scholar]
- 2.Dorfmuller P, Gunther S, Ghigna MR, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 2014; 44: 1275–1288. [DOI] [PubMed] [Google Scholar]
- 3.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. [DOI] [PubMed] [Google Scholar]
- 4.Barnes J, Dweik RA. Is pulmonary hypertension a metabolic disease? Am J Respir Crit Care Med 2014; 190: 973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014; 115: 148–164. [DOI] [PubMed] [Google Scholar]
- 6.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heresi GA, Malin SK, Barnes JW, et al. Abnormal glucose metabolism and high-energy expenditure in idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc 2017; 14: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heresi GA, Aytekin M, Newman J, et al. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CM, McCully RB, Murphy JG, et al. Usefulness of high-density lipoprotein cholesterol to predict survival in pulmonary arterial hypertension. Am J Cardiol 2016; 118: 292–297. [DOI] [PubMed] [Google Scholar]
- 11.Richter MJ, Milger K, Haase S, et al. The clinical significance of hba1c in operable chronic thromboembolic pulmonary hypertension. PLoS One 2016; 11: e0152580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khirfan G, Tejwani V, Wang X, et al. Plasma levels of high density lipoprotein cholesterol and outcomes in chronic thromboembolic pulmonary hypertension. PLoS One 2018; 13: e0197700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kan M, Shumyatcher M, Himes BE. Using omics approaches to understand pulmonary diseases. Respir Res 2017; 18: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comhair SA, McDunn J, Bennett C, et al. Metabolomic endotype of asthma. J Immunol 2015; 195: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Peng J, Lu C, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One 2014; 9: e88727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes CJ, Ghataorhe P, Wharton J, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017; 135: 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: D92–D99. [DOI] [PubMed] [Google Scholar]
- 18.Galie N, Humbert M, Vachiery JL, et al. 2015 esc/ers guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (esc) and the European respiratory society (ers): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 19.Evans AM, DeHaven CD, Barrett T, et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009; 81: 6656–6667. [DOI] [PubMed] [Google Scholar]
- 20.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 2003; 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wulff J, Mitchell M. A comparison of various normalization methods for lc/ms metabolomics data. Adv Biosci Biotechnol 2018; 9: 339–351. [Google Scholar]
- 22.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011; 60: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedi KC, Jr., Snyder NW, Brandimarto J, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016; 133: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wentz AE, d’Avignon DA, Weber ML, et al. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 2010; 285: 24447–24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West J, Niswender KD, Johnson JA, et al. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 2013; 41: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007; 115: 1275–1284. [DOI] [PubMed] [Google Scholar]
- 27.Heresi GA, Aytekin M, Hammel JP, et al. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 2014; 43: 912–914. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Ruffenach G, Umar S, et al. Role of oxidized lipids in pulmonary arterial hypertension. Pulm Circ 2016; 6: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang YH, Piao L, Hong Z, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med (Berl) 2012; 90: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talati MH, Brittain EL, Fessel JP, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 2016; 194: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo N, Craig D, Ilkayeva O, et al. Plasma acylcarnitines are associated with pulmonary hypertension. Pulm Circ 2017; 7: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeleznik OA, Poole EM, Lindstrom S, et al. Metabolomic analysis of 92 pulmonary embolism patients from a nested case-control study identifies metabolites associated with adverse clinical outcomes. J Thromb Haemost 2018; 16: 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakos G, Kitsiouli EI, Lekka ME. Bronchoalveolar lavage alterations in pulmonary embolism. Am J Respir Crit Care Med 1998; 158: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 35.Bujak R, Garcia-Alvarez A, Ruperez FJ, et al. Metabolomics reveals metabolite changes in acute pulmonary embolism. J Proteome Res 2014; 13: 805–816. [DOI] [PubMed] [Google Scholar]
- 36.Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009; 33: 325–331. [DOI] [PubMed] [Google Scholar]
- 37.Brandt KJ, Fickentscher C, Boehlen F, et al. Nf-kappab is activated from endosomal compartments in antiphospholipid antibodies-treated human monocytes. J Thromb Haemost 2014; 12: 779–791. [DOI] [PubMed] [Google Scholar]
- 38.de Groot PG, de Laat B. Mechanisms of thrombosis in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol 2017; 31: 334–341. [DOI] [PubMed] [Google Scholar]
- 39.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol 2008; 9: 162–176. [DOI] [PubMed] [Google Scholar]
- 40.Cheng HY, Dong A, Panchatcharam M, et al. Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling. Arterioscler Thromb Vasc Biol 2012; 32: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerins DM, Koury MJ, Capdevila A, et al. Plasma s-adenosylhomocysteine is a more sensitive indicator of cardiovascular disease than plasma homocysteine. Am J Clin Nutr 2001; 74: 723–729. [DOI] [PubMed] [Google Scholar]
- 42.Bharatkumar VP, Rudreshkumar KJ, Nagaraja D, et al. Plasma s-adenosylhomocysteine: a potential risk marker for cerebral venous thrombosis. Clin Chim Acta 2016; 458: 44–48. [DOI] [PubMed] [Google Scholar]
- 43.Selley ML. A metabolic link between s-adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer’s disease. Neurobiol Aging 2007; 28: 1834–1839. [DOI] [PubMed] [Google Scholar]
- 44.Dushianthan A, Cusack R, Grocott MPW, et al. Abnormal liver phosphatidylcholine synthesis revealed in patients with acute respiratory distress syndrome. J Lipid Res 2018; 59: 1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes JW, Tian L, Heresi GA, et al. O-linked beta-n-acetylglucosamine transferase directs cell proliferation in idiopathic pulmonary arterial hypertension. Circulation 2015; 131: 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng X, Wang Y, Du L. Epigenetic modulation in the initiation and progression of pulmonary hypertension. Hypertension 2019; 74: 733–739. [DOI] [PubMed] [Google Scholar]
- 47.Kirchner S, Ignatova Z. Emerging roles of trna in adaptive translation, signalling dynamics and disease. Nat Rev Genet 2015; 16: 98–112. [DOI] [PubMed] [Google Scholar]
- 48.Pan T. Modifications and functional genomics of human transfer RNA. Cell Res 2018; 28: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drazic A, Myklebust LM, Ree R, et al. The world of protein acetylation. Biochim Biophys Acta 2016; 1864: 1372–1401. [DOI] [PubMed] [Google Scholar]
- 50.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knapp HR. Dietary fatty acids in human thrombosis and hemostasis. Am J Clin Nutr 1997; 65: 1687S–1698S. [DOI] [PubMed] [Google Scholar]
- 52.Vardon Bounes F, Mujalli A, Cenac C, et al. The importance of blood platelet lipid signaling in thrombosis and in sepsis. Adv Biol Regul 2018; 67: 66–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL890553 Supplemental material for Plasma metabolomic profile in chronic thromboembolic pulmonary hypertension by Gustavo A. Heresi, Jacob T. Mey, John R. Bartholomew, Ihab S. Haddadin, Adriano R. Tonelli, Raed A. Dweik, John P. Kirwan and Satish C. Kalhan in Pulmonary Circulation