Visual Abstract
Key Words: cardiovascular regenerative medicine, endogenous tissue regeneration, in situ tissue engineering, supramolecular polymer, tissue-engineered heart valve
Abbreviations and Acronyms: B-GLAP, bone gamma-carboxyglutamate; BMMNC, bone marrow mononuclear cells; BVG, bioresorbable vascular graft; CXCL12, stromal cell-derived factor-1α (SDF1α); ECM, extracellular matrix; IL, interleukin; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; PC-BU, polycarbonate bisurea; SMA, smooth muscle actin; TEE, transesophageal echocardiography; TEHV, tissue-engineered heart valve; TGF, transforming growth factor; TVR, transcatheter valve replacement
Highlights
-
•
Bone marrow mononuclear cell pre-seeding of polycarbonate bisurea–based tissue-engineered heart valves has detrimental effects on long-term performance and in situ remodeling and, therefore, should be avoided.
-
•
Leaflet-specific analysis revealed pronounced remodeling differences with regard to cell infiltration, scaffold resorption, and extracellular matrix deposition within the same valve explant.
-
•
The heterogeneity in remodeling of polycarbonate bisurea–based tissue-engineered heart valves may have important safety implications in terms of clinical translation.
-
•
An in-depth understanding of the mechanobiological mechanisms involved in the in situ remodeling is required to limit the risk of unpredictable (maladaptive) remodeling.
Summary
This study showed that bone marrow mononuclear cell pre-seeding had detrimental effects on functionality and in situ remodeling of bioresorbable bisurea-modified polycarbonate (PC-BU)-based tissue-engineered heart valves (TEHVs) used as transcatheter pulmonary valve replacement in sheep. We also showed heterogeneous valve and leaflet remodeling, which affects PC-BU TEHV safety, challenging their potential for clinical translation. We suggest that bone marrow mononuclear cell pre-seeding should not be used in combination with PC-BU TEHVs. A better understanding of cell–scaffold interaction and in situ remodeling processes is needed to improve transcatheter valve design and polymer absorption rates for a safe and clinically relevant translation of this approach.
Transcatheter heart valve replacement (TVR) approaches were initially introduced to treat patients considered ineligible or at too high risk for surgical valve replacement (1). Currently, TVR techniques are being extended successfully to cohorts of lower risk patients (1, 2, 3). Valve prostheses compatible with TVR techniques are, however, still based on glutaraldehyde-fixed xenogeneic materials prone to calcification and degeneration, causing a high incidence of re-operations (4). Thus, novel TVR-compatible prostheses that remain functional throughout the patient’s life are needed.
We recently proposed the use of bioresorbable polymers for the development of “off-the-shelf” available tissue-engineered heart valves (TEHVs) with in situ remodeling potential (5). In situ tissue engineering involves implanting a cell-free scaffold that should promote host cell adhesion and infiltration, and induce tissue formation by gradual substitution of the bioresorbable prosthesis by a living, native-like tissue (6). We previously showed that TEHVs based on the bioresorbable polymer bisurea-modified polycarbonate (PC-BU) retained functionality for up to 1 year in sheep when surgically implanted in a pulmonary position (5). The implanted valves exhibited gradual host cell repopulation while the polymer was partially replaced by newly synthetized extracellular matrix (ECM). This approach is suited for clinical translation due to the off-the-shelf availability of the valves, low production cost, scalability, and reduced logistical complexity. Recently, a pulmonary valve conduit based on this concept showed acceptable preclinical safety and performance as well as cell infiltration and neo-tissue formation, with partial degradation of the polymer over the 12 months’ follow-up (7); this conduit has advanced into a clinical pilot trial in children (NCT02700100, NCT03022708, and NCT03405636).
The in situ approach strongly relies, however, on the regenerative cascade induced by the inflammatory response that occurs after valve implantation (6,8). To be able to control the host’s response, bone marrow mononuclear cell (BMMNC) pre-seeding has been validated on bioresorbable vascular grafts (BVGs) in both preclinical (9,10) and clinical (11,12) settings. BVGs pre-seeded with BMMNCs exhibited accelerated cellular infiltration and better remodeling toward native-like tissue architecture in a cytokine-mediated process in various animal models (9,10,13). The cytokine monocyte chemoattractant protein-1 (MCP-1), which can be secreted by BMMNCs, has been shown to influence macrophage infiltration and favor remodeling toward a well-organized vascular tissue (10,13,14). Based on these findings, we hypothesized that pre-seeding PC-BU transcatheter TEHVs with BMMNCs would lead to a favorable remodeling response similar to the one observed in vascular grafts in small (10,13,15) and large (9,16) animal models. In addition, we previously showed that pre-seeding of BMMNC onto biodegradable polymeric scaffolds (based on polyglycolic acid) is feasible and compatible with transcatheter pulmonary (17) and aortic (18, 19, 20) valve replacement techniques.
To test this hypothesis, transcatheter TEHVs based on the supramolecular polymer PC-BU were seeded with BMMNCs within a one-step intervention (17, 18, 19, 20) and implanted as pulmonary TVR in a translational sheep model for chronic in vivo evaluation. In addition to assessing the overall feasibility and in vivo performance of such valves for up to 6 months, nonseeded TEHVs from a previous report (5) (and that were produced under the same conditions) served as control in the current study. To investigate the impact of BMMNC pre-seeding on valve remodeling, a multimodal explant analysis was performed, including a novel and in-depth individual leaflet evaluation for the explanted valves using quantitative and semi-quantitative gene expression and histological analyses.
Methods
Study design
Within this study, 16 TEHVs were manufactured by electrospinning using the supramolecular polymer PC-BU (Supplemental Materials 1.1). The resulting mesh was then sutured onto a nitinol stent. In vitro TEHV functionality was confirmed by using a pulse duplicator system followed by a durability test (n = 2) (Supplemental Materials 1.2).
After in vitro BMMNC characterization via flow cytometry (Supplemental Materials 1.3, Supplemental Table 1), 8 TEHVs were pre-seeded with 1 × 106 cells/cm2 autologous BMMNCs (Supplemental Materials 1.3) before pulmonary TVR in sheep (identified as BM-TEHV_1 to BM-TEHV_8) (Supplemental Table 2). The following time points were considered: 4 h (n = 2) to prove principal safety and feasibility, and 4 weeks (n = 2) and 24 weeks (n = 4) to assess valve functionality and remodeling potential. Nonseeded TEHVs (n = 6; TEHV_1 to TEHV_6), which were from a previous report (5) and that were produced under the same conditions, served as a control group for this study with identical time points: 4 h (n = 1), 4 weeks (n = 2), and 24 weeks (n = 3).
In vivo and ex vivo analyses were performed for all pre-seeded and control explants to assess the differences in valve remodeling via quantitative and semi-quantitative analyses (as described later).
In vivo study in sheep
Transcatheter pulmonary valve replacement
The study was approved by the local ethics committee (Veterinäramt, Gesundheitsdirektion, Kanton Zürich ZH_09_2014) and was conducted in conformity with the EU Directive 2010/63/EU for animal experiments and the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (publication no. 8023). All animals received humane care.
Transapical transcatheter pulmonary valve replacement was performed as previously described (21) and detailed in Supplemental Materials 1.4. In short, following a right-sided thoracotomy, the right cardiac ventricle was exposed, the apex was punctured, and a guidewire inserted. After crimping a diameter of 28 mm to a diameter of 10 mm, the TEHV was loaded into a custom-made, pressure-based delivery system (Carag AG, Baar, Switzerland) and positioned into the pulmonary root under fluoroscopic control.
In vivo valve functionality assessment
Transesophageal echocardiography (TEE; iE33W xMATRIX Ultrasound, Philips Healthcare, Amsterdam, the Netherlands) was used post-operatively and at 4, 12, and 24 weeks’ follow-up to verify TEHV functionality. Valve regurgitation was graded as none, trivial, mild, moderate, or severe. Invasive catheter-based pressure measurements were performed before animal euthanasia in the right ventricular outflow tract and pulmonary artery to assess the presence of stenosis (n = 8).
Explant evaluation
At the end of the planned follow-up periods (n = 11) or in case of pre-term experiment termination (n = 3) (Supplemental Table 2), the animals were euthanized. Following gross explant evaluation, longitudinally cut representative samples of every TEHV leaflet were fixed in 4% formalin, embedded in paraffin, and sliced into 2- to 5-μm sections for (immuno)histological and immunofluorescence analyses (Supplemental Materials 1.5). Tissue samples of the explanted valve leaflets were snap-frozen in liquid nitrogen and stored at –80°C for gene expression analysis of chemoattractant, anti-inflammatory and pro-inflammatory, calcification, and ECM-related genes (Supplemental Materials 1.6, Supplemental Table 3).
Assessment of TEHV leaflet dimensions
For each leaflet of the valves explanted after 18 and 24 weeks (Supplemental Table 2), valve thickness and leaflet length were measured by using Pannoramic Viewer software (3DHistech, Ltd., Budapest, Hungary). For each leaflet, thickness measures were obtained from 3 distinct histological sections and in 4 locations per section: lower wall, leaflet base, leaflet middle region (leaflet mid), and leaflet tip. For each location, a total of 12 measures were obtained. The results were then compared versus the average scaffold thickness at the time of implantation (406 ± 41 μm; n = 10).
Possible changes in leaflet length were measured from the histological sections (n = 3) of each leaflet by calculating the difference in percentage between the length of the visible polymeric scaffold in the leaflet, shown as an approximation of the initial leaflet length at implantation, and the actual leaflet length upon remodeling. Changes in leaflet length were not compared between the different leaflets.
Semiquantitative evaluation of histology
For each leaflet of the valves explanted after 18 and 24 weeks (Supplemental Table 2), 2 independent observers performed a semi-quantitative evaluation of the histology by grading different remodeling (neointimal growth, scaffold remnants, elastogenesis, calcifications, and vascularization) and phenotype (endothelial cells, contractile cells, interstitial cells, and macrophages) characteristics of the explants from 0 (no expression) to 5 (high expression). For each valve leaflet, the semi-quantification was performed in 4 locations per section: lower wall, leaflet base, leaflet middle region (leaflet mid), and leaflet tip. The average value obtained from the 2 evaluators was then reported for each leaflet location.
Statistical analysis
Data in the text are represented as mean ± SD, unless stated otherwise. Leaflet thickness measures (n = 12) were evaluated as repeated measures 2-way analysis of variance, with Greenhouse-Geisser sphericity correction followed by Tukey post hoc tests to correct for testing of all pairwise comparisons. Differences between the leaflet length measured for the neo-tissue (n = 3) and scaffold (n = 3) remnants were evaluated as repeated measures 2-way analysis of variance followed by the Šidák multiple comparison test to correct for testing of scaffold length and neo-tissue length within the same leaflet. Prism software version 8 (GraphPad Software, Inc, San Diego, California) was used for the analyses.
Results
Transcatheter pulmonary valve replacement procedure using PC-BU TEHVs in combination with BMMNC pre-seeding
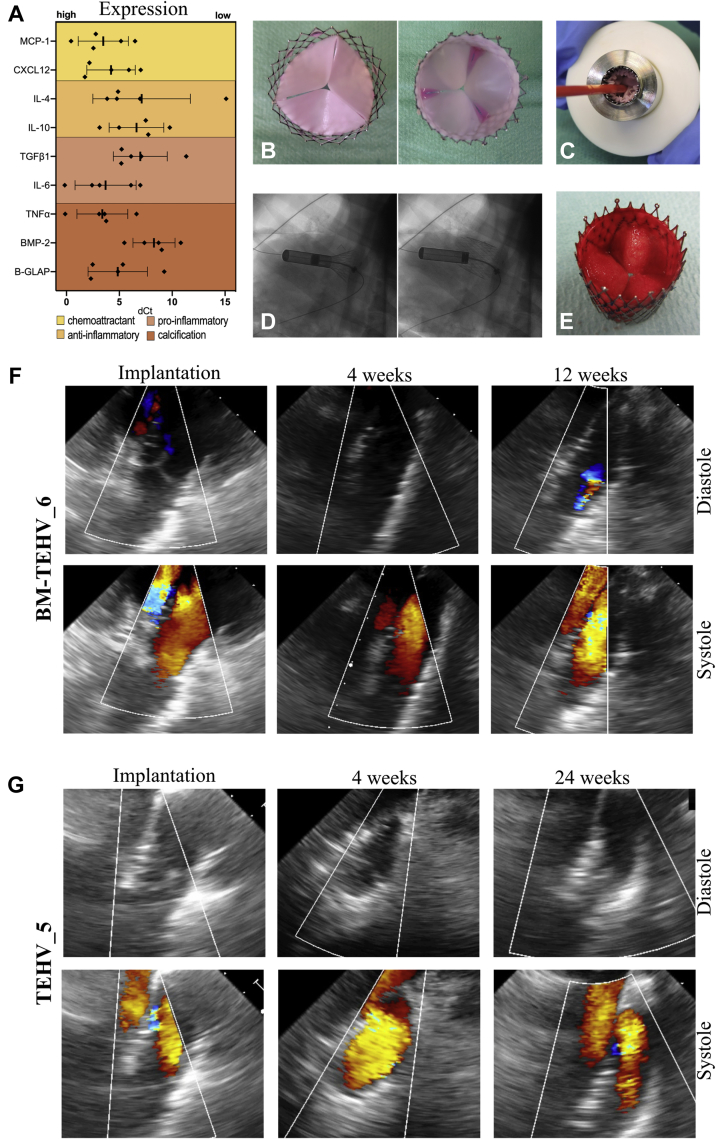
After valve manufacturing, in vitro valve functionality tests were performed (Supplemental Figures 1A to 1E, Supplemental Materials 2.1) to determine leaflet mobility and confirm a lack of stenosis or regurgitation and evaluate valve durability (Supplemental Table 5). BMMNCs were characterized by flow cytometry by using a 5-marker panel. The results confirmed consistent phenotypic marker expression among the different donors (n = 5) (Supplemental Figures 1F to 1K, Supplemental Materials 2.2). In addition, BMMNC gene expression analysis showed high expression of MCP-1 and CXCL12 (chemoattractants), interleukin (IL)-6 (pro-inflammatory), and bone gamma-carboxyglutamate (B-GLAP) and tumor necrosis factor-α (pro-calcification) (n = 5) (Figure 1A).
Figure 1.
In Vivo Functionality of Transcatheter TEHV as Pulmonary Valve Replacement
(A) Scatter plot (single events; mean ± SD) representing the gene expression profile of bone marrow mononuclear cells (n = 5) before seeding. Values are displayed as dCt and normalized on the average expression levels of the housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase and β-actin). (B) Top and bottom view of the bone marrow mononuclear cell pre-seeded tissue-engineered heart valve (TEHV). (C) Representative image of the crimped valve loaded into the delivery capsule. (D) Movie stills of valve deployment in pulmonary position performed under fluoroscopy. (E) Bottom view of the explanted TEHV after 4 h in vivo. (F) Representative trans-esophageal echocardiography images for BM-TEHV_6 exhibit sufficient early valve functionality with progressive worsening leading to moderate regurgitation at 12 weeks and pre-term animal euthanasia at 18 weeks. (G) Representative transesophageal echocardiography images for TEHV_5 (control valve, as reported previously in Kluin et al. [5]) show sustained functionality at every time point. B-GLAP = bone gamma-carboxyglutamate; BMP = bone morphogenetic protein; CXCL12 = C-X-C Motif Chemokine Ligand 12; dCt = difference between the gene cycle threshold and the housekeeping gene cycle threshold; IL = interleukin; MCP = monocyte chemoattractant protein; TGF = transforming growth factor; TNF = tumor necrosis factor.
After autologous BMMNC isolation, PC-BU TEHVs were homogeneously seeded with the cells (pre-seeded, n = 8) (Figure 1B) or nonseeded (n = 6), crimped down to 10 mm in diameter and loaded into the delivery capsule (Figure 1C). Valve deployment in the pulmonary position, performed under fluoroscopy (Figure 1D), was successful for every valve (n = 14). In all animals, angiographic assessment proved adequate valve positioning, fully excluding the native pulmonary valve leaflets. The majority of the animals (n = 13) had a swift recovery and did not exhibit perioperative complications or post-operative mortality. However, 1 animal (BM-TEHV_8) was euthanized 48 h after implantation due to severe chest bleeding. The cause of bleeding was the perforation of the pulmonary artery by the stent. This animal was therefore excluded from further analysis.
Acute in vivo performance
Acute functionality was sufficient for all animals during the 4-h follow-up, with no stenosis and no central or paravalvular leakage independent of the pre-seeding. The corresponding explants showed intact, pliable leaflets covered by a fibrin deposit and an absence of macroscopic damage related to the crimping procedure (Figure 1E).
Immunofluorescence imaging of these explants revealed fibrin deposition all over the leaflet. As expected, BMMNC pre-seeded valves exhibited a more abundant presence of (clustered) cells and greater expression of MCP-1 compared with the nonseeded group (Supplemental Figure 2).
Chronic in vivo performance
At implantation and after 4 weeks, the BM-TEHVs presented with sufficient valve functionality, without stenosis or regurgitation (Figure 1F). TEE-assessed mean and peak transvalvular gradient ranges were low (Supplemental Table 6). Two animals (BM-TEHV_6 and BM-TEHV_7) were euthanized pre-term after 18 weeks because TEE indicated worsening performance over time, starting at 12 weeks’ post-implantation, with moderate to severe regurgitation but no signs of stenosis. BM-TEHV_5 showed good early functionality, without stenosis and regurgitation, and correct valve positioning up to the 12-week follow-up. However, owing to valve migration into the right ventricle, incorrect valve positioning and paravalvular leakage were detected after 24 weeks (Supplemental Figure 3).
All the animals receiving nonseeded PC-BU TEHVs completed their designated follow-up. For these valves, post-operative and 4-week follow-up TEE showed functional valves in all animals (Figure 1G), with normal leaflet movement and excellent coaptation with no (n = 4) or only trivial (n = 1) regurgitation and low transvalvular pressure gradients (Supplemental Table 6). Two animals exhibited excellent valve function with no regurgitation after 24 weeks, whereas 1 animal (TEHV_4) had mild to moderate central regurgitation. Paravalvular leakage and stenosis were not present.
Gross explant evaluation
BMMNC pre-seeded and nonseeded TEHVs explanted after 4 weeks showed signs of stent integration with the pulmonary artery with intact, pliable leaflets (Supplemental Figures 4A and 4D). Remarkably, tissue overgrowth with partial fusion of adjacent leaflets at commissure points was evident in the explanted BM-TEHVs but not in the nonseeded TEHVs.
The BM-TEHVs explanted pre-term after 18 weeks (n = 2) showed good stent integration with the native pulmonary artery and pliable leaflets. In these explants, we observed fusion of the leaflets at the commissures (Figure 2A, Supplemental Figure 4B), which most likely caused the increasing regurgitation over time (Figure 1F). After 24 weeks, BM-TEHV_5 exhibited poor integration and stiff, fragile leaflets (Supplemental Figure 4C).
Figure 2.
Macroscopic Appearance and Histological Analyses of Longitudinal Transection of BM-TEHV_6 Explant After 18 Weeks
(A) View of the top, bottom, and longitudinally dissected valve explant. Arrows indicate tissue overgrowth, and triangles point to leaflet fusion at the commissures. (B) Tile scan of the elastica Van Gieson staining shows de novo collagen deposition in particular in the lower wall and hinge regions. A calcified nodule is marked by an asterisk. Little extracellular matrix is present on the leaflet. (C) Details of the remodeling process in the lower wall, hinge, and 2 different leaflet regions (identified by the insets in A) by means of different (immuno-)histological staining. Arrows indicate CD31-positive endothelial cells; “v” indicates vascularization. H&E = hematoxylin and eosin; SMA = smooth muscle actin; Vim = vimentin.
Nonseeded TEHVs explanted after 24 weeks showed good stent integration with the surrounding tissues and pliable leaflets (Supplemental Figure 4E). Two valves presented with small holes (1 to 2 mm) in the central part of the leaflets’ free edges but retained good coaptation. TEHV_6 had an additional hole (0.7 to 1 cm) in the belly region of one leaflet, an artifact caused by the catheter used for invasive pressure measurement performed before euthanization, as only trivial regurgitation was observed on TEE throughout the observation period.
Histological evaluation
Histological evaluation of samples harvested after 4 weeks confirmed cellularization and neo-collagen formation mainly in the lower wall region for both pre-seeded (Supplemental Figures 5A and 5B) and nonseeded valves (Supplemental Figures 5C and 5D). For both pre-seeded and nonseeded valves, vimentin-positive host cells were detected at the interface between the pannus and the scaffold material. Early signs of scaffold degradation were observed in the lower wall and hinge regions and were associated with tissue thickening and α-smooth muscle actin (SMA)-positive and vimentin-positive cells. CD31-positive cells were sparse and endothelialization incomplete at 4 weeks. Early signs of calcification were detected in the BM-TEHV_3 explant, with intracellular calcium deposits in the lower wall, hinge region, and at the base of the leaflet (Supplemental Figures 6A and 6B) but not on the nonseeded valves.
The BM-TEHVs explanted pre-term after 18 weeks (n = 2) and the nonseeded TEHVs harvested after 24 weeks (n = 3) exhibited neo-tissue formation with thickening of the lower wall and hinge region, presence of elastic fibers, and progressed scaffold reabsorption (including macrophages and giant cells) (Figures 2B and 2C, Supplemental Figures 7A and 7B). In addition, the lower wall region was highly vascularized (Supplemental Figure 8). In both study groups, the leaflet tip was fully repopulated by cells and usually characterized by little collagenous tissue, mostly within the polymeric scaffold fibers. In few explants (2 leaflets of 9, for both the pre-seeded and nonseeded groups), leaflet tip encapsulation leading to shortening was observed. For both pre-seeded and nonseeded TEHVs, αSMA-positive cells were abundant in the hinge and leaflet regions, whereas vimentin-expressing cells were mostly present in the lower wall and hinge region. Complete endothelialization, identified by the continuous monolayer of CD31-positive cells, was detected in few of the nonseeded valve leaflets (i.e., TEHV_4 and TEHV_6) (Supplemental Figure 9). However, the majority of the valve leaflets showed spot-dependent endothelial cell coverage (i.e., good CD31-positive cell coverage on the fibrosa side of the hinge region; absence of endothelial coverage toward the tip) (Figure 2C, Supplemental Figure 7B). BM-TEHV_5, however, showed a lack of collagen deposition on the scaffold, which was in line with poor stent integration and with over-time migration of the implant into the ventricle (Supplemental Figure 10). Macroscopic calcified nodules were detected in 4 of 9 leaflets of the BM-TEHVs (Supplemental Figures 6C and 6D), whereas macroscopic calcium deposits were not observed in any of the nonseeded TEHVs.
Gene expression profile of pre-seeded and nonseeded TEHVs
The gene expression profile of the 3 leaflets of each valve was evaluated via reverse transcription quantitative polymerase chain reaction (Figure 3). The heatmap shows differences between the pre-seeded and nonseeded valves, with a higher expression of chemoattractant (CXCL12), pro-inflammatory (transforming growth factor [TGF]-β1), pro-calcification (bone morphogenetic protein-2 and B-GLAP), and matrix remodeling (matrix metalloproteinase [MMP]-1 and MMP-9) genes in the BM-TEHVs explants compared with the nonseeded valves. Intervalve and interleaflet variability was observed as well, with differential gene expression profiles between valves of the same group (e.g., IL-4 expression in pre-seeded valves, IL-10 expression in nonseeded valves) and between leaflets of the same valve (e.g., TGF-β1 and IL-10 in TEHV_4; IL-4 in BM-TEHV_5).
Figure 3.
Heatmaps Representing the Gene Expression Profile of Pre-Seeded and Nonseeded TEHVs Explanted After 18 or 24 Weeks
The gene expression profile of each leaflet (L1, L2, and L3) of pre-seeded (n = 3) or nonseeded (n = 3) TEHVs is displayed in dCt and normalized on the average expression level of the housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase and β-actin). High expression levels are represented in dark green. The different genes of interest are grouped in accordance with their function in valve remodeling. MMP = matrix metalloproteinase; other abbreviations as in Figure 1.
Assessment of differential leaflet remodeling within the same valve explant
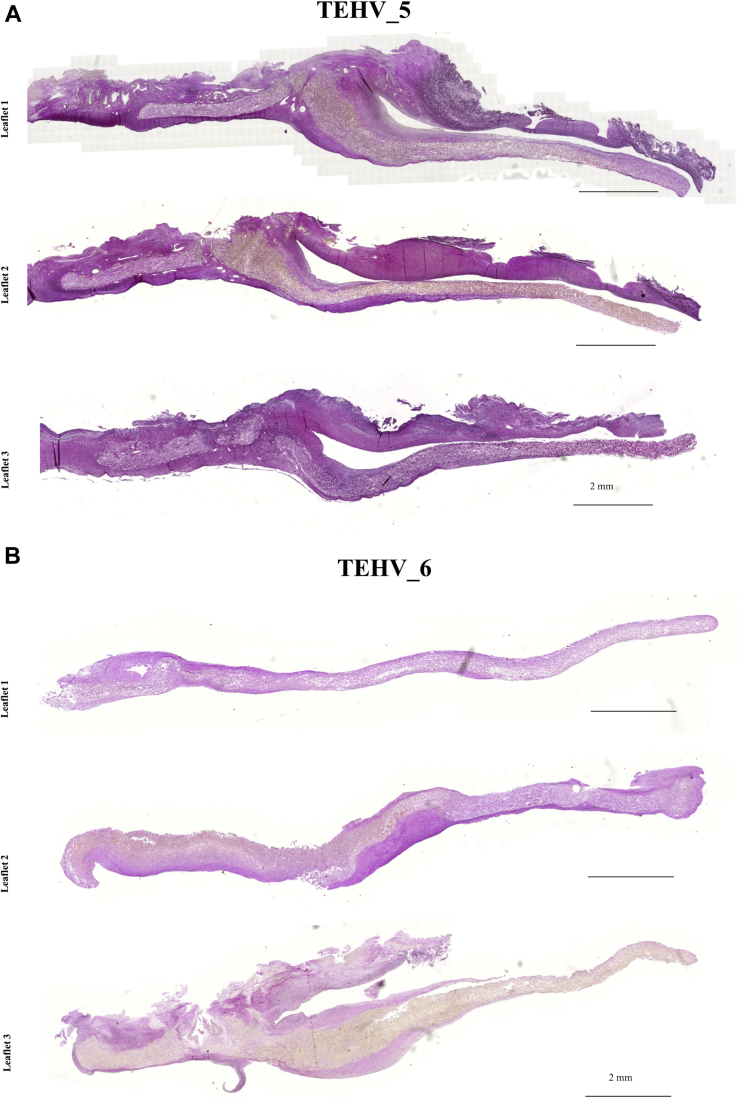
PC-BU TEHVs remodeling, evaluated via immunohistochemistry and summarized in Figure 4, proved to be animal- and leaflet-specific, independent of BMMNC pre-seeding (Figure 5, Supplemental Figures 9A, 10, 11A, and 12A). Figure 5A shows TEHV_5, which presented similar remodeling of all 3 leaflets, with comparable thickening and ECM deposition, polymer reabsorption in the hinge region, and uncovered leaflet tips (Figure 5A). Conversely, TEHV_6 showed a completely different remodeling of each of the 3 leaflets: leaflet 1 presented minimal ECM deposition in the lower wall, with no tissue formation in the leaflet; leaflet 2 underwent more substantial remodeling with thickening of the hinge region and encapsulation of the leaflet tip; and leaflet 3 showed advanced polymer degradation combined with thickening of the leaflet base (Figure 5B). Differential leaflet remodeling was also observed in the gene expression profile of each single leaflet harvested from pre-seeded or nonseeded TEHVs (Figure 3) and in the leaflet thickness (Figure 6) and length (Figure 7).
Figure 4.
Semi-Quantitative Evaluation of BMMNC Pre-Seeded and Nonseeded TEHV Remodeling After 18 and 24 Weeks
Data are presented as color-coded expression of the 3 leaflets (1, 2, and 3) for different valve locations (lower wall, hinge, leaflet base, mid, and tip). BMMNC = bone marrow mononuclear cell; TEHV = tissue-engineered heart valve.
Figure 5.
Gross Evaluation and Histological Analyses of the 3 Leaflets of TEHV_5 and TEHV_6 Explanted After 24 Weeks
(A) Tile scan of the elastica Van Gieson staining for the 3 leaflets of TEHV_5, showing similar remodeling outcome. Hematoxylin and eosin staining for leaflet one was previously reported in Kluin et al. (5). (B) Tile scan of the elastica Van Gieson staining for the 3 leaflets of TEHV_6, showing differential remodeling ranging from little (leaflet 2) to no (leaflet 1) neo-tissue formation, to thickening of the wall, hinge, and leaflet base regions (leaflet 3).
Figure 6.
Quantitative Evaluation of Leaflet Thickness
Thickness quantification for each of the 3 valve leaflets (1 to 3) in 4 different locations of the valve: lower wall (black), leaflet base (dark red), leaflet mid-region (green), and leaflet tip (blue). The gray dotted line at 400 μm indicates the average value of scaffold thickness at implantation. The cutoff for statistical significance was considered to be p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001).
Figure 7.
Quantitative Evaluation of Leaflet Length
Neo-tissue (blue bars) and scaffold (gray bars) leaflet length quantification for each of the 3 valve leaflets. The percentage value indicates leaflet shortening, mainly caused by the in-folding of the leaflet due to the neointimal tissue. The cutoff for statistical significance was considered to be p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001).
Generally, thickening (thickness value >1,000 μm) was observed in the lower wall and hinge areas of the leaflets, whereas minimal tissue deposition was observed toward the free edges. However, explant thickness measured in different leaflet regions proved to be significantly different among leaflets of the same valve (Figure 6). Effective neo-tissue leaflet length (Supplemental Figure 11C) was compared with that of the visible scaffold for each leaflet of the valve. Due to leaflet encapsulation by neointimal tissue, considerable leaflet shortening (>8%) was observed on both nonseeded and pre-seeded valves (Figure 7). Notably, valve BM-TEHV_6 showed leaflet shortening caused by folding of the leaflet tip toward the fibrosa side and fusion with the underlying tissue (Supplemental Figure 12).
Discussion
Bioresorbable, fully synthetic polymeric TEHVs have been suggested as an attractive concept for heart valve replacement, incorporating the advantages of using cell-free polymeric materials (i.e., tailored structural and mechanical properties, scalability, customized degradation profile, reduced cost and complexity). By relying on the principle of in situ tissue engineering, which comprises the infiltration of host cells and the replacement of the initial scaffold with endogenous ECM, the final goal of the approach is to achieve a living valve replacement via an inflammation-mediated process (6). The concept may become even more valuable when combined with TVR techniques, given the increasing application of minimally invasive valve replacement procedures in clinical practice (1, 2, 3).
The current study tested the feasibility of influencing the initial host response by pre-seeding the PC-BU TEHVs with autologous BMMNCs in a one-step approach. The results were compared with nonseeded TEHVs from a previous report (5), which served as the control group for the current study. Other than valve functionality, we comparatively analyzed the remodeling of each valve leaflet by using both quantitative and qualitative assessments to further gain insight into the remodeling processes of these bioresorbable valves.
Impact of BMMNC pre-seeding on TEHV functionality and remodeling
BMMNCs are an accessible cell source already used in clinical settings to pre-seed BVGs for the treatment of congenital single-ventricle anomalies (11,12). The safety of using such an autologous cell source in combination with BVGs in pediatric cardiovascular surgery has thus been established.
Next, pre-seeding of BMMNCs onto biodegradable polyglycolic acid (PGA)-based scaffolds proved to be feasible and compatible with transcatheter pulmonary valve replacement in non-human primates (17) and transcatheter aortic valve replacement in sheep (18, 19, 20). In line with the current study, Weber et al. (17) reported adequate valve functionality with mild to moderate regurgitation and detected substantial cellular infiltration and tissue remodeling, whereas any macroscopic calcifications were absent after 4 weeks in vivo (terminal time point of the experiment).
Moreover, Emmert et al. (20) showed the principal feasibility of a transcatheter TEHV implantation combined with a one-step BMMNC pre-seeding procedure also in the aortic circulation for up to 2 weeks in sheep. The long-term effects of BMMNC pre-seeding onto such PGA-based valves have yet to be elucidated.
Interestingly, the results presented in this study suggest detrimental consequences of BMMNC on long-term performance and tissue remodeling when pre-seeded onto PC-BU valves. Here, BMMNC isolation and phenotypic characterization were comparable to what was previously reported (17,18,20), with similar marker expression for CD45, CD11b, CD31, CD34, and CD44 as reported by Emmert et al. (20). However, the current study substantially differs from the aforementioned investigations (17,18,20) in terms of the following: 1) the valve material and design (PC-BU valve obtained from a tubular electrospun conduit vs. a poly-4-hydroxybutyrate–coated PGA nonwoven mesh cut and shaped into a valve construct); 2) follow-up period (4 and 24 weeks vs. 2 or 4 weeks); and 3) the animal model used (sheep vs non-human primates used by Weber et al. [17]). Together, these differences could have significantly affected long-term outcomes regarding valve performance after BMMNC pre-seeding. Importantly, these data again highlight the necessity of long-term in vivo studies when validating the safety and efficacy of a specific type of polymeric heart valve concept toward potential clinical translation.
The current study therefore discourages the use of BMMNC pre-seeding when combined with PC-BU TEHVs because of the observed regurgitation and maladaptive remodeling. Specifically, we observed fusion of neighboring leaflets that ultimately resulted in increasing valve insufficiency. At the tissue level, calcium deposits developed into pathological calcifications within the hinge regions after 18 weeks. Interestingly, nonseeded PC-BU TEHVs did not present calcified nodules, even at later time points (5). This outcome suggests that the presence of calcific deposits observed here in the BM-TEHVs was induced by the pre-seeding (22) and not by the material itself, as observed in other studies using other supramolecular polymers (7).
This hypothesis is also supported by the gene expression analysis, in which the genes involved in the activation of valve interstitial cells (TGF-β1 and IL-6), in calcification (B-GLAP and bone morphogenetic protein-2), and in matrix remodeling of regurgitant and stenotic valves (MMP-3 and MMP-9) (23) were more highly expressed in pre-seeded valves compared with the nonseeded TEHVs. We hypothesize that although the local release of MCP-1 proved to be beneficial for integration and remodeling of BVGs (10,14), it may lead to undesired outcomes in heart valve replacements because high levels of this cytokine were found in the calcified aortic valve sections (24). The cellular mechanisms by which BMMNC pre-seeding seems to inversely influence the remodeling of vessels and valves remain to be elucidated. One distinguishing factor may be the difference in hemodynamic loading, as previous in vitro studies have shown that the chemotactic effects of both pre-seeded BM-derived mesenchymal stromal cells and MCP-1 on monocyte-derived macrophages depend on hemodynamic loading (25,26).
Differential valve and leaflet remodeling
Both the explanted pre-seeded and nonseeded TEHVs displayed substantial variability in the remodeling outcomes that were not only pre-seeding dependent but also valve and leaflet dependent. The current study provides, for the first time, an in-depth overview of the remodeling of each valve leaflet by using quantitative and semi-quantitative analyses. Notably, signs of undesired leaflet remodeling (e.g., leaflet encapsulation and shortening, presence of αSMA-positive cells) were observed in both study groups.
Impact of valve positioning on tissue remodeling
Native valves are subjected to leaflet-dependent hemodynamic loading (27,28). Hence, the variability in leaflet remodeling may have been influenced by the nonphysiological orientation of the TEHV leaflets in the native annulus. In the current study, transcatheter implantation of PC-BU TEHVs was entirely based on imaging guidance during the intervention, using a stent that did not include any system for optimizing prosthesis alignment with the native valve leaflets. It is therefore possible that the TEHV leaflets have some degree of misalignment with the native leaflets. This may have resulted in nonphysiological hemodynamic loading of the valve, which represents a risk factor for undesired maladaptive remodeling phenomena (29,30). Previous studies have indeed shown how the hemodynamic loading can influence different remodeling processes, such as macrophage polarization (26,31) and elastogenesis (32). Therefore, controlling the hemodynamic variables may be the key to obtaining durable TEHVs with consistent remodeling potential.
Impact of valve design on tissue remodeling
We have shown how valve design can affect the mechanical loading on the leaflets and, therefore, affect valve functionality and tissue remodeling (33,34). TEHVs based on an in vitro grown tissue-engineered matrix depleted of cells, with a computational modeling–inspired geometry to prevent tissue compaction in a radial direction (35), exhibited sustained valve functionality for up to 1 year in sheep and physiological-like leaflet remodeling (33). Within this study, we solved the problem of leaflet shortening observed in most of the previously reported TEHVs (25,36, 37, 38) by controlling valve design. Valve design also dictates the opening and closing motion of the leaflets. Compared with the design of surgical valves (5), the transcatheter PC-BU TEHVs resulted in incongruent in vitro leaflet motion under hemodynamic loading, with a nonphysiological bending of the central part of the leaflet, which most likely caused the formation of the observed holes (Supplemental Figure 1C). This result suggests that, by influencing leaflet motion, valve design can significantly affect tissue remodeling and questions the long-term performance of these transcatheter PC-BU TEHVs.
Study limitations
For the in vitro valve characterization, the durability assessment did not take into account the remodeling potential of the prostheses, therefore significantly underestimating valve durability. For the animal study, a limited number of animals for each time point and group were used, thereby restraining the possibility of obtaining significant differences in the groups and affecting data variability. Moreover, due to unforeseen pre-term animal euthanization, not all animals reached the desired follow-up of 24 weeks. Finally, complete valve remodeling was not achieved, as confirmed by the abundant polymeric remnants of the original implant still visible after 24 weeks. Hence, further longer term in vivo experiments (i.e., ≥52 weeks) are warranted and should be performed in future studies.
Conclusions
This study reports the detrimental effects associated with autologous BMMNC pre-seeding onto PC-BU–based polymeric TEHVs used as transcatheter pulmonary valve replacement in sheep. Remarkably, opposing our hypothesis, the presented results discourage the use of BMMNC pre-seeding onto PC-BU TEHVs, simplifying the in situ heart valve tissue engineering approach using bioresorbable polymeric valves. In addition, we reported differential valve and leaflet remodeling independent of BMMNC pre-seeding. To date, it remains unclear whether these results can be extended to other polymers (i.e., PGA), but differences in valve remodeling were already observed in other surgical supramolecular polymer-based valves (5,7). Such heterogeneity may affect the safety of these supramolecular TEHVs, challenging their potential clinical translation (39). We therefore suggest the need to further investigate and understand the in situ remodeling potential of these transcatheter supramolecular TEHVs by gaining in-depth knowledge of the biological remodeling processes (e.g., by using in-silico and in-vitro models [40], and by comprehensive sheep-specific histopathological analysis [41]). In addition, ensuring valve antithrombogenicity is fundamental to prevent thrombotic and thromboembolic adverse events. In the current study, and similarly to what was previously reported for surgically implanted PC-BU TEHVs at 6 months’ follow-up (5), incomplete leaflet endothelialization was observed in the majority of the valve leaflets, with the presence of scaffold-exposed areas (particularly toward the leaflet-free edge). Finally, further research should focus on ensuring leaflet alignment with the native valve, improving valve design to control the hemodynamic loadings, and tuning scaffold degradation in vivo until complete reabsorption occurs. Only then will it be possible to make this in situ TEHV approach safe and clinically relevant.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The observed heterogeneity in the remodeling capacity of these bioresorbable elastomeric TEHVs may affect the predictability and reproducibility of the valve performance, calling into question their clinical translatability.
TRANSLATIONAL OUTLOOK: First, considering the differential leaflet remodeling observed, an in-depth analysis of each valve leaflet should be included in the guidelines (e.g., International Standards Organization norms) for assessing the remodeling of TEHV prostheses. Second, because the remodeling processes associated with these TEHVs heavily depend upon the restorative capacities of the host cells, a tailored-approach using computational modeling tools and valve-on-a-chip technologies should be considered for recipients with different regeneration potentials (e.g., with co-morbidities, elderly subjects). Third, novel comprehensive monitoring and surveillance strategies should be developed for all patients who receive a TEHV. A continuous clinical follow-up will be essential to detect early signs of malfunction or failure. Finally, long-term data will be needed to validate this supramolecular polymer-based concept with particular regard to the risk of potential unpredictable (maladaptive) remodeling upon complete polymer degradation, which is not yet fully controllable or understood
Acknowledgments
The financial contributions of the Dutch Heart Foundation and Swiss National Science Foundation is gratefully acknowledged.
Footnotes
This research forms part of Project P1.01 iValve of the research program of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs (grant number DHF-2008T089), and of project iValve-II, powered by Health∼Holland, top sector of Life Science and Health (grant number TTTI1403B), supported by the Dutch Ministry of Economic Affairs. Dr. Fioretta was partly supported by the Swiss National Science Foundation (grant number PZ00P3_180138). Drs. Bouten and Hoerstrup are shareholders of Xeltis BV. Drs. Söntjens and Janssen are employed by SyMO-Chem B.V. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For an expanded Methods section as well as the supplemental tables and figures, please see the online version of this paper.
Contributor Information
Maximilian Y. Emmert, Email: maximilian.emmert@irem.uzh.ch.
Simon P. Hoerstrup, Email: simon.hoerstrup@irem.uzh.ch.
Appendix
References
- 1.Arora S., Misenheimer J.A., Ramaraj R. Transcatheter aortic valve replacement: comprehensive review and present status. Texas Heart Inst J. 2017;44:29–38. doi: 10.14503/THIJ-16-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack M.J., Leon M.B., Thourani V.H. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Popma J.J., Deeb G.M., Yakubov S.J. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 4.Manji R.A., Lee W., Cooper D.K.C. Xenograft bioprosthetic heart valves: Past, present and future. Int. J. Surg. 2015;23:280–284. doi: 10.1016/j.ijsu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Kluin J., Talacua H., Smits A.I.P.M. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—from material design to 12 months follow-up in sheep. Biomaterials. 2017;125:101–117. doi: 10.1016/j.biomaterials.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Wissing T.B., Bonito V., Bouten C.V.C., Smits A.I.P.M. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. NPJ Regen Med. 2017;2:18. doi: 10.1038/s41536-017-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennink G., Torii S., Brugmans M. A novel restorative pulmonary valved conduit in a chronic sheep model: mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg. 2018;155:2591–2601. doi: 10.1016/j.jtcvs.2017.12.046. e3. [DOI] [PubMed] [Google Scholar]
- 8.Fioretta E.S.E.S., Dijkman P.E.P.E., Emmert M.Y.M.Y., Hoerstrup S.P.S.P. The future of heart valve replacement: recent developments and translational challenges for heart valve tissue engineering. J Tissue Eng Regen Med. 2018;12:e323–e335. doi: 10.1002/term.2326. [DOI] [PubMed] [Google Scholar]
- 9.Brennan M.P., Dardik A., Hibino N. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248:370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh J.D., Sawh-Martinez R., Brennan M.P. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibino N., McGillicuddy E., Matsumura G. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. doi: 10.1016/j.jtcvs.2009.09.057. e2. [DOI] [PubMed] [Google Scholar]
- 12.Shin’oka T., Matsumura G., Hibino N. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129 doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Fukunishi T., Best C.A., Ong C.S. Role of bone marrow mononuclear cell seeding for nanofiber vascular grafts. Tissue Eng Part A. 2018;24:135–144. doi: 10.1089/ten.tea.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talacua H., Smits A.I.P., Muylaert D.E.P. In situ tissue engineering of functional small-diameter blood vessels by host circulating cells only. Tissue Eng Part A. 2015;21:2583–2594. doi: 10.1089/ten.TEA.2015.0066. [DOI] [PubMed] [Google Scholar]
- 15.Miyachi H., Reinhardt J.W., Otsuru S. Bone marrow-derived mononuclear cell seeded bioresorbable vascular graft improves acute graft patency by inhibiting thrombus formation via platelet adhesion. Int J Cardiol. 2018;266:61–66. doi: 10.1016/j.ijcard.2018.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura G., Miyagawa-Tomita S., Shin’oka T., Ikada Y., Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 17.Weber B., Scherman J., Emmert M.Y. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. 2011;32:2830–2840. doi: 10.1093/eurheartj/ehr059. [DOI] [PubMed] [Google Scholar]
- 18.Emmert M.Y., Weber B., Behr L. Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves. J Am Coll Cardiol Intv. 2011;4:822–823. doi: 10.1016/j.jcin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Emmert M.Y., Weber B., Behr L. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardio-Thoracic Surg. 2014;45:61–68. doi: 10.1093/ejcts/ezt243. [DOI] [PubMed] [Google Scholar]
- 20.Emmert M.Y., Weber B., Wolint P. Stem cell–based transcatheter aortic valve implantation. J Am Coll Cardiol Intv. 2012;5:874–883. doi: 10.1016/j.jcin.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Dijkman P.E., Driessen-Mol A., de Heer L.M. Trans-apical versus surgical implantation of autologous ovine tissue-engineered heart valves. J Heart Valve Dis. 2012;21:670–678. [PubMed] [Google Scholar]
- 22.Vincentelli A., Wautot F., Juthier F. In vivo autologous recellularization of a tissue-engineered heart valve: Are bone marrow mesenchymal stem cells the best candidates? J Thorac Cardiovasc Surg. 2007;134:424–432. doi: 10.1016/j.jtcvs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Fondard O., Detaint D., Iung B. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 24.Li X.F., Wang Y., Zheng D.D. M1 macrophages promote aortic valve calcification mediated by microRNA-214/TWIST1 pathway in valvular interstitial cells. Am J Transl Res. 2016;8:5773–5783. [PMC free article] [PubMed] [Google Scholar]
- 25.Smits A.I.P.M., Ballotta V., Driessen-Mol A., Bouten C.V.C., Baaijens F.P.T. Shear flow affects selective monocyte recruitment into MCP-1-loaded scaffolds. J Cell Mol Med. 2014;18:2176–2188. doi: 10.1111/jcmm.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballotta V., Smits A.I.P.M., Driessen-Mol A., Bouten C.V.C., Baaijens F.P.T. Synergistic protein secretion by mesenchymal stromal cells seeded in 3D scaffolds and circulating leukocytes in physiological flow. Biomaterials. 2014;35:9100–9113. doi: 10.1016/j.biomaterials.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Chester A.H., El-Hamamsy I., Butcher J.T., Latif N., Bertazzo S., Yacoub M.H. The living aortic valve: from molecules to function. Glob Cardiol Sci Pract. 2014;2014:52–77. doi: 10.5339/gcsp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayoub S., Ferrari G., Gorman R.C., Gorman J.H., Schoen F.J., Sacks M.S. Heart valve biomechanics and underlying mechanobiology. Compr Physiol. 2016;6:1743–1780. doi: 10.1002/cphy.c150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzzardi D.G., Barker A.J., van Ooij P. Valve-related hemodynamics mediate human bicuspid aortopathy: insights from wall shear stress mapping. J Am Coll Cardiol. 2015;66:892–900. doi: 10.1016/j.jacc.2015.06.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes B.A.A., Camargo G.C., Santos J.R.L.D. Influence of the tilt angle of percutaneous aortic prosthesis on velocity and shear stress fields. Arq Bras Cardiol. 2017;109:231–240. doi: 10.5935/abc.20170115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonito V., Smits A.I.P.M., Goor O.J.G.M. Modulation of macrophage phenotype and protein secretion via heparin-IL-4 functionalized supramolecular elastomers. Acta Biomater. 2018;71:247–260. doi: 10.1016/j.actbio.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Aikawa E., Whittaker P., Farber M. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 33.Emmert M.Y., Schmitt B.A., Loerakker S. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan4587. [DOI] [PubMed] [Google Scholar]
- 34.Sanders B., Loerakker S., Fioretta E.S.E.S. Improved geometry of decellularized tissue engineered heart valves to prevent leaflet retraction. Ann Biomed Eng. 2016;44:1061–1071. doi: 10.1007/s10439-015-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loerakker S., Ristori T., Baaijens F.P.T.T. A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. J Mech Behav Biomed Mater. 2016;58:173–187. doi: 10.1016/j.jmbbm.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Syedain Z., Reimer J., Schmidt J. 6-Month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials. 2015;73:175–184. doi: 10.1016/j.biomaterials.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motta S.E., Lintas V., Fioretta E.S., Hoerstrup S.P., Emmert M.Y. Off-the-shelf tissue engineered heart valves for in situ regeneration: current state, challenges and future directions. Expert Rev Med Devices. 2018;15:35–45. doi: 10.1080/17434440.2018.1419865. [DOI] [PubMed] [Google Scholar]
- 38.Weber B., Dijkman P.E., Scherman J. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013;34:7269–7280. doi: 10.1016/j.biomaterials.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 39.Emmert M.Y., Fioretta E.S., Hoerstrup S.P. Translational challenges in cardiovascular tissue engineering. J Cardiovasc Transl Res. 2017;10:139–149. doi: 10.1007/s12265-017-9728-2. [DOI] [PubMed] [Google Scholar]
- 40.Fioretta E.S., von Boehmer L., Motta S.E., Lintas V., Hoerstrup S.P., Emmert M.Y. Cardiovascular tissue engineering: From basic science to clinical application. Exp Gerontol. 2019;117:1–12. doi: 10.1016/j.exger.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Dekker S., van Geemen D., van den Bogaerdt A.J., Driessen-Mol A., Aikawa E., Smits A.I.P.M. Sheep-specific immunohistochemical panel for the evaluation of regenerative and inflammatory processes in tissue-engineered heart valves. Front Cardiovasc Med. 2018;5:105. doi: 10.3389/fcvm.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.