Corresponding Author

Key Words: liposomes, percutaneous coronary intervention, restenosis, ultrasound
The introduction of percutaneous coronary intervention with stent implantation has revolutionized the treatment of patients with obstructive coronary artery disease since the first balloon angioplasty performed by Andreas Grüntzig in 1977.
With the widespread use of bare metal stent implantation, the angioplasty-induced complications such as arterial dissection and elastic recoil could be resolved, but this came at the cost of augmented neointimal hyperplasia within the stented vascular segment. This issue prompted the development of drug-eluting stents (DES), which successfully targeted the problem of neointimal overgrowth by sustained release of antiproliferative substances.
However, persistence of these drugs resulted in substantial delay of vascular healing, the major pathologic mechanism underlying late and very late stent thrombosis. More recently, in-stent neoatherosclerosis (i.e., progression of atherosclerosis within the stented vascular segment) was identified as additional consequence of delayed vascular healing after DES implantation and associated with late stent failure (1).
Ever since the inception of research related to finding solutions to overcome the problem of restenosis, systemic pharmacological therapy became highly attractive (2). Yet, most of these approaches were later abandoned because of unacceptable side effects at drug dosages needed to prevent restenosis (3).
In this issue of JACC: Basic to Translational Science, Kee et al. (4) report the results of a preclinical study investigating a novel site-directed pharmacologic approach to reduce in-stent restenosis following stent implantation in a hypercholesterolemic porcine model.
They used a combined endovascular ultrasound (EUS) and delivery system to ensure local application of therapeutics from echogenic liposomes (ELIPs) into stented peripheral arteries. The ELIP infusion consisted of an initial nitric oxide (NO) dose to trigger immediate antioxidative and antiplatelet effects and to increase vessel wall permeability for improved delivery of the peroxisome proliferator-activator receptor (PPAR)-γ agonist pioglitazone (PGN) coupled to anti–intercellular adhesion molecule (ICAM)–directed ELIP. Animals assigned to ELIP payload delivery were randomly allocated to EUS activation or control (absence of EUS activation). Neointimal growth was assessed by intravascular ultrasound imaging and histology 8 weeks after bare-metal stent implantation. To show payload delivery, a separate group of animals underwent balloon dilatation to simulate stent implantation, followed by application of fluorescently labelled ELIP. Intravascular ultrasound imaging showed reduction of neointimal volume in the stented arteries treated by NO-activated and anti–ICAM-1-PGN-ELIP activated by ultrasound compared to the vessels that lacked ultrasound activation. Histologic analysis confirmed reduced neointima formation in the vessels treated with NO- and anti–ICAM-PGN-ELIP with ultrasound activation compared to those without.
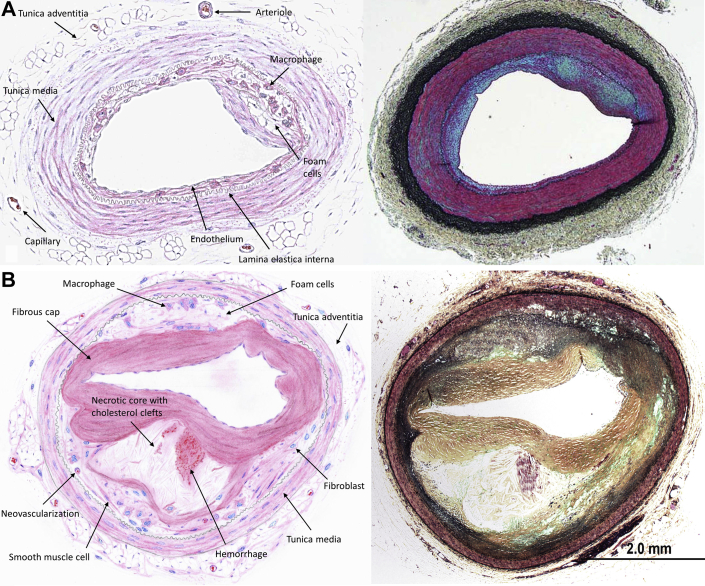
First and foremost, the authors must be congratulated for pursuing tremendous effort to establish novel, site-directed pharmacologic approaches to inhibit in-stent restenosis in a highly translational setting. Preclinical studies using diseased and nondiseased animal models remain an important step during translation of novel therapeutic strategies in cardiovascular medicine (5). Although nondiseased animal models prevail in device-based safety studies because of lower variability in vascular response, diseased animal models represent an important cornerstone during evaluation of pharmacologic therapies against cardiovascular disease conditions, especially atherosclerosis. Despite the predominance of genetically engineered mouse models in atherosclerosis research, use of large animal models such as hypercholesterolemic swine or rabbit models remains an important opportunity for studying local and systemic effects of therapeutic agents in the presence of lesions resembling human atherosclerosis, especially in the setting of human-sized vascular stents. Despite the successful delivery of 2 sequential liposome formulations aimed at molecular targeting of inflammatory reactions as shown in the current study, a general concern remains whether such artificial environment (i.e., atherosclerotic lesions secondary to vascular injury in combination with supraphysiological cholesterol diet) enables to study novel site-directed pharmacologic therapy under conditions close enough to human atherosclerosis to allow prediction of vascular safety and efficacy. In particular, expression of inflammatory adhesion molecules such as ICAM-1 in this model is rapidly induced by artificial vascular injury combined with hypercholesterolemic diet unlike human atherosclerosis, where local expression of inflammatory adhesion molecules is upregulated in early stages of atherosclerosis and is subject to substantial interindividual dynamics during progression of atherosclerosis. Consequently, it remains unclear to what extend artificially induced atherosclerosis in the porcine model, which only displays early stages of atherosclerosis, may be representative for human disease conditions (Figures 1A and 1B).
Figure 1.
Early Atherosclerotic Lesion in an Animal Model Versus Late-Stage Human Fibroatheroma
(A) Early-stage atherosclerotic lesion (schematic and Movat’s pentachrome stain) in the Yucatan miniswine (modified from Kim et al. [7]). (B) Late-stage human fibroatheroma (schematic and Movat‘s pentachrome stain).
Most importantly, relative efficacy of a new technology can only be recognized when appropriate control is incorporated into study design, which is an important prerequisite for successful clinical translation. Despite the elegant demonstration of payload delivery into target vascular segments in combination with reduced neointimal hyperplasia when ultrasound was applied, the authors unfortunately did not compare their novelty against established treatment such as coronary DES, which would have enabled us to understand its relative merits before translation into clinic (5). Moreover, no specific study group was applied to control for the active pharmacologic component (i.e., PGN), which has known anti-inflammatory and antiproliferative properties upon activation of the PPAR-γ receptor. PGN is available as an oral pill and can be customized and provided as powder for preclinical applications. Inclusion of an additional study group to receive oral PGN at concentrations known to prevent in-stent restenosis might have enlightened our comparative understanding of pharmacologic efficacy (6). Yet, it must be recognized that implementation of preclinical studies based on ideal study design is often difficult to achieve and frequently yields to pragmatic and feasible study flow determined by prior knowledge and financial constraints. Consequently, the authors of the current study are encouraged to continue their worthwhile investigation of site-directed pharmacologic approaches to reduce the burden of in-stent restenosis since only recently late accrual of adverse cardiovascular events including late catch-up phenomena of restenosis and progression of atherosclerosis within the stented vascular segments (i.e., neoatherosclerosis) were recognized as important and deleterious consequences of delayed arterial healing after implantation of conventional DES (1). Furthermore, site-directed pharmacologic targeting of in-stent restenosis using EUS activation may enable payload delivery using a diversity of different pharmacologic substances that may exhibit synergistic effects.
We have come a long way to study the pathophysiology of in-stent restenosis and yet got lost during search for causative treatment, while transitioning into an era of tremendous commercial interest with the introduction of DES. Endeavors such as the current one create important momentum to get back on track with finding enduring solution for this clinically impactful dilemma.
Footnotes
Dr. Joner has received research grants from Biotronik; and has received lecture fees from Orbus Neich, Biotronik, Coramaze, and Bayer. Dr. Lahmann has reported that she has no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Otsuka F., Byrne R.A., Yahagi K. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36:2147–2159. doi: 10.1093/eurheartj/ehv205. [DOI] [PubMed] [Google Scholar]
- 2.Marx N., Wohrle J., Nusser T. Pioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation. 2005;112:2792–2798. doi: 10.1161/CIRCULATIONAHA.105.535484. [DOI] [PubMed] [Google Scholar]
- 3.Hausleiter J., Kastrati A., Mehilli J. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with in-stent restenosis: the Oral Sirolimus to Inhibit Recurrent In-stent Stenosis (OSIRIS) trial. Circulation. 2004;110:790–795. doi: 10.1161/01.CIR.0000138935.17503.35. [DOI] [PubMed] [Google Scholar]
- 4.Kee P.H., Moody M.R., Huang S.-L. Stabilizing peri-stent restenosis using a novel therapeutic carrier. J Am Coll Cardiol Basic Trans Science. 2020;5:1–11. doi: 10.1016/j.jacbts.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joner M., Byrne R.A. The importance of preclinical research in contemporary interventional cardiology. EuroIntervention. 2010;6:19–23. [PubMed] [Google Scholar]
- 6.Joner M., Farb A., Cheng Q. Pioglitazone inhibits in-stent restenosis in atherosclerotic rabbits by targeting transforming growth factor-beta and MCP-1. Arterioscler Thromb Vasc Biol. 2007;27:182–189. doi: 10.1161/01.ATV.0000251021.28725.e8. [DOI] [PubMed] [Google Scholar]
- 7.Kim H., Kee P.H., Rim Y. Nitric oxide improves molecular imaging of inflammatory atheroma using targeted echogenic immunoliposomes. Athereosclerosis. 2013;231:252–260. doi: 10.1016/j.atherosclerosis.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]