In this study, Singh et al. set out to define mechanisms for H3:CENP-A exchange at centromeric regions in S. pombe. Using a combination of biochemical, molecular, and genetic approaches, the authors show that Hap2–Ino80 destabilizes H3 nucleosomes on centromere DNA through transcription-coupled histone H3 turnover, driving the replacement of resident H3 nucleosomes with CENP-A nucleosomes.
Keywords: CENP-A, centromere, chromatin, chromosome segregation, epigenetic, fission yeast, histone, kinetochore, remodeling, transcription
Abstract
Centromeres are maintained epigenetically by the presence of CENP-A, an evolutionarily conserved histone H3 variant, which directs kinetochore assembly and hence centromere function. To identify factors that promote assembly of CENP-A chromatin, we affinity-selected solubilized fission yeast CENP-ACnp1 chromatin. All subunits of the Ino80 complex were enriched, including the auxiliary subunit Hap2. Chromatin association of Hap2 is Ies4-dependent. In addition to a role in maintenance of CENP-ACnp1 chromatin integrity at endogenous centromeres, Hap2 is required for de novo assembly of CENP-ACnp1 chromatin on naïve centromere DNA and promotes H3 turnover on centromere regions and other loci prone to CENP-ACnp1 deposition. Prior to CENP-ACnp1 chromatin assembly, Hap2 facilitates transcription from centromere DNA. These analyses suggest that Hap2–Ino80 destabilizes H3 nucleosomes on centromere DNA through transcription-coupled histone H3 turnover, driving the replacement of resident H3 nucleosomes with CENP-ACnp1 nucleosomes. These inherent properties define centromere DNA by directing a program that mediates CENP-ACnp1 assembly on appropriate sequences.
The accurate delivery of all chromosomes to both resulting nuclei during mitotic cell division is required for eukaryotic cell viability and to prevent aneuploidy, a hallmark of cancer (Kops et al. 2005). The centromere region of chromosomes mediates their attachment to spindle microtubules for normal mitotic chromosome segregation (Fukagawa and Earnshaw 2014). In many organisms, centromeres are assembled on repetitive elements such as α-satellite repeats, minor satellite repeats, cen180/CentC/CentO repeats, and retroelements at human, mouse, plant, and Drosophila centromeres, respectively (Kipling et al. 1991; Grady et al. 1992; Cheng et al. 2002; Chang et al. 2019). Although such centromere repeats lack sequence similarity, in many cases their introduction as naked DNA into cells triggers de novo kinetochore assembly (Baum et al. 1994; Okada et al. 2007).
The underlying conserved feature at eukaryotic centromeres is the assembly of nucleosomes containing the histone H3 variant CENP-A (also generally known as cenH3, and specifically as CID in Drosophila, Cse4 in Saccharomyces cerevisiae, and Cnp1 in Schizosaccharomyces pombe) in place of canonical H3 to direct kinetochore assembly on such repetitive elements. Moreover, it is known that following deletion of an endogenous centromere, CENP-A incorporation and neocentromeres can arise at novel noncentromeric DNA locations (Ishii et al. 2008; Ketel et al. 2009; Shang et al. 2013). CENP-A chromatin has also been shown to be sufficient to trigger kinetochore assembly (Barnhart et al. 2011; Mendiburo et al. 2011; Hori et al. 2013; Chen et al. 2014). Thus, CENP-A deposition and not the primary sequence of centromere DNA determines the position of centromere formation. However, centromere DNA itself may harbor properties that favor CENP-A and kinetochore assembly (Baum et al. 1994; Fachinetti et al. 2015; Logsdon et al. 2019).
Three fission yeast species possess complex regional centromeres in which CENP-ACnp1 and kinetochores are assembled over a nonrepetitive central domain of ∼10 kb in S. pombe, S. octosporus, and S. cryophilus (Tong et al. 2019). Although flanking gene order is preserved, the central domain sequence is not conserved among these species. Despite this lack of similarity, central domain DNA from S. octosporus and S. cryophilus, as well as S. pombe, can direct de novo CENP-ACnp1 and kinetochore assembly in S. pombe (Folco et al. 2008; Catania et al. 2015; Tong et al. 2019). This suggests that these nonhomologous centromere DNAs possess innate features that program events that preferentially trigger the assembly of CENP-ACnp1 in place of H3 nucleosomes.
During replication, parental CENP-A has been shown to distribute equally to nucleosomes on duplicated DNA of both sister centromeres, thus halving the amount of CENP-A at each centromere (Jansen et al. 2007; Schuh et al. 2007). Replenishment by deposition of newly synthesized CENP-A is temporally separated from DNA replication-coupled H3 chromatin assembly. The timing of new CENP-A deposition differs in various species; mitosis/late telophase/early G1 in human and Drosophila (Schuh et al. 2007; Erhardt et al. 2008; Mellone et al. 2011), S phase in S. cerevisiae (Pearson et al. 2004), and G2 in plants and S. pombe (Lermontova et al. 2006; Shukla et al. 2018).
Several studies have reported the association of RNA polymerase II (RNAPII) with and/or transcription from CENP-A-associated DNA so that noncoding transcription has become an apparent integral feature of centromeres (Duda et al. 2017). RNAPII has been detected at human artificial chromosome (HAC) (Bergmann et al. 2011), human metaphase (Chan et al. 2012), Drosophila (Bobkov et al. 2018), and S. cerevisiae (Ohkuni and Kitagawa 2011) centromeres, and also the central domains of fission yeast centromeres (Choi et al. 2011; Catania et al. 2015). Disruption of centromere transcription appears to hinder CENP-A loading and/or maintenance (Nakano et al. 2008; Bergmann et al. 2011; Chen et al. 2015; McNulty et al. 2017; Ling and Yuen 2019). Moreover, increased centromere transcription results in the rapid loss of CENP-A and centromere function (Hill and Bloom 1987; Bergmann et al. 2012). The central CENP-ACnp1 domains from S. pombe centromeres contain numerous RNAPII transcriptional start sites and promoters (Choi et al. 2011; Catania et al. 2015). In addition, ectopically located central domain DNA, which lacks CENP-ACnp1 chromatin, exhibits high rates of histone H3 turnover (Shukla et al. 2018). Such observations suggest that transcription-coupled chromatin remodeling events might drive the eviction of H3 and its replacement with CENP-ACnp1. Consistent with this view, the accumulation of elongating RNAPII on ectopic centromere DNA during G2 coincides with the eviction of H3 and deposition of new CENP-A (Shukla et al. 2018). However, it remains to be determined which transcription-associated chromatin remodeling factors provoke the replacement of H3 with CENP-A on naïve centromere DNA.
Various ATP-dependent chromatin remodeling complexes provide access to the underlying DNA of chromatin-coated templates. Their activities enable transcription by disassembling nucleosomes, sliding nucleosomes, or replacing nucleosomal histone subunits with transcription-promoting variants (Clapier and Cairns 2009). The remodeling and spacing factor (RSF) interacts with CENP-A chromatin in mid-G1, its depletion reduces CENP-A levels at centromeres (Perpelescu et al. 2009), and tethering of RSF1 to centromere repeats promotes histone turnover/exchange resulting in both H3.3 and CENP-A deposition (Ohzeki et al. 2016). In S. pombe, Hrp1 (ortholog of chromo-helicase DNA-binding protein 1 [CHD1]) is enriched at centromeres and is required to maintain normal CENP-ACnp1 levels at centromeres (Walfridsson et al. 2005; Choi et al. 2011). Loss of the histone chaperone facilitates chromatin transcription (FACT) results in promiscuous CENP-ACnp1 assembly at noncentromeric locations ACnp1 (Choi et al. 2012), suggesting that CENP-ACnp1 may be titrated away from centromeres by loss of such factors. Moreover, inducible ectopic centromeres in Drosophila requires FACT-mediated RNAPII-dependent transcription of underlying DNA, indicating a necessity for transcription during CENP-A assembly (Chen et al. 2015).
Ino80 is a Snf2 family ATPase evolutionarily conserved from yeast to humans that participates in transcription, DNA replication, and DNA repair (Conaway and Conaway 2009). The Ino80 complex (Ino80C) can slide nucleosomes in an ATP-dependent manner (Chen et al. 2011) and can space multiple nucleosomes on longer DNA fragments (Udugama et al. 2011). Ino80C may also remove H2A.Z–H2B dimers from nucleosomes, replacing them with H2A–H2B dimers (Papamichos-Chronakis et al. 2011; Brahma et al. 2017). H2A.Z is enriched in +1 nucleosomes downstream from promoters of many active genes, and loss of Ino80 function affects transcription (Hogan et al. 2010; Marques et al. 2010; Xue et al. 2015). Individual Ino80C subunits make up three modules that associate with the main Ino80 ATPase subunit (Supplemental Table S1; Chen et al. 2011). S. pombe Ino80 has been shown to influence the maintenance of CENP-ACnp1 chromatin at centromeres (Choi et al. 2017). However, it is not known whether Ino80C influences centromere DNA transcription or the establishment of CENP-A chromatin on naïve centromere DNA and, thus, centromere identity.
Here we used affinity selection of CENP-ACnp1 chromatin and mass spectrometry to identify proteins enriched in CENP-ACnp1 chromatin that may promote CENP-ACnp1 assembly. We identify Hap2 (SPCC16C4.20) as an auxiliary subunit of Ino80C that is required for the conversion of H3 chromatin to CENP-ACnp1 chromatin on naïve centromere DNA. We show that loss of Hap2 function reduces transcription and histone H3 turnover on centromere DNA. Our findings indicate that Hap2–Ino80 is required to promote transcription-associated chromatin remodelling events that drive H3 nucleosome eviction and the assembly of CENP-ACnp1 nucleosomes in their place.
Results
Hap2 is an Ino80C subunit that is enriched in CENP-ACnp1 chromatin
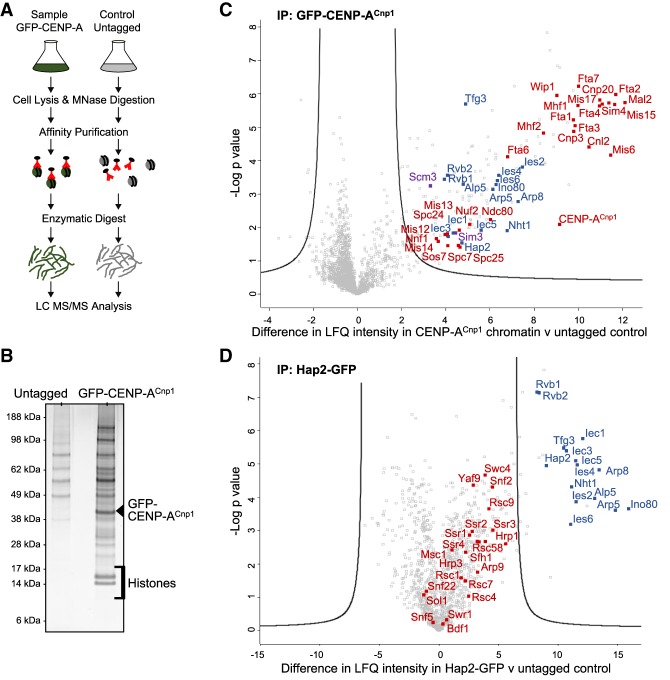
To identify proteins involved in the assembly of CENP-ACnp1 chromatin, GFP-tagged CENP-ACnp1 chromatin was affinity-purified from micrococcal nuclease (MNase)-solubilized chromatin extracts (Fig. 1A). Quantitative PCR (qPCR) analysis of the resulting enriched native chromatin revealed significant enrichment of centromeric DNA from the single-copy central CENP-ACnp1 domain of cen2 (cc2) compared with the 18 copies of flanking outer repeat (dg) sequences (Supplemental Fig. S1A). In addition, SDS-PAGE analysis showed core histones to be prevalent in this affinity-selected GFP-CENP-ACnp1 material (Fig. 1B; Supplemental Fig. S1B). Label-free quantitative mass spectrometry detected strong enrichment of all known subunits of both the inner and outer kinetochore complexes (Fig. 1C; Supplemental Tables S2, S3). As our procedure also showed association of the chaperones Scm3 (HJURP) and Sim3 (NASP) that mediate CENP-ACnp1 deposition we reasoned that other enriched, but non-centromere-specific, proteins might be involved in the incorporation of CENP-ACnp1 into centromeric chromatin.
Figure 1.
Hap2 is associated with CENP-ACnp1 chromatin and it is a subunit of Ino80C. (A) Scheme for enrichment of CENP-ACnp1 chromatin associated proteins and identification by mass spectrometry. (B) Silver-stained SDS-PAGE of proteins enriched in IPs from GFP-CENP-ACnp1 or untagged control cells. (C) Volcano plot comparing label-free quantification (LFQ) intensity of proteins enriched in affinity selected GFP-CENP-ACnp1 (anti-GFP) versus untagged control. Inner and outer kinetochore proteins detected (red). Ino80C subunits (blue). CENP-ACnp1 chaperones Scm3 and Sim3 (purple). (D) Volcano plot comparing label-free quantification of proteins enriched in affinity selected Hap2-GFP (anti-GFP) versus untagged control. Ino80C subunits (blue). Swr1 complex subunits, SWI/SNF complex, RSC complex, and CHD family members (red).
It was notable that all subunits of the Ino80 chromatin remodeling complex (Ino80C) were enriched in our affinity-selected GFP-CENP-ACnp1 preparations (Fig. 1C). Also enriched in these samples was the low-molecular-weight α-helical Hap2 protein. Hap2 has previously been reported to associate with Ino80C but no characterization has been performed (Hogan et al. 2010). To confirm association of Hap2 with Ino80C, Hap2 was C-terminally tagged with GFP at its endogenous chromosomal locus (Hap2-GFP) (Supplemental Fig. S2A). Affinity selection of Hap2-GFP followed by proteomic analysis revealed that all subunits of Ino80C, but not subunits of other remodelling complexes, are enriched with Hap2-GFP (Fig. 1D; Supplemental Table S4). IP-western analysis confirmed that Hap2-GFP associates with immunoprecipitated HA-tagged Ino80 (Ino80-HA) and vice-versa, independently of DNA/chromatin association (Supplemental Fig. S1C,D). Furthermore, affinity selection of Ino80-HA enriched all known Ino80C subunits along with Hap2 (Supplemental Fig. S1E; Supplemental Table S5). We conclude that Hap2 is a noncanonical subunit of the fission yeast Ino80 complex and that all Ino80C subunits are enriched in CENP-ACnp1 chromatin.
Hap2 association with central domain DNA assembled in CENP-ACnp1 chromatin or H3 chromatin is Ies4-dependent
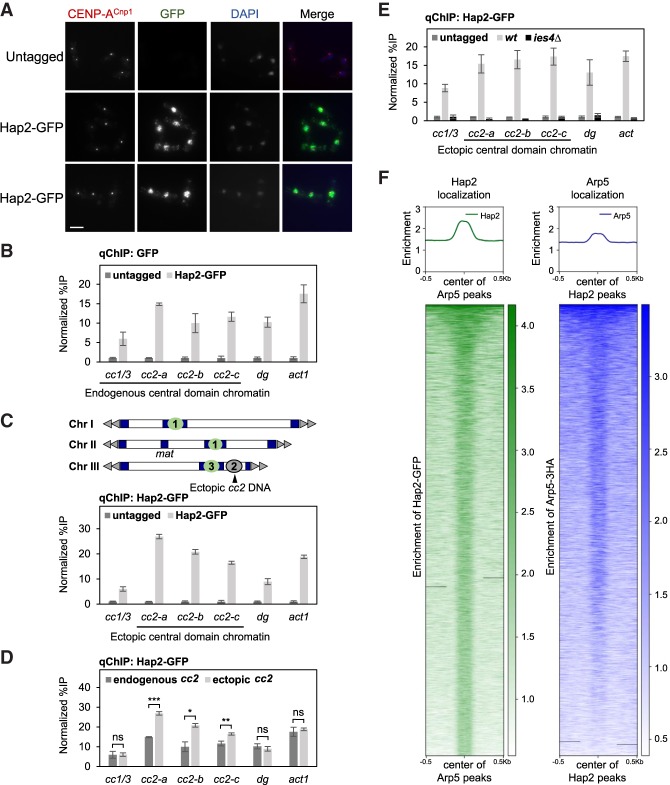
Microscopic analysis showed that Hap2-GFP localizes to the nucleus (Fig. 2A). Quantitative chromatin immunoprecipitation (qChIP) assays revealed that Hap2-GFP associates with most chromatin regions analyzed including the central CENP-ACnp1 domain of centromeres (cc2), the flanking pericentromeric outer repeats (dg) and on the highly expressed act1+ gene (Fig. 2B). When 8.5 kb of cen2 central domain DNA (cen2-cc2) is inserted at the ura4 locus on a chromosome arm (ura4:cc2), it is assembled in H3 instead of CENP-ACnp1 chromatin (Choi et al. 2012; Shukla et al. 2018). Replacement of 6.5 kb of endogenous cen2-cc2 DNA with 5.5 kb of cen1 central domain DNA (cc2Δ::cc1) allows analysis across the resulting unique ectopic copy of cen2 central domain DNA (ura4:cc2) in the absence CENP-ACnp1 and kinetochore proteins (Fig. 2C, top). qChIP analysis revealed that Hap2-GFP associates with this ectopic cc2 central domain chromatin (Fig. 2C, bottom). A noticeably higher level of Hap2-GFP was consistently detected across ectopic ura4:cc2 central domain DNA assembled in H3 chromatin relative to the same DNA sequence at the native cen2 central domain assembled in CENP-ACnp1 chromatin (Fig. 2D). Loss of Hap2 does not affect the association of Ino80-HA with these regions (Supplemental Fig. S2B), while Hap2 chromatin association is lost in the absence of Ies4 but remains unaffected in iec1Δ, ies2Δ and arp5Δ cells (Fig. 2E; Supplemental Fig. S2C,D). In budding yeast, different subunits of Ino80 have been shown to have broad interactions around NFRs and +1 nucleosomes (Yen et al. 2013). As Hap2 is a subunit of Ino80C, we examined genome-wide localization of Hap2 and Arp5. High coincidence was observed for Hap2 and Arp5 peaks (Fig. 2F). We conclude that the Ino80C subunit Hap2 is a nuclear protein that associates with noncentromeric loci and is preferentially recruited to centromeric central domain chromatin when assembled in H3 rather than CENP-ACnp1 chromatin. Hap2 chromatin association is dependent on the Ino80C subunit Ies4, and Hap2 colocalizes genome-wide with the Arp5 canonical Ino80C subunit.
Figure 2.
Hap2 associates with endogenous and ectopic central core chromatin and Ies4 dependent, Hap2 and Arp5 chromosomal distributions are coincident. (A) Immunolocalization of Hap2-GFP in cells. Representative images of wild-type and Hap2-GFP cells stained with anti-CENP-ACnp1 (red), anti-GFP (green), and DAPI (blue). Scale bar, 5 µm. (B) qChIP for Hap2-GFP at four locations within endogenous centromeres (cc1/3, cc2-a, cc2-b, and cc2-c), outer repeat heterochromatin (dg) and noncentromere locus (act1+). Error bars indicate mean ± SD (n = 3). (C, top) Diagram indicating strain with endogenous cen2-cc2 replaced with cen1 central domain DNA (cc1) at the noncentromeric ura4 locus (ura4:cc2) on Chr III. (Bottom) qChIP for Hap2-GFP at three locations within ectopic central domain H3 chromatin (cc2-a, cc2-b, and cc2-c), endogenous centromeres (cc1/3), outer repeat heterochromatin (dg), and noncentromere locus (act1+). Error bars indicate mean ± SD (n = 3). (D) Comparison of Hap2-GFP association with central domain sequence assembled in CENP-ACnp1 chromatin or H3 chromatin (data from B and C). Error bars indicate mean ± SD (n = 3). Significance of the differences observed between cells containing endogenous centromeres and ectopic central domain DNA was evaluated using Student's t-test. (*) P < 0.05; (**) P < 0.005; (***) P < 0.0005; (n.s.) not significant. (E) qChIP for Hap2-GFP at three locations within ectopic central domain H3 chromatin (cc2-a, cc2-b, and cc2-c), endogenous centromeres (cc1/3), outer repeat heterochromatin (dg) and noncentromere locus (act1+) in untagged, wild-type, and ies4Δ cells. (F) Genome-wide coincidence of Hap2-GFP and Arp5-3HA peaks (∼60%). Gradient shows enrichment of Hap2 (green) and Arp5 (blue).
Hap2 is required to maintain CENP-ACnp1 chromatin across endogenous centromeres
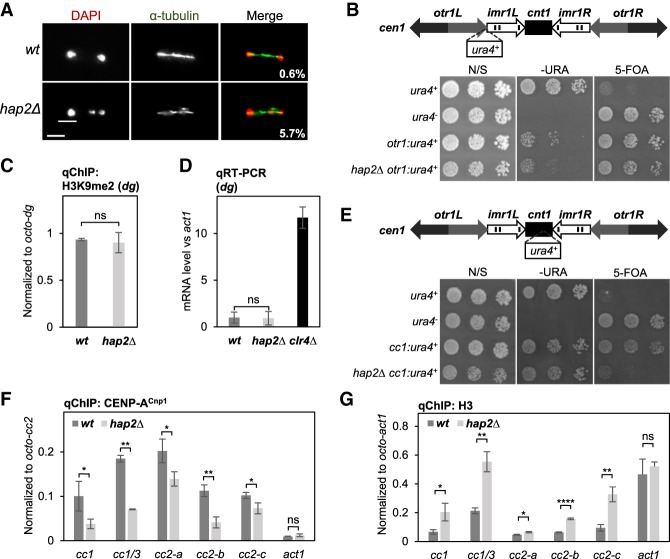
Cells lacking Hap2 exhibit an elevated frequency of lagging chromosomes during mitosis, indicating that loss of Hap2 may affect centromere function (Fig. 3A). Defective centromere function can result from reduced pericentromeric heterochromatin formation on dg/dh outer repeats or CENP-ACnp1 chromatin/kinetochore assembly and these can be sensitively detected by the use of silent ura4+ reporter genes inserted within outer repeat heterochromatin or central CENP-ACnp1 domain chromatin (Allshire et al. 1994, 1995; Partridge et al. 2000). No alleviation of heterochromatin-mediated silencing at otr1:ura4+ was detected in hap2Δ cells relative to wild type as indicated by similar poor growth on selective plates lacking uracil (−URA) and good growth on counter-selective 5-FOA plates (Fig. 3B; Supplemental Fig. S3A). Consistent with this observation, no significant change was detected in the levels of the heterochromatin H3K9me2 mark or dg transcripts produced by the underlying outer repeats in hap2Δ relative wild-type cells (Fig. 3C,D).
Figure 3.
Hap2 is required to maintain CENP-ACnp1 chromatin but not the adjacent pericentric heterochromatin. (A) Frequency of lagging chromosomes in anaphase cells. Representative images of wild-type and hap2Δ cells stained with DAPI (red) and antitubulin (green). Percentage of late anaphase cells (wild type: n = 487 and hap2Δ: n = 492) displaying lagging chromosomes is indicated. Scale bar, 5 µm. (B, top) Diagram indicating position otr1:ura4+ marker gene inserted in outer repeat heterochromatin of cen1. (Bottom) Growth assay for central domain silencing at otr1:ura4+. Fivefold serial dilution of cell cultures were spotted on nonselective (N/S), selective (−URA), or counter-selective (FOA) plates. (C) qChIP for H3K9me2 at outer repeat heterochromatin (dg). Error bars indicate mean ± SD (n = 3). (D) qRT-PCR of pericentric dg repeats performed on total RNA extracted from indicated strains. Transcript levels are shown relative to act1+ and normalized to wild type (n = 3). (E, top) Diagram indicating position cc1:ura4+ marker gene within the central CENP-ACnp1 domain cen1 (cnt1) relative to the outer repeat (otr) and innermost repeats (imr). (Bottom) Growth assay for central domain silencing at cen1:ura4+ as in B. (F,G) qChIP for CENP-ACnp1 (F) and histone H3 (G) at five locations within endogenous centromeres (cc1, cc1/3, cc2-a, cc2-b, and cc2-c) and noncentromere locus (act1+). Error bars indicate mean ± SD (n = 3). Significance of the differences observed between wild-type and hap2Δ was evaluated using Student's t-test in C, D, F, and G. (*) P < 0.05; (**) P < 0.005; (****) P < 0.00005; (n.s.) not significant.
Central CENP-ACnp1 domain chromatin is also transcriptionally repressive (Allshire et al. 1994, 1995). Defects in CENP-ACnp1 chromatin assembly alleviates this transcriptional silencing (Partridge et al. 2000; Pidoux et al. 2003). To test whether loss of Hap2 affects CENP-ACnp1-mediated silencing we examined silencing of ura4+ embedded in central CENP-ACnp1 chromatin at cen1 (cc1:ura4+). Reduced silencing in hap2Δ cells, indicated by reduced growth on counter-selective FOA plates, suggested a defect in CENP-ACnp1 chromatin integrity (Fig. 3E; Supplemental Fig. S3B). qChIP analysis detected significantly lower levels of CENP-ACnp1 and a reciprocal increase in H3 levels across the central domain of centromeres in hap2Δ relative to wild-type cells (Fig. 3F,G), consistent with the silencing defect. Importantly, loss of Hap2 does not affect the total cellular levels of GFP-CENP-ACnp1 or the expression of genes encoding proteins that are known to regulate CENP-ACnp1 loading at centromeres (Supplemental Fig. S3C,D). We conclude that loss of Hap2 specifically affects silencing through loss of CENP-ACnp1 and gain of H3 within the central domain of centromeres, thus Hap2 is required to maintain CENP-ACnp1 chromatin integrity at endogenous centromeres.
Hap2 is required for the de novo establishment of CENP-ACnp1 chromatin
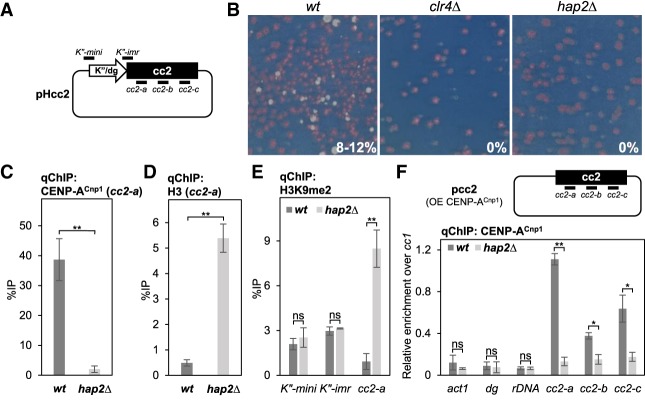
Many factors are known to assist CENP-A maintenance but little is known about the factors required for the de novo establishment of CENP-A chromatin. De novo establishment of functional centromeres can occur following the introduction of naked centromere DNA into cells (Catania et al. 2015). We first examined whether Hap2 and other subunits of Ino80C are required for the de novo establishment of centromeres on the pHcc2 minichromosome (Fig. 4A). hap2Δ cells exhibited a complete failure to establish functional centromeres, similar to clr4Δ cells that lack heterochromatin, while ies2Δ displayed increased establishment, iec1Δ and ies4Δ were less competent in establishing functional centromeres on pHcc2 (Fig. 4B; Supplemental Fig. S4A). In S. pombe, de novo CENP-ACnp1 chromatin establishment on circular plasmid-based minichromosomes requires a block of heterochromatin in close proximity to central domain DNA (Folco et al. 2008; Kagansky et al. 2009). Loss of centromere establishment could result from a failure to establish CENP-ACnp1 chromatin, and/or adjacent heterochromatin, on the minichromosome. To distinguish between these possibilities, CENP-ACnp1 levels on the plasmid-borne central domain cc2 DNA were analysed. All strains used have 6 kb of cen2 central domain DNA replaced with 5.5 kb of cen1 central domain DNA at endogenous centromeres (cc2Δ::cc1) so that the plasmid-borne cc2 is the only copy of this element. qChIP revealed that CENP-ACnp1 was not assembled over cc2 carried by pHcc2 in hap2Δ cells (Fig. 4C). Reciprocally, a high level of H3 was detected across cc2 of pHcc2 in the absence of CENP-ACnp1 assembly in hap2Δ cells (Fig. 4D). qChIP for H3K9me2 demonstrated that loss of Hap2 did not affect the establishment of heterochromatin on the plasmid-borne K″/dg repeat (Fig. 4E). Interestingly, high levels of H3K9me2 were detected within the central domain of the pHcc2 minichromosome in hap2Δ but not wild-type cells (Fig. 4E). This suggests that in the absence of CENP-ACnp1 chromatin establishment in hap2Δ cells heterochromatin may spread from the outer K″/dg repeat into the plasmid-borne central cc2 domain. To test whether Hap2 is required for the de novo establishment of CENP-ACnp1 chromatin independently from the requirement for adjacent heterochromatin, the plasmid pcc2 which carries 8.5 kb of cen2 central domain DNA, but no heterochromatin forming outer repeat sequences, was transformed into cells expressing additional GFP-CENP-ACnp1, which allows CENP-ACnp1 chromatin assembly (Fig. 4F, top; Catania et al. 2015). In contrast to wild type, hap2Δ cells did not assemble high levels of CENP-ACnp1 over the central domain of the pcc2 minichromosome (Fig. 4F, bottom). This effect was not due to altered CENP-ACnp1 protein levels in hap2Δ compared with wild-type cells (Supplemental Fig. S4B). We conclude that Hap2 is critical for the de novo establishment of CENP-ACnp1 chromatin on naïve central domain centromere DNA.
Figure 4.
Hap2 is required for the de novo establishment of CENP-ACnp1 chromatin. (A) Schematic representation of pHcc2 minichromosome K″/dg repeat adjacent to central domain 2 DNA. Position of the primer pair at the edge of K″/dg repeat and within central domain 2 DNA are indicated. (B) Transformants containing pHcc2 minichromosome plasmids were replica-plated to low adenine nonselective plate. Representative plate showing colony color and centromere establishment frequency in wild type (n = 515), hap2Δ (n = 390), and strains lacking heterochromatin clr4Δ (n = 870). (C,D) qChIP for CENP-ACnp1 and histone H3 at plasmid-borne central domain 2 DNA (cc2-a) on pHcc2 minichromosome. Error bars indicate mean ± SD (n = 3). (E) qChIP for H3K9me2 at plasmid-borne K″/dg repeat (K″-mini and K″-imr) and plasmid-borne central domain 2 DNA (cc2-a) on pHcc2 minichromosome. Error bars indicate mean ± SD (n = 3). (F, top) Schematic representation of pcc2 minichromosome without K″/dg repeat transformed in cells overexpressing CENP-ACnp1. (OE) Overexpression. (Bottom) qChIP for CENP-ACnp1 at noncentromere locus (act1+), outer repeat heterochromatin (dg) and ribosomal DNA (rDNA), and three locations within plasmid-borne central core 2 DNA (cc2-a, cc2-b, and cc2-c) on pcc2 minichromosome relative to CENP-ACnp1 levels at endogenous centromeres (cc1/3). Error bars indicate mean ± SD (n = 3). Significance of the differences observed between wild type and hap2Δ was evaluated using Student's t-test in C–F. (*) P < 0.05; (**) P < 0.005; (n.s.) not significant.
Hap2 promotes histone turnover in genomic regions prone to CENP-ACnp1 assembly
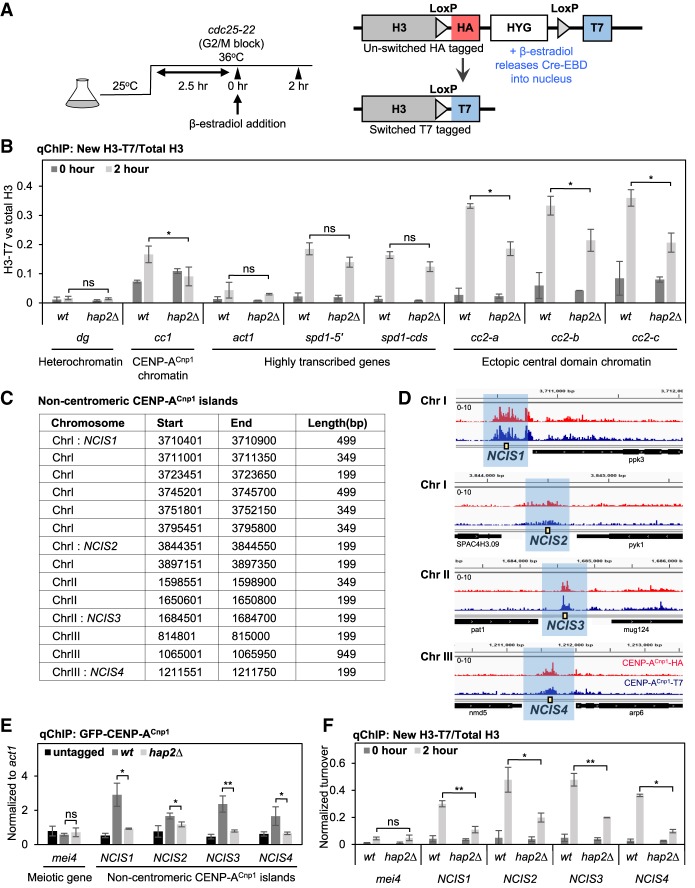
Central domain DNA inserted at a noncentromeric location on the arm of a chromosome, such as the ura4 locus on chromosome 3 (ura4:cc2), remains assembled in H3 nucleosomes and exhibits a high rate of histone H3 turnover (Shukla et al. 2018). The inherent instability of H3 nucleosomes assembled on this ectopic ura4:cc2 centromere DNA has been proposed to aid the incorporation of CENP-ACnp1 when available (Shukla et al. 2018). In S. cerevisiae, Ino80 associates with promoter-associated nucleosome-depleted regions and transcription start sites (TSS) where it mediates the turnover of +1 nucleosomes (Yen et al. 2013). The requirement for Hap2 in de novo CENP-ACnp1 assembly on central domain DNA may result from defective H3 nucleosome turnover on these centromeric sequences when assembled in H3 chromatin alone. We therefore used recombination-induced tag exchange (RITE) (Verzijlbergen et al. 2010; Shukla et al. 2018) to measure replication-independent H3 turnover in G2-arrested wild-type and hap2Δ cells on ectopic ura4:cc2, heterochromatic repeats, and highly transcribed genes. This H3.2-HA→T7 tag swap was induced in cdc25-22/G2-arrested cells and the incorporation of new histone H3.2-T7 was monitored (Fig. 5A). Importantly, the H3.2-HA → T7 tag swap efficiency was unaffected by hap2Δ relative to wild-type cells (Supplemental Fig. S5A). Compared with wild-type cells, a significant decrease in the level of H3 turnover was evident on ectopic ura4:cc2 centromere DNA in hap2Δ cells (Fig. 5B). Similarly, H3 turnover within endogenous cen1-cc1 assembled in CENP-ACnp1 chromatin was also reduced in hap2Δ cells. In contrast, H3 turnover remained unchanged within heterochromatic outer repeats (dg) and over highly transcribed genes (act1+ and spd1+). Ino80 may mediate nucleosome turnover through eviction of H2A.Z (Papamichos-Chronakis et al. 2011). However, the levels of H2A.ZPht1 associated with endogenous cc1 and ectopic ura4:cc2 were unaffected by loss of Hap2 (Supplemental Fig. S5B). We conclude that Hap2 is required to ensure H3 nucleosome instability on centromeric sequences by mediating a high frequency of H3 turnover, which may consequently allow the incorporation of CENP-ACnp1 in place of H3.
Figure 5.
Hap2 is required for high histone H3 turnover on central domain centromere DNA and islands of noncentromeric CENP-A incorporation. (A) Experimental setup to assess replication-independent H3 turnover. The cdc25-22 temperature sensitive mutation was used to block wild-type and hap2Δ cells in G2, the HA tag on H3 was swapped for the T7 tag by β-estradiol induced Cre/loxP mediated recombination. Samples were collected at 0 and 2 hr and analyzed by qChIP. (B) qChIP analysis of new H3.2-T7 incorporated in cdc25-22/G2-arrested cells at pericentromeric outer repeat heterochromatin (dg), the central CENP-ACnp1 domain of cen1 (cc1), highly transcribed genes (act1+ and spd1+) and three locations within ectopically located ura4:cc2 lacking CENP-ACnp1 (cc2-a, cc2-b, and cc2-c). (Y-axis) H3.2-T7%IP values normalized to the respective total H3%IP values represent normalized turnover for each sample. Error bars indicate mean ± SD (n = 3). (C) ChIP-nexus analysis of HA and T7 tagged CENP-ACnp1 reveals consistent noncentromeric CENP-ACnp1 islands (NCIS) of incorporation above background at 14 locations in the genome. (D) Four CENP-ACnp1 islands are shown: NCIS1, NCIS2, NCIS3, and NCIS4. (E) qChIP for GFP-CENP-ACnp1 at noncentromeric CENP-ACnp1 islands using indicated primer pairs (yellow boxes in D) and meiotic-specific gene (mei4+). Error bars indicate mean ± SD (n = 3). (F) qChIP analysis of new H3.2-T7 incorporation in cdc25-22/G2-arrested cells at NCIS1, NCIS2, NCIS3, and NCIS4. Error bars indicate mean ± SD (n = 3). Error bars indicate mean ± SD (n = 3). Significance of the differences observed between wild type and hap2Δ was evaluated using Student's t-test in B, E, and F. (*) P < 0.05; (**) P < 0.005; (n.s.) not significant.
Overexpression of CENP-ACnp1 results in low levels of promiscuous CENP-ACnp1 incorporation at noncentromeric locations (Choi et al. 2012; Castillo et al. 2013). Fission yeast CENP-ACnp1 expression is known to increase prior to that of canonical histones in advance of replication. Consequently, even without overexpression, in early S phase this natural excess of CENP-ACnp1 results in low levels of newly synthesized CENP-ACnp1 being incorporated across many gene bodies (Shukla et al. 2018). ChIP-Nexus (a modified exo-ChIP-seq protocol) (He et al. 2015) analysis allowed detection of noncentromeric genomic regions where islands of CENP-ACnp1 are retained at low levels (Fig. 5C,D). Hap2-GFP was found to associate with these noncentromeric CENP-ACnp1 islands (NCIS), while loss of Hap2 significantly decreased CENP-ACnp1 incorporation within these islands (Fig. 5E). Consistent with previous results, Hap2 association with NCIS requires Ies4, but not Iec1, Ies2, or Arp5 (Supplemental Fig. S5C,D). Interestingly, qChIP also revealed reduced histone H3 turnover within these noncentromeric CENP-ACnp1 islands in hap2Δ cells, whereas histone H3 turnover remained unchanged within the heterochromatic outer repeats, the act1+ gene, or the repressed meiosis-specific mei4+ gene (Fig. 5F). We conclude that Hap2 also promotes a high rate of H3 turnover at several noncentromeric NCIS loci prone to low-level CENP-ACnp1 incorporation. This observation reinforces the involvement of Hap2–Ino80C in mediating histone H3 turnover and the exchange of histone H3 for CENP-ACnp1-containing nucleosomes.
Hap2 facilitates transcription of central domain chromatin
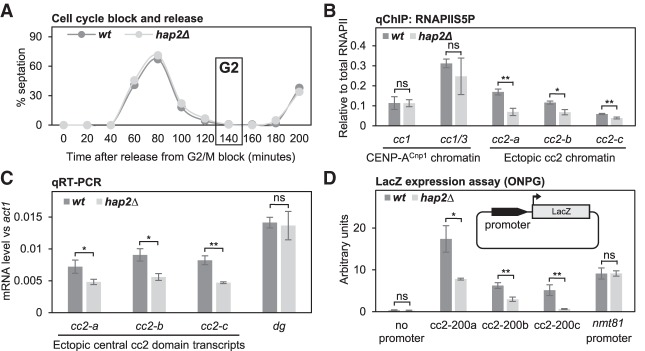
The central CENP-ACnp1 domain of S. pombe centromeres is transcribed from many TSS and the resulting RNAs are short-lived (Choi et al. 2011; Sadeghi et al. 2014; Catania et al. 2015). Transcription can provide the opportunity for histone exchange/remodeling of resident nucleosomes (Venkatesh and Workman 2015). It is therefore feasible that transcription-coupled processes also promote the exchange of H3 for CENP-ACnp1 in chromatin assembled on centromere DNA. Relatively high levels of RNAPII are detected on central domain DNA when assembled in H3 chromatin at ectopic ura4:cc2 or on the pcc2 minichromosome, yet only low levels of RNAPII are detectable within endogenous centromeric central domain CENP-ACnp1 chromatin (Catania et al. 2015; Shukla et al. 2018). Since CENP-ACnp1 is primarily deposited during G2, we investigated whether Hap2 affects transcription from ectopic ura4:cc2 in mid-G2 cells from cdc25-22 synchronized cultures (Fig. 6A). qChIP was used to measure the levels of total RNAPII, initiating RNAPIIS5P and elongating RNAPIIS2P levels across ura4:cc2. RNAPIIS5P levels were significantly lower across this ectopic central domain DNA in hap2Δ compared with wild-type cells, but no obvious difference was detected within the CENP-ACnp1 chromatin regions of endogenous centromeres (Fig. 6B). These data suggest that Hap2 promotes efficient transcriptional initiation from central domain DNA assembled in H3 chromatin (ura4:cc2). In contrast, the levels of total and elongating RNAPIIS2P associated with ectopic central domain DNA remained unchanged in hap2Δ relative to wild-type cells (Supplemental Fig. S6A,B). Consistent with reduced transcriptional initiation, lower levels of central domain transcripts were produced from ectopic ura4:cc2 in hap2Δ cells (Fig. 6C). Transcript levels from outer repeats and act1+ were unaffected. Thus, Hap2 is required for efficient transcriptional initiation and transcript production from ectopic central domain DNA assembled in H3 chromatin. The fact that no decrease in elongating RNAPIIS2P was detected across this ectopic central domain in hap2Δ cells suggests that RNAPII remains associated with the central domain template for longer when Hap2 is absent.
Figure 6.
Transcription from centromeric DNA is reduced in the absence of Hap2. (A) cdc25-22 synchronized cell populations were used to assess levels of RNAPIIS5P and central core transcripts during G2 in B and C. The septation index for wild type and hap2Δ were measured and cells were collected in G2 (T-140). (B) qChIP for initiating RNAPIIS5P at two locations within endogenous centromeres (cc1 and cc1/3) and three locations within ectopically located ura4:cc2 lacking CENP-ACnp1 (cc2-a, cc2-b, and cc2-c) in wild type and hap2Δ. Error bars indicate mean ± SD (n = 3). (C) qRT-PCR to quantify transcripts from three locations within ectopically located ura4:cc2 lacking CENP-ACnp1 (cc2-a, cc2-b, and cc2-c) and from pericentromeric outer repeat (dg) on total RNA extracted from wild-type and hap2Δ cells. Transcript levels are shown relative to act1+. Error bars indicate mean ± SD (n = 3). (D) Measurement of LacZ expression driven by three 200-bp cc2 fragments with promoter activity (cc2-200a, cc2-200b, and cc2-200c), the nmt81 promoter, and no promoter following transformation of plasmids into wild type and hap2Δ using the ONPG substrate/absorbance at 420 nm. Error bars indicate mean ± SD (n = 3). (Inset) Diagram of plasmids with different 200-bp fragments from central domain region of cen2 (cc2) placed upstream of the LacZ reporter. Significance of the differences observed between wild type and hap2Δ was evaluated using Student's t-test in B–D. (*) P < 0.05; (**) P < 0.005; (n.s.) not significant.
Regions upstream of TSS within the central domain of cen2 exhibit promoter activity (Catania et al. 2015). To determine whether Hap2 affects the transcription from these central domain promoters, the production of β-galactosidase was assessed when 200-bp promoter-containing cc2 fragments were placed upstream of lacZ (Fig. 6D, inset). Three central domain promoters exhibited significantly lower promoter activity in hap2Δ compared with wild-type cells, whereas the control nmt81 promoter was unaffected (Fig. 6D; Supplemental Fig. S6C,D). We conclude that Hap2 is required for efficient transcription from central domain promoters and that this facilitates transcription-coupled histone exchange, thereby providing an opportunity for replacement of H3 with CENP-ACnp1 to establish CENP-ACnp1 chromatin domains and assemble functional kinetochores.
Discussion
The mechanisms that contribute to the maintenance, and especially the establishment, of CENP-A chromatin remain poorly understood. To gain insight into how CENP-A chromatin is established on naïve centromere DNA, we applied proteomics to identify proteins associated with fission yeast CENP-ACnp1 chromatin. In addition to kinetochore proteins, all Ino80C subunits, including the small auxiliary subunit, Hap2, were found to be significantly enriched in solubilized CENP-ACnp1 chromatin. Hap2 and the Arp5 Ino80C subunit exhibit similar chromosomal distributions and Hap2 chromatin association depends on the Ies4 Ino80C subunit. Hap2 was found to promote CENP-ACnp1 chromatin integrity at centromeres and to be required for the de novo establishment of CENP-ACnp1 chromatin on introduced naïve centromere DNA. The requirement for Hap2 in ensuring high histone H3 turnover on endogenous centromere DNA, ectopically located centromere DNA, and noncentromeric CENP-ACnp1 islands indicates that Hap2–Ino80C drives H3 nucleosome turnover at these locations. The loss of CENP-ACnp1 incorporation from NCIS islands in the absence of Hap2 underscores the role for Hap2–Ino80C-mediated H3 turnover in stimulating CENP-ACnp1 incorporation. Strikingly, Hap2 is required for efficient transcription specifically from central domain promoters. We propose a mechanism in which Hap2–Ino80C drives the inherent instability of H3 nucleosomes on centromeric DNA via transcription-coupled nucleosome turnover, providing the opportunity for incorporation of CENP-ACnp1 in place of histone H3, when CENP-ACnp1 is available (Fig. 7).
Figure 7.
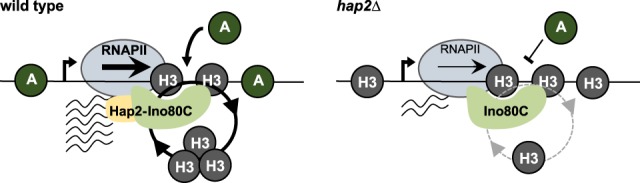
Hap2–Ino80-mediated transcription facilitates de novo establishment of CENP-ACnp1 chromatin. Model for the establishment of CENP-ACnp1 chromatin in S. pombe. (Left) Hap2 is required for transcription initiation events to maintain high H3 turnover thus renders H3 nucleosomes unstable on centromeric sequences allowing its replacement with CENP-ACnp1 nucleosomes. (Right) Cells lacking Hap2 have lower transcription initiation events that stabilize the H3 nucleosomes on centromeric sequences thus lowering its propensity to establish CENP-ACnp1 chromatin.
Studies in a variety of species have shown that Ino80C influences transcription (Cai et al. 2007; Klopf et al. 2009; Hogan et al. 2010). The noncanonical human Yin Yang 1 (YY1) transcription factor is known to associate with Ino80 and facilitate both transcriptional activation and repression (Yao et al. 2001; Cai et al. 2007). It is also known that Ino80 suppresses antisense and other noncoding transcription (Alcid and Tsukiyama 2014; Marquardt et al. 2014). The noncoding transcription of centromeric DNA by RNAPII has been implicated in CENP-A deposition in several systems (Chueh et al. 2009; Chan et al. 2012; Rošić et al. 2014; Grenfell et al. 2016; McNulty et al. 2017). However, both reduced and increased transcription of centromere DNA appears to be incompatible with CENP-A chromatin integrity and centromere function (Hill and Bloom 1987; Nakano et al. 2008; Ohkuni and Kitagawa 2011; Bergmann et al. 2012). Such observations suggest that an appropriate level and type of programmed transcription may be required to promote CENP-A assembly on centromere DNA.
Each fission yeast centromere contains a central domain of ∼10 kb assembled in CENP-ACnp1 rather than H3 nucleosomes. Multiple transcriptional start sites are detected on both strands when central domain is assembled in H3, rather than CENP-ACnp1, chromatin at an ectopic locus (Choi et al. 2011; Catania et al. 2015), indicating that these regions are pervasively transcribed. Distinct Ino80C modules undertake particular tasks such as nucleosome binding, sliding, and ATPase activity (Chen et al. 2011). The specific impact of Hap2 on Ino80C activity and how this is altered by loss of other subunits such as Ies4 remains to be determined. However, a major activity of Ino80C is to slide nucleosomes relative to sizable lengths of flanking unoccupied DNA (Udugama et al. 2011). Thus, the generation of nucleosome free regions that facilitate RNAPII recruitment, and consequently transcription, are an intrinsic facet of Ino80C function. Consequently, the loss of Hap2–Ino80C is expected to occlude central domain promoters with nucleosomes and result in reduced transcriptional initiation.
Since RNAPII recruitment to central domain chromatin during G2 phase of the cell cycle is coincident with H3 eviction and CENP-ACnp1 incorporation (Shukla et al. 2018), it is likely that these events are somehow coupled. The conserved heptad repeat composing the C-terminal domain (CTD) of RNAPII undergoes S5 phosphorylation (S5P) at promoters upon transcription initiation and S2 phosphorylation (S2P) in coding regions during transcriptional elongation (Komarnitsky et al. 2000). RNAPII stalls when it encounters obstacles such as DNA damage or natural barriers (Poli et al. 2017). Despite relatively high levels of RNAPII being detected on H3-assembled ectopic central domain, meagre levels of transcripts are produced, consistent with transcriptional stalling (Choi et al. 2011; Catania et al. 2015). Analyses in S. cerevisiae show that Ino80 is required to release stalled RNAPII from chromatin and enable its proteasomal degradation (Lafon et al. 2015). Previously, we showed that mutants (ubp3Δ, tfs1Δ) expected to increase the levels of stalled RNAPIIS2P on central domain chromatin, promote CENP-A incorporation (Catania et al. 2015). Interestingly, cells lacking Hap2 display lower initiating RNAPIIS5P over ectopic H3-assembled central domain chromatin in G2 but the levels of total RNAPII and elongating RNAPII-S2P are unaltered (Fig. 6B; Supplemental Fig. S6A,B). This indicates that, although lower levels of initiation and transcription take place within ectopic central domain chromatin in the absence of Hap2, elongating RNAPII is retained across the domain. We interpret these observations to indicate that Hap2–Ino80C is required to release elongating RNAPII that becomes trapped or stalled in central domain chromatin. We suggest that resident H3 nucleosomes are evicted by Hap2–Ino80C as part of the process involved in removing this stalled RNAPII. Thus, centromere DNA may be programmed to be pervasively transcribed and stall RNAPII over a relatively large 10-kb region in order to recruit Hap2–Ino80C and trigger H3 turnover throughout this extensive domain, thereby providing the opportunity for CENP-A incorporation. Stalling may result from collisions between converging RNAPII complexes or other obstacles such as the replication origin recognition complex (ORC) which is bound to the many AT-rich tracts present within central domain regions (Hayashi et al. 2007). Alternatively, elongating RNAPII on central domain DNA may be somehow earmarked for removal using cues analogous to those that allow Ino80 to suppresses antisense and cryptic unstable transcripts (Alcid and Tsukiyama 2014). Thus, pervasive transcription across an extensive region such as the central domain may itself provoke Ino80C recruitment.
Histone turnover rates are generally higher for nucleosomes close to promoters (Dion et al. 2007). The +1 nucleosomes exhibit high levels of turnover and Ino80C has been reported to mediate the exchange of H2A.Z for canonical H2A in such nucleosomes (Papamichos-Chronakis et al. 2011; Watanabe et al. 2013; Yen et al. 2013). However, H2A.Z turnover on transcribed genes has been shown to be not reliant on Ino80 activity (Tramantano et al. 2016). Moreover, Ino80 is also known to reduce transcription from some promoters, independently of its role in removing H2A.Z (Barbaric et al. 2007). The fact that no difference in the levels of H2A.ZPht1 was detected across the ectopic central domain in hap2Δ cells relative to wild-type (Supplemental Fig. S5B) suggests that Hap2–Ino80C does not mediate H2A.ZPht1 eviction from central domain chromatin and that it must affect some other aspect of centromere promoter function, perhaps through its known nucleosome sliding activity. Thus, Ino80C may be required to slide nucleosomes away from central domain promoters so that they are efficiently transcribed and the resulting transcriptional properties of this domain mediate H3 nucleosome turnover.
Previously, we showed that CENP-ACnp1 competes with histone H3 for incorporation into centromeric chromatin and CENP-ACnp1 incorporation is promiscuous, replacing H3 when the opportunity arises (Castillo et al. 2007; Choi et al. 2011, 2012). Thus, high histone H3 nucleosome turnover, or low nucleosome occupancy, appears to be an underlying property of many sequences that are prone to CENP-ACnp1 assembly in fission yeast. As transcription appears to be widespread at centromeres in other organisms, high turnover of resident H3 nucleosomes, stimulated by Ino80C, may be a conserved attribute of centromeric DNA that stimulates CENP-A deposition.
Materials and methods
Additional methods are described in the Supplemental Material, including lists of strains (Supplemental Table S6), plasmids (Supplemental Table S7), and primers (Supplemental Table S8) used in this study.
Affinity purification mass spectrometry analysis
Native coimmunoprecipitated samples were analyzed on an Orbitrap Fusion Lumos Tribrid mass spectrometer and on a Q Exactive (both from Thermo Fisher Scientific) both coupled online to an Ultimate 3000 RSLCnano system (Dionex, Thermo Fisher Scientific). For details of the separation, mass spectrometer parameters, and data analysis, see the Supplemental Material and Supplemental Tables S3–S5. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the Proteomics Identifications (PRIDE) (Vizcaíno et al. 2016) partner repository with the data set identifier PXD016602.
Establishment assays
Cells transformed with minichromosomes were plated on PMG-uracil-adenine plates and incubated for 5–10 d at 32°C until medium-sized colonies had grown. Colonies were replica-plated to PMG low-adenine (10 µg/mL) plates to determine the frequency of establishment of centromere function. These indicator plates allow minichromosome loss (red colony) or retention (white/pale pink colony) to be determined. In the absence of centromere establishment, minichromosomes behave as episomes that are rapidly lost. Minichromosomes occasionally integrate giving a false-positive white phenotype. To assess the frequency of such integration events and to confirm establishment of centromere segregation function, colonies giving the white/pale-pink phenotype upon replica plating were restreaked to single colonies on low-adenine plates. Sectored colonies are indicative of segregation function with low levels of minichromosome loss, whereas pure white colonies are indicative of integration into endogenous chromosomes and the establishment frequency was adjusted accordingly.
LacZ assays
LacZ assays were performed as described (Guarente 1983). Plasmids containing LacZ with upstream nmt81 promoter, 200-bp sequences from centromere 2 or no promoter were used as described (Catania et al. 2015). Plasmids were transformed into wild-type and hap2Δ strains and grown on minimal medium.
ChIP-seq and ChIP-nexus
ChIP-seq and ChIP-nexus were prepared essentially as described (Shukla et al. 2018). For details, see the Supplemental Material. The accession number of the sequencing data reported for Hap2-GFP and Arp5-3HA is GEO GSE141524 and for CENP-ACnp1 (HA and T7 tag), GEO GSE136305.
Supplementary Material
Acknowledgments
We thank members of the Allshire laboratory for advice and useful suggestions. We thank Sito Torres-Garcia for running the ChIP samples on MiniSeq system. We thank Dominik Hoelper for critical reading of the manuscript. This research was supported by award of a Darwin Trust of Edinburgh Studentship to P.P.S., a Wellcome Senior Research Fellowship (103139), and Wellcome Instrument grant (108504) to J.R.; a Wellcome Principal Research Fellow to R.C.A. (095021 and 200885); and core funding for the Wellcome Centre for Cell Biology (203149).
Author contributions: P.P.S. and R.C.A. jointly conceived the study. P.P.S., M.S., and S.A.W. performed experiments. P.P.S., M.L., and P.T. analyzed data. T.A. provided guidance in sample preparation for mass spectrometry. C.S. and J.R. performed mass spectrometry and MaxQuant analysis. P.P.S., A.L.P., and R.C.A. wrote the manuscript with input from other authors.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.332536.119.
Freely available online through the Genes & Development Open Access option.
References
- Alcid EA, Tsukiyama T. 2014. ATP-dependent chromatin remodeling shapes the long noncoding RNA landscape. Genes Dev 28: 2348–2360. 10.1101/gad.250902.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Javerzat JP, Redhead NJ, Cranston G. 1994. Position effect variegation at fission yeast centromeres. Cell 76: 157–169. 10.1016/0092-8674(94)90180-5 [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev 9: 218–233. 10.1101/gad.9.2.218 [DOI] [PubMed] [Google Scholar]
- Barbaric S, Luckenbach T, Schmid A, Blaschke D, Hörz W, Korber P. 2007. Redundancy of chromatin remodeling pathways for the induction of the yeast PHO5 promoter in vivo. J Biol Chem 282: 27610–27621. 10.1074/jbc.M700623200 [DOI] [PubMed] [Google Scholar]
- Barnhart MC, Kuich PHJL, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR. 2011. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194: 229–243. 10.1083/jcb.201012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M, Ngan VK, Clarke L. 1994. The centromeric K-type repeat and the central core are together sufficient to establish a functional Schizosaccharomyces pombe centromere. Mol Biol Cell 5: 747–761. 10.1091/mbc.5.7.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann JH, Rodríguez MG, Martins NMC, Kimura H, Kelly DA, Masumoto H, Larionov V, Jansen LET, Earnshaw WC. 2011. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J 30: 328–340. 10.1038/emboj.2010.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann JH, Jakubsche JN, Martins NM, Kagansky A, Nakano M, Kimura H, Kelly DA, Turner BM, Masumoto H, Larionov V, et al. 2012. Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function. J Cell Sci 125: 411–421. 10.1242/jcs.090639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkov GOM, Gilbert N, Heun P. 2018. Centromere transcription allows CENP-A to transit from chromatin association to stable incorporation. J Cell Biol 217: 1957–1972. 10.1083/jcb.201611087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma S, Udugama MI, Kim J, Hada A, Bhardwaj SK, Hailu SG, Lee TH, Bartholomew B. 2017. INO80 exchanges H2A.Z for H2A by translocating on DNA proximal to histone dimers. Nat Commun 8: 15616 10.1038/ncomms15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Jin J, Yao T, Gottschalk AJ, Swanson SK, Wu S, Shi Y, Washburn MP, Florens L, Conaway RC, et al. 2007. YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol 14: 872–874. 10.1038/nsmb1276 [DOI] [PubMed] [Google Scholar]
- Castillo AG, Mellonea BG, Partridge JF, Richardsonc W, Hamilton GL, Allshire RC, Pidoux AL. 2007. Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4. PLoS Genet 3: e121 10.1371/journal.pgen.0030121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo AG, Pidoux AL, Catania S, Durand-Dubief M, Choi ES, Hamilton G, Ekwall K, Allshire RC. 2013. Telomeric repeats facilitate CENP-ACnp1 incorporation via telomere binding proteins. PLoS One 8: e69673 10.1371/journal.pone.0069673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania S, Pidoux AL, Allshire RC. 2015. Sequence features and transcriptional stalling within centromere DNA promote establishment of CENP-A chromatin. PLoS Genet 11: e1004986 10.1371/journal.pgen.1004986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FL, Marshall OJ, Saffery R, Won Kim B, Earle E, Choo KHA, Wong LH. 2012. Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc Natl Acad Sci 109: 1979–1984. 10.1073/pnas.1108705109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Chavan A, Palladino J, Wei X, Martins NMC, Santinello B, Chen C-C, Erceg J, Beliveau BJ, Wu C-T, et al. 2019. Islands of retroelements are major components of Drosophila centromeres. PLoS Biol 17: e3000241 10.1371/journal.pbio.3000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Cai Y, Jin G, Florens L, Swanson SK, Washburn MP, Conaway JW, Conaway RC. 2011. Subunit organization of the human INO80 chromatin remodeling complex: an evolutionarily conserved core complex catalyzes ATP-dependent nucleosome remodeling. J Biol Chem 286: 11283–11289. 10.1074/jbc.M111.222505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Dechassa ML, Bettini E, Ledoux MB, Belisario C, Heun P, Luger K, Mellone BG. 2014. CAL1 is the Drosophila CENP-A assembly factor. J Cell Biol 204: 313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Bowers S, Lipinszki Z, Palladino J, Trusiak S, Bettini E, Rosin L, Przewloka MR, Glover DM, O'Neill RJ, et al. 2015. Establishment of centromeric chromatin by the CENP-A assembly factor CAL1 requires FACT-mediated transcription. Dev Cell 34: 73–84. 10.1016/j.devcel.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Dong F, Langdon T, Ouyang S, Buell CR, Gu M, Blattner FR, Jiang J. 2002. Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 14: 1691–1704. 10.1105/tpc.003079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ES, Strålfors A, Castillo AG, Durand-Dubief M, Ekwall K, Allshire RC. 2011. Identification of noncoding transcripts from within CENP-A chromatin at fission yeast centromeres. J Biol Chem 286: 23600–23607. 10.1074/jbc.M111.228510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ES, Strålfors A, Catania S, Castillo AG, Svensson JP, Pidoux AL, Ekwall K, Allshire RC. 2012. Factors that promote H3 chromatin integrity during transcription prevent promiscuous deposition of CENP-ACnp1 in fission yeast. PLoS Genet 8: e1002985 10.1371/journal.pgen.1002985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ES, Cheon Y, Kang K, Lee D. 2017. The Ino80 complex mediates epigenetic centromere propagation via active removal of histone H3. Nat Commun 8: 529 10.1038/s41467-017-00704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh AC, Northrop EL, Brettingham-Moore KH, Choo KHA, Wong LH. 2009. LINE retrotransposon RNA is an essential structural and functional epigenetic component of a core neocentromeric chromatin. PLoS Genet 5: e1000354 10.1371/journal.pgen.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. 2009. The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304. 10.1146/annurev.biochem.77.062706.153223 [DOI] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW. 2009. The INO80 chromatin remodeling complex in transcription, replication and repair. Trends Biochem Sci 34: 71–77. 10.1016/j.tibs.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ. 2007. Dynamics of replication-independent histone turnover in budding yeast. Science 315: 1405–1408. [DOI] [PubMed] [Google Scholar]
- Duda Z, Trusiak S, O'Neill R. 2017. Centromere transcription: means and motive BT - centromeres and kinetochores: discovering the molecular mechanisms underlying chromosome inheritance. Prog Mol Subcell Biol 56: 257–281. 10.1007/978-3-319-58592-5_11 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mellone BG, Betts CM, Zhang W, Karpen GH, Straight AF. 2008. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol 183: 805–818. 10.1083/jcb.200806038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinetti D, Han JS, McMahon MA, Ly P, Abdullah A, Wong AJ, Cleveland DW. 2015. DNA sequence-specific binding of CENP-B enhances the fidelity of human centromere function. Dev Cell 33: 314–327. 10.1016/j.devcel.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco HD, Pidoux AL, Urano T, Allshire RC. 2008. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319: 94–97. 10.1126/science.1150944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa T, Earnshaw WC. 2014. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell 30: 496–508. 10.1016/j.devcel.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady DL, Ratliff RL, Robinson DL, McCanlies EC, Meyne J, Moyzis RK. 1992. Highly conserved repetitive DNA sequences are present at human centromeres. Proc Natl Acad Sci 89: 1695–1699. 10.1073/pnas.89.5.1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell AW, Heald R, Strzelecka M. 2016. Mitotic noncoding RNA processing promotes kinetochore and spindle assembly in Xenopus. J Cell Biol 214: 133–141. 10.1083/jcb.201604029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. 1983. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol 101: 181–191. 10.1016/0076-6879(83)01013-7 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Katou Y, Itoh T, Tazumi M, Yamada Y, Takahashi T, Nakagawa T, Shirahige K, Masukata H. 2007. Genome-wide localization of pre-RC sites and identification of replication origins in fission yeast. EMBO J 26: 1327–1339. 10.1038/sj.emboj.7601585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Johnston J, Zeitlinger J. 2015. ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat Biotechnol 33: 395–401. 10.1038/nbt.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Bloom K. 1987. Genetic manipulation of centromere function. Mol Cell Biol 7: 2397–2405. 10.1128/MCB.7.7.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CJ, Aligianni S, Durand-Dubief M, Persson J, Will WR, Webster J, Wheeler L, Mathews CK, Elderkin S, Oxley D, et al. 2010. Fission yeast Iec1–Ino80-mediated nucleosome eviction regulates nucleotide and phosphate metabolism. Mol Cell Biol 30: 657–674. 10.1128/MCB.01117-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Shang WH, Takeuchi K, Fukagawa T. 2013. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol 200: 45–60. 10.1083/jcb.201210106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Ogiyama Y, Chikashige Y, Soejima S, Masuda F, Kakuma T, Hiraoka Y, Takahashi K. 2008. Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science 321: 1088–1091. 10.1126/science.1158699 [DOI] [PubMed] [Google Scholar]
- Jansen LET, Black BE, Foltz DR, Cleveland DW. 2007. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176: 795–805. 10.1083/jcb.200701066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagansky A, Diego Folco H, Almeida R, Pidoux AL, Boukaba A, Simmer F, Urano T, Hamilton GL, Allshire RC. 2009. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 324: 1716–1719. 10.1126/science.1172026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketel C, Wang HSW, McClellan M, Bouchonville K, Selmecki A, Lahav T, Gerami-Nejad M, Berman J. 2009. Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet 5: e1000400 10.1371/journal.pgen.1000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D, Ackford HE, Taylor BA, Cooke HJ. 1991. Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere. Genomics 11: 235–241. 10.1016/0888-7543(91)90128-2 [DOI] [PubMed] [Google Scholar]
- Klopf E, Paskova L, Sole C, Mas G, Petryshyn A, Posas F, Wintersberger U, Ammerer G, Schuller C. 2009. Cooperation between the INO80 complex and histone chaperones determines adaptation of stress gene transcription in the yeast Saccharomyces cerevisiae. Mol Cell Biol 29: 4994–5007. 10.1128/MCB.01858-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnitsky P, Cho EJ, Buratowski S. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev 14: 2452–2460. 10.1101/gad.824700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJPL, Weaver BAA, Cleveland DW. 2005. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5: 773–785. 10.1038/nrc1714 [DOI] [PubMed] [Google Scholar]
- Lafon A, Taranum S, Pietrocola F, Dingli F, Loew D, Brahma S, Bartholomew B, Papamichos-Chronakis M. 2015. INO80 chromatin remodeler facilitates release of RNA polymerase II from chromatin for ubiquitin-mediated proteasomal degradation. Mol Cell 60: 784–796. 10.1016/j.molcel.2015.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lermontova I, Schubert V, Fuchs J, Klatte S, Macas J, Schubert I. 2006. Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain. Plant Cell 18: 2443–2451. 10.1105/tpc.106.043174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling YH, Yuen KWY. 2019. Point centromere activity requires an optimal level of centromeric noncoding RNA. Proc Natl Acad Sci 116: 6270–6279. 10.1073/pnas.1821384116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon GA, Gambogi CW, Liskovykh MA, Barrey EJ, Larionov V, Miga KH, Heun P, Black B. 2019. Human artificial chromosomes that bypass centromeric DNA. Cell 178: 624–639.e19. doi: 10.1016/j.cell.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt S, Escalante-Chong R, Pho N, Wang J, Churchman LS, Springer M, Buratowski S. 2014. A chromatin-based mechanism for limiting divergent noncoding transcription. Cell 157: 1712–1723. 10.1016/j.cell.2014.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M, Laflamme L, Gervais AL, Gaudreau L. 2010. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics 5: 267–272. 10.4161/epi.5.4.11520 [DOI] [PubMed] [Google Scholar]
- McNulty SM, Sullivan LL, Sullivan BA. 2017. Human centromeres produce chromosome-specific and array-specific α satellite transcripts that are complexed with CENP-A and CENP-C. Dev Cell 42: 226–240.e6. 10.1016/j.devcel.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone BG, Grive KJ, Shteyn V, Bowers SR, Oderberg I, Karpen GH. 2011. Assembly of Drosophila centromeric chromatin proteins during mitosis. PLoS Genet 7: e1002068 10.1371/journal.pgen.1002068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiburo MJ, Padeken J, Fülöp S, Schepers A, Heun P. 2011. Drosophila CENH3 is sufficient for centromere formation. Science 334: 686–690. 10.1126/science.1206880 [DOI] [PubMed] [Google Scholar]
- Nakano M, Cardinale S, Noskov VN, Gassmann R, Vagnarelli P, Kandels-Lewis S, Larionov V, Earnshaw WC, Masumoto H. 2008. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev Cell 14: 507–522. 10.1016/j.devcel.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni K, Kitagawa K. 2011. Endogenous transcription at the centromere facilitates centromere activity in budding yeast. Curr Biol 21: 1695–1703. 10.1016/j.cub.2011.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzeki J, Shono N, Otake K, Martins NMC, Kugou K, Kimura H, Nagase T, Larionov V, Earnshaw WC, Masumoto H. 2016. KAT7/HBO1/MYST2 regulates CENP-A chromatin assembly by antagonizing Suv39h1-mediated centromere inactivation. Dev Cell 37: 413–427. 10.1016/j.devcel.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Ohzeki J, Nakano M, Yoda K, Brinkley WR, Larionov V, Masumoto H. 2007. CENP-B controls centromere formation depending on the chromatin context. Cell 131: 1287–1300. 10.1016/j.cell.2007.10.045 [DOI] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. 2011. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144: 200–213. 10.1016/j.cell.2010.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JF, Borgstrøm B, Allshire RC. 2000. Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev 14: 783–791. [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K. 2004. Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol 14: 1962–1967. 10.1016/j.cub.2004.09.086 [DOI] [PubMed] [Google Scholar]
- Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K. 2009. Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185: 397–407. 10.1083/jcb.200903088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Richardson W, Allshire RC. 2003. Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J Cell Biol 161: 295–307. 10.1083/jcb.200212110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli J, Gasser SM, Papamichos-Chronakis M. 2017. The INO80 remodeller in transcription, replication and repair. Philos Trans R Soc B Biol Sci 372: 20160290 10.1098/rstb.2016.0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rošić S, Köhler F, Erhardt S. 2014. Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J Cell Biol 207: 335–349. 10.1083/jcb.201404097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi L, Siggens L, Svensson JP, Ekwall K. 2014. Centromeric histone H2B monoubiquitination promotes noncoding transcription and chromatin integrity. Nat Struct Mol Biol 21: 236–243. 10.1038/nsmb.2776 [DOI] [PubMed] [Google Scholar]
- Schuh M, Lehner CF, Heidmann S. 2007. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol 17: 237–243. 10.1016/j.cub.2006.11.051 [DOI] [PubMed] [Google Scholar]
- Shang WH, Hori T, Martins NMC, Toyoda A, Misu S, Monma N, Hiratani I, Maeshima K, Ikeo K, Fujiyama A, et al. 2013. Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell 24: 635–648. 10.1016/j.devcel.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M, Tong P, White SA, Singh PP, Reid AM, Catania S, Pidoux AL, Allshire RC. 2018. Centromere DNA destabilizes H3 nucleosomes to promote CENP-A deposition during the cell cycle. Curr Biol 28: 3924–3936.e4. 10.1016/j.cub.2018.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong P, Pidoux AL, Toda NRT, Ard R, Berger H, Shukla M, Torres-Garcia J, Müller CA, Nieduszynski CA, Allshire RC. 2019. Interspecies conservation of organisation and function between nonhomologous regional centromeres. Nat Commun 10: 2343 10.1038/s41467-019-09824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramantano M, Sun L, Au C, Labuz D, Liu Z, Chou M, Shen C, Luk E. 2016. Constitutive turnover of histone H2A.Z at yeast promoters requires the preinitiation complex. Elife 5: e14243 10.7554/eLife.14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama M, Sabri A, Bartholomew B. 2011. The INO80 ATP-dependent chromatin remodeling complex is a nucleosome spacing factor. Mol Cell Biol 31: 662–673. 10.1128/MCB.01035-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, Workman JL. 2015. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16: 178–189. 10.1038/nrm3941 [DOI] [PubMed] [Google Scholar]
- Verzijlbergen KF, Menendez-Benito V, van Welsem T, van Deventer SJ, Lindstrom DL, Ovaa H, Neefjes J, Gottschling DE, van Leeuwen F. 2010. Recombination-induced tag exchange to track old and new proteins. Proc Natl Acad Sci 107: 64–68. 10.1073/pnas.0911164107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno JA, Csordas A, Del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, et al. 2016. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44: 11033 10.1093/nar/gkv1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfridsson J, Bjerling P, Thalen M, Yoo EJ, Park SD, Ekwall K. 2005. The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res 33: 2868–2879. 10.1093/nar/gki579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Radman-Livaja M, Rando OJ, Peterson CL. 2013. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340: 195–199. 10.1126/science.1229758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Van C, Pradhan SK, Su T, Gehrke J, Kuryan BG, Kitada T, Vashisht A, Tran N, Wohlschlege J, et al. 2015. The ino80 complex prevents invasion of euchromatin into silent chromatin. Genes Dev 29: 350–355. 10.1101/gad.256255.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YL, Yang WM, Seto E. 2001. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol 21: 5979–5991. 10.1128/MCB.21.17.5979-5991.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K, Vinayachandran V, Pugh BF. 2013. SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell 154: 1246–1256. 10.1016/j.cell.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.