Abstract
BACKGROUND
Gastroesophageal reflux disease (GERD) occurs when the reflux of stomach contents causes troublesome symptoms and/or complications. When medical therapy is insufficient, surgical therapy is indicated and, until now, Laparoscopic fundoplication (LF) constitutes the gold-standard method. However, magnetic sphincter augmentation (MSA) using the LINX® Reflux Management System has recently emerged and disputes the standard therapeutic approach.
AIM
To investigate the device’s safety and efficacy in resolving GERD symptoms.
METHODS
This is a systematic review conducted in accordance to the PRISMA guidelines. We searched MEDLINE, Clinicaltrials.gov, EMBASE, Cochrane Central Register of Controlled Trials CENTRAL databases from inception until September 2019.
RESULTS
Overall, 35 studies with a total number of 2511 MSA patients were included and analyzed. Post-operative proton-pump inhibitor (PPI) cessation rates reached 100%, with less bloating symptoms and a better ability to belch or vomit in comparison to LF. Special patient groups (e.g., bariatric or large hiatal-hernias) had promising results too. The most common postoperative complication was dysphagia ranging between 6% and 83%. Dilation due to dysphagia occurred in 8% of patients with typical inclusion criteria. Esophageal erosion may occur in up to 0.03% of patients. Furthermore, a recent trial indicated MSA as an efficient alternative to double-dose PPIs in moderate-to-severe GERD.
CONCLUSION
The findings of our review suggest that MSA has the potential to bridge the treatment gap between maxed-out medical treatment and LF. However, further studies with longer follow-up are needed for a better elucidation of these results.
Keywords: LINX® reflux management system, Magnetic sphincter augmentation, Gastroesophageal reflux disease, Gastroesophageal reflux disease - health - related quality of life
Core tip: Gastroesophageal reflux disease occurs when the reflux of stomach contents causes troublesome symptoms and/or complications. When medical therapy is insufficient, surgical therapy is indicated and, until now, laparoscopic fundoplication (LF) constitutes the gold-standard method. However, Magnetic sphincter augmentation (MSA) using the LINX® Reflux Management System has recently emerged and disputes the standard therapeutic approach. The findings of our review enforce the notion that MSA has the potential to bridge the treatment gap between maxed-out medical treatment and LF. However, further studies with longer follow-up are needed for a better elucidation of these results.
INTRODUCTION
Gastroesophageal reflux disease (GERD) represents the most common gastrointestinal disorder of the esophagus, with an estimated prevalence of 10%-30% in the western world[1]. According to the Montreal definition of GERD, it is defined as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications”[2]. The main underlying causal mechanism of GERD constitutes a failure in the valvular mechanism of the esophagogastric junction, which normally prevents reflux of stomach contents. This mechanism consists of six anatomic elements; the lower esophageal sphincter (LES), the diaphragmatic crura, the abdominal part of the esophagus, the acute angle of His and the Gubaroff valves[3]. GERD typically presents with regurgitation and heartburn, which constitute hallmark clinical signs[4]. However, the Montreal definition and classification of GERD describes a wide range of clinical presentation, from typical esophageal symptoms to atypical cardiac, laryngeal, and pulmonary ones[2].
Should GERD stay undertreated, a series of severe complications may occur. Erosive esophagitis, peptic stricture, aspiration pneumonia, exacerbations of chronic obstructive lung disease and lung fibrosis have been associated with reflux of gastric contents into the esophagus and the airways[5]. GERD can also cause Barrett’s esophagus (BE), a precancerous state for esophageal adenocarcinoma[6]. The initial diagnostic approach includes some combination of symptom presentation, objective testing with endoscopy, ambulatory reflux monitoring (24 h PH-Metry), and response to antisecretory therapy. The treatment approach usually starts with lifestyle modifications and antireflux medical therapy with proton-pump inhibitors (PPIs)[7]. However, 10%-40% of patients do not respond well in standard treatment. Additionally, reflux in typical treatment is not halted, because PPIs do not fundamentally address the pathophysiology of the disease and the function of the antireflux mechanism[8].
In patients not responding to standard treatment, surgical therapy is often proposed. Laparoscopic Nissen fundoplication (LNF) has been established as the gold standard treatment procedure for GERD. Additionally, in the presence of a hiatal hernia, concurrent hiatal hernia repair should be performed along with the LNF[9]. Although its long-term safety and efficacy are well documented (postoperative symptom resolution and decreased acid reflux in up to 94% of patients), the level of technical difficulty and the possible side effects have limited LNF to a specific subset of GERD patients[7,9]. In this subset of patients, it is estimated that 25%-30% of them decline LNF, mostly because they are not willing to accept its potential long-term side effects. Therefore, in absence of alternative treatment approaches, a treatment gap occurs[10]. To bridge this gap, the magnetic sphincter augmentation (MSA) device (LINX®) of the LES first appeared in 2008[11]. Considering the relative novelty of MSA devices, this review article aims to better elucidate the concept of LINX® surgical procedure, as well as to verify its potential role in GERD treatment.
LINX® device and implantation
The MSA device of the LES (LINX®, Torax Medical, Shoreview, MN) is made up of a series of magnetic beads that are interconnected by a titanium wire and allow for expansion depending to the applied pressure. The device is placed around the esophagogastric junction and applies magnetic force in order to enhance the antireflux barrier function[12]. When the beads are closed, this magnetic force is approximately 40 g, however when fully distanced they apply much less force, approximately 7 g. As a result, the device allows the bolus during swallowing to pass the esophagus and it also allows the release of elevated gastric pressure, which is associated with belching or vomiting. On the other hand, it is highly unlikely that during digestion or at rest, the stomach would generate enough force to open the device. Consequently, the LINX® device augments the LES at rest and prevents inappropriate transient relaxation[13].
Regarding technical information, the LINX® reflux management system is laparoscopically inserted at the level of the gastroesophageal junction, with the pharyngoesophageal ligament preserved. At rest, this innovative apparatus encircles the gastroesophageal junction resembling a “Roman arch” with each bead resting against its neighbor thus preventing esophageal compression. In addition, each magnetic bead can move independently of the alongside beads in an intention to imitate normal esophageal motility. This is of critical importance as this machine responds to the movements of the esophagus rather than restrains them thus averting compression that may lead to erosion. Also, it has displayed significant reproducibility, safe side effect profile and minimal disruption of anatomy. Moreover, after the procedure, fibrous tissue forms around the MSA device, outside the esophageal wall and the diaphragmatic crura, thus enabling removal without endangering esophageal damage[13]. The system is FDA approved for magnetic resonance imaging up to 1.5 T in new generation systems, while older versions are compatible with magnetic resonance imaging up to 0.7 T[14]. Patients usually stay in the hospital for 1 d, with some centers performing LINX® as an outpatient procedure. Upon discharge, patients are instructed to return on a normal diet with frequent small volume meals, chew their meals well and discontinue any previous PPI therapy[13,15].
Indications
Non-obese patients with GERD confirmed by 24 h ambulatory pH monitoring and persisting symptoms after maximized medical therapy should be offered to proceed with the LINX® surgical procedure[14]. Officially, BE exclusion in endoscopy and confirmation of normal esophageal motility in manometry, are considered strong requirements for MSA implantation. Regarding hiatal hernias (HH), those smaller than 3 cm are verified as a clear indication for the procedure[14,16].
Contraindications
Obesity [Body mass index (BMI) > 35 kg/m2] may prevent anticipated positive outcomes after LINX® implantation[17]. Therefore, patients with obesity and confirmed GERD should be advised to lose weight before LINX® becomes a viable option. Although FDA considered usage of LINX® in large HH (> 3 cm) a “precautions”, increasing evidence exist that large HH are not a contraindication, therefore more studies are needed to better elucidate these results[18]. Moreover, if a HH greater than 3 cm is detected during the operation for LINX® implantation, it is strongly recommended to repair it before device insertion[12]. Patients with advanced esophagitis or esophageal dysmotility are also excluded[13]. An allergy to titanium, stainless steel, nickel and ferrous materials is an indisputable barrier to LINX® placement[14]. LINX is a relatively new treatment option in GERD, therefore many of the contraindications mentioned are a consequence of not extensively testing the safety and effectiveness of LINX in these patient groups. Thus, as LINX® system is more and more implanted and evaluated, BE, larger HH and mild esophageal motility disorders are not considered as contraindications.
MATERIALS AND METHODS
This is a systematic review conducted in accordance to the PRISMA guidelines for reporting systematic reviews and meta-analyses[19]. This systematic literature review was performed using the MEDLINE, Clinicaltrials.gov, EMBASE and Cochrane Controlled Register of Trials (CENTRAL) databases, from inception till 15 September, 2019. The terms “LINX®” “Magnetic Sphincter Augmentation” “MSA” “Gastroesophageal Reflux Disease” and “GERD” were utilized. “Snowball sampling” by searching the references of articles retrieved was also performed, to avoid any article losses.
Regarding the eligibility criteria, all studies assessing the implementation of MSA devices were recruited. Comparative studies of MSA and laparoscopic fundoplication (LF) were also included. Data extracted include study characteristics, initial number of patients and number of patients on the follow up, demographic characteristics of patients and clinical outcomes. A total of four investigators searched and assessed the literature.
RESULTS
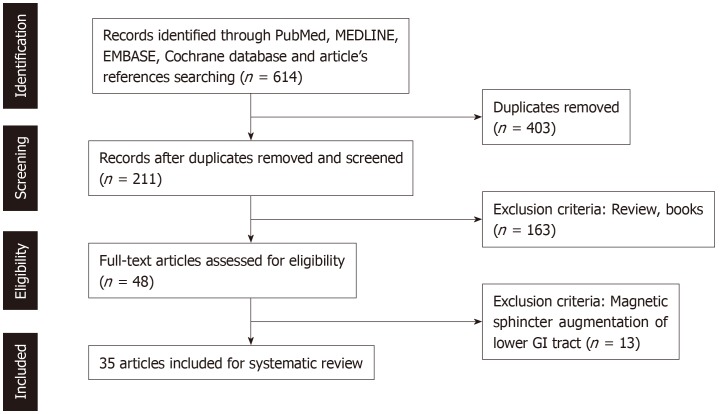
After screening 614 research articles, 579 were excluded (reviews, duplicates, articles not assessing MSA of the upper GI tract). Our literature research revealed 35 studies with a total number of 2511 MSA patients (Figure 1). Twenty of them evaluated the MSA procedure on normal indications, accounting for 1539 patients, with 1452 of them presenting on the follow up. Table 1 presents the demographic characteristics and the clinical data of patients in the 20 studies following typical MSA inclusion criteria.
Figure 1.
PRISMA flowchart.
Table 1.
Demographic and clinical characteristics of magnetic sphincter augmentation studies with typical inclusion criteria
| Ref. | Study period | Procedure | Follow-up (mo) | Study type | No. of patients (follow-up) | Mean age (yr) | Mean BMI (kg/m2) | Mean OR time (min) | DeMeester score | PPI-free | Dilation due to dysphagia | Dysphagia (%, post-op) | GERD-HRQL score (mean) | Removal in MSA or re-operation in LF | ||
| Baseline | Post-op | Baseline | Post-op | |||||||||||||
| Bonavina et al[11], 2008; Bonavina et al[20], 2010; Lipham et al[21], 2012; Saino et al[22], 2015 | Patient groups part of Bonavina et al[13], 2013 | |||||||||||||||
| Ganz et al[23], 2013 | Same patient group with Ganz et al[23], 2013 | |||||||||||||||
| Bonavina et al[24], 2013 | 2007-2012 | MSA | 12-72 | SA | 100 (95) | 44.5 | 24 | 47 | 42.3 | 11.2 | 85% | 2 | NA | 16-24 | 2 | 3 |
| Smith et al[25], 2014 | 2011-2013 | MSA | 1-18.6 | SA | 66 (66) | 53.7 | 26 | NA | NA | 83% | 4 | NA | 26 | 6 | 0 | |
| Ganz et al[26], 2016 | 2009-2014 | MSA | 60 | SA | 100 (85) | 53 | 28 | NA | NA | 75% | NA | 6% | 11-27 | 4 | 7 | |
| Warren et al[17], 2016 | 2009- 2015 | MSA | 19-60 | SA | 170 (170) | 53 | 27 | 51 | 37.9 | 15.6 | 79% | NA | NA | 26 | 5 | 1 |
| Czosnyka et al[27], 2016 | NA | MSA | 7.6 | SA | 102 (102) | 54 | 28 | NA | NA | 92% | 9 | 9% | 27 | 5 | 1 | |
| Prakash et al[28], 2017 | 2012-2015 | MSA | 12-36 | SA | 47 (47) | 53.6 | NA | 73 | NA | 83.3% | 2 | NA | 25.8 | 5.2 | 0 | |
| Schwameis et al[29], 2018 | 2012-2017 | MSA | 13 | SA | 68 (68) | 45 | 25 | 27 | NA | 87% | 2 | 16-21% | 24 | 3 | 2 | |
| Louie et al[30], 2019 | 2013-2015 | MSA | 12 | SA | 200 (182) | 48.5 | 27.4 | NA | 33.4 | 12.0 | 87.4% | 13 | 36.6% | 26 | 4 | 5 |
| Louie et al[31], 2014 | 2012-2013 | MSA | 6 | Comp. | 34 (24) | 54 | 27 | 73 | 49.5 | 14.2 | 100% | 1 | NA | 20.6 | 5.0 | 0 |
| LF | 10 | 32 (32) | 47 | 30 | 118 | 49 | 5.1 | 97% | 0 | 22.8 | 5.1 | 2 | ||||
| Sheu et al[32], 2014 | 2012-2013 | MSA | 7 | Comp. | 12 (12) | 39.3 | 26.8 | 63.7 | NA | NA | 4 | 83% | NA | NA | NA | |
| LF | 7 | 12 (12) | 43.8 | 26.8 | 90.3 | 0 | 58% | |||||||||
| Reynolds et al[33], 2015 | 2010-2013 | MSA | 12 | Comp. | 50 (47) | 53 | 26.4 | N.A | NA | 83% | 8 | 36.2% | 19.7 | 4.2 | 0 | |
| LF | 12 | 50 (47) | 54 | 26.7 | 91.5% | 5 | 31.9% | 18.8 | 4.3 | 2 | ||||||
| Riegler et al[16], 2015 | 2010- 2013 | MSA | 12 | Comp. | 202 (202) | 46.6 | 25.7 | NA | NA | 81.8% | NA | NA | 20 | 3 | 8 | |
| LF | 12 | 47 (47) | 52.8 | 26.1 | 63% | 23 | 3 | 3 | ||||||||
| Warren et al[34], 2015 | 2007-2014 | MSA | > 12 | Comp. | 201 (169) | 54 | NA | 60 | NA | 76% | NA | 44% | 21 | 3 | 2 | |
| LF | > 12 | 214 (185) | 52 | 76 | 88% | 32% | 19 | 4 | 2 | |||||||
| Reynolds et al[35], 2016 | 2010-2013 | MSA | 12 | Comp. | 52 (48) | 53 | 26 | 66 | NA | 85% | 9 | 46% | 17 | 4 | 0 | |
| LF | 12 | 67 (59) | 53 | 27 | 82 | 92% | 8 | 56% | 19 | 5 | 2 | |||||
| Asti et al[36], 2016 | 2007-2014 | MSA | 12-80 | Comp. | 135(135) | 44 | 23.94 | 42 | NA | NA | NA | NA | 21 | 0-3 | NA | |
| LF | 12-80 | 103(103) | 50 | 25.1 | 87 | 15 | 2-4 | |||||||||
| MSA total | 20 MSA | 6-80 | 13 SA | 1539 (1452) | 39.3-54 | 23.94-28 | 27-73 | 33.4-49.5 | 11.2-15.6 | 75%-100% | 8% (54/713) | 6%-83% | 11-27 | 0-6 | 2% (29/1305) | |
| NISSEN total | 7 LF | 7-80 | 7 Comp. | 525 (485) | 43.8-54 | 25.1-30 | 76-118 | 49 | 5.1 | 63%-97% | 9% (13/150) | 31.9%-58% | 15-23 | 2-5.1 | 3% (11/370) | |
NA: Not available; LF: Laparoscopic fundoplication (Nissen or Toupet); MSA: Magnetic sphincter augmentation; SA: Single-armed; Comp.: Comparative.
Due to the fact that some studies followed-up the same patient group on different time periods, only the data from the most recent study with the longest follow-up are included in the table, to avoid duplicate patient group reporting. Of the remaining 15 studies, 3 examined the efficacy of MSA on Laparoscopic Sleeve Gastrectomy patients, 3 on extended indications (e.g., large HH or increased BMI), 3 examined possible removal of MSA, 1 compared MSA with double-dose PPI medication; 2 studies of alternative surgical approaches and 1 study of esophageal erosion are also mentioned. Of the 20 studies including patients operated with normal indications, 7 were comparative between MSA and LF. Our literature research also revealed 2 meta-analyses of the comparative studies.
Studies with typical MSA inclusion criteria
Concerning the studies presented in table 1, after excluding duplicate patient populations, our literature research revealed a total of 1539 MSA patients, with 1452 of them being followed up for a period ranging between 1 and 80 mo. Most studies (15/20) had a follow-up of over 12 mo. The mean age and BMI of patients ranged between 39.3-54 years and 24-28 kg/m2, respectively. Seven studies were comparative between MSA and LF. These studies are additionally discussed in a different section bellow.
The mean OR time ranged between 27-73 min. A hospital length stay ranging between 13-100 h was reported. The most common complication was mild dysphagia, which occurred in 6%-83% of patients. In case of persistent dysphagia, balloon dilation was performed as an initial treatment approach, which occurred in 8% of patients. Additionally, in 2% of patients, device removal was required, due to dysphagia or recurrent heartburn/regurgitation or esophageal wall erosion. The device removal procedure occurred uneventfully in all of them. Regarding the results of MSA implantation as a therapeutic approach to GERD, between 75% and 100% of patients, depending on the study, stayed PPI free after surgery. Moreover, their DeMeester score ranged between 33.4 and 49.5 pre-operatively, while dropping to 11.2-15.6 post-operatively. The mean GERD health-related quality of life (GERD-HRQL) score pre-operatively was in the 11-27 range while post-operatively dropped in the 0-6 range.
Comparing MSA and LF: 2 meta-analyses
Aiolfi et al[37], conducted a meta-analysis of the 7 comparative studies mentioned in the literature. This 2018 study included a total number of 1211 patients, 686 MSA and 525 LF. There was no incidence of death in either group; however postoperative morbidity was more frequent among patients who underwent LF (0-3% in the MSA group and 0-7% in the LF group). The operative time was longer for the LF group compared to MSA group (42-73 min in the MSA group and 76-118 in the LF group). Severe dysphagia treated with endoscopic dilatation occurred in 9.3% of MSA patients and 6.6% of LF patients, a difference though not statistically significant. In addition, their results demonstrated a strong association between MSA and less bloating symptoms (P < 0.001), a greater ability to vomit (P < 0.001) and belch (P < 0.001). There was no statistically significant difference between PPI suspension and reoperation rates[37]. Similarly, in another meta-analysis of 6 comparative studies conducted in 2019, statistically significant differences occurred only in belching and bloating, whereas there were no statistically significant differences in GERD-HRQL, PPI suspension and dysphagia[38].
Assessing device removal
Aiming to examine the safety profile of the MSA device, Lipham et al[39], designed a study which analyzed all the available data of the first 1000 patients who underwent MSA at 82 institutions worldwide. Median implant duration was 274 d and the results showed that intra/perioperative complications occurred in 0.1% of patients, 1.3% needed readmission and endoscopic dilations were noted in 5.6% of patients. Furthermore, 3.4% of patients were re-operated, but no reoperation for device removal was performed emergently and there was no intraoperative complication or conversion into laparotomy. No device migrations or malfunctions were noted and erosion occurred in one patient (0.1%). The overall event rates were low and this analysis confirms the safety of this device and the MSA technique[39].
In the same direction, Smith et al[40] developed a subsequent study collecting data from the Manufacturer and User Facility Device Experience database between 2012 and 2016. The study included a total number of 3283 patients. Overall incidence of device removal was 2.7% while 88% of the removals occurred within 2 years after surgery, with no complications[40]. In addition, a single-center cohort study estimated the device’s safety examining reoperations for MSA removal out of 164 patients who underwent LINX® implantation. In total, 11 patients (6.7%) were explanted for a variety of reasons mostly between 12 and 24 mo after the index procedure. The main symptom indicating need for device removal was recurrence of heartburn or regurgitation in 46%. During device removal surgeons also performed partial fundoplication and there were no conversions to laparotomy or long-term complications[41].
Evaluating alternative surgical approaches
Upon some years of clinical application, recent studies considered and evaluated the efficacy and safety of alternative surgical strategies. Tatum et al[42] collected data of 182 patients who underwent MSA with the LINX® device at a single center between December 2012 and November 2016. Minimal hiatal dissection (MHD) at the diaphragmatic hiatus was used as the operative technique for MSA between December 2012 and September 2015 (n = 96), whereas all patients (n = 86) between September 2015 and 2016 were managed with obligatory dissection (OD). Mean follow-up time was 554 d for MHD group and 374 for OD group and mean hernia size according to intraoperative measurements was 0.77 cm for the MHD group compared to 3.95 cm for the OD group. At 1-year follow-up, both groups showed similar results in postoperative dysphagia; however, recurrent GERD symptoms were more frequent after MHD compared to OD (16.3% vs 3.6%, respectively). Recurrent hiatal hernia of 2 cm or greater occurred in 11.5% of patients in the MHD group, while no patient in OD group presented with this complication. Consequently, the study strongly indicated that OD of the hiatus during implantation of the device with crural closure has more favorable outcomes and results in decreased recurrence of GERD symptoms and hiatal hernia[34]. Moreover, Alnasser et al[42] focused on the need to obtain alternative access to implant the LINX® devices for patient with certain criteria; the authors described two cases that underwent MSA through left thoracotomy due to previous abdominal surgeries. They highlighted that a trans-thoracic approach is a feasible, alternative strategy for MSA[42].
MSA implantation on bariatric surgery patients
Laparoscopic Sleeve Gastrectomy in bariatric patients has been associated with new-onset or worsening of GERD symptoms[43]. In general, a BMI > 35 kg/m2 is negatively associated with excellent/good outcomes in MSA implantation[17]. However, upon losing weight, bariatric surgery patients become suitable candidates for LINX® procedure. Although our literature research revealed only 3 studies with a total of 33 bariatric patients (26 on follow-up) being assessed, the results seem very promising. The clinical and demographic characteristics of bariatric patients with MSA devices are presented in Table 2.
Table 2.
Demographic and clinical characteristics of bariatric patients with magnetic sphincter augmentation devices
| Ref. | Study period | No. of patients (follow-up) | Type of surgery | BMI on bariatric surgery (kg/m2, mean) | BMI loss between bariatric surgery and MSA (kg/m2, range) | BMI on MSA implan-tation (kg/m2, mean) | Mean period between surgery and MSA (mo) | Pre-operative GERD-HRQL score | Post-operative GERD-HRQL score | Compli-cations/ satis-faction |
| Desart et al[44], 2015 | 2014-2015 | 7 (7) | 7 LSG | 50.7 | 9.4-25.5 | NA | 18.1 | 17-18 | 5-6 | NA/All patients satisfied |
| Hawasli et al[45], 2018 | 2015-2017 | 13 (13) | 13 LSG | 46 | NA | 33 (21-44 range) | 43 | 47 (mean) | 12 (mean) | 1 Severe dysphagia-device removal/NA |
| Broderick et al[46], 2019 | 2014-2018 | 13 (6 with GERD-HRQL score) | 8 LSG 4 LRYGB 1 DS | NA | NA | 30.1 | NA | 15-45 | 5-13 | 2 endoscopic dilations/All patients satisfied |
| Cumu-lative Data | 2014-2018 | 33 (26) | 34 LSG 4 LRYGB 1 DS | Not enough information to compile cumulative data | ||||||
LSG: Laparoscopic sleeve gastrectomy; LRYGB: Laparoscopic Roux-en-Y gastric bypass; DS: Duodenal switch; MSA: Magnetic sphincter augmentation; N.A.: Not available.
The most common bariatric procedure was laparoscopic sleeve gastrectomy (LSG). The mean BMI of bariatric patients upon MSA implantation was reported to be 30.1 and 33 in two studies, with one of them reporting a BMI upper limit of 44, which is over the usual indications[45,46]. Moreover, one of the studies implementing LINX® on 13 LSG patients reported 100% satisfaction and a drop of GERD-HRQL score from 17-18 to 5-6[44]. In addition, although the vast majority of patients (28/33) had undergone LSG prior to MSA implantation, 1 out of 3 studies reported 4 Laparoscopic Roux-en-Y Gastric Bypass patients and 1 Duodenal Switch patient. The study reported 100% patient satisfaction rates[46]. For a better delineation of these results, further studies, with larger patient populations, are needed.
Seeking to extend the indications
Rona et al[47] reviewed a series of 192 patients with a median follow-up time of 20 months. Among these patients 52 (27%) presented with a large hiatal hernia (≥ 3 cm). These patients reported reduced postoperative PPI’s use compared to patients with smaller hernias (9.6 vs 26.6 %, respectively) and the mean GERD-HRQL score was improved (3.6 vs 5.6, respectively). In both groups, the majority of patients reported complete resolution of GERD symptoms[47]. The authors also analyzed and published the recurrent rate of hiatal hernia in a total of 47 patients with large (> 3 cm) hiatal hernia who were managed with laparoscopic repair combined with MSA. GERD-HRQL score was improved (from 20.3 to 3.1) and resolution of reflux symptoms was achieved in 97% of patients. Recurrence of HH occurred in 2 patients (4.3%) at a mean of 18 mo postoperatively[48]. In the same direction, Buckley et al[18] reviewed 200 patients with HH who were treated with MSA. 78% of patients appeared with hiatal hernia ≥ 5 cm and most of them (83%) were managed with non-permanent mesh reinforcement of the hiatus. Postoperatively, GERD-HRQL scores were significantly decreased (from 26 at baseline to 2) and complete cessation of PPI use was achieved in 94% of patients. Consequently, the authors indicated that hernia size does not affect the safety and efficacy of MSA[18].
MSA vs double-dose PPIs
A randomized controlled trial of 152 patients compared the MSA procedure with double-dose PPI medication for the treatment of moderate-to-severe GERD. Study inclusion criteria were participants aged > 21 years, having moderate-to-severe GERD and taking a daily single dose of PPI therapy for at least 8 wk. The rest of the inclusion criteria were similar to the typical indications of MSA implantation. The active seeking of participants for alternative, surgical treatments was a prerequisite. The results of the study indicated that the MSA implantation is superior to increased PPI medication, and patients with moderate-to-severe GERD should be recommended MSA implantation instead of double PPI doses[49].
Esophageal erosion
Esophageal erosion is regarded as the most dreadful complication of the LINX® procedure. A study collected data from 9453 device implantations all over the world. The data were obtained from the device manufacturer, Torax Medical and included records of devices implanted until 2017. The risk of esophageal erosion from the device increased from 0.05% at 1 year to 0.3% at 4 years. All of the devices were removed successfully, and in a median follow-up of 1.9 mo, 24/29 patients had returned to baseline and were symptom free[50].
The cost-effectiveness of LINX
Although many studies evaluating the safety and efficacy of LINX have emerged, our literature research revealed only two studies assessing the cost-effectiveness of MSA[35,51]. The first study retrieved data from 2 institutions and compared MSA with LNF regarding surgical admission charges. It concluded that the increased cost of MSA is completely counteracted by its shorter operative time and length of stay ($48491 vs $50111, P = 0.506)[35]. The second study retrieved data from patients in Western and Central Pennsylvania, the Lehigh Valley, West Virginia, and the border areas of eastern Ohio[51]. The cost analysis revealed that MSA has a higher same-day procedural payer cost than LNF ($13522 vs $13388, P = 0.02), which may partially be offset by a decreased need for hospital stay in MSA. Furthermore, in a follow-up of 12 mo, a higher reduction in disease-related costs was observed in the MSA group compared to the LNF group (65.9% vs 46%, P = 0.0001).
DISCUSSION
Even though GERD’s management is primarily conservative and involves diet modifications and acid reducing agents, there is a patient group responding only partially to this therapeutic approach. For years, LF was the usual alternative option in this patient group. However, after a decade of clinical application and with some studies reaching a 5-year follow-up, MSA appears to be a safe alternative for managing persistent GERD symptoms. Overall, the majority of patients reported complete resolution of their GERD symptoms, with post-operative PPI’s cessation rates reaching 100%. Interestingly, results were consistent even after applying MSA in patients with large HH, BMI > 35 kg/m2 and in bariatric patients. Different surgical approaches such as the left transthoracic were also introduced with success.
Although both LF and MSA appear to be safe, effective procedures, the MSA seems to have distinct advantages. First of all, the results of our review indicate MSA to be superior regarding the ability to vomit/belch and also to be associated with less bloating symptoms in comparison to LF. Moreover, it is generally considered a less technical procedure, designed to limit technical variability and provide more persistent outcomes[52]. Lastly, the procedure can be quite easily reversed through a device removal, with the same not applying to LF, which is a more interventional method, considered to have more severe complications when re-operation is deemed necessary. Most importantly, if the MSA procedure fails, LF is still a viable option after removing the device[12,26].
Interestingly, MSA also seems to take the high ground when compared with maxed-out dose of PPIs in a randomized controlled trial[49]. The promising results of this trial broaden the treatment options of patients seeking a more drastic and effective measure than doubling their dose of PPIs. Although this was the only study comparing MSA with double-dose PPIs, it could still be hypothesized that as MSA becomes an increasingly common procedure, future indications may propose the MSA procedure as a valid alternative to medical therapy in moderate-to-severe GERD.
Nonetheless, concerning complications, dysphagia appears to be the most common occurrence in both the MSA and the LF[38]. It should also be mentioned than when dysphagia occurs, some studies report that it is more severe in MSA than in LF[32]. However, this finding was not present in the 2 meta-analyses presented in our results. In addition, recent publications revealed rare and relatively serious complications such as esophageal erosion[50]. However, the device removal occurred uneventfully in these cases.
In conclusion, MSA with the LINX® device is considered a safe procedure with excellent results. When compared with the gold standard, LF, MSA seems to have similar efficacy and safety profiles. Nonetheless, it also has some distinct advantages. These include shorter operative time, less technical variability, less interventions on the normal anatomy, less bloating symptoms and a better ability to belch or vomit. Moreover, promising results comparing the MSA procedure with double-dose PPIs in moderate-to-severe GERD exist. Overall, the results of our review enforce the notion that the MSA procedure has the potential to bridge the treatment gap between maxed-out dose of medical treatment and LF.
ARTICLE HIGHLIGHTS
Research background
Gastroesophageal reflux disease (GERD) refers to the reflux of stomach contents causing troublesome symptoms and/or complications. When medical therapy is insufficient, surgical therapy is needed and, until now, Laparoscopic Fundoplication (LF) is the gold-standard method.
Research motivation
Magnetic sphincter augmentation (MSA) using the LINX® reflux management system has recently appeared and questions standard treatments.
Research objectives
The purpose of this review is to investigate the device’s safety and efficacy in resolving GERD symptoms.
Research methods
Our systematic review based on the PRISMA guidelines. From inception to September 2019, we searched Medline, Clinicaltrials.gov, EMBASE, Cochrane Central Register of Controlled Trials CENTRAL databases.
Research results
Overall, a total of 35 studies were included in a total of 2511 MSA patients. Post-operative proton-pump inhibitor (PPI) cessation rates reached 100%, with less bloating symptoms and a better ability to belch or vomit in comparison to LF. Special patient groups (e.g., bariatric or large hiatal-hernias) had promising results too. The most common postoperative complication was dysphagia ranging between 6% and 83%. Dilation due to dysphagia occurred in 8% of patients with typical inclusion criteria. Esophageal erosion may occur in up to 0.03% of patients. Furthermore, a recent trial indicated MSA as an efficient alternative to double-dose PPIs in moderate-to-severe GERD.
Research conclusions
The findings of our review suggest that MSA has the potential to bridge the treatment gap between maxed-out medical treatment and LF. However, further studies with longer follow-up are needed for a better elucidation of these results.
Research perspectives
MSA with the LINX® device is considered a safe procedure with excellent results. When compared with the gold standard, LF, MSA seems to have similar efficacy and safety profiles. Nonetheless, it also has some distinct advantages. These include shorter operative time, less technical variability, less interventions on the normal anatomy, less bloating symptoms and a better ability to belch or vomit. Moreover, promising results comparing the MSA procedure with double-dose PPIs in moderate-to-severe GERD exist. Overall, the results of our review enforce the notion that the MSA procedure has the potential to bridge the treatment gap between maxed-out dose of medical treatment and LF.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Unsolicited manuscript
Peer-review started: November 12, 2019
First decision: November 19, 2019
Article in press: December 14, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
Contributor Information
Dimitrios Schizas, 1st Department of Surgery, National and Kapodistrian University of Athens, Laikon Hospital, Athens 11527, Greece.
Aikaterini Mastoraki, 4th Department of Surgery, National and Kapodistrian University of Athens, Attikon University Hospital, Chaidari, Athens 11527, Greece.
Eleni Papoutsi, 1st Department of Surgery, National and Kapodistrian University of Athens, Laikon Hospital, Athens 11527, Greece.
Vassilis G Giannakoulis, 1st Department of Surgery, National and Kapodistrian University of Athens, Laikon Hospital, Athens 11527, Greece.
Prodromos Kanavidis, 1st Department of Surgery, National and Kapodistrian University of Athens, Laikon Hospital, Athens 11527, Greece.
Diamantis Tsilimigras, Department of Surgery, Division of Surgical Oncology, The Ohio State University Wexner Medical Center and James Cancer Hospital and Solove Research Institute, Columbus, OH 45830, United States.
Dimitrios Ntourakis, Department of Surgery, School of Medicine, European University Cyprus, Nicosia 2404, Cyprus.
Orestis Lyros, Department of Visceral, Transplant, Thoracic and Vascular Surgery, University Hospital Leipzig, Leipzig 04103, Germany.
Theodore Liakakos, 1st Department of Surgery, National and Kapodistrian University of Athens, Laikon Hospital, Athens 11527, Greece.
Dimitrios Moris, Department of Surgery, Duke University Medical Center, Duke University, Durham, NC 27705, United States. dimmoris@yahoo.com.
References
- 1.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Menezes MA, Herbella FAM. Pathophysiology of Gastroesophageal Reflux Disease. World J Surg. 2017;41:1666–1671. doi: 10.1007/s00268-017-3952-4. [DOI] [PubMed] [Google Scholar]
- 4.Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:105–112. doi: 10.4292/wjgpt.v5.i3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Hallerbäck B. Clinical manifestations and complications of gastroesophageal reflux disease (GERD) Int J Clin Pract. 2005;59:346–355. doi: 10.1111/j.1742-1241.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 6.Parasa S, Sharma P. Complications of gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2013;27:433–442. doi: 10.1016/j.bpg.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–28; quiz 329. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Boeckxstaens G, Smout AJ. Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol. 2013;27:401–414. doi: 10.1016/j.bpg.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore M, Afaneh C, Benhuri D, Antonacci C, Abelson J, Zarnegar R. Gastroesophageal reflux disease: A review of surgical decision making. World J Gastrointest Surg. 2016;8:77–83. doi: 10.4240/wjgs.v8.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triadafilopoulos G, Azagury D. How can we deal with the GERD treatment gap? Ann N Y Acad Sci. 2016;1381:14–20. doi: 10.1111/nyas.13104. [DOI] [PubMed] [Google Scholar]
- 11.Bonavina L, Saino GI, Bona D, Lipham J, Ganz RA, Dunn D, DeMeester T. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. 2008;12:2133–2140. doi: 10.1007/s11605-008-0698-1. [DOI] [PubMed] [Google Scholar]
- 12.Zadeh J, Andreoni A, Treitl D, Ben-David K. Spotlight on the Linx™ Reflux Management System for the treatment of gastroesophageal reflux disease: evidence and research. Med Devices (Auckl) 2018;11:291–300. doi: 10.2147/MDER.S113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonavina L, Saino G, Lipham JC, Demeester TR. LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol. 2013;6:261–268. doi: 10.1177/1756283X13486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telem DA, Wright AS, Shah PC, Hutter MM. SAGES technology and value assessment committee (TAVAC) safety and effectiveness analysis: LINX® reflux management system. Surg Endosc. 2017;31:3811–3826. doi: 10.1007/s00464-017-5813-5. [DOI] [PubMed] [Google Scholar]
- 15.Sheu EG, Rattner DW. Evaluation of the LINX antireflux procedure. Curr Opin Gastroenterol. 2015;31:334–338. doi: 10.1097/MOG.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 16.Riegler M, Schoppman SF, Bonavina L, Ashton D, Horbach T, Kemen M. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc. 2015;29:1123–1129. doi: 10.1007/s00464-014-3772-7. [DOI] [PubMed] [Google Scholar]
- 17.Warren HF, Brown LM, Mihura M, Farivar AS, Aye RW, Louie BE. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc. 2018;32:405–412. doi: 10.1007/s00464-017-5696-5. [DOI] [PubMed] [Google Scholar]
- 18.Buckley FP, 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc. 2018;32:1762–1768. doi: 10.1007/s00464-017-5859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 20.Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, Lipham J, Bemelman W, Ganz RA. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252:857–862. doi: 10.1097/SLA.0b013e3181fd879b. [DOI] [PubMed] [Google Scholar]
- 21.Lipham JC, DeMeester TR, Ganz RA, Bonavina L, Saino G, Dunn DH, Fockens P, Bemelman W. The LINX® reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc. 2012;26:2944–2949. doi: 10.1007/s00464-012-2289-1. [DOI] [PubMed] [Google Scholar]
- 22.Saino G, Bonavina L, Lipham JC, Dunn D, Ganz RA. Magnetic Sphincter Augmentation for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement. J Laparoendosc Adv Surg Tech A. 2015;25:787–792. doi: 10.1089/lap.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz RA, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:2039–2040. doi: 10.1056/NEJMc1303656. [DOI] [PubMed] [Google Scholar]
- 24.Bonavina L, Saino G, Bona D, Sironi A, Lazzari V. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg. 2013;217:577–585. doi: 10.1016/j.jamcollsurg.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Smith CD, DeVault KR, Buchanan M. Introduction of mechanical sphincter augmentation for gastroesophageal reflux disease into practice: early clinical outcomes and keys to successful adoption. J Am Coll Surg. 2014;218:776–781. doi: 10.1016/j.jamcollsurg.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, Horgan S, Jacobsen G, Luketich JD, Smith CC, Schlack-Haerer SC, Kothari SN, Dunst CM, Watson TJ, Peters J, Oelschlager BK, Perry KA, Melvin S, Bemelman WA, Smout AJ, Dunn D. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol. 2016;14:671–677. doi: 10.1016/j.cgh.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Czosnyka NM, Buckley FP, Doggett SL, Vassaur H, Connolly EE, Borgert AJ, Kallies KJ, Kothari SN. Outcomes of magnetic sphincter augmentation - A community hospital perspective. Am J Surg. 2017;213:1019–1023. doi: 10.1016/j.amjsurg.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Prakash D, Campbell B, Wajed S. Introduction into the NHS of magnetic sphincter augmentation: an innovative surgical therapy for reflux - results and challenges. Ann R Coll Surg Engl. 2018;100:251–256. doi: 10.1308/rcsann.2017.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwameis K, Nikolic M, Morales Castellano DG, Steindl A, Macheck S, Kristo I, Zörner B, Schoppmann SF. Results of Magnetic Sphincter Augmentation for Gastroesophageal Reflux Disease. World J Surg. 2018;42:3263–3269. doi: 10.1007/s00268-018-4608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louie BE, Smith CD, Smith CC, Bell RCW, Gillian GK, Mandel JS, Perry KA, Birkenhagen WK, Taiganides PA, Dunst CM, McCollister HM, Lipham JC, Khaitan LK, Tsuda ST, Jobe BA, Kothari SN, Gould JC. Objective Evidence of Reflux Control After Magnetic Sphincter Augmentation: One Year Results From a Post Approval Study. Ann Surg. 2019;270:302–308. doi: 10.1097/SLA.0000000000002789. [DOI] [PubMed] [Google Scholar]
- 31.Louie BE, Farivar AS, Shultz D, Brennan C, Vallières E, Aye RW. Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg. 2014;98:498–504; discussion 504-5. doi: 10.1016/j.athoracsur.2014.04.074. [DOI] [PubMed] [Google Scholar]
- 32.Sheu EG, Nau P, Nath B, Kuo B, Rattner DW. A comparative trial of laparoscopic magnetic sphincter augmentation and Nissen fundoplication. Surg Endosc. 2015;29:505–509. doi: 10.1007/s00464-014-3704-6. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds JL, Zehetner J, Wu P, Shah S, Bildzukewicz N, Lipham JC. Laparoscopic Magnetic Sphincter Augmentation vs Laparoscopic Nissen Fundoplication: A Matched-Pair Analysis of 100 Patients. J Am Coll Surg. 2015;221:123–128. doi: 10.1016/j.jamcollsurg.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Warren HF, Reynolds JL, Lipham JC, Zehetner J, Bildzukewicz NA, Taiganides PA, Mickley J, Aye RW, Farivar AS, Louie BE. Multi-institutional outcomes using magnetic sphincter augmentation versus Nissen fundoplication for chronic gastroesophageal reflux disease. Surg Endosc. 2016;30:3289–3296. doi: 10.1007/s00464-015-4659-y. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds JL, Zehetner J, Nieh A, Bildzukewicz N, Sandhu K, Katkhouda N, Lipham JC. Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc. 2016;30:3225–3230. doi: 10.1007/s00464-015-4635-6. [DOI] [PubMed] [Google Scholar]
- 36.Asti E, Bonitta G, Lovece A, Lazzari V, Bonavina L. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: Observational cohort study with propensity score analysis. Medicine (Baltimore) 2016;95:e4366. doi: 10.1097/MD.0000000000004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aiolfi A, Asti E, Bernardi D, Bonitta G, Rausa E, Siboni S, Bonavina L. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg. 2018;52:82–88. doi: 10.1016/j.ijsu.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Guidozzi N, Wiggins T, Ahmed AR, Hanna GB, Markar SR. Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and pooled analysis. Dis Esophagus. 2019:32. doi: 10.1093/dote/doz031. [DOI] [PubMed] [Google Scholar]
- 39.Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus. 2015;28:305–311. doi: 10.1111/dote.12199. [DOI] [PubMed] [Google Scholar]
- 40.Smith CD, Ganz RA, Lipham JC, Bell RC, Rattner DW. Lower Esophageal Sphincter Augmentation for Gastroesophageal Reflux Disease: The Safety of a Modern Implant. J Laparoendosc Adv Surg Tech A. 2017;27:586–591. doi: 10.1089/lap.2017.0025. [DOI] [PubMed] [Google Scholar]
- 41.Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the Magnetic Sphincter Augmentation Device: Surgical Technique and Results of a Single-center Cohort Study. Ann Surg. 2017;265:941–945. doi: 10.1097/SLA.0000000000001785. [DOI] [PubMed] [Google Scholar]
- 42.Alnasser SA, Salfity HV, Klapper JA, Hartwig MG. Left Transthoracic Approach for Magnetic Sphincter Augmentation Device LINX Implantation. Ann Thorac Surg. 2019;108:e225–e227. doi: 10.1016/j.athoracsur.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 43.El-Hadi M, Birch DW, Gill RS, Karmali S. The effect of bariatric surgery on gastroesophageal reflux disease. Can J Surg. 2014;57:139–144. doi: 10.1503/cjs.030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desart K, Rossidis G, Michel M, Lux T, Ben-David K. Gastroesophageal Reflux Management with the LINX® System for Gastroesophageal Reflux Disease Following Laparoscopic Sleeve Gastrectomy. J Gastrointest Surg. 2015;19:1782–1786. doi: 10.1007/s11605-015-2887-z. [DOI] [PubMed] [Google Scholar]
- 45.Hawasli A, Sadoun M, Meguid A, Dean M, Sahly M, Hawasli B. Laparoscopic placement of the LINX® system in management of severe reflux after sleeve gastrectomy. Am J Surg. 2019;217:496–499. doi: 10.1016/j.amjsurg.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Broderick RC, Smith CD, Cheverie JN, Omelanczuk P, Lee AM, Dominguez-Profeta R, Cubas R, Jacobsen GR, Sandler BJ, Fuchs KH, Horgan S. Magnetic sphincter augmentation: a viable rescue therapy for symptomatic reflux following bariatric surgery. Surg Endosc. 2019 doi: 10.1007/s00464-019-07096-z. [DOI] [PubMed] [Google Scholar]
- 47.Rona KA, Reynolds J, Schwameis K, Zehetner J, Samakar K, Oh P, Vong D, Sandhu K, Katkhouda N, Bildzukewicz N, Lipham JC. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc. 2017;31:2096–2102. doi: 10.1007/s00464-016-5204-3. [DOI] [PubMed] [Google Scholar]
- 48.Rona KA, Tatum JM, Zehetner J, Schwameis K, Chow C, Samakar K, Dobrowolsky A, Houghton CC, Bildzukewicz N, Lipham JC. Hiatal hernia recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate-term outcomes. Surg Endosc. 2018;32:3374–3379. doi: 10.1007/s00464-018-6059-6. [DOI] [PubMed] [Google Scholar]
- 49.Bell R, Lipham J, Louie B, Williams V, Luketich J, Hill M, Richards W, Dunst C, Lister D, McDowell-Jacobs L, Reardon P, Woods K, Gould J, Buckley FP, 3rd, Kothari S, Khaitan L, Smith CD, Park A, Smith C, Jacobsen G, Abbas G, Katz P. Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc. 2019;89:14–22.e1. doi: 10.1016/j.gie.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Alicuben ET, Bell RCW, Jobe BA, Buckley FP, 3rd, Daniel Smith C, Graybeal CJ, Lipham JC. Worldwide Experience with Erosion of the Magnetic Sphincter Augmentation Device. J Gastrointest Surg. 2018;22:1442–1447. doi: 10.1007/s11605-018-3775-0. [DOI] [PubMed] [Google Scholar]
- 51.Ayazi S, Zaidi AH, Zheng P, Chovanec K, Chowdhury N, Salvitti M, Newhams K, Levy J, Hoppo T, Jobe BA. Comparison of surgical payer costs and implication on the healthcare expenses between laparoscopic magnetic sphincter augmentation (MSA) and laparoscopic Nissen fundoplication (LNF) in a large healthcare system. Surg Endosc. 2019 doi: 10.1007/s00464-019-07021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonavina L, DeMeester TR, Ganz RA. LINX(™) Reflux Management System: magnetic sphincter augmentation in the treatment of gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2012;6:667–674. doi: 10.1586/egh.12.47. [DOI] [PubMed] [Google Scholar]