Checkpoint inhibitor therapy has shown tremendous efficacy, but also frequent and often severe side effects—especially when multiple drugs of the class are used simultaneously. Similarly, many investigational immunotherapy agents, which have shown promise in animal models, have failed in clinical trials due to dose-limiting toxicity when administered systemically. This study utilized a murine melanoma model to evaluate the efficacy of intratumoral injections of recombinant NDVs engineered to express multiple immunotherapeutic proteins with well-documented side effects in humans. Our results indicate that intratumoral administration of these recombinant NDVs, particularly when combined with systemic CTLA4 checkpoint inhibition, exerts a robust effect in treated and nontreated tumors, indicative of a systemic antitumoral response. The intratumoral delivery of rNDVs expressing immunotherapeutic proteins may be an effective method of targeting the immune cell populations most relevant for antitumoral immunity and allowing us to restrict the use of systemic immunotherapy agents.
KEYWORDS: anticancer therapy, immunotherapy, oncolytic viruses
ABSTRACT
Newcastle disease virus (NDV) is an attractive candidate for oncolytic immunotherapy due to its ability to replicate in tumor cells and potentially to overcome the inherently immunosuppressive nature of the tumor microenvironment. The advent of checkpoint blockade immunotherapy over the past few years represents a paradigm shift in cancer therapy. However, the prevalence of severe immune-related adverse events with CTLA4 and PD1 pathway blockade in clinical studies, especially in combination therapy groups, is a cause for concern. Immunotherapies with cytokines have also been extensively explored, but they have been associated with adverse events in clinical trials. Oncolytic vectors engineered to express checkpoint blockade antibodies and cytokines could provide an avenue for reducing the clinical toxicity associated with systemic therapy by concentrating the immunomodulatory payload at the site of disease. In this study, we engineered six different recombinant viruses: NDVs expressing checkpoint inhibitors (rNDV–anti-PD1 and rNDV–anti-PDL1); superagonists (rNDV–anti-CD28); and immunocytokines, where the antibodies are fused to an immunostimulatory cytokine, such as interleukin 12 (IL-12) (rNDV–anti-CD28–murine IL-12 [mIL-12], rNDV–anti-PD1–mIL-12, and rNDV–anti-PDL1–mIL-12). These six engineered viruses induced tumor control and survival benefits in both highly aggressive unilateral and bilateral B16-F10 murine melanoma models, indicative of an abscopal effect. The data represent a strong proof of concept on which further clinical evaluation could build.
IMPORTANCE Checkpoint inhibitor therapy has shown tremendous efficacy, but also frequent and often severe side effects—especially when multiple drugs of the class are used simultaneously. Similarly, many investigational immunotherapy agents, which have shown promise in animal models, have failed in clinical trials due to dose-limiting toxicity when administered systemically. This study utilized a murine melanoma model to evaluate the efficacy of intratumoral injections of recombinant NDVs engineered to express multiple immunotherapeutic proteins with well-documented side effects in humans. Our results indicate that intratumoral administration of these recombinant NDVs, particularly when combined with systemic CTLA4 checkpoint inhibition, exerts a robust effect in treated and nontreated tumors, indicative of a systemic antitumoral response. The intratumoral delivery of rNDVs expressing immunotherapeutic proteins may be an effective method of targeting the immune cell populations most relevant for antitumoral immunity and allowing us to restrict the use of systemic immunotherapy agents.
INTRODUCTION
Oncolytic immunotherapy provides a promising new way to activate the immune system to achieve a therapeutic goal in cancer treatment, especially in combination with checkpoint blockade (1). To become fully activated, T cells require the engagement of the T cell receptor (TCR) with the peptide-major histocompatibility complex (MHC), as well as costimulatory signals provided by the interaction of CD28 on T cells with its primary ligands, B7-1 (CD80) and B7-2 (CD86), on the surfaces of specialized antigen-presenting cells (APCs) (2). In addition to prompting T cell responses, T cell priming also induces critical negative-feedback loops in the form of immune checkpoints. One of these proteins, cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), acts by attenuating or preventing CD28 costimulation by competing for B7 binding (3, 4). Another major immune checkpoint, called programmed cell death 1 (PD1), is induced later during T cell activation and attenuates TCR signaling upon engagement with PDL1 or PDL2 (5 – 7).
Antibody-mediated release of these immune checkpoints has been shown to induce significant response rates in multiple types of advanced or metastatic malignancies, which has led to a paradigm shift in clinical practice (8 – 13). However, the use of immune checkpoint inhibitor therapy in the clinic is associated with an increase of immune-related adverse events (irAEs). Checkpoint blockade can induce minor irAEs in up to 70% to 90% of patients receiving CTLA4, PD1, or PDL1 blockade, but also major (grade 3 and 4) irAEs in up to 10% to 15% of patients (14, 15).
CD28 receptor is critical for cosimulation of naive T lymphocytes, and anti-CD28 superagonists capable of activating T cells have shown remarkable efficacy in several preclinical models (16, 17). However, clinical development of this antibody was abandoned as a result of adverse events in a phase 1 clinical trial when it was given systemically (18). Despite this initial failure, localized or targeted use of anti-CD28 monoclonal antibody (MAb) might have great potential for local induction of a powerful immune response within the tumor microenvironment.
Cytokines are either secreted or membrane-bound molecular messengers that can directly stimulate immune effector cells within the tumor microenvironment or recruit additional immune cells to enhance antitumor responses by cytotoxic effector cells (19).
Although immunotherapies with cytokines have been extensively explored, they have been associated with adverse events in randomized clinical trials (20, 21). Targeted delivery of the cytokine payload to the tumor microenvironment can be achieved by intratumoral delivery of recombinant oncolytic vectors expressing cytokines, but also through immunocytokines. Immunocytokines are antibody-cytokine fusion proteins consisting of a cytokine fused to monoclonal antibodies or to an antibody fragment (22 – 25).
Newcastle disease virus (NDV) is an enveloped, negative-sense, single-stranded RNA virus that is classified as an avian paramyxovirus type 1 (APMV-1) in the genus Avulavirus of the family Paramyxoviridae (26). NDV has been extensively studied as an oncolytic vector due to its ability to induce activation of the innate and adaptive antitumor responses, in addition to prompting immunogenic cell death (27, 28). Here, we show that NDVs can be engineered to express checkpoint inhibitor molecules and checkpoint inhibitor-cytokine conjugates (immunocytokines), which allows three different modalities, namely, NDVs, cytokines, and checkpoint blockade antibodies, to be combined into the same therapeutic platform. We cloned and rescued six different recombinant viruses: NDVs expressing checkpoint inhibitors (rNDV–anti-PD1 and rNDV–anti-PDL1); superagonists (rNDV–anti-CD28); and immunocytokines, where the antibodies are fused to an immunostimulatory cytokine, such as interleukin 12 (IL-12) (rNDV–anti-CD28–murine IL-12 [mIL-12], rNDV–anti-PD1–mIL-12, and rNDV–anti-PDL1–mIL-12). More importantly, we show tumor control and survival benefits by combining these recombinant NDVs expressing immunocytokines with systemic checkpoint blockade in both highly aggressive unilateral and bilateral B16-F10 tumor models.
RESULTS
Engineering rNDVs expressing antibody fragments and immunocytokines.
IL-12 is a known T and NK cell-stimulating factor (29, 30) and is a 4-bundle α-helix heterodimeric cytokine that consists of p35 and p40 subunits (31). Despite its well-documented role in the antitumor immune response, current clinical development of IL-12 has been limited due to toxicities associated with its systemic use (32). However, given its potent immune-stimulatory properties, limitations to its use for systemic therapy can be overcome by local delivery of the cytokine via an oncolytic virus engineered to express it.
Agonistic monoclonal antibodies against CD28 activate T cells both in vivo and in vitro without the requirement for TCR signaling, holding promise as a potent T cell-stimulatory platform (33). In addition to anti-CD28 agonistic antibodies (18), checkpoint inhibitors induce severe immune-related adverse events with CTLA4 and PD1 pathway blockade, which have been estimated to be as high as 55% with dual-checkpoint inhibitor therapy (34). One possible avenue to overcome the clinical toxicities associated with the systemic administration of anti-CD28, anti-PD1, and anti-PDL1 would be their delivery into the tumor microenvironment.
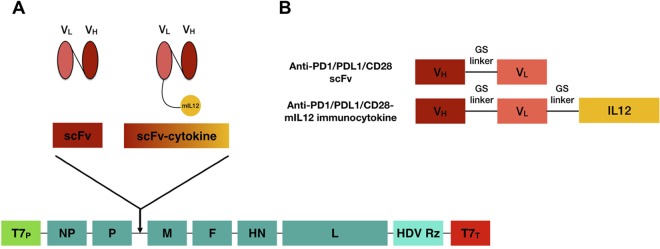
Aiming for the targeted delivery of these therapeutic regimens, we engineered recombinant full-length NDVs (rNDVs) expressing soluble single-chain variable fragments (scFvs) for anti-CD28, anti-PD1, and anti-PDL1 with the transgene inserted between the viral P and M genes (35) (Fig. 1). Furthermore, we fused these scFvs with mIL-12 via a GS linker, as shown in (Fig. 1). Rescued viruses showed no appreciable differences in their abilities to induce cell lysis at three different multiplicities of infection (MOI) in B16-F10 murine melanoma cells using a lactate dehydrogenase (LDH) release assay (data not shown) compared to wild-type NDV, suggesting that the different recombinant viruses have comparable levels of replication. In addition, mIL-12 expression from rNDVs expressing immunocytokines was verified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (data not shown).
FIG 1.
Cloning and rescue of recombinant NDVs. (A) Construction of a full-length anti-genomic NDV plasmid containing the scFv or immunocytokine. The open reading frame (ORF) for the transgene is flanked with NDV-specific transcriptional signals, followed by the Kozak sequence, and inserted into the SacII site between the P and the M ORFs. (B) Design of scFv and immunocytokine constructs. VH, variable heavy; VL, variable light.
rNDVs expressing immunocytokines enhance tumor control in the treated tumor.
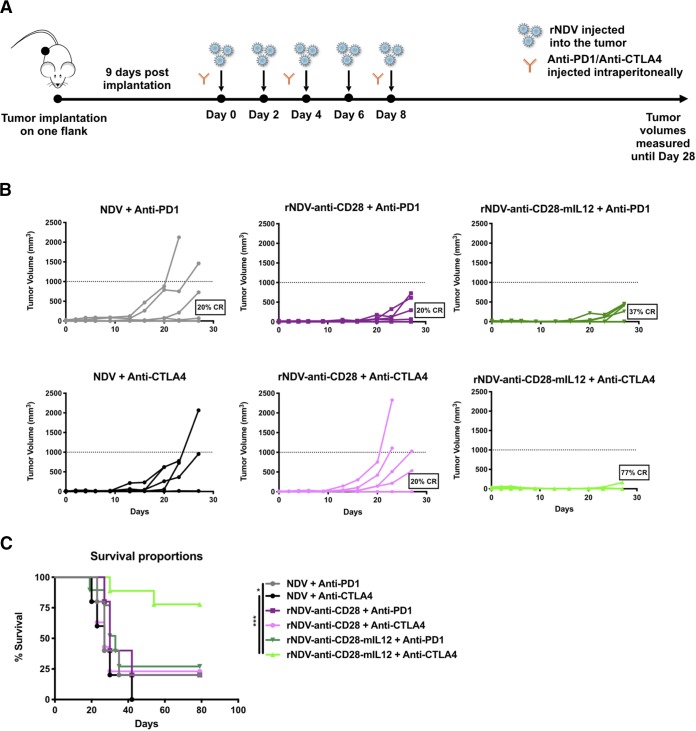
Previous studies have shown that intratumoral delivery of NDV, along with systemic checkpoint blockade, can potentiate an antitumor response in a poorly immunogenic B16-F10 tumor model (36, 37). To assess the antitumor efficacy of combining intratumoral administrations of various rNDVs expressing either anti-CD28, anti-PD1, or anti-PDL1 scFvs, as well as the immunocytokines anti-CD28–mIL-12, anti-PD1–mIL-12, and anti-PDL1–mIL-12 with systemic checkpoint blockade, B16-F10 melanoma cells (100,000) were implanted in only one flank of wild-type C57BL/6 mice. Tumors were treated 9 days postimplantation once palpable, visible tumors had been established. Intratumoral injections of rNDV were administered every 2 days with three intraperitoneal (i.p.) injections of either anti-PD1 or anti-CTLA4 MAbs administered over the course in which the oncolytic virus was given, and tumor volumes were monitored (Fig. 2A). The vast majority of mice receiving rNDV–anti-CD28–mIL-12 plus anti-CTLA4 had a complete response (CR) (77%) on the treated side compared with rNDV–anti-CD28 plus anti-CTLA4 (20%) or NDV plus anti-CTLA4 (0%). Combining rNDV–anti-CD28–mIL-12 with PD1 checkpoint blockade induced a considerably lower CR rate (37%); however, it was still higher than the CR rates observed with rNDV–anti-CD28 plus anti-PD1 (20%) or NDV plus anti-PD1 (20%) (Fig. 2B). This tumor control was again consistent with the overall survival curves, demonstrating the additive effects of combining rNDV–anti-CD28–mIL-12 with anti-CTLA4. This combination induced the most significant effect in terms of overall survival compared to other treatment regimens (Fig. 2C).
FIG 2.
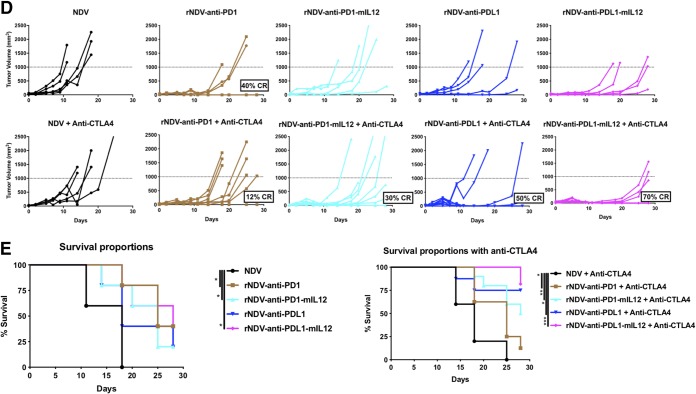
Combining rNDVs expressing immunocytokines with checkpoint inhibitor induces an antitumor response in a unilateral-flank tumor model. (A) Mouse treatment scheme. One hundred thousand B16-F10 melanoma cells were implanted in the right flanks of C57BL/6 mice (n = 5 to 9 mice per group). Nine days postimplantation, the tumors were treated with five intratumoral injections of rNDVs at 106 PFU every 2 days and three i.p. injections of anti-PD1 (200 μg) or anti-CTLA4 (100 μg) every 4 days. Tumor volumes were measured until day 28. (B) Individual tumor volume progressions with CR percentages for the rNDV–anti-CD28 and rNDV–anti-CD28–mIL-12 treatment cohorts. (C) Overall survival percentages until day 80. (D and E) Individual tumor volume progressions with CR percentages for the rNDV–anti-PD1/PDL1 and rNDV–anti-PD1/PDL1–mIL-12 treatment cohorts (D), along with the overall survival percentages (E). The data for survival were analyzed by log rank (Mantel-Cox) tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We then assessed the efficacy of intratumoral delivery of rNDV expressing inhibitors of the PD1 axis (anti-PD1/anti-PDL1) and their mIL-12 immunocytokine conjugates. We found that rNDV–anti-PDL1 and rNDV–anti-PDL1–mIL-12 in particular enhanced systemic CTLA4 checkpoint blockade and induced up to 50% and 70% CR rates, respectively (Fig. 2D). In addition, rNDV–anti-PD1–mIL-12 plus anti-CTLA4 potentiated a better antitumor response (30% CR) than rNDV–anti-PD1 with systemic anti-CTLA4 (12% CR) (Fig. 2D). This was again reiterated with the overall survival percentages showing that both rNDV–anti-PD1–mIL-12 and rNDV–anti-PDL1–mIL-12 provided a statistically significant survival benefit over NDV as a monotherapy. With systemic CTLA4 blockade, rNDV–anti-PD1–mIL-12, rNDV–anti-PDL1, and rNDV–anti-PDL1–mIL-12 provide a survival benefit over NDV plus anti-CTLA4 (Fig. 2E).
rNDVs expressing anti-CD28–mIL-12 intratumorally potentiate the efficacy of checkpoint blockade in a bilateral-flank tumor model.
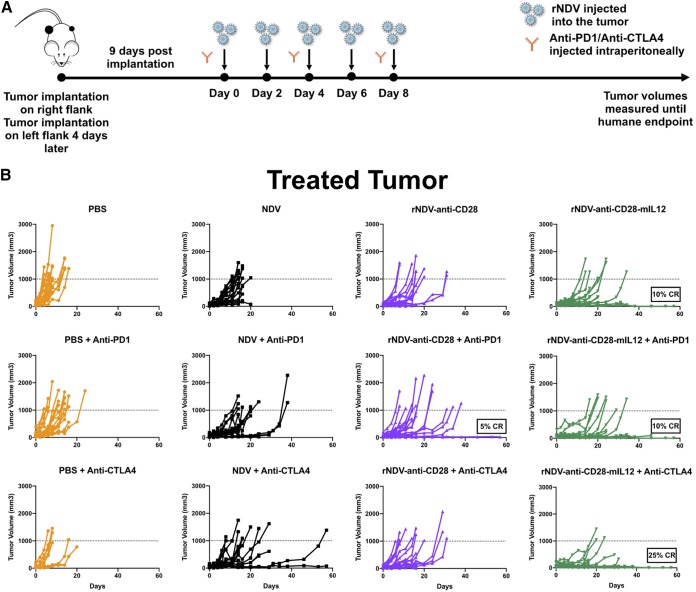
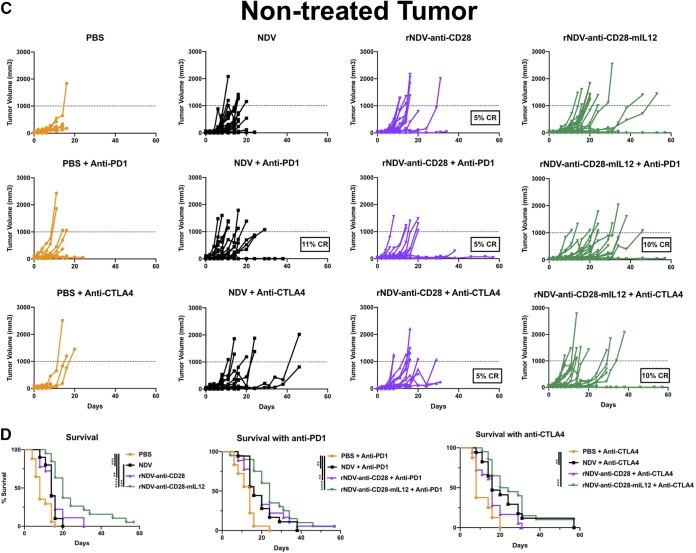
NDV treatment has been shown to induce tumor control in the treated tumor with an abscopal effect in the nontreated tumor by inducing tumor infiltration of effector T cell populations. This is further enhanced in combination with checkpoint blockade (37, 38). To assess the abilities of rNDV–anti-CD28 and rNDV–anti-CD28–mIL-12 to induce an abscopal effect with and without systemic checkpoint blockade, the highly aggressive B16-F10 melanoma tumor model was used. B16-F10 cells (200,000 and 100,000) were implanted on the right and left flanks of C57BL/6 mice 4 days apart. Nine days after the final implantation, they were treated with five intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of either anti-CTLA4 or anti-PD1 MAbs every 4 days. Tumor volumes were measured until the humane endpoint (Fig. 3A).
FIG 3.
Combining rNDVs expressing immunocytokines with checkpoint inhibitor induces an antitumor response in a bilateral-flank tumor model. (A) Mouse treatment scheme. Two hundred thousand and 100,000 B16-F10 melanoma cells were implanted in the right and left flanks of C57BL/6 mice 4 days apart. Nine days postimplantation, the tumors were treated with five intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of anti-PD1 (200 μg) or anti-CTLA4 (100 μg) every 4 days. Tumor volumes were measured until day 60. (B and C) Individual tumor volume progressions for the treated tumor (B) and the nontreated tumor (C), along with the CR percentages, for the rNDV–anti-CD28 and rNDV–anti-CD28–mIL-12 treatment cohorts. (D) Overall survival percentages for the different treatment cohorts. The data for survival were analyzed by log rank (Mantel-Cox) tests. The data represent cumulative results from 2 experiments with 8 to 20 mice per group. **, P < 0.01; ***, P < 0.001.
rNDV–anti-CD28–mIL-12 induced therapeutic control on the treated tumor, with about 10% CR observed both as a monotherapy and with PD1 checkpoint blockade. Systemic CTLA4 blockade further potentiated the antitumoral efficacy of rNDV–anti-CD28–mIL-12, inducing up to 25% CR on the treated tumor side (Fig. 3B). On the nontreated tumor side, while rNDV–anti-CD28 did seem to exert some control with anti-PD1 and anti-CTLA4 (5% CR), rNDV–anti-CD28–mIL-12 plus anti-PD1 and rNDV–anti-CD28–mIL-12 plus anti-CTLA4 induced a 10% CR rate (Fig. 3C).
The survival benefit provided by rNDV–anti-CD28–mIL-12 was further evident both as a monotherapy (compared with NDV and phosphate-buffered saline [PBS] control groups) and when combined with PD1 checkpoint blockade (relative to anti-PD1 alone) and with CTLA4 checkpoint blockade (compared to anti-CTLA4 alone) (Fig. 3D).
rNDVs expressing checkpoint inhibitor immunocytokines intratumorally enhance tumor control and survival benefits in a bilateral-flank model.
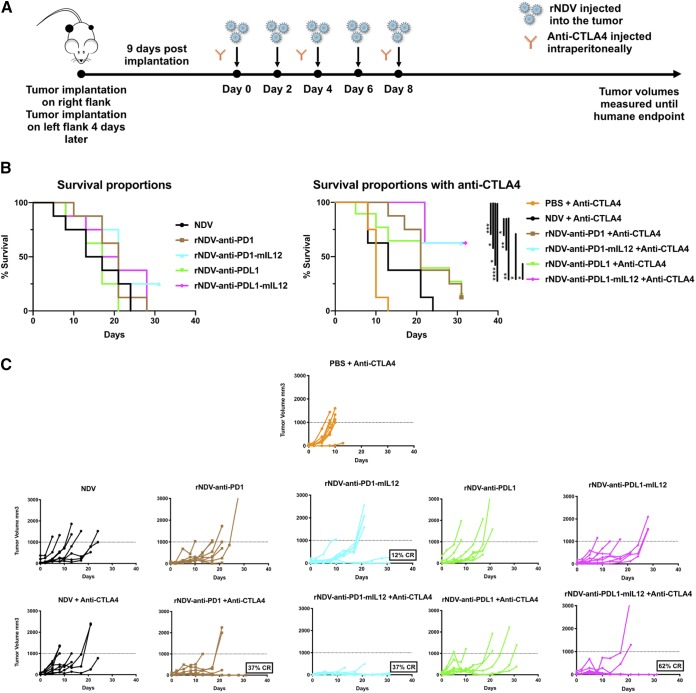
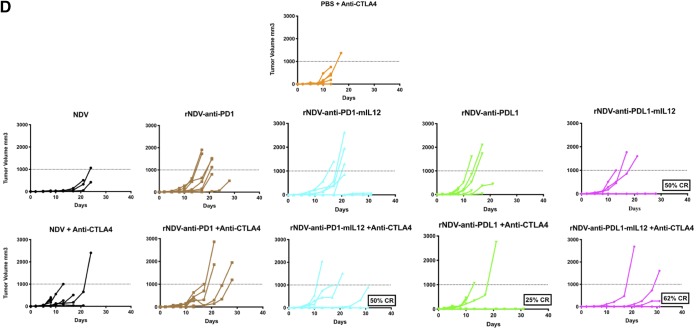
Localized delivery of both anti-CTLA4 and anti-CD40 antibodies have been shown to activate tumor-specific CD8 T cells (39, 40). Here, we asked whether localized delivery of anti-PD1 and anti-PDL1 via a virus engineered to express them induces an effect on both the treated and nontreated sides. More importantly, we also sought to address whether the therapeutic armament could be further enhanced by packaging an immunostimulatory cytokine along with the oncolytic virus and checkpoint inhibitor. To characterize the antitumor repercussions of combining rNDV–anti-PD1/PDL1 and rNDV–anti-PD1/PDL1–mIL-12 with and without systemic CTLA4 blockade, B16-F10 cells were implanted on the right (200,000) and left (100,000) flanks of C57BL/6 mice 4 days apart. Nine days postimplantation, once palpable tumors were formed, they were treated with five intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of anti-CTLA4 MAb every 4 days. Tumor volumes were measured until the humane endpoint (Fig. 4A).
FIG 4.
rNDVs expressing checkpoint inhibitor immunocytokines intratumorally enhance tumor control and survival benefits in a bilateral-flank tumor model. (A) Mouse treatment scheme. Two hundred thousand and 100,000 B16-F10 melanoma cells were implanted in the right and left flanks of C57BL/6 mice 4 days apart. Nine days postimplantation, the tumors were treated with five intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of anti-CTLA4 (100 μg) every 4 days. Tumor volumes were measured until the humane endpoint. (B) Overall survival percentages for the different treatment cohorts. (C and D) Individual tumor volume progressions for the treated tumor side (C) and the nontreated side (D), along with the CR percentages, for the rNDV–anti-PD1/PDL1 and rNDV–anti-PD1/PDL1–mIL-12 treatment cohorts. The data for survival were analyzed by log rank (Mantel-Cox) tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Combining rNDV–anti-PD1/PDL1 and rNDV–anti-PD1/PDL1–mIL-12 with anti-CTLA4 provides a statistically significant survival benefit over systemic CTLA4 blockade alone. Furthermore, rNDV–anti-PD1–mIL-12 and rNDV–anti-PDL1–mIL-12 also provide a significant added benefit over NDV with systemic checkpoint blockade, indicative of the added immunostimulatory advantage of the immunocytokine over the inherently immunogenic nature of the oncolytic virus itself (Fig. 4B). Individual tumor volume progressions showed that rNDV–anti-PD1, rNDV–anti-PD1–mIL-12, and rNDV–anti-PDL1–mIL-12 in combination with systemic CTLA4 were able to induce 37% to 62% CR rates on the treated side (Fig. 4C). On the nontreated side, which represents the abscopal response, rNDV–anti-PD1–mIL-12 and rNDV–anti-PDL1–mIL-12 were able to induce up to 50% and 62% CR rates, respectively (Fig. 4D).
Immunocytokine-expressing rNDVs combined with systemic CTLA4 blockade enhance immune stimulation within the tumor microenvironment.
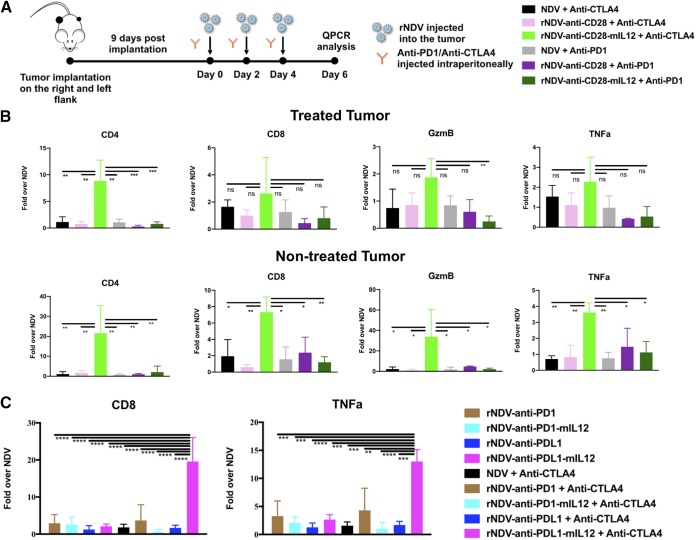
To delineate the immune landscape that mediates the antitumor effects in response to the different treatment cohorts, we performed quantitative reverse transcription (qRT)-PCR analysis on both the treated and nontreated tumors. Three hundred thousand and 150,000 cells were implanted on the right and left flanks of C57BL/6 mice to guarantee adequate tumor size for RNA extraction at the time of tumor harvest. Nine days postimplantation, once palpable tumors were formed, they were treated with three intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of either anti-PD1 or anti-CTLA4 MAbs. Tumors were harvested on day 6, and gene expression analysis was performed on extracted RNA (Fig. 5A).
FIG 5.
rNDVs expressing immunocytokines enhance immune stimulation intratumorally. (A) Mouse treatment scheme. Three hundred thousand and 150,000 B16-F10 melanoma cells were implanted in the right and left flanks, respectively, of C57BL/6 mice 4 days apart. Nine days postimplantation, the tumors were treated with three intratumoral administrations of various rNDVs at 106 PFU and three i.p. injections of anti-CTLA4 (100 μg) or anti-PD1 (200 μg) every 2 days. Tumors were harvested on day 6, and gene expression analysis was performed on extracted RNA. (B) Gene expression for the treated and nontreated tumors for rNDV–anti-CD28 and rNDV–anti-CD28–mIL-12. (C) Gene expression for the treated tumor for rNDV–anti-PD1/PDL1 and rNDV–anti-PD1/PDL1–mIL-12. The data were analyzed by one-way analysis of variance (ANOVA). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
rNDV–anti-CD28–mIL-12 with anti-CTLA4 induced significant upregulation of CD4-positive cell populations in both the treated and nontreated tumors (Fig. 5B). Interestingly, previous studies have shown that treatment with both CTLA4 checkpoint blockade and anti-CD28 antibody is associated with the expansion of CD4 effector T cells (41, 42), which is what we have observed. Significant enhancements of CD8-positive cell populations, as well as of granzyme B (GzmB) and tumor necrosis factor alpha (TNF-α) expression in nontreated tumors, were also observed when combining rNDV–anti-CD28–mIL-12 with anti-CTLA4. This might be indicative of the mechanisms underlying the enhanced survival benefit observed with this combination cohort.
Gene expression analysis of the treated tumors also showed significant upregulation of CD8 and TNF-α in the rNDV–anti-PDL1–mIL-12 plus anti-CTLA4 treatment cohort (Fig. 5C). We did not observe a similar effect in the nontreated tumor (data not shown).
DISCUSSION
The goal of any immunotherapy, such as checkpoint blockade, is to augment local and systemic responses to cancer (38). However, a primary mode of resistance to checkpoint inhibitor therapy is the inherently immunosuppressive, or “cold,” nature of a nonresponsive tumor (43 – 45). Oncolytic virotherapy using either a naturally occurring virus or a genetically modified virus to enhance tumor selectivity or immunogenicity provides an avenue to convert a cold, or nonresponsive, tumor into a “hot,” or immunologically sensitive, tumor (46). Previous studies have shown that intratumoral NDV can potentiate checkpoint blockade in a B16-F10 tumor model, leading to long-term survival and tumor rejection with an abscopal effect compared to checkpoint inhibitor monotherapy. Importantly, no virus was detected in the contralateral nontreated tumor in this model system (37).
In this study, we engineered recombinant NDVs to express checkpoint inhibitors and immunocytokines, allowing different therapeutic modalities to be packaged into one immunostimulatory platform. Notably, we utilized a very aggressive, poorly immunogenic B16-F10 murine melanoma model (36) to assess the antitumor effects of these recombinant viruses in both unilateral and bilateral tumor models. We demonstrated that combining rNDV–anti-CD28–mIL-12 with CTLA4 checkpoint blockade is particularly efficacious in inducing tumor control with an added survival benefit, which is significant over NDV with either anti-PD1 or anti-CTLA4 MAbs in a unilateral-flank model. Comparable improvements in antitumor and overall survival benefits were also observed for rNDV–anti-PDL1 and rNDV–anti-PDL1–mIL-12 with anti-CTLA4.
A bilateral tumor model was used next to assess the abilities of these recombinant viruses to induce an antitumor effect in the nontreated tumor (abscopal response), thereby enhancing the survival benefit (47 – 49). The right tumor flanks in these mice were treated as indicated, while the left tumor flanks were kept naive to virus inoculation. Reiterating the observations in the unilateral-flank studies, rNDV–anti-CD28–mIL-12 was efficacious in inducing statistically significant long-term survival as both monotherapy (compared with the PBS or NDV treatment group) and with PD1 and CTLA4 blockade. rNDV–anti-PDL1–mIL-12, rNDV–anti-PD1–mIL-12, and rNDV–anti-PD1 with CTLA4 checkpoint blockade were more efficacious than both NDV plus anti-CTLA4 and anti-CTLA4 treatment alone. Interestingly, rNDV–anti-PDL1–mIL-12 with anti-CTLA4 induced the highest number of complete remissions on both the treated and nontreated sides. Finally, we have shown that enhancement in CD4- and CD8-positive cell populations might meditate the antitumor effects seen with the rNDV–anti-CD28–mIL-12 plus anti-CTLA4 and rNDV–anti-PDL1–mIL-12 plus anti-CTLA4 cohorts. It is important to note that CTLA4 and PD1 immune checkpoints attenuate T cell responses through distinct mechanisms that are spatially and temporally separated, with CTLA4 acting during the priming phase of T cell activation and PD1 attenuating T cell responses in peripheral tissues. Therefore, it makes sense that the irAEs observed with CTLA4 and PD1 checkpoint blockade vary, with one study showing a correlation between irAEs and the overall response rate in patients with non-small-cell lung cancer (NSCLC) treated with anti-PD1 (9, 15, 50, 51). It is tempting to speculate that expansion of the CD4 effector populations with an rNDV–anti-CD28–mIL-12 plus anti-CTLA4 treatment regimen enhances the antitumor response by enhancing CD8 infiltration and cytolytic activity, as well as T cell memory formation.
Primary resistance to CTLA4 blockade has been shown to correlate with loss of gamma interferon (IFN-γ) signaling, as well as with PDL1 expression on CD4 and CD8 T cells (52, 53). Furthermore, the additive effects of combining anti-PD1 or anti-PDL1 with anti-CTLA4 have been explored in previous studies, and the combination has been found to be clinically more efficacious than monotherapy (54, 55). This has been shown to be mediated by enhanced T cell infiltration (54, 56). The enhancement in the overall response rate by combining anti-CTLA4 and anti-PD1 therapies is associated with increased prevalence of drug-related toxicity, with 53% of patients experiencing grade 3 or 4 irAEs compared to 18% in the monotherapy group (57). One possible avenue to circumvent this is to deliver one of the checkpoint modalities locally via a viral vector while the other modality is administered systemically, which is what we have shown in this study.
This multipronged approach should instigate a coordinated migration of cytotoxic T cells, as well as other immune cell populations, such as dendritic cells, into the tumor microenvironment, which might be key to achieving an abscopal effect. While it is difficult to compare the various oncolytic platforms, NDV offers some unique advantages, such as a lack of seroprevalence (58, 59), making it an attractive agent for further exploration as an immunotherapeutic modality (60, 61). Several clinical trials have demonstrated the safety of these viruses in humans, even with systemic exposure to large doses (62, 63). Moreover, the ubiquitous nature of the NDV receptor (sialic acid), as well as its ability to induce type I IFN, makes it an ideal candidate for a wide array of immunologically cold tumors (27). Finally, the ability to genetically engineer the virus to express immunostimulatory ligands directly into the tumor microenvironment while avoiding recombination or integration into the host genome also makes it an attractive candidate. Taken together, these results stand to substantially improve NDV as an oncolytic candidate and an intratumoral delivery platform for checkpoint inhibitors.
MATERIALS AND METHODS
Cell lines and antibodies.
The murine melanoma cell line (B16-F10) and Vero cells were obtained from the ATCC. BSRT7 cells (BHK-21 cells transduced to constitutively express T7 RNA polymerase) were a kind gift from Benhur Lee (Icahn School of Medicine at Mount Sinai). The B16-F10 cells were maintained in Dulbecco’s modified Eagle medium (DMEM)–F-12 medium supplemented with 10% fetal bovine serum and 1% penicillin with streptomycin. The BSRT7 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin with streptomycin. Therapeutic anti-PD1 (clone RMP1-14) and anti-CTLA4 (clone 9H10) antibodies were purchased from BioXcell (catalog no. BE0146 and BE0131).
Viruses.
The recombinant lentogenic (low-pathogenicity) NDV strain LaSota L289A was used for all experiments. Generation of recombinant NDV LaSota L289A viruses expressing anti-CD28, anti-PD1, and anti-PDL1 scFvs, as well as anti-CD28–mIL-12, anti-PD1–mIL-12, and anti-PDL1–mIL-12, was done by cloning DNA fragments encoding the respective murine scFv and scFv–mIL-12 transgenes into the SacII cloning site between the P and M genes, flanked by NDV-specific transcriptional signals. The IL-2 signal sequence (IL-2ss) (MYRMQLLSCIALSLALVTNS) was used to target the translation of the anti-PD1 and anti-PDL1 scFvs, as well as the anti-PD1–mIL-12 and anti-PDL1–mIL-12 polypeptides, into the secretory pathway. Recombinant viruses were then rescued as previously described (35). rNDV–anti-CD28, as well as the anti-CD28 sequence, was a kind gift from Dmitriy Zamarin (Memorial Sloan Kettering Cancer Center). The anti-PD1 and anti-PDL1 scFv sequences were obtained from the respective patent applications (64, 65). The p35 and p40 subunits of mIL-12 were fused with a GS linker, and the sequence for the mIL-12 transgene was obtained from Dmitriy Zamarin. Rescued viruses were grown in embryonated 9-day-old chicken eggs, and viral titers were determined by serial dilution and immunofluorescence in Vero cells.
LDH cytotoxicity assay.
B16-F10 cells in culture were infected with NDV at MOI of 0.1, 1, and 3 or mock infected. At 24 h, 48 h, 72 h, and 96 h postinfection, the cells were washed with 1 ml PBS and incubated with 1% Triton X for 20 min at 37°C. LDH activity was measured using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega; catalog no. G1780) according to the instructions provided by the manufacturer. The mean values of 3 replicates were plotted, with error bars depicting standard deviations.
Mice.
Female C57BL/6 mice were purchased from Jackson Laboratory. All animal experiments were performed in accordance with the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee guidelines. For melanoma tumor studies, 4- to 6-week-old female mice were anesthetized by i.p. injections of ketamine/xylazine (K/X). For unilateral tumor experiments, mice were implanted intradermally with approximately 1 × 105 B16-F10 cells on the right flank. For bilateral tumor experiments, mice were implanted intradermally with approximately 2 × 105 and 1 × 105 B16-F10 cells on the right and left flanks, respectively. On day 9, the mice were checked for successful tumor implantation and rehoused at random to ensure homogeneous tumor populations. Tumors were then treated with five intratumoral administrations of NDV and rNDVs at 106 PFU/dose every 2 days and three i.p. injections of anti-PD1 (200 μg) or anti-CTLA4 (100 μg) every 4 days. Tumor volumes were measured until the humane endpoint of 1,000 mm3. The animals were euthanized following signs of distress or when the total tumor volume reached 1,000 mm3. For gene expression analysis, 300,000 and 150,000 cells were implanted on the right and left flanks of C57BL/6 mice. Nine days postimplantation, once palpable tumors were formed, they were treated with three intratumoral administrations of various rNDVs at 106 PFU every 2 days and three i.p. injections of either anti-PD1 or anti-CTLA4. Tumors were harvested on day 6, and gene expression analysis was done on the extracted RNA.
qRT-PCR.
Tumor-bearing mice were euthanized, and the tumors were immediately excised and stored in RNAlater stabilization solution (Thermo Fisher Scientific; catalog no. AM7021). RNA was isolated with TRIzol reagent (Thermo Fisher Scientific; catalog no. 15596) with the protocol supplied by the manufacturer. cDNA was synthesized from the extracted RNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems; catalog no. 4368814) according to the instructions provided by the manufacturer. mRNA was quantified in a Roche LightCycler 480 according to the manufacturer’s instructions using a LightCycler 480 SYBR green I master kit (Roche; catalog no. 04707516001). The primers used are listed in Table 1.
TABLE 1.
Primers used in qRT-PCR
| Gene | Primer |
|
|---|---|---|
| Forward | Reverse | |
| TNF-α | AGAAACACAAGATGCTGGGACAGT | CCTTTGCAGAACTCAGGAATGG |
| CD4a | CTAGCTGTCACTCAAGGGAAGA | CGAAGGCGAACCTCCTCTAA |
| GZMB | CCACTCTCGACCCTACATGG | GGCCCCCAAAGTGACATTTATT |
| CD8a | CCGTTGACCCGCTTTCTGT | TTCGGCGTCCATTTTCTTTGG |
| 18S RNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Statistical analysis.
Statistical analyses were conducted using Prism software (GraphPad), and the details of the analyses are provided in the figure legends.
ACKNOWLEDGMENTS
We thank Dmitriy Zamarin (Memorial Sloan Kettering Cancer Center) for invaluable insights into the project.
The research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
G.V., S.M., and P.P. designed the research. G.V. and S.M. performed the research. G.V. and S.M. analyzed data, and G.V. and P.P. wrote the paper.
We declare no competing interests.
REFERENCES
- 1.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. 2017. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170:1109–1119 e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. 2005. The B7 family revisited. Annu Rev Immunol 23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 3.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405–413. doi: 10.1016/1074-7613(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. 1995. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 7.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. 2004. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 8.Chambers CA, Kuhns MS, Egen JG, Allison JP. 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 9.Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. 2016. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 13.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. 2016. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 14.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. 2016. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. 2018. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luhder F, Huang Y, Dennehy KM, Guntermann C, Muller I, Winkler E, Kerkau T, Ikemizu S, Davis SJ, Hanke T, Hunig T. 2003. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med 197:955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung J, Suh WK. 2014. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw 14:265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Margolin K. 2011. Cytokines in cancer immunotherapy. Cancers 3:3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA. 1993. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 21.Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, Mier JW, Canning CM, Battiato L, Tahara H, Sherman ML. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res 3:409–417. [PubMed] [Google Scholar]
- 22.Vigil A, Park M-S, Martinez O, Chua MA, Xiao S, Cros JF, Martínez-Sobrido L, Woo SLC, García-Sastre A. 2007. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res 67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 23.Vigil A, Martinez O, Chua MA, Garcia-Sastre A. 2008. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther 16:1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Janke M, Fournier P, Schirrmacher V. 2008. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res 136:75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Kiefer JD, Neri D. 2016. Immunocytokines and bispecific antibodies: two complementary strategies for the selective activation of immune cells at the tumor site. Immunol Rev 270:178–192. doi: 10.1111/imr.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Leeuw O, Peeters B. 1999. Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J Gen Virol 80:131–136. doi: 10.1099/0022-1317-80-1-131. [DOI] [PubMed] [Google Scholar]
- 27.Zamarin D, Palese P. 2012. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol 7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirrmacher V, van Gool S, Stuecker W. 2019. Breaking therapy resistance: an update on oncolytic Newcastle disease virus for improvements of cancer therapy. Biomedicines 7:E66. doi: 10.3390/biomedicines7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med 170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 32.Yuzhalin AE, Kutikhin AG. 2012. Interleukin-12: clinical usage and molecular markers of cancer susceptibility. Growth Factors 30:176–191. doi: 10.3109/08977194.2012.678843. [DOI] [PubMed] [Google Scholar]
- 33.Tacke M, Hanke G, Hanke T, Hunig T. 1997. CD28-mediated induction of proliferation in resting T cells in vitro and in vivo without engagement of the T cell receptor: evidence for functionally distinct forms of CD28. Eur J Immunol 27:239–247. doi: 10.1002/eji.1830270136. [DOI] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–21. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vijayakumar G, Zamarin D. 2020. Design and production of Newcastle disease virus for intratumoral immunomodulation. Methods Mol Biol 2058:133–154. doi: 10.1007/978-1-4939-9794-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Saffold S, Cao X, Krauss J, Chen W. 1998. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol 161:5516–5524. [PubMed] [Google Scholar]
- 37.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. 2014. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. 2018. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. 2011. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res 17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 40.Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJ. 2013. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res 19:5381–5389. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 41.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. 2016. CD28 costimulation: from mechanism to therapy. Immunity 44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, Allison JP. 2017. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribas A. 2015. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov 5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. 2019. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolytics 13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mole RH. 1953. Whole body irradiation; radiobiology or medicine? Br J Radiol 26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 48.Abuodeh Y, Venkat P, Kim S. 2016. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Fountzilas C, Patel S, Mahalingam D. 2017. Review: oncolytic virotherapy, updates and future directions. Oncotarget 8:102617–102639. doi: 10.18632/oncotarget.18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker LS, Sansom DM. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 51.Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, Nakanishi M, Yamamoto N. 2018. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P. 2016. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167:397–404.e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternes N, Jegou S, Woods DM, Sodre AL, Hansen M, Meirow Y, Sade-Feldman M, Burra A, Kwek SS, Flament C, Messaoudene M, Duong CPM, Chen L, Kwon BS, Anderson AC, Kuchroo VK, Weide B, Aubin F, Borg C, Dalle S, Beatrix O, Ayyoub M, Balme B, Tomasic G, Di Giacomo AM, Maio M, Schadendorf D, Melero I, Dreno B, Khammari A, Dummer R, Levesque M, Koguchi Y, Fong L, Lotem M, Baniyash M, Schmidt H, Svane IM, Kroemer G, Marabelle A, Michiels S, Cavalcanti A, Smyth MJ, Weber JS, Eggermont AM, Zitvogel L. 2017. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 8:592. doi: 10.1038/s41467-017-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curran MA, Montalvo W, Yagita H, Allison JP. 2010. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khair DO, Bax HJ, Mele S, Crescioli S, Pellizzari G, Khiabany A, Nakamura M, Harris RJ, French E, Hoffmann RM, Williams IP, Cheung A, Thair B, Beales CT, Touizer E, Signell AW, Tasnova NL, Spicer JF, Josephs DH, Geh JL, MacKenzie Ross A, Healy C, Papa S, Lacy KE, Karagiannis SN. 2019. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol 10:453. doi: 10.3389/fimmu.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. 2014. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. 2017. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller LT, Yates VJ. 1971. Reactions of human sera to avian adenoviruses and Newcastle disease virus. Avian Dis 15:781–788. doi: 10.2307/1588867. [DOI] [PubMed] [Google Scholar]
- 59.Charan S, Mahajan VM, Agarwal LP. 1981. Newcastle disease virus antibodies in human sera. Indian J Med Res 73:303–307. [PubMed] [Google Scholar]
- 60.Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. 2019. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov 18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 61.Russell L, Peng KW, Russell SJ, Diaz RM. 2019. Oncolytic viruses: priming time for cancer immunotherapy. BioDrugs 33:485–501. doi: 10.1007/s40259-019-00367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorence RM, Roberts MS, O'Neil JD, Groene WS, Miller JA, Mueller SN, Bamat MK. 2007. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr Cancer Drug Targets 7:157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 63.Lam HY, Yeap SK, Pirozyan MR, Omar AR, Yusoff K, Suraini AA, Al-Aziz S, Alitheen NB. 2011. Safety and clinical usage of Newcastle disease virus in cancer therapy. J Biomed Biotechnol 2011:718710. doi: 10.1155/2011/718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Genentech, Inc. 10 November 2013, filing date. U.S. patent application 8735553 B1.
- 65.Genentech, Inc. 8 December 2009, filing date. U.S. patent application 8217149 B2.