Abstract
Lung cancer immunotherapy is an effective treatment option; however, it can be hampered by adverse events, including pancreatitis, associated with excessive immune activation. Here, we report the case of a 70‐year‐old patient who presented with recurrent lung squamous carcinoma and was started with pembrolizumab treatment (200 mg every three weeks). The patient developed pembrolizumab‐induced pancreatitis. After 14 months of pembrolizumab treatment, positron emission tomography–computed tomography showed a tumour‐shaped, highly integrated lesion at the pancreatic head and significantly elevated tumour markers, including carbohydrate antigen 19‐9 (149.3 U/mL), s‐pancreas antigen‐1 (44.7 U/mL), and duke pancreatic monoclonal antigen type 2 (412 U/mL). Pembrolizumab‐induced immune‐related pancreatitis was effectively treated with prednisolone 90 mg (1 mg/kg/day). Four months later, normal levels of the three specific tumour markers were detected, with improved pancreatic enzymes and radiographic findings. To our knowledge, this is the first reported case of immune‐related pancreatitis with elevated pancreatic cancer‐specific markers.
Keywords: Immunotherapy, lung cancer, pancreatitis, pembrolizumab, tumour marker
We report a case of pembrolizumab‐induced immune‐related pancreatitis with the elevation of pancreatic tumour markers in a patient with squamous cell lung cancer.
Introduction
Currently, immunotherapy is increasingly used for the treatment of certain cancers, including various types of lung cancer. However, treatment success can be reduced by adverse immune activation events, which is not observed with classical cytotoxic agents. This overactivation can potentially affect multiple organ systems, including the gastrointestinal tract, endocrine system, liver, lungs, nervous system, skin, and pancreas.
In a previous review of toxicities of immune checkpoint inhibitor therapy, grade 2–4 pancreatitis, according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.1, was reported in nearly 1.8% of patients who received nivolumab or pembrolizumab 1. Atezolizumab, an anti‐programmed death‐ligand 1 (PD‐L1) agent, was associated with acute pancreatitis in 0.1% of 1978 patients. In asymptomatic individuals, pancreatitis was detected by positron emission tomography–computed tomography (PET–CT) after anti‐PD‐L1 therapy and confirmed to be immune‐related, not a metastatic lesion, by radiological resolution after corticosteroid use. Usually, tumour markers of pulmonary or pancreatic origin are measured to assess the malignancy of lesions for a precise diagnosis and intervention decisions.
Case Report
A 70‐year‐old Asian man with squamous cell carcinoma, who was treated with left upper lobe resection two years prior, developed mediastinal lymph node metastasis, confirmed by surgical biopsy. Immunohistochemical analysis of the PD‐L1 expression using a murine 22C‐3 antibody showed that the tumour proportion score was 85%. On the basis of this finding, pembrolizumab treatment (200 mg every three weeks) was initiated.
Fourteen months later, his serum amylase and lipase increased from normal levels to grade 2, according to the CTCAE version 4.1. A PET–CT image showed a round‐shaped, highly integrated lesion, with rough and irregular edges, at the pancreatic head. A CT scan showed a slightly swollen pancreatic parenchyma and mild pancreatic duct dilation. The patient did not have physical signs or symptoms suggesting pancreatitis.
His condition was provisionally diagnosed as pembrolizumab‐induced immune‐related pancreatitis or pancreatic cancer. Significantly elevated levels of pancreatic tumour markers were detected, including carbohydrate antigen 19‐9 (CA19‐9) of 149.3 U/mL (normal range: 0–36.9 U/mL), s‐pancreas antigen‐1 (SPan‐1) of 44.7 U/mL (0–30 U/mL), and duke pancreatic monoclonal antigen type 2 (DUPAN‐2) of 412 U/mL (0–150 U/mL). To confirm the diagnosis of pembrolizumab‐induced immune‐related pancreatitis and rule out that of pancreatic cancer, prednisolone 90 mg (1 mg/kg/day) was administered. Fifteen days after the initiation of prednisolone, PET–CT showed decreased integration at the pancreatic head, with no mass‐like lesion. The size of the whole pancreas was also normalized, as observed by CT (Fig. 1). The pancreatic tumour markers gradually decreased, and four months after treatment initiation, their levels became normal (Fig. 2). Pancreatic exocrine enzymes and radiographic findings also normalized at the same time.
Figure 1.
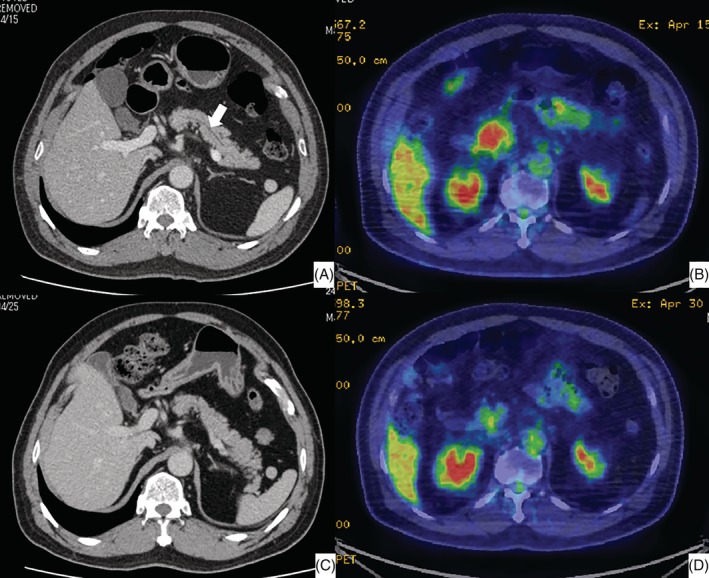
(A) Enhanced computed tomography (CT) of the abdomen after the patient developed pancreatitis. The pancreatic parenchyma is slightly swollen. The arrow indicates the mildly dilated pancreatic duct. (B) Abdominal positron emission tomography (PET)–CT after the patient developed pancreatitis. A highly integrated lesion, with a round shape and rough and irregular edges, was observed at the pancreatic head. (C) Enhanced CT of the abdomen performed 15 days after the initiation of prednisolone. The swelling of the pancreatic parenchyma and the dilation of the pancreatic duct are resolved. (D) PET–CT of the abdomen performed 15 days after the initiation of prednisolone. The highly integrated lesion at the pancreatic head is diminished.
Figure 2.
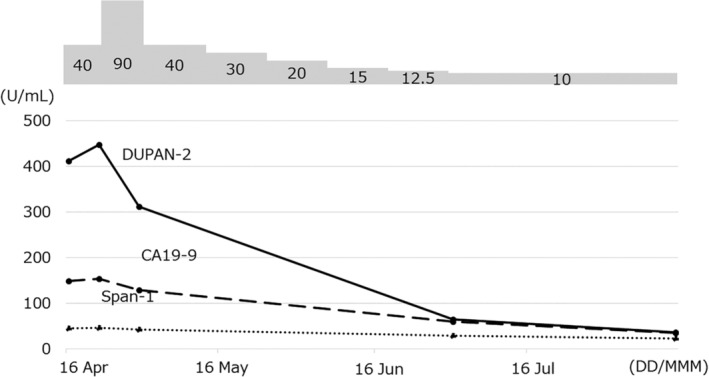
Treatment course. Tumour markers, especially duke pancreatic monoclonal antigen type 2 (DUPAN‐2), were elevated at the time of pancreatitis detection, and their levels gradually normalized after the initiation of prednisolone treatment.
Discussion
In this case, the elevation of pancreatic enzymes during treatment with pembrolizumab led us to suspect immune‐related adverse events; yet, the PET–CT results and high levels of pancreatic tumour markers suggested a malignant‐like lesion at the pancreatic head. Corticosteroid treatment was initiated in advance, resulting in the improvement of radiographic and serological findings.
SPan‐1 and DUPAN‐2 are widely used for the differential diagnosis between benign and malignant pancreatic diseases. Regardless of the disease duration, they are generally in the normal range in patients with benign diseases such as pancreatitis. SPan‐1 is detected at low levels in the normal pancreatic parenchyma, renal tubular epithelium, biliary duct epithelium, and tracheal epithelium. DUPAN‐2 is a precursor of CA19‐9, which is also produced in the normal epithelium of the pancreatic duct, gastrointestinal tract, biliary duct, and trachea. These pancreatic cancer‐associated carbohydrate antigens can be elevated in patients with stagnation of the pancreatic juice due to a pancreatic obstruction or stenosis of the main pancreatic duct. Mishima et al. reported a case of autoimmune pancreatitis with a high level of DUPAN‐2, which improved after corticosteroid therapy 2. In their case, severe stenosis of the pancreatic duct possibly destroyed the epithelium of the pancreatic and bile ducts and led to DUPAN‐2 release into the bloodstream, whereas improvement in ductal stenosis resulted in parallel reduction of the tumour marker. Moreover, there are some other case reports of benign or malignant diseases, aside from pancreatic cancer, with high levels of SPan‐1 or DUPAN‐2.
PD‐L1 expression in the pancreas is observed in the parenchyma, mainly in islets 3, and the elevation of SPan‐1 is consistent with the immune‐related inflammation reaction targeting the pancreatic parenchyma. To our knowledge, there is no report of PD‐L1 expression in the pancreatic duct epithelium. It is uncertain whether T cells, which are activated by pembrolizumab, directly affect epithelial cells of the pancreatic duct as a CD8 regulatory effect. However, certain organs, such as the kidney and colon, express PD‐L1 in epithelial cells 4. Furthermore, the histopathological features of anti‐PD‐1‐associated colitis indicate an active inflammation, observed as a mucosal injury with lymphocyte infiltration, increased apoptosis, and crypt atrophy and dropout 5, suggesting possible expression of PD‐L1 in epithelial cells of the pancreatic duct, making it a target of an immune‐related reaction.
In our case, a mild dilation of the pancreatic duct and slight swelling of the parenchyma were observed on enhanced CT, with a highly integrated lesion at the pancreatic head observed on PET–CT. It is possible that peripheral ducts, which are difficult to recognize on CT images, were constricted by diffuse inflammation, caused by an immune‐related reaction in both pancreatic parenchyma and epithelial cells of the pancreatic duct, increasing SPan‐1 and DUPAN‐2 levels in this benign condition.
In conclusion, we report a case of pembrolizumab‐induced immune‐related pancreatitis with the elevation of pancreatic tumour markers in a patient with squamous cell lung cancer.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgment
We would like to thank Editage (http://www.editage.com) for English language editing.
Kakuwa, T , Hashimoto, M , Izumi, A , Naka, G , Takeda, Y , Sugiyama, H . (2020) Pembrolizumab‐related pancreatitis with elevation of pancreatic tumour markers. Respirology Case Reports, 8(2), e00525 10.1002/rcr2.525
Associate Editor: James Ho
References
- 1. Hofmann L, Forschner A, Loquai C, et al. 2016. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti‐PD‐1 therapy. Eur. J. Cancer 60:190–209. [DOI] [PubMed] [Google Scholar]
- 2. Mishima S, Mizuta Y, Yamao T, et al. 2007. Autoimmune pancreatitis with extreme elevation of DUPAN‐2. Intern. Med. 46:377–381. [DOI] [PubMed] [Google Scholar]
- 3. Yoneda S, Imagawa A, Hosokawa Y, et al. 2019. T‐lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care 42:e116–e118. [DOI] [PubMed] [Google Scholar]
- 4. Nakazawa A, Dotan I, Brimnes J, et al. 2004. The expression and function of costimulatory molecules B7h and B7‐H1 on colonic epithelial cells. Gastroenterology 26:1347–1357. [DOI] [PubMed] [Google Scholar]
- 5. Karamchandani DM, and Chetty R. 2018. Immune checkpoint inhibitor‐induced gastrointestinal and hepatic injury: pathologists' perspective. J. Clin. Pathol. 71:665–671. [DOI] [PubMed] [Google Scholar]


