Abstract
MicroRNA24-2 (miR24-2) is associated with human tumorigenesis; however, its molecular mechanisms are poorly understood. Herein, our findings demonstrate that miR24-2 promotes the proliferation ability in vitro and the tumorigenic ability in vivo in human liver cancer stem cells (hLCSCs). Mechanically, the miR24-2 targets for 3′ UTR (2,627–2,648) of protein arginine methyltransferase 7 (PRMT7) inhibit the translational ability of prmt7 gene. Moreover, miR24-2 inhibits the di-/tri-methylation of histone H4 arginine 3 by reducing PRMT7 and then promotes the expression of Nanog via long noncoding RNA HULC. Notably, miR24-2 inhibits histone deacetylase HDAC3 through miR675, which promotes the acetylation of histone H4 at lysine 16. Subsequently, miR24-2 enhances the interaction between LC3 and ATG4 dependent on PI3K and triggers cellular autophagy. Strikingly, miR24-2 inhibits the degradation of pyruvate kinase M1 via autophagosome-P62 in hLCSCs. Furthermore, miR24-2 enhances the activity of Src by promoting the binding of PKM1 to the Src promoter regions in hLCSCs. In particular, our results also indicate that src gene determines the oncogenic functions of miR24-2. These results provided a valuable theoretical basis for the discovery of liver cancer therapeutic targets and diagnosis markers based on miR24-2.
Keywords: miR24-2, liver cancer stem cells, tyrosine protein kinase sarcoma gene Src, histone epigenetic modification, autophagy
Wang et al. indicate that miR24-2 promotes the growth of human liver cancer stem cells. In particular, the src gene determines the oncogenic functions of miR24-2. These results provided a valuable theoretical basis for human liver cancer.
Introduction
It has been found that human stem cells can be differentiated into malignant stem cells in an unfavorable microenvironment.1,2 Although most studies currently support malignant tumors that originate from malignant transformation of stem cells, there is still controversy about the mechanism of stem cell deterioration.3,4 At present, extensive research has been conducted on the mechanism of driving stem cell deterioration; for example, METTL3-eIF3h-mediated mRNA circulation promotes stem cell deterioration, etc.5 Studies indicate that liver cancer stem cell proliferation and differentiation are deregulated in hepatocarcinogenesis.6,7 Therefore, targeting liver cancer stem cells may bring hope to curing hepatocellular carcinoma.8. So far, it is not clear at any time what causes the accumulation of liver stem cell genetic errors, chromatin programming, and chromosomal instability, and eventually evolves into malignant liver cancer stem cells.
MicroRNA24-2 (miR24-2) is expressed in various tissues of the human body and participates in various physiological processes, e.g., erythropoiesis,9 lipogenesis,10 T cell senescence,11 osteoblast differentiation,12 cell growth and proliferation, 13, 14, 15 cancer cell invasion, and hepatic metastasis.16 Notably, miR24-2-5p can silence expression of several important genes by targeting the protein arginine methyltransferase 7 (PRMT7).17 Another study showed that inhibition of miR24-2 significantly altered embryonic stem cell (ESC) differentiation.18 However, the functions of miR24-2 on liver cancer stem cells are still unclear.
Furthermore, cell proliferation can be altered in response to cellular stress or oncogenic signaling by regulating miR675.19 And HULC is highly upregulated in hepatocellular carcinoma and is a driver of tumor proliferation, migration, and invasion.20,21 It is worth mentioning that PRMT7 can regulate the histone methylation modification, especially H4R3me2 and H3K4me3.22,23 Therefore, it is worthwhile to study how miR24-2 works through miR675, HULC, and PRMT7 in human liver cancer stem cells (hLCSCs).
In this study, it is confirmed that miR24-2 is a microRNA with cancerous function, which is shown at least in hLCSCs. We have also demonstrated that miR24-2 can alter several complex signaling pathways and tumor-associated protein kinase functions in hLCSCs by affecting histone H3/4 epigenetic modification and cellular autophagy events.
Results
miR24-2 Promotes the Growth of hLCSCs
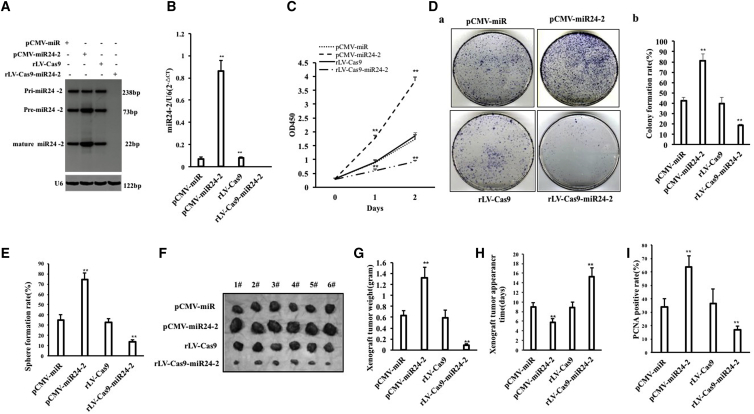
To investigate the effects of miR24-2 on hLCSCs, hLCSCs were isolated from hLCSC line (Figures S2A–S2D; Supplemental Results). In the hLCSCs, the pCMV-miR and pCMV-miR24-2 were separately transfected and rLV-Cas9 and rLV-Cas9-miR24-2 were separately infected. The GFP or Green was expressed in four stable cell lines (pCMV-miR-hLCSCs, pCMV-miR24-2-hLCSCs, rLV-Cas9-hLCSCs, rLV-Cas9-miR24-2-hLCSCs), respectively (Figure S2A). In rLV-Cas9-miR24-2-hLCSCs, the 18 cases of hLCSCs stable cell lines were analyzed and showed that #2, #3, #6, #8, #11, #14, #15, and #16 were positive miR24-2 knockout cell lines (Figure S3). miR24-2 and cyclic miR24-2 were significantly increased in pCMV-miR24-2 group compared to the pCMV-miR group and reduced in rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figures 1A and 1B; Figures S2B and S2C). As shown in Figure 1A, the size of mature miR24-2 is 22 bp, the size of pre-miR24-2 is 73 bp, the size of pri-miR24-2 is 238 bp. Moreover, the knockout of miR-24-2 did not affect the expression of miR-23a and miR-27a in LCSCs (Figures S4A and S4B). Next, the cell proliferation index was significantly increased in pCMV-miR24-2 group compared with the pCMV-miR group (24 h, p = 0.0056; 48 h, p = 0.0037), and reduced in rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (24 h, p = 0.0082; 48, p = 0.0051) (Figure 1C). The colony formation rate was significantly increased in pCMV-miR24-2 compared with the pCMV-miR group (42.63% ± 3.03% versus 81.26% ± 6.61%, p = 0.009978) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (39.82% ± 5.69% versus 18.41% ± 1.36%, p = 0.00773) (Figure 1D, a and b). The sphere formation rate was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (34.84% ± 5.23% versus 74.52% ± 6.51%, p = 0.0082) and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (32.56% ± 3.79% versus 13.78% ± 2.08%, p = 0.0098) (Figure 1E). Furthermore, miR24-2 increased the bromodeoxyuridine (BrdU)-positive rate (Figure S2D; Supplemental Results) and the average width ratio of scratches (Figure S2E; Supplemental Results). Moreover, the average weight of the transplanted tumors was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (0.63 ± 0.09 g versus 1.315 ± 0.194 g, p = 0.0008042) and decreased in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (0.598 ± 0.14 g versus 0.092 ± 0.0194 g, p = 0.000202) (Figures 1F and 1G). The average appearance time of transplanted tumors was significantly reduced in the pCMV-miR24-2 group compared with the pCMV-miR group (9.01 ± 0.89 days versus 5.83 ± 0.75 days, p = 0.0000037) and increased in the rLV-Cas9-miR24-2 group compared to rLV-Cas9 group (8.83 ± 1.17 days versus 15.33 ± 1.86 days, p = 0.00094) (Figure 1H). The poorly differentiated cancer cells were increased in the pCMV-miR24-2 group compared with the pCMV-miR group and decreased in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure S2F). Furthermore, the proliferating cell nuclear antigen (PCNA)-positive rate was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (33.88% ± 6.31% versus 63.65% ± 8.46%, p = 0.00019) and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (36.56% ± 10.69% versus 76.82% ± 3.03%, p = 0.00343) (Figure 1I; Figure S2G). Moreover, the similar results were obtained in hLCSCs infected with rLV-tet on-miR24-2, including DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (1 μg/mL) group, and DOX (2 μg/mL) group (Figures S2H–S2L; Supplemental Results). Collectively, these observations suggest that miR24-2 accelerates the growth of liver cancer stem cells in vitro and in vivo.
Figure 1.
miR24-2 Promotes Growth of hLCSCs
(A) pCMV-miR or pCMV-miR24-2 were transfected into and the rLV-Cas9 or rLV-Cas9-miR24-2 were infected hLCSCs, respectively. Northern blotting was used to detect the miR24-2. U6 was used as an internal reference. (B) Quantitative RT-PCR was used to detect the miR24-2. U6 was used as an internal reference. (C) Cell proliferation ability was measured by the CCK8 method. **p < 0.01, *p < 0.05. (D) The plate colony formation ability of cells. (a) Photograph of plate colonies. (b) Determination of cell plate colony formation rate. **p < 0.01, *p < 0.05. (E) The sphere formation ability of cells. (F) hLCSCs cells were inoculated into BALB/c nude mice for 1 month. The photograph of the dissected xenograft is shown. (G) Comparison of the size (g) of transplanted tumors in nude mice (n = 6), **p < 0.01, *p < 0.05. (H) Comparison of time (days) of transplanted tumors in nude mice. (I) 4% formalin-fixed, paraffin-embedded nude mouse transplanted tumor tissue sections (4 μm) were subjected to the immunohistochemical staining of anti-PCNA. Comparison of PCNA-positive rates. **p < 0.01, *p < 0.05.
miR24-2 Targets PRMT7
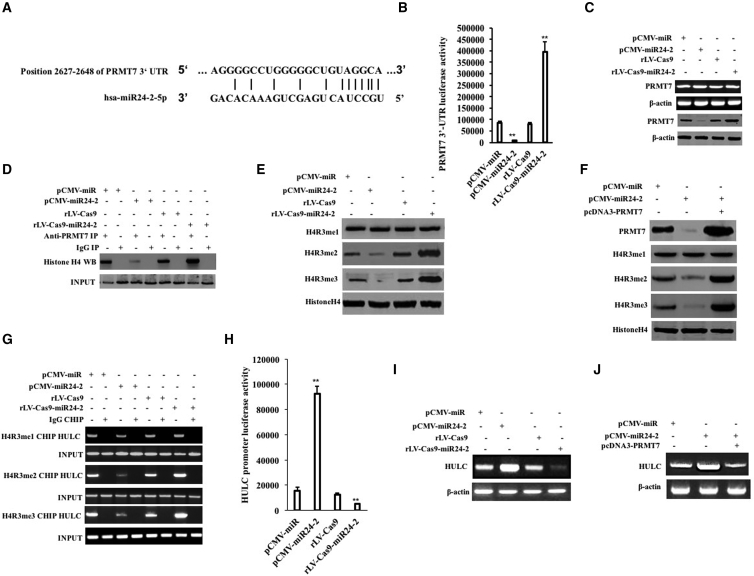
To investigate whether miR24-2 targets PRMT7 in hLCSCs, we constructed four stable cell lines (pCMV-miR-hLCSCs, pCMV-miR24-2-hLCSCs, rLV-Cas9-hLCSCs, rLV-Cas9-miR24-2-hLCSCs) (Figure S5A, a and b). Bioinformatics analysis revealed that the mature sequence of miR24-2 binds to the 3′ UTR of PRMT7 mRNA (2627-2648) via a 12-base complementary seed sequence (Figure 2A). Compared with the pCMV-miR group, the pEZX-MT-PRMT7 3′ UTR-Luc luciferase reporter gene activity was significantly reduced in the pCMV-miR24-2 group (p = 0.00126) and increased in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (p = 0.00581) (Figure 2B). However, there was no significant change of the pEZX-MT-PRMT7 3′ UTR(mutante)-Luc reporter gene activity among the four groups (p > 0.05) (Figure S5B). Although there was no significant change in the transcription level of PRMT7, the translational level of PRMT7 was significantly reduced in the pCMV-miR24-2 group compared to the pCMV-miR group and increased in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 2C). Furthermore, the similar results were obtained in hLCSCs infected with rLV-tet on-miR24-2, including DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (1 μg/mL) group, and DOX (2 μg/mL) group (Figures S5C–S5G; Supplemental Results). Taken together, these results suggest that miR24-2 targets PRMT7 3′ UTR and inhibits the expression of the PRMT7 in hLCSCs.
Figure 2.
miR24-2 Targets PRMT7 and Inhibits HULC in hLCSCs
(A) Bioinformatics method to analyze binding seed sequences of mature miR24-2 to PRMT7 mRNA 3′ UTR. (B) The cells were tested for pEZX-MT-PRMT7 3′ UTR-Luc dual luciferase reporter gene activity. **p < 0.01, *p < 0.05. (C) The PRMT7 was detected by RT-PCR and western blotting. β-actin was used as an internal reference gene. (D) CoIP with anti-PRMT7 was performed and the precipitate was analyzed by western blotting with anti-histone H4. Immunoglobulin G (IgG) coIP was used as a negative control and western blotting anti-PRMT7 was subjected to as INPUT. (E) Western blotting using anti-H4R3me, anti-H4R3me2, and anti-H4R3me3 was performed, and β-actin was used as an internal reference gene. (F) Western blotting using anti-H4R3me, anti-H4R3me2, and anti-H4R3me3 was performed, and β-actin was used as an internal reference gene. (G) ChIP using anti-H4R3me, anti-H4R3me2, and anti-H4R3me3. PCR amplification was carried out using primers designed according to the HULC promoter. IgG ChIP was used as a negative control and the product amplified by the primer designed by the HULC promoter was used as an internal reference (INPUT). (H) The pGL4-HULC-Luc luciferase reporter gene activity was measured. **p < 0.01, *p < 0.05. (I) HULC was analyzed by RT-PCR. (J) HULC was analyzed by RT-PCR. β-actin was used as an internal reference gene.
miR24-2 Enhances the Expression of Nanog
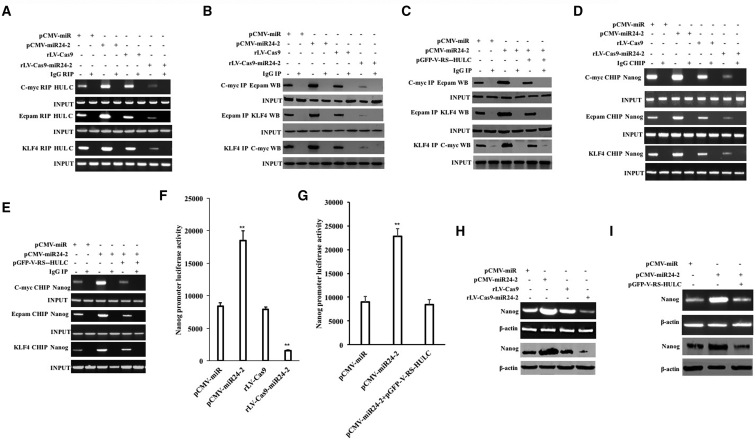
Given that miR24-2 inhibits the expression of PRMT7, we consider whether miR24-2 reduces H4R3 methylation modification in hLCSCs. The interaction between histone H4 and PRMT7 was attenuated in the pCMV-miR24-2 group compared with the pCMV-miR group and enhanced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 2D). The modification of H4R3me2 or H4R3me3 was significantly reduced in the pCMV-miR24-2 group compared with the pCMV-miR group and increased the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group. However, the modification of H4R3me was not significantly changed in these groups (Figure 2E). Moreover, the modification of H4R3me2 or H4R3me3 was significantly not altered in the pCMV-miR24-2+pcDNA3-PRMT7 group compared to pCMV-miR group (Figure 2F). Furthermore, the modification of dimethylation and trimethylation of arginine-3 of histone H4 on the long noncoding RNA HULC promoter region was significantly reduced in the pCMV-miR24-2 group compared with the pCMV-miR group and increased in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 2G). Notably, miR24-2 affects the modification of trimethylation of arginine-3 of histone H4 on the HULC promoter region specifically (Figure S6; Supplemental Results). Furthermore, the pGL4-HULC-Luc luciferase reporter activity was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p = 0.00183) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (p = 0.00447) (Figure 2H). Therefore, the HULC was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 2I). However, the HULC was significantly not altered in the pCMV-miR24-2+pcDNA3-PRMT7 group compared to pCMV-miR group (Figure 2J). In particular, the binding ability of KLF4, C-myc, and Epcam to HULC was significantly enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and weakened in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 3A). Moreover, the binding ability of Ecamp to C-myc, C-myc to Epcam, and KLF4 to C-myc was significantly enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and weakened in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group, respectively (Figure 3B). However, the binding ability of Ecamp to C-myc, C-myc to Epcam, and KLF4 to C-myc was significantly not altered in the pCMV-miR24-2+pGFP-V-RS-HULC group compared with the pCMV-miR group (Figure 3C). Thereby, the binding ability of C-myc, Epcam, and KLF4 to the Nanog promoter was significantly enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and weakened the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 (Figure 3D). However, there was no significant change in the binding ability of C-myc, Epcam, and KLF4 to the Nanog promoter in the pCMV-miR24-2+pGFP-V-RS-HULC group compared to the pCMV-miR group, respectively (Figure 3E). Furthermore, the pEZX-MT-Nanog-Luc luciferase reporter gene activity was significantly enhanced in the pCMV-miR24-2 group compared to the pCMV-miR group (p < 0.01) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (p < 0.01) (Figure 3F). However, there was no significant difference between the pCMV-miR24-2+pGFP-V-RS-HULC group and the pCMV-miR group (8,938.45 ± 1,261.59 versus 8,444.96 ± 1,055.63, p > 0.05) (Figure 3G). Ultimately, the expression of Nanog was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 3H). However, there was no significant difference between the pCMV-miR24-2+pGFP-V-RS-HULC group and the pCMV-miR group (Figure 3I). Collectively, these results suggest that miR24-2 enhances the expression of Nanog in hLCSCs.
Figure 3.
miR24-2 Promotes the Expression of Nanog in Human Liver Stem Cells
(A) The RIP using anti-KLF7, anti-C-myc, anti-Epcam. The HULC sequence was designed to amplify the HULC by RT-PCR. IgG RNA immunoprecipitation (RIP) was used as a negative control. (B) Immunoprecipitation using anti-C-myc, anti-Epcam, and anti-KLF4, respectively. (C) The coIP with anti-C-myc, anti-Epcam, and anti-KLF4, respectively. (D) ChIP using anti-C-myc, anti-Epcam, and anti-KLF4. IgG ChIP was used as a negative control. (E) The ChIP using anti-C-myc, anti-Epcam, and anti-KLF4. (F) The pEZX-MT-Nanog-Luc luciferase reporter gene activity was assayed. (G) The pEZX-MT-Nanog-Luc luciferase reporter gene activity was detected. **p < 0.01, *p < 0.05. (H) The Nanog was analyzed by RT-PCR and western blot. β-actin was used as an internal reference gene. (I) The Nanog was analyzed by RT-PCR and western blotting. β-actin was used as an internal reference gene.
miR24-2 Promotes the Expression and Maturation of miR675 via Nanog
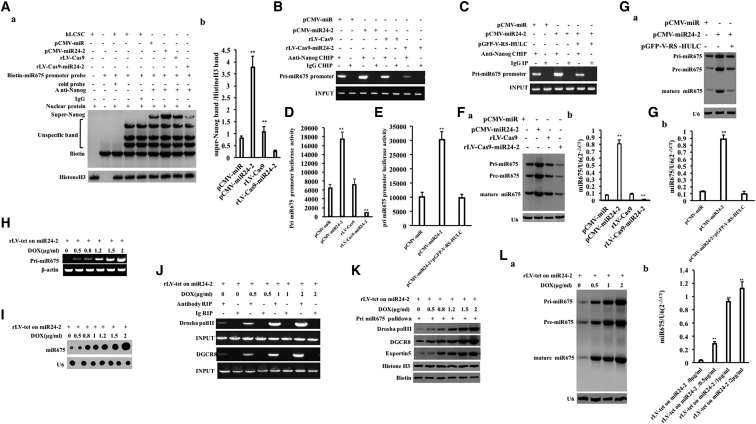
To analyze whether miR24-2 affects the binding ability of Nanog to the miR675 precursor promoter, we first analyzed this binding ability of Nanog to the miR675 promoter cis element using a super-gel migration assay. The binding ability of Nanog to the pri-miR675 promoter cis-element probe was significantly enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and reduced the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 4A, a and b). Moreover, the binding ability of Nanog to pri-miR675 promoter was significantly enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and attenuated in rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 4B). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-HULC group and the pCMV-miR group (Figure 4C). Furthermore, the pri-miR675 promoter luciferase reporter gene activity was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p = 0.0009519) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (p = 0.007702) (Figure 4D). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-HULC group compared to the pCMV-miR group (p = 0.400512 > 0.05) (Figure 4E). Therefore, the pri-miR675, pre-miR675, and mature miR675 were significantly increased in the pCMV6-miR24-2 group compared with the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 4F, a and b). However, there was no significant change between pCMV-miR24-2+pGFP-V-RS-HULC group and the pCMV-miR group (Figure 4G, a and b). Moreover, in hLCSCs infected with rLV-tet on-miR24-2, the transcriptional activity of pri-miR675 was significantly increased with increasing DOX concentration (Figures 4H and 4I; Figures S7A–S7G; Supplemental Results). Furthermore, the binding ability of Drosha pol III, DGCR8, and Exportin5 to pri-miR675 was significantly increased (Figures 4J and 4K) and pri-miR675, pre-miR675, and mature miR675 were significantly increased with increasing DOX concentration (Figure 4L, a and b). Collectively, these results suggest that miR24-2 increases miR675 through Nanog hLCSCs.
Figure 4.
miR24-2 Promotes Expression and Maturation of miR675
(A) (a) Super-DNA-protein complex gel migration assay using biotin-labeled pri-miR675 promoter cis-element probe and anti-Nanog, anti-Biotin. IgG super-EMSA was used as a negative control. (b) Grayscale scan analysis of positive bands. (B) The ChIP using anti-Nanog. The PCR amplification was carried out using a primer designed according to the pri-miR675 promoter. (C) The ChIP using anti-Nanog. (D) The pEZX-MT-pri-miR675-Luc luciferase reporter activity was assayed. **p < 0.01, *p < 0.05. (E) The pEZX-MT-pri-miR675-Luc luciferase reporter gene activity was detected. (F) (a) Northern blotting was used to detect the miR675. U6 was used as an internal reference gene. (b) Quantitative RT-PCR analysis. (G) (a) Northern blotting was used to detect the miR675. U6 was used as an internal reference gene. (b) Quantitative RT-PCR. (H) The pri-miR675 was analyzed by RT-PCR. β-actin is used as an internal reference. (I) The miR675 was analyzed by dot blotting. U6 is used as an internal reference. (J) RIP with anti-Drosha pol III and anti-DGCR8 was performed. IgG RNA immunoprecipitation was used as a negative control. (K) RNA pulldown was performed. Biotin was used as INPUT and histone H3 was as an internal reference. (L) (a) miR675 was detected by Northern blotting. U6 serves as an internal reference gene. (b) Detection of miR675 by quantitative RT-PCR. U6 was used as an internal reference gene. **p < 0.01, *p < 0.05.
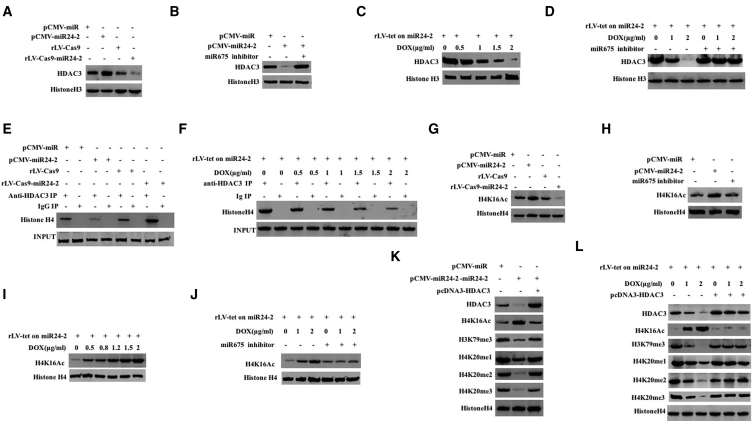
miR24-2 Enhances Acetylation on H4K16 and Inhibits Methylation on H4K20
Given that miR675 targets HDAC3 3′ UTR and and inhibits expression of the HDAC3 gene in hLCSCs (Figures S8A–S8F; Supplemental Results), we consider whether miR24-2 promotes acetylation of the 16th lysine of histone H4 and inhibits methylation of the 20th lysine of histone H4 via miR675. The expression of HDAC3 was significantly reduced in the pCMV6-miR24-2 group compared to the pCMV-miR group and increased in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 5A). However, there was no significant change between the pCMV-miR24-2+miR675 inhibitor group compared to the pCMV-miR group (Figure 5B). The expression of HDAC3 was significantly reduced as the concentration of increased DOX in the DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (1 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figure 5C). However, there was no significant change as the increasing DOX concentration among the hLCSCs of DOX (0 μg/mL)/miR675 inhibitor group, DOX (1 μg/mL)/miR675 inhibitor group, and DOX (2 μg/mL)/miR675 inhibitor group (Figure 5D). Furthermore, the interaction of histone H4 with HDAC3 was significantly attenuated in the pCMV-miR24-2 group compared to the pCMV-miR group and enhanced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 5E). In stable hLCSCs infected with rLV-tet on-miR24-2, the interaction between histone H4 and HDAC3 was significantly reduced with increasing DOX concentration (Figure 5F). The level of H4K16Ac was significantly increased in the pCMV6-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 5G). However, there was no significant change of H4K16Ac between the pCMV6-miR24-2+miR675 inhibitor group and pCMV-miR group (Figure 5H). The H4K16Ac was significantly increased with increasing DOX concentration in DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (0.8 μg/mL) group, DOX (1.2 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figure 5I). However, H4K16Ac did not change significantly with increasing DOX concentration among DOX (0 μg/mL)/miR675 inhibitor group, DOX (1 μg/mL)/miR675 inhibitor group, and DOX (2 μg/mL)/miR675 inhibitor group (Figure 5J). Furthermore, the level of H4K16Ac was significantly increased and the levels of HDAC3, H4K20me1/2/3, and H3K79me3 H3K79me3 were significantly decreased in the pCMV-miR24-2 group compared with the pCMV-miR group. However, there was no significant change between pCMV-miR24-2+pcDNA3-HDAC3 group and the pCMV-miR group (Figure 5K). However, HDAC3, H4K16Ac, H4K20me1/2/3, and H3K79me3 H3K79me3 did not significantly change with increasing DOX concentration in the DOX (0 μg/mL)/pcDNA3-HDAC3 group, the DOX (1 μg/mL)/pcDNA3-HDAC3 group, and the DOX (2 μg/mL)/pcDNA3-HDAC3 group (Figure 5L). Collectively, these results suggest that miR24-2 promotes acetylation on the 16th lysine of histone H4 and inhibits methylation on the 20th lysine of histone H4 by miR675-HDAC3.
Figure 5.
miR24-2 Promotes Acetylation of the 16th Lysine of Histone H4 and Inhibits Methylation of the 20th Lysine of Histone H4 in hLCSCs
(A) HDAC3 was detected by western blotting. β-actin was used as an internal reference gene. (B) HDAC3 was detected by western blotting. (C) HDAC3 was detected by western blotting. β-actin was used as an internal reference gene. (D) The HDAC3 was detected by western blotting. β-actin is used as an internal reference gene. (E) CoIP was performed using anti-HDAC3. IgG coIP was used as a negative control. (F) CoIP was performed using anti-HDAC3. (G) Western blotting was used to detect the H4K16Ac. (H) The H4K16Ac was detected by western blotting. (I) The H4K16Ac was detected by western blotting. Histone H4 serves as an internal reference gene. (J) The H4K16Ac was detected by western blotting. (K) Western blotting was used to detect HDAC3, H4K16Ac, H4K20me1/2/3, and H3K79me3 H3K79me3. (L) Western blotting was used to detect HDAC3, H4K16Ac, H4K20me1/2/3, and H3K79me3 H3K79me3. Histone H4 was used as an internal reference gene.
miR24-2 Enhances the Expression of Phosphatidylinositol 3-Kinase
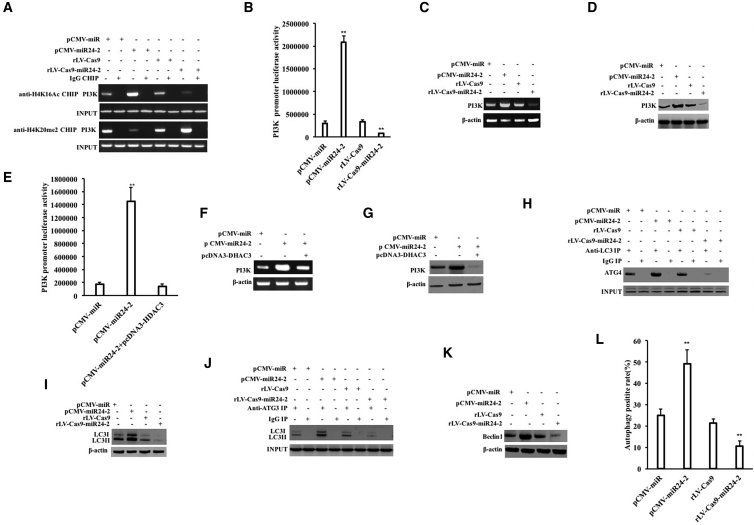
To analyze whether miR24-2 affects the modification of histone H4K16Ac and H4K20me2 on the phosphatidylinositol 3-kinase (PI3K) promoter region, we first performed chromatin immunoprecipitation (ChIP) analysis in hLCSCs. The modification of H4K16Ac in the PI3K promoter region was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group, and the modification of H4K20me2 in the PI3K promoter region was significantly decreased in the pCMV-miR24-2 group compared with the pCMV-miR group and increased in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 6A). In hLCSCs infected with rLV-tet on-miR24-2, the modification of H4K16Ac in the PI3K promoter region was significantly increased and the modification of H4K20me2 in the PI3K promoter region was significantly decreased with increasing DOX concentration (Figure S9A). The pGL4-PI3K-Luc luciferase reporter gene activity was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p = 0.00195) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (p = 0.0052453) (Figure 6B). The PI3K luciferase reporter gene activity was increased with increasing DOX concentration in the DOX (0 μg/mL) group, the DOX (0.5 μg/mL) group, DOX (1.0 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (p < 0.01) (Figure S9B). The PI3K expression was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figures 6C and 6D). And the expression of PI3K was significantly increased as the increasing DOX concentration In DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (0.8 μg/mL) group, DOX (1.2 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figures S9C and S9D). Furthermore, the binding ability of H3K20me2 to the PI3K promoter cis-element probe was significantly reduced with increasing DOX concentration in hLCSCs infected with rLV-tet on-miR24-2. However, the binding ability of H3K20me2 to the PI3K promoter cis-element probe did not significantly change with increasing DOX concentration among rLV-tet on-miR24-2/DOX (0 μg/mL) +pcDNA3-HDAC3 group, rLV-tet on-miR24-2/DOX (1 μg/mL) +pcDNA3-HDAC3 group, and rLV-tet on-miR24-2+pcDNA3-HDAC3/DOX (2 μg/mL) group (Figure S10A, a and b). Moreover, the binding capacity of RNA polII to PI3K promoter cis-element probes was significantly increased with increasing DOX concentration. However, the binding ability of RNA polII to the PI3K promoter cis-element probe did not significantly change with increasing DOX concentration in rLV-tet on-miR24-2/DOX (0 μg/mL) +pcDNA3-HDAC3 group, rLV-tet on-miR24-2/DOX (1 μg/mL) +pcDNA3-HDAC3 group, and rLV-tet on-miR24-2/DOX (2 μg/mL)+pcDNA3-HDAC3 group (Figure S10B, a and b). The pGL4-PI3K-Luc luciferase reporter gene activity was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p = 0.004232). However, there was no significant difference between the pCMV-miR24-2+pcDNA3-HDAC3 group (p = 0.223619) (Figure 6E). The PI3K promoter luciferase reporter gene activity was significantly increased with increasing DOX concentration among rLV-tet on-miR24-2/DOX (0 μg/mL) group, rLV-tet on-miR24-2/DOX (1 μg/mL) group, and rLV-tet on-miR24-2/DOX (2 μg/mL) group (p < 0.01). However, the PI3K promoter luciferase reporter gene activity did not change significantly with increasing DOX concentration in rLV-tet on-miR24-2/DOX (0 μg/mL) +pcDNA3-HDAC3 group, rLV-tet on-miR24-2/DOX (1 μg/mL) +pcDNA3-HDAC3 group, rLV-tet on-miR24-2/DOX (2 μg/mL) +pcDNA3-HDAC3 group (p > 0.05) (Figure S10C). Moreover, the expression of PI3K transcriptional capacity was significantly increased in the pCMV6-miR24-2 group compared to the pCMV-miR group. However, there was no significant change between the pCMV-miR24-2+pcDNA3-HDAC3 group and the pCMV-miR group (Figures 6F and 6G). The expression of PI3K was significantly increased in the DOX (2 μg/mL) group compared to the DOX (0 μg/mL) group. However, there was no significant change between the rLV-tet on-miR24-2/DOX (2 μg/mL) +pcDNA3-HDAC3 group and the rLV-tet on-miR24-2/DOX (0 μg/mL) (Figures S9E and S9F). The expression of PI3K/H4K16Ac was significantly increased and H3K20me2/KAT8 was significantly decreased, and SET8 did not significantly change in the pCMV-miR24-2 group compared with the pCMV-miR group. However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-KAT8 group or the pCMV-miR24-2+pcDNA3-SET8 group compared with the pCMV-miR group (Figures S10D and S10F). The expression of PI3K/H4K16Ac was significantly increased and H3K20me2/KAT8 was significantly decreased and SET8 did not change in the DOX (2 μg/mL) group compared with the DOX (0 μg/mL) group. However, there was no significant change in rLV-tet on-miR24-2/DOX (2 μg/mL) +pGFP-V-RS-KAT8 or rLV-tet on-miR24-2/DOX (2 μg/mL) +pcDNA3-SET8 group compared with the rLV-tet on-miR24-2/DOX (0 μg/mL) group (Figures S10E and S10G). Collectively, these results suggest that miR24-2 promotes expression of PI3K via HDAC3-H4K16Ac -H4K20me2 in hLCSCs.
Figure 6.
miR24-2 Enhances PI3K and Affects Autophagy in hLCSCs
(A) The ChIP using anti-H4K6Ac and anti-H4K20me2. IgG ChIP was used as a negative control. (B) The pGL4-PI3K-Luc luciferase reporter gene activity was tested. (C) RT-PCR was performed using PI3K primers. (D) Western blotting using anti-PI3K. β-actin was used as an internal reference gene. (E) The pGL4-PI3K-Luc luciferase reporter gene activity was detected. **p < 0.01, *p < 0.05. (F) The RT-PCR was performed. (G) The total protein was subjected to western blotting using anti-PI3K. β-actin was used as an internal reference gene. (H) The coIP with anti-LC3. IgG coIP was used as a negative control. (I) Western blotting using anti-LC3. β-actin as an internal reference gene. (J) The coIP with anti-ATG3. (K) Western blotting using anti-Beclin1. β-actin as an internal reference gene. (L) The infection of adenovirus rAd-Cherry-GFP-LC3 can monitor the autophagy through fluorescence microscopy directly. The occurrence of autophagy was observed (red marker Cherry-LC3). Comparison of the incidence of autophagy. **p < 0.01, *p < 0.05.
miR24-2 Promotes Cellular Autophagy Dependent on PI3K
Given that miR24-2 promotes the expression of PI3K and that PI3K can cause cellular autophagy in several cells, we consider whether miR24-2 promotes autophagy of hLCSCs. First, the interaction between the autophagy structural protein LC3 and the LC3 cleavage protein ATG4 was enhanced in the pCMV-miR24-2 group compared to the pCMV-miR group and weakened in rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 6H). In the hLCSCs infected with rLV-tet on-miR24-2, the interaction of LC3 with ATG4 was significantly increased as the increasing concentration of DOX (Figure S9G). The expression of LC3I and LC3II was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group to the rLV-Cas9 group (Figure 6I). Moreover, the expression of LC3I and LC3II increased significantly as the DOX concentration increased in DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (0.8 μg/mL) group, DOX (1.2 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figure S9H). And the interaction between the LC3 and ATG3 was enhanced in the pCMV-miR24-2 group compared to the pCMV-miR group and significantly attenuated in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 6J). Moreover, the interaction of LC3 with ATG3 was significantly increased with the increasing concentration of DOX in DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, and DOX (1 μg/mL) group, DOX (2 μg/mL) group (Figure S9I). The expression of the autophagy marker protein Beclin1 was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and significantly reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 6K). The expression of Beclin1 was significantly increased with increasing DOX concentration significantly (Figure S9J). The incidence of autophagy was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (24.85% ± 3.01% versus 49.04% ± 6.59%, p = 0.00762688) and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (21.3% ± 2.06% versus 10.47% ± 2.46%, p = 0.0223) (Figure 6L; Figure S9K, b). Furthermore, the incidence rate of autophagy was significantly increased with increasing DOX concentration in the DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) (p < 0.01 or p < 0.05) (Figure S9L, a and b). Moreover, PI3K knockdown abrogates these actions of miR24-2 for autophagy (Figures S11A–S11I; Supplemental Results). Collectively, these results suggest that miR24-2 promotes autophagy dependent on PI3K in hLCSCs.
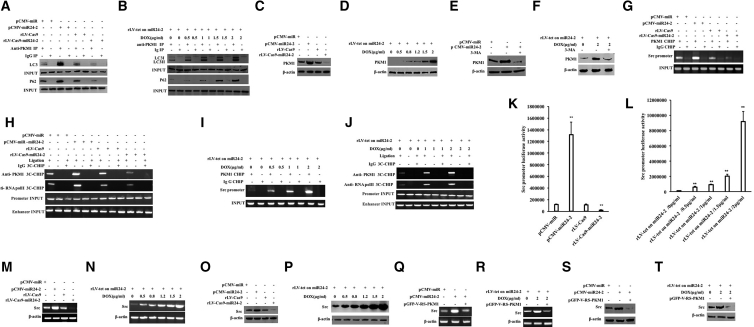
miR24-2 Promotes the Expression of PKM1 Dependent on Cellular Autophagy
In order to investigate the role of miR24-2-dependent autophagy in affecting the expression of PKM1, we first explored whether miR24-2 can enhance the interaction between PKM1 and autophagosome functional proteins in LCSCs. The interaction of PKM1 with the LC3I/II or the autophagy functional protein P62 was enhanced in the pCMV-miR24-2 group compared with the pCMV-miR group and attenuated in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 7A). The interaction between PKM1 and LC3I/II, and PKM1 and protein P62 were significantly enhanced with increasing DOX concentration in DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (1 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figure 7B). The expression of PKM1 was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figure 7C). Moreover, the expression of PKM1 was significantly increased as the DOX concentration increased in the DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (0.8 μg/mL) group, DOX (1.2 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figure 7D). The expression of PKM1 was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group. However, there was no significant change between the pCMV-miR24-2+3-MA group and the pCMV-miR group (Figure 7E). The expression of PKM1 was significantly increased in the DOX (2 μg/mL) treated group compared to the DOX (0 μg/mL) group. However, there was no significant change between the rLV-tet on-miR24-2/DOX (2 μg/mL) +3-MA group and the DOX (0 μg/mL) group (Figure 7F). Collectively, these results suggest that miR24-2 enhances the expression of PKM1 dependent on autophagy in hLCSCs.
Figure 7.
miR24-2 Promotes the Expression of the Tyrosine Protein Kinase Sarcoma Gene Src by Enhancing PKM1 in hLCSCs
(A) The coIP with anti-PKM1 was performed and the precipitates were analyzed by western blotting with anti-LC3 or anti-P62. IgG coIP was used as a negative control. (B) The coIP with anti-PKM1. (C) Western blotting using anti-PKM1. (D) Western blotting with anti-PKM1was performed. β-actin as an internal reference gene. (E) The total protein was subjected to western blotting using anti-PKM1. (F) Western blotting was performed using anti-PKM1. β-actin was used as an internal reference gene. (G) ChIP was performed using anti-PKM1. IgG ChIP was used as a negative control. (H) The binding ability of PKM1 to the Src promoter-enhancer loop was analyzed by chromosomal conformation capture (3C)-ChIP. IgG ChIP-3C was used as a negative control and the products amplified by independent primers designed by Src promoter and enhancer were used as internal reference (INPUT). (I) ChIP was performed. (J) 3C-ChIP was performed. (K) The pGL4-Src-Luc luciferase reporter activity was detected. (L) The pGL4-Src-Luc luciferase reporter gene activity was detected. (M) RT-PCR was used to detect Src. β-actin was used as an internal reference gene. (N) RT-PCR was used to detect Src. β-actin was used as an internal reference gene. (O) Western blotting was performed using anti-Src. (P) Western blotting was performed using anti-Src. (Q) RT-PCR was used to detect Src. (R) RT-PCR was used to detect Src. β-actin was used as an internal reference gene. (S) Western blotting was performed using anti-Src. (T) Western blotting was performed using anti-Src. β-actin was used as an internal reference gene.
miR24-2 Enhances the Expression of Tyrosine Protein Kinase Sarcoma Gene Src by Enhancing PKM1
To analyze whether miR24-2 affects the expression of the tyrosine protein kinase sarcoma gene Src through PKM1, we performed a series of assays in LCSCs. The binding ability of PKM1 to Src promoter was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 7G). The binding ability of PKM1 and RNA polII to the Src promoter-enhancer loop were significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared with the rLV-Cas9 group (Figure 7H). Furthermore, the binding ability of PKM1 to Src promoter was significantly increased with the increasing DOX concentration (Figure 7I). The binding ability of PKM1, RNApolII, and Src promoter-enhancer loop was significantly increased with increasing DOX concentration in the DOX (0 μg/mL) group, the DOX (1 μg/mL) group, and the DOX (2 μg/mL) group (Figure 7J). The pGL4-Src-Luc luciferase reporter gene activity was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p = 0.004693) and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (p = 0.0061157) (Figure 7K). The Src promoter luciferase reporter gene activity was increased with increasing DOX concentration (p < 0.01) (Figure 7L). Therefore, the expression of Src was significantly increased in the pCMV-miR24-2 group compared to the pCMV-miR group and reduced in the rLV-Cas9-miR24-2 group compared to the rLV-Cas9 group (Figures 7M and 7O). Moreover, the expression of Src was increased significantly as the increasing concentration of DOX in DOX (0 μg/mL) group, DOX (0.5 μg/mL) group, DOX (0.8 μg/mL) group, DOX (1.2 μg/mL) group, DOX (1.5 μg/mL) group, and DOX (2 μg/mL) group (Figures 7N and 7P). Moreover, the expression of Src was significantly increased in the pCMV-miR24-2 groups compared to the pCMV-miR group. However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-PKM1 group and pCMV-miR (Figures 7Q and 7S). Although the expression of Src was significantly increased in the DOX (2 μg/mL) group compared to the DOX (0 μg/mL) group, there was no significant change between the rLV-tet on-miR24-2/DOX (2 μg/mL) +pGFP-V-RS-PKM1 group compared to the DOX (0 μg/mL) group (Figures 7R and 7T). Collectively, these observations suggest that miR24-2 promotes the expression of Src through enhancing PKM1 in hLCSCs.
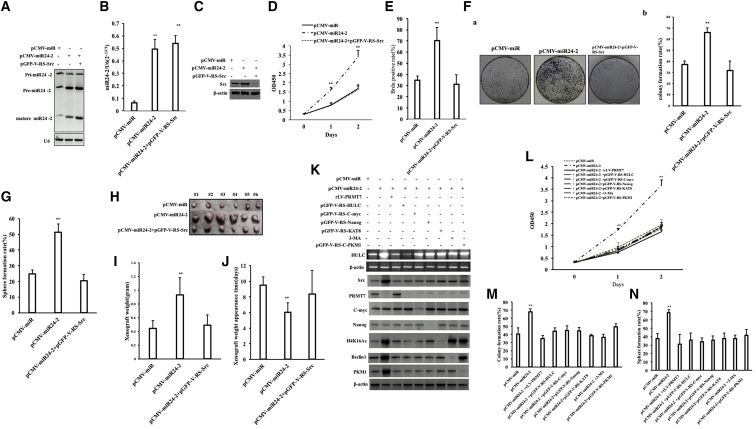
The Oncogenic Functions of miR24-2 Were Regulated by Src in Liver Cancer Stem Cells
To investigate whether the Src plays an important role in the malignant growth of hLCSCs triggered by miR24-2, hLCSCs were transfected with pCMV-miR, pCMV-miR24-2, and pCMV-miR24-2+pGFP-V-RS-Src, respectively. The excessive miR24-2 was produced in the pCMV-miR24-2 group and the pCMV-miR24-2+pGFP-V-RS-Src group (Figures 8A and 8B). And the expression of Src was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group and reduced in the pCMV-miR24-2+pGFP-V-RS-Src group (Figure 8C). The proliferation ability of hLCSC was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (p < 0.01); however, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (p > 0.05) (Figure 8D). The BrdU-positive rate was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (34.91% ± 3.86% versus 70.15% ± 12.11%, p = 0.0089263). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (34.91% ± 3.86% versus 31.08% ± 2.503%, p = 0.3234176) (Figure 8E). The colony formation rate was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (37.24% ± 3.22% versus 37.24% ± 3.22%, p = 0.0080199). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (37.24% ± 3.22% versus 31.63% ± 8.72%, p = 0.109668) (Figure 8F). The sphere formation rate was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (24.68% ± 2.68% versus 51.41% ± 5.31%, p = 0.00998). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (24.68% ± 2.68% versus 20.31% ± 4.23%, p = 0.0515) (Figure 8G). Although the average weight of transplanted tumors was significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group (0.44 ± 0.12 g versus 0.93 ± 0.25 g, p = 0.0015983), there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (0.44 ± 0.12 g versus 0.49 ± 0.15 g, p = 0.1487) (Figures 8H and 8I). And the average appearance time of transplanted tumors was significantly reduced in the pCMV-miR24-2 group compared with the pCMV-miRs group (9.5 ± 1.05 days versus 6 ± 1.26 days, p = 0.0017085). However, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (9.5 ± 1.05 days versus 8.83 ± 3.06 days, p = 0.3475961) (Figure 8J). Although the poorly differentiated tumor cells were significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group, there was no significant change between the pCMV-miR24-2+pGFP-V-RS-Src group and pCMV-miR group (Figure S12). Notably, although the expression of Src, the cell proliferation ability, colony formation rate, and sphere formation rate were significantly increased in the pCMV-miR24-2 group compared with the pCMV-miR group, there was no significant change in the pCMV-miR24-2+pGFP-V-RS-HULC group, pCMV-miR24-2+rLV-PRMT7 group, pCMV-miR24-2+pGFP-V-RS-C-myc group, pCMV-miR24-2+pGFP-V-RS-Nanog group, pCMV-miR24-2+3-MA group, pCMV-miR24-2+pGFP-V-KAT8 group, and pCMV-miR24-2+pGFP-V-RS-PKM1 group compared to pCMV-miR group, respectively (Figures 8K–8N). Collectively, these results suggest that Src regulates and controls the oncogenic functions of miR24-2 in hLCSCs positively.
Figure 8.
Src Affects miR24-2-Induced Malignant Growth of hLCSCs
(A) The miR24-2 was detected by northern blot. U6 was used as an internal reference gene. (B) The miR24-2 was detected by RT-PCR. (C) Western blotting was used to detect the Src. β-actin as an internal reference gene. (D) The cell proliferation ability was determined by the CCK8 method. (E) Determination of the S phase percentage of hLCSCs cells by BrdU staining. (F) Determination of plate colony forming ability of cells. (a) Photograph of plate colonies. (b) The analysis of cell plate colony formation rate. (G) The assay of cell sphere formation ability. (H) The hLCSCs were inoculated into the BALB/c nude mice for 1 month. Photography of xenografts. (I) Comparison of the size (g) of transplanted tumors in nude mice (n = 6). **p < 0.01,*p < 0.05. (J) The comparison of appearance time (days) of transplanted tumors in nude mice (n = 6). **p < 0.01, *p < 0.05. (K) RT-PCR was used to detect HULC, and western blotting was used to detect the expression of Src, PRMT7, C-myc, Nanog, Beclin1, H4K16Ac, and PKM1. β-actin was used as an internal reference gene. (L) The cell proliferation ability was determined by the CCK8 method. (M) The analysis of cell plate colony formation rate. (N) The assay of cell sphere formation ability. **p < 0.01, *p < 0.05.
Discussion
At the present, we clearly demonstrate that miR24-2 enhances the expression and function of the tyrosine protein kinase sarcoma gene Src in LCSCs and promotes the malignant growth of LCSCs (Figure S13). In this study, it was confirmed that miR24-2 is a microRNA with cancerous function, which is shown at least in human liver cancer. Moreover, Nanog, HDAC3, and PI3K are key players in this signaling pathway mediated by miR24-2 specifically.
Studies have reported that miR24-2 is involved in tumorigenesis. For example, miRNA24-2 promotes the development of tumors such as gastric cancer and breast cancer by enhancing the expression of oncogenes such as c-Myc.24,25 However, there are some reports that miR24-2 negatively regulates the growth of tumor cells. For example, miR24-2 can regulate different apoptotic pathways and induce apoptosis.16,26 Therefore, we believe that miR24-2 is likely to play a specific role through different mechanisms in different tumor cells and its corresponding environments.
It is well known that epigenetic modifications mainly include histone modifications and nucleic acid modifications, which play an important role in the regulation of gene expression, and epigenetic disorders are common features of most cancers.27 Our findings suggest that miR24-2 alters the expression of various histone H3/4 epigenetic modifications, indicating that miR24-2 can play a regulatory role in the development of liver cancer epigenetically. Our findings suggest that miR24-2 inhibits the expression of PRMT7. It was reported that PRMT7 can promote methylation of arginine at position 3 of histone H428 and inhibits the expression of certain genes by upregulating the symmetrical dimethylation level of H4R3.17,29,30 We demonstrate that miR24-2 can target PRMT7 and reduce the bi/trimethylation of histone H4R3 and then miR24-2 causes the changes in the function of some important genes, such as HULC, in liver cancer cells. Notably, studies have also reported that H4R3me2 is a marker of transcriptional repression.31
Furthermore, miR24-2-dependent miR675 inhibits the expression of histone deacetylase HDAC3, which plays an important role in the regulation of histone modification and is generally considered to be a locus-specific co-suppressor, which is recruited to the promoter.32,33 Moreover, miR24-2 increased the H4K16Ac modification by inhibiting the expression of HDAC3, which in turn altered the expression of certain genes. Surprisingly, miR24-2 also triggers a change in the methylation level of histone H4 lysine 20 (H4K20) and histone H3 lysine 79 (H3K79). Methylation of H4K20 plays a key role in regulating high-level chromosome structure and X chromosome gene expression,34 and H3K79me2 is a histone marker associated with transcriptional active genes.35 In this study, the methylation levels of H4K20 and H3K79 did not change after knockdown of H4K16Ac-specific acetyltransferase KAT8 in miR24-2 overexpressing cells. It was shown that miR24-2 altered the methylation modification of H4K20 and H3K79 dependent on H4K16Ac. Importantly, these changes in epigenetic modifications further cause altering of certain genes, such as PI3K.
Notably, our findings indicate that miR24-2 can play a carcinogenic role together with other noncoding RNAs including miRNA and long noncoding RNAs (lncRNAs) such as miR675, HULC, etc. This study found that miR24-2 could increase the transcriptional activity and maturation of pri-miR675 by promoting the binding of Nanog to the miR675 precursor (pri-miR675) promoter in hLCSCs. miR675 plays a different role in tumors, and this inhibits DNA damage repair and regulates abnormal expression of cell-cycle-related genes.36, 37, 38 Although miR24-2 may play a carcinogenic role through miR675, its detailed mechanism needs further to be confirmed. Importantly, our results identify that miR24-2 can increase the transcriptional activity of HULC by inhibiting the modification of H4R3me2/3 to the HULC promoter region. HULC is dysregulated in many types of cancer and promotes proliferation, invasion, migration, and angiogenesis of cancer cells.20,39,40 Our study indicates that miR24-2 enhances the binding of HULC to some molecules, such as KLF4, c-Myc, Epcam, etc., which enhances the expression of Nanog and promotes autophagy through downstream signaling pathway. It can be seen that lncRNA HULC plays a key role in the carcinogenesis triggered by miR24-2.
Strikingly, our results demonstrate that the function of miR24-2 is related to autophagy in hLCSCs. First, miR24-2 increases the expression and lipidation of the autophagy structural protein LC3 in hLCSCs. During autophagy, LC3 (LC3-I) in the cytoplasm combines with phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited to the autophagosome membrane. Therefore, the autophagosome marker LC3-II reflects autophagy activity.41,42 In this study, miR24-2 promotes the expression of LC3 and the formation of LC3-II, indicating that miR24-2 is involved in the regulation of autophagy and promotes the production of autophagy. Second, miR24-2 enhances the interaction between LC3 and the LC3 cleavage protein ATG4 in hLCSCs. Studies have shown that ATG4 is essential for autophagy and highly specific.43 During autophagy, LC3 undergoes two steps, namely proteolytic cleavage of LC3 and delipidation of LC3-PE in autophagosomes, both catalyzed by ATG4.44 This study suggests that miR24-2 enhances the function of ATG4, leads to the cleavage of LC3, and further forms activated LC3II, which is a key step in the process of autophagy, further indicating that miR24-2 is involved in the regulation of autophagy. Third, miR24-2 increases the interaction of LC3II with the activated LC3II processing protein ATG3. ATG3 is an E2-like enzyme required for LC3 lipidation during autophagy.45 When autophagy occurs, ATG3 binds to LC3 and lipidation of LC3 occurs, a process that is the initial step in autophagy. Thus, this study suggests that miR24-2 may affect the initiation of autophagy through the binding of ATG3 to LC3II. Fourthly, miR24-2 increased the expression of the autophagy marker protein Beclin1, indicating that miR24-2 promotes autophagy in hLCSCs. It is reported that Beclin1 is a coiled-coil protein that interacts with Bcl-2 and promotes autophagy.46 In this study, the expression of Beclin1 was elevated in hLCSCs overexpressing miR24-2, further confirming that miR24-2 regulates the expression of Beclin1 to promote autophagy. Finally, miR24-2 enhances PI3K expression by altering histone modifications such as H4K16Ac, H4K20me2/3, etc. PI3K plays an important role in autophagy, membrane trafficking, and cell signaling47 and interacts with Beclin1 to participate in autophagy.48,49 In this study, after knocking down PI3K in miR24-2 overexpressing hLCSCs, the function of miR24-2 to promote autophagy was fully abolished, indicating that miR24-2-dependent PI3K promotes autophagy.
It is worth noting that protein kinases can be involved in most of the signal transduction in eukaryotic cells and regulate many cellular processes.50,51 In this study, miR24-2 affected the expression and function of several protein kinases, including PKM1, and Src, indicating that miR24-2 exerts its carcinogenic function at least in part by altering protein kinase activity in hLCSCs. PKM1 is a protein kinase that activates glucose catabolism and stimulates autophagy, thereby enhancing the malignant growth of tumor cells.52 miR24-2-dependent autophagy enhanced the expression and function of PKM1 in hLCSCs, indicating that PKM1 plays an important role in the carcinogenic functions of miR24-2. Furthermore, the function of miR24-2 is associated with the tyrosine protein kinase sarcoma gene Src. Studies have shown that the Src kinase family is a class of non-receptor tyrosine kinases.53 In particular, Src plays a key role in tumor development and is involved in the regulation of proliferation, survival, migration, invasion, and metastasis of various tumor cells.54 In this study, miR24-2-dependent PKM1 promotes the expression of Src, and knockdown of Src substantially abolishes the tumorigenic ability of miR24-2, indicating that Src may decide the oncogenic action of miR24-2 at least in LCSCs.
So far, our study clearly reveals some of the mechanisms by which miR24-2 plays a carcinogenic role in human LCSCs, but the detailed mechanisms of specific processes remain to be further studied. These interesting findings will provide a valuable theoretical basis for the discovery of liver cancer therapeutic targets and diagnosis markers based on miR-24-2.
Materials and Methods
Bioinformatics Analysis
Bioinformatics analysis was performed by MirTarget scanning software, RNA22 software, BLAST tools, miRanda, RNA hybrid and PicTar, and TCGA miRNA expression profile.
hLCSC Sorting
CD133/CD44/CD24/EpCAM MicroBead Kits were purchased from Miltenyi Biotec (Boston, USA) and MACS Technology and performed the operation according to the manufacturer.
Cell Lines, Lentivirus, and Plasmids
hLCSCs were maintained in DMEM (GIBCO BRL Life Technologies) supplemented with 10% fetal bovine serum (Sigma) in a humidified atmosphere of 5% CO2 incubator at 37°C. rLV were purchased from Wu Han viraltherapy Technologies. pCMV-miR was purchased from Origene (Rockville, MD, USA).
RT-PCR
Total RNA was purified using Trizol (Invitrogen) according to the manufacturer’s instructions. cDNA was prepared by SuperScript First-Strand Synthesis System (Invitrogen). PCR analysis was performed according to the manufacturer’s instructions.
Western Blotting
The samples containing cellular proteins were separated on a 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Amersham). The blots were incubated with antibody at 4°C overnight. Following three washes, membranes were then incubated with secondary antibody at 4°C overnight. Signals were visualized by ECL System (Amersham).
CoIP
Cells were lysed in the whole-cell extract buffer A (50 mM pH 7.6 Tris-HCl, 150 mM NaCl, 1% NP40, 0.1mM EDTA,1.0 mM DTT,0.2 mM PMSF, 0.1 mM Pepstatine,0.1 mM Leupeptine, 0.1 mM Aproine). 500 μL of cell lysates was used in immunoprecipitation with antibody. Western blot was performed with another related antibody.
RNA Immunoprecipitation
Ribonucleoprotein particle-enriched lysates were incubated with protein A/G-plus agarose beads (Santa Cruz, Biotechnology, CA) together with specific antibody for 4 hours at 4°C. Beads were subsequently washed four times with 50 mM Tris-HCl (pH 7.0), 150 mM NaCl, 1 mM MgCl2, and 0.05% NP-40, and twice after addition of 1 M urea. mRNAs were then isolated and purified for RT-PCR according to the manufacturer’s instructions.
Chromosome Conformation Capture-ChIP
Chromatin bound to the antibody-protein-A/G-Sepharose beads were resuspended and the ChIP-chromosome conformation capture (3C) material was detected for long-range interaction with specific primers according to the manufacturer’s instructions.
Super-EMSA (Gel-Shift)
Cells were washed and scraped in ice-cold PBS to prepare nuclei for electrophoretic gel mobility shift assay with the use of the gel shift assay system modified according to the manufacturer’s instructions.
Cells Proliferation CCK8 Assay
The cell proliferation reagent CCK8 was purchased from Roche and the operation was performed according to the manufacturer’s instruction.
Colony-Formation Efficiency Assay
Cells were plated on a six-well plate and the DMEM containing 10% FBS was added into each well of the three replicates. Cell colonies were stained with Crystal Violet.
Xenograft Transplantation In Vivo
The male athymic BALB/c mice were injected with hLCSCs at the armpit area subcutaneously. The mice were observed for 4 weeks and then sacrificed to recover the tumors. The use of mice for this work was reviewed and approved by the institutional animal care and use committee in accordance with China National Institutes of Health guidelines.
Author Contributions
D.L. conceived the study and participated in the study design, performance, coordination, and manuscript writing. L.W., X.L., W.Z., Y.Y., Q.M., C.W., X.X., X.J., S.S., Y.L., H.P., X.G.,T.L., J.X., J.L., and S.J. performed the research. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (NCSF grant numbers 81572773 and 81773158) and by a grant from the Science and Technology Commission of Shanghai Municipality Basic Research Field Project (19JC1415200).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.10.015.
Supplemental Information
References
- 1.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Rosental B., Kowarsky M., Seita J., Corey D.M., Ishizuka K.J., Palmeri K.J., Chen S.Y., Sinha R., Okamoto J., Mantalas G. Complex mammalian-like haematopoietic system found in a colonial chordate. Nature. 2018;564:425–429. doi: 10.1038/s41586-018-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messal H.A., Alt S., Ferreira R.M.M., Gribben C., Wang V.M., Cotoi C.G., Salbreux G., Behrens A. Tissue curvature and apicobasal mechanical tension imbalance instruct cancer morphogenesis. Nature. 2019;566:126–130. doi: 10.1038/s41586-019-0891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-Danés A., Larsimont J.C., Liagre M., Muñoz-Couselo E., Lapouge G., Brisebarre A., Dubois C., Suppa M., Sukumaran V., del Marmol V. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature. 2018;562:434–438. doi: 10.1038/s41586-018-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe J., Lin S., Zhang W., Liu Q., Wang L., Ramirez-Moya J., Du P., Kim W., Tang S., Sliz P. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma S., Chan K.W., Hu L., Lee T.K., Wo J.Y., Ng I.O., Zheng B.J., Guan X.Y. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z.F., Ho D.W., Ng M.N., Lau C.K., Yu W.C., Ngai P., Chu P.W., Lam C.T., Poon R.T., Fan S.T. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Lui S.K., Vilchez V., Gedaly R. Liver Cancer Stem Cells: A New Paradigm for Hepatocellular Carcinoma Treatment. J. Stem Cell Res. Ther. 2015;5:283. [Google Scholar]
- 9.Wang D., Si S., Wang Q., Luo G., Du Q., Liang Q., Guo X., Zhang G., Feng J., Leng Z. MiR-27a Promotes Hemin-Induced Erythroid Differentiation of K562 Cells by Targeting CDC25B. Cell. Physiol. Biochem. 2018;46:365–374. doi: 10.1159/000488436. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Zhang Y., Su X., Wang H., Yang W., Zan L. Cooperative and Independent Functions of the miR-23a∼27a∼24-2 Cluster in Bovine Adipocyte Adipogenesis. Int. J. Mol. Sci. 2018;19:E3957. doi: 10.3390/ijms19123957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teteloshvili N., Dekkema G., Boots A.M., Heeringa P., Jellema P., de Jong D., Terpstra M., Brouwer E., Pawelec G., Kok K. Involvement of MicroRNAs in the Aging-Related Decline of CD28 Expression by Human T Cells. Front. Immunol. 2018;9:1400. doi: 10.3389/fimmu.2018.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H.C., Bae Y., Dawson B.C., Chen Y., Bertin T., Munivez E., Campeau P.M., Tao J., Chen R., Lee B.H. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-β signalling in osteoblasts. Nat. Commun. 2017;8:15000. doi: 10.1038/ncomms15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng A.M., Byrom M.W., Shelton J., Ford L.P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin E.C., Elliott S., Rhodes L.V., Antoon J.W., Fewell C., Zhu Y., Driver J.L., Jodari-Karimi M., Taylor C.W., Flemington E.K. Preferential star strand biogenesis of pre-miR-24-2 targets PKC-alpha and suppresses cell survival in MCF-7 breast cancer cells. Mol. Carcinog. 2014;53:38–48. doi: 10.1002/mc.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manvati S., Mangalhara K.C., Kalaiarasan P., Srivastava N., Bamezai R.N. miR-24-2 regulates genes in survival pathway and demonstrates potential in reducing cellular viability in combination with docetaxel. Gene. 2015;567:217–224. doi: 10.1016/j.gene.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Liu X., Xu W., Zhou P., Gao P., Jiang S., Lobie P.E., Zhu T. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2. J. Biol. Chem. 2013;288:18121–18133. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S.H., Chen T.Y., Dhar S.S., Gu B., Chen K., Kim Y.Z., Li W., Lee M.G. A feedback loop comprising PRMT7 and miR-24-2 interplays with Oct4, Nanog, Klf4 and c-Myc to regulate stemness. Nucleic Acids Res. 2016;44:10603–10618. doi: 10.1093/nar/gkw788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musto A., Navarra A., Vocca A., Gargiulo A., Minopoli G., Romano S., Romano M.F., Russo T., Parisi S. miR-23a, miR-24 and miR-27a protect differentiating ESCs from BMP4-induced apoptosis. Cell Death Differ. 2015;22:1047–1057. doi: 10.1038/cdd.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G., Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He D., Wang J., Zhang C., Shan B., Deng X., Li B., Zhou Y., Chen W., Hong J., Gao Y. Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol. Cancer. 2015;14:73. doi: 10.1186/s12943-015-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panzitt K., Tschernatsch M.M., Guelly C., Moustafa T., Stradner M., Strohmaier H.M., Buck C.R., Denk H., Schroeder R., Trauner M., Zatloukal K. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Yao R., Jiang H., Ma Y., Wang L., Wang L., Du J., Hou P., Gao Y., Zhao L., Wang G. PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 2014;74:5656–5667. doi: 10.1158/0008-5472.CAN-14-0800. [DOI] [PubMed] [Google Scholar]
- 23.Jain K., Jin C.Y., Clarke S.G. Epigenetic control via allosteric regulation of mammalian protein arginine methyltransferases. Proc. Natl. Acad. Sci. USA. 2017;114:10101–10106. doi: 10.1073/pnas.1706978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua K., Chen Y.T., Chen C.F., Tang Y.S., Huang T.T., Lin Y.C., Yeh T.S., Huang K.H., Lee H.C., Hsu M.T. MicroRNA-23a/27a/24-2 cluster promotes gastric cancer cell proliferation synergistically. Oncol. Lett. 2018;16:2319–2325. doi: 10.3892/ol.2018.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He H.W., Wang N.N., Yi X.M., Tang C.P., Wang D. Low-level serum miR-24-2 is associated with the progression of colorectal cancer. Cancer Biomark. 2018;21:261–267. doi: 10.3233/CBM-170321. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava N., Manvati S., Srivastava A., Pal R., Kalaiarasan P., Chattopadhyay S., Gochhait S., Dua R., Bamezai R.N. miR-24-2 controls H2AFX expression regardless of gene copy number alteration and induces apoptosis by targeting antiapoptotic gene BCL-2: a potential for therapeutic intervention. Breast Cancer Res. 2011;13:R39. doi: 10.1186/bcr2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad H.P., Barbash O., Creasy C.L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat. Med. 2019;25:403–418. doi: 10.1038/s41591-019-0376-8. [DOI] [PubMed] [Google Scholar]
- 28.Jelinic P., Stehle J.C., Shaw P. The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation. PLoS Biol. 2006;4:e355. doi: 10.1371/journal.pbio.0040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain K., Clarke S.G. PRMT7 as a unique member of the protein arginine methyltransferase family: A review. Arch. Biochem. Biophys. 2019;665:36–45. doi: 10.1016/j.abb.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang B., Chen Y., Zhao Y., Bruick R.K. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 31.Dhar S.S., Lee S.H., Kan P.Y., Voigt P., Ma L., Shi X., Reinberg D., Lee M.G. Trans-tail regulation of MLL4-catalyzed H3K4 methylation by H4R3 symmetric dimethylation is mediated by a tandem PHD of MLL4. Genes Dev. 2012;26:2749–2762. doi: 10.1101/gad.203356.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones P.L., Shi Y.B. N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. Curr. Top. Microbiol. Immunol. 2003;274:237–268. doi: 10.1007/978-3-642-55747-7_9. [DOI] [PubMed] [Google Scholar]
- 33.Bhaskara S., Knutson S.K., Jiang G., Chandrasekharan M.B., Wilson A.J., Zheng S., Yenamandra A., Locke K., Yuan J.L., Bonine-Summers A.R. Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell. 2010;18:436–447. doi: 10.1016/j.ccr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brejc K., Bian Q., Uzawa S., Wheeler B.S., Anderson E.C., King D.S., Kranzusch P.J., Preston C.G., Meyer B.J. Dynamic Control of X Chromosome Conformation and Repression by a Histone H4K20 Demethylase. Cell. 2017;171:85–102.e23. doi: 10.1016/j.cell.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernt K.M., Zhu N., Sinha A.U., Vempati S., Faber J., Krivtsov A.V., Feng Z., Punt N., Daigle A., Bullinger L. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y., Song S., Jiang X., Meng Q., Wang C., Li X., Yang Y., Xin X., Zheng Q., Wang L. miR675 Accelerates Malignant Transformation of Mesenchymal Stem Cells by Blocking DNA Mismatch Repair. Mol. Ther. Nucleic Acids. 2019;14:171–183. doi: 10.1016/j.omtn.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang W.P., Ng E.K., Ng S.S., Jin H., Yu J., Sung J.J., Kwok T.T. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Meng Q., Wang C., Li X., Lu Y., Xin X., Zheng Q., Lu D. MicroRNA 675 cooperates PKM2 to aggravate progression of human liver cancer stem cells induced from embryonic stem cells. J. Mol. Med. (Berl.) 2018;96:1119–1130. doi: 10.1007/s00109-018-1687-9. [DOI] [PubMed] [Google Scholar]
- 39.Chen S., Wu D.D., Sang X.B., Wang L.L., Zong Z.H., Sun K.X., Liu B.L., Zhao Y. The lncRNA HULC functions as an oncogene by targeting ATG7 and ITGB1 in epithelial ovarian carcinoma. Cell Death Dis. 2017;8:e3118. doi: 10.1038/cddis.2017.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D., Liu X., Zhou J., Hu J., Zhang D., Liu J., Qiao Y., Zhan Q. Long noncoding RNA HULC modulates the phosphorylation of YB-1 through serving as a scaffold of extracellular signal-regulated kinase and YB-1 to enhance hepatocarcinogenesis. Hepatology. 2017;65:1612–1627. doi: 10.1002/hep.29010. [DOI] [PubMed] [Google Scholar]
- 41.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanida I., Ueno T., Kominami E. LC3 and Autophagy. Methods Mol. Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 43.Maruyama T., Noda N.N. Autophagy-regulating protease Atg4: structure, function, regulation and inhibition. J. Antibiot. (Tokyo) 2017;71:72–78. doi: 10.1038/ja.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pengo N., Agrotis A., Prak K., Jones J., Ketteler R. A reversible phospho-switch mediated by ULK1 regulates the activity of autophagy protease ATG4B. Nat. Commun. 2017;8:294. doi: 10.1038/s41467-017-00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radoshevich L., Murrow L., Chen N., Fernandez E., Roy S., Fung C., Debnath J. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell. 2010;142:590–600. doi: 10.1016/j.cell.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang X.H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 47.Miller S., Tavshanjian B., Oleksy A., Perisic O., Houseman B.T., Shokat K.M., Williams R.L. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327:1638–1642. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He C., Wei Y., Sun K., Li B., Dong X., Zou Z., Liu Y., Kinch L.N., Khan S., Sinha S. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154:1085–1099. doi: 10.1016/j.cell.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 51.Leopold A.V., Chernov K.G., Verkhusha V.V. Optogenetically controlled protein kinases for regulation of cellular signaling. Chem. Soc. Rev. 2018;47:2454–2484. doi: 10.1039/c7cs00404d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita M., Sato T., Nomura M., Sakamoto Y., Inoue Y., Tanaka R., Ito S., Kurosawa K., Yamaguchi K., Sugiura Y. PKM1 Confers Metabolic Advantages and Promotes Cell-Autonomous Tumor Cell Growth. Cancer Cell. 2018;33:355–367.e7. doi: 10.1016/j.ccell.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Ishizawar R., Parsons S.J. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S., Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol. Sci. 2012;33:122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.