Abstract
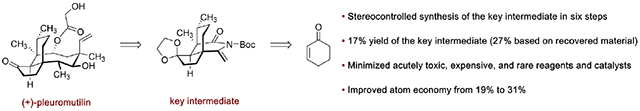
An improved synthesis of an eneimide, which is a useful precursor to pleuromutilin-based antibiotics, is reported. This synthesis proceeds in six steps and 17% overall yield (27% based on recovery of a key hydrindenone intermediate) and requires two fewer chromatography steps and five fewer days of reaction time than the previously reported route. The use of expensive, acutely toxic, and precious metal reagents or catalysts has been minimized.
Graphical Abstract
The diterpene metabolite (+)-pleuromutilin (1)1 and various semisynthetic derivatives (Scheme 1A) inhibit the growth of Gram-positive pathogens (GPPs) by binding the peptidyl transferase center of the bacterial ribosome.2 These antibiotics bind via an induced fit mechanism whereby the macrocyclic core blocks the A-site of the large ribosomal subunit and the C14 side chain occupies the P-site. Researchers have focused on obtaining new pleuromutilins by semisynthesis, primarily by modification of the C14 side chain.1e,3 The approval of retapamulin (2) in 2007 as a topical agent for the treatment of impetigo marked the entry of pleuromutilins into widespread use.4 Retapamulin (2) and other derivatives elicit low mutational frequencies,3b and clinical resistance to 2 had not been reported as of 2014.5 The C14 derivative lefamulin (3) is in phase III clinical trials for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections (ABSSSI) by oral and IV administration.3b Its success would mark a second important milestone for this class of antibiotics.
Scheme 1.
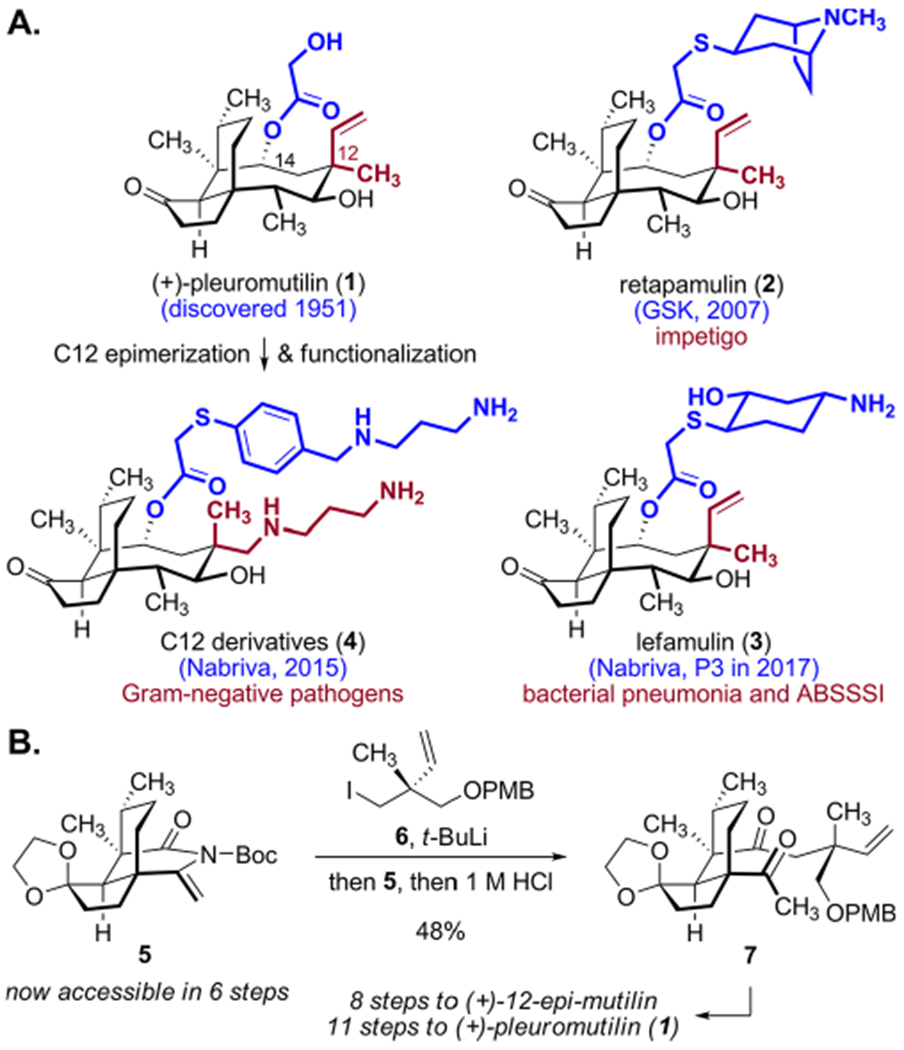
(A) Structures of Selected Pleuromutilins; (B) Key Fragment Coupling en Route to Pleuromutilins
While alterations to the C14 side chain of 1 have been extensively investigated and lead to increased potency against GPPs, studies suggest that modification of the macrocyclic core can provide agents with extended-spectrum activity in vitro and in vivo.6 For example, epimerization of the C12 position7 followed by functionalization of the transposed alkene provides derivatives (e.g., 4) with activity against drug-resistant Gram-negative pathogens (GNPs).6 Incorporating C12 modifications appear to hamper drug efflux mediated by the AcrAB–TolC pump in GNPs.6b,8
The eneimide 5 bears the pleuromutilin hydrindanone core and was used in a 2-fold neopentylic coupling reaction with the iodoether 6 to access pleuromutilins and 12-epi-pleuromutilins (Scheme 2B).9,10 Analogs with diverse substituents within the macrocycle, including those with polar functional groups and heteroatomic substitution, are envisioned to be accessible by employing derivatives of 6 in the coupling. To maximize the feasibility of this approach, a more efficient synthesis of 5 was desired. The published synthesis of the eneimide 5 proceeds in eight steps and 20% yield from cyclohex-2-ene-1-one (8).9 Herein we report an alternative route to 5 that improves the step count and atom economy11 of the synthesis and minimizes the use of reagents and catalysts that are expensive, acutely toxic, or precious metal-based.
Scheme 2.
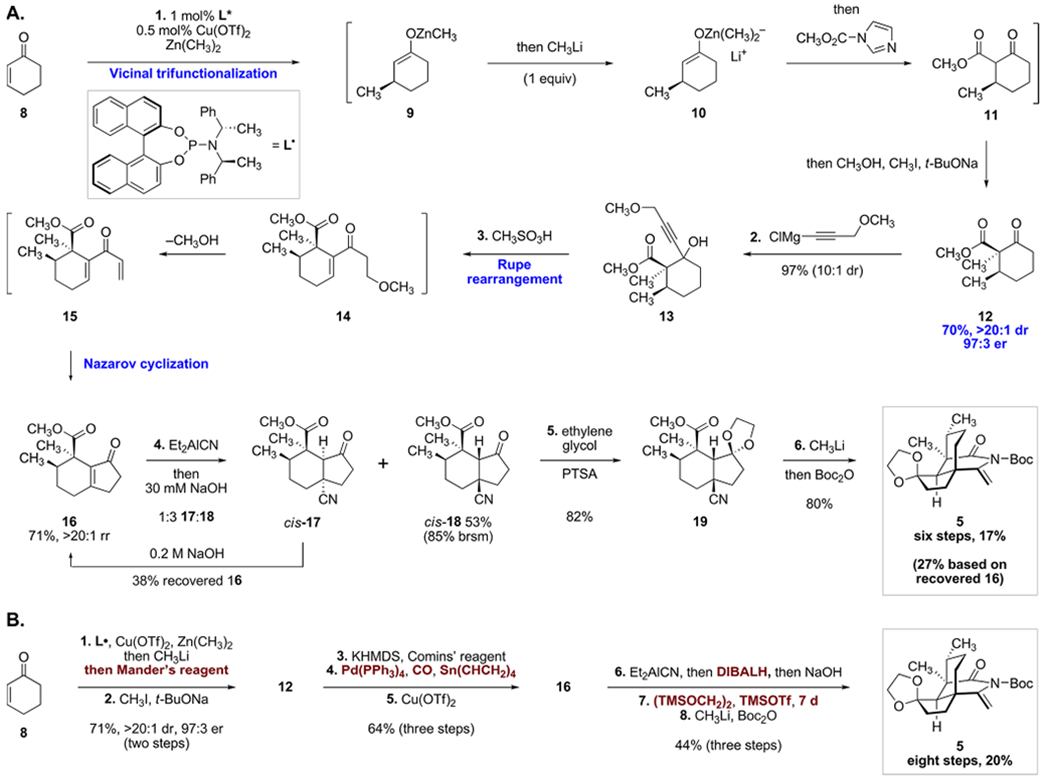
(A) Improved Synthesis of the Eneimide 5 in Six Steps;a (B) Prior Synthesis of the Eneimide 5 in Eight Steps
aSee the Supporting Information for synthetic details and characterization data.
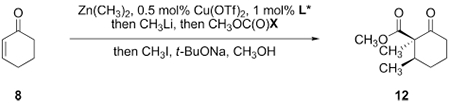
Our optimized synthetic route is shown in Scheme 2A, whereas the original route, for comparison, is abbreviated in Scheme 2B. Our original synthetic route involved conversion of cyclohex-2-ene-1-one (8) to the α-methyl-β-ketoester 12 by a two-step sequence. This involved stereoselective copper-catalyzed 1,4-addition of dimethylzinc to 8, in situ activation of the resulting zinc enolate with methyllithium (1 equiv),12 and C-acylation with methyl cyanoformate (Mander’s reagent).13 In a second step, the α-methyl-β-ketoester 12 was obtained by stereoselective alkylation of 11 with iodomethane (71%; see also entry 1, Table 1). While this sequence consistently provided gram quantities of 12, we sought to avoid the use of an expensive acylating reagent (USD 12/g),14 the generation of stoichiometric cyanide waste, and the need to isolate the volatile β-ketoester 11.
Table 1.
Optimization of the Synthesis of 12
 | |||
|---|---|---|---|
| no. | X | protocol | yield of 12 (%) |
| 1 | CN | two-step | 71 |
| 2 | Cl | two-step | 28a,b |
| 3 | OCH3 | two-step | <5a,b |
| 4 | Imc | two-step | 75 |
| 5 | Imc | telescopedd | 70 |
Yield of the conjugate addition–acylation product 11.
Determined by 1H NMR analysis.
Im = imidazole.
CH3OH added directly to the reaction mixture in the telescoped procedure.
To improve the safety and economy of the synthesis, we first sought to replace methyl cyanoformate with a benign and inexpensive acylating reagent. Substituting with methyl chloroformate resulted in a diminished yield of the conjugate addition–acylation product (28%, entry 2, Table 1) while dimethylcarbonate was not an effective acylating reagent (entry 3). Employing the Heller–Sarpong reagent [N-(carbomethoxy)-imidazole], originally developed for esterification of carboxylic acids,15 resulted in smooth acylation of the zincate enolate to provide, after diastereoselective α-alkylation, the desired α-methyl-β-ketoester 12 in 75% yield (two steps, >20:1 dr, 97:3 er, entry 4). The Heller–Sarpong reagent is commercially available and can be conveniently prepared from the inexpensive reagents imidazole (USD 0.15/g) and methyl chloroformate (USD 0.13/g).14 To further expedite the preparation of 12, we investigated telescoping the conjugate addition–acylation and methylation steps. We found that the sequential addition of methanol (to consume residual alkylzinc species), sodium tert-butoxide, and iodomethane following the acylation step provided the alkylation product 12 directly (entry 5). This vicinal trifunctionalization provided access to the α-methyl-β-ketoester 12 in one flask and 70% yield (>20:1 dr, 97:3 er) on multigram scale, eliminated the production of cyanide waste, reduced the cost and time of synthesizing 12, and shortened the step count of the route.
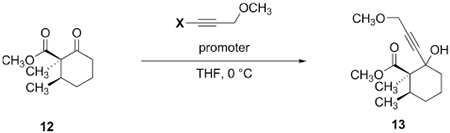
The cyclopentenone ring was originally constructed by a three-step procedure (12 → 16, Scheme 2B) comprising ketone triflation, palladium-catalyzed carbonylative Stille coupling with tetravinyltin,16 and copper-catalyzed Nazarov cyclization17 (64% overall). This approach poses several issues including poor atom economy,11 the use of lateral manipulations, the use of acutely toxic reagents and catalysts (carbon monoxide, tetravinyltin, palladium), and difficulties associated with removing tin impurities that sporadically inhibited the Nazarov cyclization. To address this, we pursued a 1,2-addition–Rupe rearrangement–Nazarov cyclopentannulation strategy developed by Raphael.18,19 We first investigated the addition of the acetylide derived from methyl propargyl ether to 12 (Table 2). Surprisingly, the yield of the propargylic alcohol 13 was highly dependent on the counterion of the acetylide. Lithium and magnesium bromide acetylides provided moderate yields of product (60% and 65%, entries 1 and 2, respectively). The addition of the magnesium chloride acetylide in the presence of zinc chloride (10 mol %) as a promoter20 was more efficient and generated the product 13 in 88% yield (entry 3). However, the addition of the magnesium chloride acetylide in the absence of zinc chloride proceeded in 97% yield (entry 4). In the case of entry 4, the product 13 was formed as a 10:1 mixture of diastereomers (stereochemistry not assigned).
Table 2.
Optimization of the Acetylide Addition to 12
NMR yield.
13 was isolated as a 10:1 mixture of diastereomers.
We initially treated the unpurified addition product 13 with ethanolic sulfuric acid to affect conversion to the hydrindenone 16. Unfortunately, the hydrindenone 16 was formed in only 35% yield and was accompanied by unidentified decomposition products. Whereas trifluoroacetic acid was not an effective promoter, methanesulfonic acid in dichloromethane at 0 °C lead to the formation of 16 in 71% isolated yield after purification by flash-column chromatography. The mechanism of this transformation likely involves a Rupe rearrangement21 of 13 to the β-methoxyketone 14, elimination of methanol to form the dienone 15, and acid-mediated Nazarov cyclization to produce the hydrindenone 16. Use of this two-step method for annulation decreases the cumulative reaction time of the synthesis by approximately two days, eliminates two chromatography steps, and improves the step efficiency and overall yield.
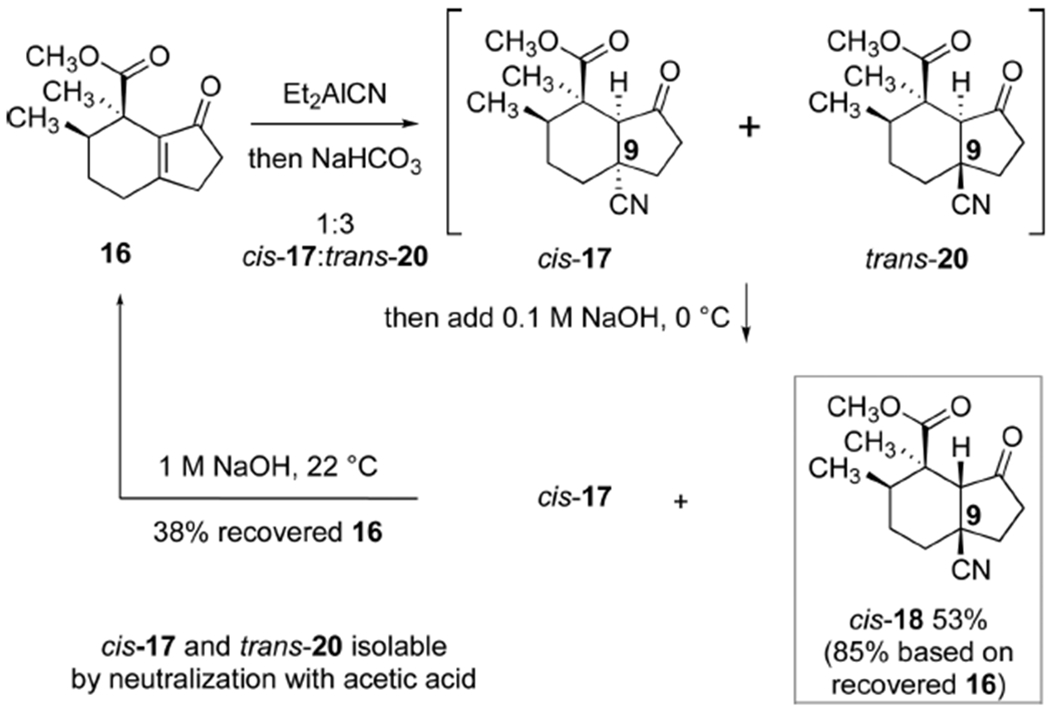
1,4-Hydrocyanation22 of the hydrindenone 16 was a challenging step that required extensive experimentation. 1,4-Addition of diethylaluminum cyanide followed by acidification with acetic acid provided a 1:3 mixture of the hydrindanones cis-17 and trans-20 which possess the undesired and desired C9-stereochemistry, respectively (Scheme 3). Careful addition of dilute (100 mM) sodium hydroxide allowed for selective epimerization of trans-20 to cis-18 without E1cb elimination. In our previous approach we resolved these diastereomers by selective reduction of the undesired hydrindanone with diisobutylaluminum hydride (DIBALH, Scheme 2B). In our improved approach, we found conditions to separate the two hydrindanones cis-17 and cis-18 on multigram scale using flash-column chromatography (53% yield of isolated cis-18). Treatment of the undesired diastereomer cis-17 with sodium hydroxide in methanol (1 M) induced E1cb elimination to reform 38% of the starting hydrindenone 16. Thus, this approach allows us to efficiently recycle the undesired diastereomer formed in the hydrocyanation step.
Scheme 3.
Synthesis of the cis-Hydrindanones 17 and 18
Next, the hydrindanone cis-18 was converted to the ethylene glycol ketal 19. The ketone of cis-18 is sterically encumbered and difficult to protect. Initially, ketalization of cis-18 under Noyori’s conditions23 [TMSOTf, (TMSOCH2)2] was one of the only protocols that provided efficient conversion. While effective on <20 mg scales, the reaction time increased dramatically to 7 days or greater when larger amounts of substrate were employed, despite the use of excess reagents. Ultimately, we found that treating cis-18 with ethylene glycol and substoichiometric amounts of para-toluenesulfonic acid (PTSA, 2 mol%) was a reliable, scalable, and inexpensive method for ketal formation providing an 82% yield of the ketal 19 in four fewer days of reaction time. Finally, the nitrile addition–cyclization–activation cascade was carried out as before to provide the eneimide 5 in 80% overall yield.
In summary, the eneimide 5 is a useful precursor to (+)-pleuromutilin (1) and (+)-12-epi-pleuromutilin derivatives. Pleuromutilins are clinical agents with activity against GPPs, while 12-epi-pleuromutilins have yielded promising preclinical results for the treatment of Gram-negative and drug-resistant pathogens. We have described an improved synthesis of the eneimide 5 that proceeds in six steps, 17% yield (27% based on recovery of 16), and 31% atom economy and requires two fewer chromatography steps and five fewer days of reaction time than our previously reported route. The new steps reported here have been conducted on scales of ca. 0.5–10 g. We envision that this approach will further enable the production of a large number of pleuromutilin antibiotics.
Supplementary Material
ACKNOWLEDGMENTS
Financial support from Yale University, the National Institute of General Medical Sciences (R01GM110506), and the National Sciences and Engineering Research Council of Canada (postdoctoral fellowship to S.K.M.) is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b02476.
Experimental procedures and characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Isolation and structure determination:; (a) Kavanagh F; Hervey A; Robbins WJ Proc. Natl. Acad. Sci. U. S. A 1951, 37, 570. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kavanagh F; Hervey A; Robbins WJ Proc. Natl.Acad. Sci. U. S. A 1952, 38, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Birch AJ; Holzapfel CW; Rickards RW Tetrahedron 1966, 22, 359. [Google Scholar]; (d) Arigoni D Pure Appl. Chem 1968, 17, 331. [DOI] [PubMed] [Google Scholar]; For a recent review, see:; (e) Fazakerley NJ; Procter DJ Tetrahedron 2014, 70, 6911. [Google Scholar]
- (2).(a) Schlunzen F; Pyetan E; Fucini P; Yonath A; Harms JM Mol. Microbiol 2004, 54, 1287. [DOI] [PubMed] [Google Scholar]; (b) Davidovich C; Bashan A; Auerbach-Nevo T; Yaggie RD; Gontarek RR; Yonath A Proc. Natl. Acad. Sci. U. S. A 2007, 104, 4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For reviews, see:; (a) Novak R Ann. N. Y. Acad. Sci 2011,1241,71. [DOI] [PubMed] [Google Scholar]; (b) Paukner S; Riedl R Cold Spring Harbor Perspect. Med 2017, 7, a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Daum RS; Kar S; Kirkpatrick P Nat. Rev. Drug Discovery 2007, 6, 865. [DOI] [PubMed] [Google Scholar]
- (5).Walsh CT; Wencewicz TA J. Antibiot 2014, 67, 7. [DOI] [PubMed] [Google Scholar]
- (6).(a) Wicha WW; Ivezic-Schoenfeld Z In Vivo Activity of Extended Spectrum Pleuromutilins in Murine Sepsis Model. In 24th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Barcelona, Spain, 2014. [Google Scholar]; (b) Paukner S; Strickmann DB; Ivezic-Schoenfeld Z Extended Spectrum Pleuromutilins: Mode-of-action Studies. In 24th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Barcelona, Spain, 2014. [Google Scholar]; (c) Paukner S; Wicha WW; Heilmayer W; Thirring K; Riedl R, Extended Spectrum Pleuromutilins: Potent Translation Inhibitors with Broad-Spectrum Antibacterial Activity In Vitro an In Vivo. In Interscience Conference of Antimicrobial Agents and Chemotherapy/International Congress of Chemotherapy and Infection (ICAAC/ICC), San Diego, CA, 2015. [Google Scholar]; (d) Thirring K; Heilmayer W; Riedl R; Kollmann H; Ivezic-Schoenfeld Z; Wicha W; Paukner S; Strickmann D Preparation of 12-Epi-pleuromutilin Derivatives as Antimicrobial Agents. Patent WO2015110481A1, July 30, 2015.; (e) Paukner S; Kollmann H; Riedl R; Ivezic-Schoenfeld Z Kill Curves of the Novel Extended-Spectrum Pleuromutilin Antibiotic BC-9529. In 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark, 2015. [Google Scholar]; (f) Paukner S; Wicha WW; Thirring K; Kollmann H; Ivezic-Schoenfeld Z In Vitro and In Vivo Efficacy of Novel Extended Spectrum Pleuromutilins Against S. aureus and S. pneumoniae. In 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark, 2015. [Google Scholar]; (g) Wicha WW; Paukner S; Strickmann DB; Thirring K; Kollmann H; Heilmayer W; Ivezic-Schoenfeld Z Efficacy of Novel Extended Spectrum Pleuromutilins Against E. coli In Vitro and In Vivo. In 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark, 2015. [Google Scholar]
- (7).Berner H; Vyplel H; Schulz G; Schneider H Monatsh. Chem 1986, 117, 1073. [Google Scholar]
- (8).Hergenrother and co-workers recently suggested that the presence of a primary amine and an amphiphilic structure are useful elements to promote the accumulation of antibiotics in Gram-negative bacteria. While the durability of these prediction rules remains to be fully determined, it is interesting to note that derivatives such as 4 possess these features.; Richter MF; Drown BS; Riley AP; Garcia A; Shirai T; Svec RL; Hergenrother PJ Nature 2017, 545, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Murphy SK; Zeng M; Herzon SB Science 2017, 356, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For prior syntheses of pleuromutilin (1), see:; (a) Gibbons EG. J. Am. Chem. Soc 1982, 104, 1767. [Google Scholar]; (b) Boeckman RK; Springer DM; Alessi TR J. Am. Chem. Soc 1989, 111, 8284. [Google Scholar]; (c) Fazakerley NJ; Helm MD; Procter DJ Chem. - Eur. J 2013,19, 6718. [DOI] [PubMed] [Google Scholar]; For synthetic studies, see:; (d) Kahn M Tetrahedron Lett. 1980, 21, 4547. [Google Scholar]; (e) Paquette LA; Wiedeman PE; Page PCB J. Org. Chem 1988, 53, 1441. [Google Scholar]; (f) Paquette LA; Page PCB; Pansegrau PD; Wiedeman PE J. Org. Chem 1988, 53, 1450. [Google Scholar]; (g) Paquette LA; Pansegrau PD; Wiedeman PE; Springer JP J. Org. Chem 1988, 53, 1461. [Google Scholar]; (h) Bacque E; Pautrat F; Zard SZ Org. Lett 2003, 5, 325. [DOI] [PubMed] [Google Scholar]; (i) Findley TJK; Sucunza D; Miller LC; Helm MD; Helliwell M; Davies DT; Procter DJ Org. Biomol. Chem 2011,9,2433. [DOI] [PubMed] [Google Scholar]; (j) Lotesta SD; Liu J ; Yates EV; Krieger I; Sacchettini JC; Freundlich JS; Sorensen EJ Chem. Sci 2011, 2, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Liu J; Lotesta SD; Sorensen EJ Chem. Commun 2011, 47, 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Trost BM Science 1991, 254, 1471. [DOI] [PubMed] [Google Scholar]
- (12).Murphy SK; Zeng M; Herzon SB Org. Lett 2016, 18, 4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mander LN; Sethi SP Tetrahedron Lett. 1983, 24, 5425. [Google Scholar]
- (14).Pricing from Sigma–Aldrich; retrieved 3/15/2017.
- (15).(a) Heller ST; Sarpong R Org. Lett 2010, 12, 4572. [DOI] [PubMed] [Google Scholar]; The success of this reagent may be due to the formation of a stable tetrahedral intermediate in solution, as has previously been observed in additions to N-acyl imidazoles. See:; (b) Evans DA; Borg G; Scheidt KA Angew. Chem., Int. Ed 2002, 41 , 3188. [DOI] [PubMed] [Google Scholar]
- (16).Crisp GT; Scott WJ; Stille JK J. Am. Chem. Soc 1984, 106, 7500. [Google Scholar]
- (17).He W; Sun X; Frontier AJ J. Am. Chem. Soc 2003,125, 14278. [DOI] [PubMed] [Google Scholar]
- (18).(a) MacAlpine GA; Raphael RA; Shaw A; Taylor AW; Wild H-JJ Chem. Soc. Chem. Commun 1974, 834. [Google Scholar]; (b) Karpf M; Dreiding AS Helv. Chim. Acta 1976, 59, 1226. [Google Scholar]; (c) Hiyama T; Shinoda M; Saimoto H; Nozaki H Bull. Chem. Soc. Jpn 1981, 54, 2747. [Google Scholar]
- (19).This cyclopentannulation strategy was also applied by Paquette and co-workers in their pleuromutilin studies; see ref 10e.
- (20).Hatano M; Suzuki S; Ishihara K J. Am. Chem. Soc 2006, 128, 9998. [DOI] [PubMed] [Google Scholar]
- (21).For a review, see:; Swaminathan S; Narayanan KV Chem. Rev 1971, 71,429. [Google Scholar]
- (22).For a review, see:; Nagata W; Yoshioka M Hydrocyanation of Conjugated Carbonyl Compounds In Organic Reactions; Dauben WG, Ed.; John Wiley & Sons, Inc.: New York, 2004; Vol. 25, pp 255–476. [Google Scholar]
- (23).Tsunoda T; Suzuki M; Noyori R Tetrahedron Lett. 1980, 21, 1357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.