Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2003.
Allergic rhinitis is a common condition which can significantly impair quality of life. Immunotherapy by injection can significantly reduce symptoms and medication use but its use is limited by the possibility of severe systemic adverse reactions. Immunotherapy by the sublingual route is therefore of considerable interest.
Objectives
To evaluate the efficacy and safety of sublingual immunotherapy for allergic rhinitis in adults and children.
Search methods
We searched the Cochrane ENT Group Trials Register; CENTRAL (2010, Issue 3); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT and additional sources for published and unpublished trials. The date of the most recent search was 14 August 2009.
Selection criteria
Randomised, double‐blind, placebo‐controlled trials of sublingual immunotherapy in adults or children. Primary outcome measures were symptom and medication scores. We also collected adverse event data.
Data collection and analysis
Two independent authors selected studies and assessed risk of bias. One author extracted data which was rechecked by two other authors. We used the standardised mean difference (SMD) with a random‐effects model to combine data.
Main results
We included a total of 60 randomised controlled trials in the review. Forty‐nine were suitable for pooling in meta‐analyses (2333 SLIT, 2256 placebo participants). Overall, we found a significant reduction in symptoms (SMD ‐0.49; 95% confidence interval (CI) ‐0.64 to ‐0.34, P < 0.00001) and medication requirements (SMD ‐0.32; 95% CI ‐0.43 to ‐0.21, P < 0.00001) in participants receiving sublingual immunotherapy compared to placebo. None of the trials included in this review reported severe systemic reactions or anaphylaxis, and none of the systemic reactions reported required the use of adrenaline.
Authors' conclusions
This updated review reinforces the conclusion of the original 2003 Cochrane Review that sublingual immunotherapy is effective for allergic rhinitis and has been proven to be a safe route of administration.
Plain language summary
Sublingual immunotherapy for allergic rhinitis (including hay fever)
Allergic rhinitis is characterised by red, itchy eyes, a blocked and runny nose, and sneezing. The most common causes of allergic rhinitis are different pollens (grass and tree), house dust mites, mould and animal dander. Allergic rhinitis can be intermittent (such as hay fever) or persistent (all year round). The treatment of allergic rhinitis depends on its severity and duration, and is usually based on the use of antihistamines and nasal corticosteroids. If these drugs cannot control symptoms immunotherapy is recommended. Immunotherapy involves the administration of gradually increasing doses of the allergen over a period of time to desensitise the patient. It is the only known treatment that modifies the immune response and treats the cause rather than the symptoms.
In reviewing 60 trials we found a significant reduction in symptom and medication scores in patients treated with sublingual immunotherapy compared to placebo. There were no serious adverse reactions reported in the included trials and no patient needed the use of adrenaline. This updated Cochrane Review therefore reinforces the conclusions of the earlier review in confirming the efficacy and safety of sublingual immunotherapy.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2003.
Allergic rhinitis is a common condition, affecting between 10% and 40% of people worldwide. The typical clinical features are sneezing, watery rhinorrhoea, nasal blockage, itchy, watery eyes and itchy throat. In clinical trials the severity of allergic rhinitis is usually assessed by numerical validation of nasal and eye symptoms, which takes into account subjective intensity, and whether the condition interferes with everyday life or school and work performance.
The ARIA guidelines (ARIA 2001; ARIA 2008) recommend allergen avoidance as first‐line treatment, followed by pharmacotherapy aimed at symptom control (mainly antihistamines and topical nasal corticosteroids). For patients with more severe disease, who do not respond to usual therapy, specific immunotherapy is recommended.
Subcutaneous injection immunotherapy has been used for decades. The exact mechanism of action is not fully understood, but involves changes in serum antibody levels (Jutel 1995; Rossi 2004) and a number of cellular changes, including alteration of the T cell response, from Th2 to Th1 (Wachholz 2002). More recent work suggests that regulatory mechanisms could also play an important role (Francis 2003; Jutel 2003). This immunomodulation results in significant reductions in symptoms and medication requirements (Calderon 2010).
Though proven to be efficacious, the subcutaneous route can be uncomfortable and time‐consuming. Local adverse events such as injection site itch or swelling are fairly common and, although rare, systemic reactions can be severe. For this reason alternative routes for the delivery of immunotherapy, with a better safety profile, were sought. In the last two decades attention has focused on the sublingual route.
A Cochrane Review of sublingual immunotherapy for allergic rhinitis was published in 2003 (Wilson 2003) and included 22 randomised, placebo‐controlled trials identified up to September 2002. Analysis of symptom and medication scores proved sublingual immunotherapy to be efficacious. Adverse events reported in these trials were minor and local, and no systemic reactions were reported.
Research in the field of sublingual immunotherapy has continued since 2002, resulting in the publication of many additional studies with increased numbers of participants. This review updates the original to give a more comprehensive evaluation of the efficacy and safety of sublingual immunotherapy.
Objectives
To evaluate the efficacy of sublingual immunotherapy compared with placebo in:
reducing symptoms and/or medication requirements during naturally occurring allergic rhinitis;
altering immunological markers in blood and immunological markers and allergen sensitivity in target organs (nose, eye, skin).
To evaluate the safety of sublingual immunotherapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, placebo‐controlled clinical trials.
Types of participants
Studies with participants of any age (children and adults). All patients had a history of allergic rhinitis, with or without allergic conjunctivitis, and with or without allergic asthma. In all studies the allergen was clearly identified. Patients’ sensitivity was proven by positive skin prick tests and/or high specific IgE to a particular allergen. The existence of other clinically relevant sensitivities was one of the exclusion criteria in the majority of studies.
We excluded trials dealing with asthma only from the review.
Types of interventions
Included studies were those investigating the efficacy and safety of sublingual immunotherapy. We analysed all trials regardless of treatment dose, duration, or whether the allergen was swallowed or spat out.
Types of outcome measures
Primary outcomes
Symptom scores, however recorded (either daily or weekly, via symptom score diaries, visual analogue scales, number of well days or overall assessment).
Medication scores referring to the use of relevant anti‐allergic medications, however recorded and scored.
Secondary outcomes
Measurement of serum IgE and IgG (total and specific).
Assessment of allergen sensitivity (eye, nose or skin).
Quality of life.
Adverse event reports.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 14 August 2009 following original searches in September 2002.
Electronic searches
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2009, Issue 3); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI (China National Knowledge Infrastructure); mRCT (Current Controlled Trials); ICTRP (International Clinical Trials Registry Platform) and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, Box 6.4.b. (Handbook 2008)).
CENTRAL search strategy
#1 MeSH descriptor Immunotherapy explode all trees #2 MeSH descriptor Desensitization, Immunologic explode all trees #3 MeSH descriptor Allergens explode all trees with qualifiers: AD,IM #4 immunotherap* #5 ((allergen* OR immunologic*) AND (hyposensitiz* OR hyposensitis* OR desensitiz* OR desensitis*)) #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Administration, Sublingual explode all trees #8 (SUBLINGUAL* OR ORAL* OR TONGUE OR MUCOSA) #9 (#7 OR #8) #10 (#6 AND #9) #11 SLIT #12 (#10 OR #11) #13 MeSH descriptor Rhinitis, Allergic, Perennial explode all trees #14 MeSH descriptor Rhinitis, Allergic, Seasonal explode all trees #15 MeSH descriptor Rhinitis explode all trees #16 rhinti* #17 MeSH descriptor Hypersensitivity explode all trees #18 allerg* OR hypersensitiv* #19 (( #15 OR #16 ) AND ( #17 AND #18 )) #20 (perennial:ti OR persistent:ti OR nonseasonal:ti OR nose:ti OR nasal:ti OR cat:ti OR fur:ti OR hair*:ti OR dander:ti OR dust*:ti OR mite*:ti OR pet*:ti OR dog*:ti OR cockroach*:ti OR seasonal:ti OR intermittent:ti OR spring:ti OR summer:ti OR pollen:ti OR grass*:ti OR birch:ti OR ragweed:ti OR tree*:ti OR weed*:ti OR mugwort:ti OR willow:ti OR alder:ti) #21 (( #17 OR #18 ) AND #19) #22 (hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR OR PAR) #23 (#13 OR #14 OR #19 OR #21 OR #22) #24 (#12 AND #23)
Search strategies for other key databases including PubMed are shown in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and Audiology, and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials. We sought abstracts from conference proceedings via the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
We identified additional trials through discussion with specialist allergists, or other professionals with an interest in the area.
We used papers written in English and other languages; translations were performed by the Cochrane ENT Group.
Data collection and analysis
Selection of studies
Two authors (SR and MC) independently screened the search results and selected studies which appeared to meet the review inclusion criteria. We obtained all such studies in full text for further assessment. Any disagreements about which studies to include in the review were resolved by further discussion with the other two authors (SRD and DW) where necessary.
Data extraction and management
We extracted data from the included studies onto a standard form, covering study type and methodology, number and description of participants, details of type, dosage, schedule, duration of sublingual immunotherapy used, as well as the results, types, timing and method of outcome measures. One author (SR) extracted all data and values were checked by MC and SRD.
Where published manuscripts did not report data sufficiently or in suitable format for meta‐analysis we sought further information directly from the authors.
As all the authors were previously familiar with the content of most of the studies, we did not remove the study author names before assessment and data extraction.
Assessment of risk of bias in included studies
Three authors (SR, MC and DW) assessed the identified studies separately and compared the results.
For the original 2003 review, quality assessment of the trials was performed using the Jadad scale (Moher 1998). For the 2010 update, all the originally included studies were re‐assessed by SR and MC, and scored again with the Jadad scale. We only included double‐blind, placebo‐controlled trials with a Jadad score > 3/5 in the review.
For the 2010 update, two authors (SR and MC) also assessed all included studies for risk of bias using the Cochrane Collaboration 'Risk of bias' tool as guided by The Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2008).
The following were taken into consideration:
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We described each of these domains as reported in the trial and then assigned a judgement about the adequacy of each entry. This involved answering a pre‐specified question whereby a judgement of ‘Yes’ indicates low risk of bias, ‘No’ indicates high risk of bias, and ‘Unclear’ indicates unclear or unknown risk of bias.
Data synthesis
Apart from adverse events, all the outcome data analysed were continuous. The most common way of recording data was through daily diary cards, recording and scoring symptoms (nasal, eye or less frequently chest) and medication use (antihistamine tablets, nasal sprays, eye drops). The data were subsequently totalled and averaged.
A wide range of different scoring systems and scales were employed by trial authors for both primary and secondary outcomes. This creates problems of significant heterogeneity but is unavoidable.
The outcome data extracted from the included studies were entered into RevMan 5 by SR and MC for the statistical analysis (RevMan 2008). A wide variety of scoring systems were used therefore we performed the analysis using the standardised mean difference (SMD) (the difference in means between two treatment groups, immunotherapy and placebo, in units of pooled standard deviation).
We used random‐effect models for statistical analysis of the overall efficacy of sublingual immunotherapy. We presented the results as SMDs with the 95% confidence intervals (CI).
We analysed heterogeneity between studies using the Chi² test, with a P value of < 0.1 indicating the significant heterogeneity between studies, and the I² statistic which describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. We used the threshold values recommended in the Cochrane Handbook for Systematic Reviews of Interventions (0% to 40%: might not be important; 30% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; 75% to 100%: considerable heterogeneity).
We carried out subgroup analysis according to the review protocol as follows:
seasonal versus perennial allergens;
children versus adults;
dosage of major allergen (< 5 mcg of major allergen protein versus 5 to 20 mcg versus > 20 mcg;
duration of immunotherapy (< 6 months versus 6 to 12 months versus 12 months, to cover pre‐seasonal, perennial and prolonged treatment);
sublingual spit versus sublingual swallow; and
sublingual drops versus tablets.
We analysed adverse events (AE) as discontinuous data, therefore we present only descriptive analysis.
Results
Description of studies
The original 2003 version of this review included 22 studies; the 2010 update includes 60 studies.
Results of the search
The updated searches in 2009 identified 628 papers of potential interest. We discarded 498 papers after reading the abstracts (review articles or descriptive studies, papers investigating other routes of immunotherapy or not investigating allergic rhinitis). We therefore evaluated 130 papers in detail. Among these, 12 references were matched to studies already included or excluded from the review and these are grouped under the 'primary' reference for the study.
Following this evaluation we discarded another 68 papers.
Included studies
We thus identified 62 papers as potentially appropriate for the review and meta‐analysis. We identified two studies as ongoing (Ingels 2002; O'Hehir 2005).
A total of 60 studies are now included in the 2010 update of this review, of which 49 studies are included in the updated meta‐analyses. Eleven studies that did not contain efficacy data eligible for meta‐analysis contained useful adverse event data.
Allergen
Most trials were performed with grass pollen (23 studies). Other allergens used were Parietaria (five trials), ragweed (two trials), trees (nine trials: two olive, three cypress, two birch pollen, two mixed trees), house dust mite (eight trials) and cat (one trial).
One of the trials investigated the efficacy of grass and birch pollen immunotherapy.
Participants
Thirty‐four studies were performed in adults and 15 investigated efficacy and safety in children.
Treatment duration
Treatment lasted for less than six months in 17 studies; six to 12 months in 16 studies and longer than 12 months in 16 studies.
Dose of allergen
Of the 49 studies, 32 reported the major allergen dose in a manner suitable for meta‐analysis. The rest of the trials either did not provide the sufficient data or reported the cumulative dose (weekly, monthly or a total cumulative dose over the complete treatment). Eight trials used daily doses of less than 5 mcg, in 12 studies the dose was between 5 and 20 mcg per day, and 12 papers reported a daily dose of more than 20 mcg.
Allergen reactivity
Nine trials reported data on skin sensitivity, but only six (skin prick test after treatment) could be included in the meta‐analysis. Seven trials reported data eligible for meta‐analysis of nasal reactivity. Data on conjunctival reactivity were not sufficient for meta‐analysis.
Excluded studies
At the 2010 update we excluded 14 further studies. We also excluded three studies that had been previously included in the original 2003 review and which did not satisfy the new risk of bias assessment criteria (D'Ambrosio 1996; Mungan 1999; Quirino 1996). Two formerly excluded studies (Clavel 1998; Sabbah 1994) are now included in the review. These two studies did not contain sufficient efficacy data, but they satisfied the review inclusion criteria and their adverse event data were analysed. A total of 24 studies are excluded from this updated version of the review (see Characteristics of excluded studies). Studies listed as excluded are those which satisfied the majority but not all of our inclusion criteria.
Risk of bias in included studies
All included studies were double‐blind, placebo‐controlled trials of parallel‐group design. Concealment of treatment allocation was considered adequate in all studies, based on statements made by the original authors. Blinding of study subjects and investigators was almost universally maintained by the use of identical placebo preparations. It should, however, be noted that most investigators reported high levels of minor oral side effects (tingling, itching and swelling beneath the tongue) in actively treated subjects, which could influence blinding.
Full risk of bias assessments can be found in the Characteristics of included studies table. A 'Risk of bias' summary of our judgements about each risk of bias item for each included study is presented in Figure 1 and as a graph in Figure 2.
1.
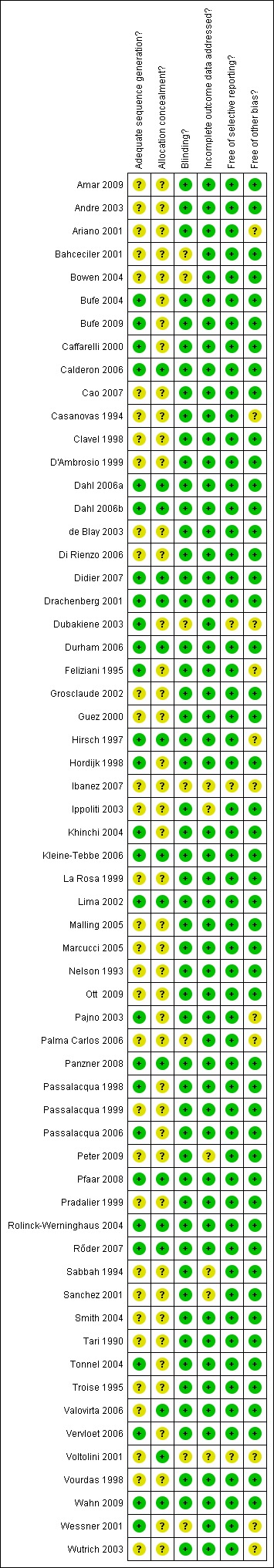
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
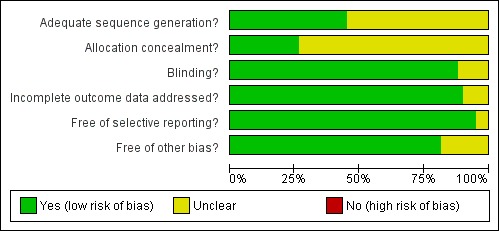
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Symptom scores
A total of 49 studies were included in the meta‐analysis. In total 2333 active (sublingual immunotherapy ‐ SLIT) and 2256 placebo patients were included. The combined standardised mean difference (SMD) following sublingual immunotherapy (SLIT) was SMD ‐0.49 (95% confidence interval (CI) ‐0.64 to ‐0.34) favouring active treatment (P < 0.00001). There was significant heterogeneity between the studies (Chi² = 256.76, P < 0.00001, I² = 81%) (Analysis 1.1).
1.1. Analysis.
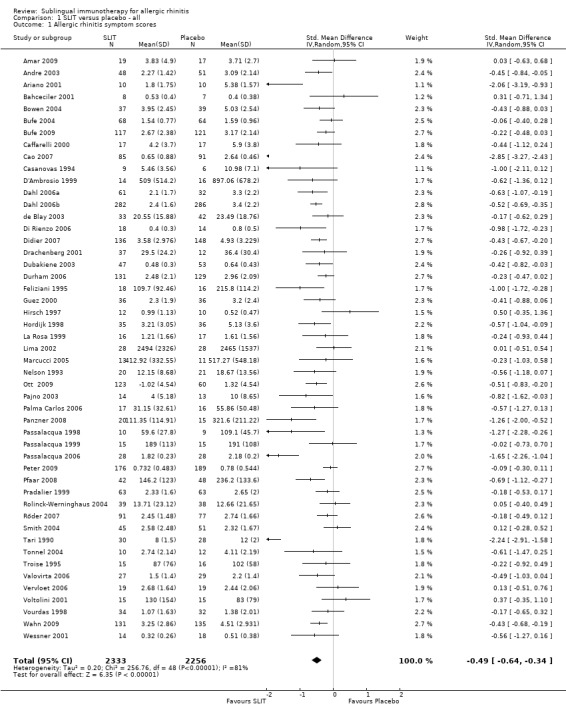
Comparison 1 SLIT versus placebo ‐ all, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: seasonal and perennial allergens
We performed the first subgroup analysis in the two biggest subgroups: seasonal (39 trials) and perennial allergens (10 trials).
In the seasonal allergens group there were 2081 participants in the SLIT and 2003 in the placebo group. The combined SMD was ‐0.34 (95% CI ‐0.44, ‐0.25, P < 0.00001). Significant heterogeneity between studies was indicated (Chi² = 68.54, P = 0.002, I² = 45%) (Analysis 2.1).
2.1. Analysis.
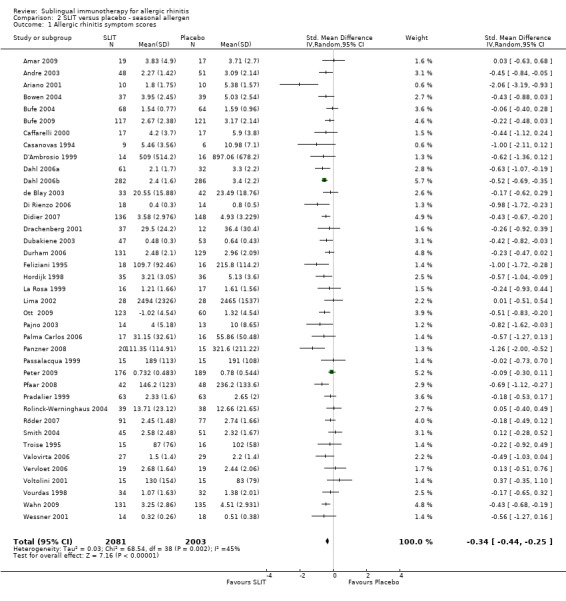
Comparison 2 SLIT versus placebo ‐ seasonal allergen, Outcome 1 Allergic rhinitis symptom scores.
The perennial allergen studies involved 252 SLIT and 253 placebo patients, with SMD ‐0.93 (95% CI ‐1.69 to ‐0.17, P = 0.02). Significant heterogeneity between studies was indicated (Chi² 115.91, P < 0.00001, I²= 92%) (Analysis 3.1).
3.1. Analysis.
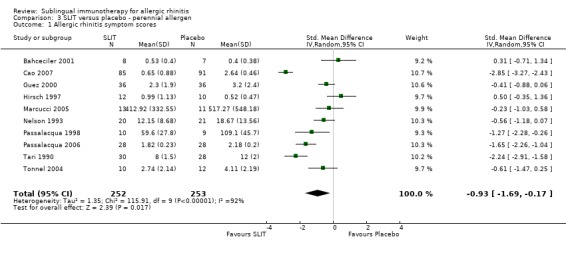
Comparison 3 SLIT versus placebo ‐ perennial allergen, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: age
We performed the age subgroup analysis for adults and children in eight studies. In studies with a mixed population the participants were considered as adults if the median age was ≥ 20, otherwise they were not included in the age subgroup analysis.
Thirty‐four studies were performed in adults, 1631 participants received SLIT and 1566 placebo. The combined SMD was ‐0.44 (95% CI ‐0.56 to ‐0.31, P < 0.00001). Significant heterogeneity was indicated between studies (Chi² 77.81, P = 0.0001, I² = 58%) (Analysis 4.1).
4.1. Analysis.
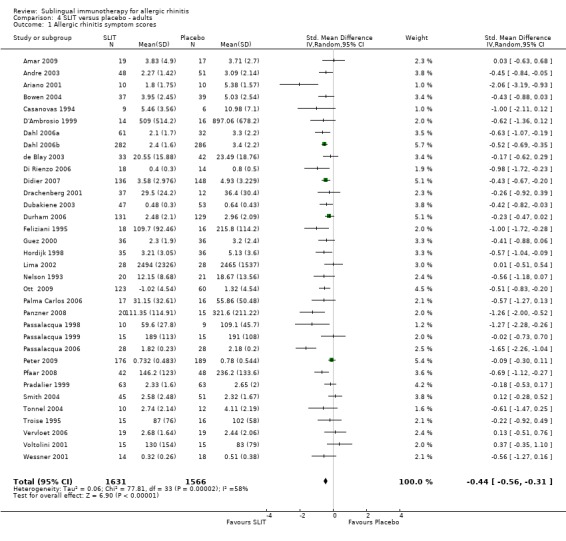
Comparison 4 SLIT versus placebo ‐ adults, Outcome 1 Allergic rhinitis symptom scores.
Fifteen studies were identified in children; 702 participants were included in the SLIT and 690 in the placebo group. The combined SMD was ‐0.52 (95% CI ‐0.94 to ‐0.10, P = 0.02). Highly significant heterogeneity between studies was indicated (Chi² = 177.60, P < 0.00001, I² = 92%) (Analysis 5.1).
5.1. Analysis.
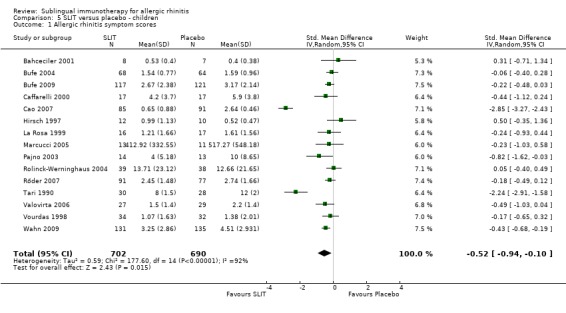
Comparison 5 SLIT versus placebo ‐ children, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: treatment duration
Seventeen trials reported treatment duration for less than six months; 890 patients received SLIT and 882 received placebo treatment. The combined SMD in this group was ‐0.54 (95% CI ‐0.86 to ‐0.21, P = 0.001). There was an indication of highly significant heterogeneity between these trials (Chi² 157.82 , P = 0.00001, I² = 90%) (Analysis 6.1).
6.1. Analysis.
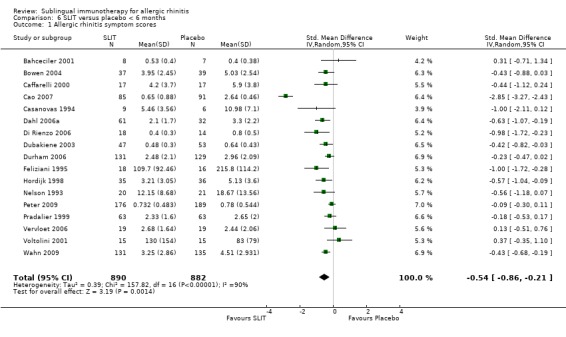
Comparison 6 SLIT versus placebo < 6 months, Outcome 1 Allergic rhinitis symptom scores.
In 16 studies the treatment duration was between six and 12 months; 867 participants were in the SLIT and 869 in the placebo group. The SMD in this group was ‐0.31 (95% CI ‐0.46 to ‐0.16, P < 0.0001). A significant level of heterogeneity was indicated (Chi² = 25.47, P = 0.04, I² = 41%) (Analysis 7.1).
7.1. Analysis.
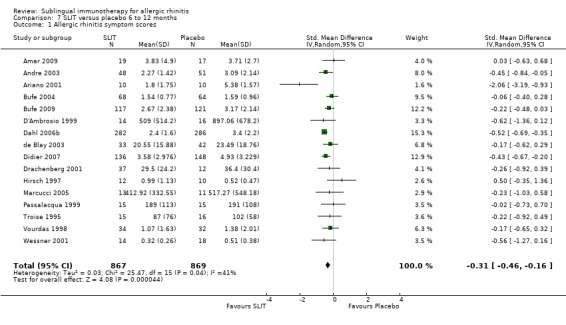
Comparison 7 SLIT versus placebo 6 to 12 months, Outcome 1 Allergic rhinitis symptom scores.
Treatment lasted for longer than 12 months in 16 studies; 580 received SLIT and 509 placebo. The SMD was ‐0.63 (95% CI ‐0.92 to ‐0.34, P < 0.0001). Significant heterogeneity among the studies was indicated (Chi² = 74.88, P < 0.00001, I² = 80%) (Analysis 8.1).
8.1. Analysis.
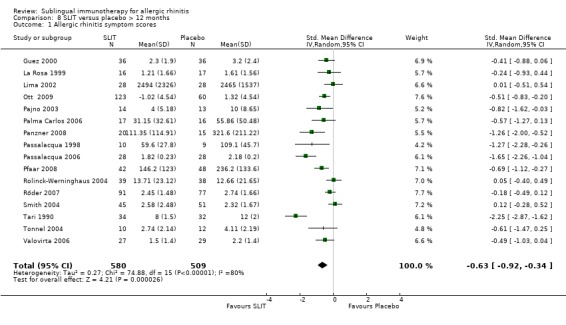
Comparison 8 SLIT versus placebo > 12 months, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: major allergen dose
Major allergen content < 5 mcg
Eight trials in this group reported symptom score results; 141 patients received SLIT and 134 placebo. The SMD was ‐0.32 (95% CI ‐0.69 to 0.05, P = 0.09). Significant heterogeneity was shown between studies (Chi² = 15.18, P = 0.03, I²= 54%) (Analysis 9.1).
9.1. Analysis.
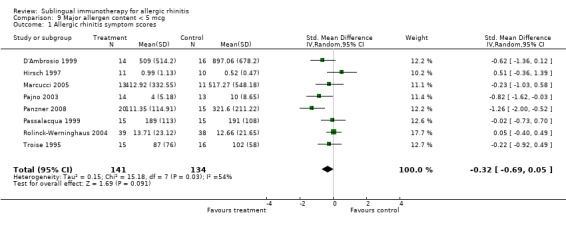
Comparison 9 Major allergen content < 5 mcg, Outcome 1 Allergic rhinitis symptom scores.
Major allergen content 5 to 20 mcg
There were 12 studies included in this subgroup, with 1006 patients receiving SLIT and 966 placebo. The SMD was ‐0.34 (95% CI ‐0.45 to ‐0.24, P < 0.00001). A lack of heterogeneity between studies was indicated (Chi² was 13.52, P = 0.26, I²= 19%) (Analysis 10.1).
10.1. Analysis.
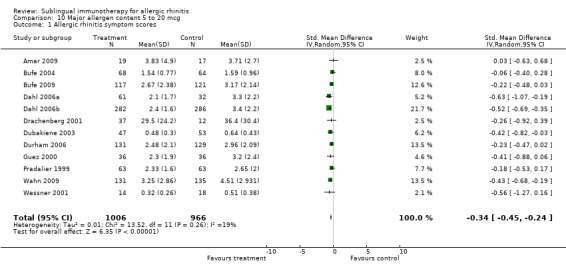
Comparison 10 Major allergen content 5 to 20 mcg, Outcome 1 Allergic rhinitis symptom scores.
Major allergen content > 20 mcg
There were 12 studies in this subgroup, with 541 receiving SLIT and 500 placebo. The SMD in this group was ‐0.33 (95% CI ‐0.49 to ‐0.17, P < 0.0001). No significant heterogeneity between studies was demonstrated (Chi² = 15.19, P = 0.17, I² = 28%) (Analysis 11.1).
11.1. Analysis.
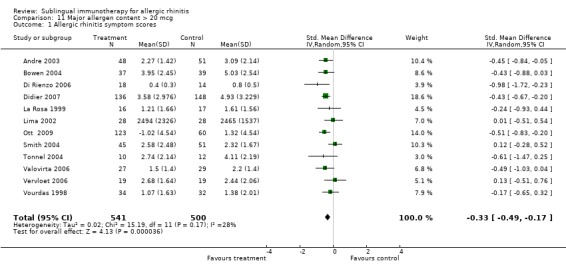
Comparison 11 Major allergen content > 20 mcg, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: individual allergens
It was possible to perform individual subgroup analysis for one perennial (house dust mite) and four seasonal (grass pollen, Parietaria, ragweed, tree) allergens because they were investigated in more than one study.
Nine studies investigated house dust mite, with 232 patients in the SLIT and 232 in the placebo group. The SMD was ‐0.97 (95% CI ‐1.80 to ‐0.13, P = 0.02). There was an indication of highly significant heterogeneity between studies (Chi² = 110.42, P < 0.00001, I² = 93%) (Analysis 13.1).
13.1. Analysis.
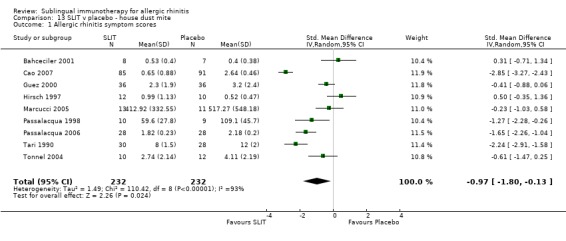
Comparison 13 SLIT v placebo ‐ house dust mite, Outcome 1 Allergic rhinitis symptom scores.
Twenty‐three studies were performed with grass pollen allergen. There were 1549 in the SLIT and 1464 patients in the placebo group. The combined SMD was ‐0.35 (95% CI ‐0.45 to ‐0.24, P < 0.00001). Significant heterogeneity was indicated among studies (Chi² = 39.58, P = 0.01, I² = 44%) (Analysis 14.1).
14.1. Analysis.
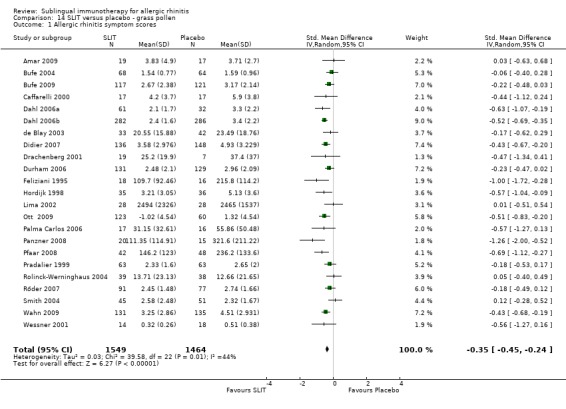
Comparison 14 SLIT versus placebo ‐ grass pollen, Outcome 1 Allergic rhinitis symptom scores.
There were only two studies investigating ragweed, involving 85 participants in the SLIT and 90 in the placebo group. The SMD was ‐0.44 (95% CI ‐0.74 to ‐0.14, P = 0.004). There was no heterogeneity shown between the studies (Chi² = 0.00, P = 0.96, I² = 0%) (Analysis 15.1).
15.1. Analysis.
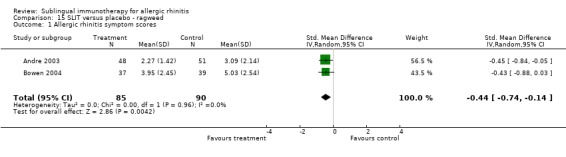
Comparison 15 SLIT versus placebo ‐ ragweed, Outcome 1 Allergic rhinitis symptom scores.
Parietaria was investigated in five trials, with 74 patients in the SLIT and 77 in the placebo group. The SMD was ‐0.36 (95% CI ‐0.69 to ‐0.04, P = 0.03). There was no heterogeneity shown between studies (Chi² = 2.95, P = 0.57, I² = 0%) (Analysis 16.1).
16.1. Analysis.
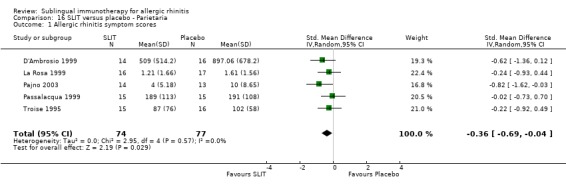
Comparison 16 SLIT versus placebo ‐ Parietaria, Outcome 1 Allergic rhinitis symptom scores.
Nine different studies involved trees (two mixed trees, two birch, two olive and three cypress); 197 participants received SLIT and 183 placebo. The SMD was ‐0.42 (95% CI ‐0.77 to ‐0.06, P = 0.02). Significant heterogeneity was indicated among studies (Chi² = 20.17, P = 0.01, I² = 60%) (Analysis 17.1).
17.1. Analysis.
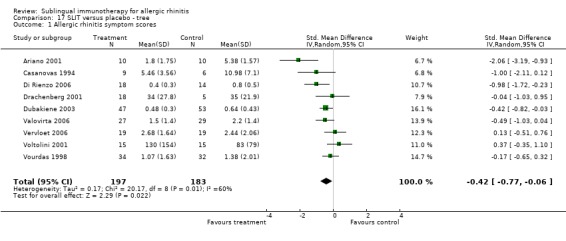
Comparison 17 SLIT versus placebo ‐ tree, Outcome 1 Allergic rhinitis symptom scores.
Subgroup analysis: medication preparation
Sublingual tablets
Sublingual tablets were used in 11 studies, with 945 participants in the SLIT and 936 in the placebo group. The SMD in this subgroup was ‐0.48 (95% CI ‐0.58 to ‐0.38, P < 0.00001). A significant level of heterogeneity between studies was indicated (Chi² = 39.07, P < 0.0001, I² = 74%) (Analysis 20.1).
20.1. Analysis.
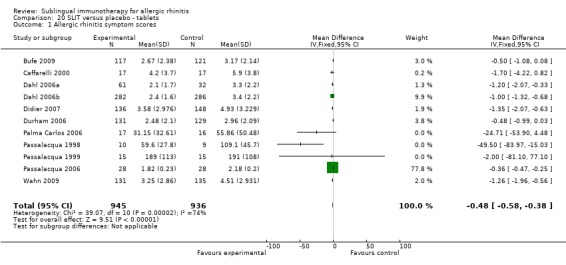
Comparison 20 SLIT versus placebo ‐ tablets, Outcome 1 Allergic rhinitis symptom scores.
Sublingual drops
There were 35 studies included in this subgroup, with 1270 patients receiving SLIT and 1194 placebo. The SMD was ‐0.35 (95% CI ‐0.42 to ‐0.28, P < 0.00001). Highly significant heterogeneity between studies was indicated (Chi² = 436.12, P < 0.00001, I² = 92%) (Analysis 21.1).
21.1. Analysis.
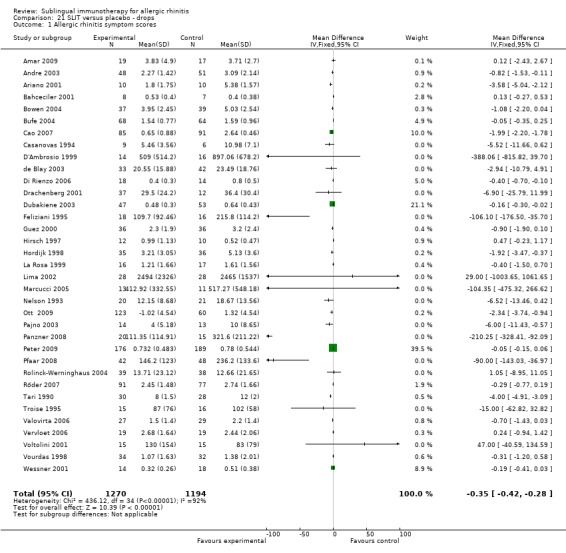
Comparison 21 SLIT versus placebo ‐ drops, Outcome 1 Allergic rhinitis symptom scores.
Three studies used both drops and tablets and were not included in the analysis.
Medication scores
Thirty‐eight trials reported medication score results, with a total of 1737 patients in the SLIT group and 1642 in the placebo group. The combined SMD was ‐0.32 (95% CI ‐0.43 to ‐0.21, P < 0.00001). Significant heterogeneity was indicated (Chi² = 73.32, P = 0.0003, I² = 50%) (Analysis 1.2).
1.2. Analysis.
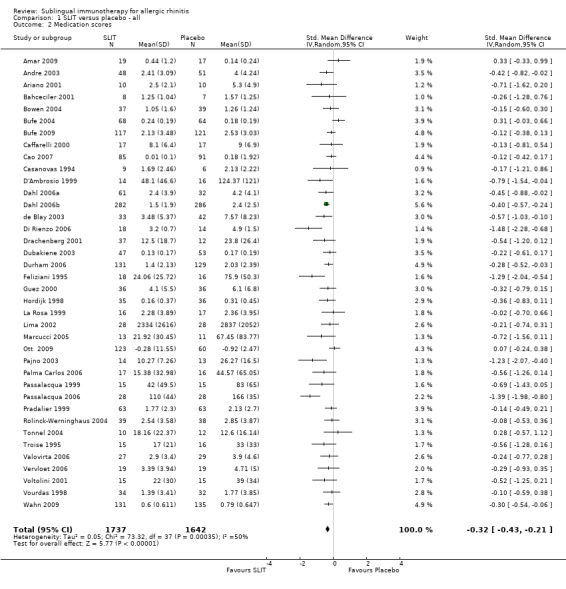
Comparison 1 SLIT versus placebo ‐ all, Outcome 2 Medication scores.
Subgroup analysis: seasonal and perennial allergens
In the seasonal allergens group 32 trials reported medication score, with 1557 patients receiving SLIT and 1457 placebo. The SMD was ‐0.30 (95% CI ‐0.41 to ‐0.19, P < 0.00001). Significant heterogeneity among studies was indicated (Chi² = 55.80; P = 0.004, I² = 44%) (Analysis 2.2).
2.2. Analysis.
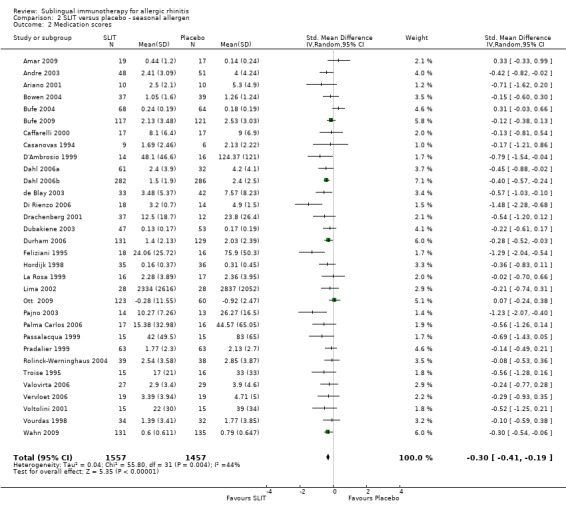
Comparison 2 SLIT versus placebo ‐ seasonal allergen, Outcome 2 Medication scores.
Six studies with perennial allergens involved 180 SLIT and 185 placebo patients. The SMD was ‐0.43 (95% CI ‐0.89 to 0.02, P = 0.06). Significant heterogeneity between studies was indicated (Chi² = 17.21, P = 0.004, I² = 71%) (Analysis 3.2).
3.2. Analysis.
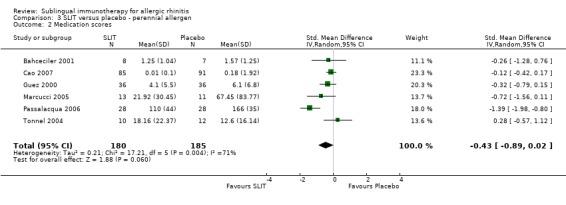
Comparison 3 SLIT versus placebo ‐ perennial allergen, Outcome 2 Medication scores.
Subgroup analysis: age
Twenty‐six studies which were performed in adults reported the medication score; 1168 participants received SLIT and 1067 placebo. The combined SMD was ‐0.40 (95% CI ‐0.53 to ‐0.26, P < 0.00001). Significant heterogeneity was indicated (Chi² = 47.76, P = 0.004, I²= 48%) (Analysis 4.2).
4.2. Analysis.
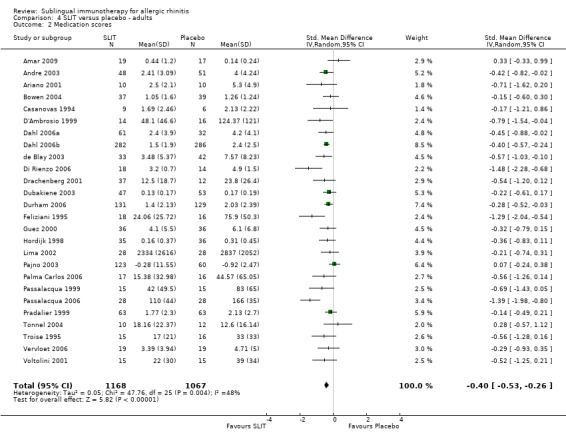
Comparison 4 SLIT versus placebo ‐ adults, Outcome 2 Medication scores.
Twelve studies in children reported the medication score; 569 patients in the SLIT and 575 in the placebo group. The combined SMD was ‐0.16 (95% CI ‐0.32 to 0.00, P = 0.06). Heterogeneity was non‐significant (Chi² = 17.31, P = 0.10, I² = 36%) (Analysis 5.2).
5.2. Analysis.
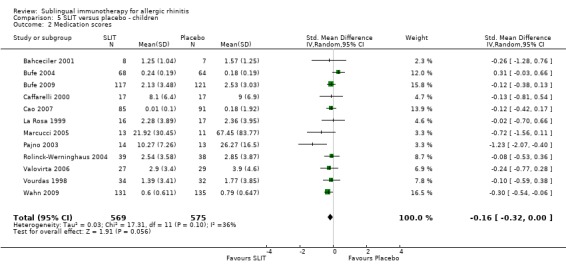
Comparison 5 SLIT versus placebo ‐ children, Outcome 2 Medication scores.
Subgroup analysis: treatment duration
Fifteen out of 17 trials with a treatment duration of less than six months reported medication scores; 694 participants received SLIT and 672 placebo. The SMD was ‐0.32 (95% CI ‐0.45 to ‐0.18, P < 0.00001). A lack of heterogeneity between studies was indicated (Chi² = 19.09; P = 0.16, I² = 27%) (Analysis 6.2).
6.2. Analysis.
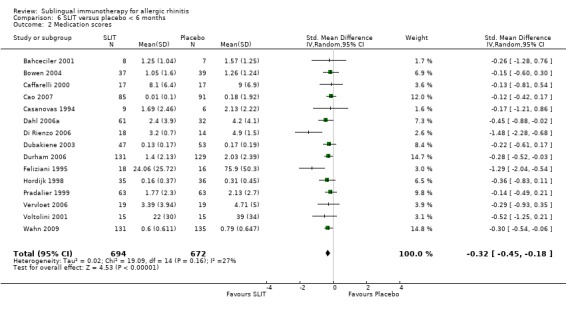
Comparison 6 SLIT versus placebo < 6 months, Outcome 2 Medication scores.
Medication score was reported in 13 studies with a treatment duration between six and 12 months. A total of 705 active and 693 placebo participants took part in these trials. The SMD was ‐0.31 (95% CI ‐0.50 to ‐0.12, P = 0.002). A significant level of heterogeneity was indicated (Chi² = 26.90, P = 0.008, I² = 55%) (Analysis 7.2).
7.2. Analysis.
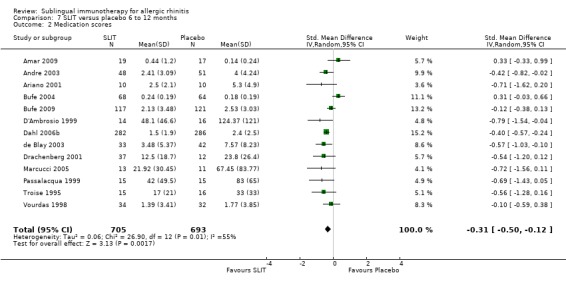
Comparison 7 SLIT versus placebo 6 to 12 months, Outcome 2 Medication scores.
Treatment lasted for longer than 12 months in 10 studies reporting medication scores; 338 patients received SLIT and 277 placebo. The SMD was ‐0.34 (95% CI ‐0.64 to ‐0.04, P = 0.03). Significant heterogeneity among the studies was indicated (Chi² = 27.13, P = 0.001, I² = 67%) (Analysis 8.2).
8.2. Analysis.
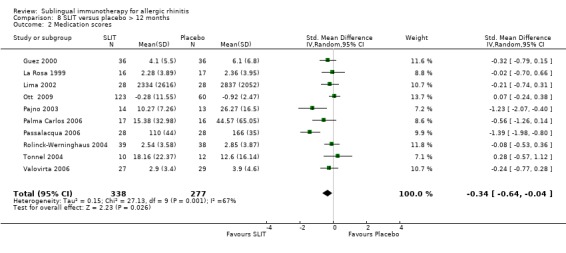
Comparison 8 SLIT versus placebo > 12 months, Outcome 2 Medication scores.
Subgroup analysis: major allergen dose
Major allergen content < 5 mcg
Six trials in this group reported medication score; 110 patients received SLIT and 109 placebo. The SMD was ‐0.59 (95% CI ‐0.94 to ‐0.24, P = 0.0008). A lack of heterogeneity between studies was indicated (Chi² = 7.43, P = 0.19, I² = 33%) (Analysis 9.2).
9.2. Analysis.
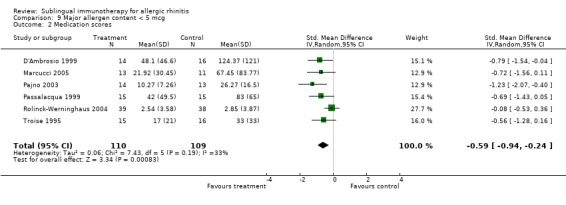
Comparison 9 Major allergen content < 5 mcg, Outcome 2 Medication scores.
Major allergen content 5 to 20 mcg
Eleven studies were included in this subgroup analysis, with total of 992 patients receiving SLIT and 948 placebo. The SMD was ‐0.21 (95% CI ‐0.35 to ‐0.07, P = 0.003). Significant heterogeneity between studies was indicated (Chi² = 19.84, P = 0.03, I² = 50%) (Analysis 10.2).
10.2. Analysis.
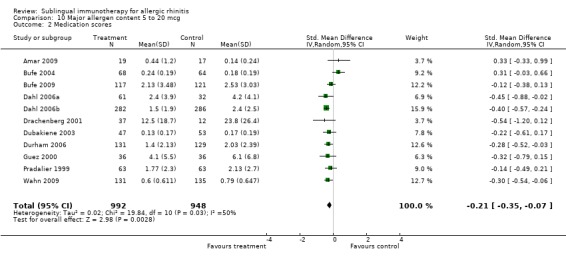
Comparison 10 Major allergen content 5 to 20 mcg, Outcome 2 Medication scores.
Major allergen content > 20 mcg
Ten studies from this group reported medication scores; 360 participants in the SLIT and 301 in the placebo group. The SMD in this group was ‐0.22 (95% CI ‐0.43 to 0.00, P = 0.05). Significant heterogeneity between studies was indicated (Chi² = 15.70, P = 0.07, I² = 43%) (Analysis 11.2).
11.2. Analysis.
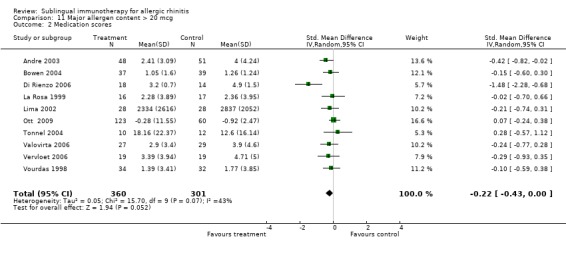
Comparison 11 Major allergen content > 20 mcg, Outcome 2 Medication scores.
Subgroup analysis: individual allergens
As with symptom scores, we performed meta‐analysis for medication scores for one perennial (house dust mite) and four seasonal (grass pollen, Parietaria, ragweed, tree) allergens.
Medication scores were reported in five trials involving house dust mite; 95 patients received SLIT and 94 received placebo. The SMD was ‐0.52 (95% CI ‐1.09 to 0.05, P = 0.07). Highly significant heterogeneity was indicated between studies (Chi² test = 12.92, P = 0.01, I² = 69%) (Analysis 13.2).
13.2. Analysis.
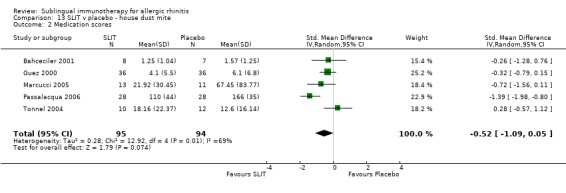
Comparison 13 SLIT v placebo ‐ house dust mite, Outcome 2 Medication scores.
Seventeen studies were performed with grass pollen allergen. There were 1201 patients in the SLIT and 1107 in the placebo group. The combined SMD was ‐0.23 (95% CI ‐0.37 to ‐0.10, P = 0.0008). Significant heterogeneity among studies was indicated (Chi² = 34.55, P = 0.005, I² = 54%) (Analysis 14.2).
14.2. Analysis.
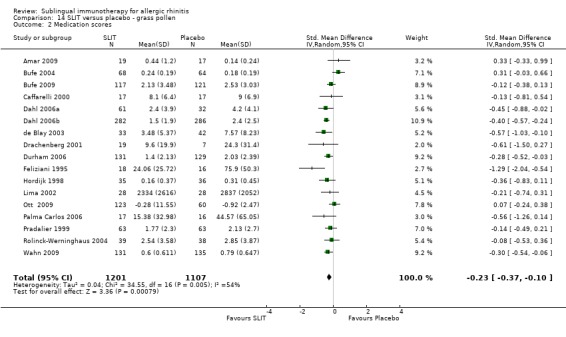
Comparison 14 SLIT versus placebo ‐ grass pollen, Outcome 2 Medication scores.
Medication scores were also reported in two studies investigating ragweed, involving 85 patients in the SLIT and 90 in the placebo group. The SMD was ‐0.30 (95% CI ‐0.60 to 0.00, P = 0.05). No heterogeneity was shown between studies (Chi² 0.82, P = 0.37, I² = 0%) (Analysis 15.2).
15.2. Analysis.
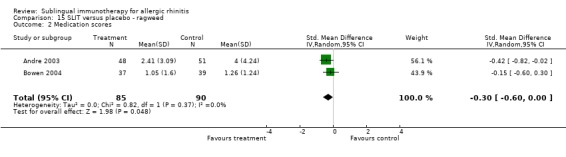
Comparison 15 SLIT versus placebo ‐ ragweed, Outcome 2 Medication scores.
Parietaria was investigated in five trials, with 74 patients in the SLIT and 77 in the placebo group. The SMD was ‐0.62 (95% CI ‐1.00 to ‐0.24, P = 0.001). No heterogeneity was shown between studies (Chi² = 5.30, P = 0.26, I² = 25%) (Analysis 16.2).
16.2. Analysis.
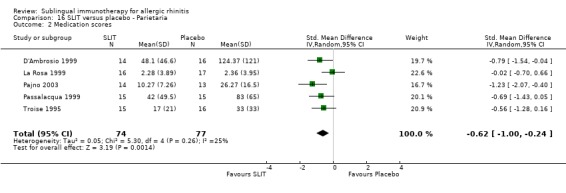
Comparison 16 SLIT versus placebo ‐ Parietaria, Outcome 2 Medication scores.
Nine different studies involved trees, with 197 participants receiving SLIT and 183 placebo. The SMD was ‐0.38 (95% CI ‐0.62 to ‐0.13, P = 0.002). There was a lack of significant heterogeneity among studies (Chi² = 10.24, P = 0.25, I² = 22%) (Analysis 17.2).
17.2. Analysis.
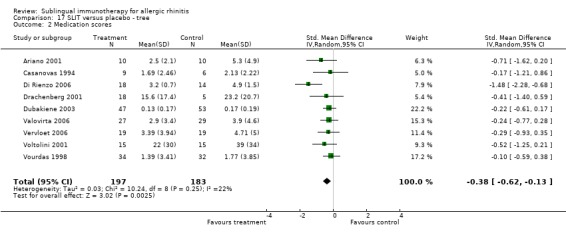
Comparison 17 SLIT versus placebo ‐ tree, Outcome 2 Medication scores.
Subgroup analysis: medication preparation
Sublingual tablets
Sublingual tablets were used in nine trials, with 799 participants in the SLIT group and 779 in the placebo group. The SMD was ‐0.33 (95% CI ‐0.46 to ‐0.20, P < 0.00001). Highly significant heterogeneity was shown between studies (Chi² = 50.39, P < 0.00001, I² = 84%) (Analysis 20.2).
20.2. Analysis.
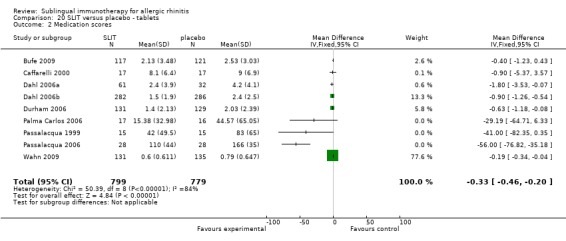
Comparison 20 SLIT versus placebo ‐ tablets, Outcome 2 Medication scores.
Sublingual drops
Medication score was reported in 27 studies using sublingual drops; 865 SLIT and 788 placebo participants took part in these trials. The SMD was ‐0.01 (95% CI ‐0.05 to 0.04, P = 0.74). A significant level of heterogeneity was indicated (Chi² = 82.89, P < 0.00001, I² = 69%) (Analysis 21.2).
21.2. Analysis.
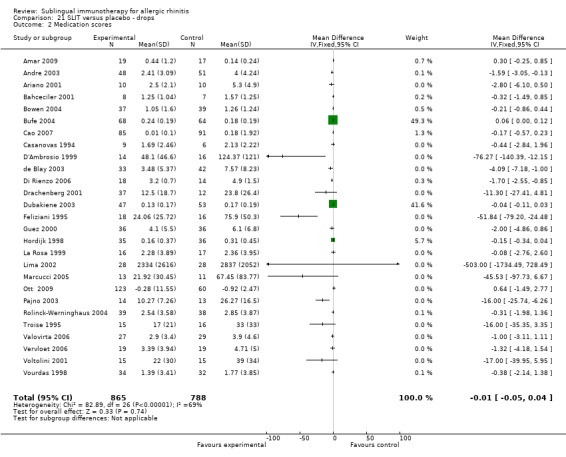
Comparison 21 SLIT versus placebo ‐ drops, Outcome 2 Medication scores.
Specific serum antibodies
Fourteen studies reported increases in serum‐specific IgE levels in a manner suitable for the meta‐analysis, with 675 participants in the SLIT and 659 in the placebo group. The combined SMD was 0.27 (95% CI ‐0.01 to 0.55, P = 0.05). Significant heterogeneity was indicated between studies (Chi² = 68.63, P < 0.00001, I² = 81%) (Analysis 12.1).
12.1. Analysis.
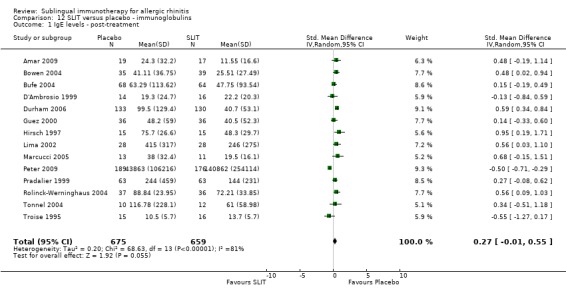
Comparison 12 SLIT versus placebo ‐ immunoglobulins, Outcome 1 IgE levels ‐ post‐treatment.
Total serum‐specific IgG was measured in three studies, with 286 participants in the SLIT and 304 in the placebo group. The combined SMD was 0.95 (95% CI 0.78 to 1.12, P < 0.00001). There was no significant heterogeneity between studies (Chi² = 1.01, P = 0.60, I² = 0%) (Analysis 12.2).
12.2. Analysis.
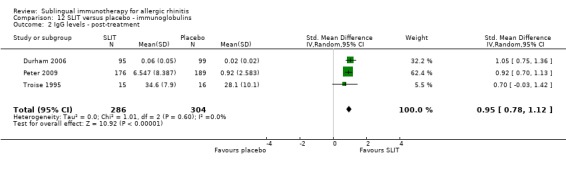
Comparison 12 SLIT versus placebo ‐ immunoglobulins, Outcome 2 IgG levels ‐ post‐treatment.
Serum‐specific IgG4 was measured in 13 trials, with a total number of 588 in the SLIT and 599 in the placebo group. The total SMD was 0.46 (95% CI 0.29 to 0.63, P < 0.00001). Highly significant heterogeneity was indicated (Chi² = 174.24, P < 0.00001, I² = 93%) (Analysis 12.3).
12.3. Analysis.
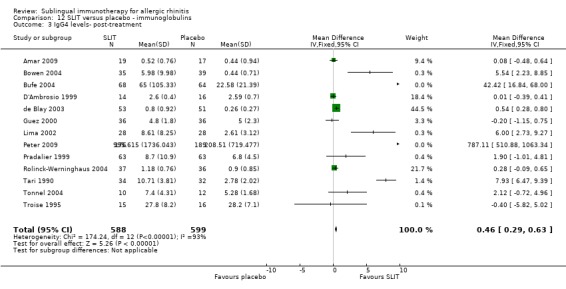
Comparison 12 SLIT versus placebo ‐ immunoglobulins, Outcome 3 IgG4 levels‐ post‐treatment.
Allergen sensitivity
Six studies included skin reactivity data (skin prick test or early phase reaction) that were eligible for the meta‐analysis; 183 participants were in the SLIT and 148 in the placebo group. The SMD was 0.12 (95% CI ‐0.26 to 0.51, P = 0.53). Significant heterogeneity was indicated among studies (Chi² was 12.47, P = 0.03, I² = 60%) (Analysis 18.1).
18.1. Analysis.
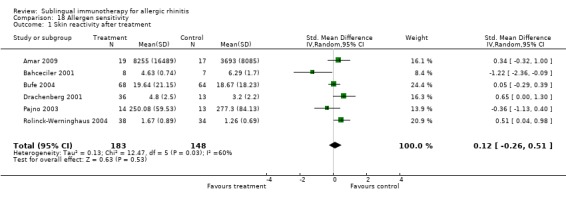
Comparison 18 Allergen sensitivity, Outcome 1 Skin reactivity after treatment.
The results of nasal reactivity were reported in 10 studies, but only seven were suitable for meta‐analysis. The analysis involved 111 participants in the SLIT and 109 participants in the placebo group. The SMD was 0.32 (95% CI ‐0.13 to 0.78, P = 0.17). Highly significant heterogeneity was indicated between studies (Chi² = 16.39, P = 0.01, I² = 63%) (Analysis 18.2).
18.2. Analysis.
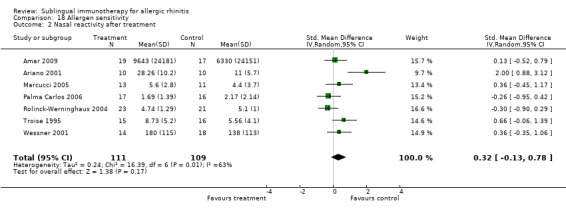
Comparison 18 Allergen sensitivity, Outcome 2 Nasal reactivity after treatment.
We identified only one study each assessing conjunctive and bronchial provocation tests which were eligible for meta‐analysis, therefore we could not perform these analyses.
Adverse events
We included 60 studies in our systematic review and all were analysed for safety results. Adverse events were the only discontinuous data analysed in our review.
Twenty‐five studies reported data in a manner not suitable for our analysis (insufficient data, adverse events reported by system organ classification, data expressed in percentages, etc.) (Table 22).
1. Adverse events: data not suitable for analysis.
| Study ID | Sublingual immunotherapy | Placebo | Additional comments |
| N | N | ||
| Bowen 2004 | 43 | 40 | Report by SOC |
| Casanovas 1994 | 9 | 6 | Grading according to EAACI |
| Cao 2007 | 85 | 91 | Insufficient data |
| Dahl 2006a | 61 | 32 | AE reported as percentage of patients |
| de Blay 2003 | 33 | 42 | AE reported as percentage of patients |
| Di Rienzo 2006 | 19 | 15 | Insufficient data |
| Drachenberg 2001 | 49 | 19 | AE reported as difference between SLIT and placebo group (P values) |
| Dubakiene 2003 | 59 | 60 | Insufficient data |
| Durham 2006 | AE reported by severity | ||
| Feliziani 1995 | 18 | 16 | Insufficient data |
| Guez 2000 | 36 | 36 | Insufficient data |
| Hirsch 1997 | 15 | 15 | Insufficient data |
| Hordijk 1998 | 27 | 30 | AE reported SOC |
| Ippoliti 2003 | 47 | 39 | Insufficient data |
| Lima 2002 | 28 | 28 | Data reported in percentages |
| Malling 2005 | 36 | 11 | Trial design Data reported in percentages |
| Marcucci 2005 | 13 | 11 | Insufficient data |
| Mungan 1999 | 15 | 11 | Insufficient data |
| Ott 2009 | 142 | 67 | Insufficient data |
| Palma Carlos 2006 | 17 | 16 | Insufficient data |
| Panzner 2008 | 20 | 15 | Insufficient data |
| Passalacqua 1998 | 10 | 10 | Insufficient data |
| Sanchez 2001 | 20 | 20 | Insufficient data |
| Tari 1990 | 34 | 32 | Insufficient data |
| Voltolini 2001 | 15 | 15 | Insufficient data |
Six studies with 125 participants in the SLIT and 126 in the placebo group reported no adverse events during the trials (Table 23).
2. No adverse events reported.
| Study ID | Sublingual immunotherapy | Placebo |
| N | N | |
| Ariano 2001 | 10 | 10 |
| Bahceciler 2001 | 8 | 7 |
| D'Ambrosio 1999 | 14 | 16 |
| Passalacqua 1998 | 15 | 15 |
| Passalacqua 1999 | 15 | 15 |
| Pradalier 1999 | 63 | 63 |
Twenty‐two studies reported different local and systemic reactions as a total number of single adverse events during the whole trial, hence we analysed these data.
Twenty‐five studies reported different local events. Buccal pruritus was reported in 21 trials: 1126 participants experienced a total number of 1798 events in the SLIT group and 1075 participants experienced 492 events in the placebo group. Labial oedema was reported in 11 studies, with 604 participants/55 events in the SLIT group and 526 participants/7 labial swelling episodes in the placebo group.
Bucco‐lingual oedema was reported in eight trials, with 648 participants/143 events in the SLIT group, and 606 participants/2 episodes in the placebo group. Throat irritation was reported in 10 trials, including 770 participants in the SLIT and 747 in the placebo group, with 243 and 29 episodes respectively. Local adverse events were also reported as oral non‐specified in three and local non‐specified in three trials (Table 24).
3. Adverse events ‐ local reactions.
| Type of reaction | No of studies reported the event | Sublingual immunotherapy | Placebo | ||
| Total No of patients | Total No of events | Total No of patients | Total No of events | ||
| Labial oedema | 11 | 604 | 55 | 536 | 7 |
| Buccal pruritus | 21 | 1126 | 1798 | 1075 | 492 |
| Bucco‐ lingual oedema | 8 | 648 | 143 | 606 | 2 |
| Throat irritation | 10 | 770 | 243 | 747 | 29 |
| Oral ‐ non‐specified | 3 | 68 | 143 | 71 | 24 |
| Non‐specified | 3 | 119 | 7 | 116 | 3 |
Systemic reactions were reported by 18 studies (Table 25). Rhinitis was reported in 16 trials, with 965 participants/1403 events in the SLIT group and 912 participants/1034 events in the placebo group. Conjunctivitis alone was reported by eight trials, with 262 participants and 774 conjunctivitis episodes in the SLIT and 238 participants and 786 events in the placebo group. Cough was reported in eight trials: 337 participants treated with SLIT experienced 313 events and 304 participants in the placebo group reported 211 episodes of cough. Gastro‐intestinal symptoms were described by 20 trials with 630 participants in the SLIT and 561 in the placebo group, with 88 and 10 events respectively. None of the studies reported anaphylaxis.
4. Adverse events ‐ systemic reactions.
| Type of reaction | No of studies reported the event | Sublingual immunotherapy | Placebo | ||
| Total No of patients | Total No of events | Total No of patients | Total No of events | ||
| Urticaria | 8 | 204 | 7 | 199 | 9 |
| Pruritus/rash | 10 | 363 | 13 | 222 | 9 |
| Conjunctivitis | 8 | 262 | 774 | 238 | 786 |
| Rhinitis | 16 | 965 | 1403 | 912 | 1034 |
| Rhino‐conjunctivitis | 6 | 184 | 60 | 176 | 58 |
| Asthma/wheezing | 15 | 488 | 51 | 450 | 42 |
| Cough | 8 | 337 | 313 | 304 | 211 |
| Gastro‐intestinal | 20 | 630 | 88 | 561 | 10 |
| Headache | 6 | 535 | 70 | 548 | 68 |
| Anaphylaxis | 6 | 291 | 0 | 288 | 0 |
| Systemic ‐ non‐specified | 5 | 330 | 4 | 36 | 0 |
Fifteen studies reported adverse events which led to treatment discontinuation. In the SLIT group, 41 out of 824 patients and 12 out of 861 placebo participants were withdrawn because of adverse events. Troublesome local reactions were the most common cause, although systemic reactions were described (Table 26).
5. Adverse events leading to treatment discontinuation.
| Study ID | Sublingual immunotherapy | Placebo | ||||
| N | n | AE description | N | n | AE description | |
| Andre 2003 | 53 | 4 | Sublingual burning Oral pruritus, vomiting, headache Pruritus, lingual oedema, gastralgia, diarrhoea Asthma and gastralgia |
53 | 1 | Gastralgia |
| Dahl 2006b | 316 | 5 | Angioedema on the base of the tongue Inferior lip angioedema pharyngeal hyperemia, cough, mild dyspnoea Pharynx oedema, voice changes Swelling throat Angioedema of lips |
318 | 0 | Not applicable |
| Durham 2006 | 141 | 8 | Not described | 136 | 1 | Not described |
| Khinchi 2004 | 23 | 3 | Pain in fingers and visible veins Gastrointestinal complaints Itching in the mouth |
24 | 1 | Pain and weakness in both arms |
| La Rosa 1999 | 20 | 4 | Not described | 21 | 1 | Not described |
| Lima 2002 | 28 | 1 | Troublesome local side effects | 28 | 0 | Not applicable |
| Malling 2005 | 12 | 1 | Sting and blisters in the mouth | 11 | 1 | Mouth itching |
| Pajno 2003 | 15 | 1 | Systemic reaction (abdominal pain, shortness of breath, wheezing) | 15 | 0 | Not applicable |
| Pradalier 1999 | 63 | 2 | Worsening symptoms Marked reactions |
63 | 2 | Worsening symptoms Marked reactions |
| Rolinck‐Werninghaus 2004 | 49 | 1 | Acute asthma exacerbation needed hospitalisation | 48 | 3 | Not described |
| Smith 2004 | 44 | 7 | 4 patients with systemic but not life‐threatening AE | 45 | ‐ | Insufficient data |
| Tonnel 2004 | 15 | 1 | Itching/burning of the mouth | 17 | 1 | Respiratory tract infection |
| Vervloet 2006 | 38 | 1 | Gastric pain and vomiting | 38 | 0 | Not applicable |
| Vourdas 1998 | 33 | 1 | Worsening of allergic disease | 29 | 0 | Not applicable |
| Voltolini 2001 | 15 | 1 | Exacerbation of rhinitis and OAS | 15 | 1 | Dyspnoea |
Five studies reported adverse events by their severity, but not all of them considered the link with the treatment. The majority of adverse events were mild to moderate and did not require any treatment. None of the reactions required the administration of adrenaline.
Quality of life
Quality of life was reported by three studies, but assessment in those studies differed greatly and we considered that these data could not be included in our analysis.
Discussion
This systematic review of sublingually administered allergen immunotherapy (SLIT) represents an update of a review first published in The Cochrane Library in 2003 (Wilson 2003). The original review included data from 22 randomised controlled trials (979 patients) and demonstrated the efficacy of this form of treatment based on meta‐analysis of symptom severity scores (standardised mean difference (SMD) ‐0.42; 95% confidence interval (CI) ‐0.69 to ‐0.15). Ongoing research in this area has been considerable and this review has now been updated to include studies published since 2003. The number of studies included has almost trebled to 60 (with 49 being suitable for pooling in meta‐analyses) and the number of patients in meta‐analysis has increased over four‐fold, reflecting a trend towards larger, better designed and more powerful trials.
The overall results of the meta‐analysis differ little from those seen in 2003, with the overall effect for symptom scores (SMD ‐0.49; 95% CI ‐0.64 to ‐0.34) being of a similar magnitude, with tighter confidence intervals reflecting the greatly increased number of study subjects. The same is true for the analysis of medication scores, with SMD ‐0.32 (95% CI ‐0.43 to ‐0.21). These data continue to support the clinical efficacy of sublingual immunotherapy for allergic rhinitis.
In contrast to the original review, the greater number of studies has allowed more meaningful analyses of some of the pre‐determined subgroups. In particular there are now 15 studies looking exclusively at children, some of which are large studies in their own right (Bufe 2009; Wahn 2009). The treatment effect within this subgroup of trials appears to be similar to that seen in adults, especially when considering symptom scores. SLIT represents a particularly attractive alternative to injection immunotherapy in this patient group and our findings are entirely consistent with those reported elsewhere (Calderon 2008).
The protocol for the original review reflected the then classification of aero‐allergens into seasonal and perennial. The ARIA classification (ARIA 2008) now uses the terms intermittent and persistent but for this review this change makes no difference. In this meta‐analysis there does appear to be a greater effect with perennial allergens (predominantly house dust mite) when compared to seasonal allergens although this is based on fewer studies. More studies of perennial rhinitis are needed to confirm or exclude this possibility.
It is not possible to differentiate between different doses on the basis of this meta‐analysis. The difficulty in determining dose in terms of micrograms of major allergen, and standardising this information across a range of studies utilising allergen extracts from different sources, was acknowledged in the original review and remains problematic.
Although the difference is small, this review has shown a trend in symptom score reduction in trials which lasted for longer than 12 months, when compared with shorter treatment periods. Indeed, SLIT is now given for longer time periods (over 12 months in 32% of included studies compared with 19% in 2003), and more recent studies have shown that treatment for longer than 12 months provides consistent clinical improvement in symptom and medication scores (Dahl 2008; Durham 2010). These data are encouraging and should be taken into consideration in future recommendations or guidelines for the use of SLIT in allergic rhinitis.
Looking at the total effect and SMD (95% CI) of sublingual immunotherapy for individual allergens, house dust mite appears to be more effective than treatment with other types of allergen, and even more effective than treatment with grass pollen. However, the majority of these trials are small, with five (out of nine) trials involving fewer than 20 participants. Heterogeneity in this group is amongst the highest in all the meta‐analyses; when comparing P values for the overall effect, the level of significance appears to be lower than for the majority of other allergens. We therefore conclude that this finding should be interpreted with caution.
This review has shown that SLIT provokes significant changes in terms of allergen‐specific IgG and IgG4 antibodies, which coincide with a clinical response in terms of symptom and medication scores. These findings are in complete concordance with the findings of the previous SLIT review (Wilson 2003), as well as injection immunotherapy (Calderon 2007). Unfortunately, changes detected in IgG and IgG4 values were not supported by changes in allergen sensitivity, and meta‐analysis of data for skin and nasal reactivity after treatment showed no difference between the immunotherapy and placebo group. The exact role of IgG and IgG4 is still not completely clear. Although they are likely to have a certain 'protective' role, there is still an ongoing debate as to whether this increase in (particularly) IgG4 is just a consequence of exposure to a high dose of allergen or the real immunomodulatory effect of sublingual immunotherapy. This review could not draw a definitive conclusion and only further mechanistic studies can enable us to answer this question.
This review explored the possible differences between different sublingual preparations (i.e. sublingual drops versus tablets). Although tablets proved to be more effective in terms of medication scores, and had similar efficacy in terms of symptoms, overlapping confidence intervals and the substantial heterogeneity between studies did not allow us to draw any firm conclusions. It seems both preparations are similarly effective.
An increasing number of studies report quality of life as a primary or secondary outcome measure as is the case in clinical trials in general. Nevertheless, there were big differences in quality of life scoring systems such that we were unable to analyse these data by meta‐analysis.
It was acknowledged in the original review that many of the studies included were small, early publications that did not conform to the CONSORT (1996) guidelines for the publication of randomised controlled trials (Begg 1996; Moher 2001). The methodological quality of the studies included on this occasion has been scrutinised more closely using the new Cochrane criteria, including assessment of randomisation and allocation concealment.
The possible confounder of publication bias that exists in all meta‐analysis (Calderon 2008; Nieto 2009) was acknowledged in the original review and has again been addressed on this occasion through extensive consultation with those active in the field of SLIT research. We are confident that no data have been excluded purely on the basis of negative outcome.
Funnel plot evaluations for the two main outcomes (Figure 3; Figure 4) showed that the plots were reasonably symmetrical and there did not appear to be a paucity of smaller trials with small or absent symptom reduction effect.
3.
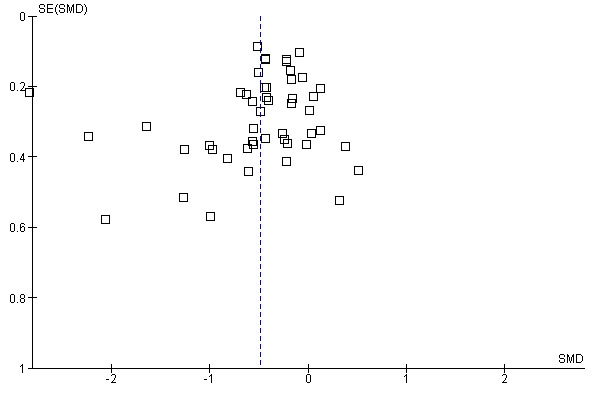
Funnel plot of comparison: 1 SLIT versus placebo ‐ all, outcome: 1.1 Allergic rhinitis symptom scores.
4.
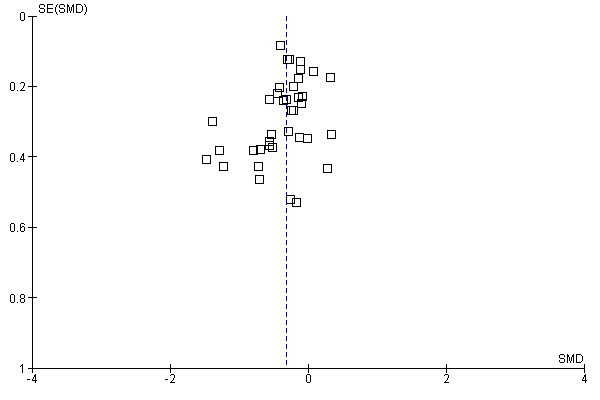
Funnel plot of comparison: 1 SLIT versus placebo ‐ all, outcome: 1.2 Medication scores.
In the current review we withdrew three studies (D'Ambrosio 1996; Mungan 1999; Quirino 1996) that had been included in the 2003 review as they did not satisfy the new Cochrane criteria for randomised, double‐blind studies.
Heterogeneity between studies was acknowledged as a significant problem in the last review, and is a known problem in systematic reviews. This results largely from methodological and clinical heterogeneity (i.e. differences in scoring systems, sample sizes, type and dose of allergen, age groups, etc.) used across studies. Selection of the studies for this review was defined in our protocol and studies which satisfied our criteria were chosen. The method used by The Cochrane Collaboration for assessing heterogeneity has however changed and can now be expressed as an I2 statistic. It remains the case that studies in this field are heterogeneous and data are expressed in a wide variety of different ways. However, certain subgroup analyses (e.g. seasonal allergens, individual allergens, subgroup analysis for major allergen content) have shown the significant reduction in heterogeneity even though these groups were prespecified by a protocol. This means that there will always be a degree of interpretation required when amalgamating studies in meta‐analysis and further subgroup analysis could be performed in order to address better the problem of heterogeneity. Despite this, there is remarkable consistency in the outcomes of related systematic reviews (Casale 2009; Cox 2006; Wilson 2005).
Adverse events
Although considered as a secondary outcome, we felt analysis of adverse events to be crucial as a low incidence confers advantage on SLIT as an alternative to injection immunotherapy. Adverse event data were, by their nature, non‐continuous. Authors mainly reported data as total number of events for a number of patients, rather than number of certain events per patient. Some papers reported their most common events as a percentage of total events, or as a percentage of patients who experienced particular events. These were therefore not suitable for meta‐analysis and we were able to perform only descriptive analysis. A further problem is that unlike for the subcutaneous route there is currently no internationally standardised methodology for reporting local adverse events of sublingual immunotherapy.
With these reservations, local reactions are again shown to be common and reported much more frequently in SLIT recipients than in those receiving placebo. These are clearly unavoidable but are usually seen as an inconvenience and cause little distress and have no lasting effect, though rarely these adverse events were distressing enough to warrant withdrawal of treatment.
Systemic reactions were again largely confined to the upper respiratory tract and associated organs (rhinitis, conjunctivitis or rhinoconjunctivitis) and were more frequent in the SLIT than in the placebo groups. Asthma or wheeze was no more likely in SLIT recipients than in placebo recipients. Gastrointestinal effects (non‐specified) were rare but more apparent in SLIT recipients and were largely confined to paediatric trials. None were considered serious.
There were no reports in clinical trials of severe systemic reactions or anaphylaxis and none of the systemic reactions needed the use of adrenaline. No fatalities were reported.
This review evaluates a large number of double‐blind, placebo‐controlled studies which, in total, give us a large number of doses. No incidences of life‐threatening reactions were reported in the studies analysed. We conclude that SLIT remains a safe treatment with an extremely low incidence of significant side effects. Systemic adverse events were predominantly mild to moderate and their causality was unlikely to be related to SLIT. We have been unable to correlate adverse events with allergen dose.
There are no reports of fatalities following sublingual immunotherapy. Six isolated cases of severe reactions have been reported independently of clinical trials and all involved deviation from current recommended practice according to international guidelines (Antico 2006; Blazowski 2008; de Groot 2009; Dunsky 2006; Eifan 2007). Numbers are too few to allow identification of risk factors for severe systemic reactions, whereas it can be noted that five out of six occurred in young females, five out of six had asthma, and two out of six had previously experienced severe reactions during subcutaneous immunotherapy. These data should be viewed in the context of the number of doses of sublingual immunotherapy that have been prescribed and administered worldwide.
Since the original systematic review in 2003 SLIT has become established as an effective and low‐risk alternative to allergen injection immunotherapy, which carries a significant morbidity and a requirement for delivery within specialist centres capable of meeting CSM recommendations. SLIT is recommended to be initiated in secondary care and the first dose taken under medical supervision whereas maintenance treatment is recommended to be self‐administered in the patient’s home.
Only two studies in the original review compared injection immunotherapy with sublingual immunotherapy directly (Mungan 1999; Quirino 1996). In the current review only one study compared injection immunotherapy versus SLIT. Although comparison of those two treatment options was not the objective of this review, the search process enabled us to identify papers comparing the efficacy of these treatment options. We found very few such papers: there appears to be insufficient data available to draw any conclusions and more definitive head‐to‐head trials are needed.
A Cochrane Review of allergen injection immunotherapy, which included 51 trials with 2871 participants (Calderon 2007), showed a SMD of ‐0.73 (95% CI ‐0.97 to ‐0.5) for symptom scores compared to placebo and a SMD of ‐0.57 (95% CI ‐0.82 to ‐0.33) for medication scores. Although the SMDs are numerically different, the confidence intervals overlap with those for SLIT, indicating no apparent difference between the two therapies on this basis. However, it is not correct to perform a direct statistical comparison between these two meta‐analyses. These data raise the importance of future double‐blind, double‐dummy trials that directly compare these two routes of immunotherapy.
Injection immunotherapy exerts its long‐lasting effects through modulation of the response of the immune system upon allergen exposure, altering from an allergic response to one of immune tolerance as evidenced by alterations in Th cell cytokine profiles, numbers of effector cells at target sites and changes in humoral responses, including increases in putative blocking IgG4 class antibodies (Wilson 2001). In the 2003 review there were only six studies that reported immunological outcomes, in terms of immunoglobulin levels, that were suitable for meta‐analysis but available data did suggest consistent increases in allergen‐specific IgG4 during SLIT treatment. Eleven studies (doubling the number of subjects) in the current review contained data on IgG4 levels and a consistent and significant two‐fold increase in IgG4 levels was observed. The role of allergen‐specific IgG4 antibodies remains controversial but the evidence points towards similar immunological mechanisms underlying the two forms of therapy. Further discussion on mechanisms is outside the scope of this review.
Authors' conclusions
Implications for practice.
This systematic review and meta‐analysis is more powerful than the original review published in 2003, with over four times the number of patients. The data again strongly support the efficacy of sublingual immunotherapy compared with placebo in terms of a reduction in rhinitis symptom scores and anti‐allergic medication requirements. Furthermore, the data now more strongly support the use of sublingual immunotherapy in children and in allergic rhinitis due to all aero‐allergens.
Sublingual immunotherapy is now established as a viable alternative to allergen injection immunotherapy, with a significantly lower risk profile and, on the basis of meta‐analyses, little difference in overall efficacy.
Implications for research.
The optimum dose and duration of therapy remains an unanswered question and it is unlikely that meta‐analysis will provide the answers. Ongoing clinical trials of prolonged therapy and increasing use of standardised tablet products may do.
The mechanism of action of both injection and sublingual immunotherapy remain under investigation, and injection immunotherapy has been proven to lead to long‐term changes in the immunological response to allergen that may persist for years following discontinuation.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2010 | Amended | Contact details and conflict of interest statement updated. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 11 May 2010 | New citation required and conclusions have changed | We included 38 new studies in the review, strengthening the conclusions. The authorship has also changed. |
| 11 May 2010 | New search has been performed | New searches run 14 August 2009. |
| 28 February 2009 | Amended | Converted to new review format. |
| 23 February 2004 | Amended | Correction submitted for Issue 2, 2004. |
| 25 November 2003 | Feedback has been incorporated | Feedback and authors' response incorporated. |
Acknowledgements
Grateful thanks are extended to the authors of individual studies who provided additional data to allow meaningful meta‐analysis to be performed.
Appendices
Appendix 1. Search strategies
| PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 "IMMUNOTHERAPY" [MeSH] OR "DESENSITIZATION, IMMUNOLOGIC" [MeSH] #2 ("Allergens/administration and dosage"[Mesh] OR "Allergens/immunology"[Mesh]) #3 ALLERGEN* [tiab] OR IMMUNOLOGIC [tiab]) AND (HYPOSENSITIZ* [tiab] OR HYPOSENSITIS* [tiab] OR DESENSITIZ* [tiab] OR DESENSITIS* [tiab]) #4 #1 OR #2 OR #3 #5 "ADMINISTRATION, SUBLINGUAL" [Mesh] #6 (SUBLINGUAL* [tiab] OR ORAL* [tiab] OR TONGUE [tiab] OR MUCOSA [tiab]) #7 #5 OR #6 #8 #4 AND #7 #9 (SLIT [tiab] OR (SUBLINGUAL* [tiab] AND IMMUNOTHERAP* [tiab])) #10 #8 OR #9 #11 (((("rhinitis, allergic, perennial"[Mesh]) OR ("rhinitis, allergic, seasonal"[Mesh]) OR ((("rhinitis"[Mesh]) OR (rhinit*[tiab])) AND (allerg*[tiab] OR "hypersensitivity"[Mesh])) OR (((("rhinitis"[Mesh]) OR (rhinit*[tiab])) OR (allerg*[tiab] OR "hypersensitivity"[Mesh])) AND ((perennial[ti] OR persistent[ti] OR nonseaosnal[ti] OR nose[ti] OR nasal[ti] OR cat*[ti] OR fur[ti] OR hair*[ti] OR dander[ti] OR dust*[ti] OR mite*[ti] OR pet*[ti] OR dog*[ti] OR cockroach*[ti]) OR (seasonal[ti] OR intermittent[ti] AND spring[ti] OR summer[ti] OR pollen[ti] OR grass*[ti] OR birch[ti] OR ragweed[ti] OR tree*[ti] OR weed*[ti] OR mugwort[ti] OR willow[ti] OR alder[ti]))) OR (hayfever[tiab] OR "hay fever"[tiab] OR pollenosis[tiab] OR pollinosis[tiab] OR SAR [tiab] OR PAR [tiab])) #12 #10 AND #11 | 1 IMMUNOTHERAPY/ or IMMUNOLOGICAL TOLERANCE/ or exp IMMUNOMODULATING AGENT/ or exp IMMUNOSUPPRESSIVE TREATMENT/ 2 ((ALLERGEN* or IMMUNOLOGIC) and (HYPOSENSITIZ* or HYPOSENSITIS* or DESENSITIZ* or DESENSITIS*)).tw. 3 1 or 2 4 (SUBLINGUAL* or ORAL* or TONGUE or MUCOSA).tw. 5 4 and 3 6 (SLIT or (SUBLINGUAL* and IMMUNOTHERAP*)).tw. 7 6 or 5 8 exp Allergic Rhinitis/ 9 Rhinitis/ 10 Rhinit*.tw. 1110 or 9 12 exp Hypersensitivity/ 13 allerg*.tw. 14 13 or 12 15 11 and 14 16 (perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*).ti. 17 (seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder).ti. 18 17 or 16 19 11 or 14 20 18 and 19 21 (hayfever or "hay fever" or pollenosis or pollinosis or SAR).tw. 22 15 or 8 or 20 or 21 23 22 and 7 | S1 (MH "Rhinitis, Allergic, Perennial") or (MH "Rhinitis, Allergic, Seasonal") S2 (MH "Rhinitis") S3 TX rhinit* S4 S2 or S3 S5 TX allerg* S6 (MH "Hypersensitivity") S7 S5 or S6 S8 S4 and S7 S9 TI perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach* S10 TI seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder S11 S9 or S10 S12 S5 OR S6 S13 S11 and S12 S14 TX hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR S15 S1 or S8 or S13 or S14 S16 (MH "Immunotherapy") S17 (MH "Desensitization, Immunologic") S18 TX ( ALLERGEN* OR IMMUNOLOGIC* ) and TX ( HYPOSENSITIZ* OR HYPOSENSITIS* OR DESENSITIZ* OR DESENSITIS* ) S19 S16 or S17 or S18 S20 (MH "Administration, Sublingual") S21 TX SUBLINGUAL* OR ORAL* OR TONGUE OR MUCOSA S22 S20 or S21 S23 S19 and S22 s24 TX SLIT OR TX (sublingual AND immunotherap*) S25 S23 OR S24 S26 S15 AND S25 |
| Web of Science | BIOSIS Previews/CAB Abstracts (Ovid) | mRCT |
| #1 TS=rhiniti* #2 TS=(allerg* OR hypersensitivty) #3 #2 AND #1 #4 TI=(perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*) #5 TI=(seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder) #6 #5 OR #4 #7 #6 AND #2 #8 TS=(hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR OR PAR) #9 #3 OR #7 OR #8 #10 TS=((ALLERGEN* OR IMMUNOLOGIC*) AND (HYPOSENSITIZ* OR HYPOSENSITIS* OR DESENSITIZ* OR DESENSITIS* )) #11 TS=(immunotherap*) #12 #10 OR #11 #13 TS=(SUBLINGUAL* or ORAL* or TONGUE or MUCOSA) #14 #12 AND #13 #15 SLIT #16 #14 OR #15 #17 #9 AND #16 | 1 IMMUNOTHERAPY/ or IMMUNOLOGICAL TOLERANCE/ or exp IMMUNOMODULATING AGENT/ or exp IMMUNOSUPPRESSIVE TREATMENT/77 2 ((ALLERGEN* or IMMUNOLOGIC) and (HYPOSENSITIZ* or HYPOSENSITIS* or DESENSITIZ* or DESENSITIS*)).tw. 3 1 or 2 4 (SUBLINGUAL* or ORAL* or TONGUE or MUCOSA).tw. 5 4 and 3 6 (SLIT or (SUBLINGUAL* and IMMUNOTHERAP*)).tw. 7 6 or 5 8 Rhinitis/ 9 Rhinit*.tw. 10 9 or 10 11 exp Hypersensitivity/ 12 allerg*.tw. 13 11 or 12 14 10 and 13 15 (perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*).ti. 16 (seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder).ti. 17 15 or 16 18 13 AND 17 19 (hayfever or "hay fever" or pollenosis or pollinosis or SAR).tw. 20 10 or 18 OR 19 21 7 AND 20 | (rhinit% OR hayfever OR allerg%) AND (sublingual% OR oral% OR tongue OR mucosa) |
Data and analyses
Comparison 1. SLIT versus placebo ‐ all.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 49 | 4589 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.64, ‐0.34] |
| 2 Medication scores | 38 | 3379 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.43, ‐0.21] |
Comparison 2. SLIT versus placebo ‐ seasonal allergen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 39 | 4084 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.44, ‐0.25] |
| 2 Medication scores | 32 | 3014 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.41, ‐0.19] |
Comparison 3. SLIT versus placebo ‐ perennial allergen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 10 | 505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.69, ‐0.17] |
| 2 Medication scores | 6 | 365 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.89, 0.02] |
Comparison 4. SLIT versus placebo ‐ adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 34 | 3197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.56, ‐0.31] |
| 2 Medication scores | 26 | 2235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.53, ‐0.26] |
Comparison 5. SLIT versus placebo ‐ children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 15 | 1392 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.94, ‐0.10] |
| 2 Medication scores | 12 | 1144 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.32, 0.00] |
Comparison 6. SLIT versus placebo < 6 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 17 | 1772 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.86, ‐0.21] |
| 2 Medication scores | 15 | 1366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.45, ‐0.18] |
Comparison 7. SLIT versus placebo 6 to 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 16 | 1736 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.46, ‐0.16] |
| 2 Medication scores | 13 | 1398 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.50, ‐0.12] |
Comparison 8. SLIT versus placebo > 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 16 | 1089 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.92, ‐0.34] |
| 2 Medication scores | 10 | 615 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.64, ‐0.04] |
Comparison 9. Major allergen content < 5 mcg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 8 | 275 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.69, 0.05] |
| 2 Medication scores | 6 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.94, ‐0.24] |
Comparison 10. Major allergen content 5 to 20 mcg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 12 | 1972 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.45, ‐0.24] |
| 2 Medication scores | 11 | 1940 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.07] |
Comparison 11. Major allergen content > 20 mcg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 12 | 1041 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.49, ‐0.17] |
| 2 Medication scores | 10 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.43, 0.00] |
Comparison 12. SLIT versus placebo ‐ immunoglobulins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IgE levels ‐ post‐treatment | 14 | 1334 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.01, 0.55] |
| 2 IgG levels ‐ post‐treatment | 3 | 590 | Std. Mean Difference (IV, Random, 95% CI) | 0.95 [0.78, 1.12] |
| 3 IgG4 levels‐ post‐treatment | 13 | 1187 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.29, 0.63] |
Comparison 13. SLIT v placebo ‐ house dust mite.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 9 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.80, ‐0.13] |
| 2 Medication scores | 5 | 189 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.09, 0.05] |
Comparison 14. SLIT versus placebo ‐ grass pollen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 23 | 3013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.45, ‐0.24] |
| 2 Medication scores | 17 | 2308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.37, ‐0.10] |
Comparison 15. SLIT versus placebo ‐ ragweed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.74, ‐0.14] |
| 2 Medication scores | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
Comparison 16. SLIT versus placebo ‐ Parietaria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 5 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.69, ‐0.04] |
| 2 Medication scores | 5 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.00, ‐0.24] |
Comparison 17. SLIT versus placebo ‐ tree.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 9 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.77, ‐0.06] |
| 2 Medication scores | 9 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.62, ‐0.13] |
Comparison 18. Allergen sensitivity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Skin reactivity after treatment | 6 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.26, 0.51] |
| 2 Nasal reactivity after treatment | 7 | 220 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.13, 0.78] |
Comparison 19. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adults | 2 | 397 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.73, ‐0.12] |
19.1. Analysis.
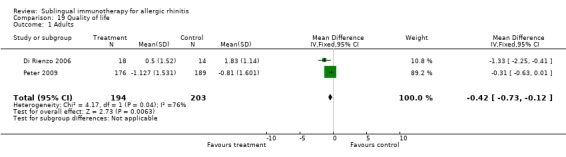
Comparison 19 Quality of life, Outcome 1 Adults.
Comparison 20. SLIT versus placebo ‐ tablets.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 11 | 1881 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.58, ‐0.38] |
| 2 Medication scores | 9 | 1578 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.46, ‐0.20] |
Comparison 21. SLIT versus placebo ‐ drops.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allergic rhinitis symptom scores | 35 | 2464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.42, ‐0.28] |
| 2 Medication scores | 27 | 1653 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amar 2009.
| Methods | Randomised DBPC trial | |
| Participants | Adults 58 participants randomised 53 participants analysed Timothy grass monotherapy 19; losses to f/u 0 Mixed grass 17; losses to f/u 3 Placebo 17; losses to f/u 2 |
|
| Interventions | 10 months SLIT Monotherapy: timothy grass 680 mcg/ml Phl p5 Multiple allergen group: timothy grass, maple, ash, juniper, American elm, cottonwood, kochia, ragweed, sagebrush, Russian thistle Daily maintenance dose 19 mcg Phl p5; cumulative monthly dose 571 mcg Phl p5 | |
| Outcomes | Symptom scores, medication scores, titrated skin prick tests, titrated nasal challenge, allergen‐specific IgE and IgG4 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | This was not stated in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Caramelised sugar was added to placebo and timothy grass monotherapy extract in order to mimic the colour of the active treatment groups. |
| Incomplete outcome data addressed? All outcomes | Low risk | Additional information was sought and obtained from the authors |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Andre 2003.
| Methods | Randomised DBPC trial | |
| Participants | Adults and children Age range 7 to 55 years 55 active (22 m); losses to f/u 12 55 placebo (23 m); losses to f/u 6 | |
| Interventions | 7.5 months Pre‐seasonal (start at March) SLIT Sublingual drops and tablets Standardised ragweed extract Dose: 100 IR/ml solution = 1 tablets = 160 mcg of Amb a 1 Maintenance dose 1, 2 or 3 tablets 3 times a week, depending on patient's tolerance Cumulative dose over maintenance phase: 1300 to 30,500 Max maintenance dose 480 mcg/day 3 times a week | |
| Outcomes | Symptom scores based on diary cards (nose, eyes) Medication scores Global assessment Skin prick test Allergen‐specific IgE and IgG4 | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | It was not stated |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Ariano 2001.
| Methods | Randomised DBPC trial | |
| Participants | Adults 10 active (5 m) 10 placebo (4 m) |
|
| Interventions | 8 months SLIT 250,000U RAST | |
| Outcomes | Diary scores Nasal provocation | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | This was not stated |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | All patients completed the study |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Bahceciler 2001.
| Methods | Randomised DBPC trial | |
| Participants | 8 active (4 m) 7 placebo (4 m) Children | |
| Interventions | 5 months SLIT 0.56 mg DP 0.98 mg DF | |
| Outcomes | Diary scores SPT Total IgE | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | This was not stated in the paper |
| Blinding? All outcomes | Unclear risk | Investigators and patients were blinded to treatment but how this was done was not stated |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants completed treatment |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Bowen 2004.
| Methods | Randomised DBPC trial | |
| Participants | Adults and children 43 active; loses to follow up 15 40 placebo; losses to follow up 11 |
|
| Interventions | 3.5 months SLIT, 2 weeks pre‐seasonal Sublingual drops Dose 116 mcg Amb 1/100 IR Max dose: 314 mcg Amb 1 | |
| Outcomes | Symptom scores (rhinitis and conjunctivitis) Number of days with asthma symptoms Medication score Overall evaluation Levels of ragweed‐specific IgE and IgG4 Adverse events description | |
| Notes | Jadad scale 5/5 15 patients asthma; 9 in treatment and 6 in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | This was not stated in paper |
| Blinding? All outcomes | Unclear risk | Investigators and patients were blinded to treatment but how this was done was not stated |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Bufe 2004.
| Methods | Randomised DBPC trial | |
| Participants | Children 83 active; losses to follow up 15 78 placebo; losses to follow up 14 |
|
| Interventions | 1 year Pre‐seasonal period: Sublingual drops Maintenance dose 2500 AU˜ 9.1 mcg Cumulative dose 2,625,000 AU (9.6 mg of Phl p 5) | |
| Outcomes | Clinical symptoms assessed by questionnaire in a structured interview by an independent person by phone at 6 different points (nose, eye and lung symptoms) Visual analogue scale recorded by patients Medication score (medication index) | |
| Notes | Jadad scale 5/5 Asthma symptoms 68; 35 in treatment and 33 in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block‐wise randomisation was performed |
| Allocation concealment? | Unclear risk | Not stated in paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Bufe 2009.
| Methods | Randomised DBPC trial | |
| Participants | Children Active: 126 (83 male); data analysed for 117 participants; withdrawn 12 Placebo: 127 (83 male); data analysed for 121 participants; withdrawn 7 |
|
| Interventions | 6 months 8 to 23 weeks pre‐seasonal (mean 17.1 weeks) Sublingual tablets grass pollen (Phleum pratense) Daily dose 75000 SQ; 15 mcg Phl p 5 | |
| Outcomes | Symptom score Medication score Specific IgE, IgG4 and IgE blocking factor Adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed by stratification according to trial centre |
| Allocation concealment? | Unclear risk | Not stated in paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Described the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. Stated whether attrition and exclusions were reported, the numbers in each intervention group and reasons for attrition/exclusions where reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Caffarelli 2000.
| Methods | Randomised DBPC trial | |
| Participants | Children 24 active (12 m); losses to follow up 0 20 placebo (13 m); losses to follow up 4 |
|
| Interventions | 3.5 months All pre‐seasonal Sublingual tablets ‐ allergoid (mixture 33% Holcus lanatus; 33% Phleum pratense, 33% Poa pratense) Max dose of allergen: 1000 AU Cumulative dose 37,250 AU | |
| Outcomes | Symptom scores on daily dairy cards (nasal, eye and bronchial symptoms) Medication score Adverse events description ECP | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Children were randomly assigned by a computer‐generated list to receive either grass‐pollen allergoid oral soluble tablets or placebo |
| Allocation concealment? | Unclear risk | Stated in a paper that neither the investigators nor the patients were aware of the treatment assignments, but no details were provided in the paper |
| Blinding? All outcomes | Low risk | Placebo tablets were indistinguishable from active treatment |
| Incomplete outcome data addressed? All outcomes | Low risk | Described the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. Attrition and exclusions were reported, the numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Calderon 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults Active 9 (6 male); withdrawn 0 Placebo 11 (6 male); withdrawn 0 |
|
| Interventions | 28 days; all pre‐seasonal
Sublingual tablet Grass pollen (Phleum pratense) 75000 SQ‐T Daily dose 15 mcg Phl p 5 |
|
| Outcomes | Adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Participants were randomly assigned by a computer‐generated list |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | All randomised patients completed the trial and the adverse events were reported |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Cao 2007.
| Methods | Randomised DBPC trial | |
| Participants | Children Active 139 Placebo 139 Age range 4 to 18 |
|
| Interventions | SLIT drops Dermatophagoides farinae 25 weeks | |
| Outcomes | Rhinitis symptom score Medication score for rhinitis Asthma symptom scores Lung function tests Skin sensitivity Mite‐specific IgE and IgG4 | |
| Notes | Full paper published in Chinese, but authors have provided data for total symptom and medication scores for rhinitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | Not stated in paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Casanovas 1994.
| Methods | Randomised DBPC trial | |
| Participants | Adults 9 active (3 m) 6 placebo (1 m) Minimum age 18 |
|
| Interventions | 2 months pre‐seasonal SLIT Dose n/s | |
| Outcomes | Diary scores SPT | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | Not stated in paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists. Small number of patients across each trial group. |
Clavel 1998.
| Methods | Randomised DBPC trial | |
| Participants | Adults 62 active 58 placebo Age range 27 +/‐ 10 years |
|
| Interventions | 6 months SLIT 5 mixed grasses (orchard grass, meadow grass, rye grass, sweet vernal grass, timothy grass) Cumulative dose 2.6 mg timothy grass Updosing period 25 days | |
| Outcomes | Daily dairy: symptom scores Medication scores Specific IgE and IgG4 Adverse events | |
| Notes | Jadad scale 3/5. Data insufficient for meta‐analysis. Included in the systematic review only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but it details were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Placebo consisted of glycerol‐saline diluent. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data for each main outcome completed and attrition and exclusions were reported; the numbers in each intervention group and reasons for attrition/exclusions were reported |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
D'Ambrosio 1999.
| Methods | Randomised DBPC trial | |
| Participants | Adults 14 active (7 m) 16 placebo (7 m) |
|
| Interventions | 9 months SLIT 12.77 mcg Parj 1 0.12 mcg/day solution of standardised extract | |
| Outcomes | Diary scores Ig | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Dahl 2006a.
| Methods | Randomised DBPC trial Multicentre | |
| Participants | Adults 74 active (53 m); dropped out 13 40 placebo (24 m); dropped out 8 |
|
| Interventions | 3 to 3.5 months SLIT Sublingual tablet Grass pollen 75000 SQ‐T daily Plp p5 15 mcg Pre‐seasonal 10 to 14 weeks | |
| Outcomes | Asthma symptom score (pre‐seasonal and in season) Asthma medication score (pre‐seasonal and in season) Rhinoconjunctivitis symptom scores and medication score; all reported through daily diaries Number of well days Adverse events reports | |
| Notes | Jadad scale 5/5 All patients had mild to moderate seasonal asthma and rhinoconjunctivitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation sequence was generated by the sponsoring company and blinded for the investigators |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Dahl 2006b.
| Methods | Randomised DBPC trial; multicentre | |
| Participants | Adults 316 active (179 m); losses to f/u 42 318 placebo (193 m); losses to f/u 46 |
|
| Interventions | 12 months SLIT Sublingual tablet Grass pollen 75 000 SQ‐T Phl p 5 15 mcg Daily | |
| Outcomes | Rhinoconjunctivitis symptom score Medication score Global assessment Adverse events | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation sequence was generated by the sponsoring company and blinded for the investigators |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Unblinded efficacy and safety assessments on subject level were available only for a biostatistician at the Contract Research Organization. All personnel associated with the study and participants remained blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
de Blay 2003.
| Methods | Randomised DBPC trial; multicentre study | |
| Participants | Adults and children 61 active 57 placebo Mean age 24.9 +/‐ 7.6 29 mild persistent |
|
| Interventions | 10 months SLIT (8 months pre‐season) Drops 3 grass pollen extract: Dactylis glomerata, Phleum pratense and Lolium perenne Average cumulative dose: 2.75 mg | |
| Outcomes | Clinical score Rhinitis score Conjunctivitis score Rescue medication score | |
| Notes | Jadad scale 3/5 ITT 104 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but not stated in the paper how was it done |
| Allocation concealment? | Unclear risk | Not stated in paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Di Rienzo 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults 19 active; losses to f/u 1 15 placebo; losses to f/u 1 |
|
| Interventions | 4 months SLIT Drops Juniperus ashei extract Maintenance 8 drops of 300 IR/ml Dose of major allergen 70 mcg/ml of Jun a1 at 100 IR conc | |
| Outcomes | Symptom score Medication score Quality of life Adverse events | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but the details about randomisation were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Placebo treatment consisted of identical vials containing only the diluent (a glycero‐saline solution) |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Didier 2007.
| Methods | Randomised DBPC trial Multicentre |
|
| Participants | Adults (age range 18 to 45 years) Active 155 (ITT 136) Active discontinued 22 Placebo 156 (ITT 148) Placebo discontinued 10 | |
| Interventions | Sublingual tablets mixed grass (orchard, meadow, perennial rye, sweet vernal, timothy grass) 4 months pre‐seasonal and co‐seasonal treatment Mean dosage 25 mcg/ml of the group 5 major allergens | |
| Outcomes | Symptom score Quality of life Grass pollen specific IgE | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list utilised |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Both investigators and participants were blinded to allocation. To maintain the blinding, patients took 2 tablets per day during the first 5 days of titration and 1 tablet per day from day 6 until the end of treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Drachenberg 2001.
| Methods | Randomised DBPC trial | |
| Participants | Children and adults 49 active (28 m): 21 birch, 28 grass/rye pollen 19 placebo (9 m) |
|
| Interventions | 6 months SLIT
SL drops
27 days of updosing phase maintenance dose: 16 drops of 100,000 SOU/ml for both allergens Cumulative dose of major allergen For grass pollen: Phl p 1: 1913.6 mcg For birch pollen: Bet v 1: 1535.23 mcg |
|
| Outcomes | Combined symptom/medication score Titrated SPTs Adverse events | |
| Notes | Jadad scale 5/5 Placebo group 19 Active treatment: Grass/rye 28 Birch 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation in blocks |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | The placebo consisted of the allergen‐free solution |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Dubakiene 2003.
| Methods | Randomised DBPC trial | |
| Participants | Adults 59 active; losses to f/u 12 60 placebo; losses to f/u 7 3 trees |
|
| Interventions | 4 months SLIT 15 days updosing Sublingual drops tree pollen (birch, hazel, alder) Maintenance dose 2500 AU/ day Bet v 1 13 mcg/day | |
| Outcomes | Rhinoconjunctivitis Clinical Index Score (combined from symptom and medication score) Adverse events | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation in blocks of 4 was performed (2 SLIT and 2 placebo) |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Investigators and patients were blinded to treatment but how this was done was not stated in the paper |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were analysed |
| Free of selective reporting? | Unclear risk | Not reported in the abstract |
| Free of other bias? | Unclear risk | This paper has not been published |
Durham 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults 141 active (84 m) 136 placebo (89 m) |
|
| Interventions | 18 weeks No updosing phase Sublingual tablets 75,000 SQ‐T 15 mcg Phl p 5 | |
| Outcomes | Rhinoconjunctivitis symptom score Medication score Post‐treatment specific IgE levels Post‐treatment specific IgG Number of well days | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation sequence was generated by the sponsoring company and blinded for the investigators (computer‐generated schedule) |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Tablet similar in taste, smell and appearance. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Feliziani 1995.
| Methods | Randomised DBPC trial | |
| Participants | Adults 18 active 16 placebo |
|
| Interventions | 3 months SLIT Dose n/s in mcg Maintenance dose 20 BU daily 3 times a day | |
| Outcomes | Diary scores Rhinoconjunctivitis symptom score Medication scores for rhinoconjunctivitis Asthmatic symptoms Overall symptoms Overall drug consumption | |
| Notes | Jadad scale 4/5 The data published in previous review reporting overall symptom and medication score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Treatments were coded according to a key unknown to the clinician and to the patient and they were assigned randomly to each patient |
| Allocation concealment? | Unclear risk | Not reported in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Grosclaude 2002.
| Methods | Randomised DBPC trial | |
| Participants | Children and adults 47 active: 15; 16; 16 (29 m) 15 placebo (11 m) |
|
| Interventions | 8 months SLIT 5 mixed grasses pollen tablets and drops 5 months pre‐seasonal 3 different updosing regimens 18 days updosing Maintenance 300 IR 3 times a day, drops Average cumulative dose 18,000 IR; 0.88 mg Lol p 1 | |
| Outcomes | Adverse events | |
| Notes | Jadad scale 3/5 The study included in the review but not the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but no details were provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | The placebo consisted of tablets (cellulose, magnesium stearate, lactose) and drops (glycerinated saline) that were indistinguishable from active treatment |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Guez 2000.
| Methods | Randomised DBPC trial | |
| Participants | Adults and children 36 active (14 m) 36 placebo (15 m) |
|
| Interventions | 24 months SLIT Sublingual drops 2.2 mg D.Pt 1.7 mg D.f |
|
| Outcomes | Diary scores SPT Ig | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but details were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | The placebo preparation was identical to active treatment in terms of composition, appearance, presentation, taste and colour, but did not contain allergens |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Hirsch 1997.
| Methods | Randomised DBPC trial | |
| Participants | Children 15 active (10 m) 15 placebo (10 m) |
|
| Interventions | 12 months SLIT cumulative dose 570 mcg Derp 1 | |
| Outcomes | Diary scores SPT | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Subjects were randomised according to a code provided by the manufacturer |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow‐up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Hordijk 1998.
| Methods | Randomised DBPC trial | |
| Participants | Adults 27 active (14 m) 30 placebo (13 m) |
|
| Interventions | SLIT drops mixed grasses 3 months pre‐seasonal Dose n/s | |
| Outcomes | Diary scores Ig | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Stratified randomisation |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. The placebo consisted of the non‐active ingredients. |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Ibanez 2007.
| Methods | Randomised DBPC trial | |
| Participants | Children Active 45 (28 male); withdrawn 2 Placebo 15 (12 male); withdrawn 0 |
|
| Interventions | 28 days (pre‐seasonal) Grass pollen (Phleum pratense) Sublingual tablet 75,000 SQ‐T Daily dose 15 mcg Phl p 5 | |
| Outcomes | Adverse events | |
| Notes | This paper involves 2 studies performed with the identical study protocols | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but no details were provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Unclear risk | A placebo tablet similar in taste, smell and appearance was used |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Unclear risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | 2 studies were pooled |
Ippoliti 2003.
| Methods | Randomised DBPC trial | |
| Participants | Children Active 47 (28 m); losses to f/u 0 Placebo 39 (22 m); losses to f/u 0 |
|
| Interventions | 6 months Sublingual drops Dose: 2.4 mcg/ml of Der p 1 and 1.2 mcg/ml of Der p2 per week | |
| Outcomes | Symptom scores from diary cards Expression of CD40 on B cells Serum IL‐13, ECP, prolactin Adverse events description | |
| Notes | Jadad scale 3/5 33 children with rhinoconjunctivitis 18 in treatment 15 in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but the full details were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Placebo consisted of the same glycerine/phenol diluent used in active group |
| Incomplete outcome data addressed? All outcomes | Unclear risk | It is not clear if any patients lost during follow up occurred |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Khinchi 2004.
| Methods | Randomised, double‐blind, double‐dummy, placebo‐controlled trial | |
| Participants | Active SLIT 23; losses to f/u 9 Active SCIT 24; losses to f/u 5 Placebo 19; losses to f/u 9 | |
| Interventions | 2 years Bet v 1 SLIT drops Max dose: SLIT 49.2 mcg every second day SCIT 3.38 mcg monthly | |
| Outcomes | Symptom scores Medication score Adverse events | |
| Notes | Jadad scale 4/5 3 groups of patients: SLIT, SCIT and placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed by minimisation based on disease severity during the baseline season, gender and age |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | All study personal and participants were blinded to treatment assignment for the 2‐year duration of treatment in the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Kleine‐Tebbe 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults Active 63; no losses to f/u Placebo 21; no losses to f/u |
|
| Interventions | 4 weeks SLIT tablets Grass pollen 25,000 SQ‐T, 75,000 SQ‐T, 150,000 SQ‐T, 300,000 SQ‐T, 500,000 SQ‐T, 750,000 SQ‐T, 1,000,000 SQ‐T and placebo 100,000 SQ‐T correspond to 20 mcg Phl p 5 | |
| Outcomes | Adverse events | |
| Notes | Jadad scale 5/5 Study included in review, but not meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list was provided by a manufacturer |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | All randomised subjects received intervention and all completed the trial |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
La Rosa 1999.
| Methods | Randomised DBPC trial | |
| Participants | Children 20 active (13 m) 21 placebo (12 m) Losses to f/u 4 |
|
| Interventions | 24 months SLIT 52.5 mg Parj1 | |
| Outcomes | Rhinitis symptom scores Medication score Ig SPT Conjunctival | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Lima 2002.
| Methods | Randomised DBPC trial | |
| Participants | Adults 28 active 28 placebo |
|
| Interventions | 12 to 18 months SLIT 900 mcg Phlp5 per month | |
| Outcomes | Diary scores Ig | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation to active or placebo treatment was performed by the manufacturer of the grass pollen vaccine using a system of computer‐generated random numbers in blocks of 12 |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | The treatment schedule and assessments were performed double‐blind, with treatment allocations kept in sealed envelopes by the principal investigator |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Malling 2005.
| Methods | Randomised DBPC trial | |
| Participants | Adults 5 updose groups: 2500, 25,000, 75,000, 125,000, 375,000 SQ‐T |
|
| Interventions | SLIT tablets Maximum dose 375000 SQ‐T | |
| Outcomes | Phleum pratense specific: IgE, IgA and IgG Adverse events description ‐ local and systemic | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but no details were provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Data completed for each main outcome, including attrition and exclusions from the analysis. Attrition and exclusions were reported, the numbers in each intervention group and reasons for attrition/exclusions where reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Marcucci 2005.
| Methods | Randomised DBPC trial | |
| Participants | Children Active 13 (6 m); losses to f/u 0 Placebo 11 (10m); losses to f/u 0 |
|
| Interventions | 12 months SLIT Sublingual drops Maintenance dose: 0.8 mcg of mite allergen group 1 and 0.4 mcg of mite allergen group 2 | |
| Outcomes | Symptom scores for rhinitis and asthma Medication scores Nasal tryptase Nasal IgE Specific nasal challenge test Nasal ECP Sputum ECP Sputum tryptase Serum IgE | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but no details about randomisation provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | The placebo preparation was identical to active treatment in terms of composition, appearance, presentation, taste and colour, but did not contain allergens |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Nelson 1993.
| Methods | Randomised DBPC trial | |
| Participants | Adults 20 active (7 m) 21 placebo (6 m) |
|
| Interventions | 65 days SLIT drops 450‐900 Feld1 units | |
| Outcomes | Cat room scores Ig Titrated SPT | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but the details were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Those randomised to the placebo group received histamine phosphate (1 mg/ml histamine base in 50% glycerine with caramelised sugar for colour). |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported; the numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Ott 2009.
| Methods | Randomised DBPC trial | |
| Participants | Adults Total mean age 33.3+/‐ 10.4 yrs (7.9 to 64.7) Active total 142 Active full analysis set (FAS) 123 Active ITT 99 Active per protocol 58 Active losses to f/u 43 Placebo total 67 Placebo full analysis set (FAS) 60 Placebo ITT 46 Placebo per protocol 33 Placebo losses to f/u 21 |
|
| Interventions | 3 consecutive grass pollen seasons SLIT grass pollen (mixture of 5 grasses; orchard, meadow, perennial rye, sweet vernal and timothy grass) Sublingual drops Rush updosing of 30, 90, 150 and 300 IR at 20 minutes intervals, followed by a daily intake of 300 IR for the duration of pollen season 300 IR/ml = 21 mcg/ ml og Phl p 5 major allergen | |
| Outcomes | Symptom score Medication score Combined symptom/medication score Specific IgE and IgG4 Compliance Safety | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were stated |
| Blinding? All outcomes | Low risk | Investigators and patients were blinded to treatment |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Pajno 2003.
| Methods | Randomised DBPC trial | |
| Participants | Children 15 active (7 m); losses to f/u 1 15 placebo (6 m); losses to f/u 2 |
|
| Interventions | 13 months SLIT Parietaria pollen Sublingual drops Maintenance dose 5 drops of 10 BU/ ml sol Cumulative dose of major allergen 20.3 mcg Par j 1 ˜ 0.15 mcg/day | |
| Outcomes | Total symptom score Chest symptom score Nose symptom score Medication score Early and late‐phase skin reaction | |
| Notes | Active group: SLIT+ fluticasone
Placebo group: placebo + fluticasone
Control group: asthma medications as needed only Jadad scale 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomisation to the active, placebo or control group was obtained by means of a computer‐generated key‐code |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment. The placebo was indistinguishable from the active treatment in appearance, colour and taste. |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | A second drug other than SLIT was considered in the study design |
Palma Carlos 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults Active 17; loss to follow up 4 Placebo 16; loss to follow up 9 |
|
| Interventions | 24 months sublingual tablets Allergoid mixture (33% Holcus lanatus, 33% Phleum pratense, 33% Poa pratensis) | |
| Outcomes | Symptom score Medication score Nasal reactivity (allergen nasal challenge) Adverse events | |
| Notes | Major allergen protein dose not possible to measure as this is an allergoid | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were stated in the paper |
| Blinding? All outcomes | Unclear risk | Investigators and patients were blinded to treatment but how this was done was not stated |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | 39.4% of participants withdrew during follow up |
Panzner 2008.
| Methods | Randomised DBPC trial | |
| Participants | Adults and children Total mean age 19.5 Age range 7 to 50 years Active sublingual 20 (11 m) Placebo sublingual 15 (9 m) |
|
| Interventions | Sublingual and supralingual drops 6 grasses: oat grass (Arrhenatherum elatius), orchard grass (Dactylis glomerata), fescue (Festuca sp.), rye grass (Lolium sp.), timothy grass (Phleum pratense) and rye (Secale cereale) 1 year Updosing scheme from 1 to 10 drops. Maximal dose 10 drops of 10,000 JSK/ml (1.265 mcg of Lol p1) |
|
| Outcomes | Symptom scores (nasal, ocular and bronchial) Medication scores Skin prick tests Grass pollen specific IgE and IgG | |
| Notes | Mixed grasses drops were delivered sublingually or supralingually. Only sublingual immunotherapy data were used for the purpose of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Central randomisation. The randomisation key was generated by the GraphPad Software. |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Passalacqua 1998.
| Methods | Randomised DBPC trial | |
| Participants | Adults 10 active (3 m) 10 placebo (4 m) |
|
| Interventions | 24 months SLIT Tablets Dose n/s | |
| Outcomes | Diary scores | |
| Notes | Jadad scale 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random codes were used |
| Allocation concealment? | Unclear risk | It was not stated in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Passalacqua 1999.
| Methods | Randomised DBPC trial | |
| Participants | Adults 15 active (10 m) 15 placebo (3 m) |
|
| Interventions | 6 months SLIT 16 mcg Parj1 | |
| Outcomes | Diary scores Nasal challenge | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | It was not stated in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Passalacqua 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults 34 active (11 m); losses to f/u 6 34 placebo (11 m); losses to f/u 6 |
|
| Interventions | 25 months SLIT allergoid Soluble tablets 1000 AU | |
| Outcomes | Symptom score Medication score Quality of life questionnaire Adverse events | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A computer‐generated list was used |
| Allocation concealment? | Unclear risk | Not stated in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Peter 2009.
| Methods | Randomised DBPC trial | |
| Participants | Adults Active 176; lost to f/u unknown Placebo 189; lost to f/u unknown |
|
| Interventions | SLIT drops Grass pollen mixture (Lolium perenne, Phleum pratense and Poa pratensis) 16 weeks pre‐seasonal | |
| Outcomes | Clinical index score Symptom score Medication score Specific IgE for grass pollen mixture Specific IgE for Phl p 1 and Phl p 5 Specific IgG and IgG4 for grass pollen mixture Specific IgG and IgG4 for Phl p 1 and Phl p 5 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details were provided in the paper |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Pfaar 2008.
| Methods | Randomised DBPC trial | |
| Participants | Adults Active 49; losses to f/u 7 Placebo 55; losses to f/u 7 |
|
| Interventions | Sublingual immunotherapy with 6 grasses pollen mixture (Holcus lanatus, Dactylis glomerata, Lolium perenne, Phleum pratense, Poa pratensis and Festuca elatior) Sublingual drops Dose escalation was performed at the day 1 of the treatment, with doubling of a dose every 60 minutes. Initial dose, 1 drop, 2 drops, 4 drops (100%), corresponding to 10, 20 and 40 mcg of the group 5 grass allergen The group 5 allergen content of the maintenance dose was 40 mcg (ELISA) and 20 mcg (CREATE) Once daily | |
| Outcomes | Symptom score Medication score Specific IgE Specific IgG1 and IgG4 Compliance Adverse events | |
| Notes | A block randomisation was performed, and there was a stratification procedure with respect to asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A block randomisation was performed, and there was a stratification procedure with respect to asthma |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were completed for each main outcome. Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Pradalier 1999.
| Methods | Randomised DBPC trial | |
| Participants | Adults 63 active (29 m) 63 placebo (36 m) |
|
| Interventions | 5 months pre‐seasonal SLIT drops and tablets Cumulative dose 0.935 mg Phlp5 Daily maintenance dose ˜ 8.5 mcg | |
| Outcomes | Diary scores SPT Ig | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Rolinck‐Werninghaus 2004.
| Methods | Randomised DBPC trial | |
| Participants | Children Age range 3 to 14 Active 49 (30 m); losses to f/u 11 Placebo 48 (35 m); losses to f/u 11 |
|
| Interventions | 32 months SLIT sublingual drops Mixed grasses (Dactylis glomerata, Festuca pratensis, Lolium perenne, Phleum pratense and Poa pratensis) Maintenance dose: 5 drops of 1000 STU/ml solution 3 times a week Cumulative dose 188 mcg of major allergens The potency in STU; 1000 STU equivalent to 25 BU (2.5 mcg of major allergens) Study started in January 1999 | |
| Outcomes | Symptom scores (nasal, eyes, lung) Medication scores Total IgE, specific IgE and IgG4 Skin prick test Conjunctival provocation test Nasal provocation test Lung function test Exhaled nitric oxide concentrations SCORAD Adverse events descriptions | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was conducted for age and history of asthma in consecutive order at inclusion |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Rőder 2007.
| Methods | Randomised DBPC trial | |
| Participants | Children Age range 6 to 18 Active 108 (91 ITT); losses to follow up 26 Placebo 96 (77 ITT); losses to follow up 24 |
|
| Interventions | Sublingual drops ‐ mixed grasses 5 grasses extract (Lolium perenne, Phleum pratense, Dactylis glomeratein, Anthoxatum odoratum, Holcus lanatus) Treatment duration 2 years. Maintenance dose 21 mcg of Lol p 5 twice a week. | |
| Outcomes | Symptom score Percentage of symptom‐free days Percentage of medication‐free days Quality of life evaluation Safety | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A computer‐generated randomisation list stratifying for symptom score and participating general practice was utilised |
| Allocation concealment? | Low risk | A pharmacist allocated medication in accordance with a computer‐generated randomisation list stratifying for symptom score and participating general practice. Participants, parents, investigators and caregivers were unaware of the group assignment and could not make a distinction between verum and placebo treatment. |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Sabbah 1994.
| Methods | Randomised DBPC trial | |
| Participants | Children and adults Age range 13 to 51 Active 29 Placebo 29 |
|
| Interventions | Sublingual drops ‐ 5 grass pollen extracts or placebo The updosing phase duration was 40 days. It started with 1 drop of 1 IR/ml up to 10 drops on day 10, while on days 11 to 20, 1 to 10 drops of 10 IR/ml were given. Finally, from days 20 to 40, the patients took 1 to 20 drops of 100 IR/ml. Once this dose of 20 drops was reached, maintenance treatment was continued every day for 30 days and then every 2 days for the following 30 days. The cumulative dosage received by the patients was therefore 4500 IR. |
|
| Outcomes | Symptom scores Medication scores Safety | |
| Notes | Study included in review, but not meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but no details were provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details were provided in the paper |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Sanchez 2001.
| Methods | Randomised DBPC trial | |
| Participants | Adults 40 participants Mean age: 24.5 years old Male 16 and female 24 Active 20 patients Placebo 20 patients |
|
| Interventions | SLIT: active therapy contained an aqueous extract of the major cat allergen Fel d 1 (CBF‐Leti). Five vials were provided containing: 0.00032, 0.0016, 0.008, 0.04, 0.02 and 1 HEP equivalent/mL. Saline solution, glycerine 50% and phenol 0.4% was used as vehicle. SLIT was provided daily and kept under the tongue during 3 minutes. After 1 year of treatment the cumulative dose was 3.6 μg of Fel d 1. Placebo: saline solution, glycerine 50% and phenol 0.4% |
|
| Outcomes | Symptom scores Medication scores Skin prick tests Nasal provocation tests IgE and IgG4 Eosinophil cationic protein | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details were provided in the paper |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Smith 2004.
| Methods | Randomised DBPC trial | |
| Participants | Adults 44 active treatment for 2 years (21 m) 45 active treatment then placebo (22 m) 45 placebo in both years (27 m) |
|
| Interventions | 2 years SLIT from February until 31 July each year Sublingual drops and tablets for maintenance Mixed grasses (orchard, meadow, rye, sweet vernal and timothy grass) 100 IR contained 24 mcg Lolp1 and 14 mcg Dacg5 Cumulative annual dose 26.100 IR 6264 mcg Lolp1 3654 mcg Dacg5 | |
| Outcomes | Symptom scores; daily diary Medication scores; dairy cards Conjunctival provocation threshold Skin tests Specific IgE and IgG4 Side effects reported by patients | |
| Notes | Jadad scale 5/5 Participants were randomised into 3 groups Active treatment for 2 years Active treatment for a year, then placebo and placebo for 2 years Treatment every year from February until 31 July | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | Authors stated that groups were checked for homogeneity regarding demographics, symptom scores and relevant clinical parameters |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Placebo tablets and drops were physically identical to active medication. |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Tari 1990.
| Methods | Randomised DBPC trial | |
| Participants | Adults 34 active 32 placebo |
|
| Interventions | 18 months SLIT 2340 drops of 5BU/ml mcg dose n/s | |
| Outcomes | Diary scores Ig | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, but further details were not provided |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | The placebo contained only the phosphate buffered physiological solution |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Tonnel 2004.
| Methods | Randomised DBPC trial | |
| Participants | Adults and children 15 active (8 m); losses to f/u 5 17 placebo (10 m); losses to f/u 9 |
|
| Interventions | 24 months SLIT Der p 1 and Der f 1 50/50 drops and tablets Progression phase for 2 weeks Max dose 100 IR tablet Mean cumulative dose 47500 IR 1.28 mg Der p1 1.47 mg Der f 1 | |
| Outcomes | Symptom scores Medication scores Skin prick tests Nasal provocation test Levels of specific IgE and IgG4 Adverse events reports and descriptions | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random computer‐generated code was used |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Blinding was done using solutions and tablets of identical appearance |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition and exclusions were reported. The numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Troise 1995.
| Methods | Randomised DBPC trial | |
| Participants | Adults 15 active (6 m) 16 placebo (6 m) |
|
| Interventions | 10 months SLIT Parietaria extract 6.3 mcg Parj1 | |
| Outcomes | Diary scores | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | It was not specified in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods. The specified outcomes in the methodology were reported in the results section. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Valovirta 2006.
| Methods | Randomised DBPC trial | |
| Participants | Children 35 active Dose group one 33 patients (19 m); losses to f/u 1 Dose group 2: 32 patients (13 m); losses to f/u 7 33 placebo (18 m); losses to f/u 6 |
|
| Interventions | 18 months SLIT Group 1: accumulated weekly dose 3.6 mcg Bet v 1/ Aln g 1/ Cor a 1 Group 2: accumulated weekly dose 30 mcg of Bet v 1/ Aln g 1/ Cor a 1 5 weeks updosing schedule | |
| Outcomes | Symptom score Medication score | |
| Notes | Jadad scale 5/5 2 treatment groups Mixed tree pollen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | The study was conducted under double‐blind conditions. There was no difference in colour and viscosity between the study drug and placebo. All blinding procedures were performed by the Quality Assurance Department at ALK‐Abello A/S. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data for each main outcome completed, including attrition and exclusions from the analysis. Attrition and exclusions were reported; the numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Vervloet 2006.
| Methods | Randomised DBPC trial | |
| Participants | Adults 38 active (22 m); losses to f/u 2 38 placebo (17 m); losses to f/u 4 |
|
| Interventions | 4 months SLIT Sublingual drops Ultra rush treatment Maintenance dose 300 IR daily Dose of major allergen 228 mcg Jun a 1 | |
| Outcomes | Symptom scores Medication scores Adverse events reports | |
| Notes | Jadad scale 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation in blocks was used |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Voltolini 2001.
| Methods | Randomised DBPC trial | |
| Participants | Adults 15 active (7 m) 15 placebo (4 m) |
|
| Interventions | Rush pre‐seasonal and co‐seasonal maintenance
445 mcg Bet v 1 Tree pollen extract |
|
| Outcomes | Diary scores Ig SPT | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Low risk | No details were provided in the paper |
| Blinding? All outcomes | Unclear risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Placebo was prepared as saline solution in vials with exactly the same appearance, colour and taste, but without allergens, in order to guarantee the double‐blind design of the trial. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Unclear risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Vourdas 1998.
| Methods | Randomised DBPC trial | |
| Participants | Children 34 active (25 m) 32 placebo (24 m) |
|
| Interventions | 6 months SLIT per year for 2 years
4.05 mg Olee 1 Olive pollen extract solution |
|
| Outcomes | Diary scores SPT | |
| Notes | Jadad scale 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised but how this was done was not stated |
| Allocation concealment? | Unclear risk | No details were provided in the paper |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. The placebo was a glycerinated phenolated saline solution with an appearance similar to that of the active agent. |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Wahn 2009.
| Methods | Randomised DBPC trial | |
| Participants | Children Active 131 (65.6% male); withdrawn 8 Placebo 135 (63.0% male); withdrawn 8 |
|
| Interventions | < 6 months Sublingual tablets Mixed grasses (Dactylis glomerata, Poa pratensis, Lolium perenne, Anthoxanthum odoratum, Phleum pratense) Daily dose 300 IR; 20 mcg of group 5 major allergens | |
| Outcomes | Symptom score Medication score Timothy grass specific IgE Grass pollen specific IgG4 Adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomisation list was stratified by study centre and organised in blocks |
| Allocation concealment? | Low risk | Central allocation |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study. Excipients used in both active and placebo tablets include lactose, sodium stearate and sodium croscarmellose. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data for each main outcome completed, including attrition and exclusions from the analysis. Attrition and exclusions were reported; the numbers in each intervention group and reasons for attrition/exclusions were reported. |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Low risk | No other sources of bias were detected or suspected |
Wessner 2001.
| Methods | Randomised DBPC trial | |
| Participants | 22 active; losses to f/u 8 23 placebo; losses to f/u 5 | |
| Interventions | 12 months SLIT drops maintenance dose 2000 AU per day 8 mcg major allergen per day | |
| Outcomes | Symptom scores Daily cards Nasal provocation tests | |
| Notes | Jadad scale 5/5 The study was performed for 2 years Only first year was performed as a DBPC trial; in the second year all participants received verum therapy for another year Data analysed only for the first year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation blocks of 4 containing 2 of each treatment |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Unclear risk | No details were provided |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | The study has not been published after its presentation at a meeting in 2001 |
Wutrich 2003.
| Methods | Randomised DBPC trial | |
| Participants | Children
Age range 4 to 11 years Active 14 (7 m); losses to f/u 2 Placebo 14 (9 m); losses to f/u 0 |
|
| Interventions | 24 months SLIT Sublingual drops Mixed grasses (Dactylis glomerata, Festuca pratensis, Lolium perenne, Phleum pratense, Poa pratensis) Build‐up phase 30 days ‐ cumulative dose of major allergen was 1.88 mcg Maintenance phase ‐ cumulative dose of major allergen 6 mcg The first year the cumulative dose was 67.88 mcg and 139.88 mcg of major allergen in total | |
| Outcomes | Conjunctival provocation test Skin prick test Symptom scores ‐ diary cards Medication scores ‐ diary cards | |
| Notes | Jadad scale 5/5 4 children were not included in data analysis because of their incomplete diary cards | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised |
| Allocation concealment? | Unclear risk | No details were provided |
| Blinding? All outcomes | Low risk | Investigators and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with patients followed to designated follow‐up periods |
| Free of selective reporting? | Low risk | The specified outcomes in the methodology were reported in the results section |
| Free of other bias? | Unclear risk | Small number of patients across each trial group |
DBPC = double‐blind, placebo‐controlled; f/u= follow up; mcg = microgram; ITT = intention‐to‐treat; m = male; n/s = not specified; SCIT = subcutaneous (injection); immunotherapy; SL = sublingual; SLIT = sublingual immunotherapy; SPT = skin prick test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernardis 1996 | Not randomised |
| Black 2002 | Insufficient data available for the review |
| Corthay 1996 | Insufficient data available for the review |
| D'Ambrosio 1996 | Open study design; not a randomised DBPC trial |
| Donato 1997 | Prospective study; not a randomised DBPC trial |
| Feliziani 1993 | Not randomised |
| Gammeri 2004 | Not placebo‐controlled |
| Gozalo 1997 | Not randomised, controlled or blinded |
| Hansen 2004 | Other outcomes investigated (food allergy) |
| Horak 1998 | No symptom data. Not seasonal exposure. |
| Inal 2009 | Insufficient data available for the review |
| Karakoc 2003 | Not randomised, not blinded and not placebo‐controlled |
| Mitsch 1996 | Open study |
| Mungan 1999 | Not a randomised DBPC trial |
| Nanda 2004 | SCIT trial |
| Okubo 2008 | Additional data not available |
| Quirino 1996 | Not truly randomised |
| Radjenovic 2004 | Not DBPC trial; prospective parallel‐group study |
| Russello 2004 | Not blinded |
| Sabbah 1993 | Duplicate study (same as Sabbah 1994) |
| Tonnel 2002 | Data published in Tonnel 2004 |
| Troise 2009 | Insufficient data available for the review |
| Van Niekerk 1987 | Not sublingual immunotherapy |
| Yuksel 1999 | This study does not investigate outcomes evaluated by this review |
DBPC = double‐blind, placebo‐controlled; SCIT = subcutaneous immunotherapy
Characteristics of ongoing studies [ordered by study ID]
Ingels 2002.
| Trial name or title | A placebo controlled, double‐blind, randomized study to assess efficacy of sublingual immunotherapy in patients with grass pollen allergy through assessment of its immunological effects on the mucosal tissue of the nose |
| Methods | Randomised, placebo‐controlled, double‐blind |
| Participants | Expected 38 |
| Interventions | Sublingual immunotherapy with Oralgen Further procedures: Nasal biopsy Nasal washing PNIF |
| Outcomes | Decrease of IgE specific cells and Th2 mediator release Increase in Th 1 mediator release Rescue medication Determining the effects on decongestion Assessment of treatment compliance |
| Starting date | Study start 2002 |
| Contact information | — |
| Notes | — |
O'Hehir 2005.
| Trial name or title | A trial of immunological outcomes of sublingual immunotherapy for house dust mite (D. pteronyssinus) |
| Methods | Randomised, placebo‐controlled, double‐blind |
| Participants | Expected enrolment 30 |
| Interventions | Sublingual immunotherapy for house dust mite |
| Outcomes | Immunoregulatory cytokine production and T cell phenotype and function Symptom diary Medication use Visual analogue score Disease‐specific rhinoconjunctivitis Quality of life questionnaire |
| Starting date | November 2005 |
| Contact information | Allesandra Sandrini MD, PhD a.sandrini@alfred.org.au +61 3 9276 2000 ext 2350 |
| Notes | — |
Differences between protocol and review
Evaluation of safety has been noted as a primary objective of the review.
The I² statistic has now been used to quantify heterogeneity.
The Cochrane 'Risk of bias' tool has now been used to assess the methodological quality of the included trials.
Contributions of authors
SUZANA RADULOVIC: Lead review author, searching for trials, quality assessment of trials, design of data extraction form, data extraction, data analysis.
MOISES CALDERON: Review author, searching for trials, quality assessment of trials, data analysis, input at all other stages of review.
DUNCAN WILSON: Review author, protocol development, quality assessment of trials, design of data extraction form, data extraction, data analysis.
STEPHEN DURHAM: Protocol development, input at all other stages of review.
Declarations of interest
The lead review author, Dr Suzana Radulovic, has received financial support from the ITN (Immune Tolerance Network) as an employee of the Paediatric Allergy Research Department at King's College London, UK.
The Department of Upper Respiratory Medicine, National Heart & Lung Institute, London, UK, headed by Professor Durham, has received financial support from ALK Abello, Horsholm, Denmark ‐ manufacturers of allergen extracts. Stephen Durham has received consultancy and lecture fees and research grants from Alk Abello, a manufacturer of allergy vaccines, via Imperial College.
There are no other conflicts of interest to be declared.
Edited (no change to conclusions)
References
References to studies included in this review
Amar 2009 {published and unpublished data}
- Amar SM, Harbeck RJ, Sills M, Silveira LJ, O'Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in multiallergen extract. Journal of Allergy and Clinical Immunology 2009;124(1):150‐6. [DOI] [PubMed] [Google Scholar]
Andre 2003 {published data only}
- Andre C, Perrin‐ Fayolle M, Grosclaude M, Couturier P, Basset D, Cornillon J, et al. A double‐ blind placebo‐ controlled evaluation of sublingual immunotherapy with standardized ragweed extract in patients with seasonal rhinitis. International Archives of Allergy and Immunology 2003;131:111‐18. [DOI] [PubMed] [Google Scholar]
Ariano 2001 {published data only}
- Ariano R, Spadolini I, Panzani RC. Efficacy of sublingual immunotherapy in Cupressaceae allergy using an extract of Cupressus arizonica. A double blind study. Allergologia et Immunopathologia 2001;29:238‐44. [DOI] [PubMed] [Google Scholar]
Bahceciler 2001 {published and unpublished data}
- Bahceciler NN, Isik U, Barlan IB, Basaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double blind, placebo‐controlled study. Pediatric Pulmonology 2001;32:49‐55. [DOI] [PubMed] [Google Scholar]
Bowen 2004 {published and unpublished data}
- Bowen T, Greenbaum J, Charbonneu Y, Hebert J, Filderman R, Sussman G, et al. Canadian trial of sublingual swallow immunotherapy for ragweed rhinoconjuctivitis. Annals of Allergy, Asthma & Immunology 2004;93:425‐30. [DOI] [PubMed] [Google Scholar]
Bufe 2004 {published and unpublished data}
- Bufe A, Ziegler‐Kirbach E, Stoeckman E, Heidemann P, Gehlhar K, Holland‐Letz T, et al. Efficacy of sublingual swallow immunotherapy in children with severe grass pollen allergic symptoms, a double‐blind, placebo‐controlled study. Allergy 2004;59:498‐504. [DOI] [PubMed] [Google Scholar]
- Bufe A, Ziegler‐Kirbach E, Stoekmann E, Heidemann P, Braun W. Specific sublingual immunotherapy in children: results of double‐blind, placebo controlled clinical trial [Abstract]. XX Congress of the European Academy of Allergology and Clinical Immunology. 2001:Abstract No 829.
Bufe 2009 {published data only (unpublished sought but not used)}
- Bufe A, Eberle P, Franke‐Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ‐standardized grass allergen tablet for sublingual immunotherapy. Journal of Allergy and Clinical Immunology 2009;123(1):167‐73. [DOI] [PubMed] [Google Scholar]
Caffarelli 2000 {published and unpublished data}
- Caffarelli C, Sensi LG, Marcucci F, Cavagni G. Preseasonal local allergoid immunotherapy to grass pollen in children : a double‐blind, placebo‐controlled, randomized trial. Allergy 2000;55:1142‐7. [DOI] [PubMed] [Google Scholar]
Calderon 2006 {published data only}
- Calderon MA, Rak S, Durham SR. Grass pollen tablets for sublingual immunotherapy in seasonal allergic rhinitis [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S65. [Google Scholar]
- Calderón M, Essendrop M. Specific immunotherapy with high dose SQ standardized grass allergen tablets was safe and well tolerated. Journal of Investigational Allergology and Clinical Immunology 2006;16(6):338‐44. [PubMed] [Google Scholar]
Cao 2007 {published and unpublished data}
- Cao LF, Lu Q, Gu HL, Chen YP, Zhang Y, Lu M, et al. Clinical evaluation for sublingual immunotherapy of allergic asthma and atopic rhinitis with Dermatophagoides Farinae Drops. Zhonghua Er Ke Za Zhi. 2007;45(10):736‐41. [PubMed] [Google Scholar]
Casanovas 1994 {published and unpublished data}
- Casanovas M, Guerra F, Moreno C, Miguel R, Maranon F, Daza JC. Double‐blind, placebo‐controlled clinical trial of preseasonal treatment with allergenic extracts of Olea europaea pollen administered sublingually. Journal of Investigational Allergology & Clinical Immunology 1994;4(6):305‐14. [PubMed] [Google Scholar]
Clavel 1998 {published data only}
- Clavel R, Bousquet J, Andre C. Clinical efficacy of sublingual‐swallow immunotherapy: a double‐blind, placebo‐controlled trial of a standardized five‐grass‐pollen extract in rhinitis. Allergy 1998;53:493‐8. [DOI] [PubMed] [Google Scholar]
D'Ambrosio 1999 {published and unpublished data}
- Purello‐D'Ambrosio F. Sublingual immunotherapy: a double‐blind, placebo‐controlled trial with Parietaria judaica extract standardised in mass units in patients with rhinoconjunctivitis, asthma, or both. Allergy 1999;54:968‐73. [DOI] [PubMed] [Google Scholar]
Dahl 2006a {published data only}
- Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy 2006;61:185‐90. [DOI] [PubMed] [Google Scholar]
Dahl 2006b {published data only}
- Dahl R, Kapp A, Colombo G, Monchy JGR, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. Journal of Allergy and Clinical Immunology 2006;118:434‐40. [DOI] [PubMed] [Google Scholar]
- Dahl R, Kapp A, Ribel M, Durham SR. Sublingual immunotherapy with SQ standardized grass allergen tablets [Abstract]. Annual Meeting of the AAAACI. 2006.
- Durham SR, Riis B. Grass allergen tablet immunotherapy relieves individual seasonal eye and nasal symptoms, including nasal blockage. Allergy 2007;62(8):954‐7. [DOI] [PubMed] [Google Scholar]
- Emminger W, Dahl R, Kapp A, et al. The long‐term efficacy and safety of a grass allergen tablet for sublingual immunotherapy: findings from a 3‐year clinical trial. Journal of Allergy and Clinical Immunology 2008;121(2):S75. [DOI] [PubMed] [Google Scholar]
de Blay 2003 {published and unpublished data}
- Blay F, Purohit A, Kanny G, Sellin J, Wessel F, Leynadier F, et al. Sublingual‐swallow immunotherapy with standardized 3‐grass pollen extract: a double‐blind placebo controlled study [Abstract]. XXII Congress of the European Academy of Allergology and Clinical Immunology (EAACI). 2003:780.
Di Rienzo 2006 {published and unpublished data}
- Rienzo V, Pucci S, D'Alo S, Cara G, Incorvaia C, Frati F, et al. Effects of high‐dose sublingual immunotherapy on quality of life in patients with cypress‐induced rhinitis: a placebo‐controlled study. Clinical and Experimental Allergy Reviews 2006;6:67‐70. [Google Scholar]
Didier 2007 {published data only}
- Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, et al. Optimal dose, efficacy, and safety of once‐daily sublingual immunotherapy with a 5‐grass pollen tablet for seasonal allergic rhinitis. Journal of Allergy and Clinical Immunology 2007;120(6):1338‐45. [DOI] [PubMed] [Google Scholar]
Drachenberg 2001 {published data only}
- Drachenberg KJ, Pfeiffer P, Urban E. Sublingual immunotherapy ‐ results from a multi‐centre, randomised, double‐blind, placebo‐controlled study with a standardised birch and grass/rye pollen extract. Allergologie 2001;24:525‐34. [Google Scholar]
Dubakiene 2003 {published and unpublished data}
- Dubakiene R, Kleinjans HAJ. Effectivness of specific sublingual IT (SLIT) with tree pollen. A placebo controlled study in 119 patients [Abstract]. XXII Congress of European Academy of Allergology and Clinical Immunology. 2003.
Durham 2006 {published and unpublished data}
- Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once‐daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. Journal of Allergy and Clinical Immunology 2006;117(4):802‐9. [DOI] [PubMed] [Google Scholar]
Feliziani 1995 {published and unpublished data}
- Feliziani V, Lattuada G, Parmiani S, Dall'Aglio PP. Safety and efficacy of sublingual rush immunotherapy with grass allergen extracts. A double blind study. Allergologia et Immunopathologia (Madrid) 1995;23(5):224‐30. [PubMed] [Google Scholar]
Grosclaude 2002 {published data only}
- Grosclaude M, Bouillot P, Alt R, Leynadier F, Scheimann P, Rufin P, et al. Safety of various dosage regimens during induction of sublingual immunotherapy: a preliminary study. International Archives of Allergy & Immunology 2002;129:248‐53. [DOI] [PubMed] [Google Scholar]
Guez 2000 {published and unpublished data}
- Guez S, Vatrinet C, Fadel R, Andre C. House‐dust‐mite sublingual‐swallow immunotherapy (SLIT) in perennial rhinitis: a double‐blind, placebo‐controlled study. Allergy 2000;55:369‐75. [DOI] [PubMed] [Google Scholar]
Hirsch 1997 {published and unpublished data}
- Hirsch T, Sahn M, Leupold W. Double‐blind placebo‐controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatric Allergy and Immunology 1997;8(1):21‐7. [DOI] [PubMed] [Google Scholar]
Hordijk 1998 {published data only}
- Hordijk GJ, Antvelink JB, Luwema RA. Sublingual immunotherapy with a standardised grass pollen extract: a double‐blind, placebo‐controlled study. Allergologia et Immunopathologia (Madrid) 1998;26[5]:234‐40. [PubMed] [Google Scholar]
Ibanez 2007 {published data only}
- Ibañez MD, Kaiser F, Knecht R, Armentia A, Schöpfer H, Tholstrup B, et al. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatric Allergy and Immunology 2007;18(6):516‐22. [DOI] [PubMed] [Google Scholar]
Ippoliti 2003 {published data only}
- Ippoliti F, Santis W, Volterrani A, Lenti L, Canitano N, Lucarelli S, et al. Immunomodulation during sublingual therapy in allergic children. Pediatric Allergy and Immunology 2003;14:216‐21. [DOI] [PubMed] [Google Scholar]
Khinchi 2004 {published data only}
- Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen‐specific immunotherapy: a randomized, placebo‐. Allergy 2004;59:45‐53. [DOI] [PubMed] [Google Scholar]
Kleine‐Tebbe 2006 {published data only}
- Kleine‐Tebbe J, Herold DA, Ribel M. Clinical safety of grass pollen allergen tablet for specific immunotherapy [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S266. [Google Scholar]
- Kleine‐Tebbe J, Ribel M, Herold DA. Safety of SQ‐standardized grass allergen tablet for sublingual immunotherapy: a randomized, placebo controlled trial. Allergy 2006;61:181‐4. [DOI] [PubMed] [Google Scholar]
La Rosa 1999 {published and unpublished data}
- Rosa M, Ranno C, Andre C, Carat F, Tosca MA, Canonica GW. Double‐blind placebo‐controlled evaluation of sublingual‐swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. Journal of Allergy and Clinical Immunology 1999;104:425‐32. [DOI] [PubMed] [Google Scholar]
Lima 2002 {published and unpublished data}
- Lima M, Wilson DR, Roberts A, Walker SM, Durham SR. Grass pollen sublingual immunotherapy (SLIT) for seasonal rhinoconjunctivitis: a randomised controlled trial. Clinical and Experimental Allergy 2002;32(4):507‐14. [DOI] [PubMed] [Google Scholar]
Malling 2005 {published and unpublished data}
- Malling HJ, Lund L, Ipsen H, Poulsen L. Safety and immunological changes during sublingual immunotherapy with standardized quality grass allergen tablets. Journal of Investigational Allergology and Clinical Immunology 2006;16:162‐8. [PubMed] [Google Scholar]
- Malling HJ, Lund L, Ipsen H, Poulsen LK. Safety and immunological changes during tablet based specific immunotherapy [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S161. [Google Scholar]
Marcucci 2005 {published and unpublished data}
- Marcucci F, Sensi L, Cara G, Salvatori S, Bernini M, Pecora S, et al. Three‐year follow‐up of clinical and inflammation parameters in children monosensitized to mites undergoing sub‐lingual immunotherapy. Pediatric Allergy and Immunology 2005;16:519‐26. [DOI] [PubMed] [Google Scholar]
- Marcucci F, Sensi L, Frati F, Bernardini R, Novembre E, Barbato A, et al. Effects on inflammation parameters of a double‐blind, placebo controlled one‐year course of SLIT in children monosensitized to mites. Allergy 2003;58:657‐62. [DOI] [PubMed] [Google Scholar]
Nelson 1993 {published data only}
- Nelson HS, Oppenheimer J, Vatsia GA, Buchmeier A. A double‐blind, placebo‐controlled evaluation of sublingual immunotherapy with standardized cat extract. Journal of Allergy and Clinical Immunology 1993;92(2):229‐36. [DOI] [PubMed] [Google Scholar]
Ott 2009 {published data only}
- Ott H, Sieber J, Brehler R, Fölster‐Holst R, Kapp A, Klimek L, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy 2009;64(1):179‐86. [DOI] [PubMed] [Google Scholar]
Pajno 2003 {published and unpublished data}
- Pajno GB, Vita D, Parmiani S, Caminiti L, Grutta S, Barberio G. Impact of sublingual immunotherapy on seasonal asthma and skin reactivity in children allergic to Parietaria pollen treated with inhaled fluticasone propionate. Clinical Experimental Allergy 2003;33:1641‐7. [DOI] [PubMed] [Google Scholar]
Palma Carlos 2006 {published and unpublished data}
- Palma‐Carlos AG, Santos AS, Branco‐Ferreira M, Pregal AL, Palma‐Carlos ML, Bruno ME, et al. Clinical efficacy and safety of preseasonal sublingual immunotherapy with grass pollen carbamylated allergoid in rhinitis patients. A double‐blind, placebo‐ controlled study. Allergologia et Immunopathologia (Madrid) 2006;34(5):194‐8. [DOI] [PubMed] [Google Scholar]
Panzner 2008 {published and unpublished data}
- Panzner P, Petrás M, Sýkora T, Lesná I. Double‐blind, placebo‐controlled evaluation of grass pollen specific immunotherapy with oral drops administered sublingually or supralingually. Respiratory Medicine 2008;102(9):1296‐304. [DOI] [PubMed] [Google Scholar]
Passalacqua 1998 {published and unpublished data}
- Passalacqua G, Albano M, Fregonese L, Riccio A, Pronzato C, Mela GS, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite‐induced rhinoconjunctivitis. Lancet 1998;351:629‐32. [DOI] [PubMed] [Google Scholar]
Passalacqua 1999 {published and unpublished data}
- Passalacqua G, Albano M, Riccio A, Fregonese L, Puccinelli P, Parmiani S, et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: a double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 1999;104:964‐8. [DOI] [PubMed] [Google Scholar]
Passalacqua 2006 {published and unpublished data}
- Lombardi C, Passalaqua G, Ariano R, Pasquali M, Baiardini I, Giardini A, et al. A 3‐year randomized controlled study with sublingual immunotherapy in mite‐induced respiratory allergy [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S207. [Google Scholar]
- Passalacqua G, Pasquali M, Ariano R, Lombardi C, Giardini A, Baiardini I, et al. Randomized double‐blind controlled study with sublingual carbamylated allergoid immunotherapy in mild rhinitis due to mites. Allergy 2006;61:849‐54. [DOI] [PubMed] [Google Scholar]
Peter 2009 {published and unpublished data}
- Peter R, Kleinjans H, Hecker H. Significant increase in IgG(4) levels after slit grass treatment. A double‐blind, placebo‐controlled, multi‐center study (twin grasses study). Allergy 2009;90:352‐3. [Google Scholar]
Pfaar 2008 {published and unpublished data}
- Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high‐dose sublingual grass pollen preparation: a double‐blind, placebo‐controlled trial. Annals of Allergy, Asthma, and Immunology 2008;100(3):256‐63. [DOI] [PubMed] [Google Scholar]
Pradalier 1999 {published data only}
- Pradalier A, Basset D, Claudel A, Couturier P, Wessel F, Galvain S, et al. Sublingual‐swallow immunotherapy (SLIT) with a standardised five‐grass‐pollen extract (drops and sublingual tablets) versus placebo in seasonal rhinitis. Allergy 1999;54:819‐28. [DOI] [PubMed] [Google Scholar]
Rolinck‐Werninghaus 2004 {published and unpublished data}
- Rolinck‐Werninghaus C, Wolf H, Liebke C, Baars JC, Lange J, Kopp MV. A prospective, randomized, double‐blind, placebo‐controlled multi‐centre study on the efficacy and safety of sublingual immunotherapy (SLIT) in children with seasonal allergic rhino‐conjuctivitis to grass pollen. Allergy 2004;59:1285‐93. [DOI] [PubMed] [Google Scholar]
Rőder 2007 {published and unpublished data}
- Röder E, Berger MY, Hop WC, Bernsen RM, Groot H, Gerth van Wijk R. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. Journal of Allergy and Clinical Immunology 2007;119(4):892‐8. [DOI] [PubMed] [Google Scholar]
Sabbah 1994 {published data only}
- Sabbah A, Hassoun S, Sellin J, Andre C, Sicard H. A double‐blind, placebo‐controlled trial by the sublingual route of immunotherapy with a standardized grass pollen extract. Allergy 1994;49:309‐13. [DOI] [PubMed] [Google Scholar]
Sanchez 2001 {published data only}
- Sanchez Palacios A, Schamann F, Garcia JA. Sublingual immunotherapy with cat extract, personal experience. Allergologia et Immunopathologia (Madrid) 2001;29:60‐5. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith H, White P, Annila I, Poole J, Andre C, Frew A. Randomized controlled trial of high‐ dose sublingual immunotherapy to treat seasonal allergic rhinitis. Journal of Allergy and Clinical Immunology 2004;114:831‐7. [DOI] [PubMed] [Google Scholar]
- Smith HE, White PJ, Poole J, Annila I, Andre C, Frew AJ. Sublingual immunotherapy for hay fever: a 2‐year double‐blind placebo‐controlled trial. Allergy 2002;57. [Google Scholar]
Tari 1990 {published and unpublished data}
- Tari MG, Mancino M, Madonna F, Buzzoni L, Parmiani S. Immunologic evaluation of 24 month course of sublingual immunotherapy. Allergologia at Immunopathologia 1994;22:209‐16. [PubMed] [Google Scholar]
- Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite: a double‐blind study. Allergologia et Immunopathologia (Madrid) 1990;18:277‐84. [PubMed] [Google Scholar]
Tonnel 2004 {published and unpublished data}
- Tonnel AB, Scherpereel A, Douay B, Mellin B, Leprince D, Goldstein N, et al. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy 2004;59:491‐7. [DOI] [PubMed] [Google Scholar]
Troise 1995 {published and unpublished data}
- Troise C, Voltolini S, Canessa A, Pecora S, Negrini AC. Sublingual immunotherapy in Parietaria pollen‐induced rhinitis: a double‐blind study. Journal of Investigational Allergology & Clinical Immunology 1995;5:25‐30. [PubMed] [Google Scholar]
Valovirta 2006 {published data only}
- Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy 2006;61:1177‐83. [DOI] [PubMed] [Google Scholar]
Vervloet 2006 {published data only}
- Vervloet D, Birnbaum J, Laurent P, Hugues B, Fardeau MF, Massabie‐ Bouchat YP, et al. Safety and efficacy of Juniperus Ashei sublingual‐swallow ultra‐rush pollen immunotherapy in cypress rhinoconjunctivitis: a double‐blind, placebo‐controlled study. International Archives of Allergy and Immunology 2007;142(3):239‐46. [DOI] [PubMed] [Google Scholar]
Voltolini 2001 {published and unpublished data}
- Voltolini S, Modena P, Minale P, Bignardi D, Troise C, Puccinelli P, et al. Sublingual immunotherapy in tree pollen allergy. Double‐blind, placebo‐controlled study with a biologically standardised extract of three pollens (alder, birch and hazel) administered by a rush schedule. Allergologia et Immunopathologia (Madrid) 2001;29(4):103‐10. [DOI] [PubMed] [Google Scholar]
Vourdas 1998 {published and unpublished data}
- Vourdas D, Syrigou E, Potamianou P, Carat F, Batard T, Andre C, et al. Double‐blind, placebo‐controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy 1998;53(7):662‐72. [DOI] [PubMed] [Google Scholar]
Wahn 2009 {published data only}
- Wahn U, Tabar A, Kuna P, Halken S, Montagut A, Beaumont O, et al. Efficacy and safety of 5‐grass‐pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. Journal of Allergy and Clinical Immunology 2009;123(1):160‐6. [DOI] [PubMed] [Google Scholar]
Wessner 2001 {published and unpublished data}
- Wessner DB, Wessner S, Mohrnschlager M, Rakoski J, Ring J. Efficacy and safety of sublingual immunotherapy in adults with allergic rhinoconjunctivitis: results after 2 years of controlled trial [Abstract]. Allergy 2001;56(Suppl 68):88. [Google Scholar]
Wutrich 2003 {published and unpublished data}
- Wutrich B, Bucher C, Jorg W, Bircher A, Eng P, Schneider Y, et al. Placebo‐ controlled study with sublingual immunotherapy in children with seasonal allergic rhinitis to grass pollen. Journal of Investigational Allergology and Clinical Immunology 2003;13:145‐8. [PubMed] [Google Scholar]
- Wutrich B, Eigenmann P, Bircher A, Schneider F, Jorg W. Double‐blind, placebo‐controlled study with sublingual immunotherapy in children sensitized to grass pollen efficacy and tolerance [Abstract]. XX Congress of the European Academy of Allergology and Clinical Immunology (EAACI). 2001:Abstract No. 256. [PubMed]
References to studies excluded from this review
Bernardis 1996 {published data only}
- Bernardis P, Agnoletto M, Puccinelli P, Parmiani S, Pozzan M. Injective versus sublingual immunotherapy in Alternaria tenuis allergic patients. Journal of Investigational Allergology & Clinical Immunology 1996;6(1):55‐62. [PubMed] [Google Scholar]
Black 2002 {published and unpublished data}
- Black PN, Douglas R, Brodie S, Fitzharris P. Controlled trial of sublingual immunotherapy with grass pollen allergoids for the treatment of seasonal allergic rhinitis [Abstract]. XXI Congeress of the European Academy of Allergology and Clinical Immunology (EAACI). 2002:Abstract No. 72.
Corthay 1996 {published data only}
- Corthay P, Gumowski PI, Bodmer R, Clot B. Efficacy of sublingual versus subcutaneous immunotherapy to pollen allergens after 3 consecutive years of treatment. Annual Meeting of the Swiss Society for Allergology and Immunology. 1996.
D'Ambrosio 1996 {published data only}
- D'Ambrosio FP, Ricciardi L, Isola S, Savi E, Parmiani S, Puccinelli P. Rush sublingual immunotherapy in Parietaria allergic patients. Allergologia et Immunopathologia (Madrid) 1996;24(4):146‐51. [PubMed] [Google Scholar]
Donato 1997 {published data only}
- Donato RM, Gutierezz NA, Pinilla V, Bagliano A. Sublingual immunotherapy (SLIT) as non‐invasive treatment for allergic rhinitis in children [Abstract]. International Journal of Immunopathology and Pharmacology 1997;10:228. [Google Scholar]
Feliziani 1993 {published data only}
- Feliziani V, Marfisi RM, Parmiani S. Rush immunotherapy with sublingual administration of grass allergen extract. Allergologia et Immunopathologia (Madrid) 1993;21:173‐8. [PubMed] [Google Scholar]
Gammeri 2004 {published data only}
- Gammeri E. Safety and tolerability of ultra‐rush (20 minutes) allergoid sublingual immunotherapy in patients with allergic rhinitis and/or asthma [Abstract]. Journal of Allergy and Clinical Immunology 2004;2:S108. [Google Scholar]
Gozalo 1997 {published data only}
- Gozalo F, Martin S, Rico P, Alvarez E, Cortes C. Clinical efficacy and tolerance of two year Lolium perenne sublingual immunotherapy. Allergologia et Immunopathologia (Madrid) 1997;25(5):219‐27. [PubMed] [Google Scholar]
Hansen 2004 {published data only}
- Hansen KS, Khinchi MS, Skov PS, Bindslev JC, Poulsen Lars K, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Molecular Nutrition & Food Research 2004;48:441‐8. [DOI] [PubMed] [Google Scholar]
Horak 1998 {published data only}
- Horak F, Stubner P, Berger UE, Marks B, Toth J, Jager S. Immunotherapy with sublingual birch pollen extract. A short‐term double‐blind placebo study. Journal of Investigational Allergology & Clinical Immunology 1998;8(3):165‐71. [PubMed] [Google Scholar]
Inal 2009 {published and unpublished data}
- Inal A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc G. Effect of subcutaneous and sublingual immunotherapy on clinical improvement and quality of life in children with rhinitis and asthma monosensitised to house dust mite: a randomised, placebo‐controlled, double‐blind, double‐dummy study. Allergy 2009:461. [Google Scholar]
Karakoc 2003 {published and unpublished data}
- Karakoc GK, Altintas D, Yilmaz M, Kendirli SG. Conventional immunotherapy versus sublingual immunotherapy with standardized five‐grass pollen in children with seasonal allergic rhinoconjuctivitis [Abstract]. Allergy and Clinical Immunology International 2003;1(Suppl 1):Abstract No. P‐15‐23. [Google Scholar]
Mitsch 1996 {published data only}
- Mitsch A, Drachenberg KJ. Positive results in a primary multicentric study, specific immunotherapy administered sublingually. TW Padiatrie 1996;9:628‐31. [Google Scholar]
Mungan 1999 {published data only}
- Mungan D, Misirligil Z, Gurbuz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite‐sensitive patients with rhinitis and asthma: a placebo controlled study. Annals of Allergy, Asthma & Immunology 1999;82(5):485‐90. [DOI] [PubMed] [Google Scholar]
Nanda 2004 {published data only}
- Nanda A, O'Connor M, Anand M, Dreskin SC, Zhang L, Hines B, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. Journal of Allergy and Clinical Immunology 2004;114(6):1339‐44. [DOI] [PubMed] [Google Scholar]
Okubo 2008 {published data only (unpublished sought but not used)}
- Okubo K, Gotoh M, Fujieda S, Okano M, Yoshida H, Morikawa H, et al. A randomized double‐blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergology International 2008;57(3):265‐75. [DOI] [PubMed] [Google Scholar]
Quirino 1996 {published data only}
- Quirino T, Lemoli E, Siciliani E, Parmiani S, Milazzo F. Sublingual versus injective immunotherapy in grass pollen allergic patients: a double blind (double dummy) study. Clinical and Experimental Allergy 1996;26(11):1253‐61. [PubMed] [Google Scholar]
Radjenovic 2004 {published data only}
- Radjenovic G. Rhinitis allergica due to house dust mite in children: long lasting effect of sublingual immunotherapy [Abstract]. 5th European Congress of Oto Rhino Laryngology Head and Neck Surgery. 2004; Vol. 209:PP151.
Russello 2004 {published and unpublished data}
- Russello M, Maurol M, Incorvaia C, D'Ingianna E, Gazzola GB. Subcutaneous and sublingual immunotherapy in birch pollinosis: a comparison of efficacy and safety [Abstract]. XXIII Congress of the European Academy of Allergology and Clinical Immunology (EAACI). 2004:Abstract No. 256.
Sabbah 1993 {published data only}
- Sabbah A, Lesellin J, Hassoun S, Sicard H, Andre C. Sublingual specific immunotherapy for rhinoconjunctivitis caused by grass pollens. Allergie et Immunologie (Paris) 1993;25(6):241‐7. [PubMed] [Google Scholar]
Tonnel 2002 {published data only}
- Tonnel A, Vannimenus C, Douay B, Mellin B, Deroubaix C, Trochu G, et al. Rhinite aux acariens: evaluation de L'efficacite de L'immunotherapie allergenique par voie sublinguale. Allergie et Immunolgie 2002;34:60‐1. [Google Scholar]
Troise 2009 {published data only}
- Troise C, Voltolini S, Incorvaia C, Grutta S, Frati F. Efficacy and safety of sublingual immunotherapy with high dose birch extract: placebo‐controlled study. Allergy 2009:465. [Google Scholar]
Van Niekerk 1987 {published data only}
- Niekerk CH, Wet J. Efficacy of grass‐maise pollen oral immunotherapy in patients with seasonal hay‐fever: a double blind study. Clinical Allergy 1987;17:507‐13. [DOI] [PubMed] [Google Scholar]
Yuksel 1999 {published data only}
- Yuksel H, Tanac R, Gousseinov A, Demir E. Sublingual immunotherapy and influence on urinary leukotrienes in seasonal pediatric allergy. Journal of Investigational Allergology and Clinical Immunology 1999;9(5):305‐13. [PubMed] [Google Scholar]
References to ongoing studies
Ingels 2002 {published and unpublished data}
- A placebo controlled, double‐blind, randomized study to assess efficacy of sublingual immunotherapy in patients with grass pollen allergy through assessment of its immunological effects on the mucosal tissue of the nose. Ongoing study Study start 2002.
O'Hehir 2005 {published and unpublished data}
- A trial of immunological outcomes of sublingual immunotherapy for house dust mite (D. pteronyssinus). Ongoing study November 2005.
Additional references
Antico 2006
- Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy 2006;61:1236‐7. [DOI] [PubMed] [Google Scholar]
ARIA 2001
- Bousquet J, Cauwenberge P, Khaltaev N, Aria Workshop Group, World Health Organization. Allergic Rhinitis and it impact on Asthma. Journal of Allergy and Clinical Immunology 2001;108(Suppl 5):S147‐334. [DOI] [PubMed] [Google Scholar]
ARIA 2008
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63(Suppl 86):S8‐160. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Blazowski 2008
- Blazowski L. Anaphylactic shock because of sublingual immunotherapy overdose during third year of maintenance dose. Allergy 2008;63:374. [DOI] [PubMed] [Google Scholar]
Calderon 2007
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD001936. DOI: 10.1002/14651858.CD001936.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderon 2008
- Calderon MA. Meta‐analyses of specific immunotherapy trials. Drugs Today (Barc) 2008;44(Suppl B):31‐4. [PubMed] [Google Scholar]
Calderon 2010
- Calderon M, Mösges R, Hellmich M, Demoly P. Towards evidence‐based medicine in specific grass pollen immunotherapy. Allergy 2010;65:420‐34. [DOI] [PubMed] [Google Scholar]
Casale 2009
- Casale TB, Canonica GW, Bousquet J, Cox L, Lockey R, Nelson HS, et al. Recommendations for appropriate sublingual immunotherapy clinical trials. Journal of Allergy and Clinical Immunology 2009;124(4):665‐70. [DOI] [PubMed] [Google Scholar]
Cox 2006
- Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. Journal of Allergy and Clinical Immunology 2006;117(5):1021‐35. [DOI] [PubMed] [Google Scholar]
Dahl 2008
- Dahl R, Kapp A, Colombo G, Monchy JG, Rak S, Emminger W, et al. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. Journal of Allergy and Clinical Immunology 2008;121:512‐18. [DOI] [PubMed] [Google Scholar]
de Groot 2009
- Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy 2009;64(6):963‐4. [DOI] [PubMed] [Google Scholar]
Dunsky 2006
- Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy 2006;61:1235. [DOI] [PubMed] [Google Scholar]
Durham 2010
- Durham SR, Emminger W, Kapp A, Colombo G, Monchy JG, Rak S, et al. Long‐term clinical efficacy in grass pollen‐induced rhinoconjunctivitis after treatment with SQ‐standardized grass allergy immunotherapy tablet. Journal of Allergy and Clinical Immunology 2010;125(1):131‐8.e1‐7. [DOI] [PubMed] [Google Scholar]
Eifan 2007
- Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy 2007;62:567‐8. [DOI] [PubMed] [Google Scholar]
Francis 2003
- Francis N, Till SJ, Durham SR. Induction of IL‐10 + CD4 + CD25 + T cells by grass pollen immunotherapy. Journal of Allergy and Clinical Immunology 2003;111(6):1255‐61. [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jutel 1995
- Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Muller UR. Bee venom immunotherapy results in decrease of IL‐4 and IL‐5 and increase of IFN‐gamma secretion in specific allergen‐stimulated T cell cultures. Journal of Immunology 1995;154(8):4187‐94. [PubMed] [Google Scholar]
Jutel 2003
- Jutel M, Akdis M, Budak F, Aebischer‐Casaulta C, Wrzyszcz M, Blaser K, et al. IL‐10 and TGF‐b cooperate in regulatory T cell response to mucosal allergens in normal immunity specific immunotherapy. European Journal of Immunology 2003;33:1205‐14. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Ba' Pham, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;14(357):1191‐4. [PubMed] [Google Scholar]
Mungan 1999
- Mungan D, Misirligil Z, Gürbüz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite‐sensitive patients with rhinitis and asthma‐a placebo controlled study. Annals of Allergy, Asthma, and Immunology 1999;82(5):485‐90. [DOI] [PubMed] [Google Scholar]
Nieto 2009
- Nieto A, Mazon A, Pamies R, Bruno L, Navarro M, Montanes A. Sublingual immunotherapy for allergic respiratory diseases: an evaluation of meta‐analyses. Journal of Allergy and Clinical Immunology 2009;124(1):157‐161.e1‐32. [DOI] [PubMed] [Google Scholar]
Quirino 1996
- Quirino T, Lemoli E, Siciliani E, Parmiani S, Milazzo F. Sublingual versus injective immunotherapy in grass pollen allergic patients: a double blind (double dummy) study. Clinical and Experimental Allergy 1996;26(11):1253‐61. [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rossi 2004
- Rossi RE, Monasterolo G. Evaluation of recombinant and native timothy pollen (rPhl p 1, 2, 5, 6, 7, 11, 12 and nPhl p 4)‐specific IgG4 antibodies induced by subcutaneous immunotherapy with timothy pollen extract in allergic patients. International Archives of Allergy and Immunology 2004;135:44‐53. [DOI] [PubMed] [Google Scholar]
Wachholz 2002
- Wachholz PA, Nouri‐Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology 2002;105(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2001
- Wilson DR, Irani AM, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clinical and Experimental Allergy 2001;31(11):1705‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wilson 2003
- Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database of Systematic Reviews 2003, Issue 2. [Art. No.: CD002893. DOI: 10.1002/14651858.CD002893] [DOI] [PubMed] [Google Scholar]
Wilson 2005
- Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta‐analysis. Allergy 2005;60(1):4‐12. [DOI] [PubMed] [Google Scholar]


