Abstract
The second most common type of tumor worldwide is prostate cancer (PCa). Certain genetic factors contribute to a risk of developing PCa of as much as 40%. BRCA1 and BRCA2 mutations have linked with an increased risk for breast, ovarian, and PCa. However, BRCA2 is the most common gene found altered in early-onset of PCa in males younger than 65. BRCA2 mutation has a higher chance of developing an advanced stage of the disease, resulting in short survival time. This review aimed to describe the genetic changes in BRCA2 that contribute to the risk of PCa, to define its role in the early diagnosis in a man with a strong family history, and to outline the purpose of genetic testing and counseling. Also, the review summarizes the impact of BRCA2 gene mutation in localized PCa, and the treatment strategies have used for PCa patients with a BRCA2 modification.
Keywords: BRCA2, hereditary, prostate cancer, mutation
Epidemiology and etiology. In 2018, prostate cancer (PCa) found to be the second highest tumor type with fifth foremost etiology of cancer-related mortality among males worldwide.1 Approximately 1.3 million new PCa cases and 359,000 associated deaths occurred worldwide in 2018, most commonly in sub-Saharan Africa and the Caribbean. Prevalence rates are higher in countries such as, New Zealand, Australia, Europe (north and west), and North America.1 In Nordic, countries; however, the incidence has either dropped or steadied since 2000.2 In Saudi Arabia, PCa is the fifth most common cancer among all age groups. In 2001, the highest age-standardized rate (ASR) was found in the eastern region, at 11.3/100,000, while the lowest seen in the Asir region, at 4.9/100,000.3 Prostate cancer prevalence is lesser in the Arab population than in North American Society,4 and this lower incidence due to small prostatic specific antigen (PSA) level.5 Approximately 25% of newly diagnosed cancers in men are PCa. Middle Eastern, North African, and Asian men were found to have a lower prevalence of PCa and commonly diagnosed in Swedish men.6 The low incidence of PCa among the first generation of descendants of Middle Eastern immigrants has been evident in places such as Sweden,7 the Netherlands,8 and California (USA).9 However, with each new generation, the incidence gradually increases. Conventionally, it believed that high saturated fat consumption, PCa was still low.10 Genetic contributions in the pathogenesis of PCa have well described, but knowledge and awareness related to inherent pathology are continuously changing. There are some positive aspects, predominantly with genetic associations, those that occur within PCa: 1) early-onset PCa in those aged <55 years. 2) PCa males associated along with a breast, pancreas, or tumor of ovaries. 3) Numerous pretentious first-degree relatives (FDRs) with PCa.10
Prostate cancer is very rare under 40 years of age, but its prevalence afterward, this age rises promptly. Men of age 49, 50-59, 60-69, and 70 or more, the likelihood of being diagnosed PCa is one in 437, one in 59, one in 22, and one in 13, respectively.11 Men who develop PCa below the age of 55 categorized as having an early onset of PCa and the rate of presence of such cases is growing. Further, some of these cases are highly aggressive, typically due to the manifestation of a germline mutation.12 Those patients present with a positive family history of PCa, they are at increased 60% chances of developing PCa.13 Some of the familial PCa clusterings has been described, as an association with breast and colonic cancer.14,15 Approximately 10% of PCa cases are mostly due to inherited factors or PCa predilection genes. Published data has reported that strong family history is one of the highest risk factors for PCa development.16 Familial accretions are due to more PCa checking in relatives supposed to be at high risk.17
Prostate cancer is a natural component of heritable breast carcinoma, where the genetic affinity interconnected to BRCA1 and BRCA2 gene mutation.18 The overall PCa chances are stated to be up to 3.8-fold or 8.6-fold for those who are carriers of BRCA1 and BRCA2 genes, respectively.19 Additionally, BRCA2 mutations have been reported to result in an aggressive pattern of disease with a reduced survival rate. BRCA2 mutations have been present in approximately 5% of patients with progressive PCa.20-22 Germline BRCA changes can increasingly be measured in metastatic castration-resistant PCa (mCRPC). As such, genetic assessment and testing are critical to cancer risk assessment, screening, and treatment.23-25
This study aims to review the pathologic and genetic changes in PCa that can interfere with the treatment approach, guidelines, and clinical considerations for the management of PCa. It also analyzed the case and prognosis of a patient with history of PCa in relatives. The knowledge of genetic testing and genetic counseling for men in BRCA2 families may be helpful for risk assessment and prevention. We believe this review on an emerging BRCA2 gene mutation will be helpful in early detection of PCa.
Molecular and genetic variations in prostate cancer. The interface between multiple genes and environmental factors results in complex molecular pathogenesis in the development of PCa, and these genetic and epigenetic changes can occur at various stages. Transformations in BRCA1 and BRCA2 have recognized the factor for progression of poor-risk PCa.26 Prostate cancer is known to have an abnormally different genetic process, including somatic copy number alterations, point mutation, chromosomal number changes, and structural modifications.27 Somatic copy number alterations may found in around 90% of PCa cases. Primary PCa often shows deletions on different chromosome numbers like 6q, 8p, 10q,13q, and other specific genes, including NKX3-1, PTEN, BRCA2, and RB1. In mCRPC, the augmentation of chromosome x, 7, 8q, and 9q have been identified, as well as that of androgen receptor pathway genes and the MYC oncogene.27
Prostate cancer includes somatic mutation, as do breast cancer and ovarian cancer.28-30 In PCa gene mutation, at least 20 mutations likely result in protein function interruption. RNASEL is a tumor suppressor gene that has a crucial role in PCa.31 Hereditary PCa gene BRCA2 found on chromosome 17p, which codes a protein that similar to the family of DNA cross-link repair enzymes, and these enzymes included in biosynthesis.32 The Arg293X and Asp175Ty genes were the first identified for familial PCa in a man from European origin and in an African American populations respectively.33 The variants of HOXB13 G84E have identified in high risk of PCa patient after the screening of more than 200 genes.34
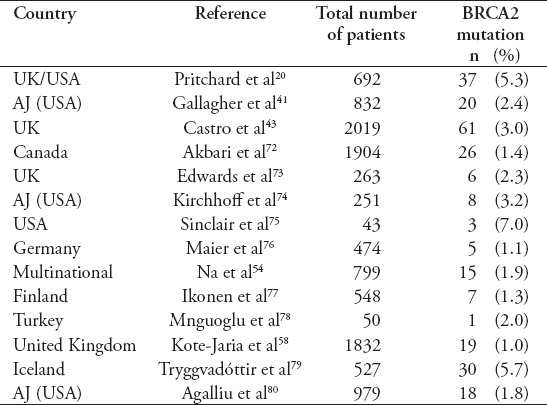
Table 1 summarize the prevalence and frequency of BRCA2 carriers in PCa.
Table 1.
The frequency of BRCA2 mutations in patients with prostate cancer.
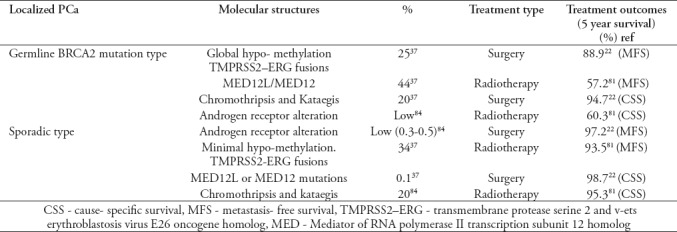
The impact of BRCA2 mutation on localized prostate cancer. Patients with germline BRCA2 gene mutation and diagnosed with localized PCa have reduced cancer-specific and metastasis-free survival than non-carriers.22 Those patients were having the affiliation of intra-ductal PCa and a germline BRCA2 mutation and undergoing surgery, and they have an inferior prognosis due to a reason of intraductal carcinoma.35,36 Recently, Taylor et al37 published an article on localized PCa patients, those were found with germline BRCA2 mutations in spite of those having sporadic BRCA2 transformations. They have mentioned that the localized PCa from BRCA2 alteration carriers has clinical and molecular structures that are more alike to be an aggressive and progressive tumor from non-carriers than to localized sporadic tumors from non-carriers. As like, the molecular abnormalities existent in growths from new detected BRCA2 mutation carriers with management early localized PCa is similar to individuals appreciated man having metastatic castrate-resistant PCa, those who have received numerous type of treatment. And this result is persistent with the assessment that BRCA2 altered localized cancers are already on a destructive route at the time of diagnosis. Furthermore, the presence of the intra-ductal PCa sub pathology is another sign of poor prognosis. Intra-ductal PCa, origin from a typical familial copy, which is described by more genomic uncertainty, which comparatively describes the reasons of having a BRCA2 mutation carriers have unfortunate consequences when intra-ductal PCa exists. The occurrence of germline BRCA2 alterations in PCa patient is somewhat low (1%). Still, the destructive natural pattern of localized cancer may explain any variation in medical treatment to further strengthened the methods as compared presently worked.37 Table 2 summarize the outcomes of germline and sporadic BRCA2-mutation in the localized PCa. The DNA damage path and its consequence is very crucial and significant, which confirms the existence of regular and malignant prostate cells. And includes the breast cancer predisposition BRCA1/2, ataxia-telangiectasia altered and the partner and localizer of BRCA2 genes.38
Table 2.
Outcomes of germline and sporadic BRCA2-mutation in the localized prostate cancer (PCa)
BRCA2 gene and its role in PCa
BRCA2 is an autosomal dominant inheritance cancer suppressor gene along with an essential function in the preservation of genomic control. The heterozygous germline mutation entities in the BRCA2 gene are at greater risk of dropping the effective allele due to secondary harmful effects.23 These harmful effects can occur due to many reasons, such as alkylating drugs, ionizing radiotherapy, oxygen radical’s types, and chemical mutagens.23 BRCA2 codes 3418, an amino acid, which comprises 8 BRCA replications, a DNA-binding domain, and a nuclear localization signal. BRCA2 correlates with RAD51 through the BRCA recurrences and the RAD51-binding domain at its C-terminus as a fragment of the double-strand break restoration mechanism.23 Recent studies39 have reported that 10% of primary cancer and 25% of advanced from PCa anchorage the DNA damage repair defects that are associated with progressive BRCA2 deficiencies. These BRCA2 gene mutations have linked with advanced disease with poor clinical outcomes.40-43
Recently, poly-ADP ribose polymerase (PARP) blockers or chemotherapy such as platinum based treatment options identified for some somatic and germline DNA damage repair defects.44-47 Robinson et al23 have reported in their data that germline changes for DNA damage repair genes are known factors linked a higher chance for developing of metastatic PCa. Pritchard et al20 published a data of 84 germline DNA-repair gene changes, recognized as very highly notorious, and these found in 82 patients (11.8%). Alterations recognized in 16 different genes, as well as BRCA2 in 37 men (5.3%) and others such as ATM 1.6%, CHECK2 1.9% of 534 analysis of patients, BRCA1 0.9%, RAD5ID 0.4%, and PALB2 0.4%.20 The prevalance of BRCA2 germline mutation is greater (1.2%) than BRCA1 gene (0.4%).48 Authors reported in a landmark study, and they detect the altered condition of 20 DNA repair genes 692 patients present with metastatic PCa.20 The authors recognized a peak incidence for germline changes in several genes tested, also BRCA2, RAD51 paralog D, checkpoint kinase 2, BRCA1, and BRCA2 partner and localizer.
Table 1 summarized the prevalence and frequency of BRCA2 carriers in PCa.
When the patient should be referred for genetic counseling and testing. As per recommendations by the American College of Medical Genetics that men should seek for genetic testing when there is a history of prostate cancer: 1) Three or more first-degree relatives, 2) Two or more first-degree relatives those who identified for PCa, when their age was below 55 years, 3) a Gleason grade more than 7, and 4) a family history of more than 2 persons with breast, ovaries, or cancer of pancraes.49
The National Comprehensive Cancer Network has proposed that person must referred for genetic testing if they have any of the following: 1) If Gleason’s grade is more than 7, regardless of age and more than one close relative having breast carcinoma with younger than 50 years of age. 2) If Gleason grade more magnificent than 7 and at least 2 relatives with a history of a breast, pancreas, or PCa with Gleason grade more than 7, recognized at any age. 3) Individual history of advanced PCa, diagnosed by prostate biopsy or by radiology work up.50
Johns Hopkins suggested that, in terms of the concerns of the familial PCa, the patient must be referred for genetic testing: 1) Three or more closest family relatives with history of PCa, 2) PCa in 3 generations or 3) 2 close families with PCa diagnosed at or younger than 55-years of age.51 Recently, Zhen et al52 studied the importance of age at the time of diagnosis when assessing hereditary risk. Notably, the bottommost decile of PCa cases diagnosed in men below the age of 55 years should be referred for genetic testing and consultation.
The importance of BRCA2 genetic testing in young males with a positive family history. BRCA1 and BRCA2 specify clinically subclass, which results in a higher percentage of lethal tumors, which highly suggested for genetic testing in an initial stage to diagnose the PCa. Updated research studies have stated that more than 10% of men with metastatic PCa have genetic alterations in DNA repair genes,53-55 and these mutations are also found in 5% of men at high risk for localized PCa, with a low incidence rate of low-risk indolent tumors. There is also robust indication signifying that these mutations may disturb the response of PARP inhibitors and platinum-based chemotherapy treatment.56
Giri et al57 recently, they have reported the results of 200 patients in the genetic evaluation of men study. Of 200 patients, 11 (5.5%) were identified a pathogenic change by using a 25-gene cancer predisposition panel, with 9 of those mutations were occurred in the group of 125 men affected with PCa (7.2%) and 2 variations happened in the group of 75 men at high risk but unaffected. In 63.6% of cases studied, the alterations linked to DNA repair genes, including BRCA1, BRCA2, and others. Over one-third of men in the study had variants of uncertain consequences.
Kote-Jarai et al58 have reported the importance of BRCA2 in PCa risk in a male of fewer than 65 years’ age. BRCA2 gene analyzed for 1832 samples. Nineteen protein-truncating mutations identified (16 frame shifts and 3 were non-sense mutations). Furthermore, they noticed 3 in structure losses one new and 69 missense modifications 13 new of indeterminate consequence, one common non-sense alteration, and 3 ends of the protein, considered as benign, 31 identical changes, 5 new, and 35 intronic alterations. Harmful mutations noticed patients those present at 65 years or lesser of age; no truncating mutations well known in those patients who diagnosed PCa at age of 65 or more than 65-year-old. The deleterious mutations were recognized throughout the gene, in exon 10 (4 mutations), 11 (13 mutations), 22 (1 mutation) and 24 (1 mutation). The DNA sample types taken from the families, obtainable for 3 of the mutation carriers, and suitable fragments sequenced from these families. One patient was found with 2 affected brothers, of whom one carried the same mutation at 69 years. Another case was more sibling, who suffered from variations, when he was 66 years old. Twelve out of 19 of the harmful mutation harbors have died earlier 1-11 years, 8 of these mortality identified, and 7 patients are still alive.58
Identification of males with a hereditary susceptibility for PCa. The focused selection of BRCA1/2 altered carriers and control is a worldwide association of 62 centers in 20 countries, these accessing to rule out the PCa in male population with BRCA2 mutations.49 Bancroft et al,49 reported the selection outcomes for males, who enrolled in the research study. They enrolled all males between the age from 40 to 69 years’ associates germline BRCA1/2 alterations and a group, who have checked as negative for the pathogenesis of BRCA1 or BRCA2 mutation recognized in relatives.49 Prostatic specific antigen (PSA) requested for all and >3 ng/ml value offered biopsy. A total of 2481 men were included in the study, 791 BRCA1 carriers, 531 BRCA1 controls; 731 BRCA2 carriers, and 428 BRCA controls.
Of the 199 men, 8% had PSA more than 3.0 ng/ml, 162 biopsies completed, 59 prostate cancers diagnosed in 18 BRCA1 carriers, 10 BRCA1 controls; 24 BRCA2 carriers, and 7 BRCA2 controls. Approximately 66% of the tumors grouped were in a high-risk or intermediate disease.49 The predictive value to be positive for tissue diagnosis at the PSA value was 3.0 ng/ml BRCA2 mutation harbors 48% higher the predictive value reported in screening group studies. The most important change noticed the high or intermediate risk disease BRCA2 positive patients. Approximately 95% of males were white, therefore, the outcomes not seen as global in cultural population.49 The authors conclude that the impact screening system is helpful for targeted PCa work up in males associated with germline genetic risk variants. Initial outcomes help of selective PSA screening established the BRCA genotype expresses, these screening produces highly life threatening condition.49
Limitations of genetic testing and counselling. The American Society of Clinical Oncology emphasized on informed consent and counseling for patients prior to have any genetic testing.59 Genetic testing and advice may be costly for the patient. Besides the interview with the psychotherapist, fees charged for collection of specimens (either a blood test or saliva) and specific test ordered. Cost of the genetic analysis depends on the genes tested; either one gene or many genes test may take. There are several testing laboratories, those who agreed to submit an approval for a financier covering company after delivery of the sample and prior to start any process with the test. After receiving the laboratory tests, patient always informed about the details of covering costs. The high price of evaluating individuals with a personal and family history may prevent the search of genetic therapy or testing in patients who are unable to pay the charges.52
Bancroft et al60 recently published a study on the psychosocial issues in PCa work up for BRCA1/2 mutations. They reported the outcome of a longitudinal psychosocial survey. Many centers carried out investigation of focused PCa workup among males recognized pathogenesis germline of BRCA1/ BRCA2 genes.60
All enrolled males in the IMPACT study were asked to answers the questionnaire at different group work sites before their yearly checkup appointment. The survey measures include anxiety depression scale in hospital, impact of event scale, 36 item short-form health assessment, memorial anxiety scale for PCa, cancer worry scale, and risk awareness.60
Total 432 men finalized questionnaires: BRCA1 gene mutation was identified in 98 and BRCA2 gene mutation in 160 men. One hundred seventy-four were controls (no familial mutation) member’s observation for PCa risk subjective by hereditary position. The level of awareness was high and not free for hereditary conditions. The scores for the hospital anxiety depression measurements and 36 elements of short form health checkup started for general people. The impact of event scale scores were within standard range. Impact of Event Scale says interruption and escaping totals found high in BRCA1/BRCA2 carriers than in controls and more in men with better PCa risk awareness. At the level of multivariate, risk impression shared greater to the variance in the impact of event scale scores than genetic status. The authors conclude that no practical alarming levels of general or cancer-specific distress or reduced quality of life noticed in the group.60 Some of the contributors saw the difficulty and signifying the essential for primary healthcare specialists, proposing PCa screening to review the risk factors and to support the men seeking PCa screening.60
Role of immunotherapy and targeted therapy in BRCA2-mutated advanced PCa. Patients who are carriers of genetic alterations in the critical area of DNA repair pathway have considerably more period threat to the emergence of malignancy in comparison with their peers. New developments in following group of DNA sequencing skills permitted the work up for the persons those who harbor the mutation, that will lead to increases in practical risk-reduction approaches; this has justified identifying the best prevention and treatment paths for such patients at an early stage. Currently, many treatment strategies are working for PCa patients who have germline/somatic modifications in the DNA restoration pathway mechanism for BRCA1 and BRCA2, likewise precise screening strategies also the new treatment approaches including Poly-ADP ribose polymerase blocking agents or chemotherapy.61,63
Role of chemotherapy agents
Chemotherapy drugs are established to be a beneficial treatment for BRCA1/2 mutated breast and ovarian cancer.61-63 In standard practices for PCa, platinum-based chemotherapy is indicated as ideal for differentiated neuroendocrine tumors, as phase-III trials in mCRPC unsuccessful outcome in an unselected group of people.64 However, according to the findings produced by published studies, BRCA2-mutated PCa may be highly sensitive to chemotherapy.45,48 Pomerantz et al65 reported data of 141 men with mCRPC who managed with minimum of 2 cycles of carboplatin and docetaxel. They indicated that the treatment was favorable for germline BRCA2 mutation patients, 6 out of the 8 (75%) BRCA2 carriers presenting PSA decline of more than 50% within 12 weeks after starting this chemotherapy; however, this outcome achieved in only 23 of 133 (17%) non-carriers (p=0.001). A higher than 50% reduction in PSA levels was also associated with lengthier survival time approximately 19 months in BRCA2 carriers versus approximately 10 months in non-carriers with DNA repair defects.65
Role of targeted therapy
Targeted therapies, such as PARP inhibitors, are used in BRCA-linked breast and ovarian tumors and are also recommended for BRCA carriers affected by another solid cancer, also PCa. These targeted agents are nuclear DNA-binding enzyme complexes using for DNA single-strand break repair along with base excision and repair path.66 Poly-ADP ribose polymerase inhibitors are effective to treat DNA repair-poor cancers, because mentioned agents stimulate the requirement of homologous recombination (HR)-deficient tumors on a different DDR pathway.67 These drugs can also be used at various stages of clinical progress.
There is a growing awareness of such approaches resulting from the immunotherapy studies that have been completed and particularly after studies on vaccines directed to the Food and Drug Administration (FDA) approval for sipuleucel-T68 and the broader to use the PROSTVAC-VF. Immune checkpoint inhibitors have investigated for treating mCRPC, for example pembrolizumab and atezolizumab in combination with enzalutamide.69 Several Poly-ADP ribose polymerase blocking agents such as rucaparib, olaparib, niraparib, velaparib and talazoparib are still under investigation for treating several tumor types.
Clinical outcomes. This review related to BRCA2 gene mutation was conducted because of its importance in clinical practices aiming to diagnose PCa at an early age, as it linked with an active genetic component. In the present study, we assessed the importance of genetic counseling and testing. Study indicated that PCa has a genetic component and that strong family history with quick detection of PCa are the leading signs of a contributing BRCA2 gene mutation.
Men with a BRCA2 mutation are at greatest risk of death as compared with non-carriers. Therefore, it is imperative to inform primary treating physicians and patients of the importance of genetic counselors in the process of discussing the different treatment options.70 Pharmacological agents targeted at specific genes, including DNA mismatch repair, have been developed. It has also observed that the finding of somatic and germline changes after checking results shows the impact in the treatment approach in patients with BRCA2 mutations (the most frequently altered germline DDR gene). The license of harbors of genomic abnormalities permits not only for those having disease proneness to be identified but also for a better description of tumor subtypes, which can have different sensitivities to the various treatment and management options. DNA sequencing will likely change the therapeutic methodology of PCa in the future, improving the molecular arrangement of this cancer and, hence, the suitable therapeutic method. The molecular classification of PCa appears to be helpful in better determining the disease prognosis.71
Recommendations 1) The references and guidelines for genetic counseling referrals should take into account the patient’s age at PCa diagnosis and definite family cancer history pattern. Referral should consider for persons with a history of numerous affected FDRs with PCa, early-onset PCa (aged less than 55 years), metastatic PCa, or a history of another tumor, such as breast, ovaries, or pancreas). 2) Knowledge about mutation status for men in BRCA2 families may be beneficial for risk assessment and prevention. 3) Due to the link of the gene and genetic changes, it recommended that the families of pretentious persons to start standard PCa screening prior than does the general population; however, this decision must be based on physician and patient preference. 4) Genetic mutation linked to PCa can check through blood or saliva tests. If the urologist recommends genetic testing, the case may also be referred to a genetic counselor. 5) To take full advantage of the effectiveness of cancer treatments and avoid preventable adverse effects, the recognition and potential authentication of prognostic biomarkers are highly encouraged.
In conclusions, the available data indicate that the early diagnosis of PCa through genetic testing should be mandatory for patients associated who have a strong family history of PCa. Genetic testing and counseling performed by an experienced multi-disciplinary team, including a treating physician and genetic counselor, is needed for appropriate and timely management. Results from ongoing randomized controlled trials on PARP inhibitors in PCa will give further confidence for their approval in clinical practice. Additional studies related to recent updates and patient awareness in Saudi Arabia are also necessary.
Acknowledgment
The authors acknowledge Scribendi (www.scribendi.com) for English language editing.
Footnotes
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kvale R, Myklebust TA, Engholm G, Heinävaara S, Wist E, Moller B. Prostate and breast cancer in four Nordic countries:a comparison of incidence and mortality trends across countries and age groups 1975-2013. Int J Cancer. 2017;141:2228–2242. doi: 10.1002/ijc.30924. [DOI] [PubMed] [Google Scholar]
- 3.Saudi Cancer Registry. Annual Report. 2010. Available from: https://shc.gov.sa/Arabic/Pages/default.aspx .
- 4.Mosli HA. Prostate cancer in Saudi Arabia in 2002. Saudi Med J. 2003;24:573–581. [PubMed] [Google Scholar]
- 5.Kehinde EO. Age-specific reference levels of serum prostate-specific antigen and prostate volume in healthy Arab men 2005. BJU Int. 2005;96:308–312. doi: 10.1111/j.1464-410X.2005.05620.x. [DOI] [PubMed] [Google Scholar]
- 6.Alyaiya AA. Proteomics-based signature for human benign prostate hyperplasia and prostate adenocarcinoma. Int J Oncol. 2011;38:1047–1057. doi: 10.3892/ijo.2011.937. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Czene K. Cancer risks in first-generation immigrants to Sweden. Int J Cancer. 2002;99:218–228. doi: 10.1002/ijc.10322. [DOI] [PubMed] [Google Scholar]
- 8.Visser O, van Leeuwen FE. Cancer risk in first generation migrants in North-Holland/Flevoland, The Netherlands 1995-2004. Eur J Cancer. 2007;43:901–908. doi: 10.1016/j.ejca.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Nasseri K, Mills P, Allan A. Cancer incidence in the Middle Eastern population of California 1988-2004. Asian Pac J Cancer Prev. 2007;8:405–411. [PMC free article] [PubMed] [Google Scholar]
- 10.Hanash K, Al-Othaimeen A, Kattan S, Lindstedt E, Al-Zahrani H. Prostatic carcinoma:a nutritional disease?Conflicting data from the Kingdom of Saudi Arabia. J Urol. 2000;164:1570–1572. [PubMed] [Google Scholar]
- 11.American Cancer Society. Cancer Facts and Figures 2019. Atlanta (GA): American Cancer Society; 2019. [Google Scholar]
- 12.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men:an important clinical entity. Nat Rev Urol. 2014;11:317–323. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2303–2310. doi: 10.1158/1055-9965.EPI-13-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC. Family history and the risk of prostate cancer. Prostate. 1990;17:337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 15.Stanford JL, Ostrander EA. Familial prostate cancer. Epidemiol Rev. 2001;23:19–23. doi: 10.1093/oxfordjournals.epirev.a000789. [DOI] [PubMed] [Google Scholar]
- 16.Matikaine MP, Pukkala E, Schleutker J, Tammela TL, Koivisto P, Sankila R, et al. Relatives of prostate cancer patients have an increased risk of prostate and stomach cancers:a population-based, cancer registry study in Finland. Cancer Causes Control. 2000;12:223–230. doi: 10.1023/a:1011283123610. [DOI] [PubMed] [Google Scholar]
- 17.Bratt O, Garmo H, Adolfsson J, Bill-Axelson A, Holmberg L, Lambe M, et al. Effects of prostate-specific antigen testing on familial prostate cancer risk estimates. J Natl Cancer Inst. 2010;102:1336–1343. doi: 10.1093/jnci/djq265. [DOI] [PubMed] [Google Scholar]
- 18.Jacqueline Mersch, Michelle A, Jackson, Minjeong Park, Denise Nebgen, Susan K. Peterson, Claire Singletary, et al. Cancer. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–275. doi: 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giri VN, Beebe-Dimmer JL. Familial prostate cancer. Seminoncol. 2016;43:560–565. doi: 10.1053/j.seminoncol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B:A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2019;37:490–503. doi: 10.1200/JCO.18.00358. [DOI] [PubMed] [Google Scholar]
- 22.Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localized prostate cancer. Eur Urol. 2015;68:186–193. doi: 10.1016/j.eururo.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Robinson D, Eliezer M, van Allen, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joaquin Mateo, Suzanne Carreira, Shahneen Sandhu, Susana Miranda, Helen Mossop, Raquel Perez-Lopez, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasmintan A, Schrader Donavan T. Cheng, Vijai Joseph, Meera Prasad, Michael Walsh, Ahmet Zehir, et al. Germline Variants in Targeted Tumor Sequencing Using Matched Normal DNA. JAMA Oncol. 2016;2:104–111. doi: 10.1001/jamaoncol.2015.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King M-C, Marks JH, Mandell JB New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 27.Schoenborn JR, Nelson P, Fang M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin Cancer Res. 2013;19:4058–4066. doi: 10.1158/1078-0432.CCR-12-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, et al. Pan-cancer patterns of somatic copy number alteration. Nat Gen. 2013;45:1134–1139. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasso CS, Wu YM, Robinson DR, Dhanasekaran SM, Khan AP, Quist MJ, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci USA. 2011;108:17087–17092. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisbal C, Silverman RH. Diverse functions of RNase L and implications in pathology. Biochimie. 2007;89:789–798. doi: 10.1016/j.biochi.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takaku H, Minagawa A, Takagi M, Nashimotoet M. A candidate prostate cancer susceptibility gene encodes tRNA 3 processing endoribonuclease. Nucleic Acids Res. 2003;31:2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beuten J, Gelfond JAL, Franke JL, Shook S, Teresa L, Johnson-Pais, Thompson Ian M, et al. Single and multivariate associations of MSR1, ELAC2, and RNase L with prostate cancer in an ethnic diverse cohort of men. Cancer Epidemiol Biomarkers Prev. 2010;19:588–599. doi: 10.1158/1055-9965.EPI-09-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, et al. Germ line mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risbridger G. P, Taylor RA, Clouston D, Sliwinski A, Thorne H, Hunter S, et al. Patient- derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol. 2015;67:496–503. doi: 10.1016/j.eururo.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Porter LH, Lawrence MG, Ilic D, Clouston D, Bolton DM, Frydenberg M, et al. Systematic review links the prevalence of intraductal carcinoma of the prostate to prostate cancer risk categories. Eur Urol. 2017;72:492–495. doi: 10.1016/j.eururo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RA, Fraser M, Rebello RJ, Boutros PC, Murphy DG, Bristow RG, et al. The influence of BRCA2 mutation on localized prostate cancer. Nat Rev Urol. 2019;16:281–229. doi: 10.1038/s41585-019-0164-8. [DOI] [PubMed] [Google Scholar]
- 38.Castro E, Mateo J, Olmos D, de Bono JS. Targeting DNA repair:The role of PARP inhibition in the treatment of castration resistant prostate cancer. Cancer J. 2016;22:353–356. doi: 10.1097/PPO.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 39.Armenia J, Wankowicz S.A.M, Liu D, Gao J, Kundra R, Reznik E, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50:645–651. doi: 10.1038/s41588-018-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narod SA, Neuhausen S, Vichodez G, Armel S, Lynch HT, Ghadirian P, et al. Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer. 2008;99:371–374. doi: 10.1038/sj.bjc.6604453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorne H, Willems A.J, Niedermayr E, Hoh I.M, Li J, Clouston D, et al. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res. 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 43.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with a higher risk of nodal involvement, distant metastasis and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H.H, Pritchard C.C, Boyd T, Nelson P.S, Montgomery B. Biallelic Inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zafeiriou Z, Bianchini D, Chandler R, Rescigno P, Yuan W, Carreira S, et al. Genomic Analysis of Three Metastatic Prostate Cancer Patients with Exceptional Responses to Carboplatin Indicating Different Types of DNA Repair Deficiency. Eur Urol. 2019;75:184–192. doi: 10.1016/j.eururo.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomerantz MM, Spisak S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–3539. doi: 10.1002/cncr.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nombela P, Lozano R, Aytes A, Mateo J, Olmos D, Castro E. BRCA2 and Other DDR Genes in Prostate Cancer. Cancers. 2019;11:352. doi: 10.3390/cancers11030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bancroft E.K, Page E.C, Castro E, Lilja H, Vickers A, Sjoberg D, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers:Results from the initial screening round of the IMPACT study. Eur Urol. 2014;66:489–499. doi: 10.1016/j.eururo.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasivisvanathan V, Rannikko A.S, Borghi M, Panebianco V, Mynderse L.A, Vaarala M.H, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378:1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen F.C, van Overeem Hansen T, Sorensen C.S. Hereditary breast and ovarian cancer:New genes in confined pathways. Nat Rev Cancer. 2016;16:599–612. doi: 10.1038/nrc.2016.72. [DOI] [PubMed] [Google Scholar]
- 52.Zhen JT, Syed J, Nguyen KA, Leapman MS, Agarwal N, Brierley K, et al. Genetic testing for hereditary prostate cancer:Current status and limitations. Cancer. 2018;124:3105–3117. doi: 10.1002/cncr.31316. [DOI] [PubMed] [Google Scholar]
- 53.Hart SN, Ellingson MS, Schaal K, Vedell PT, Carlson RE, Sinnwell JP, et al. Determining the frequency of pathogenic germline variants from exome sequencing in patients with castrate-resistant prostate cancer. BMJ Open. 2016;6:e010332. doi: 10.1136/bmjopen-2015-010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Na R, Zheng SL, Han M, Yu H, Jiang D, Shah S, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol. 2017;71:740–747. doi: 10.1016/j.eururo.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beltran H, Eng K, Mosquera JM, Sigaras A, Romanel A, Rennert H, et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015;1:466–474. doi: 10.1001/jamaoncol.2015.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng HH, Pritchard CC, Boyd T, Nelson PS. Montgomery Bet al:Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giri VN, Obeid E, Gross L, Bealin L, Hyatt C, Hegarty SE, et al. Inherited mutations in men undergoing multigene panel testing for prostate cancer:emerging implications for personalized prostate cancer genetic evaluation. J Precis Oncol JCO Precision Oncology. 2017;1:1–17. doi: 10.1200/PO.16.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kote-Jarai Z, Leongamornlert D, Saunders E, Tymrakiewicz M, Castro E, Mahmud N, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer:implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American Society of Clinical Oncology policy statement update:genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 60.Bancroft EK, Saya S, Page EC, Myhill K, Thomas S, Pope J, et al. Psychosocial impact of undergoing prostate cancer screening for men with BRCA1 or BRCA2 mutations. BJU Int. 2019;123:284–292. doi: 10.1111/bju.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 62.Von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto;GBG 66):A randomized phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 63.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood A.K, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hager S, Ackermann C.J, Joerger M, Gillessen S, Omlin A. Anti-tumor activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol. 2016;27:975–984. doi: 10.1093/annonc/mdw156. [DOI] [PubMed] [Google Scholar]
- 65.Pomerantz MM, Spisak S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–3539. doi: 10.1002/cncr.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morales J, Li L, Fattah FJ, Dong Y, Bey EA, Patel M, et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24:15–28. doi: 10.1615/critreveukaryotgeneexpr.2013006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy:Lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–470. doi: 10.1146/annurev-med-050913-022545. [DOI] [PubMed] [Google Scholar]
- 68.Sonpavde G, Di Lorenzo G, Higano CS, Kantoff PW, Madan R, Shore ND. The role of sipuleucel-T in therapy for castration-resistant prostate cancer:a critical analysis of the literature. Eur Urol. 2012;61:639–634. doi: 10.1016/j.eururo.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 69.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of phase II randomized controlled trial of Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorne H, Willems AJ, Niedermayr E, Hoh IM, Li J, Clouston D, et al. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res. 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 71.Caffo O, Veccia A, Kinspergher S, Rizzo M, Maines F. Aberrations of DNA Repair Pathways in Prostate Cancer:Future Implications for Clinical Practice? Front Cell Dev Biol. 2018;5:61. doi: 10.3389/fcell.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akbari MR, Wallis CJ, Toi A, Trachtenberg J, Sun P, Narod SA, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br J Cancer. 2014;111:1238–1240. doi: 10.1038/bjc.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, Osin P, et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72:1–12. doi: 10.1086/345310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirchhoff T, Kauff ND, Mitra N, Nafa K, Huang H, Palmer C, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin Cancer Res. 2004;10:2918–2921. doi: 10.1158/1078-0432.ccr-03-0604. [DOI] [PubMed] [Google Scholar]
- 75.Sinclair CS, Berry R, Schaid D, Thibodeau SN, Couch FJ. BRCA1 and BRCA2 have a limited role in familial prostate cancer. Cancer Res. 2000;60:1371–1375. [PubMed] [Google Scholar]
- 76.Maier C, Herkommer K, Luedeke M, Rinckleb A, Schrader M, Vogel W. Subgroups of familial and aggressive prostate cancer with considerable frequencies of BRCA2 mutations. Prostate. 2014;74:1444–1451. doi: 10.1002/pros.22860. [DOI] [PubMed] [Google Scholar]
- 77.Ikonen T, Matikainen MP, Syrjäkoski K, Mononen N, Koivisto PA, Rökman A, et al. BRCA1 and BRCA2 mutations have no major role in predisposition to prostate cancer in Finland. J Med Genet. 2003;40:e98. doi: 10.1136/jmg.40.8.e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manguoğlu E, Güran S, Yamaç D, Colak T, Simşek M, Baykara M, et al. Germline mutations of BRCA1 and BRCA2 genes in Turkish breast, ovarian, and prostate cancer patients. Cancer Genet Cytogenet. 2010;203:230–237. doi: 10.1016/j.cancergencyto.2010.07.125. [DOI] [PubMed] [Google Scholar]
- 79.Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, Jonasson JG, Olafsdóttir EJ, Olafsdóttir GH. Prostate cancer progression, and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 80.Agalliu I, Gern R, Leanza S, Burk RD. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res. 2009;15:1112–1120. doi: 10.1158/1078-0432.CCR-08-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 82.Artibani W, Porcaro AB, De Marco V, Cerruto MA, Siracusano S. Management of biochemical recurrence after primary curative treatment for prostate cancer:a review. Urol Int. 2018;100:251–262. doi: 10.1159/000481438. [DOI] [PubMed] [Google Scholar]
- 83.Castro E, Jugurnauth-Little S, Karlsson Q, Al-Shahrour F, Piñeiro-Yañez E, Van de Poll SF, et al. High burden of copy number alterations and c- MYC amplification in prostate cancer from BRCA2 germline mutation carriers. Ann Oncol. 2015;26:2293–2300. doi: 10.1093/annonc/mdv356. [DOI] [PubMed] [Google Scholar]
- 84.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, et al. Genomic hallmarks of localized, non- indolent prostate cancer. Nature. 2017;541:359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]


